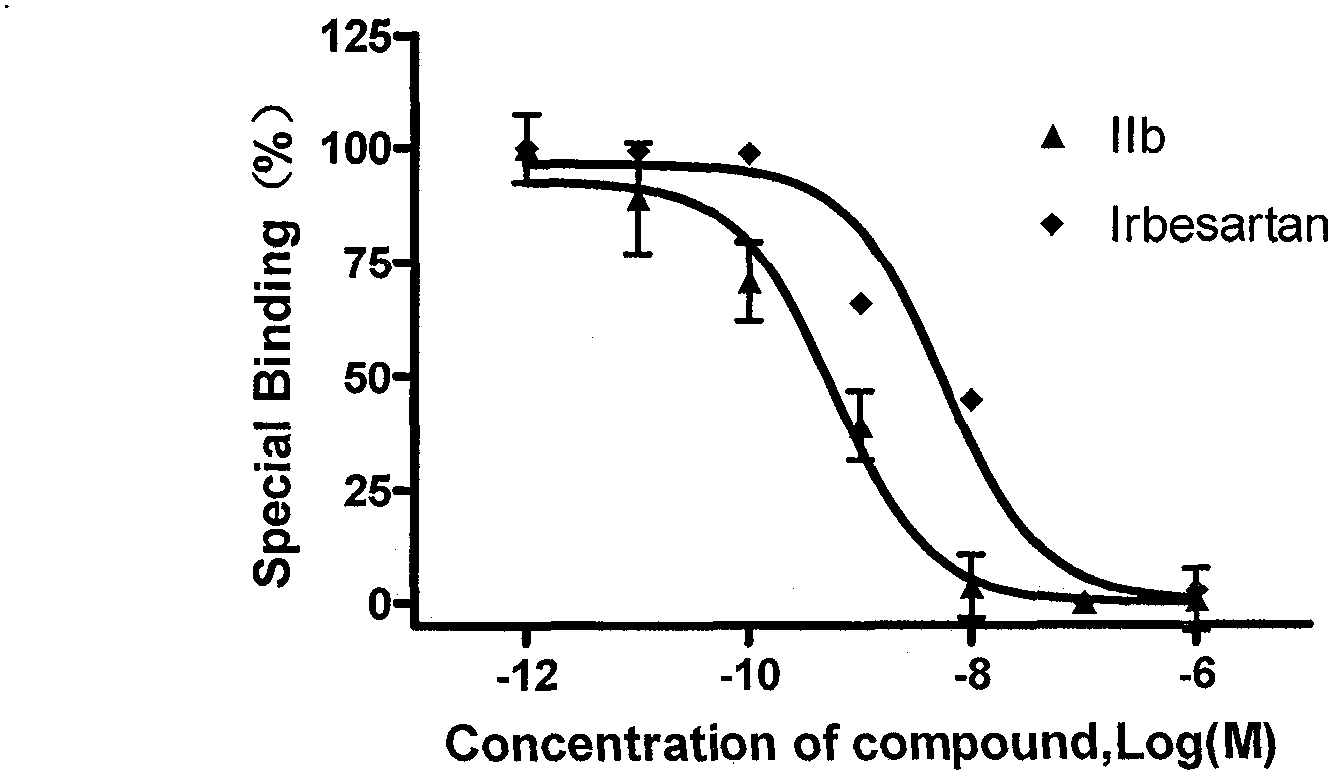
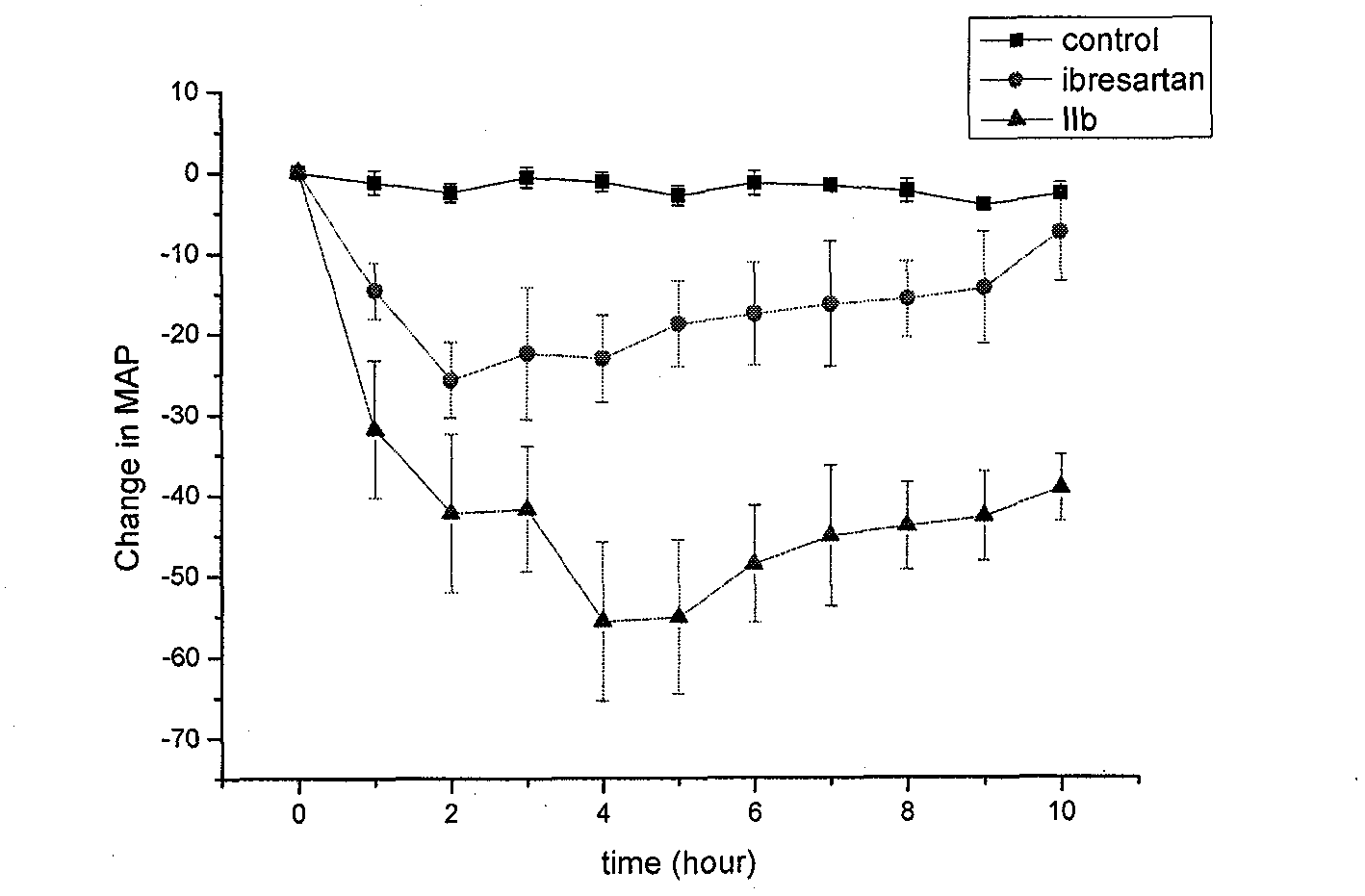
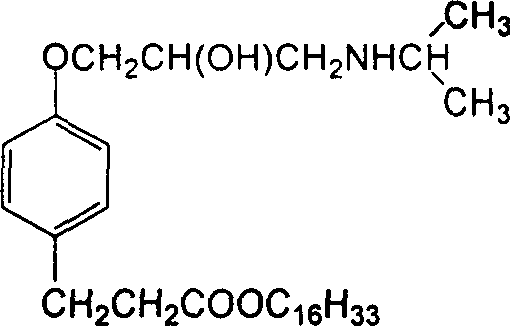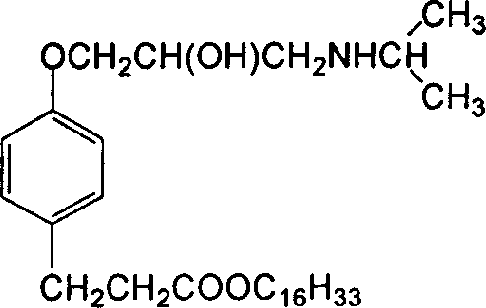Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Receptor blockade" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Receptor blockade, blocking the effect of a hormone at the cell surface. 3. Arrest of nerve impulse conduction or transmission at autonomic synaptic junctions, autonomic receptor sites, or neuromuscular junctions by various means, most often pharmacotherapy.
Use of vitamin Ds to treat kidney disease
InactiveUS20050124591A1Reduce inflammationSuppression problemBiocideUrinary disorderDiseaseAngiotensin Receptor Blockers
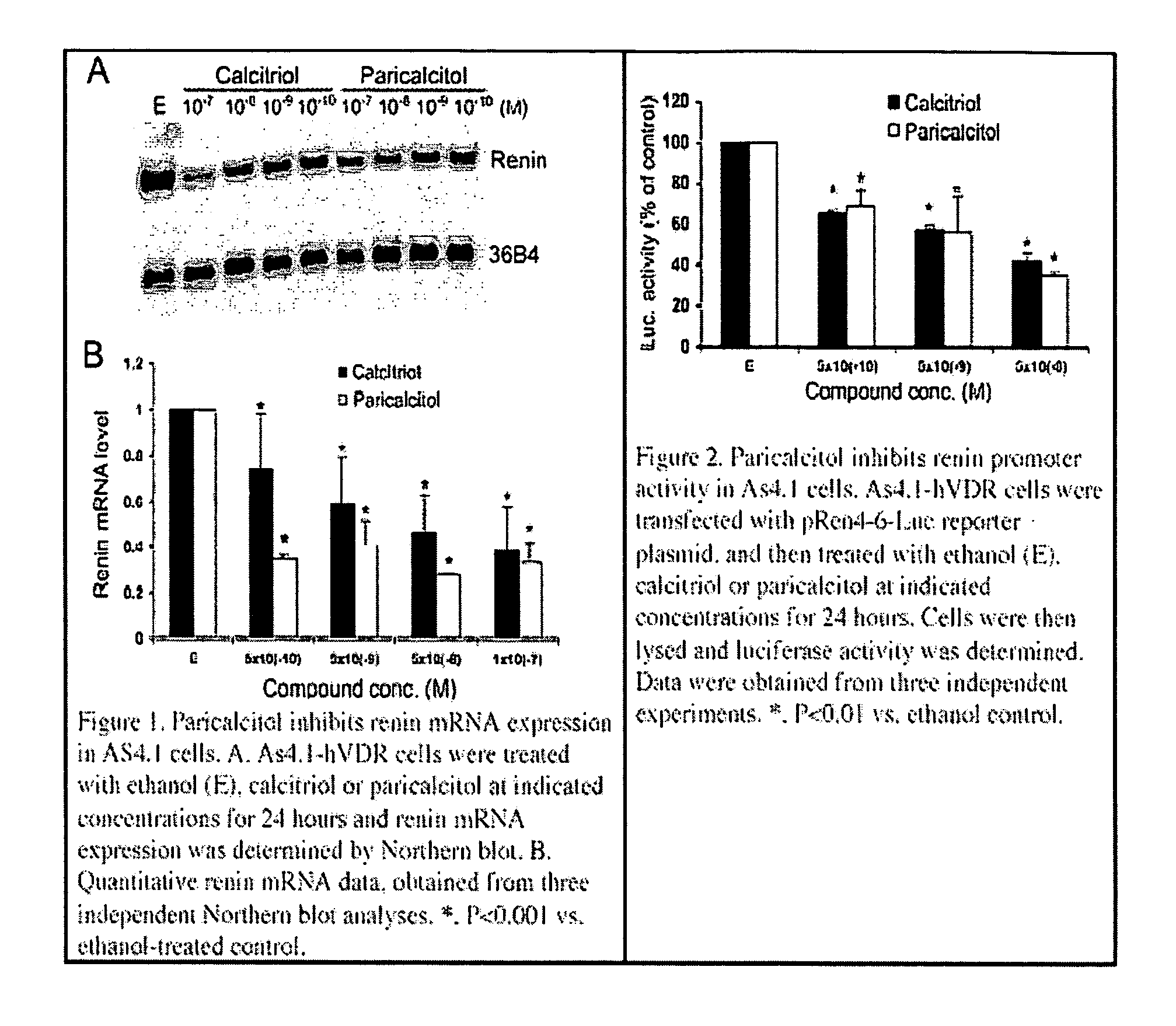
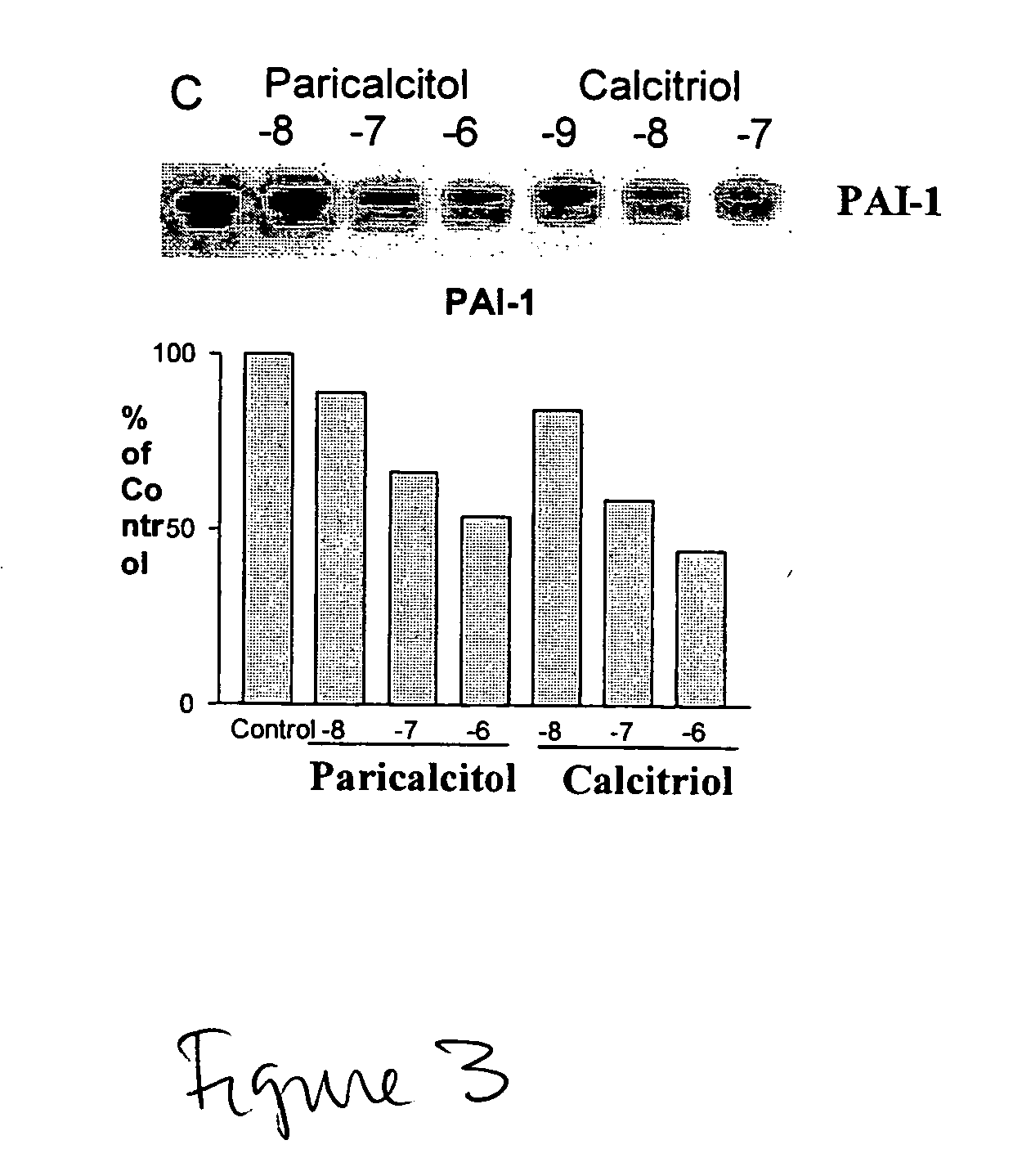
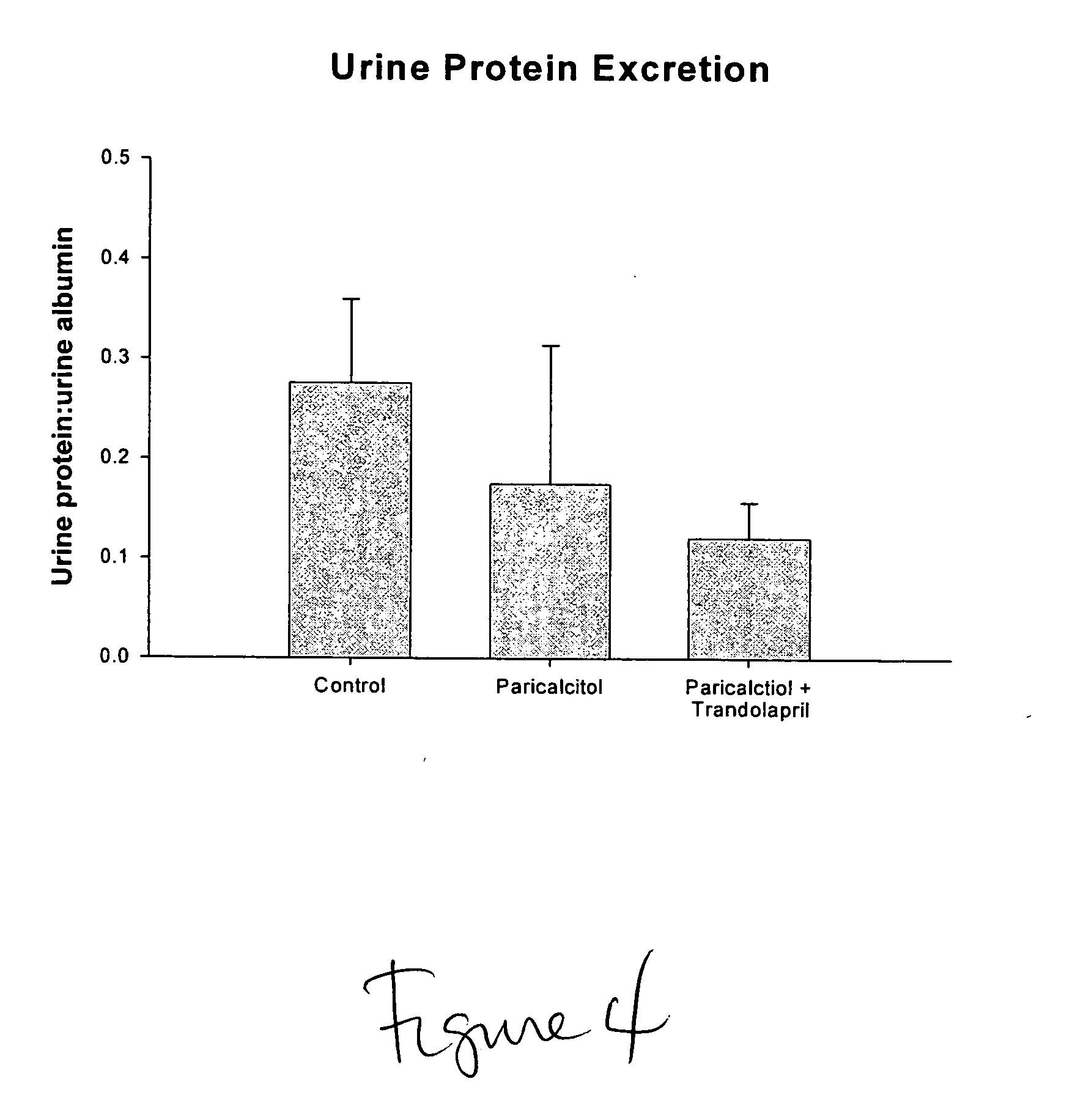
Disclosed are compositions containing a VDRA / Vitamin D analog to treat or prevent kidney disease, including chronic kidney disease. The present invention also relates to methods of treating kidney disease by administering to a patient a pharmaceutical composition containing a therapeutically effective amount of a VDRA / Vitamin D analog. Compositions according to the invention include a VDRA / Vitamin D analog and at least one of the following agents: an ACE inhibitor, an angiotensin (II) receptor blocker (ARB) and aldosterone blocker in therapeutically effective amounts to inhibit renin production or inhibit activation of the renin-angiotensin-aldosterone system. Preferred compositions contain paricalcitol with at least one of these other agents. Such combinations can avoid ACE inhibition escape and aldosterone escape with subsequent increase in angiotensin (II) and aldosterone generation.
Owner:ABBOTT LAB INC
1-(4-arylpiperazin-1-yl)- omega -[n-( alpha . omega -dicarboximido)]-alkanes useful as uro-selective alpha 1-adrenoceptor blockers
Novel piperzine derivatives substituted on one nitrogen by an aromatic system and on the other nitrogen by (2,5-dioxopyrrolidin)-1-yl) alkanes or (2,6-dioxopiperidin-1-yl) alkanes have been found to exhibit selective alpha 1A adrenergic activity. The compounds are useful for treatment of disease conditions, such as peripheral vascular disease, congestive heart failure, hypertension and especially benign prostatic hypertrophy.
Owner:RANBAXY LAB LTD
Measurement of gait dynamics and use of beta-blockers to detect, prognose, prevent and treat amyotrophic lateral sclerosis
InactiveUS20070021421A1Prevent weight lossPreventing and delaying and mitigating symptomCompounds screening/testingBiocideBeta blockerAdrenergic
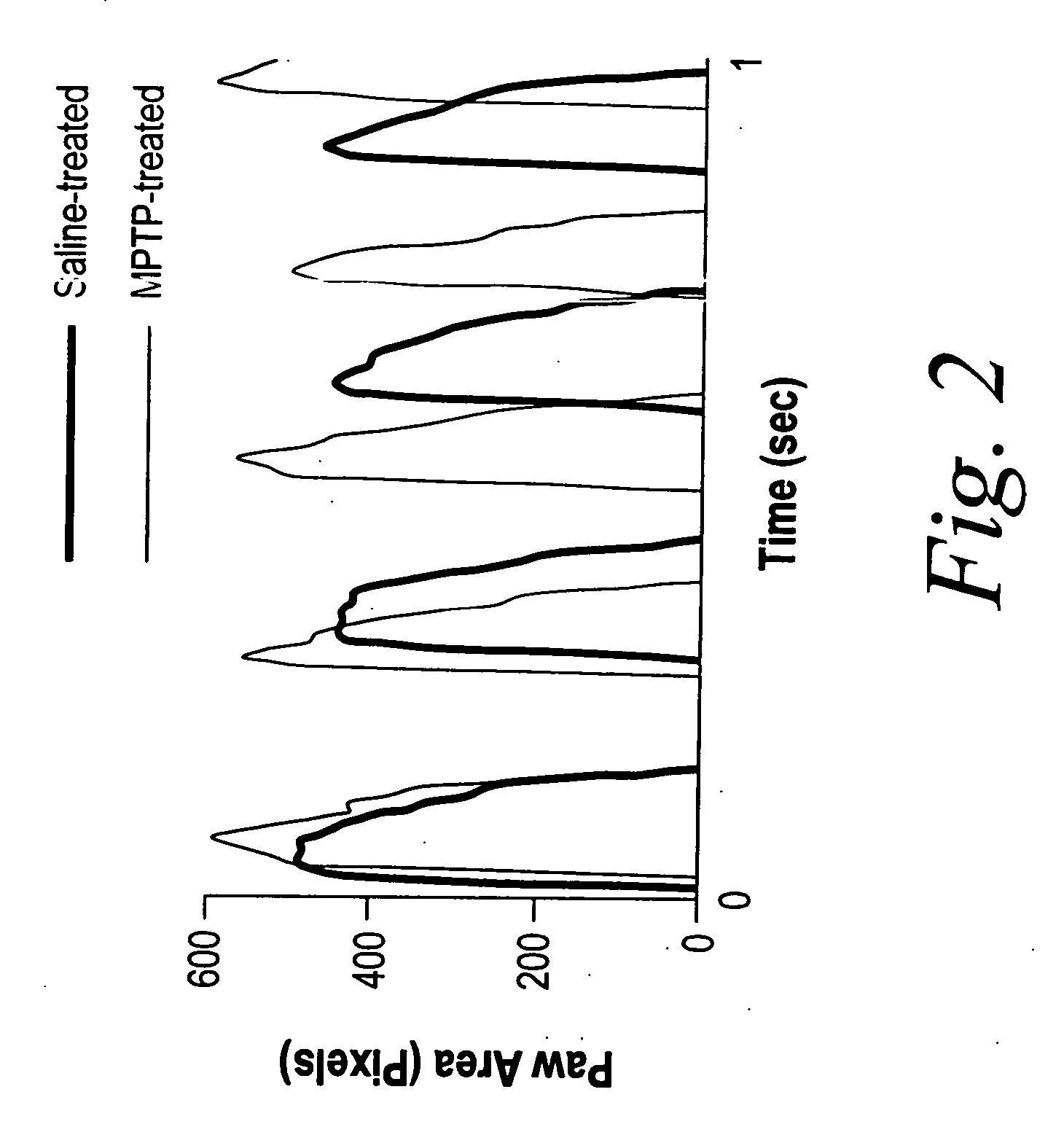
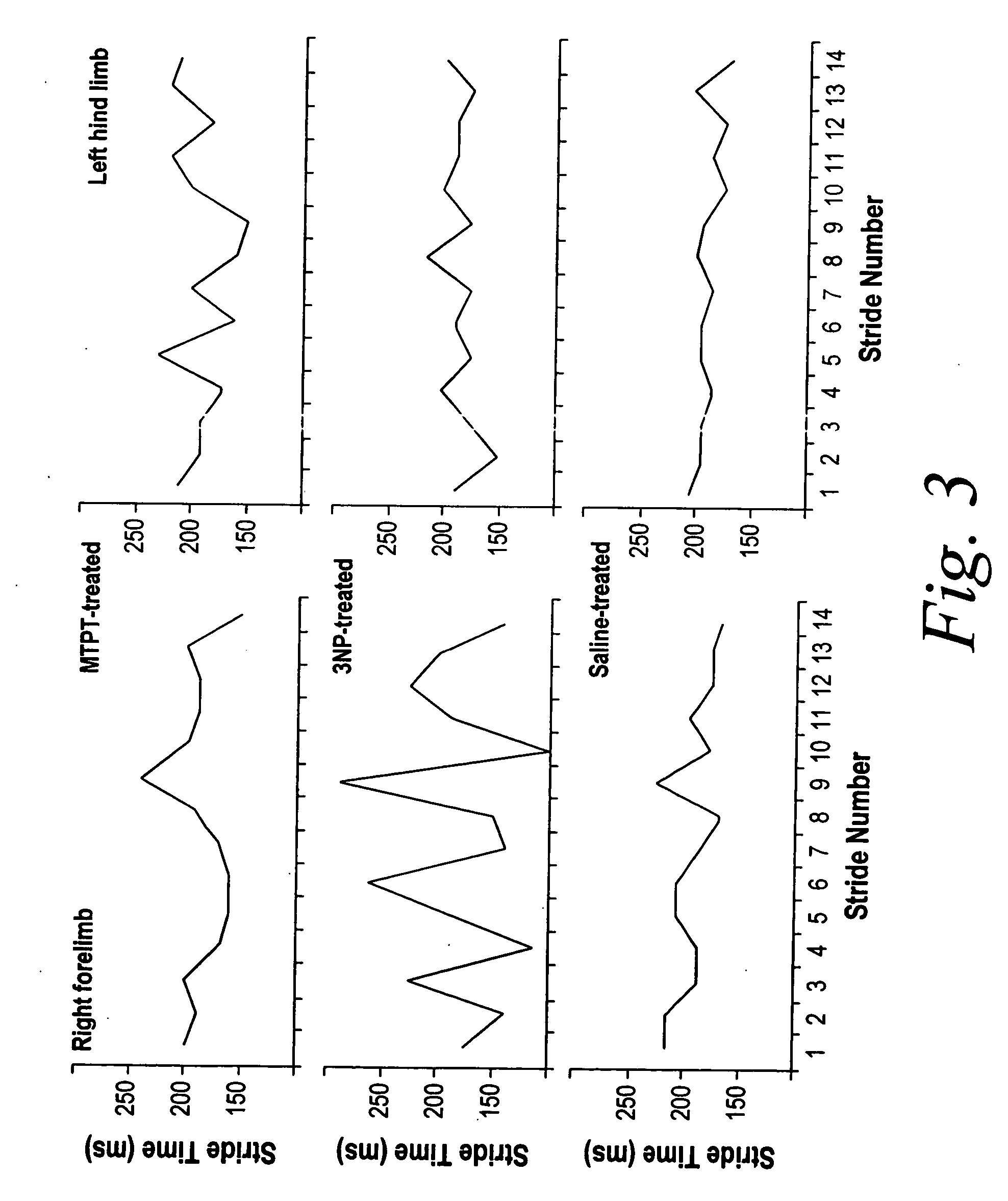
The present invention, at least in part, provides methods of improved early diagnosis of neurodegenerative disease, e.g., ALS, in a subject via measurement of the gait dynamics of the subject (e.g., via the exemplary ventral plane videography methods disclosed herein). The present invention also provides for administration of a beta-adrenergic blocking agent (beta-blocker) to a subject at risk of developing ALS (e.g., a SOD1 G93A mouse) and / or having early stages of ALS, to modulate supranormal gait characteristics and to prevent, treat and / or ameliorate the onset, advancement, severity or effects of a neurodegenerative disease, e.g., ALS, in the subject.
Owner:THE CURAVITA CORP
Controlled release complex composition comprising angiotensin-ii-receptor blockers and hmg-coa reductase inhibitors
ActiveUS20100074951A1Maximize the effect of treatmentPreventing and reducing side effectBiocideMetabolism disorderHMG-CoA reductaseCoronary artery disease
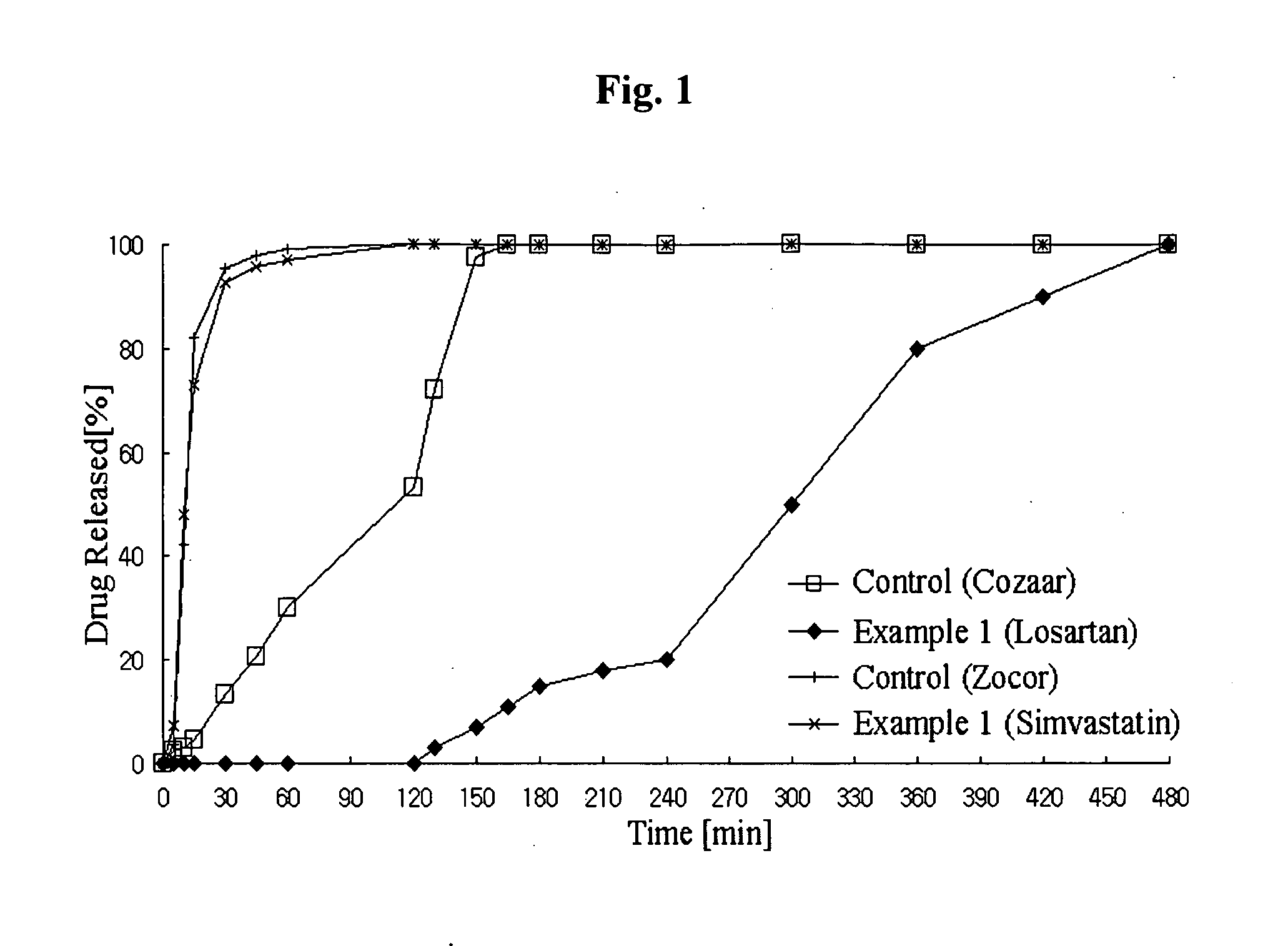
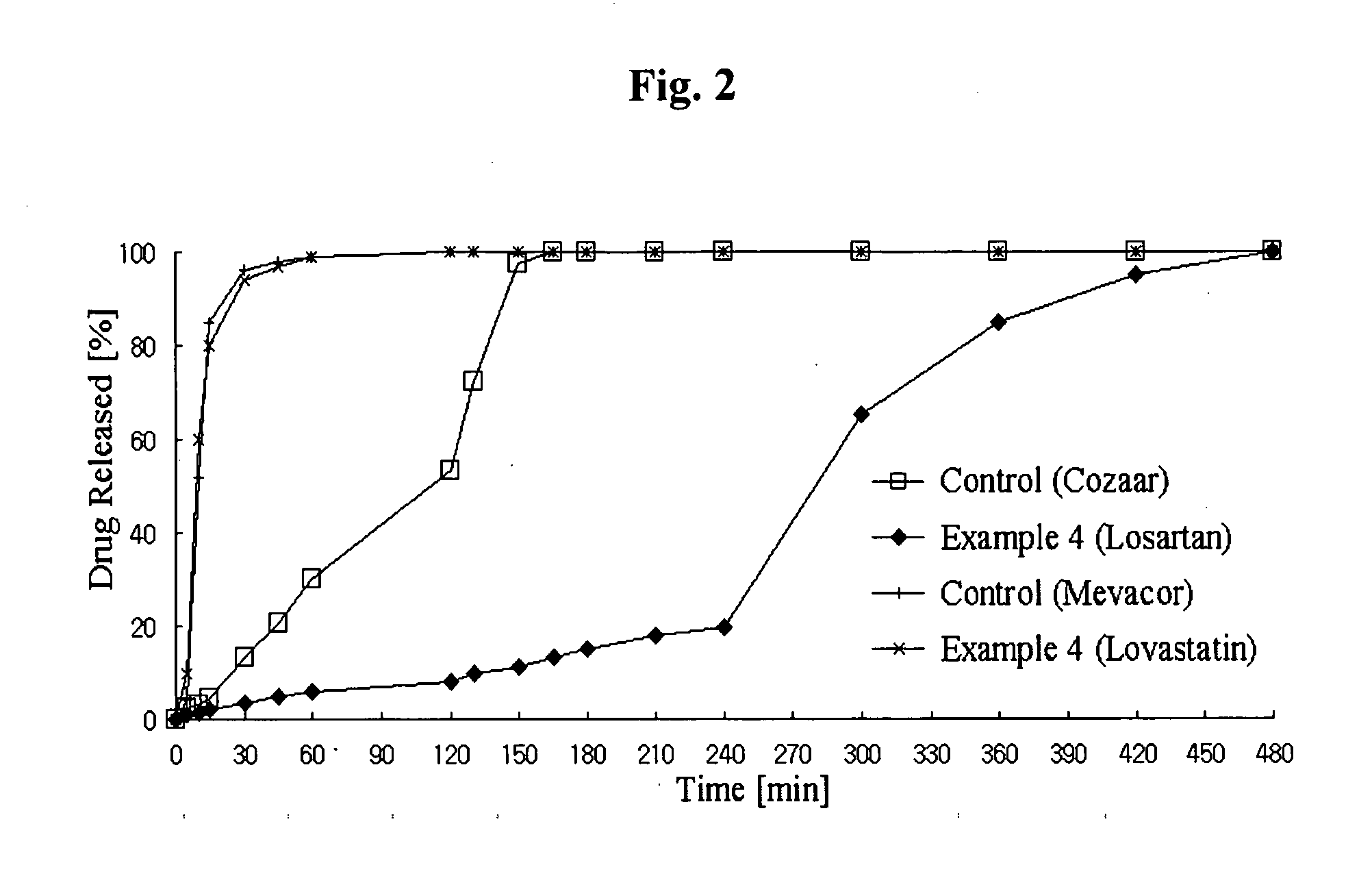
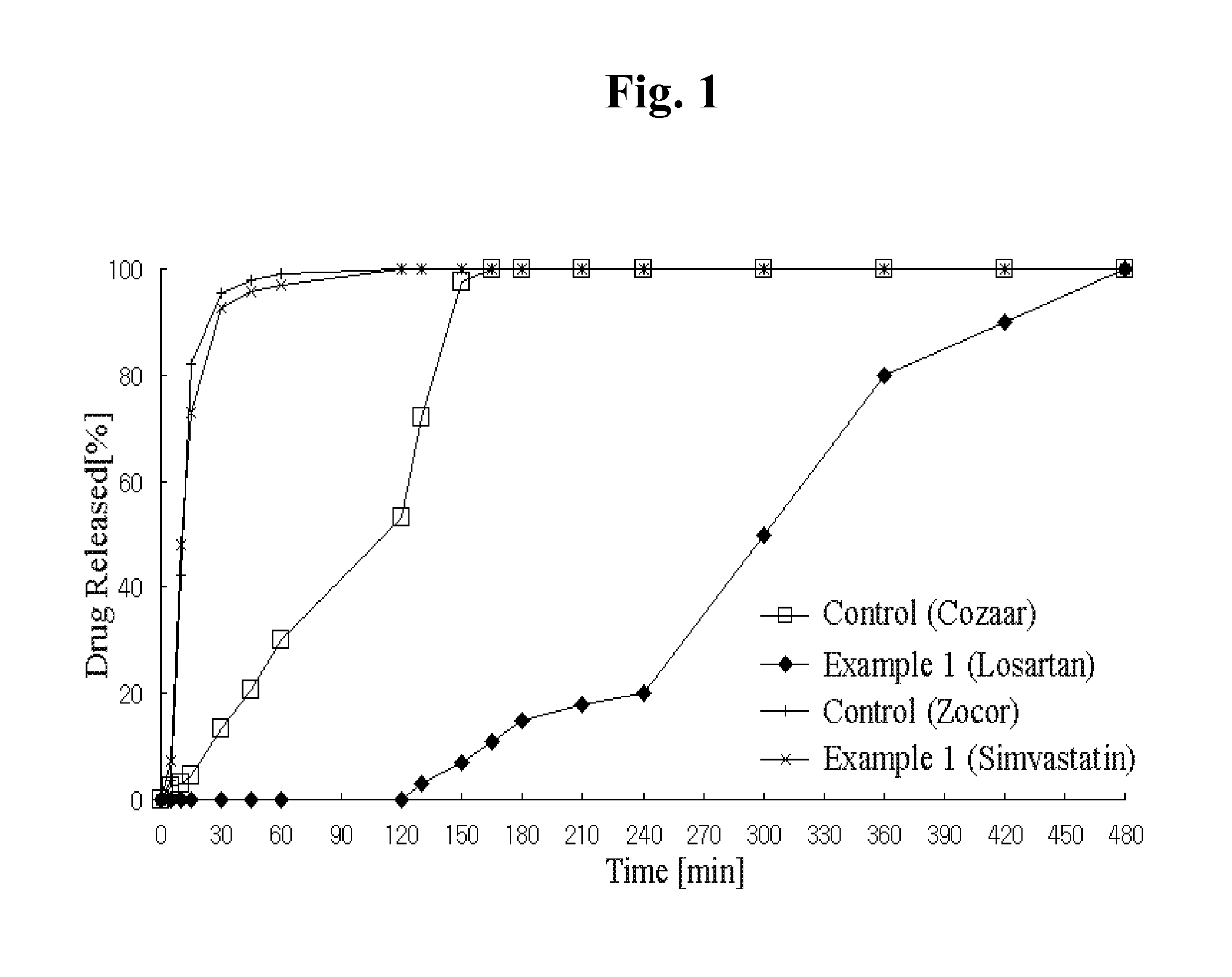
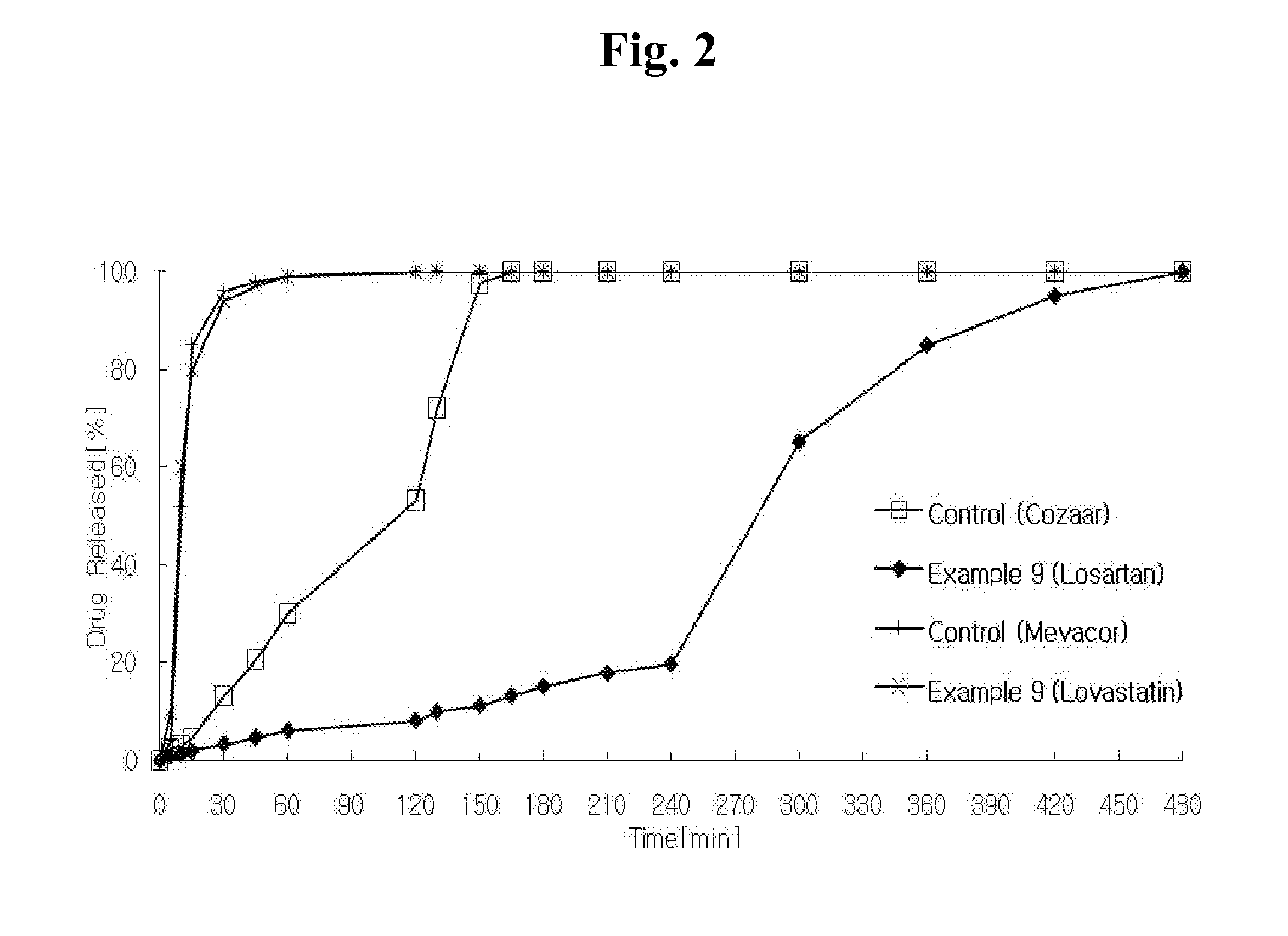
Disclosed herein is a lag time delayed-release combination pharmaceutical composition comprising of an angiotensin-II-receptor blocker and an HMG-CoA reductase inhibitor, as well as a preparation method thereof. The composition is designed based on chronotherapy in which active ingredients are administered to have different onset times, such that the release of each active ingredient of the composition in body can be lag time delayed to a specific rate. Also, the composition is very effective for the treatment of hypertension and the prevention of complications in patients having metabolic syndromes which show diabetes, obesity, hyperlipidemia, coronary artery diseases and the like. More specifically, the composition is a drug delivery system designed such that the release of each drug is controlled to a specific rate, and it can show the most ideal effect, when it is absorbed in body.
Owner:HANALL PHARMA CO LTD
Combination preparation comprising angiotensin-ii-receptor blocker and hmg-coa reductase inhibitor
InactiveUS20110212175A1Reduce effectIncreased riskBiocideMetabolism disorderSide effectEnzyme inhibitor
Disclosed herein is a combination therapy and a combination preparation of an angiotensin-II-receptor blocker and an HMG-CoA reductase inhibitor characterized in that the angiotensin-II-receptor blocker is absorbed substantially later than the HMG-CoA reductase inhibitor. As the angiotensin-II-receptor blocker and the HMG-CoA reductase inhibitor are released at different times, the present combination therapy prevents competitive inhibition between the two drugs and side effects, as well as simultaneously provides synergistic effects for each active ingredient and convenience of taking the drugs.
Owner:HARNALL PHARM CO LTD
Non-imidazole tertiary amines as histamine 3 receptor inhibitors for the treatment of cognitive and sleep disorders, obesity and other CNS disorders
This invention relates to compounds having pharmacological activity, to compositions containing these compounds, and to a method of treatment employing the compounds and compositions. More particularly, this invention concerns certain non-imidazole tertiary amine derivatives and their salts and solvates. These compounds have H3 histamine receptor antagonist activity. This invention also relates to pharmaceutical compositions containing these compounds and to a method of treating disorders in which histamine H3 receptor blockade is beneficial.
Owner:ATHERSYS
Methods for diagnosis, prediction and treatment of heart failure and other cardiac conditions based on beta1-adrenergic receptor polymorphism
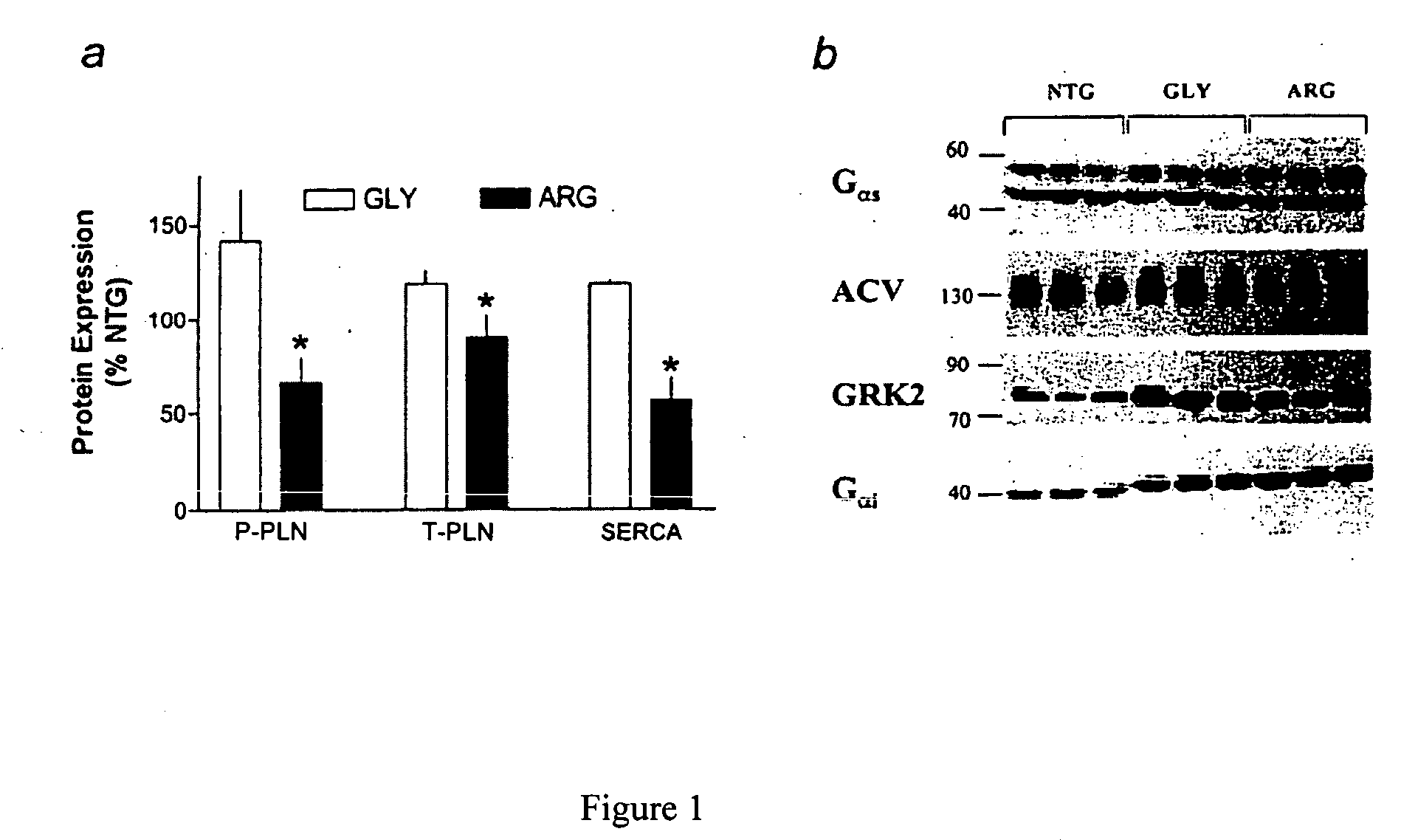
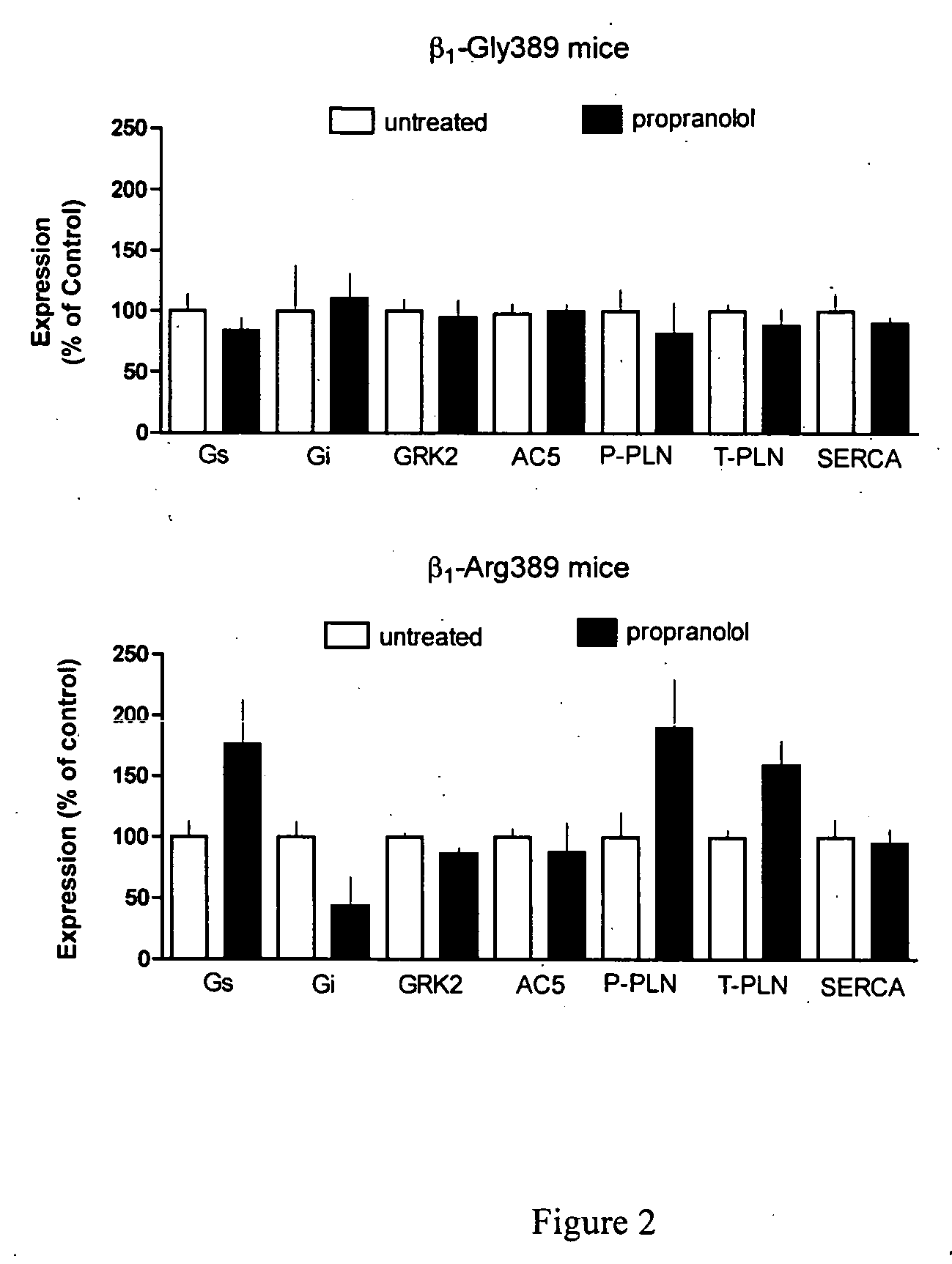
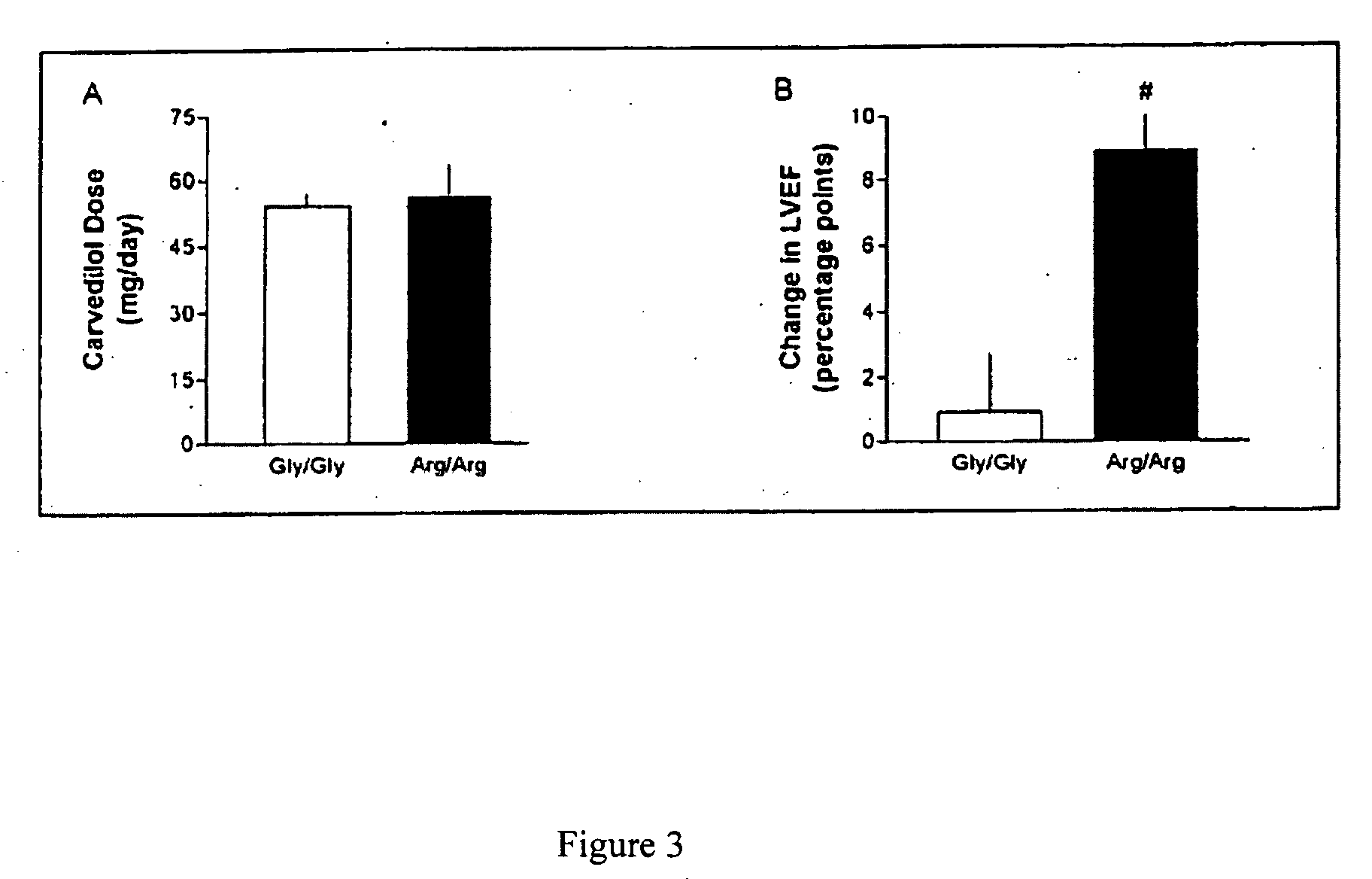
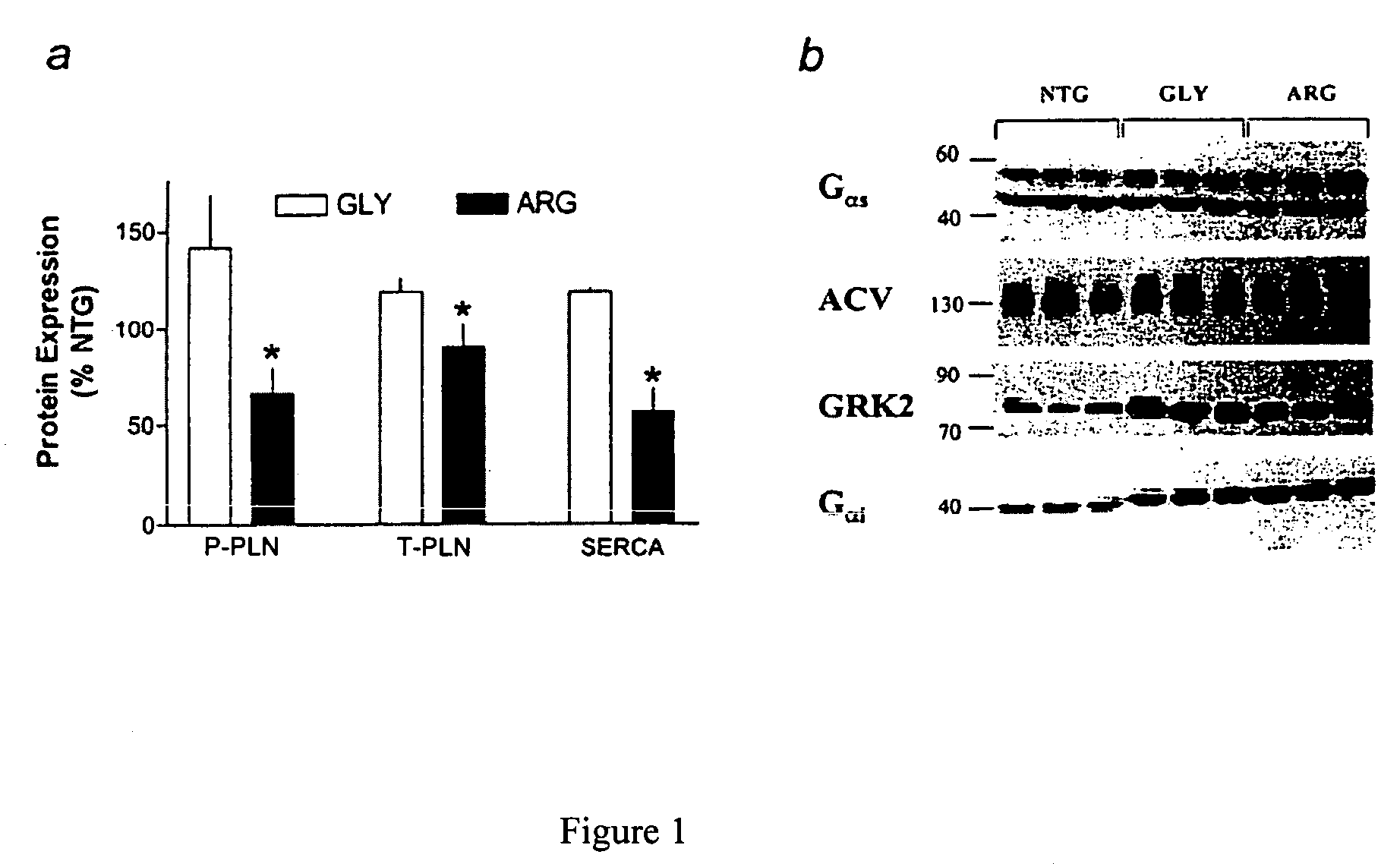
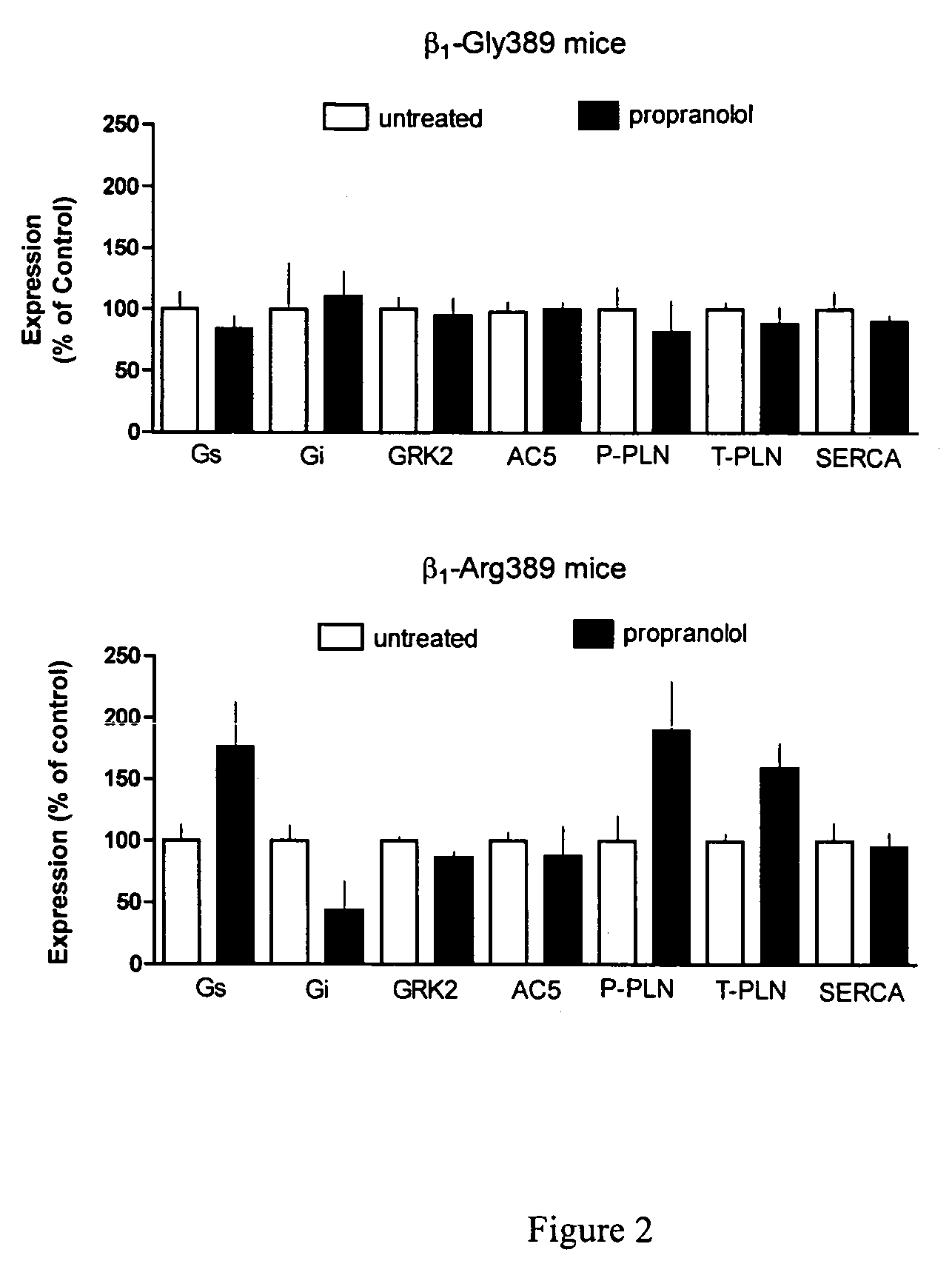
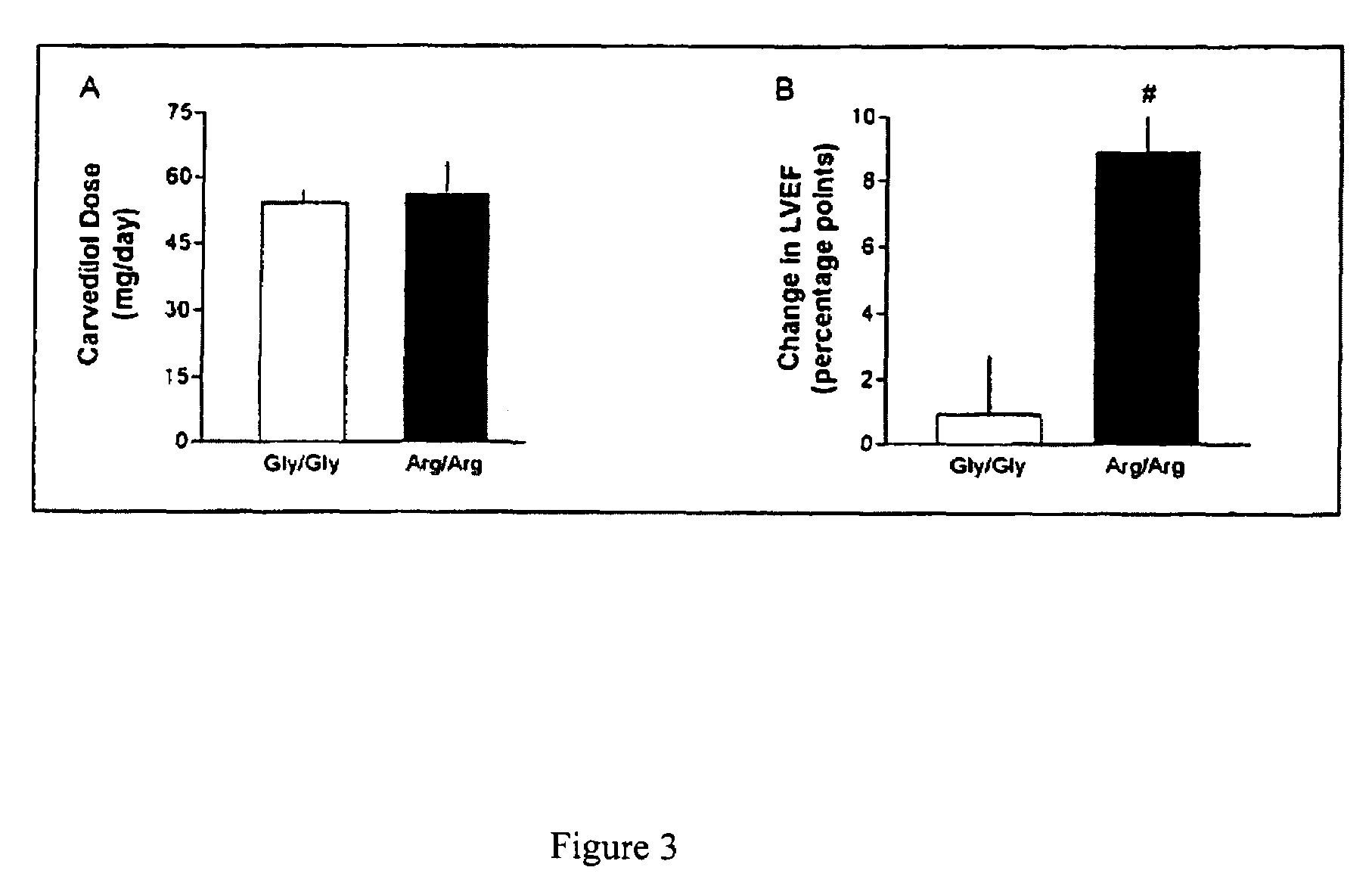
Methods and compositions for the detection, diagnosis, and prevention of cardiac conditions are provided. Polymorphisms of β1-adrenergic receptor are provided. The Gly389 β1-adrenergic receptor variants are not as responsive to treatment β blockers such as carvedilol, metoprolol or bisoprol. Thus, genotyping β1-adrenergic receptor polymorphisms is useful for predicting relative responsiveness to treatment with beta blockers. The Gly389 polymorphism also may be used, alone or in conjunction with other adrenergic receptor polymorphisms, to predict relative risk of developing cardiovascular diseases such as heart failure or to predict relative survival rate in patients with heart failure or other cardiovascular diseases. Also provided are transgenic mice and transgenic cells expressing the β1-adrenergic receptor polymorphisms, and their use in identifying therapeutic agents.
Owner:UNIVERSITY OF CINCINNATI
Methods for predicting relative efficacy of a beta blocker therapy based on a B1-adrenergic receptor polymorphism
InactiveUS7449292B2Sugar derivativesMicrobiological testing/measurementBeta blockerRelative efficacy
Methods and compositions for the detection, diagnosis, and prevention of cardiac conditions are provided. Polymorphisms of β1-adrenergic receptor are provided. The Gly389 β1-adrenergic receptor variants are not as responsive to treatment β blockers such as carvedilol, metoprolol or bisoprol. Thus, genotyping β1-adrenergic receptor polymorphisms is useful for predicting relative responsiveness to treatment with beta blockers. The Gly389 polymorphism also may be used, alone or in conjunction with other adrenergic receptor polymorphisms, to predict relative risk of developing cardiovascular diseases such as heart failure or to predict relative survival rate in patients with heart failure or other cardiovascular diseases. Also provided are transgenic mice and transgenic cells expressing the β1-adrenergic receptor polymorphisms, and their use in identifying therapeutic agents.
Owner:UNIVERSITY OF CINCINNATI
Beta-adrenoceptor genetic polymorphisms and obesity
InactiveUS20040033524A1Reducing trial and error approachShorten the time limitBiocideOrganic active ingredientsHypertension medicationsBeta blocker
The present invention provides a method for identifying a subject having a risk of developing obesity, coronary microvasular dysfunction, or hypertension, comprising detection of the presence of a single nucleotide polymorphism (SNP) in a nucleic acid encoding an element of at least one beta-adrenergic receptor from the subject. The presence of the SNP is correlated with obesity, coronary microvascular dysfunction, or hypertension, and thereby identifies the subject as having a risk of developing obesity, coronary microvascular dysfunction, of hypertension. The subject invention also provides methods of identifying patients likely to benefit from the prescription of beta blocker hypertension medications. In various embodiments, the nucleic acids detected include those genes encoding ADRB1 (beta1AR), ADRB2 (beta2AR), ADRB3 (beta3AR), GNB3 (G protein beta3 subunit), or GNAS1 (Gs protien alpha subunit). Methods of treating identified individuals are also provided.
Owner:FLORIDA UNIV OF A FLORIDA
Saccharomycete with stereoselectivity lipase liveness and application in producing S- type betaxolol hydrochloride with biological split method thereof
InactiveCN101220336ALow priceMild reaction conditions for production conversionFungiHydrolasesBacterial strainSalbutamol sulfate
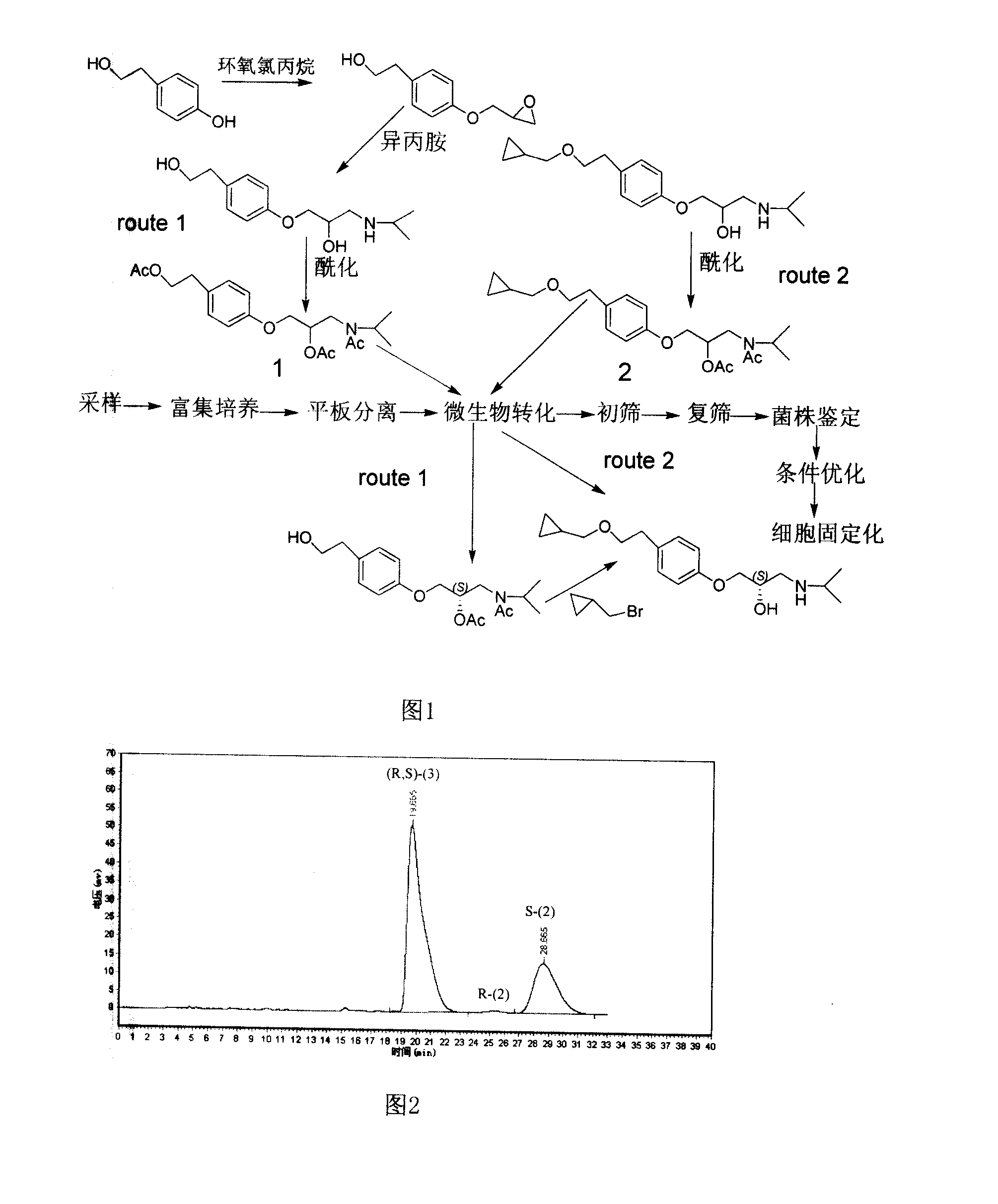
The invention discloses a yeast which has the stereoselective lipase activity and the application of the yeast in the preparation of an S-type betaxolol hydrochloride by using a biological separation method. The method selects one yeast with the stereoselective lipase activity by screening from the soil, utilizes an immobilized cell which is obtained by immobilizing the wet bacteria or sodium alginate-activated carbon-polyethylenimine as an enzyme preparation, carries out an enantiomer separation to a substrate which contains acyl group and prepares the S-type betaxolol hydrochloride. The conversion method is simple, the cost is low and the stereoselectivity is better. The usage of the bacterial strain can carry out the chiral separation of Beta-receptor blocker of betaxolol etc., ephedrine hydrochloride, epinephrine, levodropropizine, salbutamol sulfate, captopril, zofenopril and other compounds which contain hydroxy group or acyl group at the chiral center, thereby having important application value for promoting the development process of the chiral drugs of China.
Owner:ZHENGZHOU UNIV
Methods of treating hypertension with at least one angiotensin ii receptor blocker and chlorthalidone
The present invention relates to a method of treating hypertension in a subject or patient needing treatment thereof by administering to said subject or patient at least one angiotensin II receptor blocker in combination with chlorthalidone.
Owner:TAKEDA PHARMA CO LTD
Antihypertensive therapy method
InactiveUS20070293552A1Improve cardiovascular functionImproved renal functionBiocideAnimal repellantsDiseaseAnti hypertensive treatment
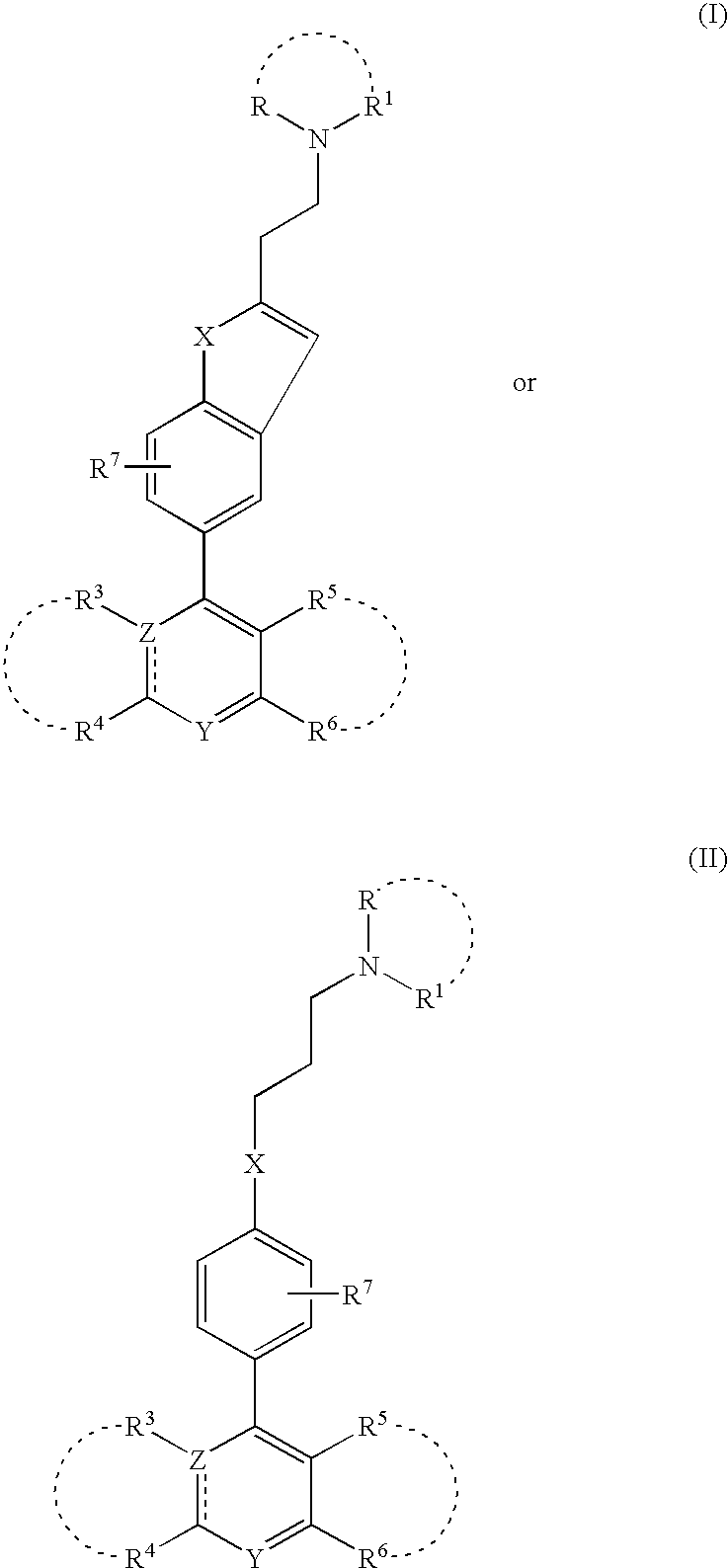
Methods and therapeutic combinations are provided for lowering blood pressure in a subject exhibiting resistance to a baseline antihypertensive therapy with one or more drugs, or a subject having diabetes and / or chronic kidney disease. A method of the invention comprises administering, in some embodiments adjunctively with a modified baseline therapy, a compound of formula (I) as defined herein. A therapeutic combination of the invention comprises a compound of formula (I); at least one diuretic; and at least one antihypertensive drug selected from ACE inhibitors, angiotensin II receptor blockers, beta-adrenergic receptor blockers and calcium channel blockers; wherein the at least one diuretic and / or the at least one antihypertensive drug are present at substantially less than a full dose.
Owner:GILEAD COLORADO
Novel composing prescription sustained-release preparation for treating high blood pressure and preparation method thereof
InactiveCN101185624AQuick-acting and long-actingReduce the frequency of takingPharmaceutical delivery mechanismHeterocyclic compound active ingredientsDissolutionDrug release
The invention relates to a sustained release preparation of a novel prescription for treating hypertension and the preparing method thereof. The novel prescription comprises a heart selective Beta1 receptor blocker metoprolol and an angiotensin II receptor antagonist (sartan drugs). The sustained release preparation consists of delayed release part and rapid release part, wherein the heart selective Beta1 receptor blocker metoprolol is the delayed release part, with first hour releasing 25-45%, fourth hour releasing 40-75% and eighth hour releasing over 75%; the angiotensin II receptor antagonist (sartan drugs) is the rapid release part, with 45 minutes dissolution over 75%. The composition has both rapid and prolonged action. The invention discloses in vitro drug release characteristics and preparation method thereof.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Treatment of heart disease using beta-blockers
The present invention relates to a method of reversing the electrophysiological cardiac remodeling of animals with heart disease. More specifically, the method includes administering to an animal in need thereof a β-adrenoceptor blocker.
Owner:BAYER ANIMAL HEALTH
Combination therapy
The invention relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof, and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof. The invention also relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof; and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof which inhibits a component of the chemokine receptor pathway other than the chemokine receptor. Oral sustained release pharmaceutical compositions comprising the pharmaceutical composition, as well as injectable sustained release pharmaceutical compositions comprising the pharmaceutical composition are described. The invention further relates to tablets, capsules, injectable suspensions, and compositions for pulmonary or nasal delivery comprising the pharmaceutical composition. Also described are: methods for assessing the efficacy of the pharmaceutical composition; methods for assessing the inhibition or partial inhibition activity of the pharmaceutical composition; methods for the treatment, amelioration or prevention of a condition or disease comprising administering to a subject a therapeutically effective amount of the pharmaceutical composition; and the use of the pharmaceutical composition for the manufacture of a dosage form for the treatment of a disease.
Owner:DIMERIX BIOSCIENCES PTY LTD
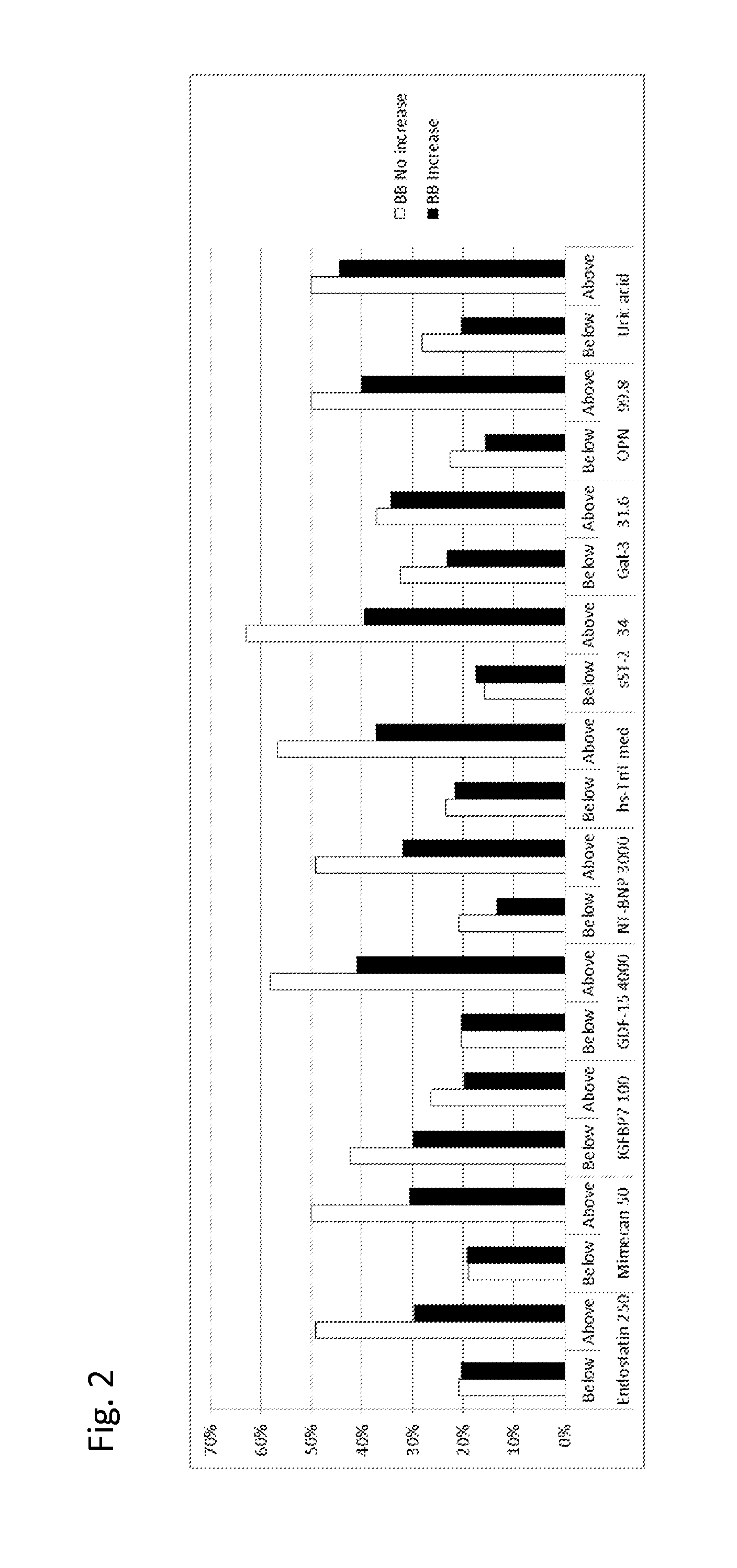
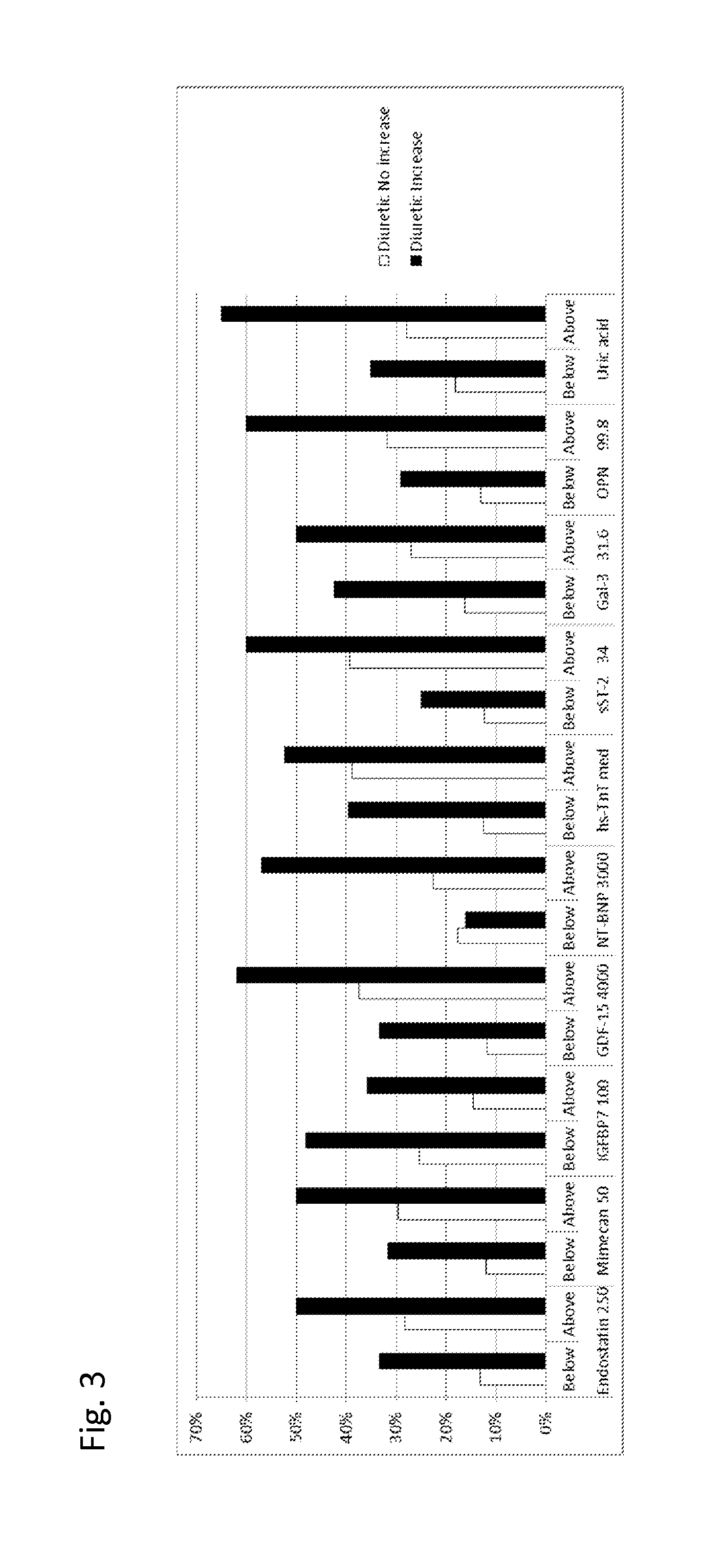
Biomarkers in the selection of therapy of heart failure
InactiveUS20150268251A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBeta blockerBiomarker (petroleum)
The present invention relates to a method for identifying a subject being eligible to the administration of at least one medicament selected from the group consisting of a beta blocker, an aldosterone antagonist, a diuretic, and an inhibitor of the renin-angiotensin system. The method is based on the determination of the amount of at least one biomarker selected from the group consisting of GDF-15 (Growth Differentiation Factor 15), endostatin, mimecan, IGFBP7 (IGF binding protein 7), a cardiac Troponin, a BNP-type peptide, uric acid, Gal3 (Galectin-3), osteopontin, sST2 (soluble ST2), PlGF, sFlt-1, P1NP, Cystatin C, Prealbumin, and Transferrin in a sample from a subject suffering from heart failure. Further, the method comprises the step of comparing the, thus, determined amount with a reference amount. Further envisaged by the present invention are kits and devices adapted to carry out the method of the present invention. The present invention also relates to a system for identifying a subject being eligible to the administration of at least one medicament as disclosed herein and to reagents and kits used in performing the methods as disclosed herein.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
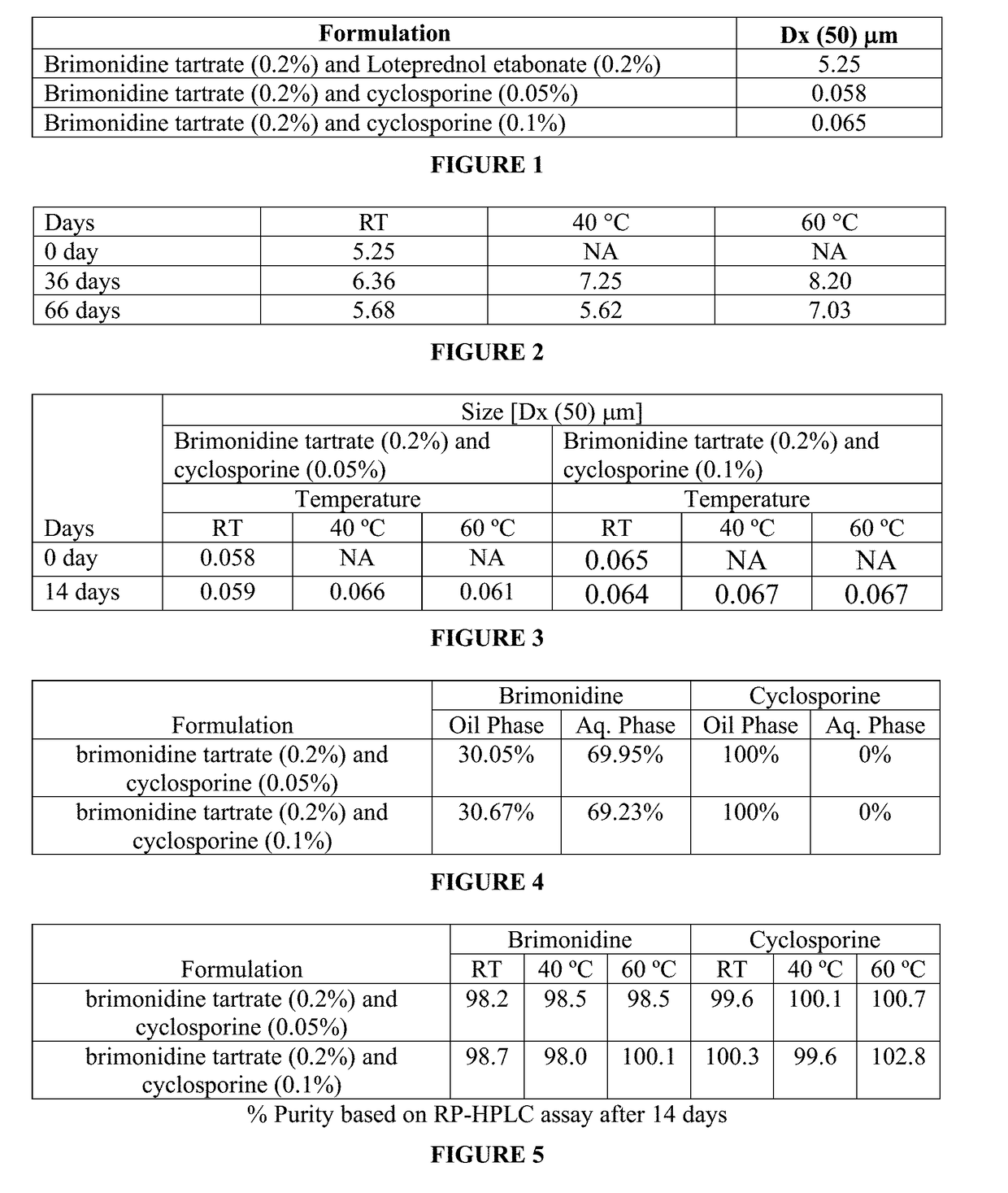
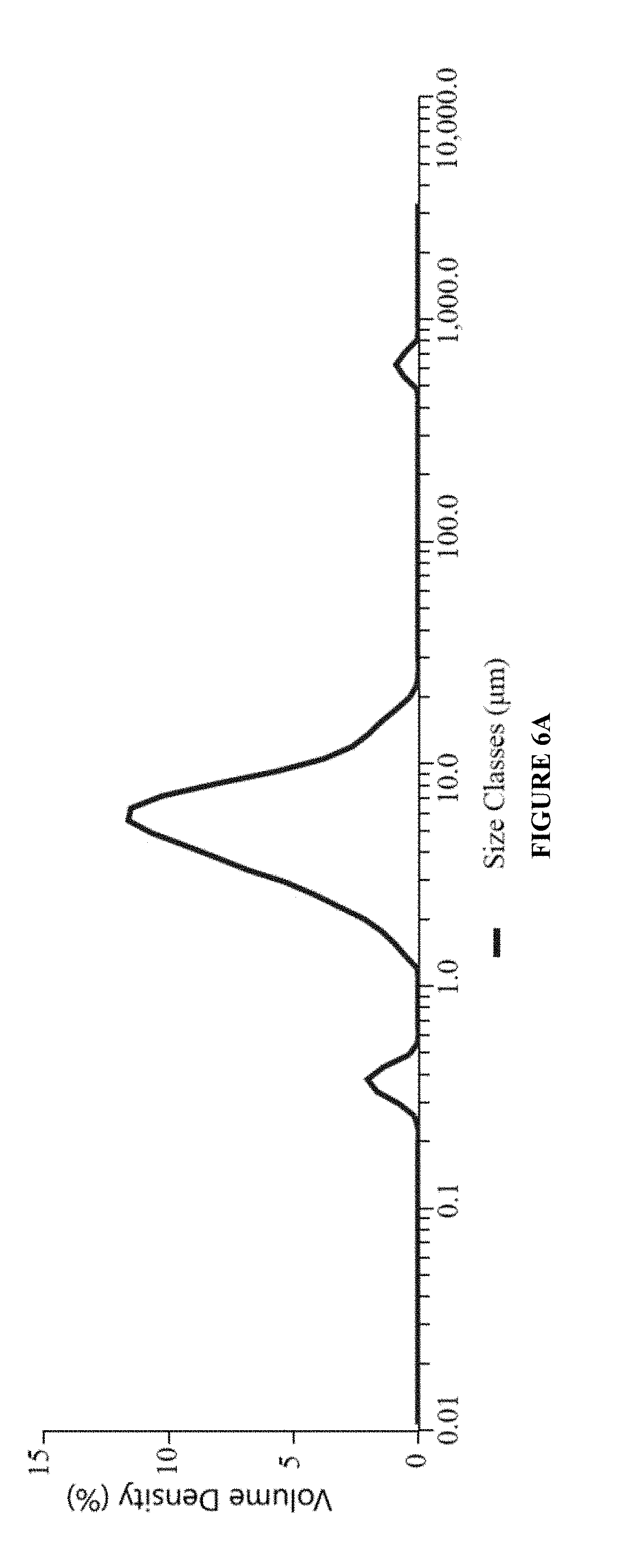
Ophthalmic compositions and methods of use
InactiveUS20190008920A1Significant comprehensive benefitsExtended releaseOrganic active ingredientsSenses disorderAntigenBeta blocker
The present invention relates to an ophthalmic composition comprising at least two active pharmaceutical ingredients. In particular, the active pharmaceutical ingredients are selected from the group consisting of: an alpha 2 adrenergic receptor agonist; a beta-adrenergic receptor agonist; an immunosuppressant; a lymphocyte associated antigen antagonist; an anti-inflammatory; a beta-blocker; a prostaglandin analog; a histamine receptor antagonist; a carbonic anhydrase inhibitor; and an antibiotic. In some embodiments, the composition of the invention is a nanoemulsion formulation. In one particular embodiment, the first active pharmaceutical ingredient is an alpha 2 adrenergic receptor agonist. The present invention also provides a method for treating various clinical conditions associated with an eye disorder or eye disease using the composition of the invention.
Owner:OCUGEN INC
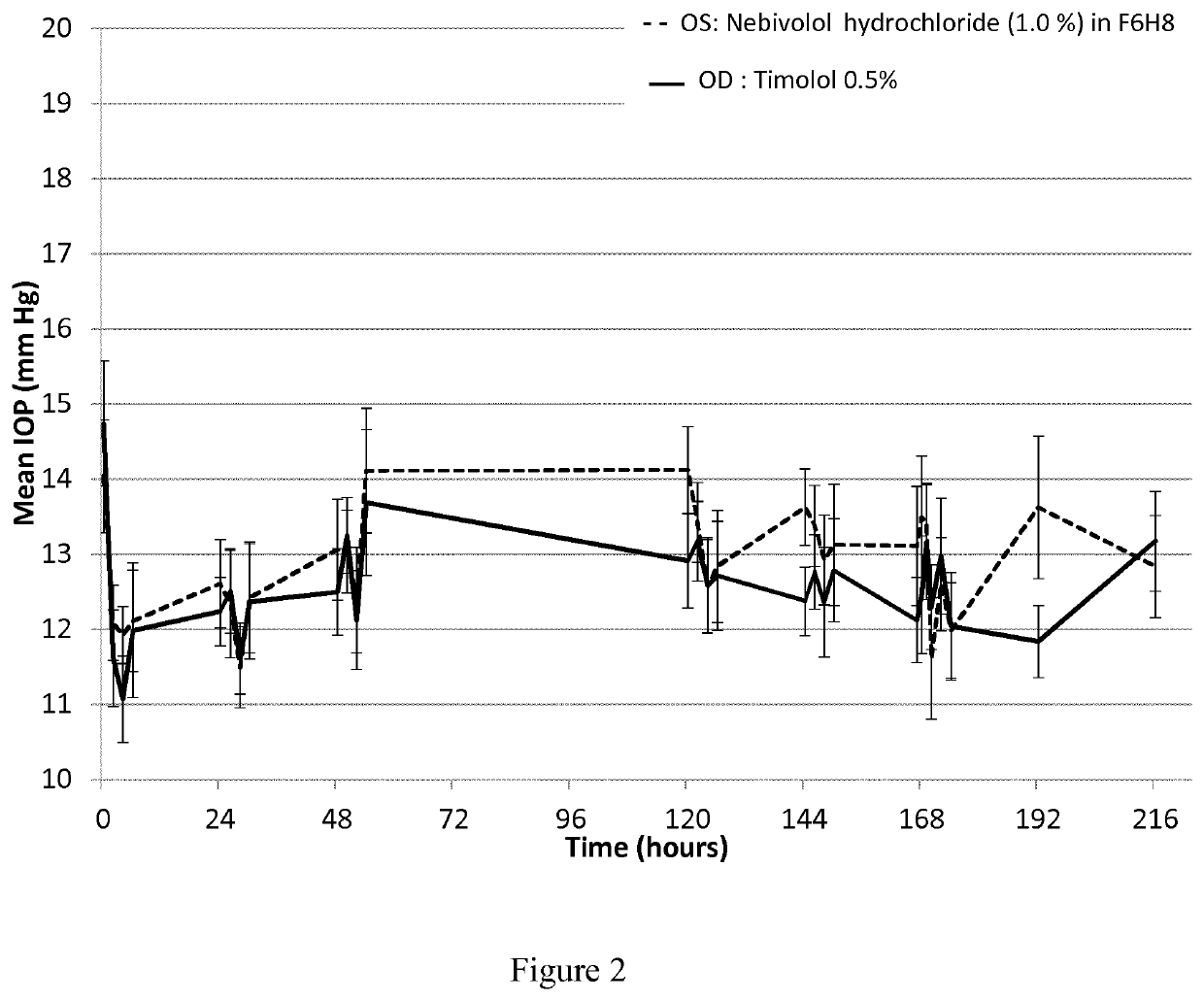
Pharmaceutical compositions comprising nebivolol
ActiveUS20210106558A1Easily not evaporateEfficient deliveryOrganic active ingredientsPowder deliveryIntra ocular pressureAlkane
The present invention relates to pharmaceutical compositions comprising the selective beta 1 (B1)-receptor blocker nebivolol and / or a pharmaceutically acceptable salt thereof and a liquid vehicle comprising a semifluorinated alkane. The pharmaceutical composition of the present invention is useful for topical administration, for example ophthalmic topical administration and for use in the treatment of glaucoma, increased intraocular pressure, ocular hypertension and / or a symptom associated therewith.
Owner:NOVALIQ GMBH
Combination therapy
ActiveUS20130289084A1Inhibits and partially inhibits inositol phosphate productionAvoid adjustmentBiocidePill deliveryDiseaseAngiotensin receptor
The invention relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof, and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof. The invention also relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof; and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof which inhibits a component of the chemokine receptor pathway other than the chemokine receptor. Oral sustained release pharmaceutical compositions comprising the pharmaceutical composition, as well as injectable sustained release pharmaceutical compositions comprising the pharmaceutical composition are described. The invention further relates to tablets, capsules, injectable suspensions, and compositions for pulmonary or nasal delivery comprising the pharmaceutical composition. Also described are: methods for assessing the efficacy of the pharmaceutical composition; methods for assessing the inhibition or partial inhibition activity of the pharmaceutical composition; methods for the treatment, amelioration or prevention of a condition or disease comprising administering to a subject a therapeutically effective amount of the pharmaceutical composition; and the use of the pharmaceutical composition for the manufacture of a dosage form for the treatment of a disease.
Owner:DIMERIX BIOSCIENCES PTY LTD
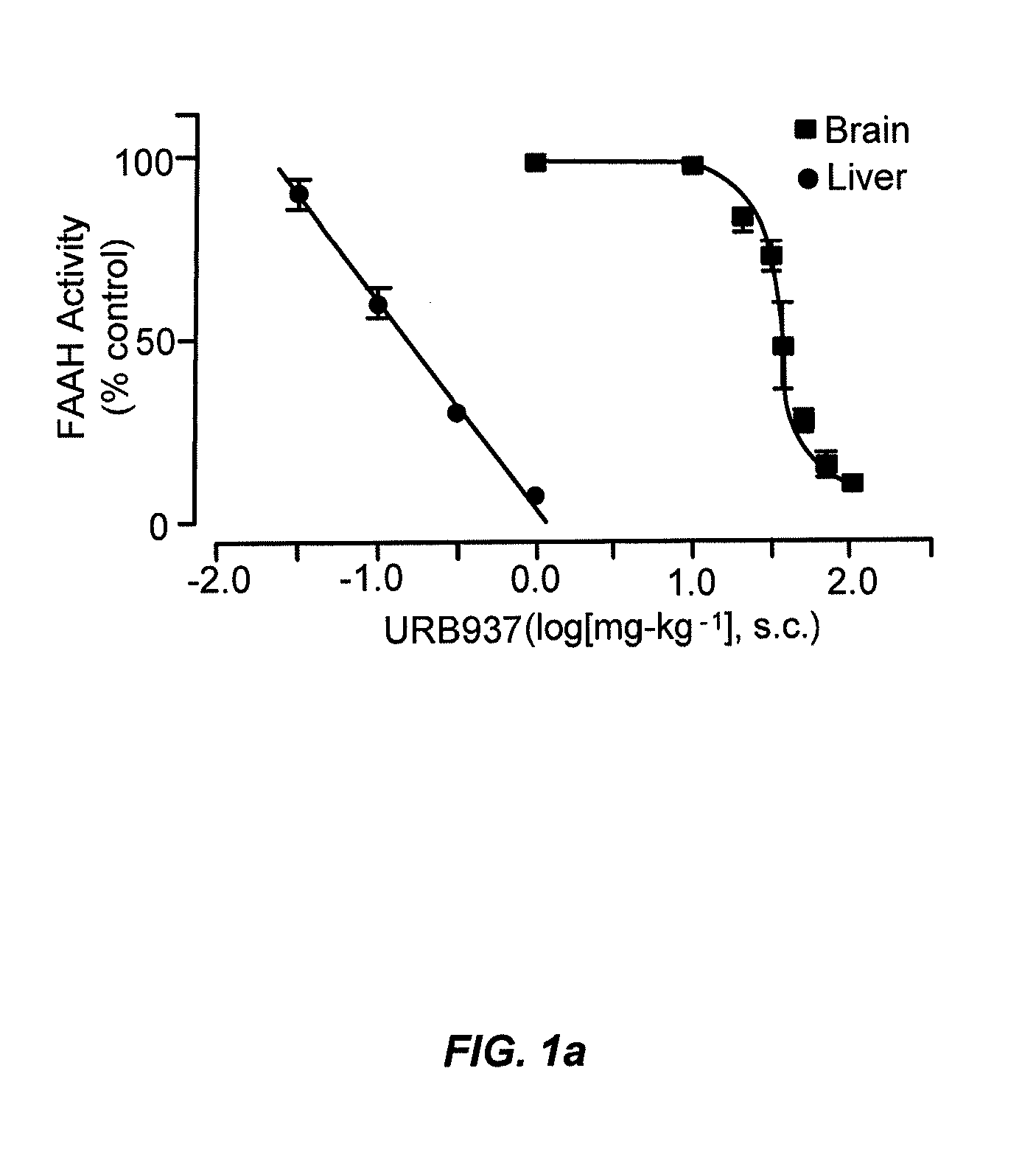
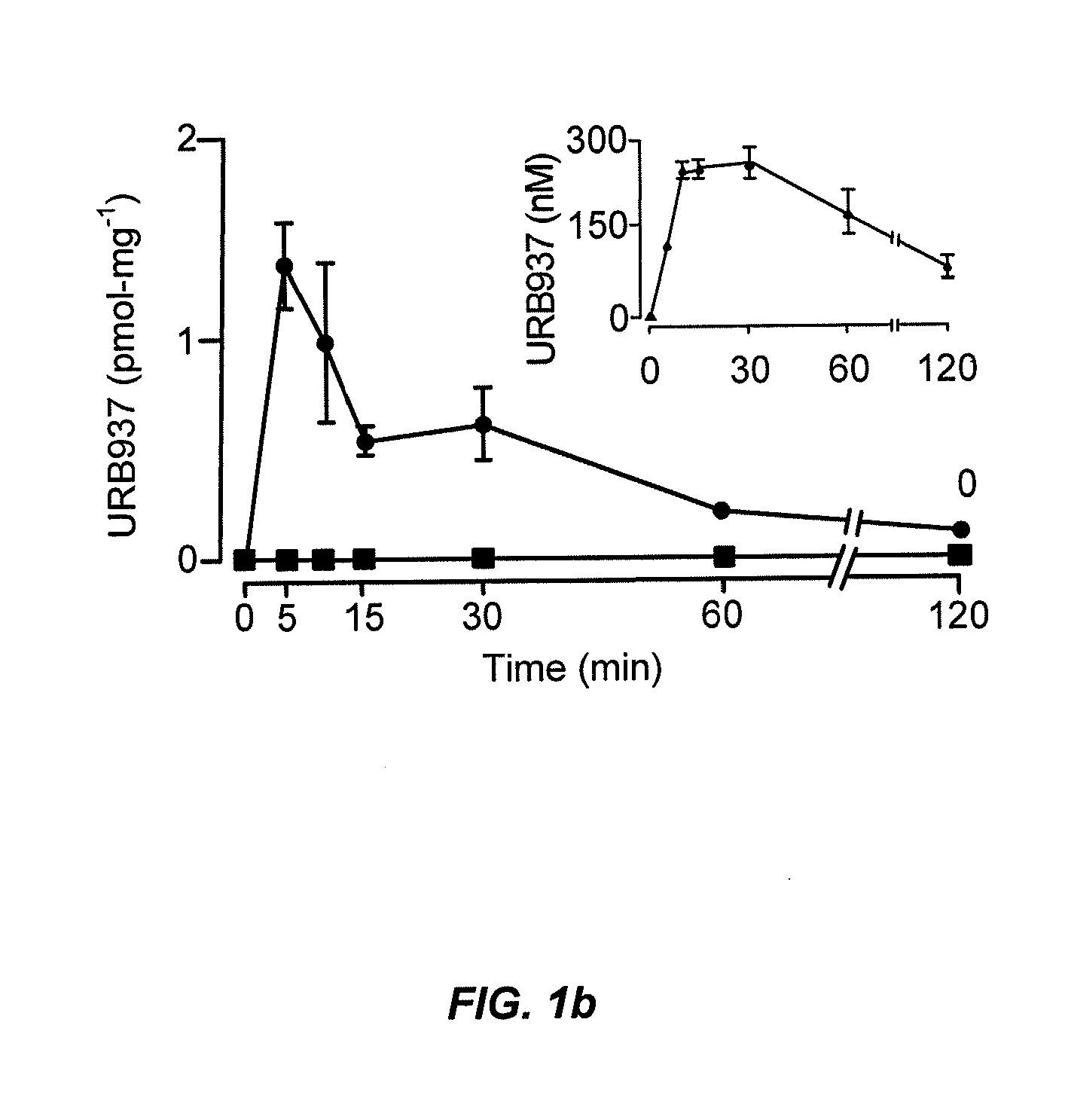
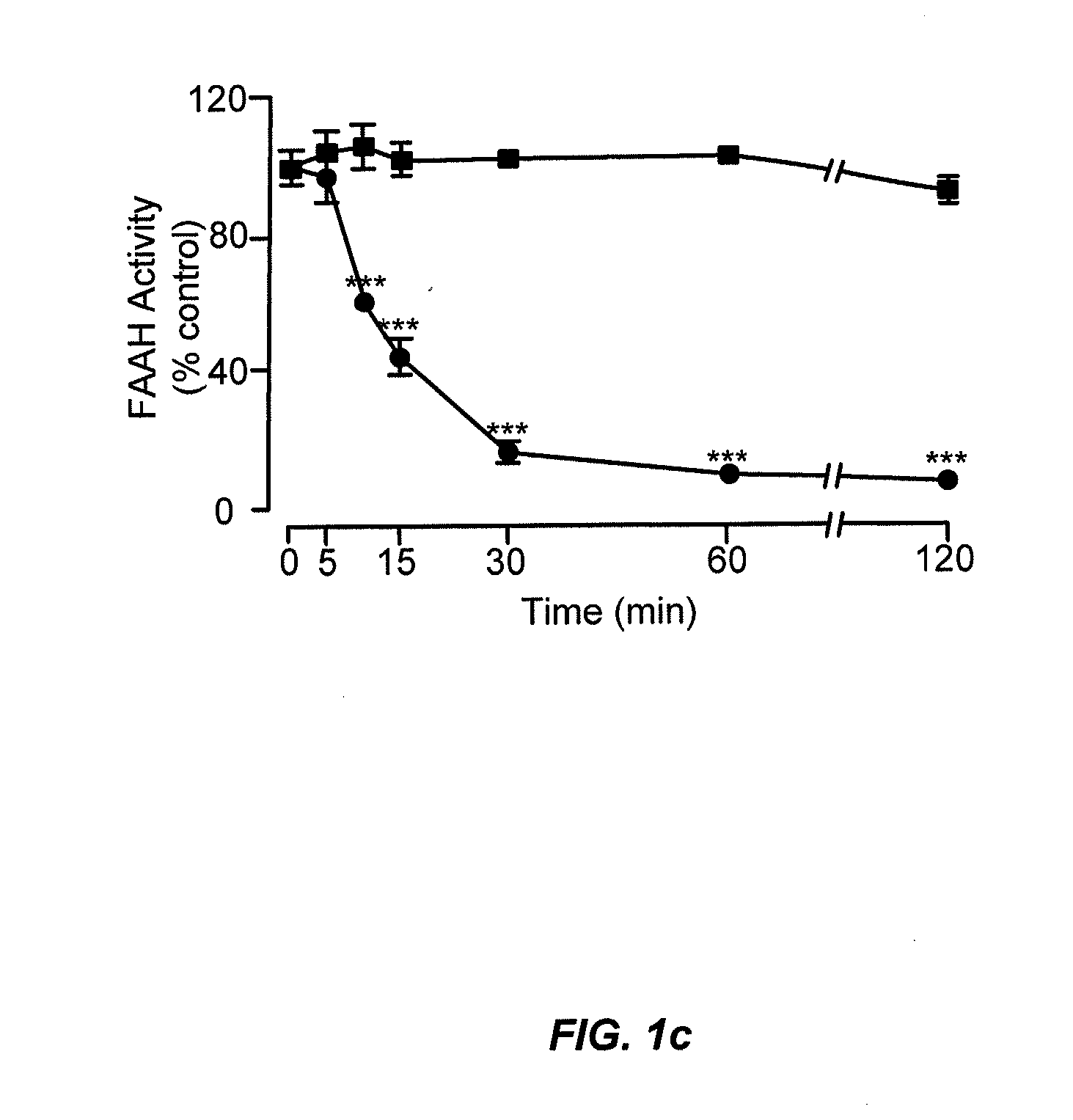
Peripherally restricted FAAH inhibitors
ActiveUS9187413B2Enhancing peripheral activityBiocideNervous disorderDepressantPharmaceutical medicine
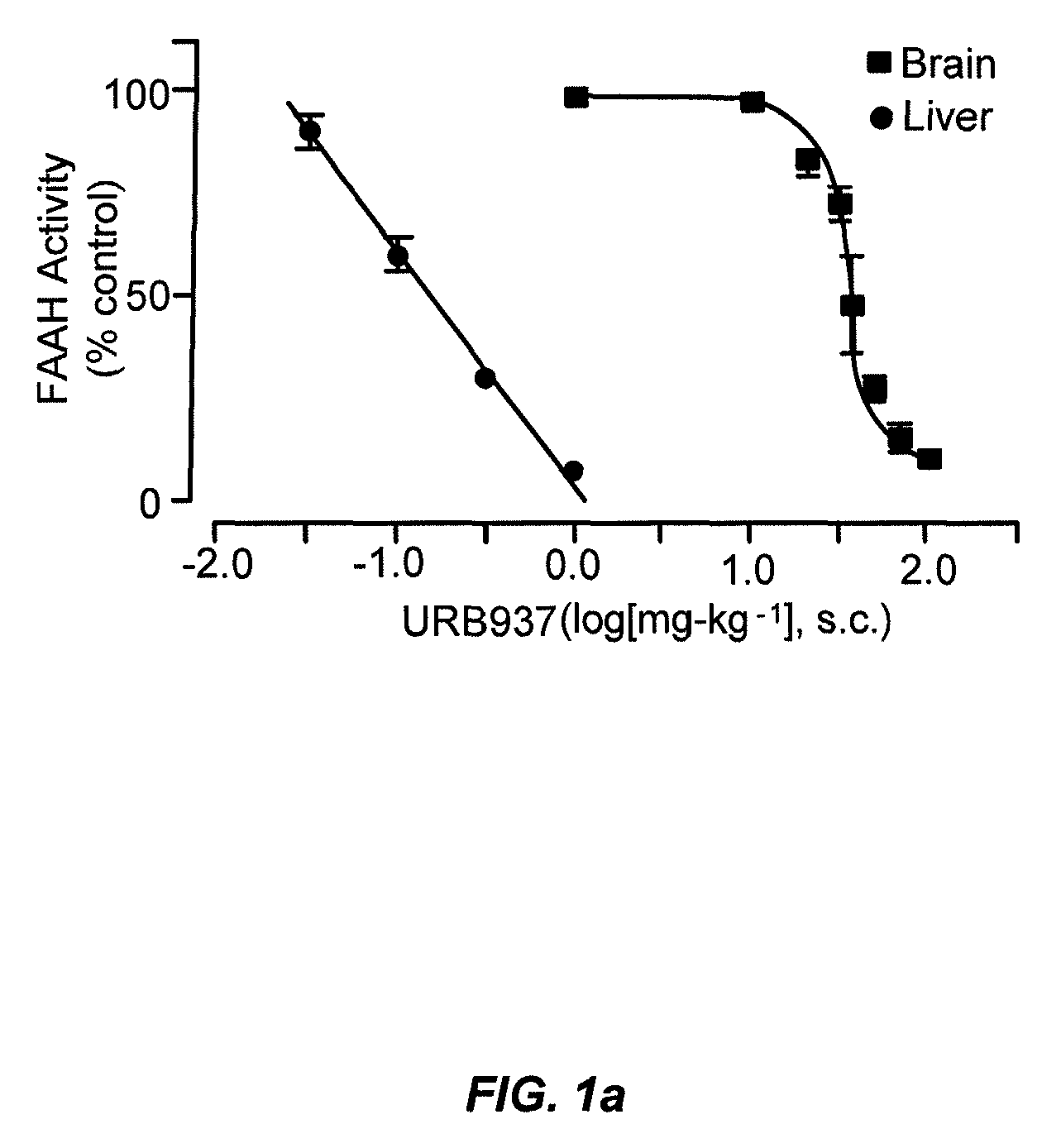
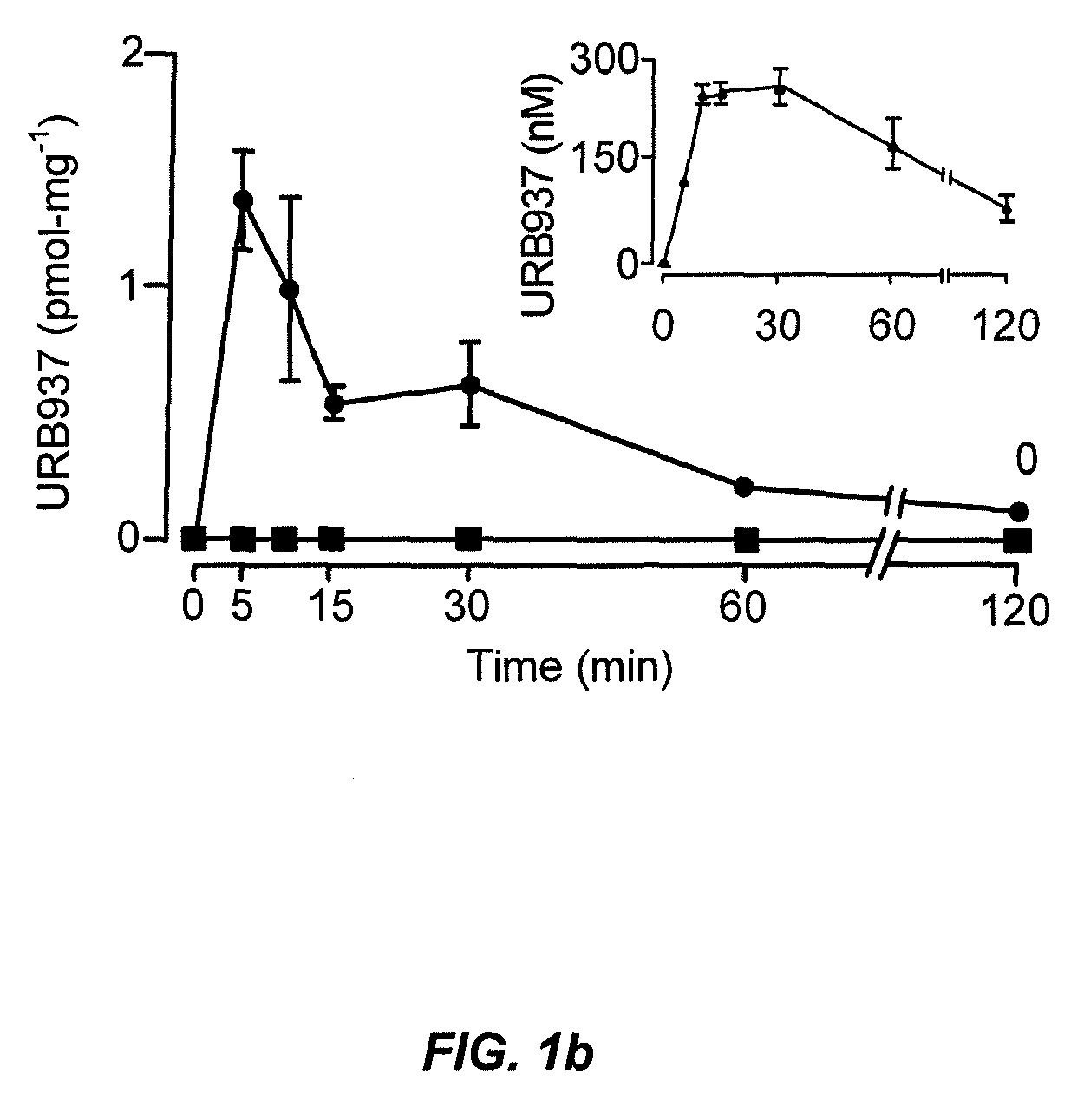
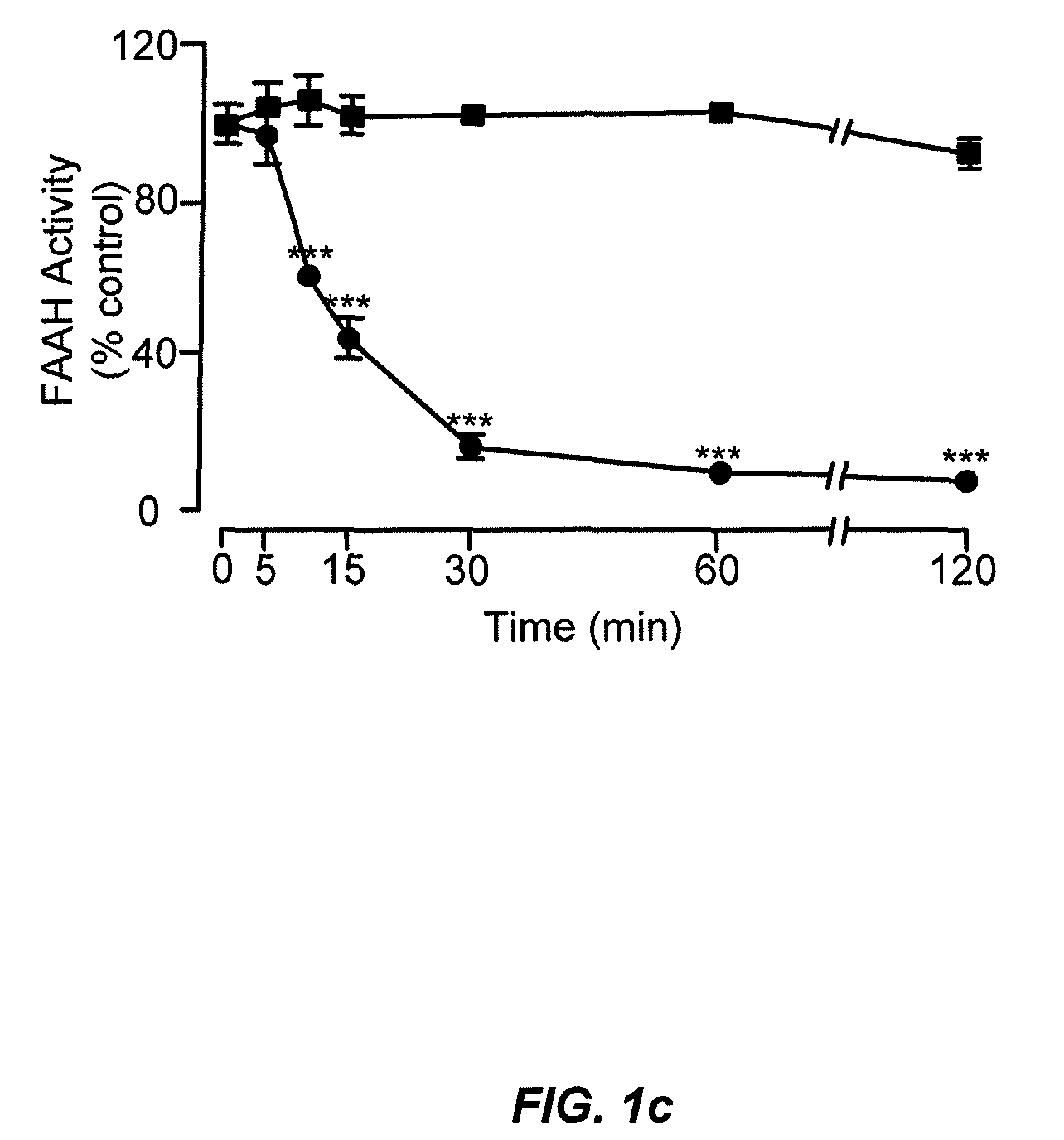
Peripherally restricted inhibitors of fatty acid amide hydrolase (FAAH) are provided. The compounds can suppress FAAH activity and increases anandamide levels outside the central nervous system (CNS). Despite their relative inability to access brain and spinal cord, the compounds attenuate behavioral responses indicative of persistent pain in rodent models of inflammation and peripheral nerve injury, and suppresses noxious stimulus-evoked neuronal activation in spinal cord regions implicated in nociceptive processing. CBi receptor blockade prevents these effects. Accordingly, the invention also provides methods, and pharmaceutical compositions for treating conditions in which the inhibition of peripheral FAAH would be of benefit. The compounds of the invention are according to the formula (I): in which R1 is a polar group. In some embodiments, R1 is selected from the group consisting of hydroxy and the physiologically hydro lysable esters thereof. R2 and R3 are independently selected from the group consisting of hydrogen and substituted or unsubstituted hydrocarbyl; each R4 is independently selected from the group consisting of halogen and substituted or unsubstituted hydrocarbyl and n is an integer from 0 to 4; each R5 is independently selected from the group consisting of halo and substituted or unsubstituted hydrocarbyl and m is an integer from 0 to 3; and R6 is substituted or unsubstituted cyclohexyl; and the pharmaceutically acceptable salts thereof.
Owner:UNIV DEGLI STUDI DI URBINO +1
Application of benzamine in preparation of drugs for preventing and treating myocardial ischemia-reperfusion injury or ischemic heart disease
InactiveCN111329861ABroaden the use of pharmaceuticalsEster active ingredientsNitrile/isonitrile active ingredientsPharmacologyReceptor blockade
The present invention discloses an application of benzamine in preparation of drugs for preventing and treating myocardial ischemia-reperfusion injury or ischemic heart disease. Through transcriptomedata analysis of peripheral blood of patients with acute coronary syndrome and based on co-expression pathways and a drug gene set enrichment analysis method, a list of drug candidates for preventingand treating the acute coronary syndrome are obtained and comprise several marketed drugs for the acute coronary syndrome, the benzamine having functions of preventing and treating the acute coronarysyndrome is not found in the list, besides, an effect of the benzamine is verified to be comparable to that of metoprolol, a common beta-receptor antagonist cardiovascular disease drug on a hypoxia-reoxygenation cell model that simulates the myocardial ischemia-reperfusion injury and ischemic heart disease, thus the benzamine can be used to prepare the drugs for preventing and treating the myocardial ischemia-reperfusion injury or the ischemic heart disease.
Owner:GENERAL HOSPITAL OF PLA
Spiro heterocyclic ketone N-phenyl indole compound, its preparation method and application in controlling cardiovascular diseases and other medicine fields
ActiveCN102796083AImprove specific binding abilityLower blood pressureOrganic active ingredientsNervous disorderTetrazoleCoronary heart disease
The invention discloses a spiro heterocyclic ketone N-phenyl indole compound, its preparation method and application in controlling cardiovascular diseases and other medicine fields. The compound is formed by organically connecting spiro heterocycle, indole, phenyl ring and tetrazole, is an AT1 type acceptor retarding agent of angiotensin II, and can be used for preventing or treating hypertension, coronary heart disease, disease in blood vessels of heart, brain and kidney, hemicrania, pulmonary hypertension and other diseases.
Owner:陈志龙
Peripherally restricted faah inhibitors
ActiveUS20130217764A1Enhancing peripheral activityBiocideNervous disorderBehavioral responseReceptor blockade
Peripherally restricted inhibitors of fatty acid amide hydrolase (FAAH) are provided. The compounds can suppress FAAH activity and increases anandamide levels outside the central nervous system (CNS). Despite their relative inability to access brain and spinal cord, the compounds attenuate behavioral responses indicative of persistent pain in rodent models of inflammation and peripheral nerve injury, and suppresses noxious stimulus-evoked neuronal activation in spinal cord regions implicated in nociceptive processing. CBi receptor blockade prevents these effects. Accordingly, the invention also provides methods, and pharmaceutical compositions for treating conditions in which the inhibition of peripheral FAAH would be of benefit. The compounds of the invention are according to the formula (I): in which R1 is a polar group. In some embodiments, R1 is selected from the group consisting of hydroxy and the physiologically hydro lysable esters thereof. R2 and R3 are independently selected from the group consisting of hydrogen and substituted or unsubstituted hydrocarbyl; each R4 is independently selected from the group consisting of halogen and substituted or unsubstituted hydrocarbyl and n is an integer from 0 to 4; each R5 is independently selected from the group consisting of halo and substituted or unsubstituted hydrocarbyl and m is an integer from 0 to 3; and R6 is substituted or unsubstituted cyclohexyl; and the pharmaceutically acceptable salts thereof.
Owner:UNIV DEGLI STUDI DI URBINO +1
Compound towards target of cardiac muscle and preparation
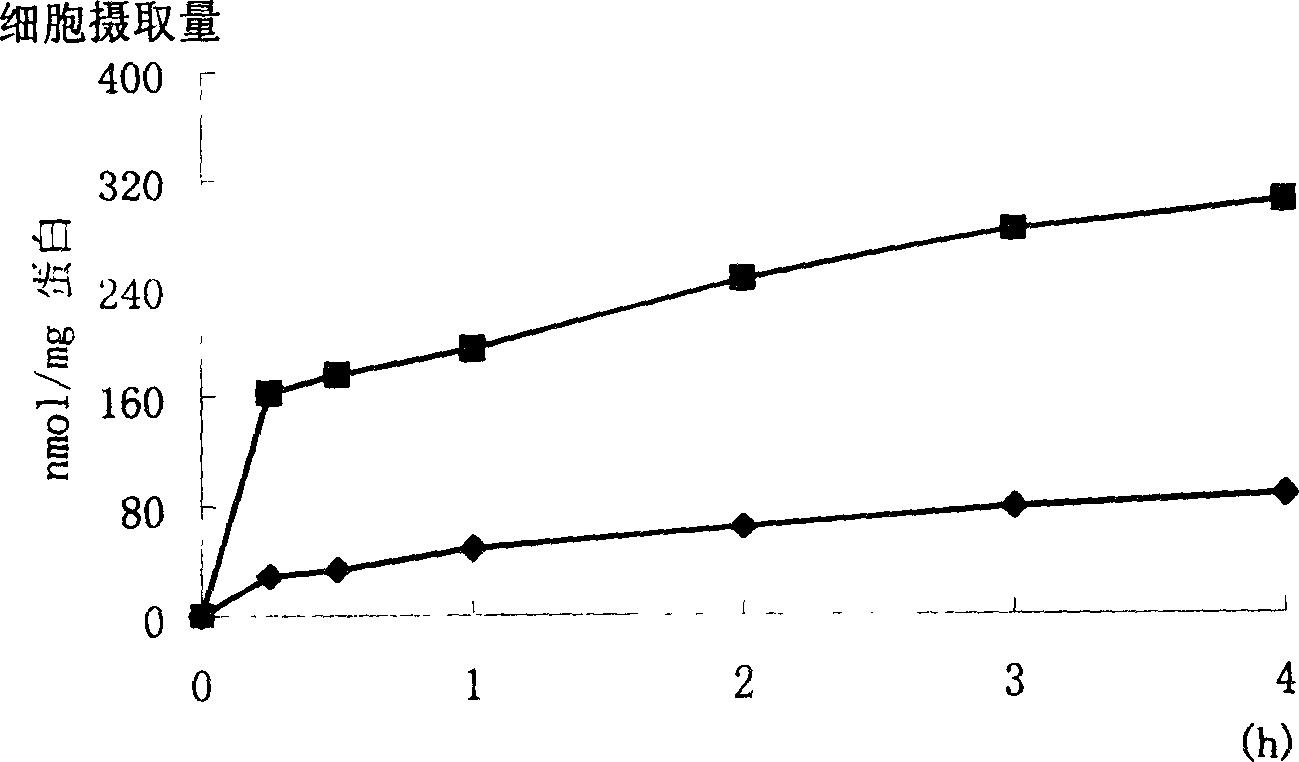
InactiveCN1660780ARaw materials are easy to getHigh affinityOrganic active ingredientsOrganic compound preparationPropionateBenzoic acid
A cardiac muscle targetable compound (+ / -)-3-{4-[2-hydroxy-(1-methyl ethylamino)propoxy]phenyl} hexadecanol propionate, which is used to modify the surface of liposome, nanoparticles or micro softgel for increaisng the affinity between medicine and cardiac muscle, is prepared from p-hydroxy benzoic acid through esterifying, o-epoxy propylation and isopropylamination.
Owner:SHENYANG PHARMA UNIVERSITY
Treatment of tachycardia
ActiveUS20200345744A1Cardiovascular disorderHeterocyclic compound active ingredientsParoxysmal AFVentricular contraction
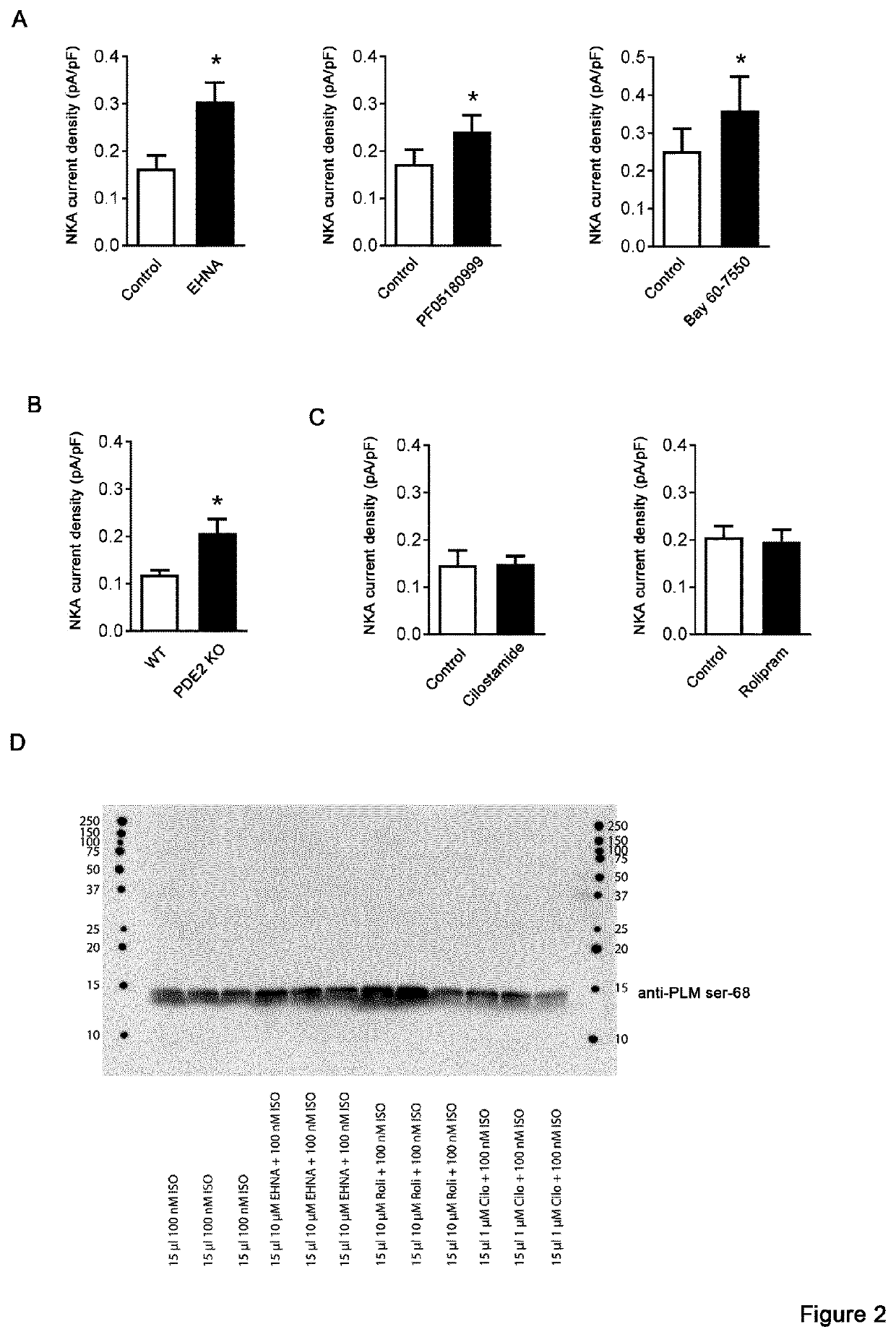
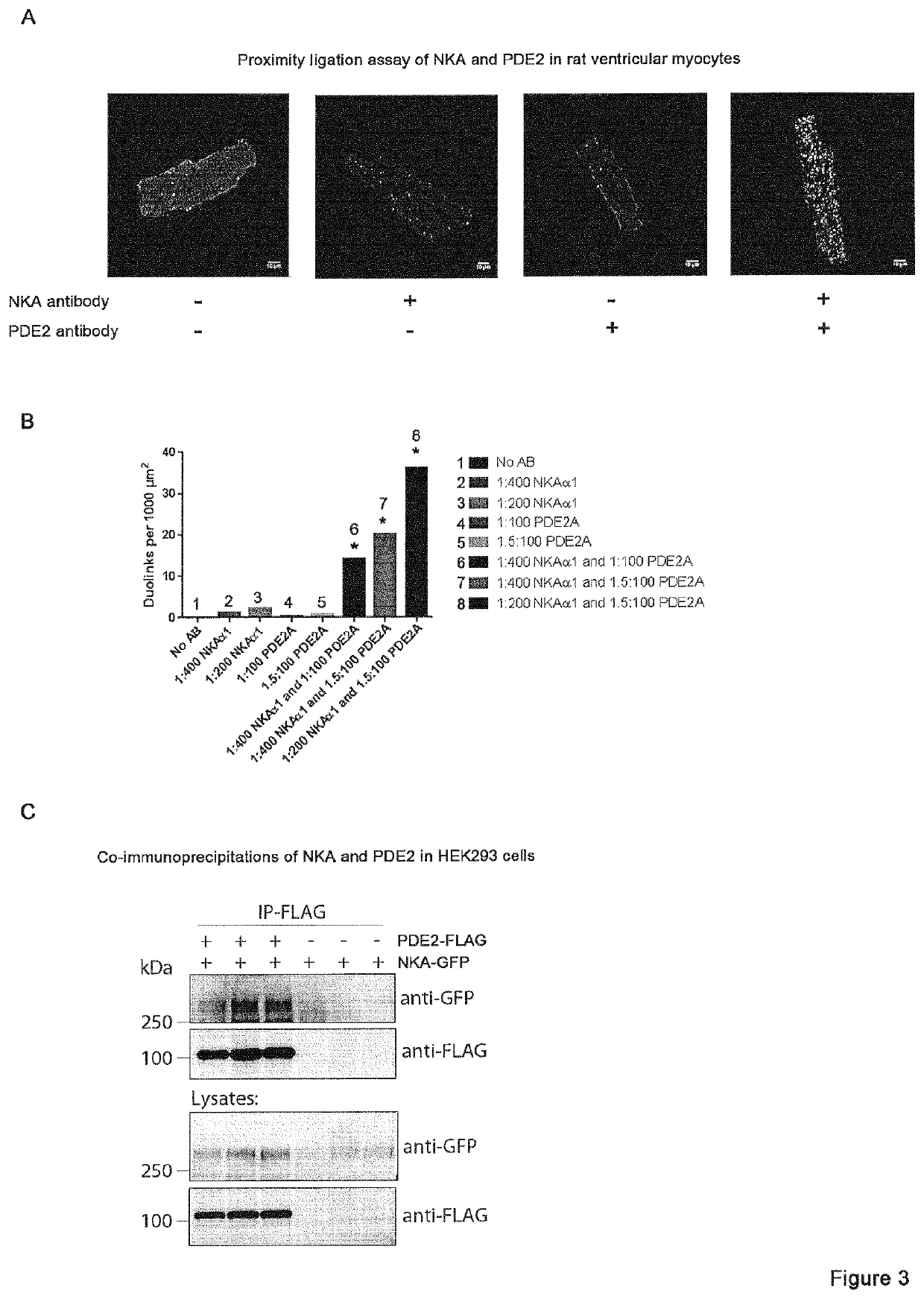
The invention provides compounds which are selective PDE2 inhibitors for use in the treatment of tachycardia or tachyarrhythmia Such compounds are particularly suitable for use in the treatment of any of the following conditions: atrial tachycardia, atrial fibrillation, atrial flutter, paroxysmal supraventricular tachycardia, premature ventricular contractions (PVCs), ventricular fibrillation and ventricular tachycardia, and may be used alone or in combination therapy with other conventional cardiovascular drugs, e.g. beta-blockers. In particular, the invention provides compounds which are selective PDE2 inhibitors for use in the treatment of ventricular tachycardia in patients who are suffering from, or who are at risk of suffering from heart failure, CPVT or long QT syndrome.
Owner:UNIV OSLO HF
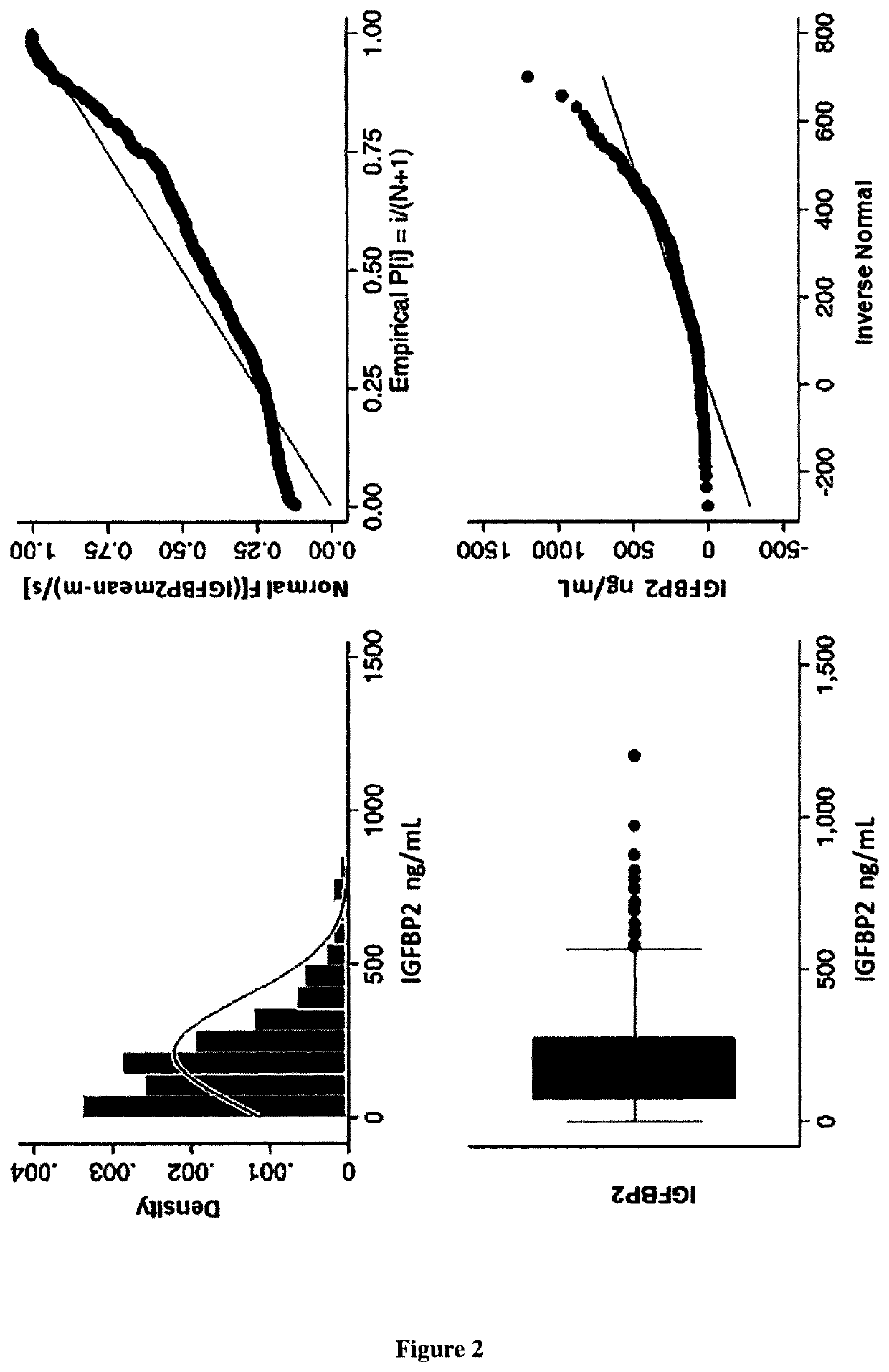
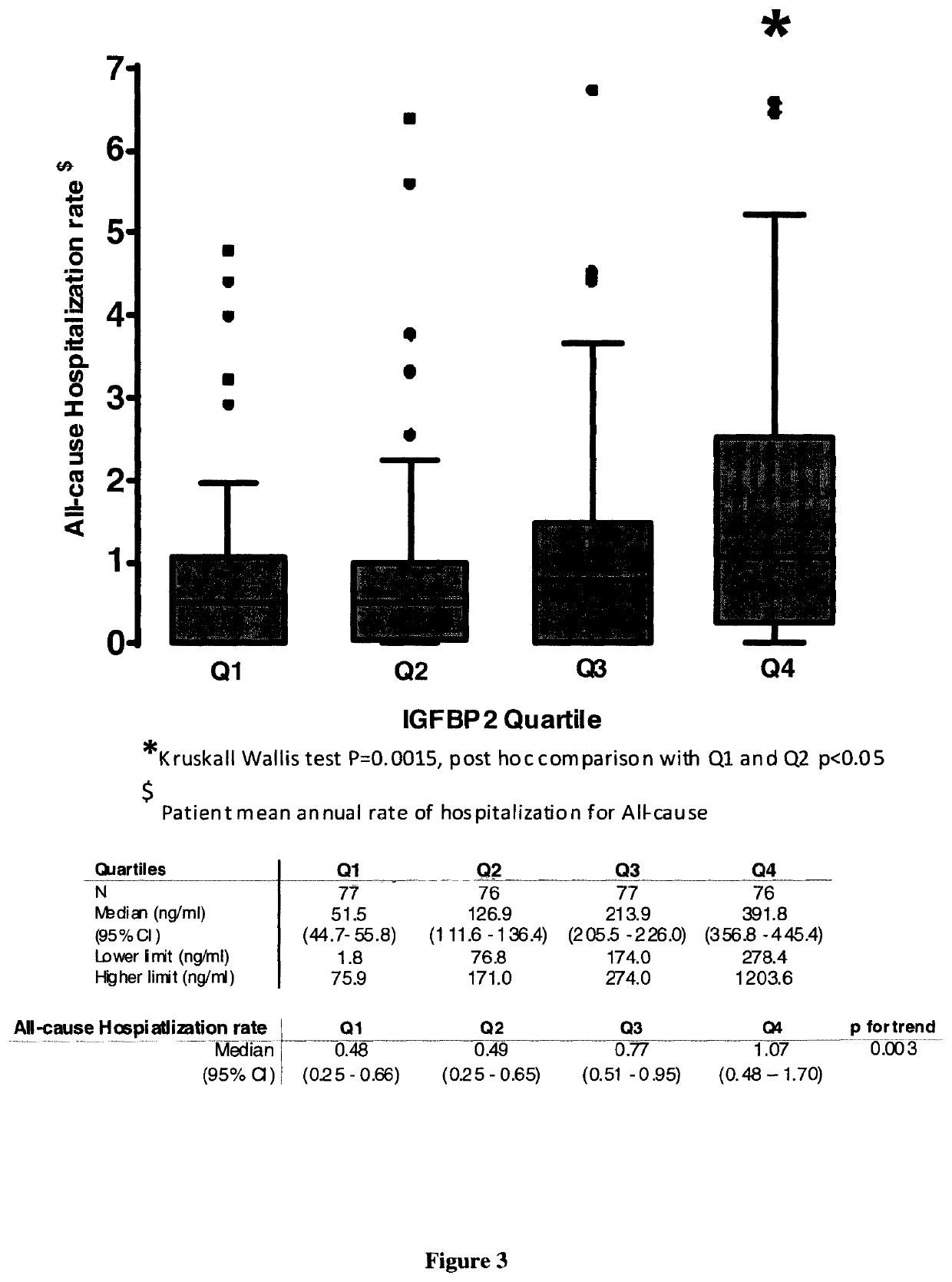
Biomarker of rehospitalization after heart failure
The invention is a method for treating a heart failure patient with the steps of measuring the concentration of IGFBP2 in a blood, plasma or urine sample obtained from the heart failure patient and comparing the patient's IGFBP2 level to a threshold value derived from IGFBP2 measured in samples taken from a group of patients with heart failure selected from the group consisting of stage I, stage stage III and stage IV heart failure, according to New York Heart Association (NYHA) classification system. A patient with an IGFBP2 level that exceeds the threshold value is admitted or readmitted to a hospital and treated with at least one treatment selected from the group consisting of administration of a beta-blocker, an angiotensin-converting enzyme inhibitor, an angiotensin receptor blocker, an aldosterone antagonist, an implantable cardiac defibrillator, a cardiac resynchronization therapy, an implantable left ventricular assist device and a heart transplant.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Promoting sleep using AT1 receptor blockers
ActiveUS10813915B2Nervous disorderHeterocyclic compound active ingredientsCandesartanSleep difficulties
Owner:UNIVERSITY OF PITTSBURGH
Therapeutic Combination Comprising a Nmda Receptors Blocker and a Narcotic Analgesic Substance
InactiveUS20080287480A1Inhibit development of toleranceBiocideNervous disorderNR1 NMDA receptorPhysical dependence
The invention relates to a medicament comprising a combination of a substance that blocks both the ion channel associated to NMDA receptors and MAO enzymes and a narcotic analgesic substance, preferably a fixed combination, pharmaceutical compositions thereof, and to the use thereof for the treatment of subjects suffering of various types of pain and / or for inhibiting the development of tolerance, and / or physical dependence on a narcotic analgesic substance.
Owner:CHIESI FARM SPA
Endothelin antagonist and beta receptor blocking agent as comblined preparation
InactiveCN1149087CBlood pressure did not dropLower blood pressureOrganic active ingredientsUrinary disorderDiseaseEndothelin Antagonists
The invention relates to a combination consisting of endothelin-antagonists and beta receptor blocking agents. Said combination is suitable for the treatment of diseases.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Treatment of infantile hemangioma
PendingUS20220218716A1Ointment deliveryPharmaceutical non-active ingredientsBeta blockerInfantile haemangioma
Owner:ROSSI THOMAS M +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com