Tenofovir disoproxil fumarate tablets
A technology of tenofovir fumarate and disoproxil, applied in the field of pharmaceutical preparations, can solve the problems of low resistance of HIV-infected patients, very sensitive to external stimuli, unstable tablet quality, etc., to ensure product stability , the effect of improving disintegration time, good compressibility and capillary properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
[0017] Filling agent is lactose 110g, mannitol 100g and microcrystalline cellulose 94g.
[0018] The weight ratio composition of the coating agent is: lactose monohydrate 40%, hypromellose 29%, titanium dioxide 22.9% and glycerol acetate 8.1%.
Embodiment 2
[0020]
[0021] The filler is 120g of lactose, 80g of mannitol and 100g of microcrystalline cellulose.
[0022] The weight ratio of the coating agent is composed of: 35% lactose monohydrate, 30% hypromellose, 25% titanium dioxide and 10% glycerol acetate.
Embodiment 3
[0024]
[0025] The fillers are lactose 90g, mannitol 120g and microcrystalline cellulose 140g.
[0026] The weight ratio composition of the coating agent is: 45% of lactose monohydrate, 25% of hypromellose, 20% of titanium dioxide and 10% of glycerol acetate.
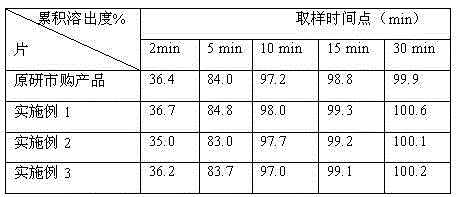
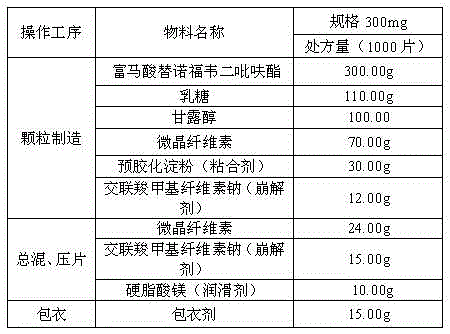
[0027] Examples 1-3 The following methods are adopted during the preparation of tenofovir disoproxil fumarate tablets:
[0028] 1) Weigh each raw material according to the prescription amount for later use;
[0029] 2) Particle manufacturing Mix tenofovir disoproxil fumarate, lactose, mannitol, 40-80% by weight of microcrystalline cellulose, binder, and 30-60% by weight of disintegrant in a CH-150 tank Mix evenly in the machine, then add purified water to make soft material, granulate with a sieve, dry and sieve for granulation;
[0030] 3) Total blending and tableting Add the remaining microcrystalline cellulose, remaining disintegrant and lubricant to the sized dry granules, mix them evenly in a SYH-200 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com