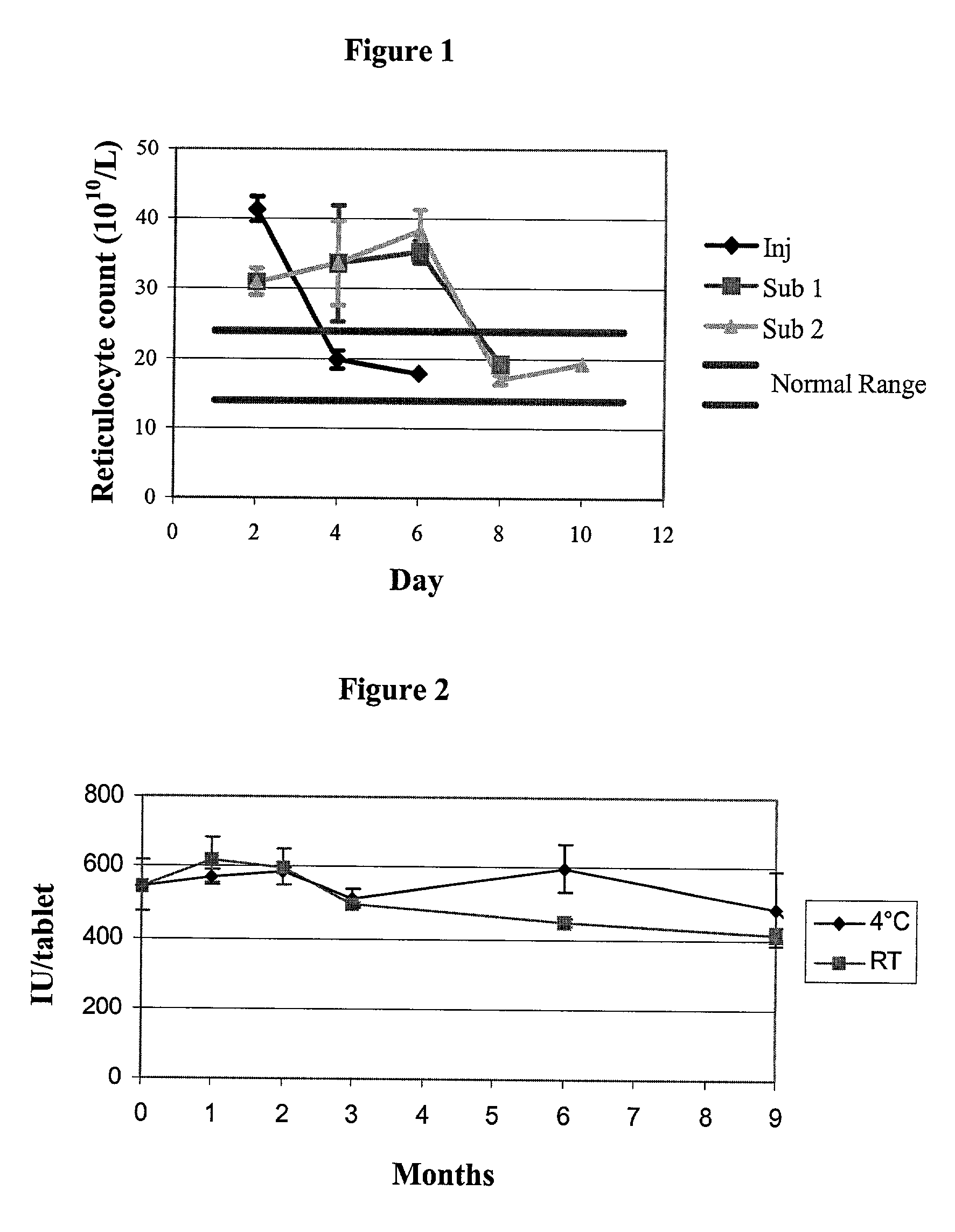
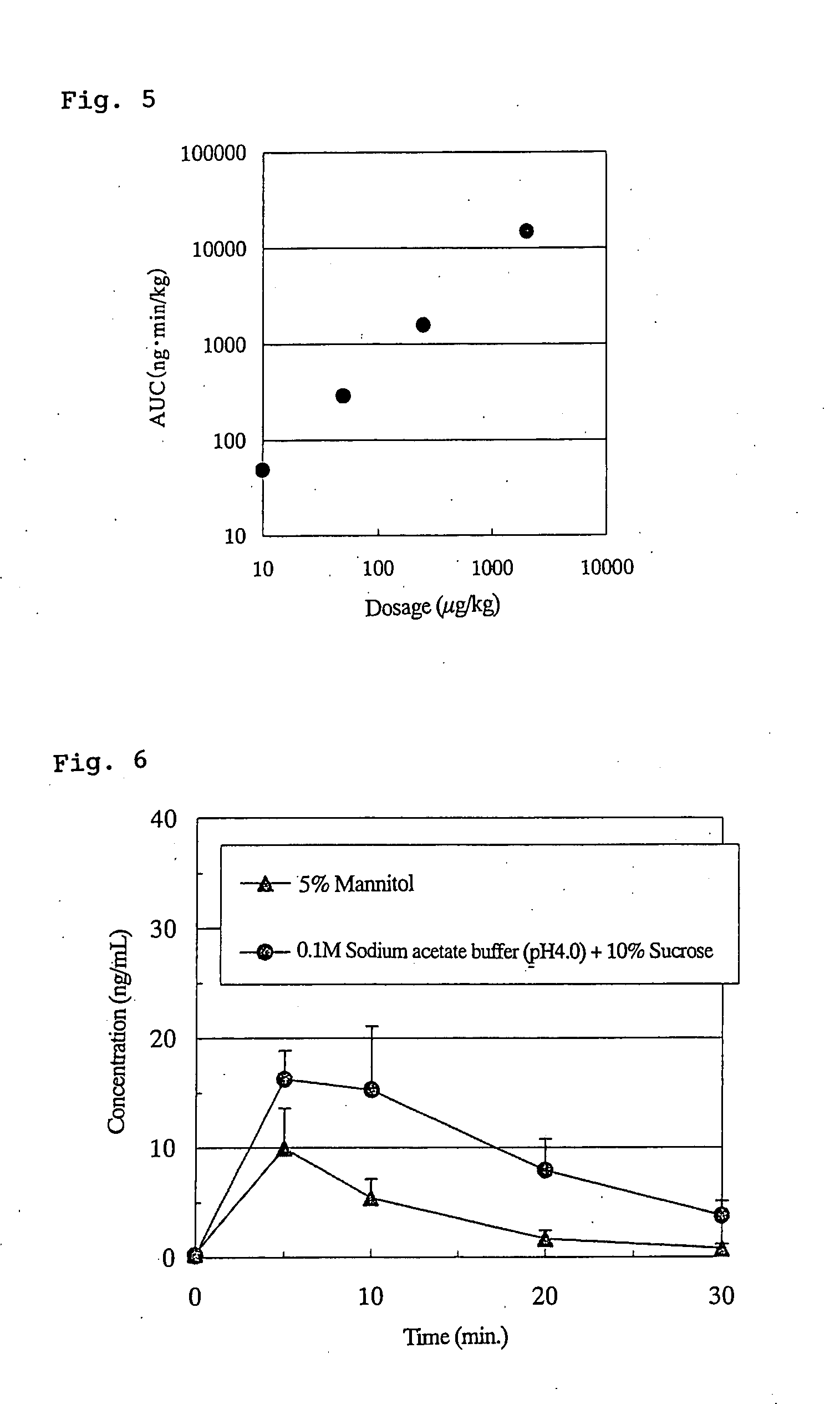
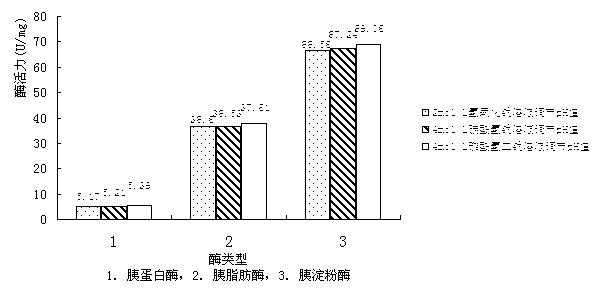
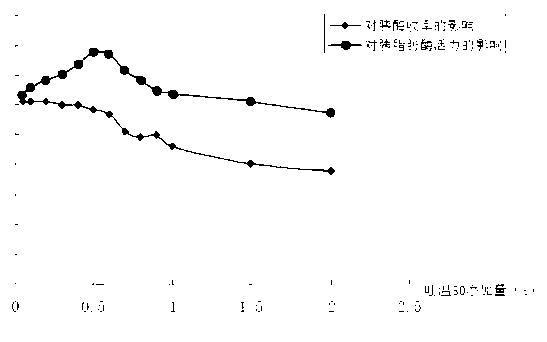
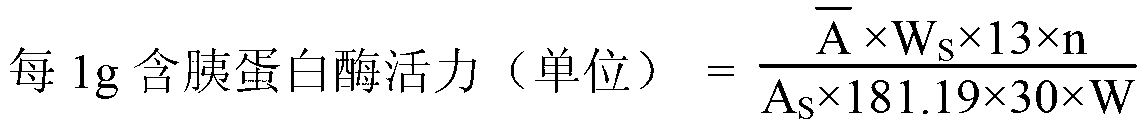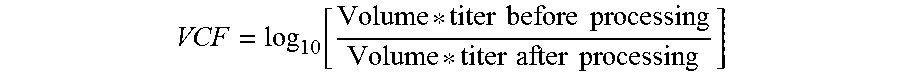Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89results about "Pancreatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
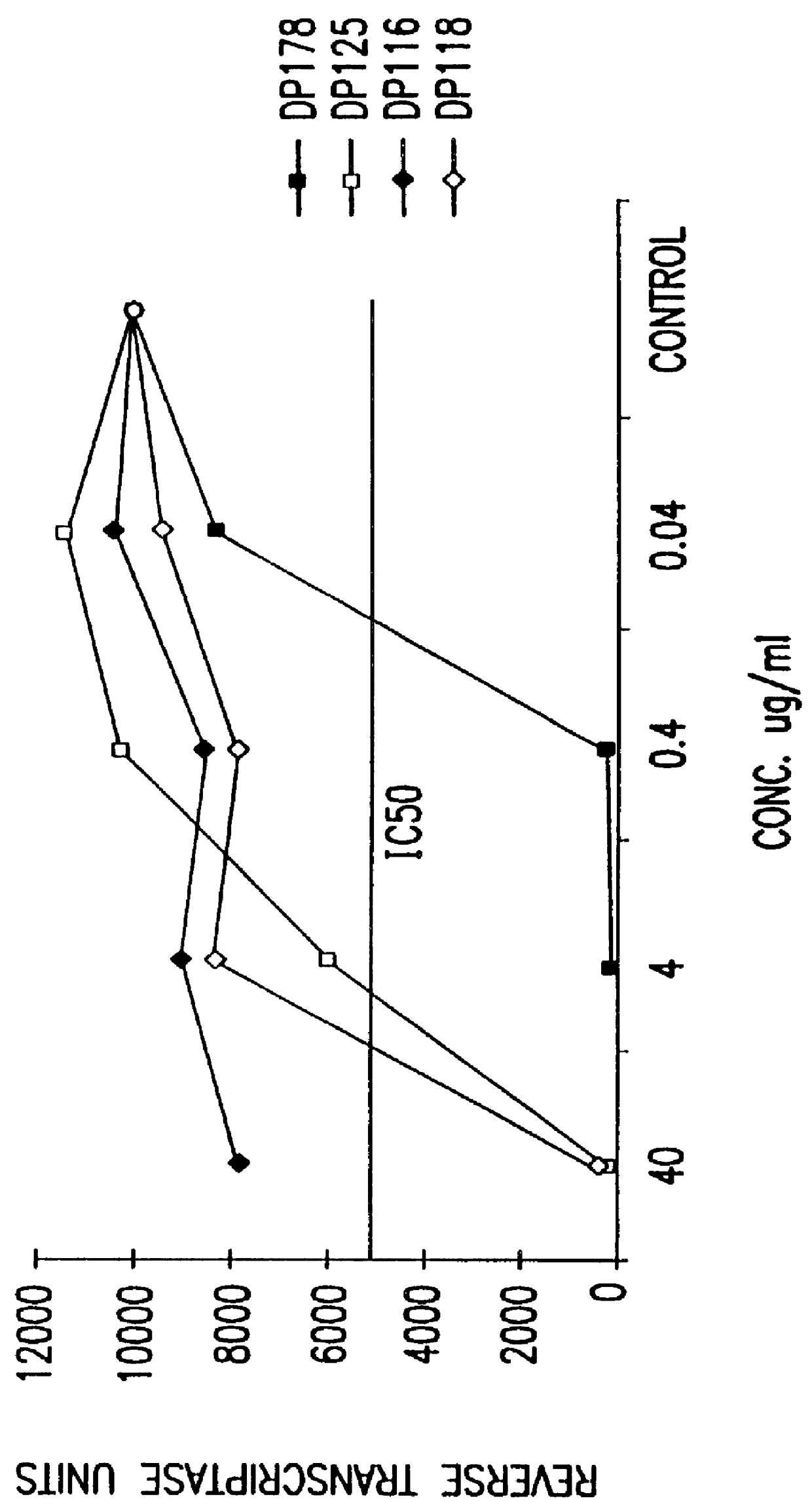
Measles virus peptides with antifusogenic and antiviral activities
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:TRIMERIS
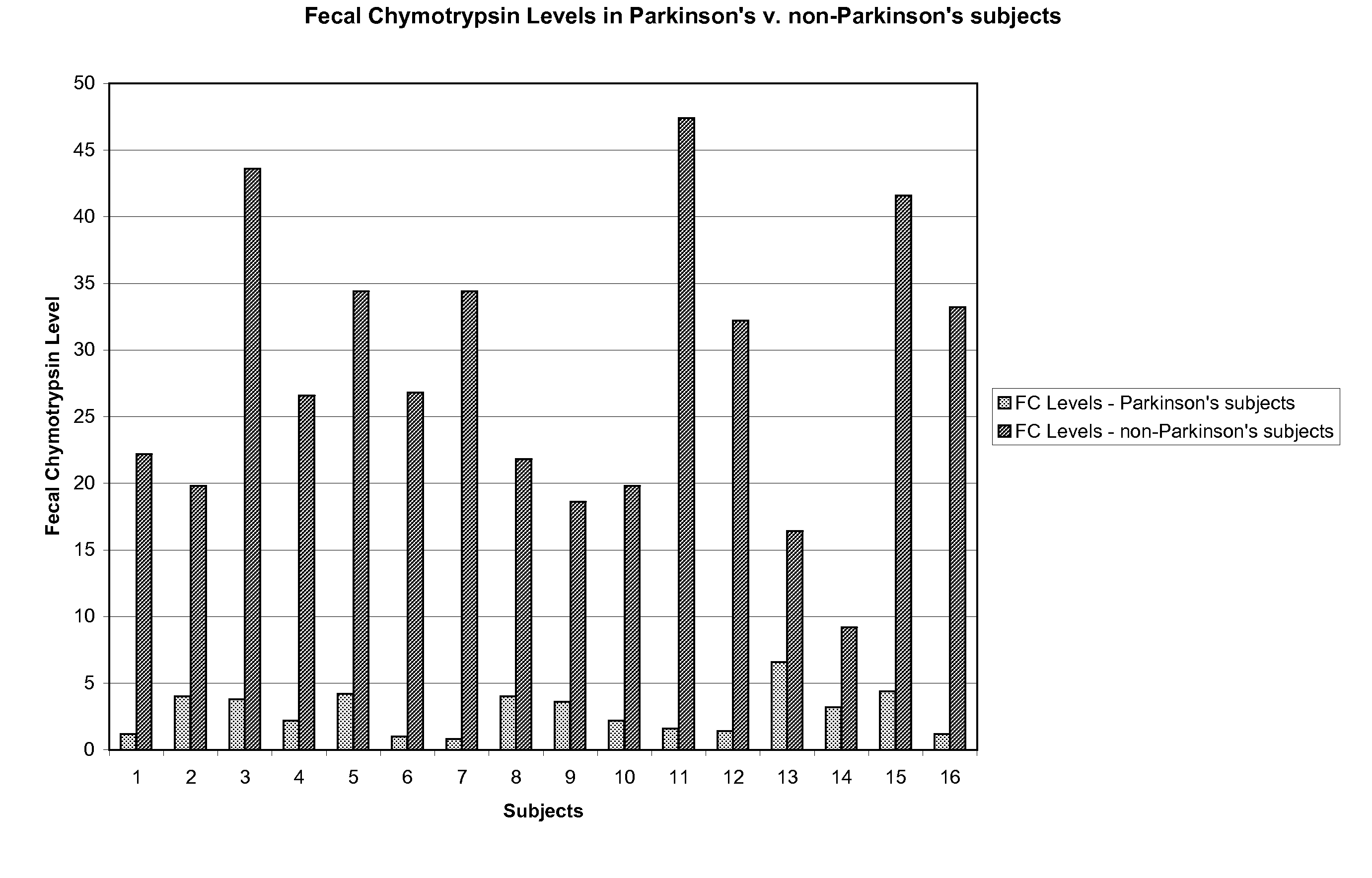
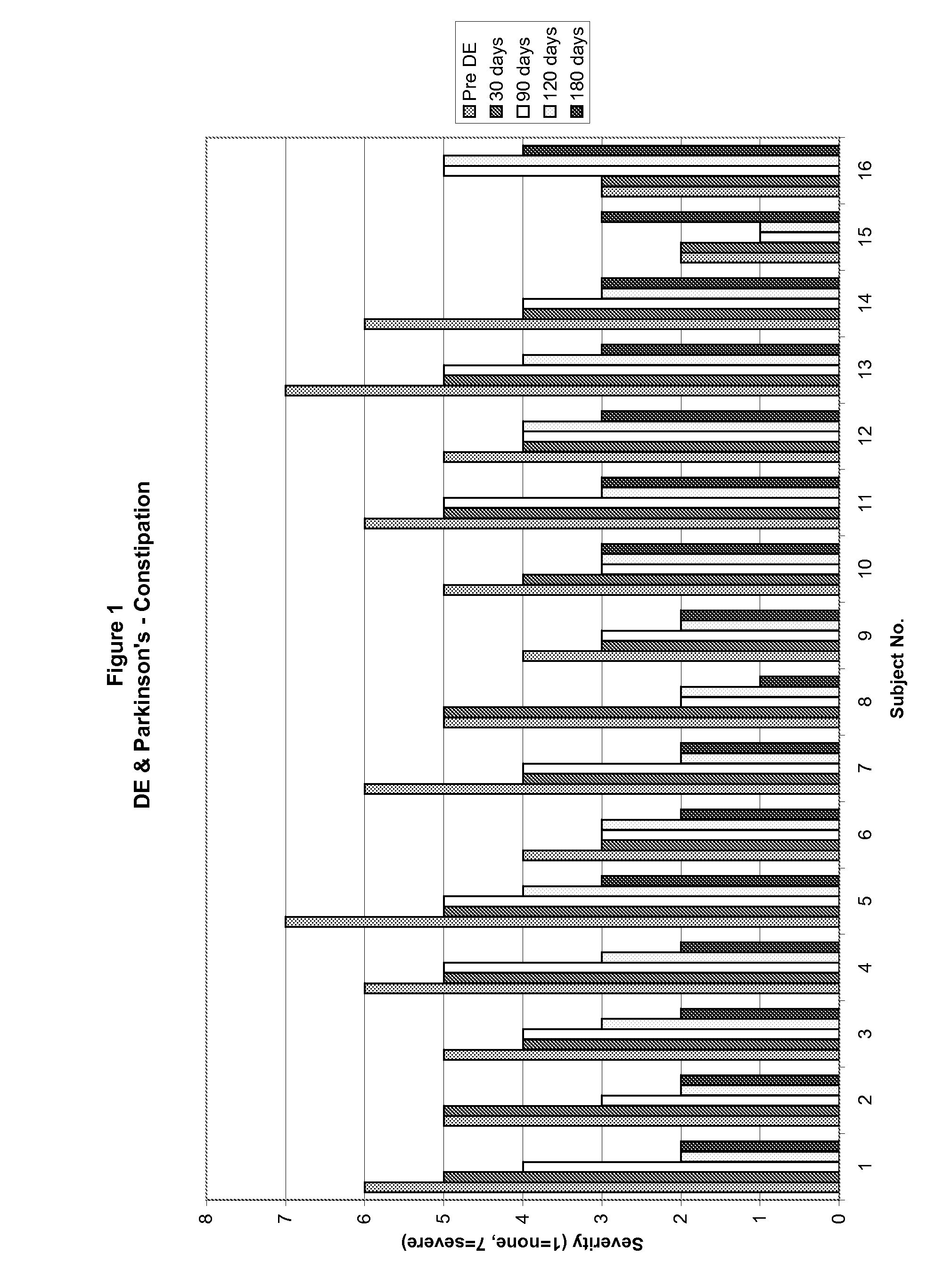
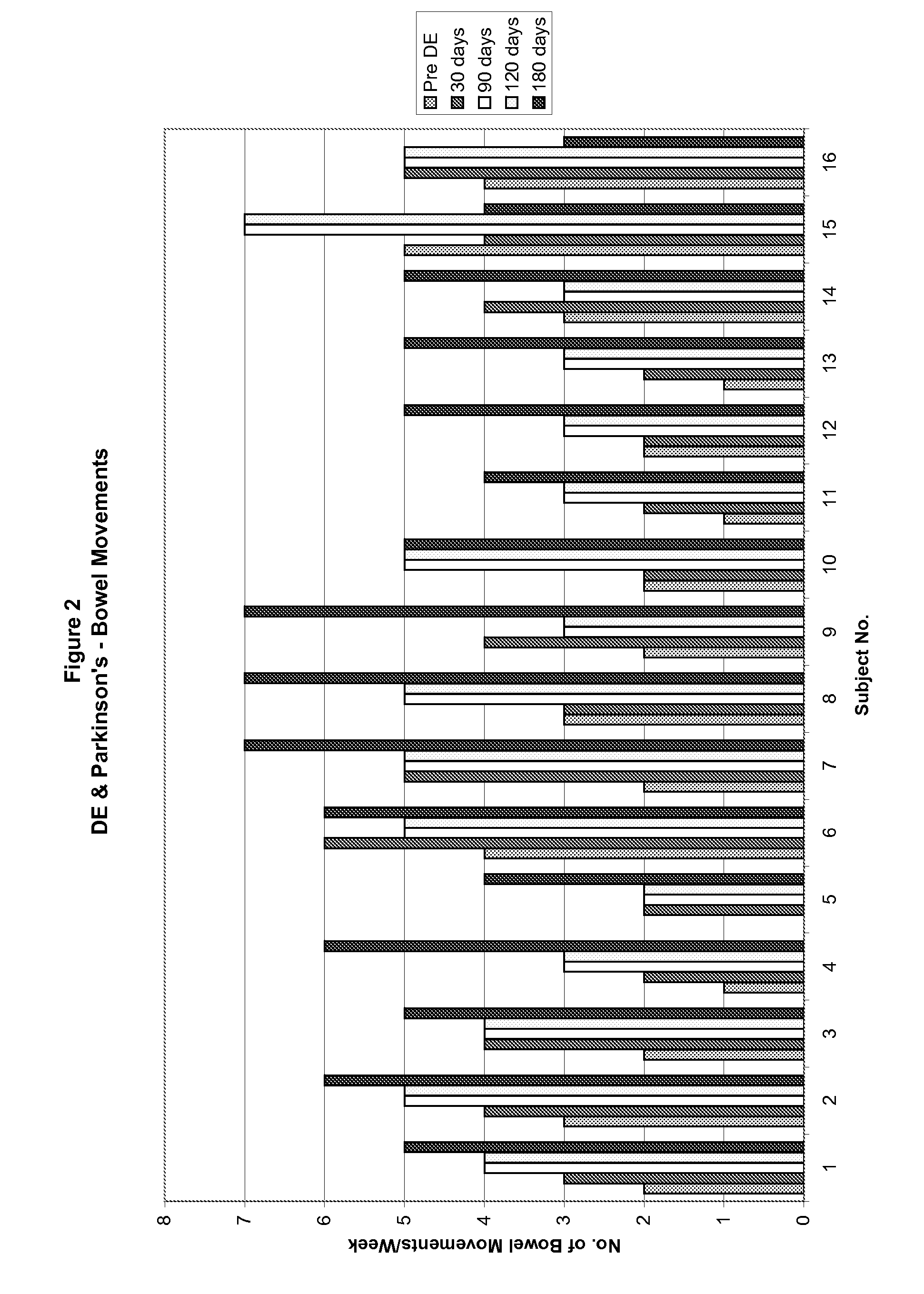
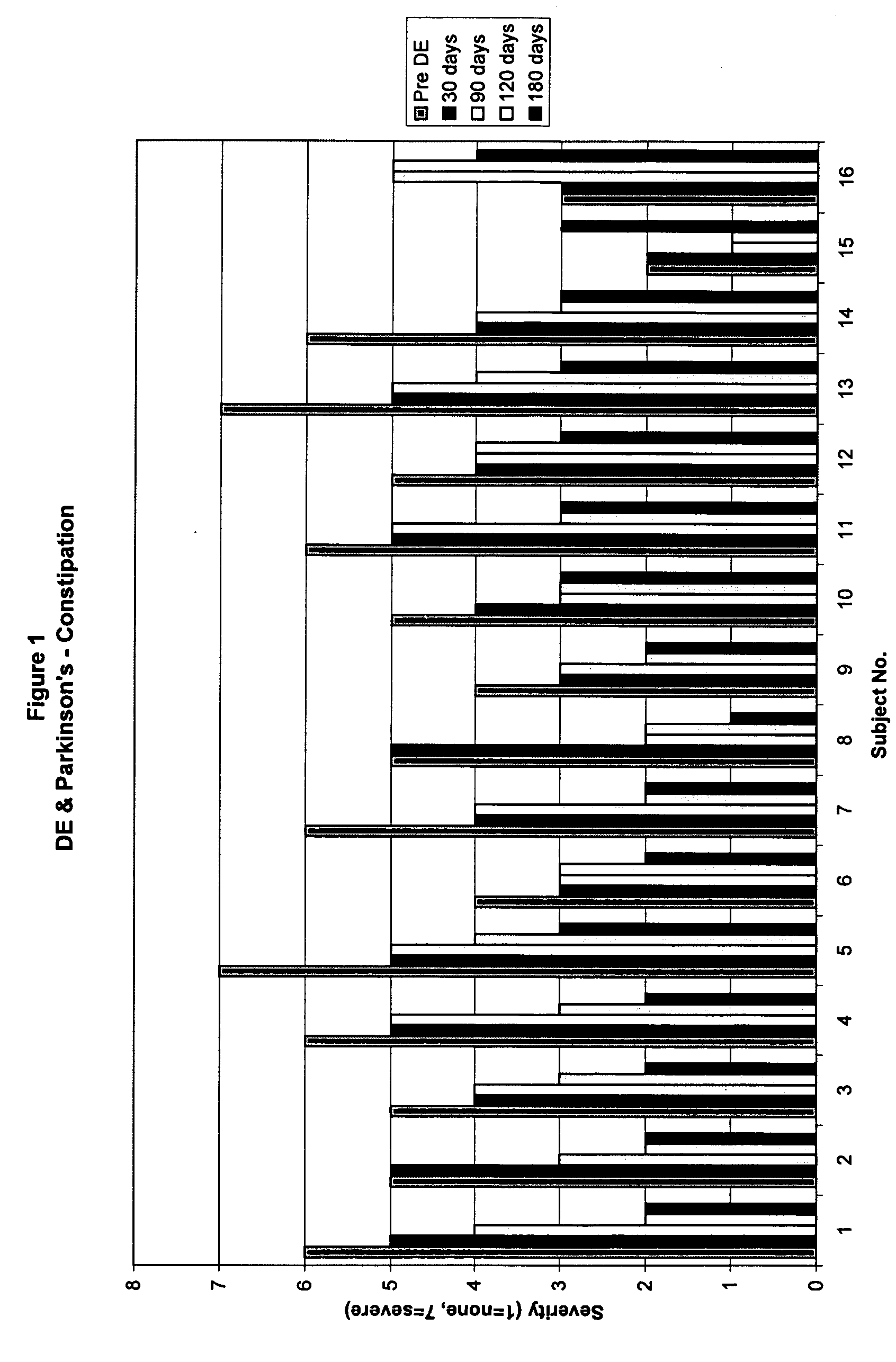
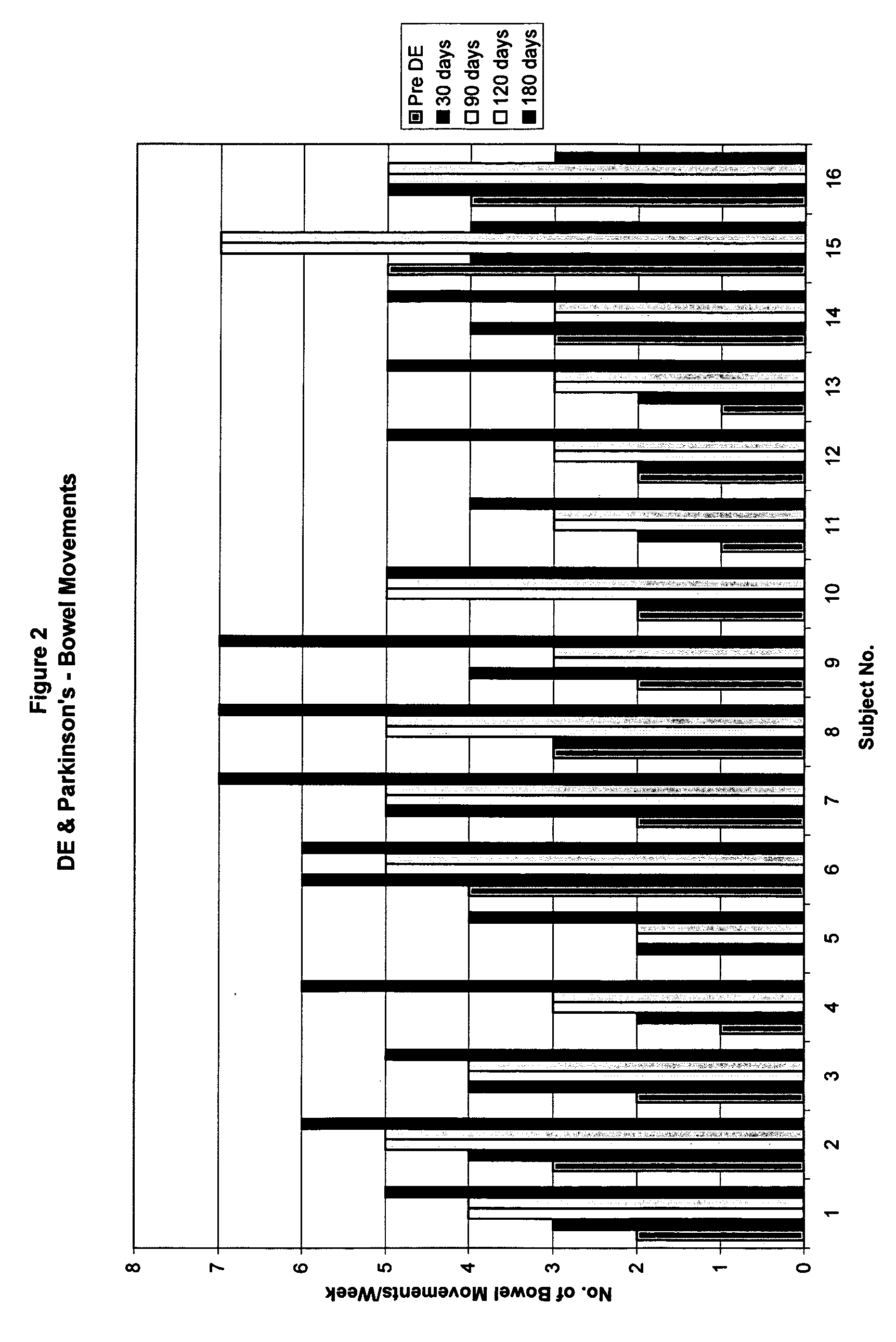
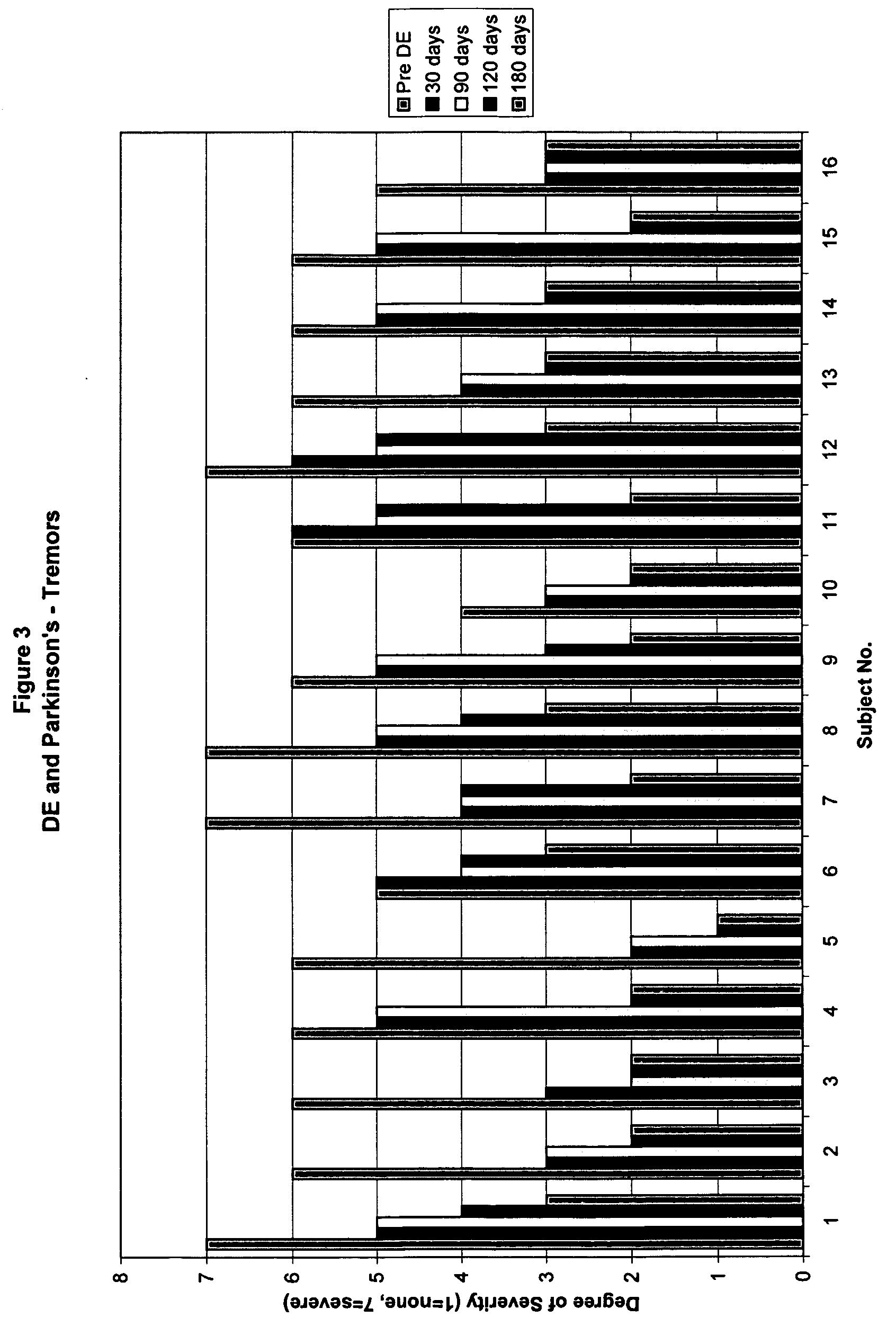
Method of treating and diagnosing parkinsons disease and related dysautonomic disorders
InactiveUS20070053895A1Symptoms improvedNervous disorderPeptide/protein ingredientsAutonomic bladder dysfunctionPsychiatry
A method for treating a Parkinson's patient with digestive / pancreatic enzymes involves administering an effective amount of digestive / pancreatic enzymes to an individual having the disorder in order to improve a symptom of the disorder. In addition, a method is provided for determining whether an individual has, or may develop, Parkinson's disease or related dysautonomic disorders and for determining whether an individual will benefit from the administration of pancreatic / digestive enzymes to treat the dysautonomic disorder.
Owner:CUREMARK
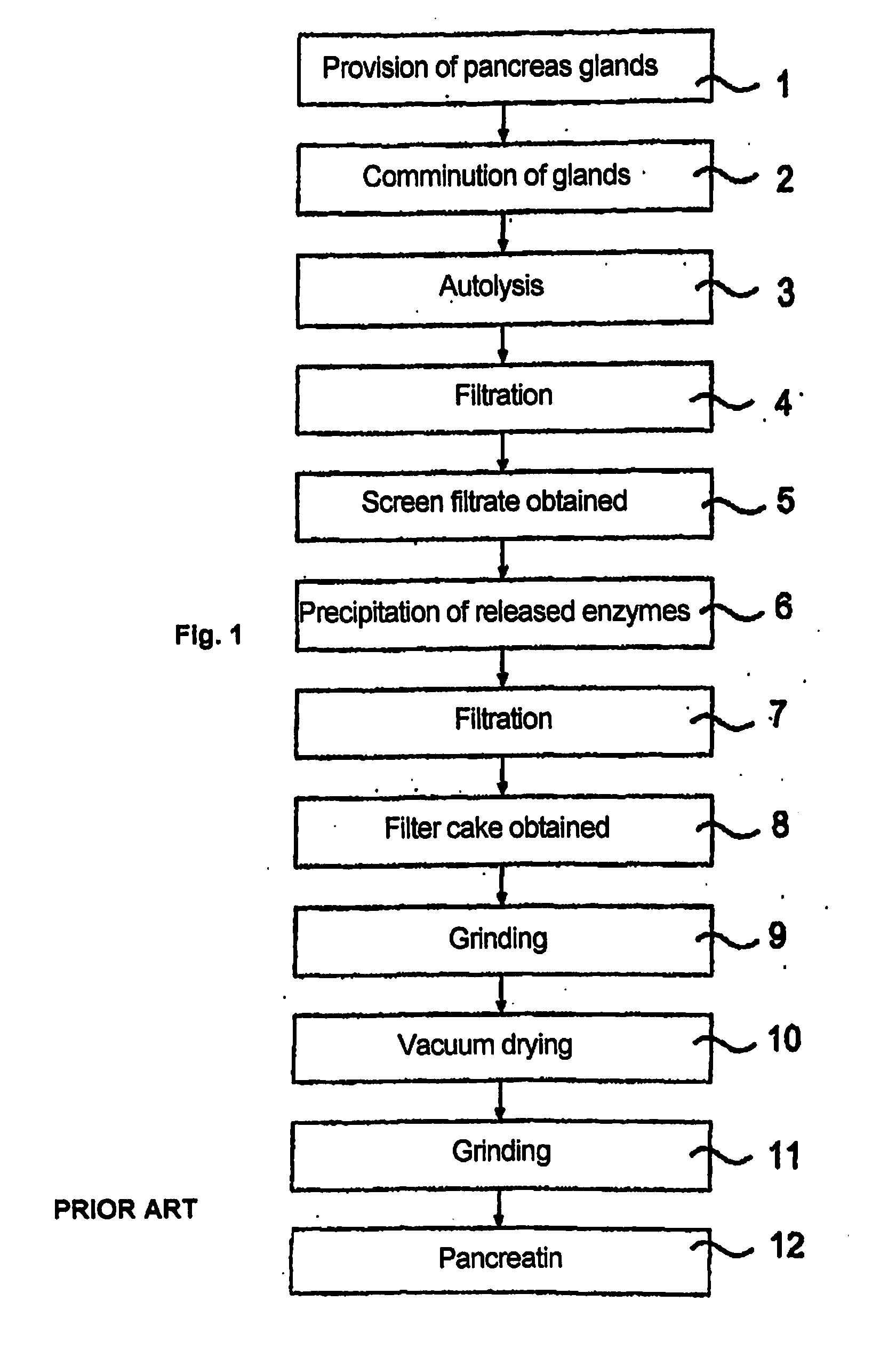
Method of preparing biological materials and preparations produced using same
InactiveUS20050019417A1Simple methodDesired characteristicOrganic active ingredientsSenses disorderMicroparticleSolid particle
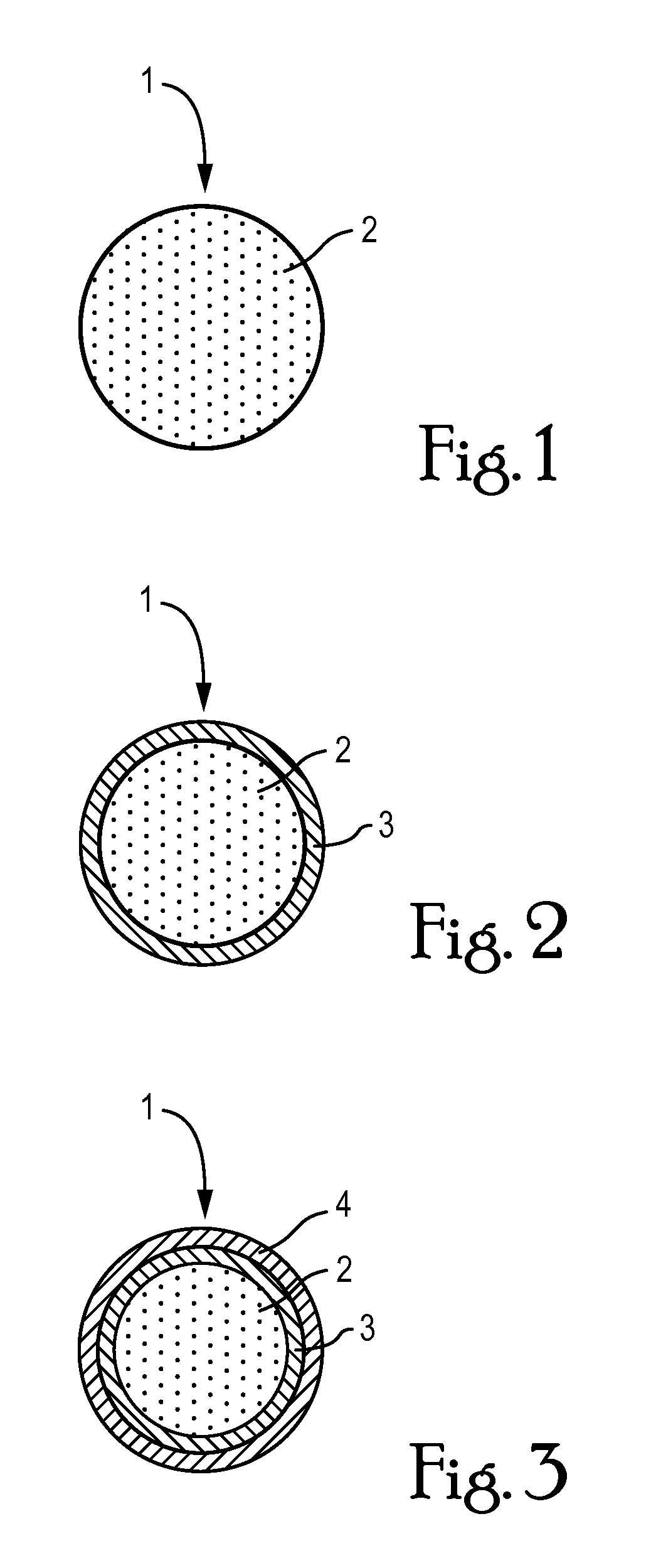
Methods for preparing products containing moisture-sensitive materials, including biological materials such as proteins, peptides or live cells, comprising at least the steps: (i) providing a coating liquid comprising at least one active, a sugar polymer and a water soluble / miscible solvent; (ii) providing a quantity of microparticles comprising at least water soluble gel forming solid particles; (iii) fluidizing said quantity of microparticles within a processing chamber of a of a suitable apparatus to form a fluidized bed of said microparticles; (iv) spraying said coating liquid onto said fluidized bed from beneath the fluidized bed to coat said microparticles therewith under saturated moisture conditions; and (vi) allowing coated microparticles to dry, are described. Also described are compositions and uses.
Owner:PFIZER INC
Methods of treating and diagnosing parkinsons disease and related dysautonomic disorders
InactiveUS20080152637A1Nervous disorderPeptide/protein ingredientsAutonomic bladder dysfunctionPsychiatry
A method for treating a Parkinson's patient with digestive / pancreatic enzymes involves administering an effective amount of digestive / pancreatic enzymes to an individual having the disorder in order to improve a symptom of the disorder. In addition, a method is provided for determining whether an individual has, or may develop, Parkinson's disease or related dysautonomic disorders and for determining whether an individual will benefit from the administration of pancreatic / digestive enzymes to treat the dysautonomic disorder.
Owner:CUREMARK
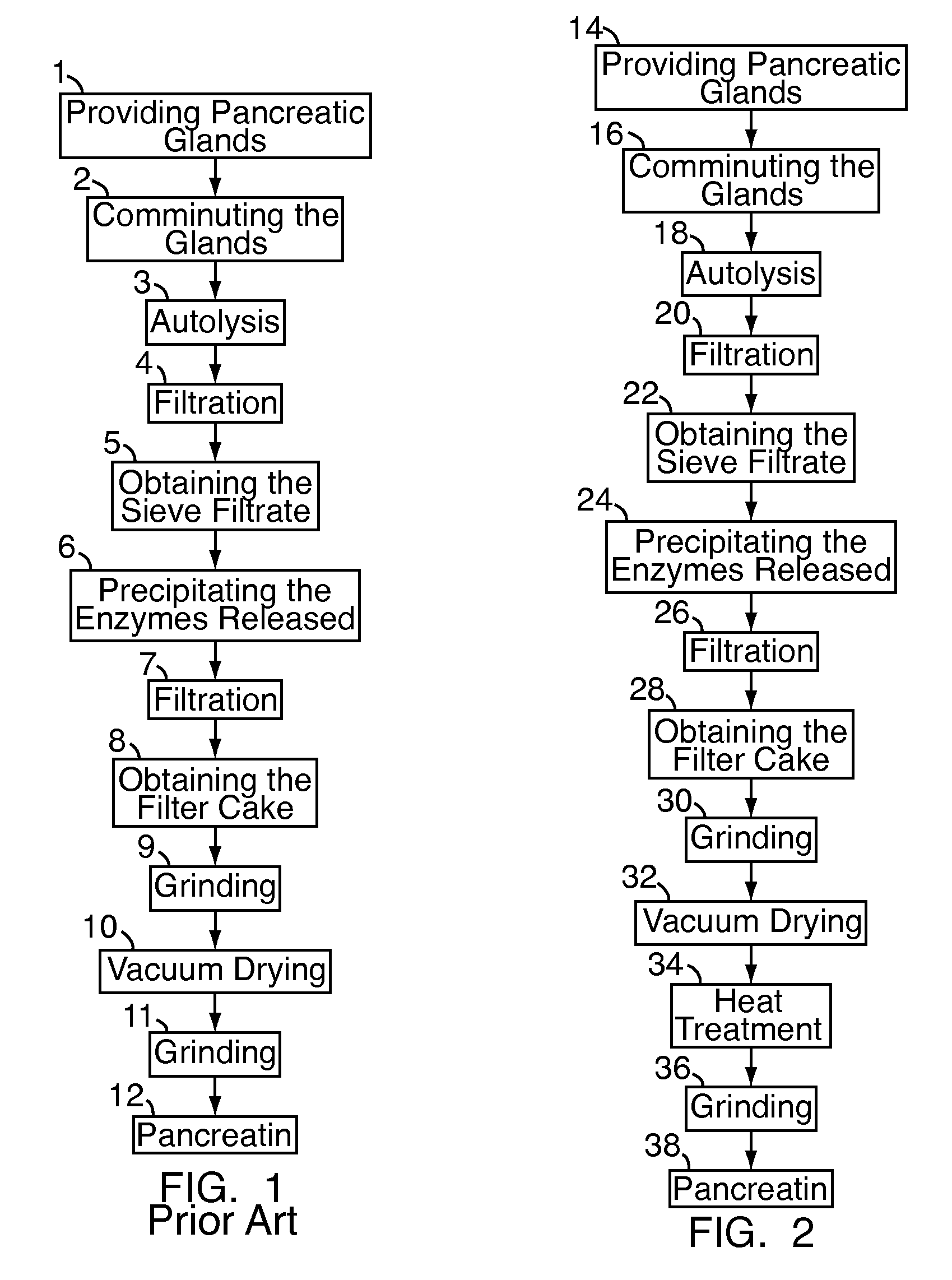
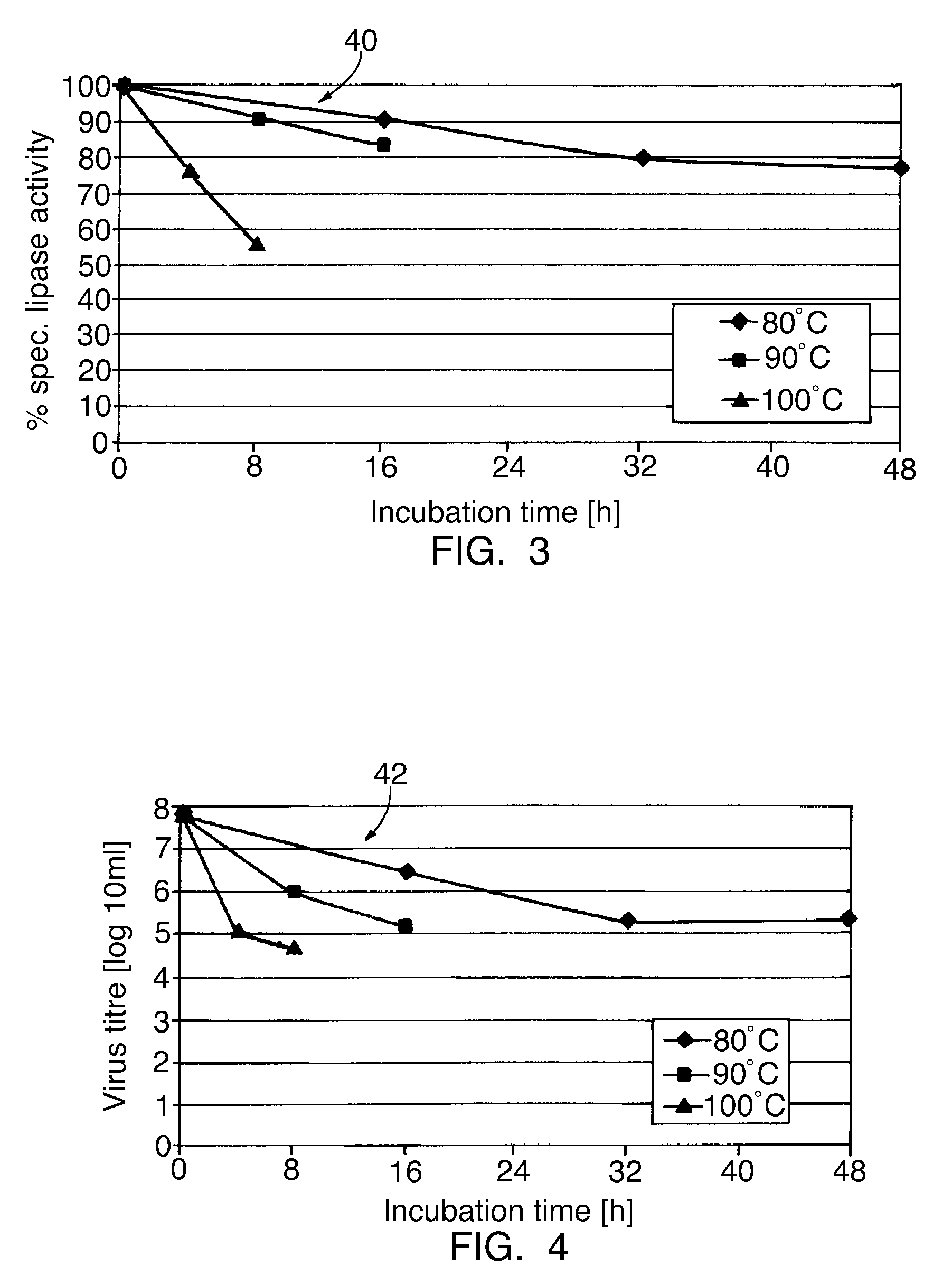
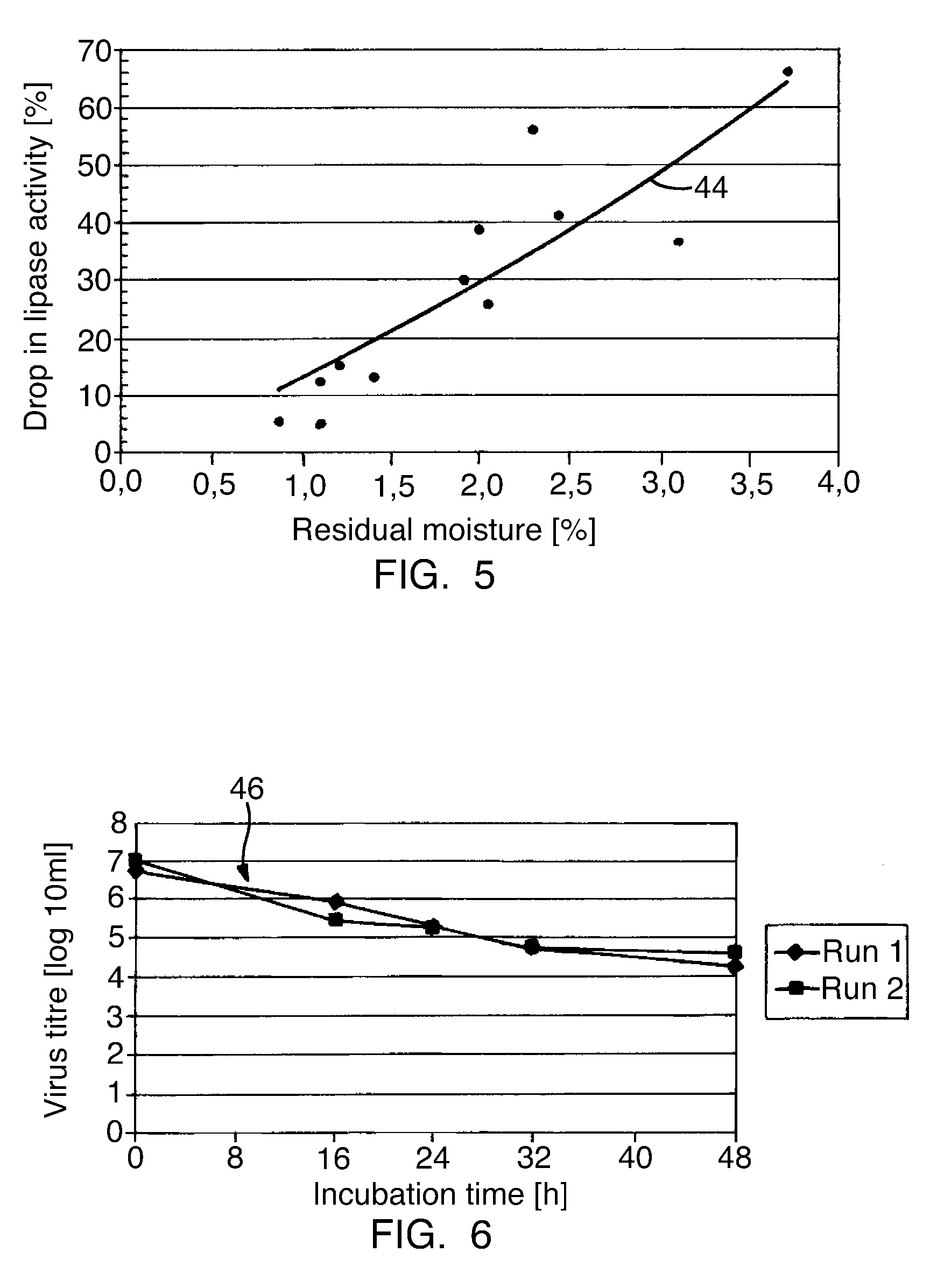
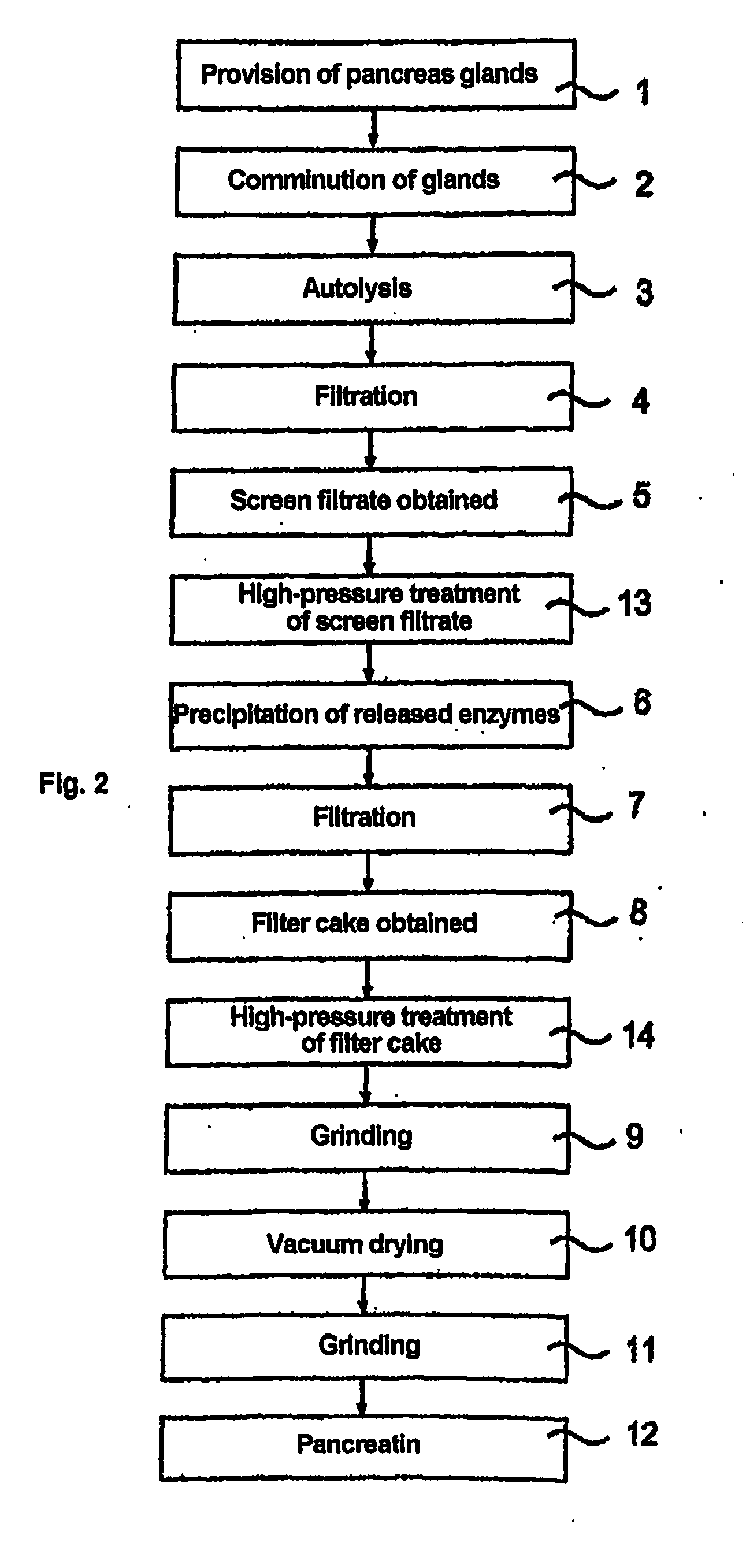
Pancreatin and method for reducing the viral and microbial contamination of pancreatin
The invention relates to a method for producing pancreatin with reduced viral and microbial contamination, comprising the steps of (a) providing the pancreatin in solid form with a residual moisture of 0.5 weight % or less, down to almost zero, based on the pancreatin provided; (b) subjecting the pancreatin provided in step (a) to a heat treatment at a temperature of 84° C., preferably 80° C. and below; wherein, the biological activity of the pancreatin obtained in step (b) corresponds to at least 50% of the biological activity of the pancreatin provided in step (a); and the viral infectiousness of the pancreatin obtained in step (b) has been reduced by a factor of more than 1 log10 in comparison with the viral infectiousness of the pancreatin provided in step (a), as well as a pancreatin produced according to this method and its use for producing a medicine or a nutritional supplement.
Owner:NORDMARK PHARMA GMBH
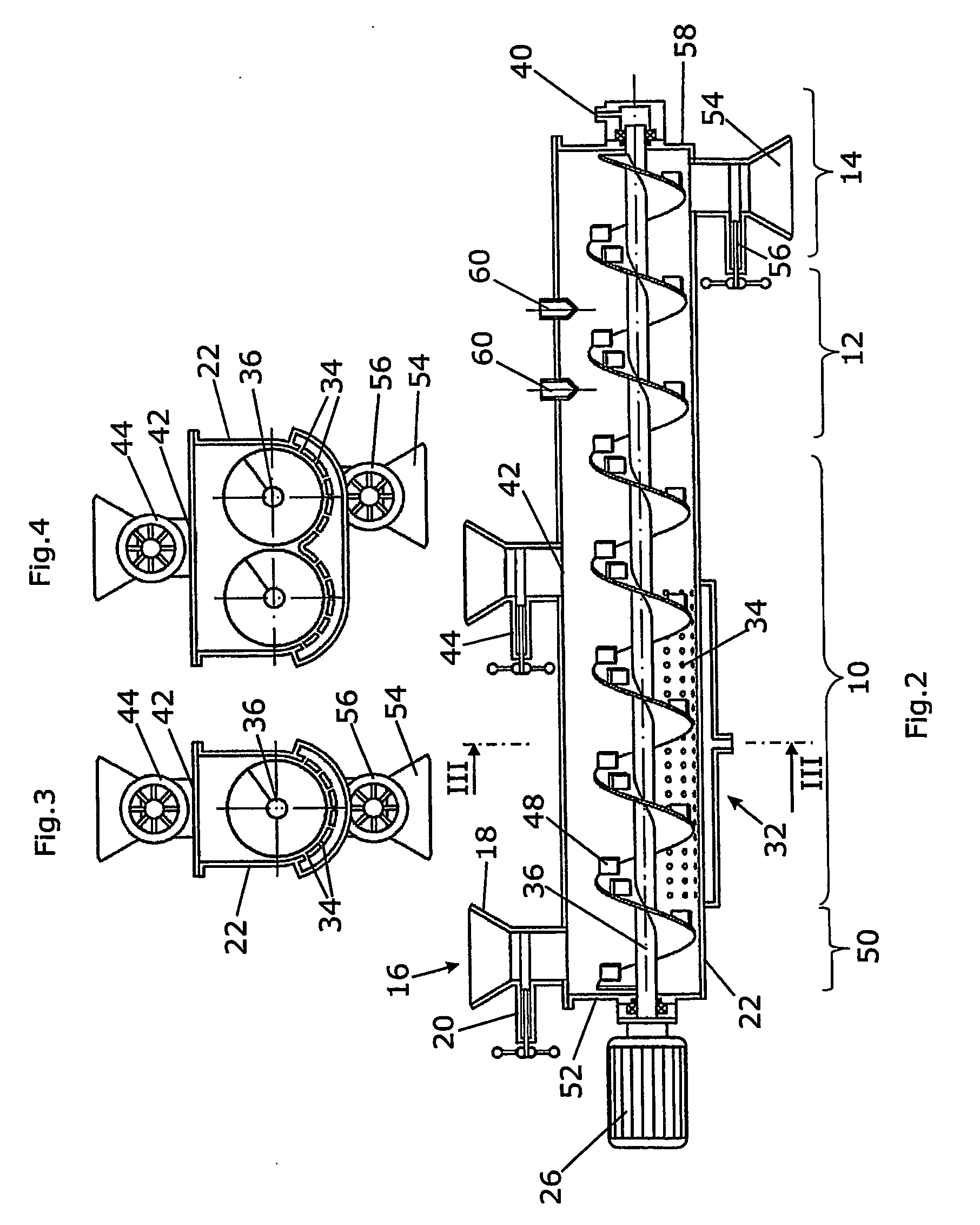
Pancreatine pellets and method of producing same
Owner:NORDMARK PHARMA GMBH
Screening assays for compounds that inhibit membrane fusion-associated events
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:TRIMERIS
Method of preparing biological materials and preparations produced using same
Methods for preparing products containing moisture-sensitive materials, including biological materials such as proteins, peptides or live cells, comprising at least the steps: (i) providing a coating liquid comprising at least one active, a sugar polymer and a water soluble / miscible solvent; (ii) providing a quantity of microparticles comprising at least water soluble gel forming solid particles; (iii) fluidizing said quantity of microparticles within a processing chamber of a of a suitable apparatus to form a fluidized bed of said microparticles; (iv) spraying said coating liquid onto said fluidized bed from beneath the fluidized bed to coat said microparticles therewith under saturated moisture conditions; and (vi) allowing coated microparticles to dry, are described. Also described are compositions and uses.
Owner:PFIZER INC
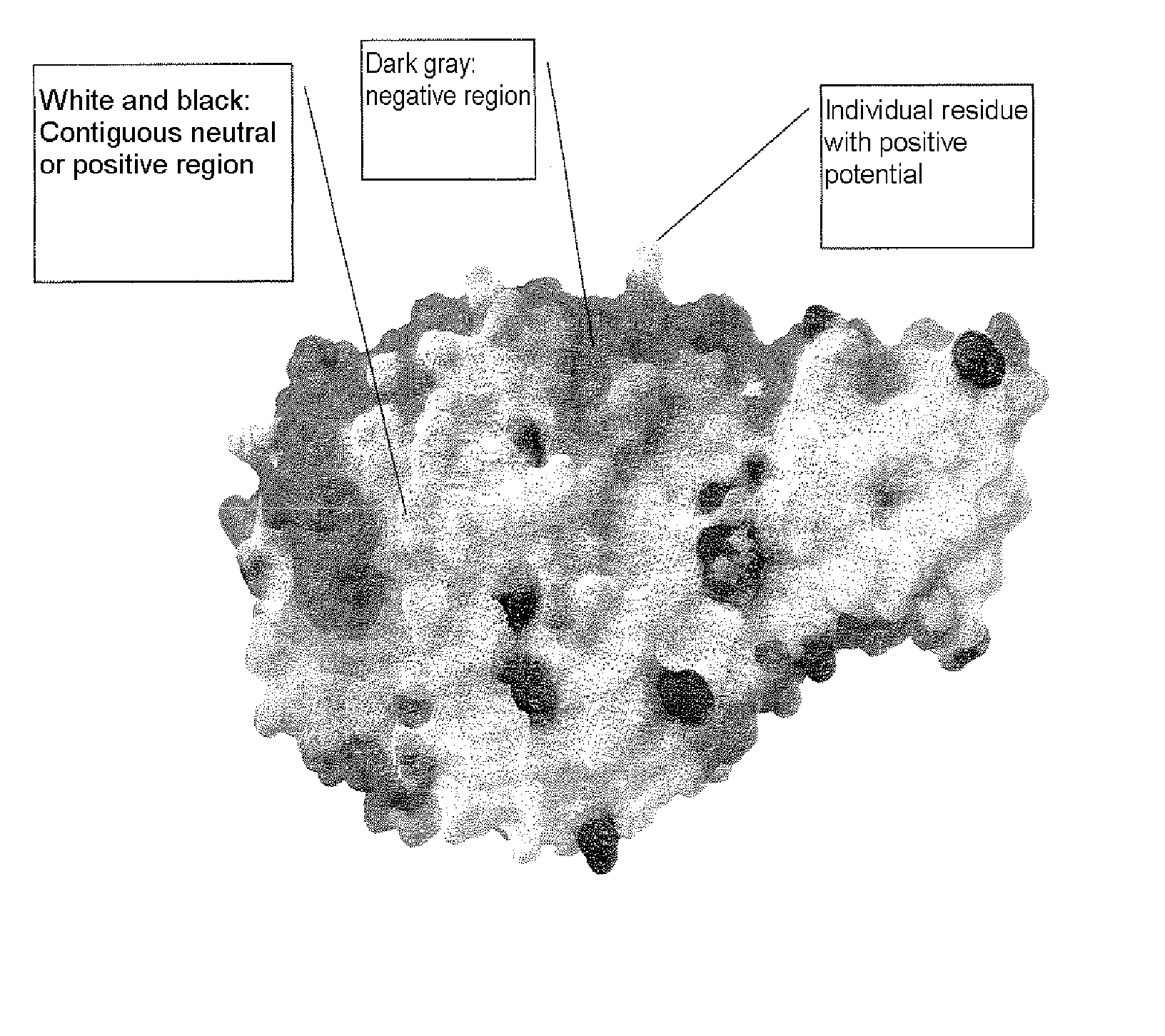
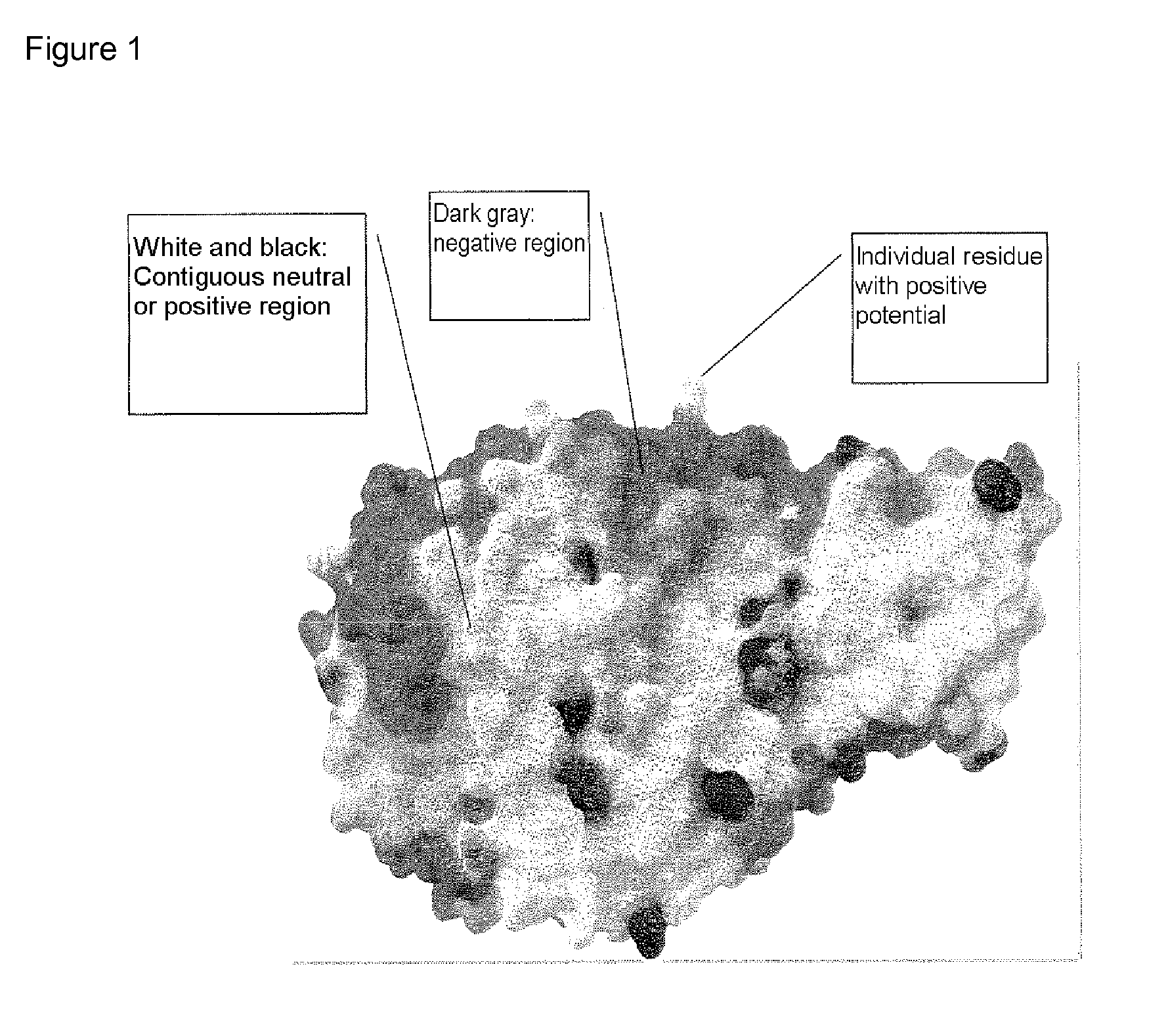
Alpha-amylase variants stabilized against dimerization and/or multimerization, method for the production thereof, and detergents and cleansers containing these alpha-amylase variants
InactiveUS20070212768A1Improve stabilityHigh homologyDetergent compounding agentsPancreatinAlpha-amylaseSolvent
The present invention relates to α-amylase variants that are stabilized to dimerization and / or multimerization, in particular at elevated temperatures or high pH, by point mutagenesis of positively polarized or charged or neutral surface amino acids to give more negatively polarized or charged amino acids. The invention further relates to methods of increasing the stability of an α-amylase to dimerization and / or multimerization brought about by electrostatic interactions whereby at least one amino acid residue on the surface of the starting molecule, which makes a neutral or positively polar or charged contribution to the electrostatic potential of said molecule, is replaced with a more negatively polar or negatively charged amino acid residue. The α-amylase variants obtained thereby exhibit better stability to influences of the solvent, increased processivity, and are suited for numerous industrial areas of use, in particular as active ingredients in detergents and cleansers.
Owner:BASF AG
Method for stabilization of enzymes during exposure to sterilizing radation
InactiveUS20060281165A1Inhibition releaseAvoid premature reactionEnzyme stabilisationOxidoreductasesL lactateZinc
The present invention provides a composition comprising an enzyme, e.g. oxidase enzyme, a source of zinc and / or ammonium ions and a source of lactate ions. The compositions are typically sterilised by exposing the compositions to sterilising radiation, e.g. gamma radiation. The incorporation of a source of zinc and / or ammonium ions and a source of lactate ions, e.g. zinc L-lactate, in the enzyme-containing composition results in an improvement in enzyme activity post-sterilisation. The presence of a source of zinc and / or ammonium ions and a source of lactate ions in the composition therefore has a protective effect on the enzyme during exposure to sterilising radiation so that good recovery of enzyme activity can be obtained.
Owner:INSENSE LIMITED
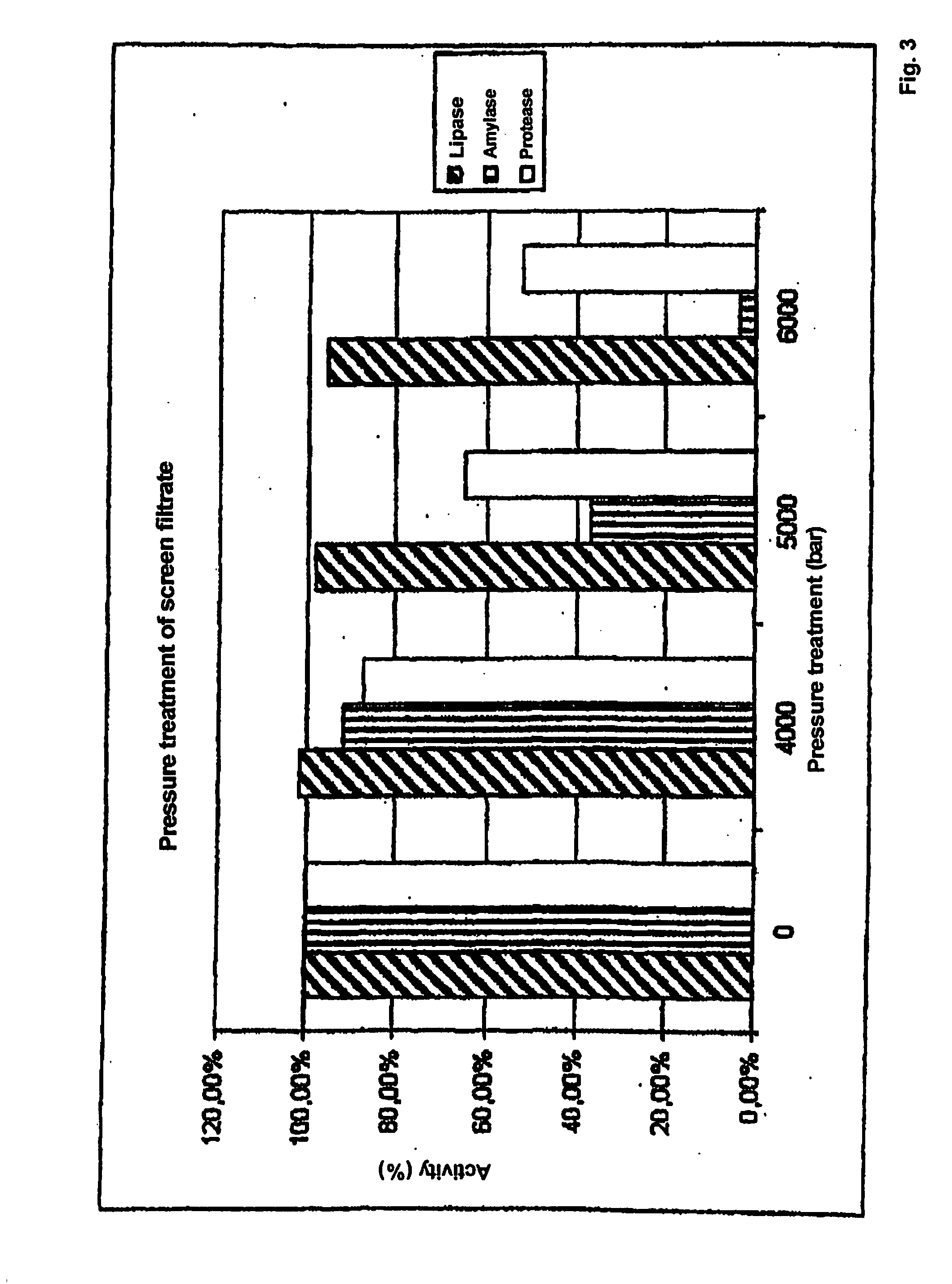
Method for reducing the viral and microbial load of biological extracts containing solids
ActiveUS20110268844A1Reduce contentEnzymic activity being substantially reducedMilk preparationPeptide/protein ingredientsBiotechnologyMicroorganism
The invention relates to a method for reducing the viral and microbial content of biological extracts which contain solids. It is provided that the method comprises the steps(a) provision of the biological extract which contains solids, comprising a biologically active substance, selected from enzymes, proteins and peptides, or a mixture of such substances; and(b) subjecting the biological extract provided in step (a) to a high-pressure treatment;wherein the biological activity of the biological extract which contains solids after the high-pressure treatment is at least 50% of the biological activity of the biological extract which contains solids before the high-pressure treatment.
Owner:NORDMARK PHARMA GMBH
Directed selective solid-phase culturing of stable microbial mixed populations for the continuous preparation of defined enzyme and metabolite mixtures
ActiveUS20070048852A1High enzyme productionShortened growth timeBioreactor/fermenter combinationsFungiMicroorganismMetabolite
The present invention provides a method for directed selective solid-phase culturing of stable microbial mixed populations for the continuous preparation of defined enzyme and metabolite mixtures, enzyme and metabolite materials obtained by such method and suitable devices therefore.
Owner:SENZYME
Silicon carbide semiconductor device and method for manufacturing same
ActiveUS20120305943A1Lower on-resistanceTransistorSemiconductor/solid-state device manufacturingDevice materialJFET
A drift layer has a thickness direction throughout which a current flows and has an impurity concentration N1d for a first conductivity type. A body region is provided on a portion of the drift layer, has a channel to be switched by a gate electrode, has an impurity concentration N1b for the first conductivity type, and has an impurity concentration N2b for the second conductivity type greater than the impurity concentration N1b. A JFET region is disposed adjacent to the body region on the drift layer, has an impurity concentration N1j for the first conductivity type, and has an impurity concentration N2j for the second conductivity type smaller than the impurity concentration N1j. N1j−N2j>N1d and N2j<N2b are satisfied.
Owner:SUMITOMO ELECTRIC IND LTD
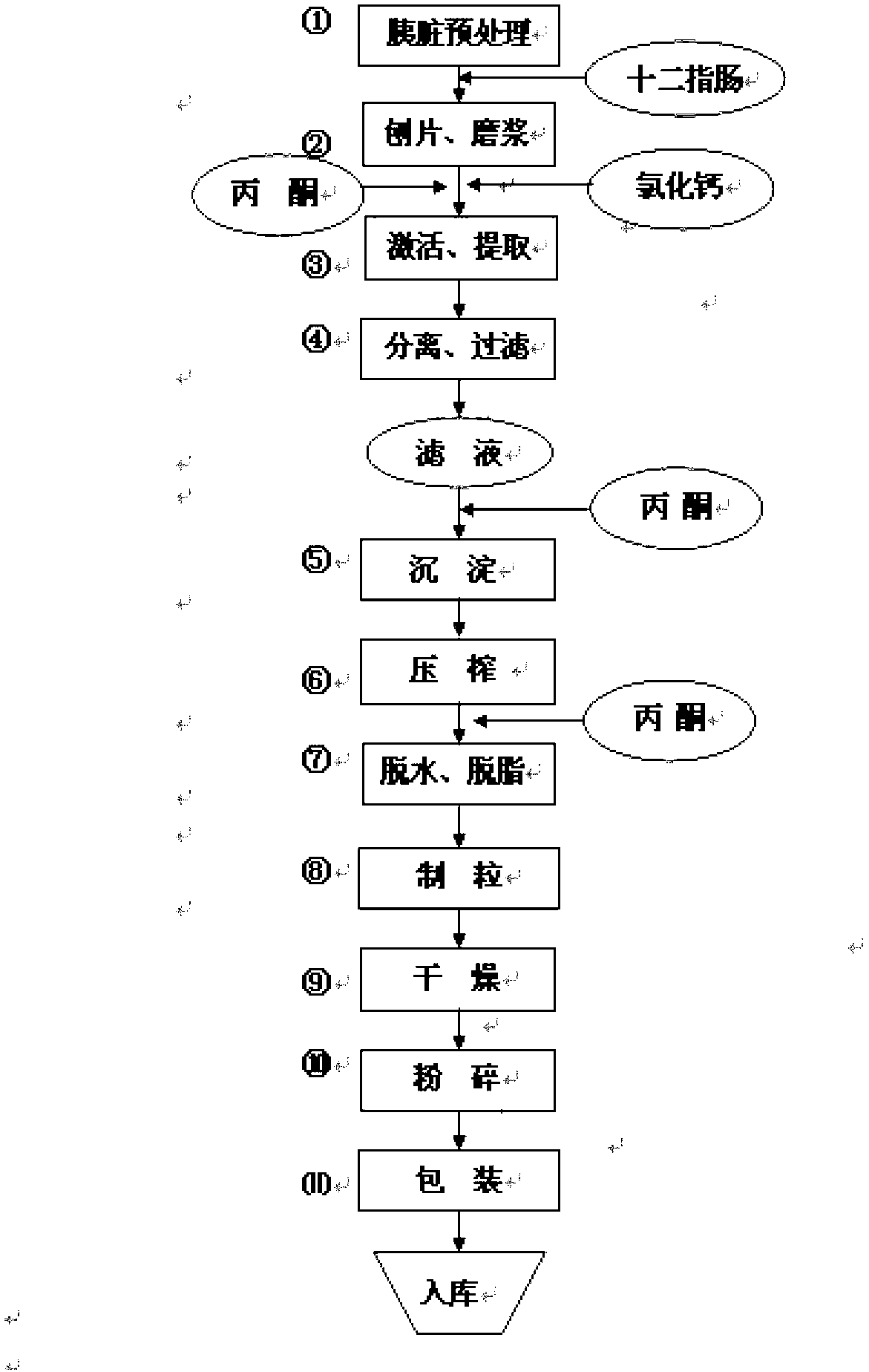
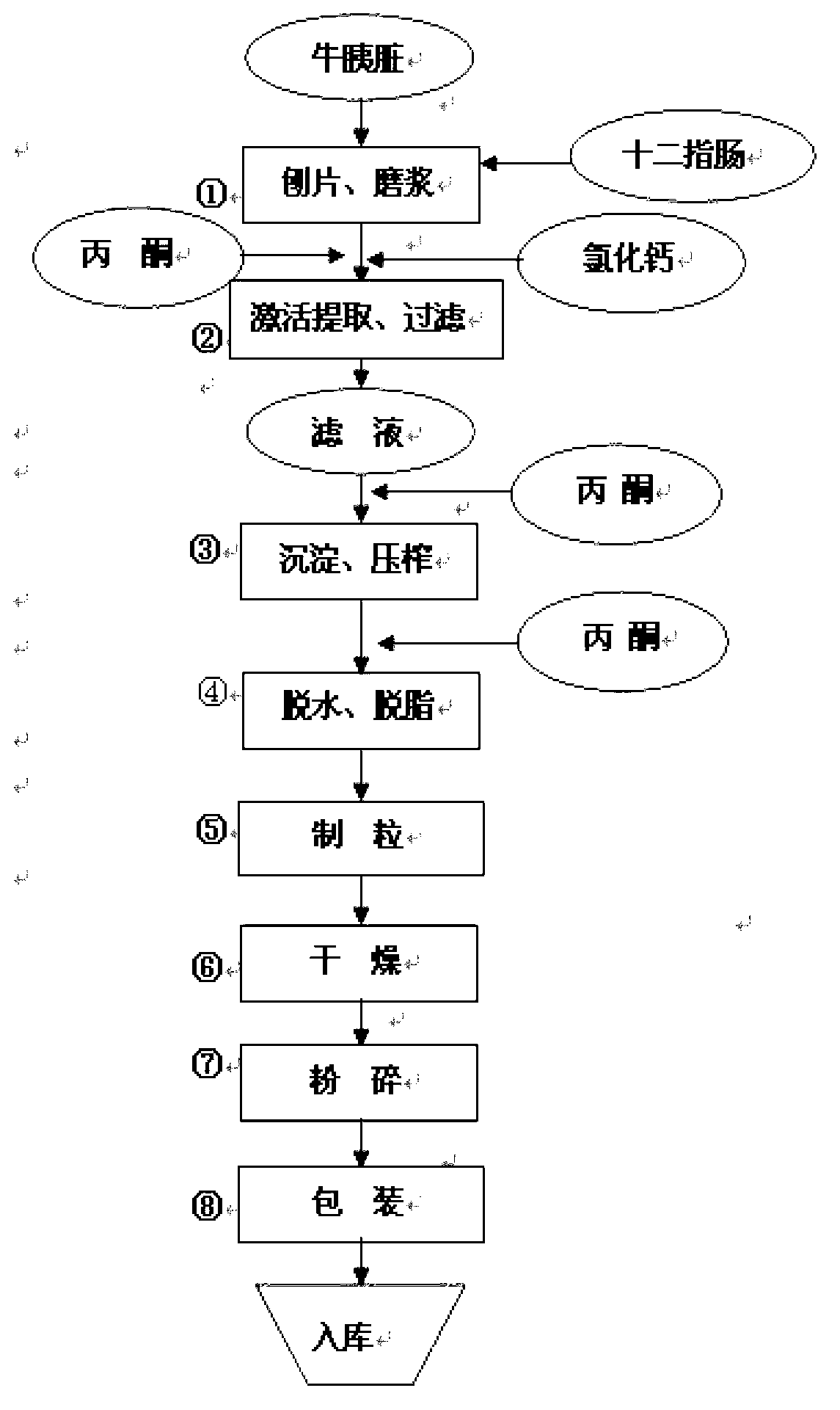
Novel production process of pancreatin
ActiveCN103215246AHigh yieldHyperamylaseDigestive systemPancreatinAqueous acetoneBiological activation
The invention discloses a novel production process of pancreatin, belonging to the technique of medicine manufacturing. The production process comprises the following steps of: unfreezing raw materials, polishing tablets, grinding into pulp, activating, separating, filtering, precipitating, squeezing, degreasing, palletizing, drying and crushing, wherein 0.2-0.5% CaCl2 is adopted as an activating agent in the step of activation, a 10-30% acetone solution is adopted for extraction, the extraction temperature is 5-15 DEG C, and the time is 8-20 hours. The productivity of pancreatin produced by using the production process is remarkably improved, and the ratios of pancreatic lipase and amylopsin are remarkably improved; and on such basis, the steps are further adjusted and optimized, so that the steps cooperate with one another so as to obtain a complete optimized production process, the pancreatin yield is about 14%, and the main indexes are that the trypsin content is about 3600 U / g, the amylopsin is about 100000 U / g, and the pancreatic lipase content is about 43000 U / g, and achieve the standard of exported pancreatin.
Owner:CHONGQING AOLI BIOPHARM
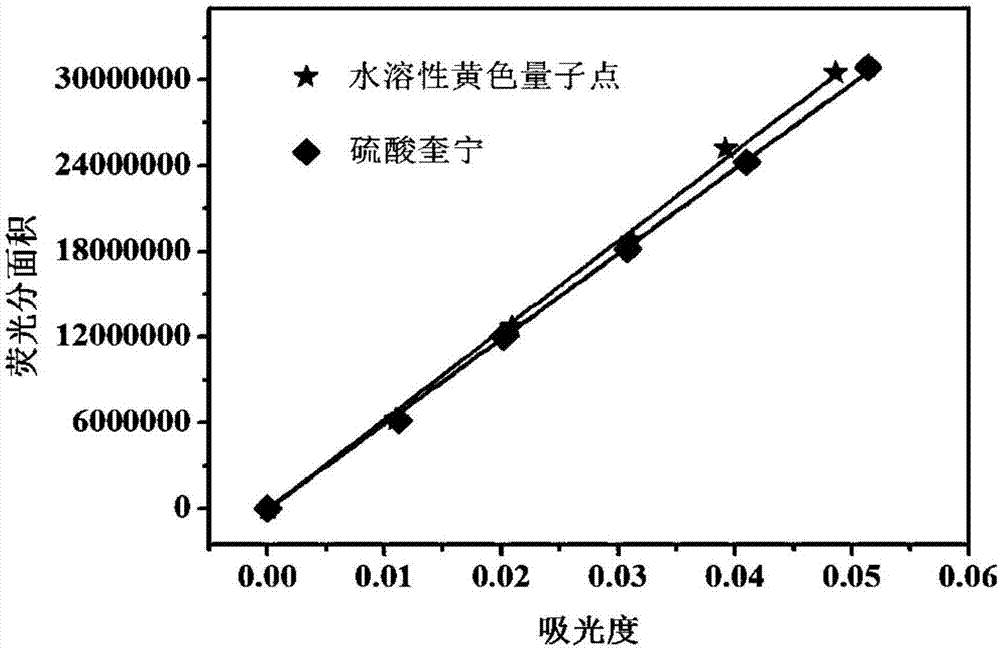
Preparation method of water-soluble yellow fluorescent carbon quantum dots
The invention discloses a preparation method of water-soluble yellow fluorescent carbon quantum dots. The preparation method includes the steps: S1 uniformly mixing pancreatic enzymes, ethylenediamine and ultra-pure water to obtain mixed liquid, and performing hydrothermal reaction on the mixed liquid to prepare reaction liquid; S2 performing centrifugation, filtration, dialysis and evaporation to prepare the water-soluble yellow fluorescent carbon quantum dots. The mass volume ratio of the pancreatic enzymes, the ethylenediamine to the ultra-pure water is 1.5g: 1mL: 9mL. The quantum dots take pancreatic enzymes and the ethylenediamine as raw materials which are natural, free from toxicity, low in cost and easy to obtain, the preparation method is simple, rapid and efficient by a hydrothermal method, and the yield of the quantum dots is high.
Owner:苏州诺维康生物科技有限公司
Method for isolating mesenchymal stem cells from placental vessel and used digestive enzyme composition
ActiveCN109234229AGood differentiation effectHigh purityCell dissociation methodsSkeletal/connective tissue cellsVascular tissueTissue fluid
The present invention relates to a method for isolating mesenchymal stem cells from placental vessels and used digestive enzyme compositions. The method comprises the following steps: the placenta issterilized with alcohol; Dissecting placental blood vessels from the placenta; Cut into pieces, wash with PBS, filter the residual blood stains to obtain placental vascular tissue; Adding mixed enzymesolution to digest; End digestion, filter and collect tissue fluid; The placental mesenchymal stem cells (P0) were obtained by centrifugation and cultured with DMEM-F12 basal medium was resuspended and the number and viability of nucleated cells were counted. The obtained cells are cryopreserved with cryopreservation protection solution so as to be re-cultured before use, or the passages and / or the mesenchymal stem cells are continued to be subjected to cell identification and / or detection, cryopreservation, library establishment and the like. The method of the invention can effectively improve the efficiency of isolating mesenchymal stem cells from the blood vessels of the placenta.
Owner:BOYALIFE
Stabilized pancreas product
InactiveUS7153504B2Peptide/protein ingredientsClimate change adaptationAnimal feed additivePancreatic structure
The present invention provides stabilized pancreas products useful, for example, as an animal feed additives. The invention also provides for feed additives and feed rations comprising a stabilized pancreas product. Further, the invention provides for methods of making stabilized pancreas products. The present invention also provides for methods of supplementing an animal feed utilizing stabilized pancreas products.
Owner:CAN TECH INC
New production technology of bovine pancreatin
ActiveCN103215245AIncrease enzyme activityImprove extraction efficiencyPancreatinManufacturing technologyPancreatin powder
The invention discloses a new production technology of bovine pancreatin and belongs to the medicine manufacturing technology. The new production technology comprises the steps of raw material treatment, activation, extraction, precipitation, degreasing, drying and grinding and is characterized in that the steps of activation and extraction further comprise the sub-steps of: adding calcium chloride accounting for 0.5-1% of the weight of the raw material and pancreatin powder accounting for 0.5-1% of the weight of the raw material into pancreatic pulp as activators; adopting 10-20% (v / v) acetone solution which is 0.5-2 times the weight of the raw material as an extractant; stirring for 30-60 minutes; extracting at 10-20 DEG C for 10-15 hours; filtering with a separator; and discarding the filter residue to obtain pancreatic milk filtrate; and then other steps are performed. The new production technology disclosed by the invention has the characteristics of high extraction efficiency, high enzyme activity, stable properties and short production period, turns waste into wealth, produces obvious economic benefits, and has good application and popularization prospect in the fields of food, leather, washing and cleaning, medicine and the like.
Owner:CHONGQING AOLI BIOPHARM
Process for producing N-acetylneuraminic acid
The present invention provides a process for economically producing N-acetylneuraminic acid without using expensive materials such as pyruvic acid and phosphoenolpyruvic acid. The process comprises: allowing (i) a culture of a microorganism having N-acetylneuraminic acid aldolase activity or N-acetylneuraminic acid synthetase activity, or a treated matter of the culture, (ii) a culture of a microorganism capable of producing pyruvic acid or a treated matter of the culture, or a culture of a microorganism capable of producing phosphoenolpyruvic acid or a treated matter of the culture, (iii) N-acetylmannosamine, and (iv) an energy source which is necessary for the formation of pyruvic acid or phosphoenolpyruvic acid to be present in an aqueous medium to form and accumulate N-acetylneuraminic acid in the aqueous medium; and recovering N-acetylneuraminic acid from the aqueous medium.
Owner:KYOWA HAKKO KOGYO CO LTD
Preparation method of pancreatin
The invention relates to the field of biological medicines, and discloses a preparation method of pancreatin. The preparation method comprises the following steps: taking freshly frozen raw materials, namely, freshly frozen pancreas and / or duodenum; carrying out slicing and freeze-drying, namely, slicing the freshly frozen raw materials, and drying the slices by adopting a freeze-drying machine till the water content in the raw materials is less than 8%; carrying out grinding for obtaining powder, namely, grinding the freeze-dried raw materials into freeze-dried powder; carrying out degreasing, namely, degreasing the freeze-dried powder, and carrying out draining to obtain degreased freeze-dried powder; carrying out activating and extracting, namely, adding an activating agent and an extracting solvent into the degreased freeze-dried powder, carrying out stirring for extracting at 0-5 DEG C for 3-6 h, thus obtaining an extracted solution; and carrying out precipitating, namely, carrying out freeze centrifugation on the extracted solution at 0-5 DEG C, taking the supernate, carrying out salting-out, and carrying out separating to obtain pancreatin sediment; carrying out squeezing and drying, namely, squeezing the pancreatin sediment till the pancreatin sediment is block-shaped, thus obtaining pancreatin blocks; drying the pancreatin blocks, and grinding the dried pancreatin blocks to obtain pancreatin powder. According to the preparation method of pancreatin, the yield of the pancreatin product is relatively high, the consumption of the solvent is greatly reduced, the activity of trypsin, amylopsin and pancrelipase in the prepared pancreatin is relatively high, and the yield of pancreatin is relatively high.
Owner:江苏麦德森制药有限公司
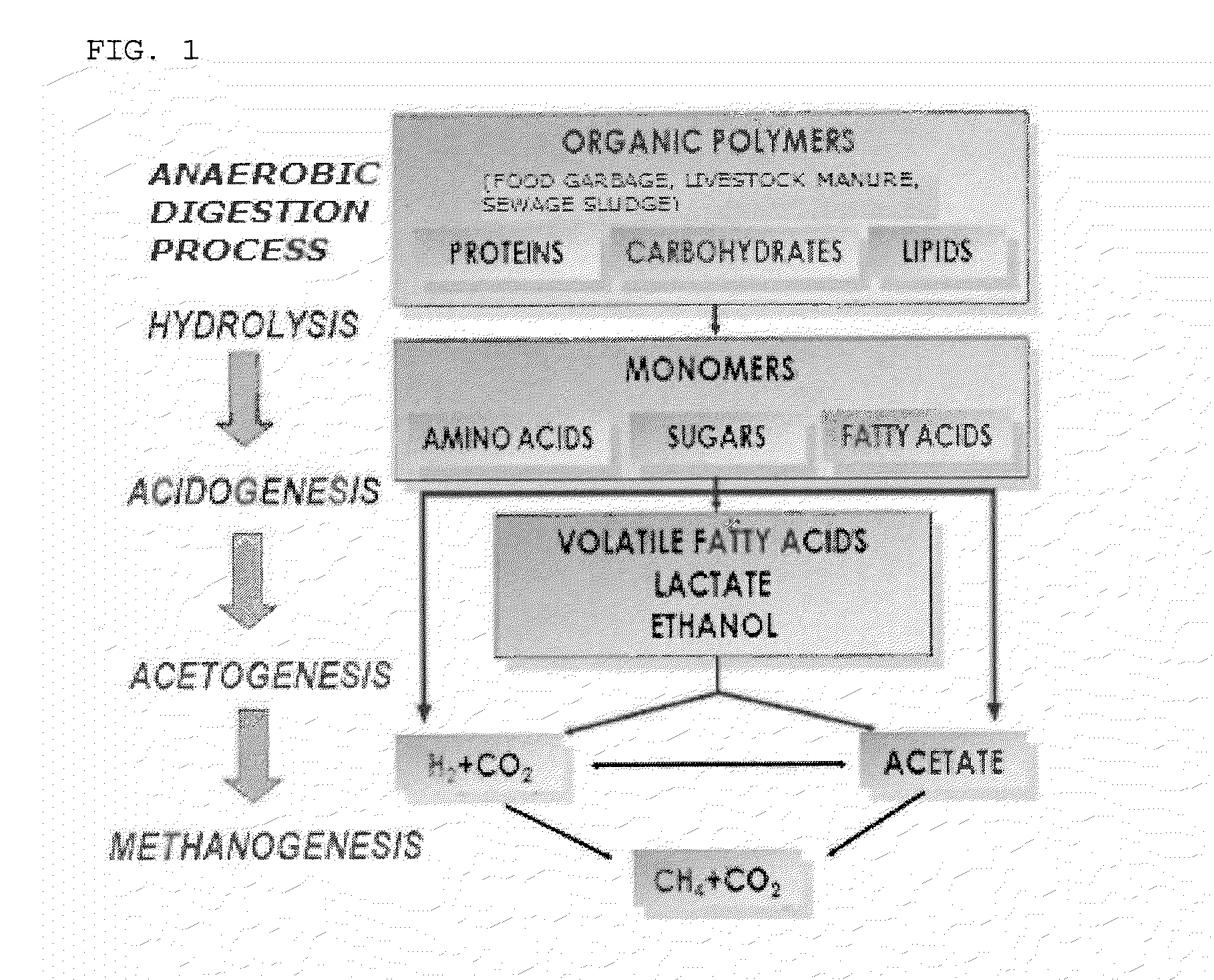
Method for Promoting Production of Biogas Using Pancreatin in an Anaerobic Digestion Process
InactiveUS20150175462A1Promote degradationImprove hydrolysis efficiencyHormonesWaste based fuelBiotechnologyMicroorganism
Disclosed is a method for promoting production of biogas using pancreatin in an anaerobic digestion process. In addition, disclosed are a composition for improving hydrolysis efficiency or promoting production of biogas, which includes pancreatin as an active ingredient, and a method for promoting (or increasing) production of biogas from organic manure of livestock using the same. The composition for improving hydrolysis efficiency or for promoting production of biogas in an anaerobic digestion process for treatment of organic waste, includes pancreatin as an active ingredient. The organic waste is livestock manure, and the composition has optimum activity at pH 7.0 to pH 8.0. The composition further includes a microorganism with excellent degradability of degradation resistant organic compounds.
Owner:KOREA ADVANCED INST OF SCI & TECH
Immobilized enzyme pickering emulsion reaction system and application thereof
ActiveCN109706141ANo pollution in the processRich varietyMaterial nanotechnologyHydrolasesEnvironmental resistancePickering emulsion
The invention discloses an immobilized enzyme pickering emulsion reaction system and an application thereof. The immobilized enzyme pickering emulsion reaction system comprises an immobilized enzyme using mesoporous nanometer materials as a carrier, and an oil phase and a water phase for forming emulsion, wherein the emulsion particle diameter of the system is 10-80[mu]m, reaction raw materials are used as the oil phase, a buffer solution is used as the water phase, and the immobilized enzyme using the mesoporous nanometer materials as a carrier is used as a catalyst and an emulsifying agent.In the pickering emulsion enzymic method reaction system, the reaction raw materials are directly used as the oil phase, and the immobilized enzyme of the mesoporous nanometer materials is used as a reaction catalyst and besides, is the emulsifying agent of pickering emulsion. Compared with a conventional emulsion added with an organic reagent or an emulsifying agent, the catalysis activity and the stability are notably improved, products are easy to separate and purify, and repeated use and scaled enlargement are facilitated, the application range is broad, and the immobilized enzyme pickering emulsion reaction system is green and environmental-friendly.
Owner:OIL CROPS RES INST CHINESE ACAD OF AGRI SCI
Liquid Preparation of Physiologically Active Peptide
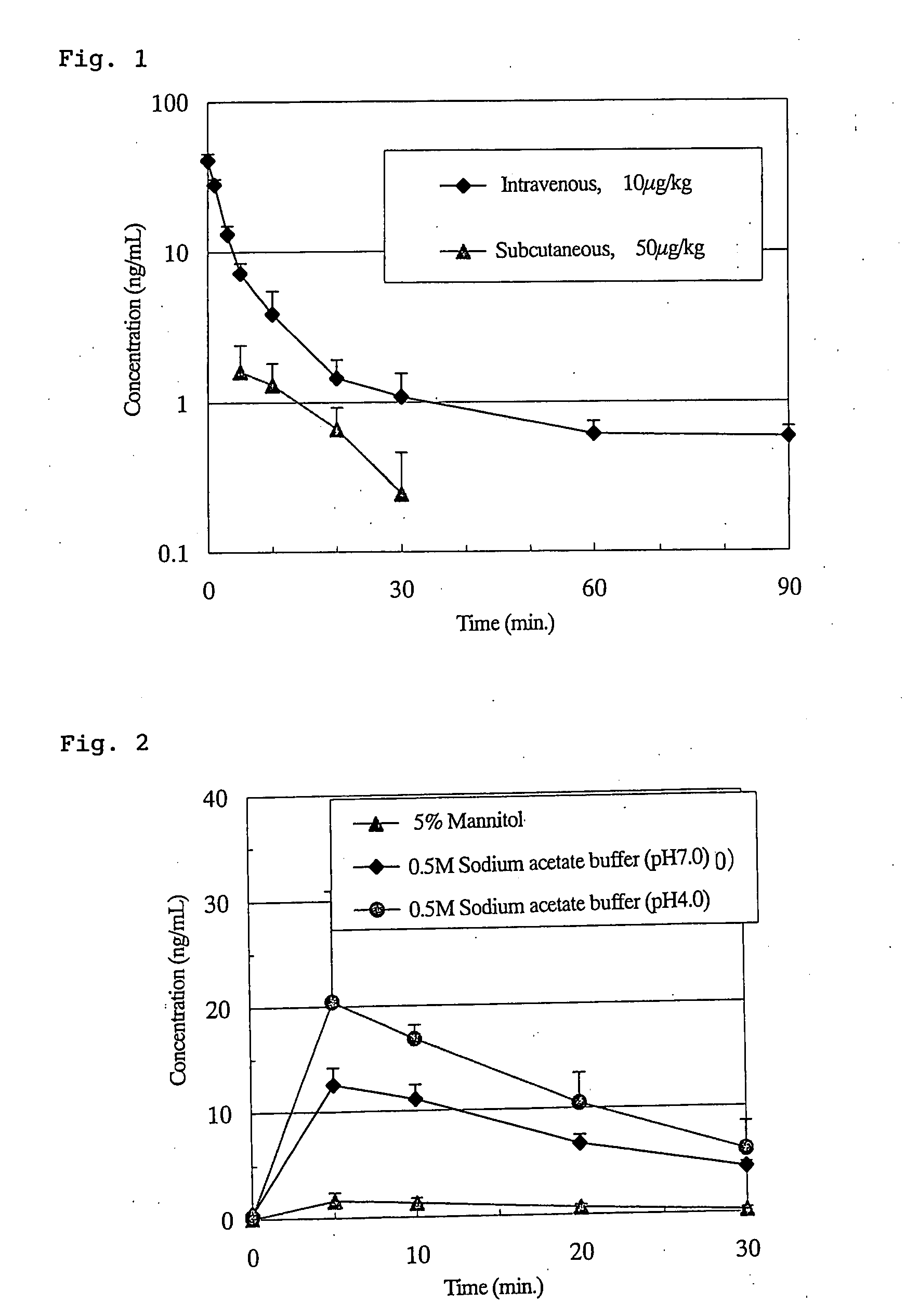
InactiveUS20080193997A1Improve bioavailabilityImproving BAPeptide/protein ingredientsMetabolism disorderBenzoic acidPropanoic acid
An effective liquid preparation achieves high bioavailability (BA) of physiologically active peptides or proteins, including ghrelins, that are administered as drugs. Also provided is a method for improving the BA of physiologically active peptides or proteins, including ghrelins, that are subcutaneously injected in aqueous solutions. The liquid preparation contains: a physiologically active peptide or protein, such as ghrelins, as an active ingredient; an acid solution including one or a combination of two or more selected form the group consisting of acetic acid, lactic acid, phosphoric acid, glycine, citric acid, hydrochloric acid, propionic acid, butyric acid, benzoic acid and salts thereof; an alcohol; and a polar organic liquid including one or a combination of two or more selected from the group consisting of N-methyl-2-pyrrolidone, dimethylformamide, dimethylsulfoxide and methylparaben.
Owner:ASUBIO PHARMA +1
Method for preparing high-activity pancreatin
The invention provides a method for preparing high-activity pancreatin. The method comprises the following steps of: by adopting porcine pancreas or the rest pancreatic slag generated by extracting insulin through neutral ethanol as a raw material; adding 2% of calcium chloride solution for activating, adding moderate NaCl, NH4Cl, sucrose, starch, glycerinum and tween 80; adjusting pH (Potential of Hydrogen) to 5.5 to 7.0 through 4mol / Lof Na2HPO4; activating for 6 hours at 30 DEG C; dividing size and filtering to remove residues; standing for cooling to reach 4 to 6 DEG C; precipitating through PEG (Polyethylene Glycol)-6000; degreasing through acetone; and drying to obtain the pancreatin. According to the method, the yield of the pancreatin is increased by 16% under the pancreatin extraction environment set in the experiment, and the activity of three enzymes is obviously improved, wherein the activity of trypsin is increased by 13%, the activity of amylopsin is increased by 26%, and the activity of pancreatic lipase is increased by 43%; the activity of the pancreatic lipase, which is easily damaged, is greatly improved.
Owner:SICHUAN UNIV
Digestive enzyme composition and application thereof, and isolated culture method of umbilical epithelial cells
InactiveCN104894088AImprove separation efficiencyAvoid damageArtificial cell constructsEmbryonic cellsDigestionHyaluronidase
The invention relates to the technical field of cell isolated culture, particularly a digestive enzyme composition and application thereof, and an isolated culture method of umbilical epithelial cells. The digestive enzyme composition comprises hyaluronidase, collagenase VIII and pancreatin. The umbilical epithelial cell primary isolation adopts the hyaluronidase, collagenase VIII and pancreatin for combined digestion. The digestive enzyme composition has the advantages of mild properties, low cell trauma and high enzymolysis rate and greatly enhances the epithelial cell isolation efficiency.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Preparation method of pancreatic enzyme powder
The invention provides a method for preparing pancreatic enzyme powder. The method comprises the following steps of (1) collecting pancreas; (2) preserving the pancreas at a low temperature; (3) carrying out thawing, impurity removing and mincing; (4) extracting the pancreas so as to obtain pancreas slurry, and filtering by utilizing a frame filter press, so as to obtain an extracting solution; (5) adding ethanol into the extracting solution, standing, filtering by utilizing the frame filter press, and collecting a filter cake; (6) washing and centrifuging the filter cake to obtain sediments; (7) drying the sediments in vacuum and crushing, so as to obtain the pancreatic enzyme powder. According to the method, the bovine pancreatic enzyme powder accordant with a Mohammedanism rule is provided, and the market blank is made up; a method for preparing the industrial pancreatic enzyme powder by virtue of pig pancreas is technically improved. The method has the advantages that the production cost is low, the pollution to a final product and the environment is avoided, and the production security is greatly improved; the operation process is simplified, the consumed time is short, the time for preparing each batch of product only lasts for 45-48 hours, and the method is easy to industrialize. The product prepared by utilizing the method has the advantages that three enzyme activities are high and the yield reaches 11.0%.
Owner:马忠仁 +1
Enzyme compositions with reduced viral and microbial contamination
ActiveUS20180036393A1Reduced viralReduce Microbial ContaminationHydrolasesPeptide/protein ingredientsMicroorganismVirus
The present invention pertains to an enzyme preparation obtained from e-beam irradiated animal tissue, such as porcine pancreas. The present invention also pertains to methods for making such enzyme preparations, pharmaceutical compositions comprising such enzymes preparations, and methods for using such pharmaceutical compositions and enzyme preparations.
Owner:ABBVIE INC
Model gut system
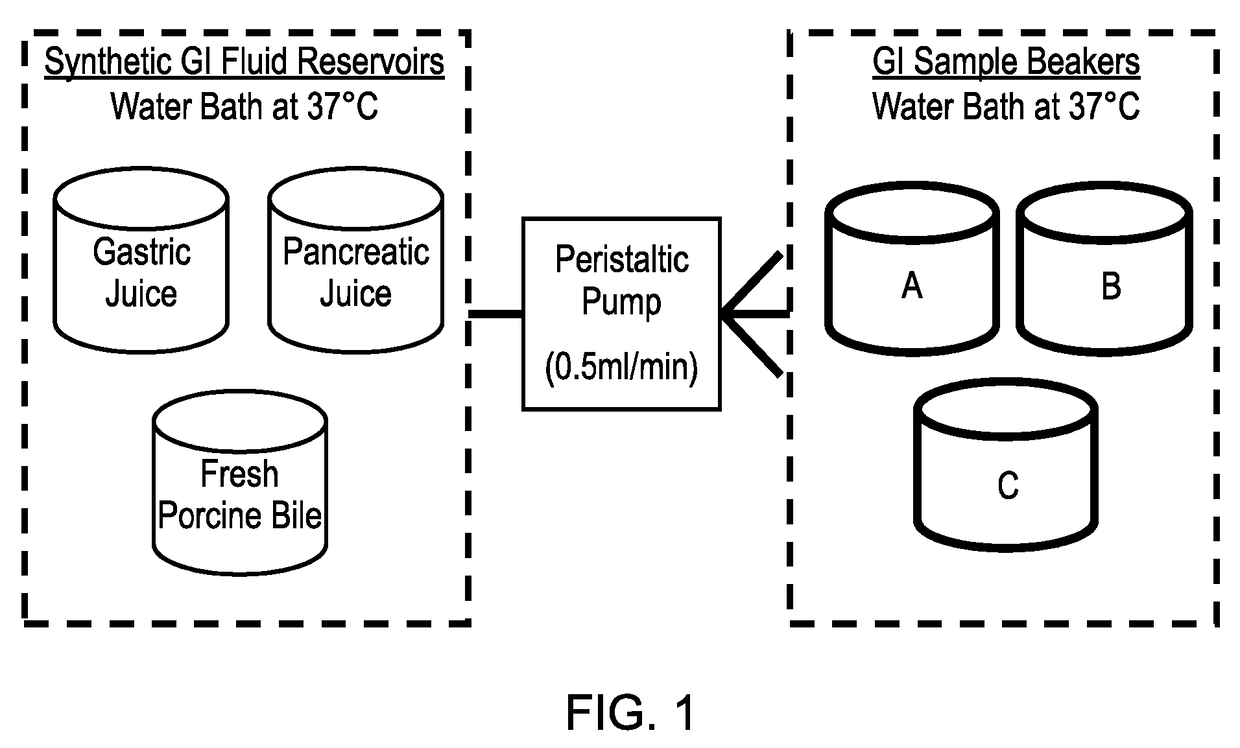
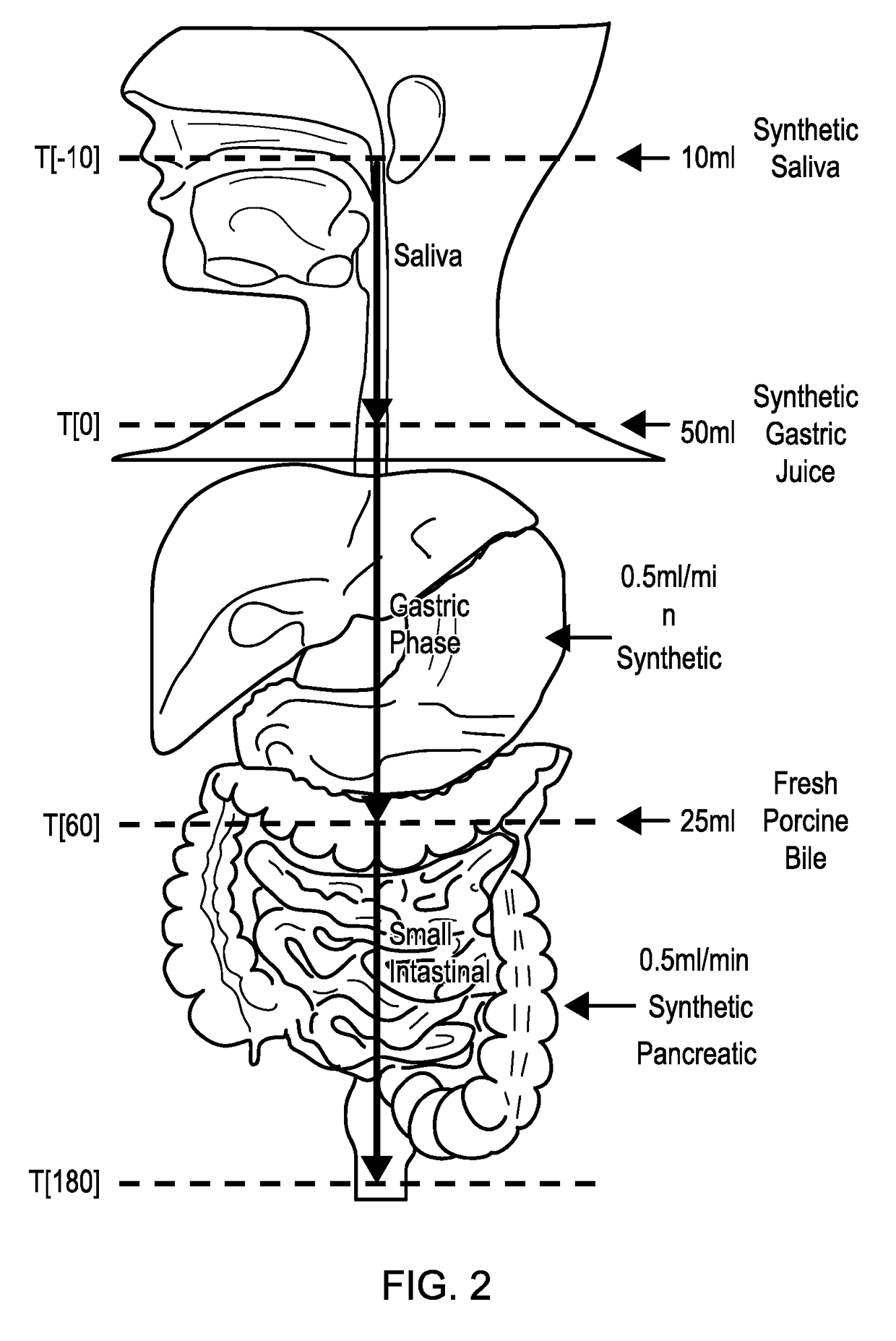
ActiveUS20170160283A1Cost prohibitiveSmall scaleCosmonautic condition simulationsMicrobiological testing/measurementPancreatic juiceDiluent
The invention relates to a Model Gut System (MGS) comprising a pancreatic phase consisting essentially of synthetic pancreatic juice comprising pancreatin and one or more suitable pancreatic diluent(s) at a pH from about 7 to about 9, preferably about 7.9 to about 8.2, and porcine bile.
Owner:NEWCASTLE UNIV
Medicine composition pancreatin enteric capsule and preparation method thereof
ActiveCN103397013AImprove stabilityLow costPeptide/protein ingredientsDigestive systemCelluloseSucrose
The invention provides pancreatin and an enteric capsule thereof. An amino acid sequence of the pancreatin is shown in SEQIDNO:2 and the like; a particle in the pancreatin enteric capsule is composed of a pill core and a coating wrapped outside the pill core, wherein the pill core is composed of the pancreatin, cane sugar, dextrin and hydroxypropylated methylcellulose, and the coating is composed of polyacrylic resin, triethyl citrate and talcum powder.
Owner:浙江华润三九众益制药有限公司
Preparation method of pancreatin
ActiveCN108795920AAchieve the purpose of inactivationEnhanced Virus SecurityPancreatinVirus inactivationVirus safety
The invention relates to a preparation method of pancreatin. The preparation method comprises following steps: pancreas is subjected to grounding, hydrogen peroxide is added so as to obtain a mixture,wherein the mass ratio of hydrogen peroxide in the mixture is controlled to be 0.1 to 2%; the mixture is stirred for 1 to 8h, catalase is added for 0.5 to 2h of reaction, and degreasing, activating,deposition, and drying are carried out so as to obtain pancreatin. In the preparation method, hydrogen peroxide is capable of realizing full contact with virus in a relatively short time, so that virus inactivation is realized; the residual hydrogen peroxide is degraded by catalase into water and oxygen, so that no hydrogen peroxide is left in pancreatin, the drug virus safety is improved greatly,and the obtained pancreatin product is capable of satisfying the strict requirements of pharmacopeia and animal-sourced biochemical medicine standards of different states.
Owner:苏州良辰生物医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com