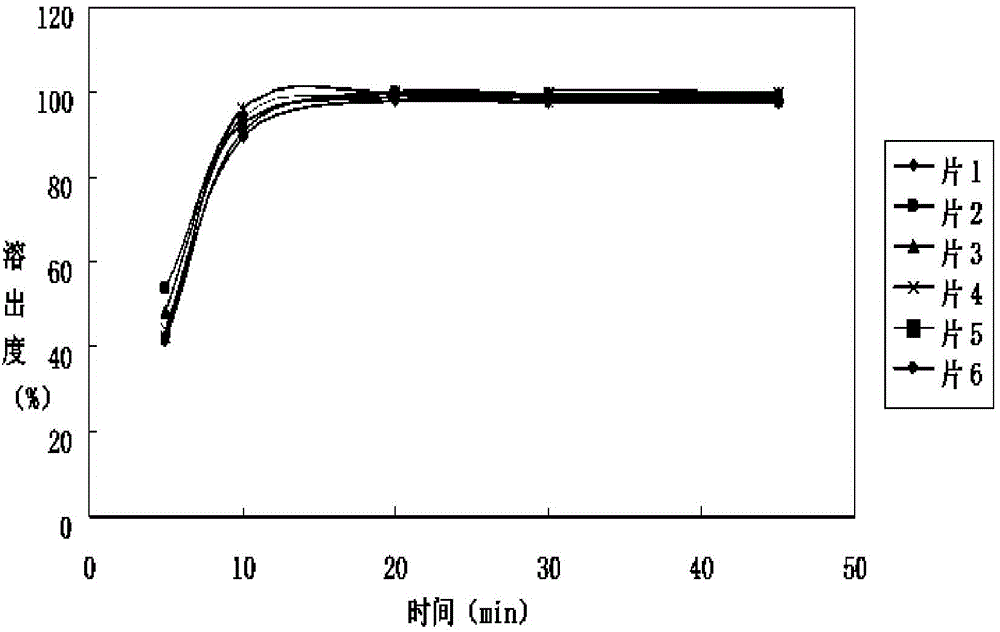
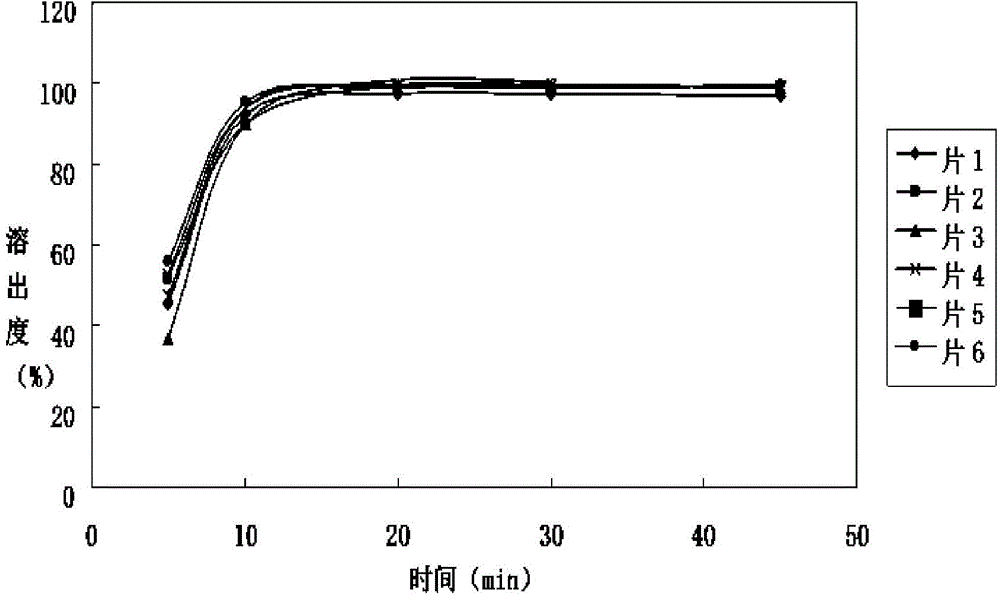
Irbesartan tablet and preparation method thereof
A technology for irbesartan tablets and irbesartan, applied in the field of medicine, can solve the problems of low bioavailability of human body, poor particle fluidity, unsatisfactory drug disintegration, etc., and reduce the types and use of excipients The effect of increasing the disintegration rate, increasing the disintegration rate and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Embodiment 1: the preparation of irbesartan tablet
[0116] Irbesartan tablets are made from the following raw materials in parts by mass:
[0117]
[0118] The preparation method is as follows:
[0119] (1) Irbesartan, sucrose, and montmorillonite are pulverized; wherein irbesartan is passed through a 90-mesh sieve for subsequent use; sucrose is passed through a 120-mesh sieve for subsequent use; montmorillonite is passed through a 350-mesh sieve for subsequent use;
[0120] (2) adding 95% (w / w) medicinal ethanol to purified water to prepare 75-80% (w / w) ethanol aqueous solution, and set aside;
[0121] (3) Take irbesartan, sucrose, and montmorillonite according to the proportioning ratio, mix well, then add the 75-80% (w / w) ethanol aqueous solution prepared in step (2), stir and mix evenly to make a soft material , and sieved to obtain wet granules;
[0122] (4) Dry the wet granules prepared in step (3) at 50-52° C. for 1.5 hours, detect the moisture content of ...
Embodiment 2
[0126] The preparation of embodiment 2 irbesartan tablets:
[0127] Irbesartan tablets are made from the following raw materials in parts by mass:
[0128]
[0129]
[0130] The preparation method is as follows:
[0131] (1) Irbesartan, sucrose, and montmorillonite are pulverized; wherein irbesartan is passed through a 80-mesh sieve for subsequent use; sucrose is passed through a 110-mesh sieve for subsequent use; montmorillonite is passed through a 400-mesh sieve for subsequent use;
[0132] (2) adding 95% (w / w) medicinal ethanol to purified water to prepare 75-80% (w / w) ethanol aqueous solution, and set aside;
[0133] (3) Take irbesartan, sucrose, and montmorillonite according to the proportioning ratio, mix well, then add the 75-80% (w / w) ethanol aqueous solution prepared in step (2), stir and mix evenly to make a soft material , and sieved to obtain wet granules;
[0134] (4) Dry the wet granules prepared in step (3) at 53-55° C. for 1 hour, detect that the moistur...
Embodiment 3
[0138] The preparation of embodiment 3 irbesartan tablets:
[0139] Irbesartan tablets are made from the following raw materials in parts by mass:
[0140]
[0141] The preparation method is as follows:
[0142] (1) Irbesartan, sucrose, and montmorillonite are pulverized; wherein irbesartan is passed through a 80-mesh sieve for subsequent use; sucrose is passed through a 130-mesh sieve for subsequent use; montmorillonite is passed through a 370-mesh sieve for subsequent use;
[0143] (2) adding 95% (w / w) medicinal ethanol to purified water to prepare 75-80% (w / w) ethanol aqueous solution, and set aside;
[0144] (3) Take irbesartan, sucrose, and montmorillonite according to the proportioning ratio, mix well, then add the 75-80% (w / w) ethanol aqueous solution prepared in step (2), stir and mix evenly to make a soft material , and sieved to obtain wet granules;
[0145] (4) Dry the wet granules prepared in step (3) at 52-54° C. for 1.2 hours, detect the moisture content of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com