Milk powder high-protein effervescent tablet and preparation method thereof
A high-protein, effervescent tablet technology, applied in dairy products, milk formulations, applications, etc., can solve the problem of unacceptable to consumers, unstable system, no evaluation of effervescent tablet preparation, disintegration state and product stability, etc. problems, to avoid the phenomenon of foam and sticking, the product shape is stable, and the disintegration time is shortened.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of milk powder high-protein effervescent tablet, comprising the following steps:
[0029] (1) After mixing 8g of skimmed milk powder, 2g of sodium bicarbonate, 2g of milk protein powder, 0.1g of monoglyceride, 0.1g of sucrose fatty acid ester, 0.1g of polyglycerol fatty acid ester, and 0.5g of maltodextrin, Granulate and dry under the effect of binder PVP 0.8g, make alkaline granule;
[0030] (2) Mix 3 g of citric acid, 0.2 g of disodium hydrogen phosphate and 0.8 g of binder PVP, then granulate, and dry to obtain acid source granules;
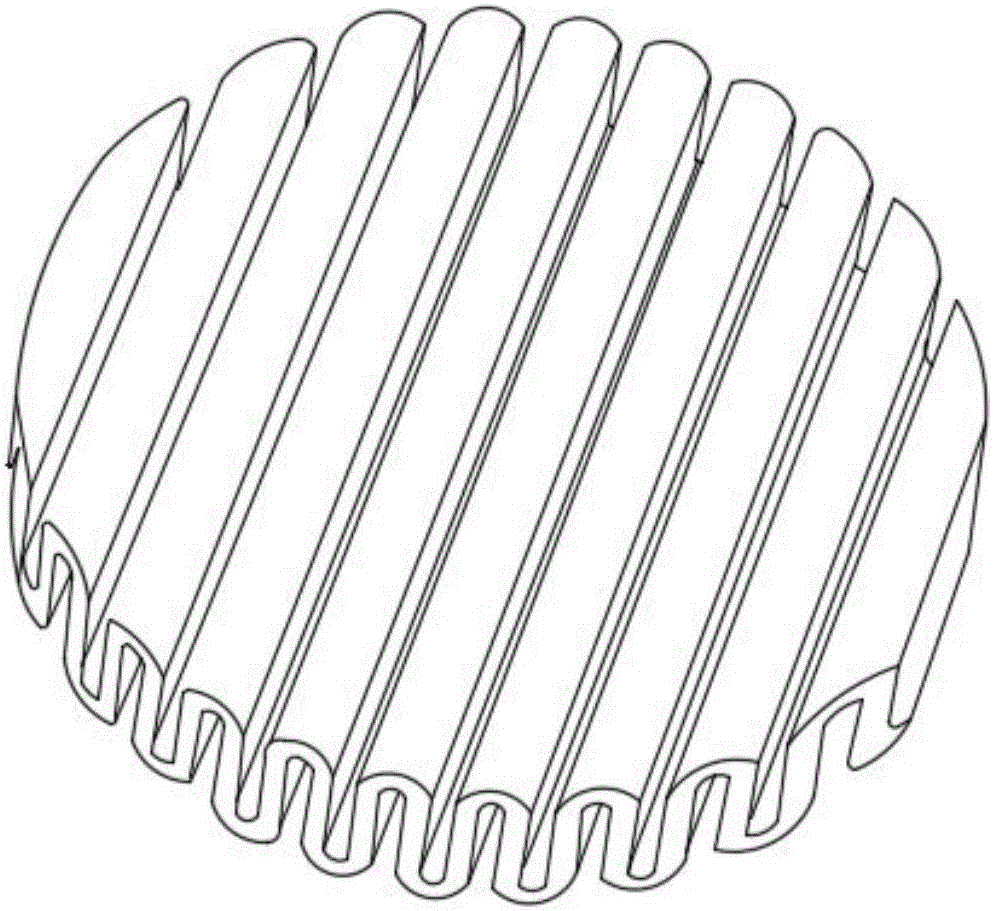
[0031] (3) After uniformly mixing the alkali source granules and the acid source granules, they are mixed with 1.5 g of polyethylene glycol PFG6000 and pressed into curved and zigzag tablets to obtain milk powder high protein effervescent tablets. The tablet has a thickness of 2 mm; a diameter of 80 mm and a height of 10 mm.
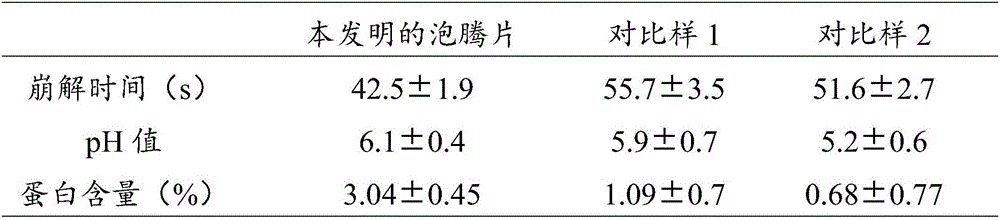
[0032] The obtained tablet is dissolved in 100ml of water, and after disintegrating for ...
Embodiment 2
[0045] A preparation method of milk powder high-protein effervescent tablet, comprising the following steps:
[0046] (1) After mixing 10g of skimmed horse milk powder, 1g of sodium bicarbonate, 1g of milk protein powder, 0.05g of monoglyceride, 0.05g of sucrose fatty acid ester, 0.05g of polyglycerol fatty acid ester, and 1g of maltodextrin, Under the action of binder PVP 1g, granulate and dry to obtain alkaline granules;
[0047] (2) Mix 2 g of citric acid, 0.5 g of disodium hydrogen phosphate and 1 g of binder PVP, then granulate, and dry to obtain acid source granules;
[0048] (3) After uniformly mixing the alkali source granules and the acid source granules, they are mixed with 1.5 g of polyethylene glycol PFG6000 and pressed into curved and zigzag tablets to obtain milk powder high protein effervescent tablets. The tablet has a thickness of 1 mm; a diameter of 50 mm and a height of 5 mm. The tablet is dissolved in 100ml of water, and after a certain time of disintegra...
Embodiment 3
[0051] A kind of preparation method of milk powder high-protein effervescent tablet comprises the following steps:
[0052] (1) After mixing 6g of skimmed camel milk powder, 3g of sodium bicarbonate, 3g of milk protein powder, 0.2g of monoglyceride, 0.25g of sucrose fatty acid ester, 0.25g of polyglycerol fatty acid ester, and 0.1g of maltodextrin, Granulate and dry under the effect of binder PVP 0.5g, make alkaline granule;
[0053] (2) 5 g of tartaric acid, 0.1 g of disodium hydrogen phosphate and 0.5 g of binder PVP are mixed and granulated, and dried to obtain acid source granules;
[0054] (3) After uniformly mixing the alkali source granules and the acid source granules, mix them with 1 g of polyethylene glycol PFG4000 and press them into arc-shaped jagged tablets to obtain milk powder high-protein effervescent tablets. The tablet has a thickness of 5 mm; a diameter of 100 mm and a height of 15 mm. The tablet is dissolved in 200ml of water, and after disintegrating for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com