Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Urea nitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Urea nitrate is a fertilizer-based high explosive that has been used in improvised explosive devices in Afghanistan, Pakistan, Iraq, and various other terrorist acts elsewhere in the world, like the 1993 World Trade Center bombings. It has a destructive power similar to better-known ammonium nitrate explosives, with a velocity of detonation between 11,155 ft/s (3,400 m/s) and 15,420 ft/s (4,700 m/s).
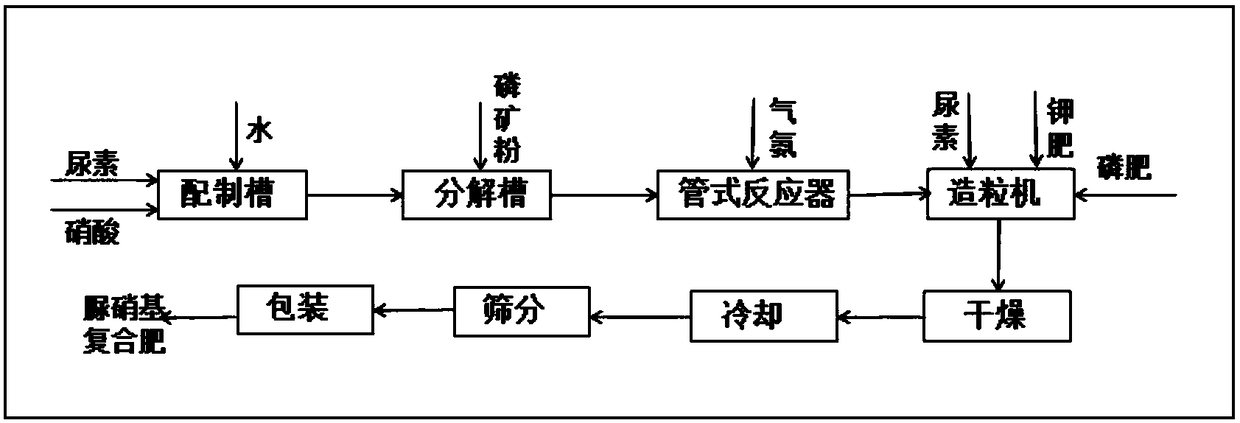
Production method of readily available and controlled release composite fertilizer containing three nitrogen elements
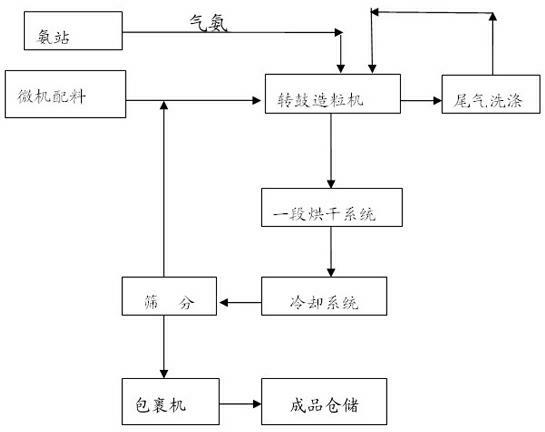
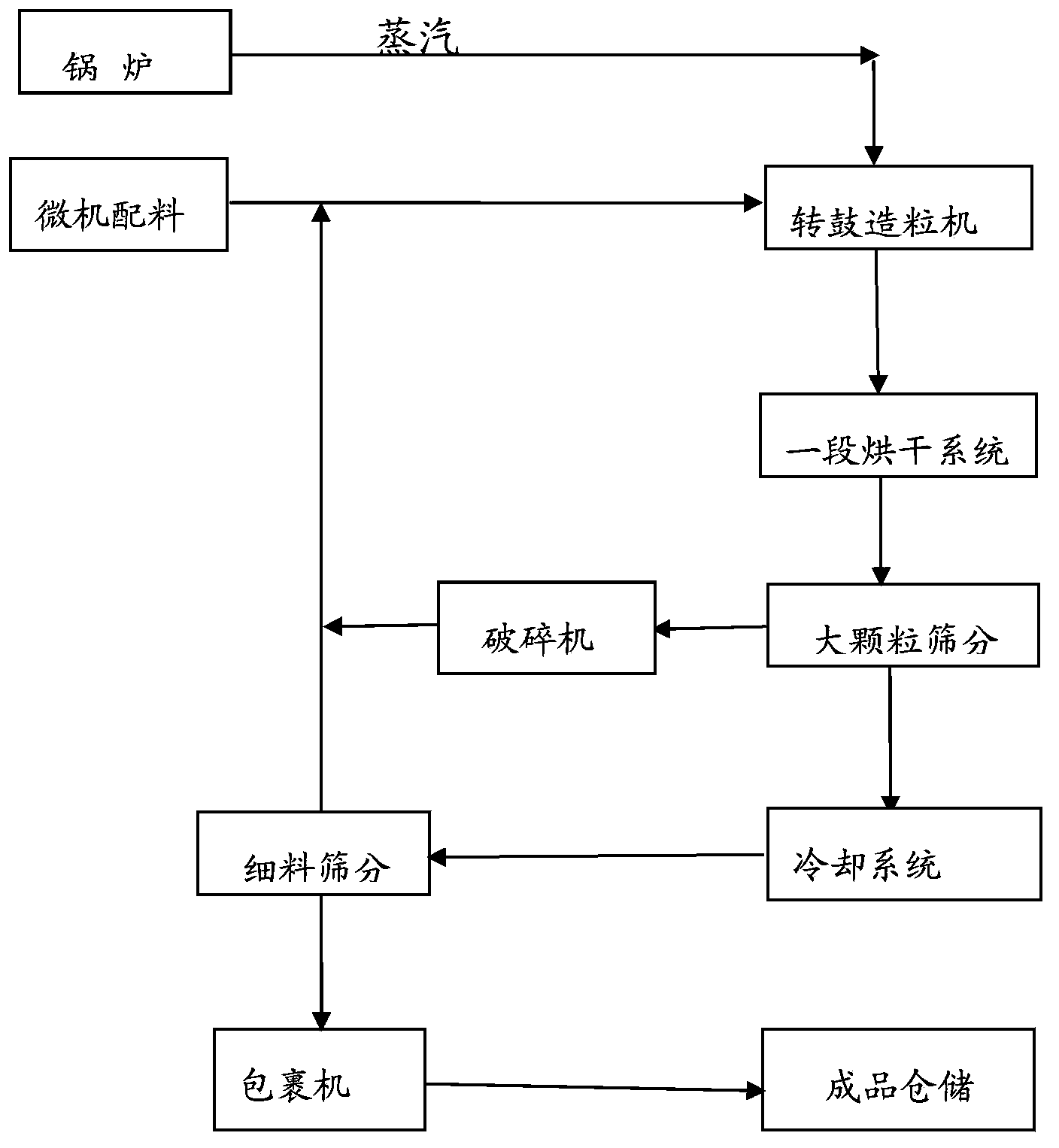
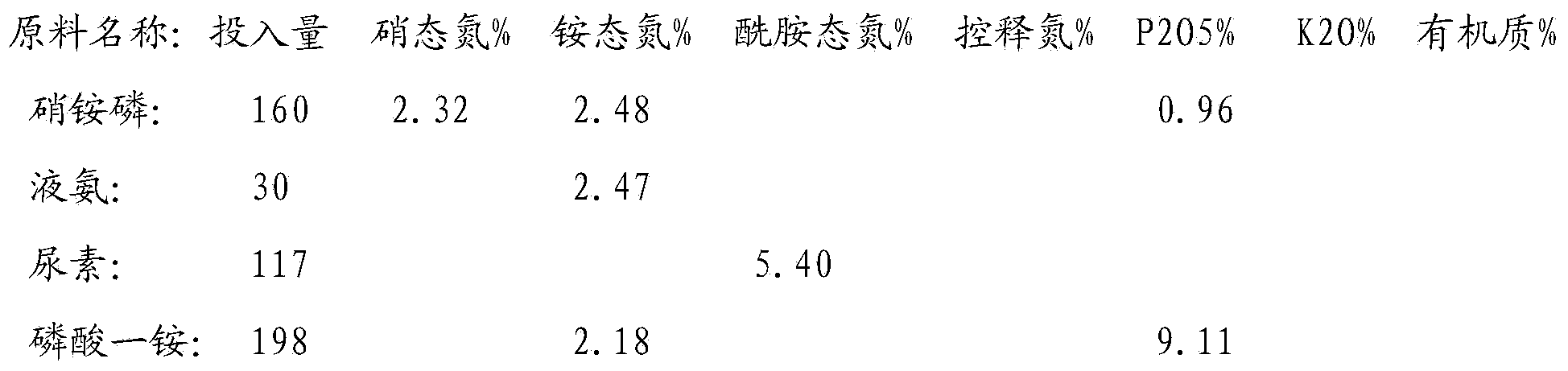
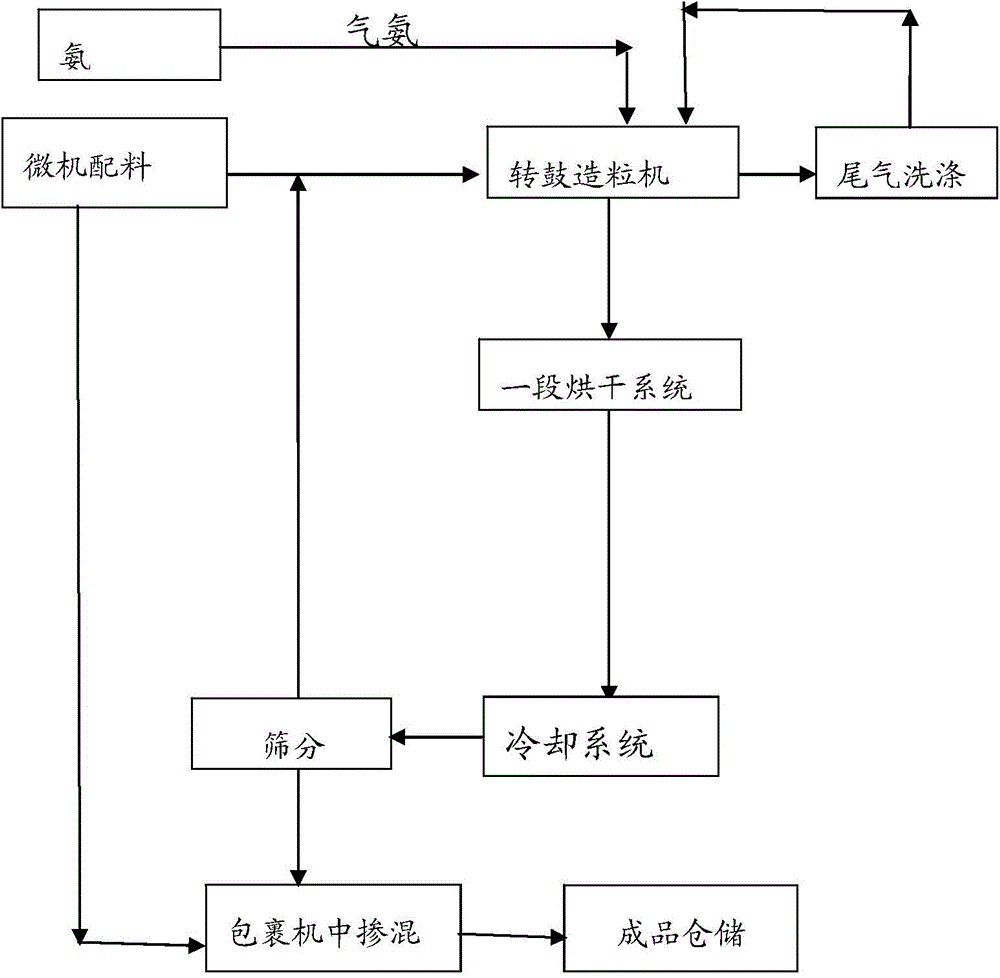
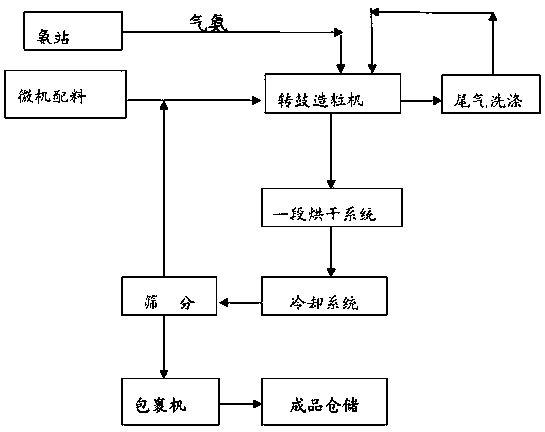
The invention discloses a production method of a readily available and controlled release composite fertilizer containing three nitrogen elements. The production method is as follows: raw materials containing nitric nitrogen, ammonium nitrogen and amido nitrogen are added in materials; in order to prevent that urea reacts with nitro phosphate fertilizer to generate too much urea nitrate and the production can not performed, ammonia gas is introduced in the granulation process according to different proportions to ammonify nitro phosphate fertilizer, reduce the generation of urea nitrate and increase the neutralization degree of monoammonium phosphate; and powdery fundamental fertilizers containing nitrogen, phosphorous and potassium are added to granulate, and then the composite fertilizer can be obtained through drying, cooling, screening and coating. In the production method of the invention, a lot of heat generated in the thermolysis of the nitro phosphate fertilizer and the neutralization heat of the reaction of ammonia gas and monoammonium phosphate are fully utilized to heat materials and increase the granulation temperature; and the viscosity of urea nitrate is utilized, the solubility of ammonium phosphate salt is increased, the quantity of the liquid phase for granulation can be satisfied, higher granulation rate can be realized without using other adhesive, the cost is reduced, the water content of the granulation material is significantly reduced, the drying energy consumption can be reduced and a large amount of energy consumption can be saved.
Owner:天津芦阳肥业股份有限公司
Quick-acting slow-released compound fertilizer and preparation method thereof
InactiveCN103193535AImprove solubilityRaise the granulation temperatureFertiliser formsFertilizer mixturesUrea nitratePotassium
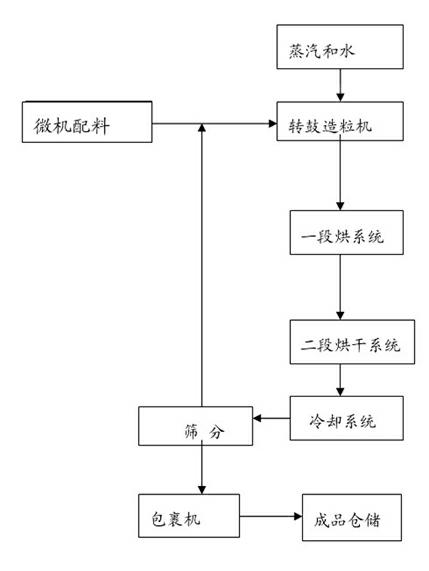
The invention discloses a quick-acting slow-released compound fertilizer and a preparation method thereof. The preparation method comprises the following steps of: adding a raw material containing nitrate nitrogen, ammonium nitrogen and amidonitrogen to materials, directly introducing gas ammonia to a material layer of a rotor drum granulator to finish ammoniation of ammonium nitrate phosphorus, and controlling the mass of urea nitrate generated by reaction. According to effective proportioning, the contents of nitrogen in the quick-acting slow-released compound fertilizer are as follows in percentage by mass: 2-5 percent of nitrate nitrogen, 6-9 percent of ammonium nitrogen and 7-11 percent of amidonitrogen. The phosphorous and potassium nutrient content is determined according to proportioning of different products. The ammonium nitrate phosphorus, containing the nitrate nitrogen, as the raw material is added by in two parts, i.e., the ammonium nitrate phosphorus containing 1-3 percent of nitrate nitrogen is added into the material layer, and the rest of ammonium nitrate phosphorus containing 1-2 percent of nitrate nitrogen is added into a part which is 1-1.5m away from the inlet of a cooler; and the surfaces of fertilizer particles subjected to drying and fine screening are wrapped, so that the quick-acting slow-released compound fertilizer has available nitrogen shells. The slow-released nitrogen urea is added in a solid manner, used as master batch for granulation and wrapped layer by layer by other material layers of nutrients, so that the quick-acting slow-released compound fertilizer has slow-released kernels.
Owner:天津芦阳肥业股份有限公司
Environment-friendly nitrogen potassium dressing fertilizer containing various nitrogen elements and preparation method thereof
InactiveCN103382140AQuality improvementSolve the problem of difficult granulationFertilizer mixturesUrea nitratePlant growth
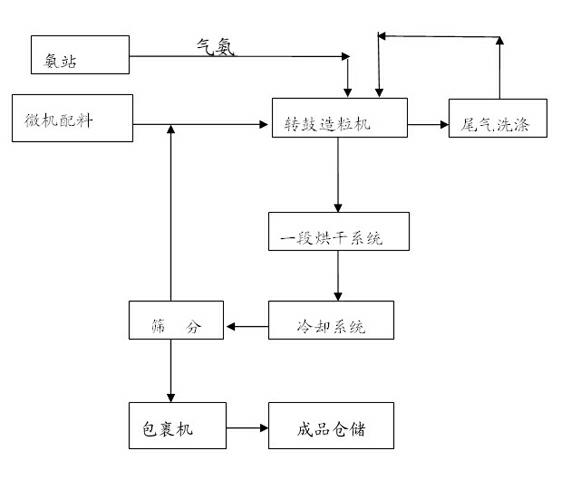
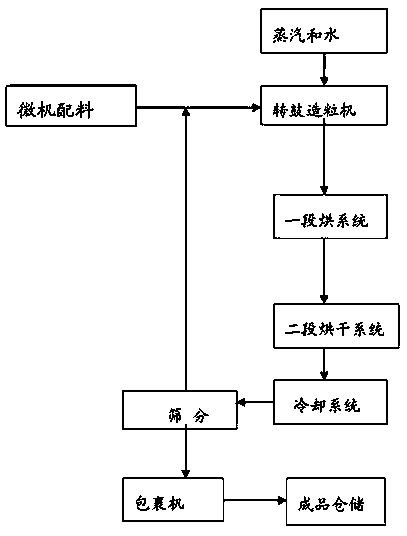
The invention discloses an environment-friendly nitrogen potassium dressing fertilizer containing various nitrogen elements and a preparation method thereof. The fertilizer comprises, by weight, at least 20% of urea, 5-7% of ammonium bicarbonate, 7-15% of ammonium sulfate, 10-14% of nitrate, at least 8% of potassium chloride, 7% of humic acid, 8-12% of sepiolite, 5-7% of calcium hydroxide, other materials containing trace elements and a plant growth regulator. In a granulating process, urea nitrate generated by reaction of nitrate and urea and sepiolite with viscosity are used as adhesive, water generated by decomposing of ammonium bicarbonate is used as a liquid phasor, steam is used for increasing the temperature of granulating materials, liquid phasor required by granulation is supplemented, and a material granulating process is finished. The final product contains nitrate nitrogen, ammonium nitrogen, amidonitrogen, organic matters, various medium trace elements and the plant growth regulator and achieves the purposes of improving soil, reducing soil salinity, improving nutrient element absorption of plants, increasing plant quality and productivity and improving plant disease resistance.
Owner:PANJIN STRONG FERTILIZER
Agent combination of safe fireworks and fire cracker
InactiveCN1431182AAvoid low temperature decomposition disadvantagesHigh melting pointExplosivesFireworksUrea nitrateFireworks
A high-safety composite charge for firecrackers or fireworks is prepared from oxidant (metallic oxides including Fe2O3, Fe3O4, copper oxide, manganese oxide, etc and organic alkali nitrate including urea nitrate, guanidine nitrate, etc.) sulfate compound, aluminium powder, pearlite powder and charcoal powder. The said sulfate compound is ferrous sulfate as gas expanding agent, which is treated through dewatering, pulverizing, surface treating, and neutralizing. Its advantages are high safety, and high performance in sound, light and colors.
Owner:刘琼吾 +1
Whole-process nutrient supply type composite fertilizer and preparation method thereof
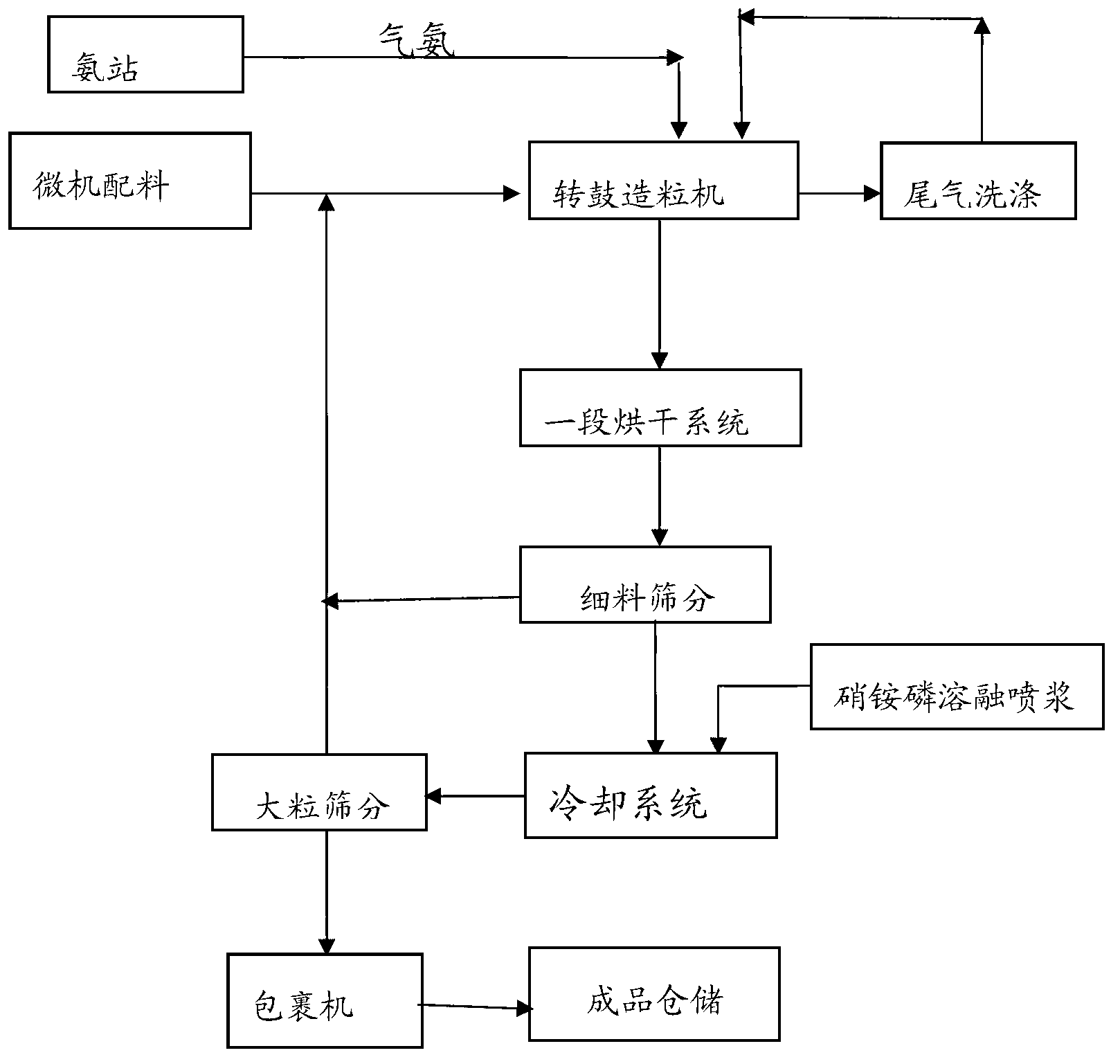
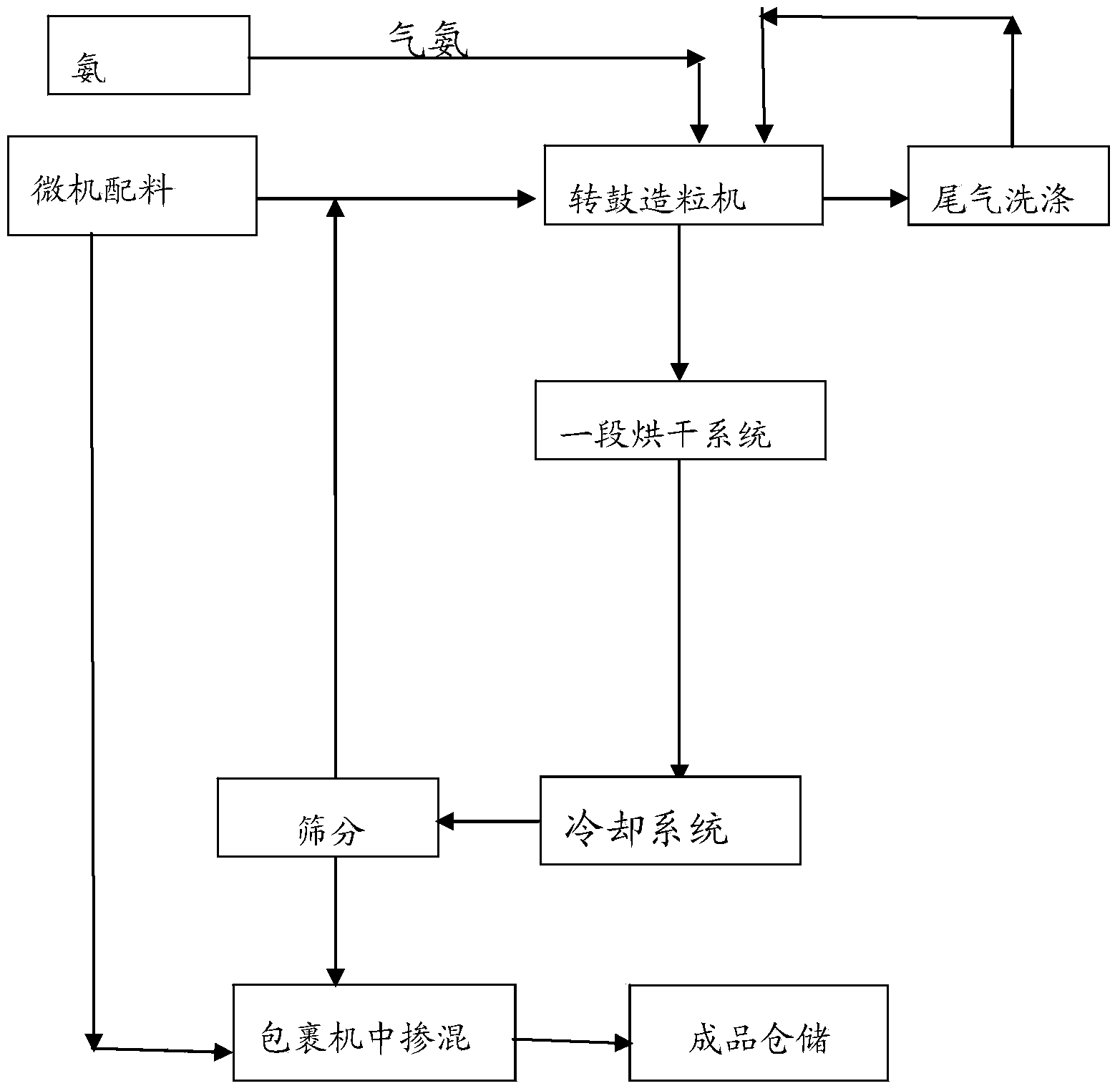
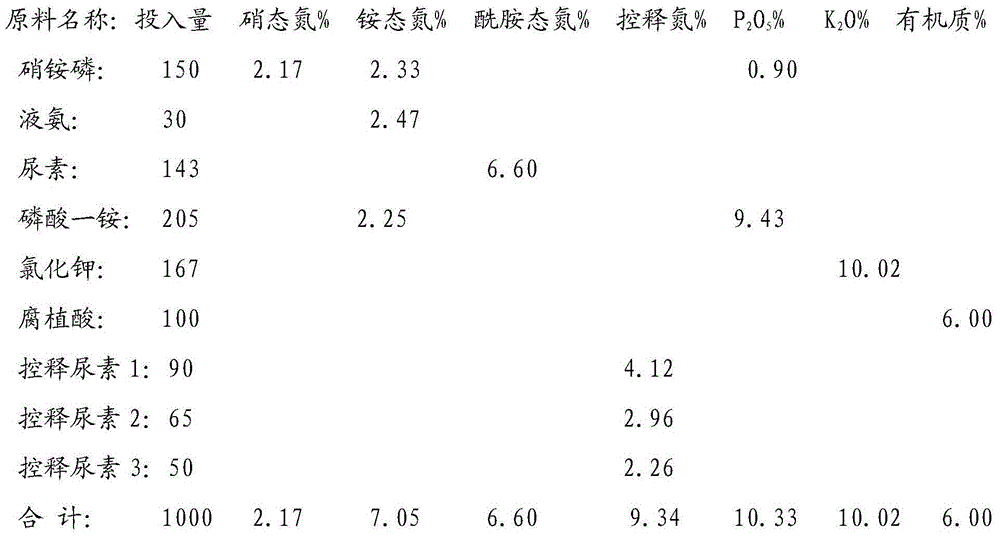
The invention discloses a whole-process nutrient supply type composite fertilizer and a preparation method thereof. A raw material containing nitrate nitrogen, ammonium nitrogen, amide nitrogen, controlled release nitrogen and organic matters is added in the material. The components of the whole-process nutrient feeding type composite fertilizer are effectively proportioned in percentage by mass on the basis of nitrogen content: 2-4% of nitrate nitrogen, 5-8% of ammonium nitrogen, 5-8% of amide nitrogen and 6-10% of controlled release nitrogen, the contents of phosphorus and potassium are determined according to different product proportions, and the content of the organic matters is not less than 6%. In the production process, the viscosity of urea nitrate generated in a reaction of ammonium nitrate phosphate and urea and granulation tail gas washing water are used as liquid phase amount necessary for granulation to finish the material granulation process, in order to prevent excessive urea nitrate from being generated in the granulation process, ammonia gas is directly introduced into the material layer of a rotor drum granulator to finish the ammoniation treatment of ammonium nitrate phosphate and control the quantity of the urea nitrate generated in the reaction. The fertilizer not only can improve the utilization rate of fertilizer nutrients and ensure the whole-process nutrient supply of crops so as to increase the yield of the crops and improve the quality, but also can avoid harm caused by escherichia coli and such toxic substances as heavy metal, improve the soil, reduce the soil salinity and fertilize the land.
Owner:天津芦阳肥业股份有限公司
Nitrogen oxide tail gas absorption solution and method for preparing powdered nitric acid with absorption solution
InactiveCN103028320AIncrease the flexibility of absorbing operationEnsure environmental complianceDispersed particle separationNitric acidUrea nitrateAbsorption efficiency
The invention discloses nitrogen oxide tail gas absorption solution which comprises the following components by weight percentage: 10-20% of urea, 3-15% of hydrochloric acid, 3-20% of nitric acid, and the balance of water. The invention further discloses a method for preparing powdered nitric acid with the nitrogen oxide tail gas absorption solution. The method comprises the steps that nitrogen oxide tail gas is supplied to a buffer tank filled with distilled water for cooling, initial absorption and tail gas flow velocity buffer; the nitrogen oxide tail gas is introduced into an absorption tower for multistage absorption; the temperature of the absorption solution is controlled at 15-25 DEG C; when urea nitrate is crystallized in the absorption solution, the absorption solution is frozen to be below 10 DEG C; a urea nitrate crystal (the powered nitric acid) is separated by crystallization; and a mother solution is recycled after consumed urea is supplemented and water volume is shifted. The nitrogen oxide tail gas absorption solution and the method have the advantages that the absorption efficiency of the nitrogen oxide tail gas is high, the urea consumption is small, the absorption solution can be recycled; the environmental protection management cost is low; powdered nitric acid is synthesized by the nitrogen oxide tail gas absorption solution; the production cost of powdered nitric acid is lowered; and the environmental pollution of the nitrogen oxide tail gas is solved.
Owner:TIANJIN VOCATIONAL INST
Hydrogenation hot gas chemical yield increasing solution component applied to low-yield low-permeability oil well
ActiveCN102942911AHigh porosityImprove permeabilityFluid removalDrilling compositionUrea nitrateMass ratio
The invention belongs to the technical field of oil exploitation, and particularly relates to a hydrogenation hot gas chemical yield increasing solution component capable of being used for increasing productivity of a low-yield low-permeability oil well. The solution component comprises first solution and second solution, the mass ratio of the first solution to the second solution is 1:1, the first solution comprises ammonium nitrate, ammonium chloride, urea, urea nitrate, glucose and water, and the second solution comprises sodium nitrite, urea, aluminum dodecaboride and carbon dichloride. The first solution and the second solution react, carry energy, penetrate through a well-cased perforating area of the oil well at a high speed and enter micro-cracks and air holes of near wellbore region rock strata, the micro-cracks can be broken through under the action of high-temperature high-pressure gas, oil-gas seepage channels are communicated, seepage resistance is decreased, drainage area is increased, accordingly, permeability of the near wellbore region rock strata is improved, and further the productivity of the individual well is increased.
Owner:吉林贯通能源科技有限责任公司
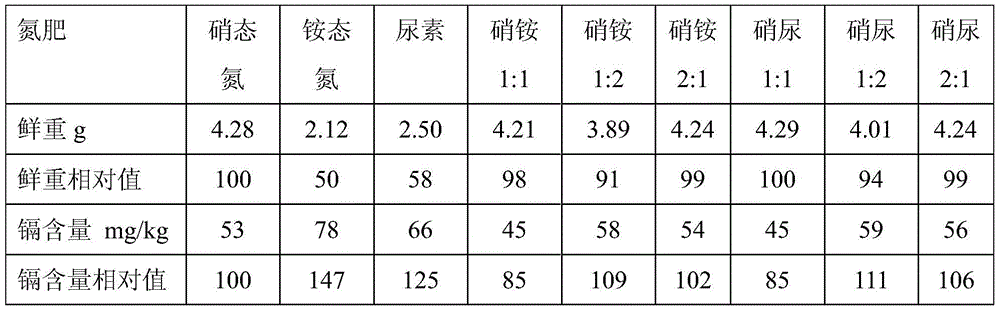
Nitrogen fertilizer management method suitable for planting vegetable crops with different buffer actions in cadmium-polluted farmland
InactiveCN104541719AReduce cadmium contentReduce outputFertilising methodsUrea nitrateNitrate nitrogen
The invention discloses a nitrogen fertilizer management method suitable for planting vegetable crops with different buffer actions in a cadmium-polluted farmland. The nitrogen fertilizer management method comprises the following steps: applying an ammonium nitrogen fertilizer or urea to the cadmium-polluted farmland when the acid buffering capacity of soil is more than or equal to 500mmol kg<-1>; applying an ammonium nitrate fertilizer or a urea nitrate fertilizer to the cadmium-polluted farmland when the acid buffering capacity of soil is more than 250mmol kg<-1> and less than 500mmol kg<-1>; applying a nitrate nitrogen fertilizer to the cadmium-polluted farmland when the acid buffering capacity of soil is less than or equal to 250mmol kg<-1>, wherein in the ammonium nitrate fertilizer, the use level ratio of the ammonium nitrogen fertilizer to the nitrate nitrogen fertilizer is 1 to 1 based on the quantity of N; in the urea nitrate fertilizer, the use level ratio of the urea to the nitrate nitrogen fertilizer is 1 to 1 based on the quantity of N. According to the nitrogen fertilizer management method, the nitrogen fertilizer suitable for the production of the planting vegetable crops in the cadmium-polluted farmland is selected according to the numerical value of acid buffering capacity of soil, so that the damage caused by cadmium to the growth of the crops is retarded, and the content of cadmium in the crops is decreased.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Slow-release type solid chlorine dioxide generating agent and preparation method thereof
The invention discloses a slow-release type solid chlorine dioxide generating agent and a preparation method thereof. The slow-release type solid chlorine dioxide generating agent has the advantages of plain and thin smell, stable concentration character, long lasting period and convenience in use. The slow-release type solid chlorine dioxide generating agent is prepared from the following components in parts by mass: 15 to 25 parts of sodium chlorite, 10 to 20 parts of urea nitrate, 40 to 50 parts of medical stone, 4 to 8 parts of magnesium sulfate, 4 to 8 parts of calcium sulfate, 3 to 8 parts of super absorbent resin and 2 to 6 parts of beta-cyclodextrin. The preparation method comprises the steps of smashing the medical stone into small particles and activating for 60 to 120 minutes at 90 to 100 DEG C to open inner holes of the medical stone; with the activated medical stone as a carrier, adding magnesium sulfate and calcium sulfate; adding sodium chlorite and urea nitrate; drying under assistance of the super absorbent resin; adding beta-cyclodextrin and curing into particle shapes; utilizing antioxidant aluminum plastic package bags to seal and package the cured particle-shaped products. The slow-release type solid chlorine dioxide generating agent can be widely applied to the field of space environment purification.
Owner:广州海音环保科技有限公司
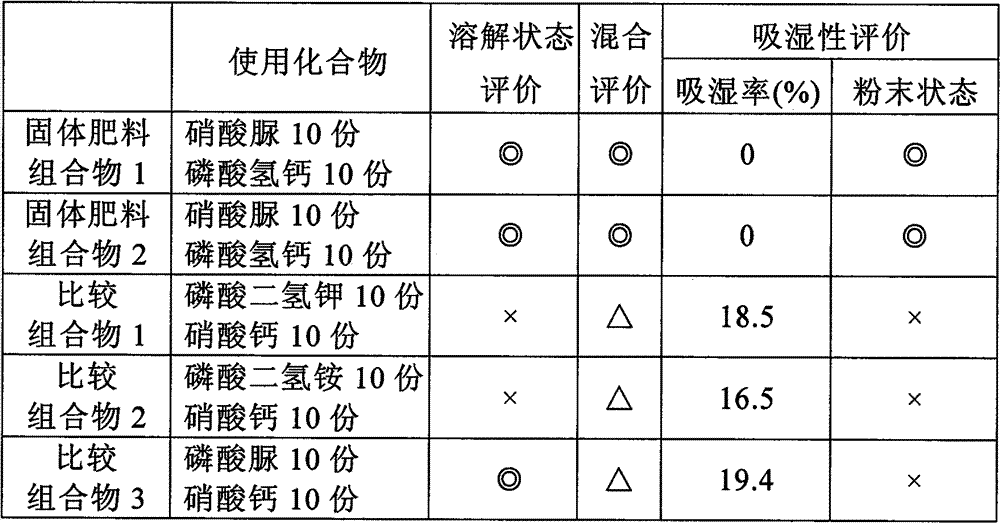
Fertilizer composition, process for producing the same and method of use thereof
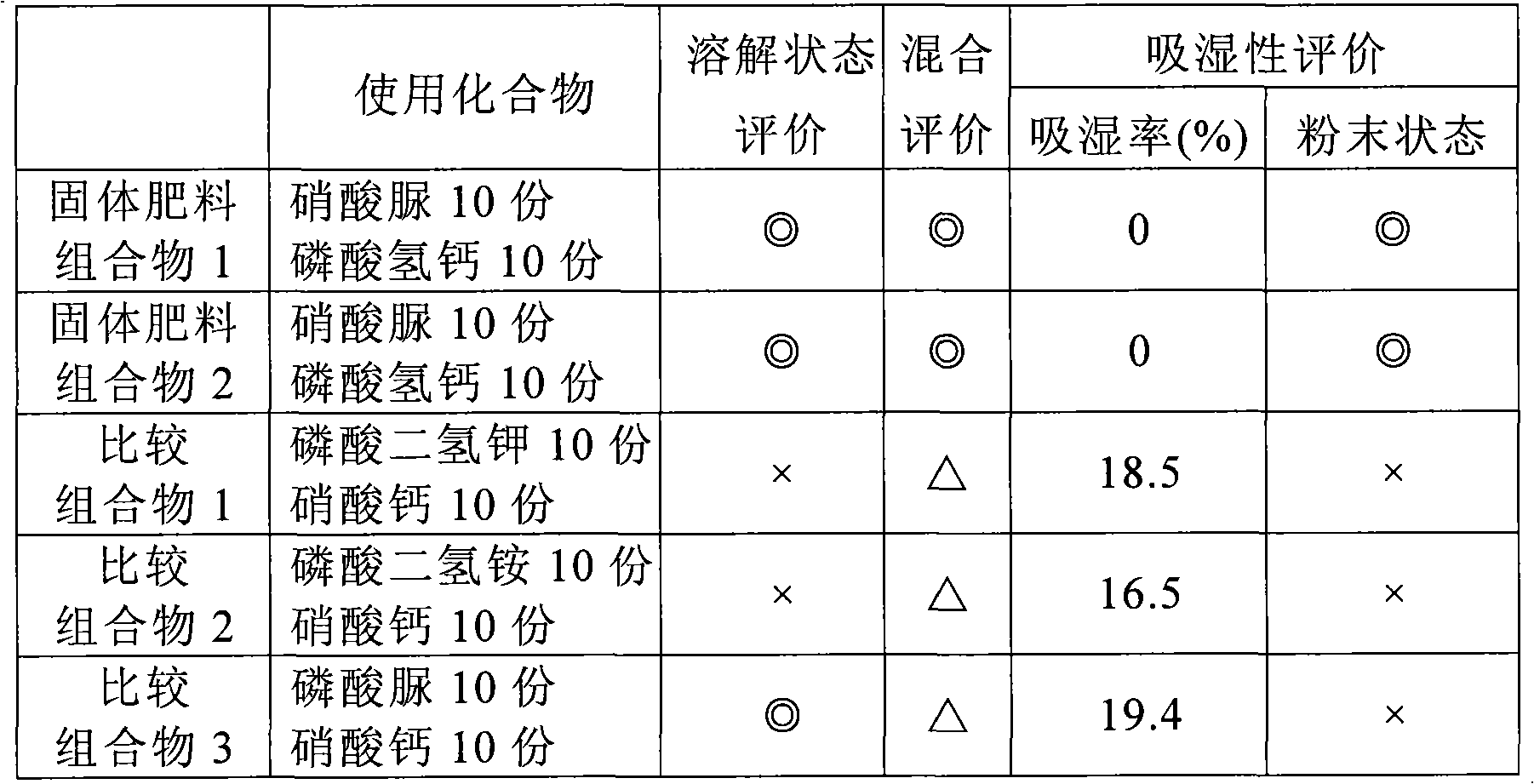
ActiveCN101405239BImprove stabilityWon't happenSuperphosphatesDi-calcium phosphate fertilisersCalcium biphosphateUrea nitrate
A solid fertilizer composition comprising urea nitrate and calcium phosphate; and a liquid fertilizer composition comprising urea nitrate and calcium phosphate both dissolved in water. These fertilizer compositions are fertilizers capable of feeding nitrogen, phosphoric acid and calcium which are crucial for the growth of plants, excelling in storage stability, and when dissolved in water, would not generate phosphate precipitates. These fertilizer compositions are suitable for use in a nutrient solution cultivation method, and, for example, the liquid fertilizer composition is applied to the stems and / or leaves of plants.
Owner:OTSUKA AGRITECHNO
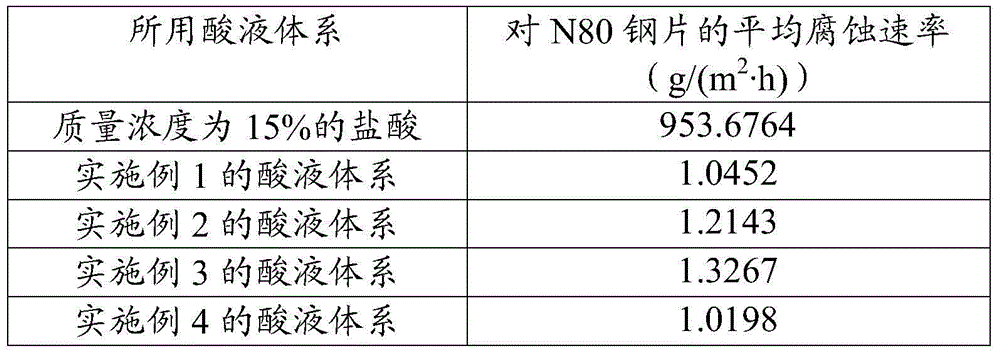
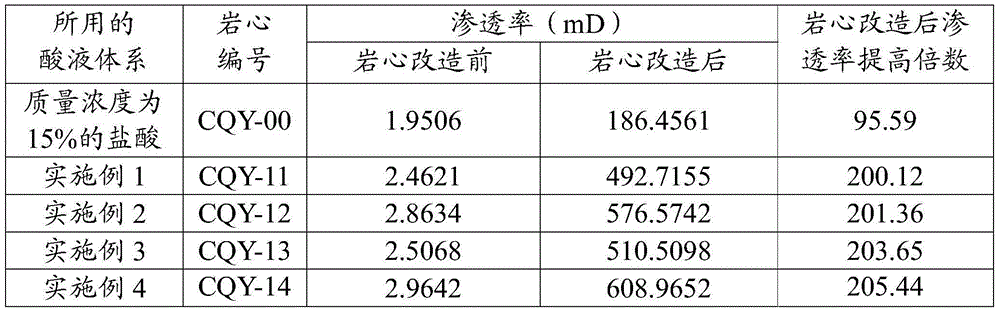
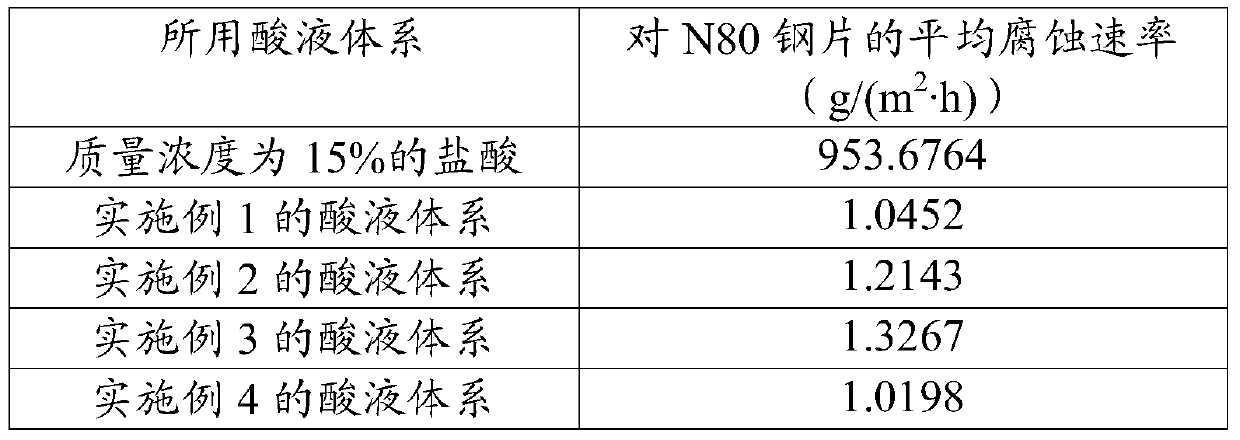
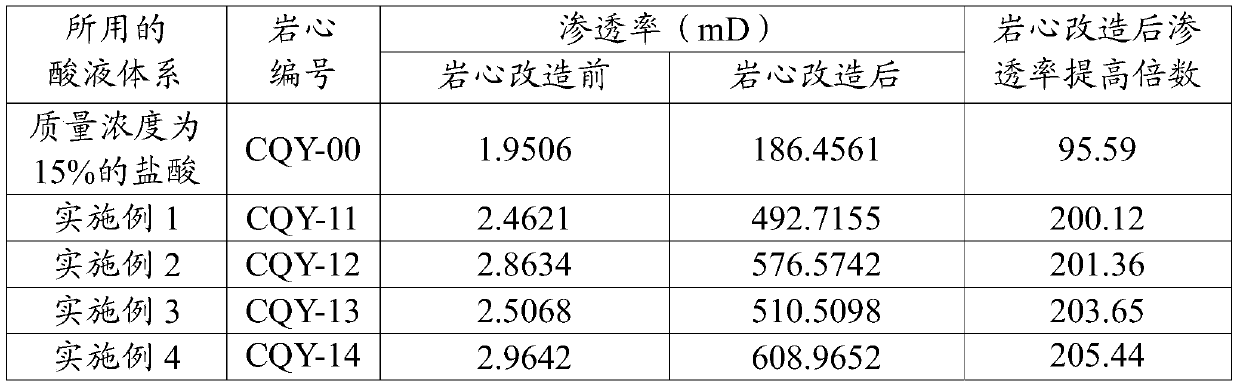
Acid solution system for acidifying carbonate reservoir rocks
The invention discloses an acid solution system for acidifying carbonate reservoir rocks, and belongs to the field of petrochemical industry. The acid solution system comprises the following components in percentages by mass: 10-50% of sulfamic acid, 10-50% of urea nitrate, 3-15% of organic weak acid, 0.1-5% of a cosolvent, 0.1-2% of a corrosion inhibitor, and the balance being water. The acid solution system has the advantages of high corrosion rate, large increasing range of the corrosion rate, low corrosion rate, and the like. The acid solution system provided by an embodiment has the advantages of simple formula, wide freight source of each component, transport convenience, and low cost; and the system is good for large scale on-site construction application.
Owner:PETROCHINA CO LTD
Neutralization method using reactive energetic materials
Formulations of reactive materials, such as aluminum, magnesium and alloys thereof, with combustible additives such as wood derivatives or charcoal, provide a composition for neutralizing energetic materials via combustion. Specifically, explosive substances such as ammonium nitrate and urea nitrate, which are commonly used as homemade explosives, are rapidly incinerated in a non-propagating manner by the contact with burning reactive material formulations.
Owner:MATSYS
Process for producing high-purity manganese dioxide
InactiveCN101428861ASolve the problem of scarringWill not remainManganese oxides/hydroxidesNitrateManganese
A method for preparing high-purity manganese dioxide is characterized in that the method comprises the following steps: adding a certain proportion of additives to high-purity manganese nitrate solution with the weight concentration being 30% to 70%; and pyrolyzing at the temperature of 150 to 250 DEG C to obtain the high-purity manganese dioxide, wherein, the selected additives include one or more of ammonium carbonate, ammonium hydrogen carbonate, ammonium nitrate, carbamide and urea nitrate; and the proportion of the additives in the manganese nitrate solution is 1% to 15% by the weight of manganese nitrate.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Method for decomposing phosphorite through urea nitrate to prepare granular urea nitrate compound fertilizer
InactiveCN108530207AReduce volatile lossOvercome the disadvantage of easy moisture absorptionAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersUrea nitrateMoisture absorption
The invention relates to a method for decomposing phosphorite through urea nitrate to prepare a granular urea nitrate compound fertilizer. The method includes the steps of preparing a urea nitrate water solution and conducting phosphorite decomposing, neutralizing, granulating and the like. The compound fertilizer is prepared through the one-step method of decomposing phosphorite through the ureanitrate solution, the temperature required for reaction is low, the volatile loss of nitrogen is reduced, the phosphorite conversion rate can reach 95% or above, the defect that a calcium nitrate product is prone to moisture absorption in the process of decomposing phosphorite through nitric acid is overcome by means of the method, the fluorine escape rate is greatly reduced, and the method is anenergy-saving and environment-friendly type urea nitrate compound fertilizer production process with diversified nitrogen nutrient form and high efficiency.
Owner:ZHENGZHOU UNIV
Liquid fertilizer for soil cultivation and method of cultivating crop
InactiveCN101410350AExcellent fertilizer effectHorticulture methodsLiquid fertilisersUrea nitratePhytotoxicity
A liquid fertilizer for soil cultivation that realizes use of urea nitrate substantially not having been utilized as a fertilizer to date and attains exertion of strikingly enhanced fertilizer effects. There is provided a liquid fertilizer for soil cultivation comprising urea nitrate wherein the content of urea nitrate is in the range of 0.006 to 1.2 wt.%. By application of this liquid fertilizer for soil cultivation onto the soil surface and / or in the earth while avoiding direct contact with the leaves of germinating crops, crop cultivation can be carried out without phytotoxicity.
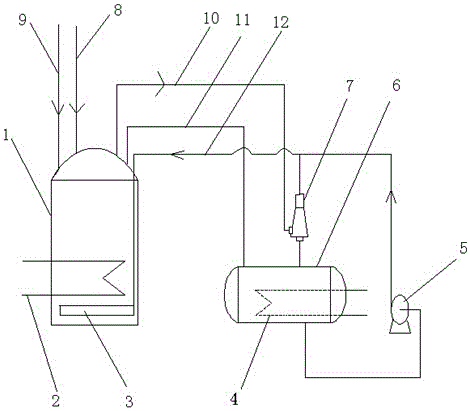
A kind of device and preparation method of urea method bleaching nitric acid solution
InactiveCN105013382BAvoid pollutionGuaranteed colorlessMixer accessoriesUrea nitrateNitrogen dioxide
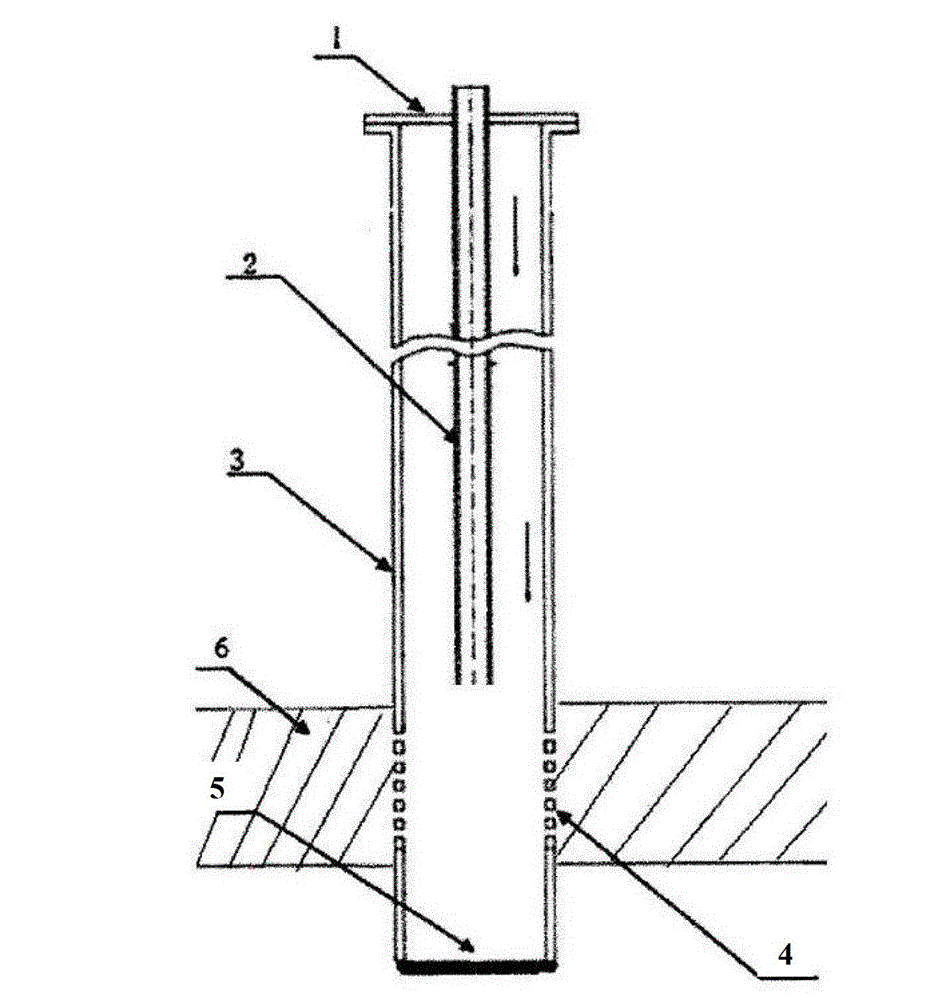
The invention provides a device for bleaching a nitric acid solution through a urea method and a preparation method therefor. The device comprises an acid preparation tank and a urea storage tank, wherein the acid preparation tank is connected with the urea storage tank through a communicating pipe; the top end of the acid preparation tank is connected with one inlet end of an ejector through a nitrogen dioxide pipe, the other inlet end of the ejector is connected with the urea storage tank, and the outlet end of the ejector is connected with a liquid distributor horizontally arranged inside the acid preparation tank through a urea pipe; and the outlet of the urea storage tank is connected with the urea pipe and the ejector respectively through a circulating conveying pump. Under micro negative pressure, NO2 in nitric acid and a urea solution both enter the ejector for reaction, and enter the acid preparation tank together with the urea solution to react with carbon dioxide generated later. Long-acting colorlessness of the nitric acid solution can be realized for a long time, so that the content of carbon dioxide in the acid preparation tank is quickly controlled, and the content overstandard problem of nitrogen dioxide is solved.
Owner:ANHUI WANBEI COAL REFCO GRP LTD HANSHAN HENGTAI NONMETALLIC MATERIALS BRANCH
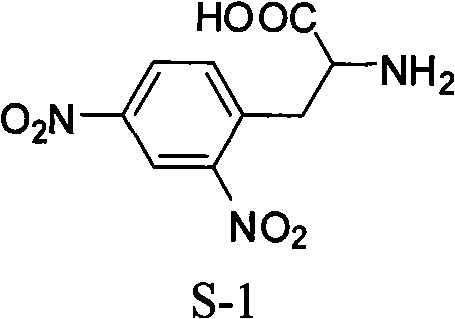
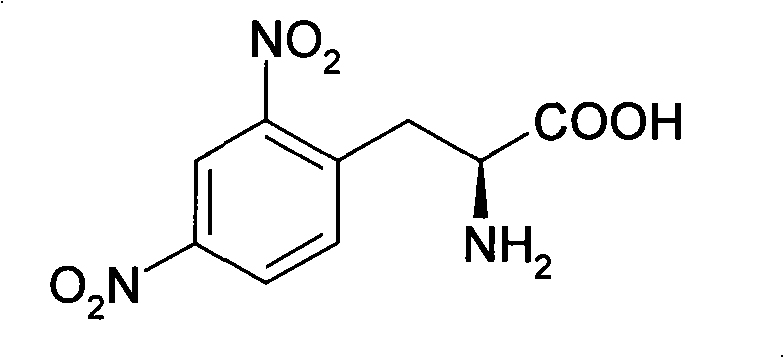
Method for synthesizing L-2,4-dinitrophenylalanine
InactiveCN101591261AHigh reactivityAvoid oxidative decarboxylationOrganic compound preparationAmino-carboxyl compound preparationUrea nitrateReaction temperature
The invention discloses a method for synthesizing L-2,4-dinitrophenylalanine. L-phenylalanine or L-4-nitrophenylalanine which is used as a raw material and urea nitrate and concentrated sulfuric acid which are used as nitration reagents are subjected to nitration reaction, the reaction temperature is between 50 and 80 DEG C, and the reaction time is 4 to 10 hours; and after the reaction is finished, the reaction solution is neutralized by alkali water solution, dehydrated and recrystallized by a solvent to form the L-2,4-dinitrophenylalanine. The method for synthesizing the L-2,4-dinitrophenylalanine has the characteristics of simple process, little side reaction, high yield, and the like.
Owner:ZHEJIANG UNIV
High-efficiency foaming agent for explosives
InactiveCN102093145BFast foamingStable chemical propertiesNon-explosive/non-thermic compositionsPhosphateUrea nitrate
The invention relates to a special sensitizer for emulsion explosives, particularly a high-efficiency foaming agent for explosives. The high-efficiency foaming agent for explosives is prepared by mixing a component A and a component B according to the weight ratio of 1:(1.2-1.5). The component A is prepared from the following components in percentage by weight: 0.5-1.5% of thiocarbamide, 3-5% of potassium nitrite, 0.9-1.5% of urea, 20-30% of sodium nitrite and 62-75.6% of water. The component B is prepared from the following components in percentage by weight: 0.7-1% of nitric acid, 7-11% of ammonium nitrate, 0.2-0.5% of ammonium chloride, 0.2-0.6% of phosphoric acid, 0.5-2% of ammonium monoacid phosphate, 0.1-2.5% of ammonium thiocyanate, 1.8-2.5% of citric acid, 0.7-1.5% of glacial acetic acid, 1.5-2.5% of urea nitrate, 0.9-1.5% of urea and 74.4-86.4% of water. The invention has the characteristics of high foaming speed, weak acidity, high storage stability, long natural storage period, small aftereffect, favorable foaming effect and the like.
Owner:马永宁
A kind of low detonation velocity mixed explosive for explosive welding and its preparation method and application
ActiveCN112876325BHigh detonation velocityImprove securityExplosive working-up apparatusNon-electric welding apparatusCarbide siliconTEX-explosive
The invention provides a low-detonation-velocity mixed explosive for explosive welding and its preparation method and application, which is specially used for explosive compounding of low-melting-point metals. The components (mass percentage) of the low-detonation-velocity mixed explosive include: 60-70% antimony-free rock Ammonium nitrate explosive (70-85% ammonium nitrate, 3-6% wood powder, 10-20% modified urea nitrate, 1.5-4% compound modifier), 15-20% calcium carbonate powder, 5-15 % silicon carbide powder and 5‑10% water. The low detonation velocity explosive is prepared by uniformly mixing the above components on site. The explosive of the present invention can significantly reduce the detonation velocity of common antimony-free rock ammonium nitrate explosives and the cost of explosive composite explosives; the present invention is easy to produce and stable in performance, and solves the problem of excessively high detonation velocity and easy destruction when conventional explosives are used to prepare explosive composite plates of low-melting-point metal materials Disadvantages of low melting point materials. Explosives with different detonation velocity requirements can be prepared on site according to different materials, the process is simple, and it is suitable for large-scale application.
Owner:HUNAN FORHOME COMPOSITE MATERIALS CO LTD
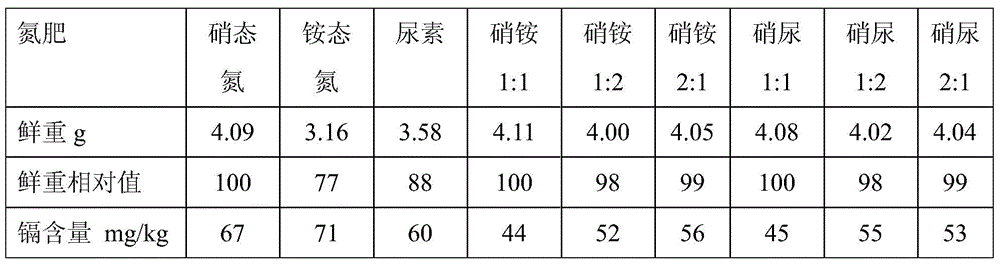
Nitrogen fertilizer management methods suitable for planting vegetable crops in different buffer cadmium polluted farmlands
InactiveCN104541719BReduce cadmium contentReduce outputFertilising methodsUrea nitrateNitrate nitrogen
The invention discloses a nitrogen fertilizer management method suitable for planting vegetable crops with different buffer actions in a cadmium-polluted farmland. The nitrogen fertilizer management method comprises the following steps: applying an ammonium nitrogen fertilizer or urea to the cadmium-polluted farmland when the acid buffering capacity of soil is more than or equal to 500mmol kg<-1>; applying an ammonium nitrate fertilizer or a urea nitrate fertilizer to the cadmium-polluted farmland when the acid buffering capacity of soil is more than 250mmol kg<-1> and less than 500mmol kg<-1>; applying a nitrate nitrogen fertilizer to the cadmium-polluted farmland when the acid buffering capacity of soil is less than or equal to 250mmol kg<-1>, wherein in the ammonium nitrate fertilizer, the use level ratio of the ammonium nitrogen fertilizer to the nitrate nitrogen fertilizer is 1 to 1 based on the quantity of N; in the urea nitrate fertilizer, the use level ratio of the urea to the nitrate nitrogen fertilizer is 1 to 1 based on the quantity of N. According to the nitrogen fertilizer management method, the nitrogen fertilizer suitable for the production of the planting vegetable crops in the cadmium-polluted farmland is selected according to the numerical value of acid buffering capacity of soil, so that the damage caused by cadmium to the growth of the crops is retarded, and the content of cadmium in the crops is decreased.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
An acid liquid system for acidifying carbonate rock reservoirs
The invention discloses an acid solution system for acidifying carbonate reservoir rocks, and belongs to the field of petrochemical industry. The acid solution system comprises the following components in percentages by mass: 10-50% of sulfamic acid, 10-50% of urea nitrate, 3-15% of organic weak acid, 0.1-5% of a cosolvent, 0.1-2% of a corrosion inhibitor, and the balance being water. The acid solution system has the advantages of high corrosion rate, large increasing range of the corrosion rate, low corrosion rate, and the like. The acid solution system provided by an embodiment has the advantages of simple formula, wide freight source of each component, transport convenience, and low cost; and the system is good for large scale on-site construction application.
Owner:PETROCHINA CO LTD
Oxidizing agent for firework and firecracker leads and preparation method thereof
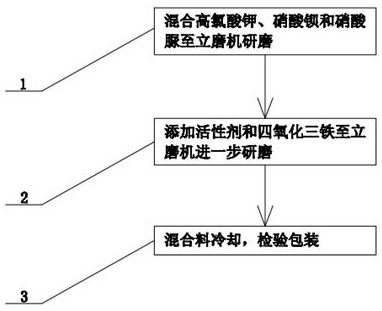
InactiveCN113372181AGood chemical stabilityImprove securityExplosivesPressure gas generationPerchlorate saltUrea nitrate
The invention discloses an oxidizing agent for firework and firecracker leads and a preparation method thereof, and relates to firework and firecracker. A certain amount of potassium perchlorate, barium nitrate and urea nitrate are taken and put into a vertical mill to be ground until the materials pass through a certain-mesh sieve, then grinding is stopped, a certain amount of an active agent and ferroferric oxide are further added into the vertical mill, then the vertical mill is started to be ground, the rotating speed of the vertical mill is adjusted, after grinding is conducted for a period of time, the obtainedmixture is sieved, and finally, the mixture is cooled to normal temperature, and inspected to be qualified. According to the invention, potassium perchlorate, barium nitrate and urea nitrate are adopted as ingredients, and potassium perchlorate, barium nitrate and urea nitrate have the characteristics of relatively high chemical stability and low sensitivity to machinery and friction, so that the prepared oxidizing agent has the characteristic of high safety.
Owner:湖南庆泰烟花制造有限公司
Production method of readily available and controlled release composite fertilizer containing three nitrogen elements
Owner:天津芦阳肥业股份有限公司
Fertilizer composition, process for producing the same and method of use thereof
ActiveCN101405239AImprove stabilityWon't happenSuperphosphatesDi-calcium phosphate fertilisersCalcium biphosphatePhosphate
The present invention provides a solid fertilizer composition comprising urea nitrate and calcium phosphate; and a liquid fertilizer composition comprising urea nitrate and calcium phosphate both dissolved in water. These fertilizer compositions are fertilizers capable of feeding nitrogen, phosphoric acid and calcium which are crucial for the growth of plants, excelling in storage stability, and when dissolved in water, would not generate phosphate precipitates. These fertilizer compositions are suitable for use in a nutrient solution cultivation method, and, for example, the liquid fertilizer composition is applied to the stems and / or leaves of plants.
Owner:OTSUKA AGRITECHNO
Hydrogenation hot gas chemical yield increasing solution component applied to low-yield low-permeability oil well
ActiveCN102942911BHigh porosityImprove permeabilityFluid removalDrilling compositionUrea nitrateMass ratio
The invention belongs to the technical field of oil exploitation, and particularly relates to a hydrogenation hot gas chemical yield increasing solution component capable of being used for increasing productivity of a low-yield low-permeability oil well. The solution component comprises first solution and second solution, the mass ratio of the first solution to the second solution is 1:1, the first solution comprises ammonium nitrate, ammonium chloride, urea, urea nitrate, glucose and water, and the second solution comprises sodium nitrite, urea, aluminum dodecaboride and carbon dichloride. The first solution and the second solution react, carry energy, penetrate through a well-cased perforating area of the oil well at a high speed and enter micro-cracks and air holes of near wellbore region rock strata, the micro-cracks can be broken through under the action of high-temperature high-pressure gas, oil-gas seepage channels are communicated, seepage resistance is decreased, drainage area is increased, accordingly, permeability of the near wellbore region rock strata is improved, and further the productivity of the individual well is increased.
Owner:吉林贯通能源科技有限责任公司
Low explosive for metal welding and preparation method
InactiveCN105130719AImprove detonation sensitivityPromote safe productionNon-explosive/non-thermic compositionsTEX-explosiveUrea nitrate
The invention provides a low explosive for metal welding. The low explosive comprises, in percent by weight, 27-30% of urea, 27-30% of nitric acid, 18-20% of ammonium nitrate, 0.10-0.15% of octadecyl primary amine, 21.90-25.85% of deposit white carbon black, and water. The low explosive is prepared according to the following preparation method: firstly adding 27-30 Kg of urea into 30-35 Kg of water according to the batches, heating to 30-40 DEG C, stirring, after dissolving, adding 27-30 Kg of nitric acid at a uniform speed within 10 min, and stirring for 30 min, so as to generate urea nitrate; adding 18-20 Kg of ammonium nitrate and 0.10-0.15 Kg of octadecyl primary amine into urea nitrate, heating with stirring, controlling the heating temperature for 100-110 DEG C, after 30 min, performing vacuum drying and crushing; and adding 21.90-25.85 Kg of deposit white carbon black, and performing grinding and uniform mixing, so as to obtain the low explosive for metal welding. The material cost is low, production is simple, the detonation sensitivity is good, the detonation velocity is stable and adjustable, the explosive detonation velocity is 2000-2400 m / s, the storage life is within 1 year, the explosive does not cake, production is safe and simple, the employed materials all do not contain toxic and harmful compositions, and the production and usage processes are harmless to contact personnel and environment.
Owner:湖北东神天神实业有限公司
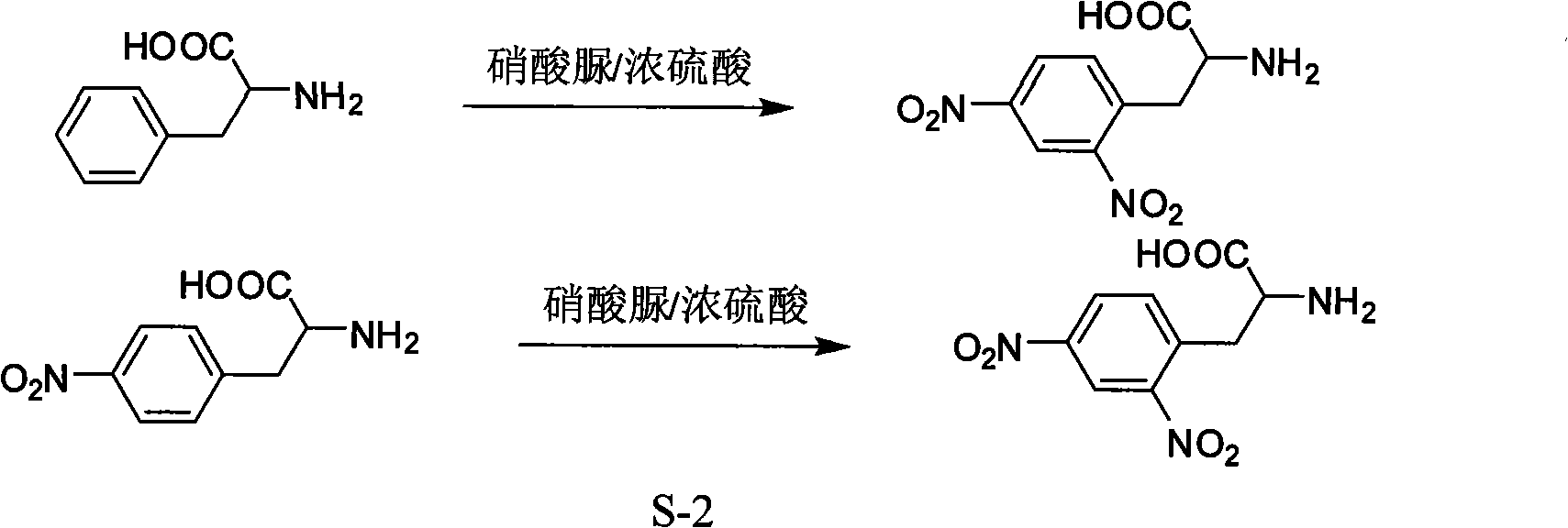
Process for synthesizing L-2,4-dinitrophenyl alanine
InactiveCN101462976BLow yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationUrea nitrateDinitrophenyl
The invention discloses a method for synthesizing L-2, 4-dinitrobenzene lactamine. L-4-nitrobenzene lactamine is dissolved in concentrated sulfuric acid, and urea nitrate or nitrourea is added to react for 1-20 hours at the temperature of 0-100 DEG C. The mol ratio of the urea nitrate or nitrourea and the L-4-nitrobenzene lactamine is 1.2-3.0:1. An obtained reaction product is put into ice water;then pH is adjusted to neutral by alkaline substance; solvent is removed; residual solid is recrystallized by saturated fatty alcohol; and separated yellow solid is the L-2, 4-dinitrobenzene lactamine. The method for producing the L-2, 4-dinitrobenzene lactamine is characterized by good safety, convenient operation and high yield.
Owner:ZHEJIANG UNIV
Neutralization method using reactive energetic materials
Formulations of reactive materials, such as aluminum, magnesium and alloys thereof, with combustible additives such as wood derivatives or charcoal, provide a composition for neutralizing energetic materials via combustion. Specifically, explosive substances such as ammonium nitrate and urea nitrate, which are commonly used as homemade explosives, are rapidly incinerated in a non-propagating manner by the contact with burning reactive material formulations.
Owner:MATSYS
Gas generating agent and preparation method thereof
ActiveCN112979395AImprove flammabilityIncrease gas productionExplosivesPotassium nitrateChromate salt
The invention relates to a gas generating agent, which comprises, by mass, 76-80% of a component A, 12-21% of a component B and 3-8% of an auxiliary agent, the component A comprises urea nitrate and guanidine nitrate according to a certain ratio, the component B comprises potassium nitrate and ethylenediamine chromate according to a certain ratio, and the auxiliary agent is sodium tetraborate. The formula has the advantages that oxygen balance is easy to achieve, the combustion performance is good, the gas production rate is high, the mechanical sensitivity is low, storage is easy and the like, gas production components are basically non-toxic CO2, N2 and water vapor, and the non-sodium-azide gas production agent is an ideal non-sodium-azide gas production agent.
Owner:ZHONGBEI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com