Sustained-release type orally disintegrating film with excellant oral mucoadhesion and the manufacturing method thereof
A technology of oral disintegration and oral mucosa, which is applied in the directions of medical preparations with non-active ingredients, medical preparations containing active ingredients, and oral care, etc. The effect of increasing the disintegration time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] [Step 1: Prepare Materials]
[0139] In addition to the material used in Comparative Example 1, chitosan was used as a positively charged product that was deacetylated with sodium hydroxide in commercially available products, more specifically, as Glycol chitosan (Glycol chitosan, GCS) purchased products from VITATRA (USA).
[0140] [Step 2: Adjust the mixing ratio]
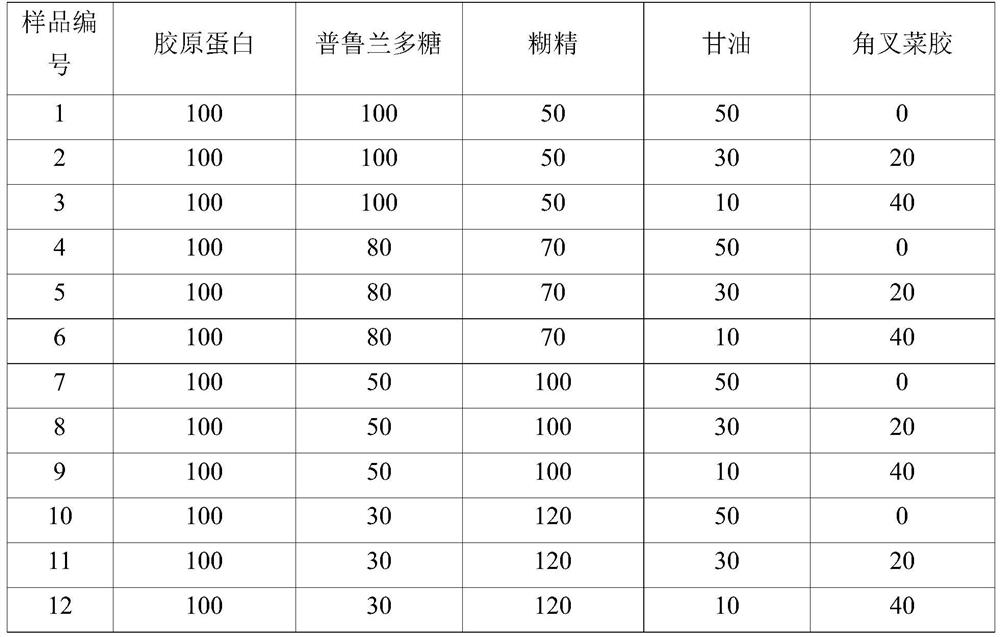
[0141] Using the prepared material, based on pure collagen content 100 mg, the basic composition ratio of the main components affecting the physical properties of a sample product containing chitosan, a film with a final weight of 300 mg, was investigated.
[0142] In Example 1, the same material composition ratio as Comparative Example 1 was used, but the physical property change during drying was first confirmed under the conditions of [Table 6] to find the optimum mixing ratio according to the content of chitosan.
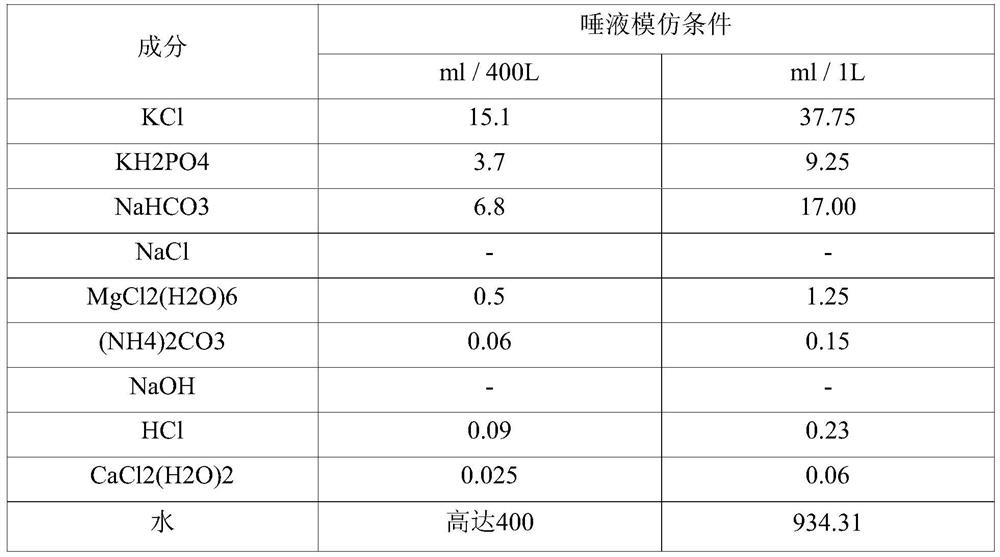
[0143] 【Table 6】
[0144] [Unit: 300mg]
[0145]
[0146] It was shown that in th...
experiment example
[0158] [Sustained release (disintegration) measurement]
[0159] Based on the compositions determined by the above-mentioned Comparative Example 1 and Example 1, 6 samples of Comparative Example and 6 samples of Example were prepared, respectively, and then three samples each of Comparative Example 1 and Example 1 were thinly coated with B Base cellulose (Ethylcellulose), so as to compare the difference in disintegration speed.
[0160] As a matter of course, all samples were prepared in the same process sequence as previously prepared, with the only difference being whether the ethylcellulose was spray coated or not.
[0161] Dissolve ethyl cellulose in purified water at a ratio of 10, put it into a small spray sprayer, spray three times at a distance of about 30 cm from the petri dish, and then completely dry it in a convection oven at 50°C for 30 minutes, so that the final Prepare samples for disintegration testing.
[0162] The device for the disintegration test is confi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com