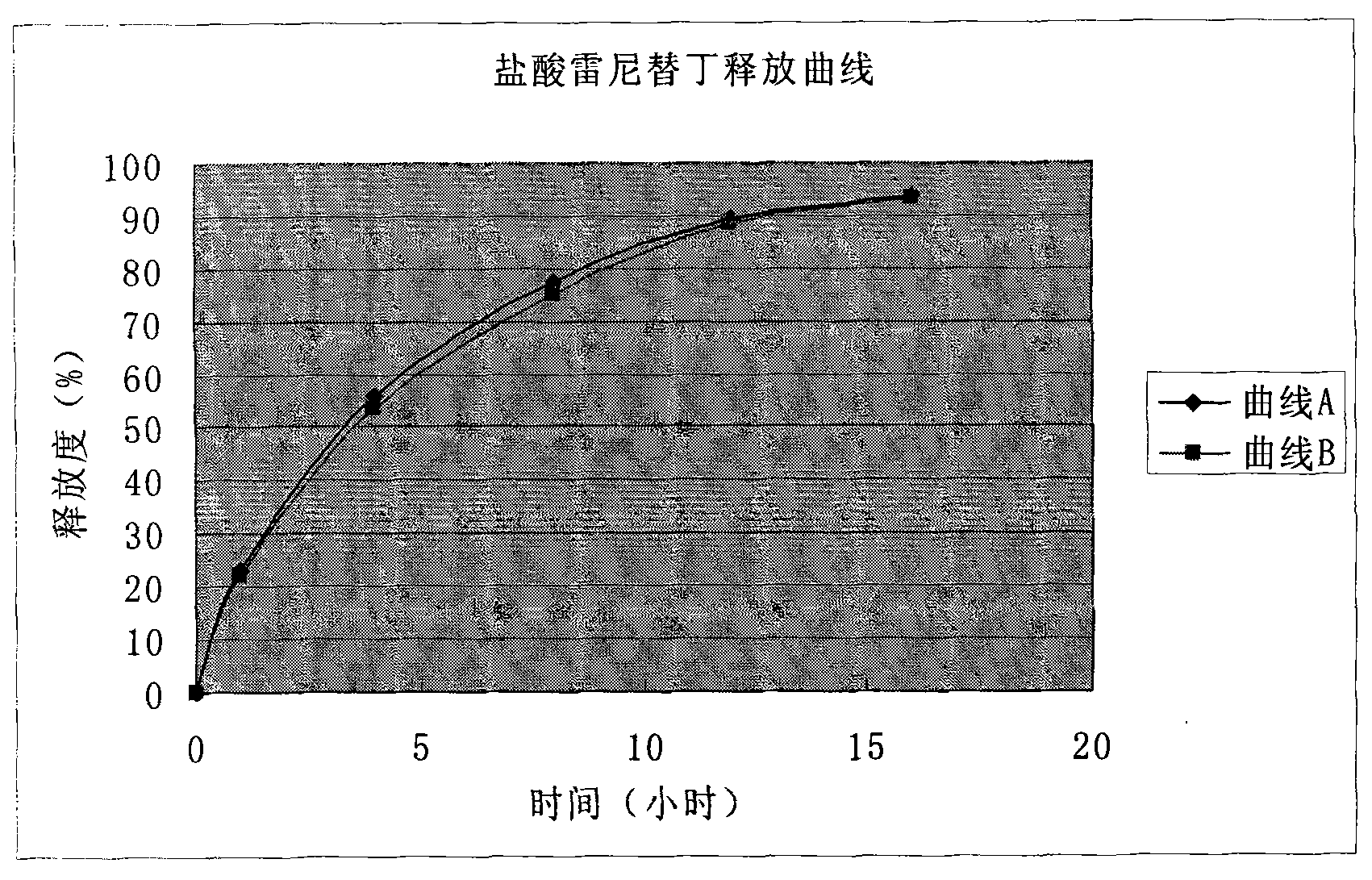
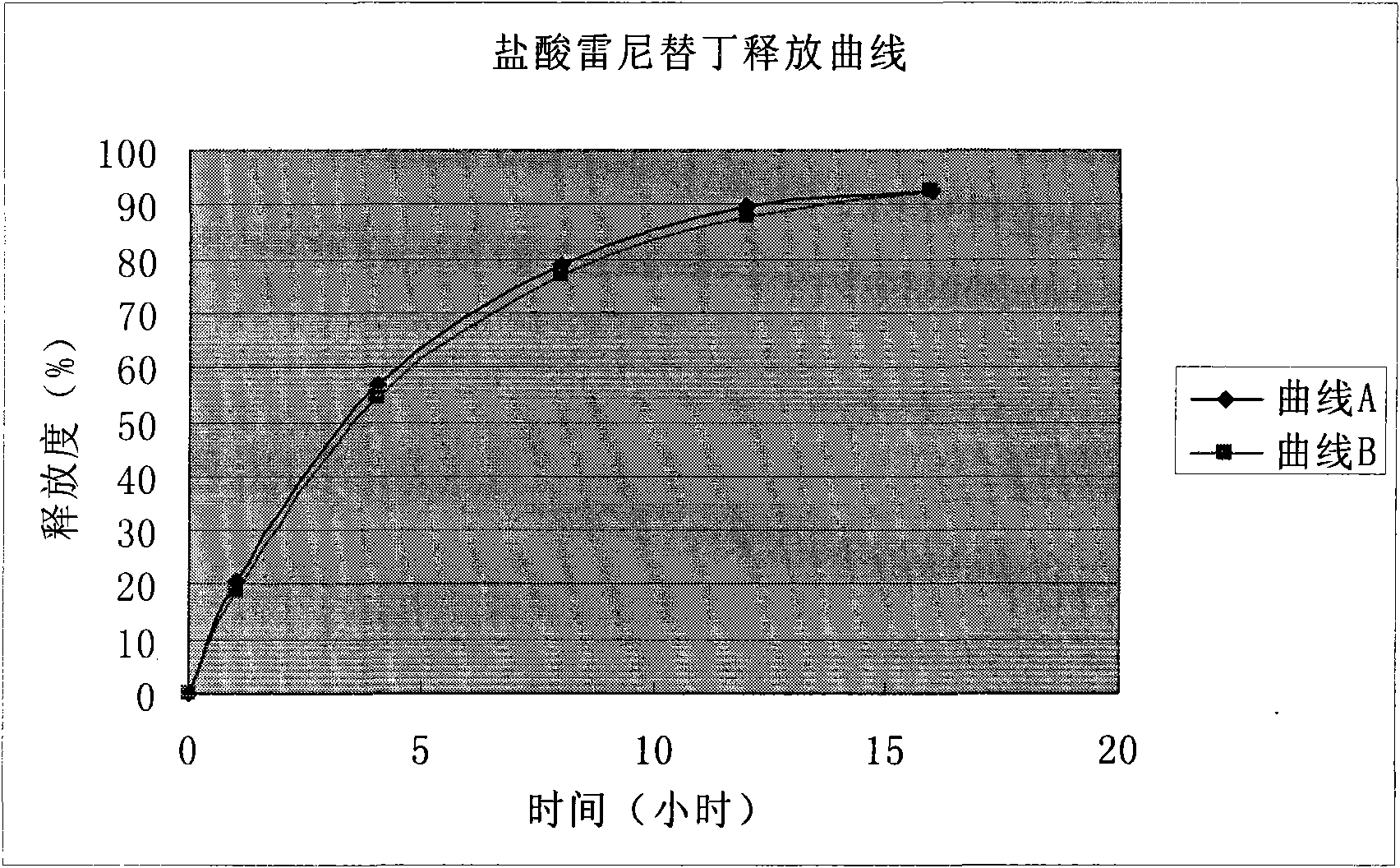
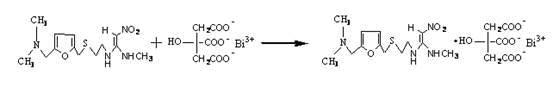
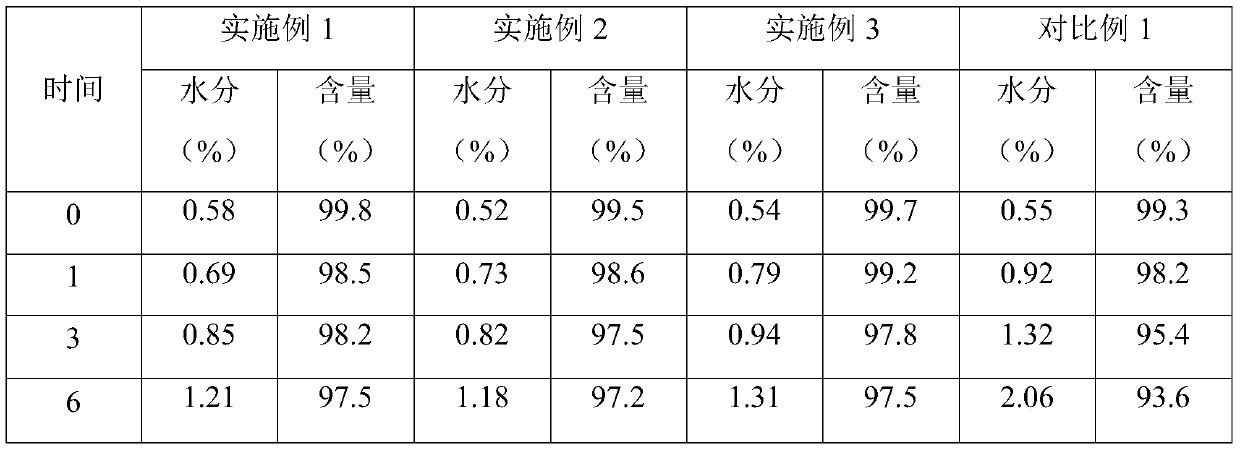
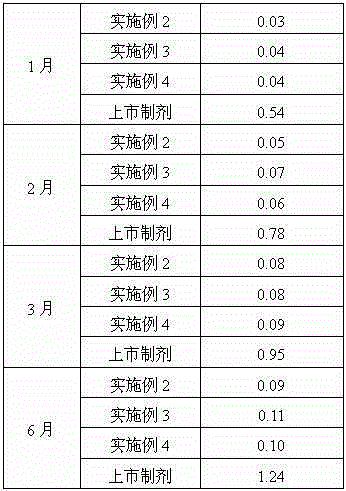
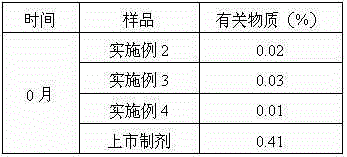
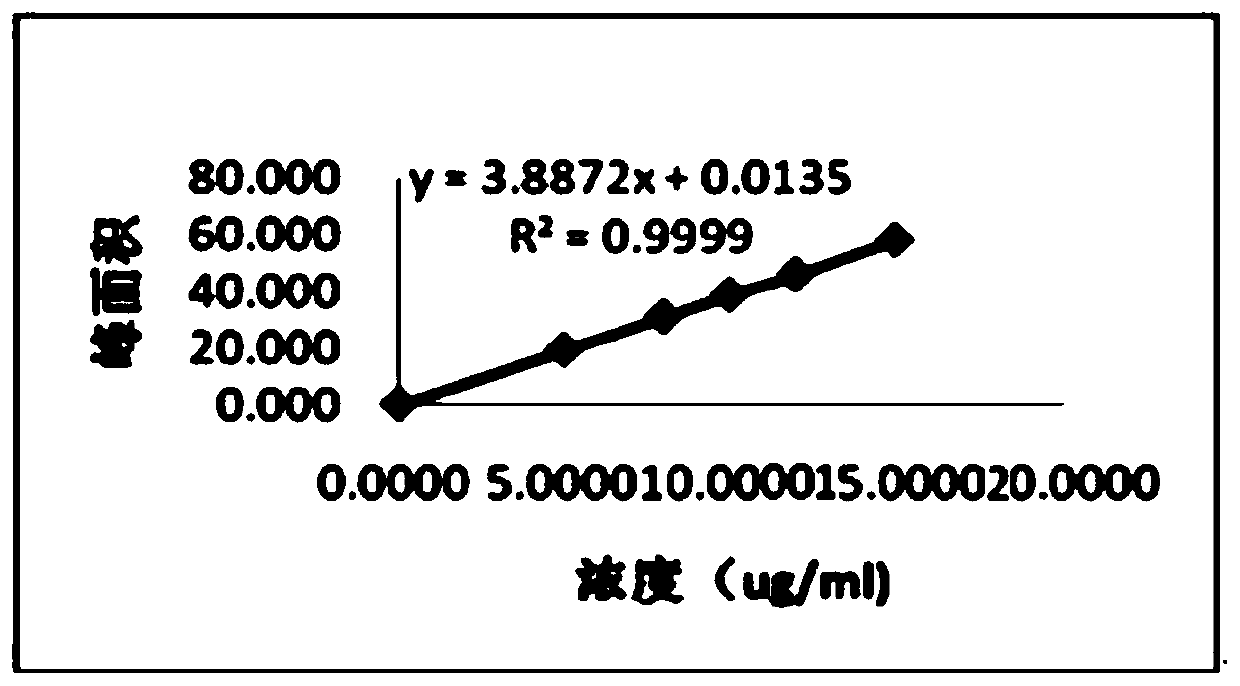
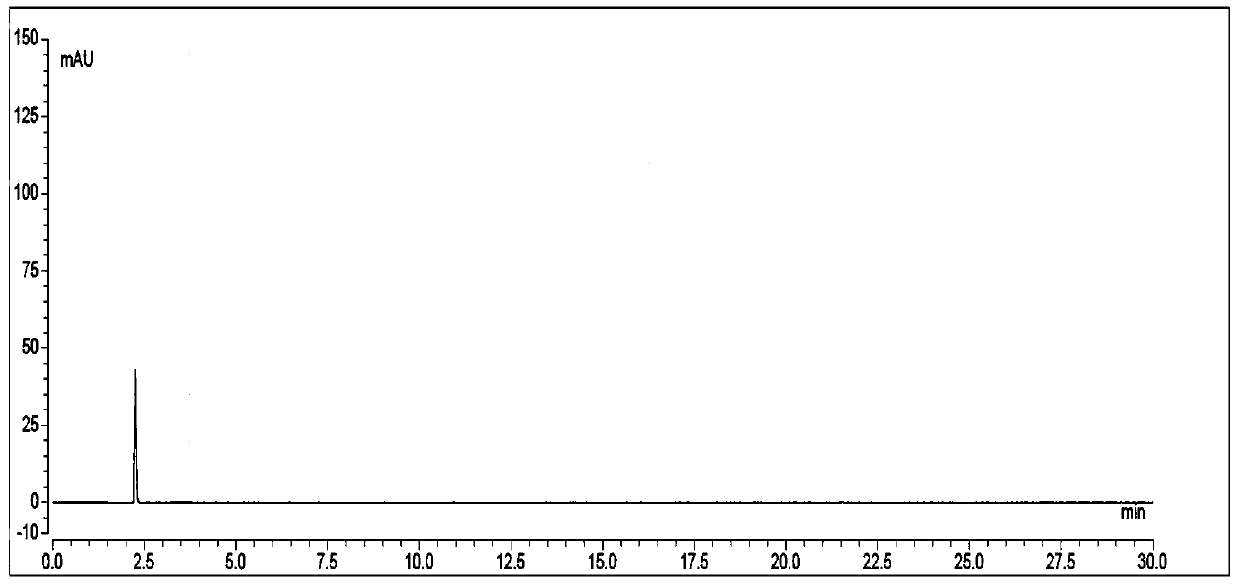
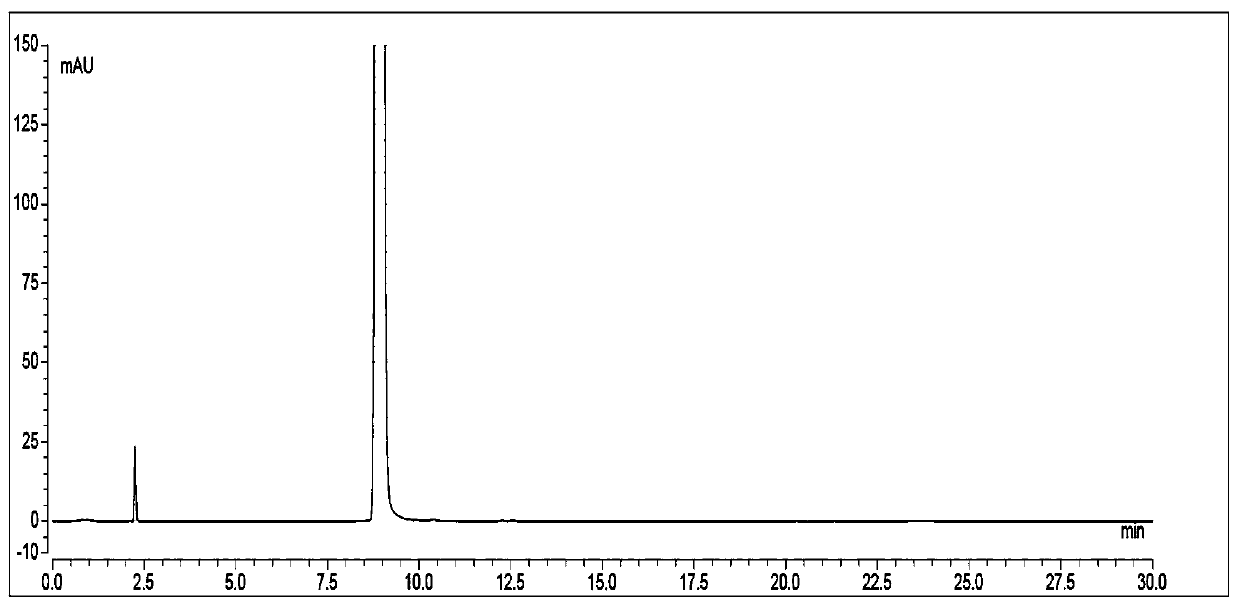
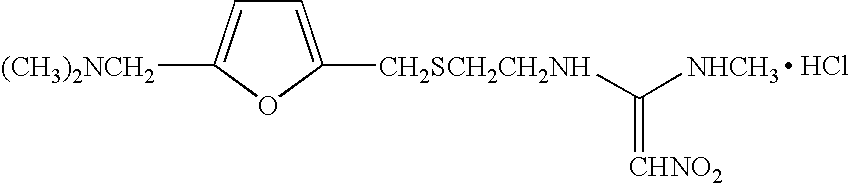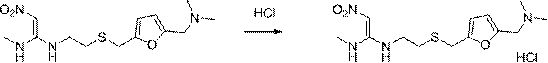Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
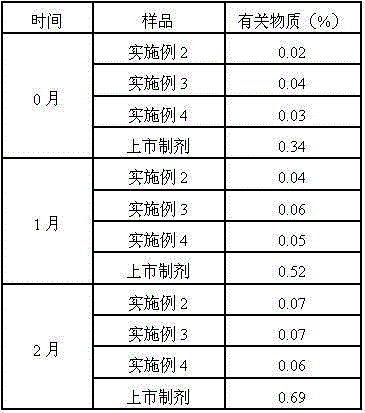
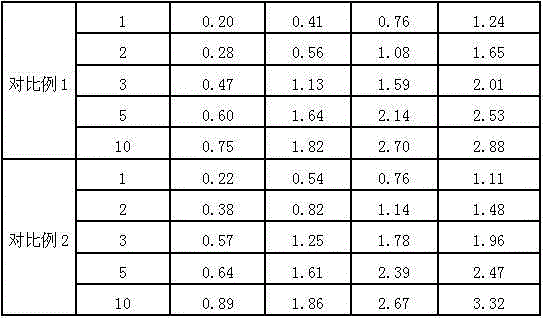
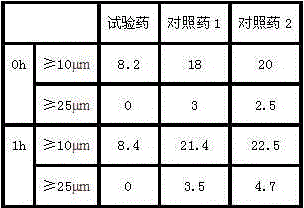
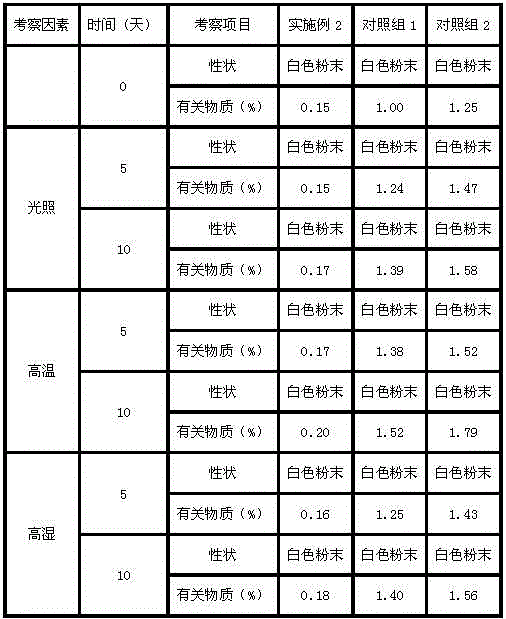
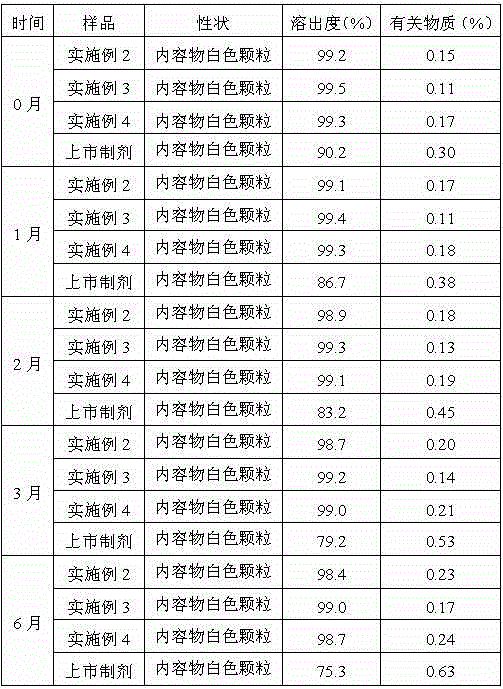
34 results about "Ranitidine hcl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Learn about Zantac (Ranitidine Hcl) may treat, uses, dosage, side effects, drug interactions, warnings, patient labeling, reviews, and related medications.
Mucoadhesive Oral Formulations of High Permeability, High Solubility Drugs
Solid oral dosage formulations, such as tablet, mini-tab, multiparticulates or osmotic delivery systems, are coated with a mucoadhesive polymeric coating or formed of a mucoadhesive polymer to increase oral bioavailability of Biopharmaceutical Classification System (BCS) Class I drugs. Representative BCS I drugs include valacyclovir, gabapentin, furosemide, levodopa, metformin, and ranitidine HCl. The inclusion of mucoadhesives in the solid oral dosage form brings the dosage form into close proximity with the target epithelium and facilitates diffusion of drug into intestinal tissue. The mucoadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. Preferred mucoadhesive polymers include poly(adipic)anhydride “P(AA)” and poly(fumaric-co-sebacic)anhydride “P(FA:SA)”. Other preferred mucoadhesive polymers include non-erodable polymers such as DOPA-maleic anhydride co polymer; isopthalic anhydride polymer; DOPA-methacrylate polymers; and DOPA-cellulosic based polymers.
Owner:JACOB JULES S +4
Ranitidine hydrochloride lipidosome capsule and new application thereof
InactiveCN101623258AImprove efficacyImprove bioavailabilityDigestive systemAntiviralsYolkHerpetic stomatitis
The invention provides a ranitidine hydrochloride lipidosome capsule and new application thereof. Particularly, certain amounts of yolk lecithin, cholesterin, natrium glycocholicum, tween 80 and an active ingredient ranitidine hydrochloride are combined and are prepared into ranitidine hydrochloride lipidosome by film dispersion technology, and the ranitidine hydrochloride lipidosome is mixed with the general accessories of medicine to prepare the capsule. The ranitidine hydrochloride lipidosome capsule better solves the problems of easy deliquescence, moisture absorption, color change and poor stability of the ranitidine hydrochloride, increases preparation medical effect and biological availability and can be used for treating herpetic stomatitis of childrem.
Owner:HAINAN MEIDA PHARMA
Solid preparation of ranitidine hydrochloride/bismuth potassium citrate medicinal composition
InactiveCN101862320AGuaranteed stabilityImprove stabilityAntibacterial agentsOrganic active ingredientsRanitidine HydrochlorideBioavailability
The invention relates to the solid preparation of a ranitidine hydrochloride / bismuth potassium citrate medicinal composition, which is prepared by mixing ranitidine hydrochloride / bismuth potassium citrate medicinal composition microcapsules and other pharmaceutical adjuvant required by the preparation of the solid preparation, wherein the ranitidine hydrochloride / bismuth potassium citrate medicinal composition microcapsules are prepared from ranitidine hydrochloride, bismuth potassium citrate, chitosan and sodium alginate. Compared with the prior art, the preparation of the invention has the characteristics of greatly improved stability and bioavailability, and stable and persistent release.
Owner:郝志艳
Ranitidine hydrochloride composition tablet medicine for treating digestive system diseases
InactiveCN104971053ASolve deliquescenceSolve moisture absorptionOrganic active ingredientsOrganic chemistrySilicic acidMedicine
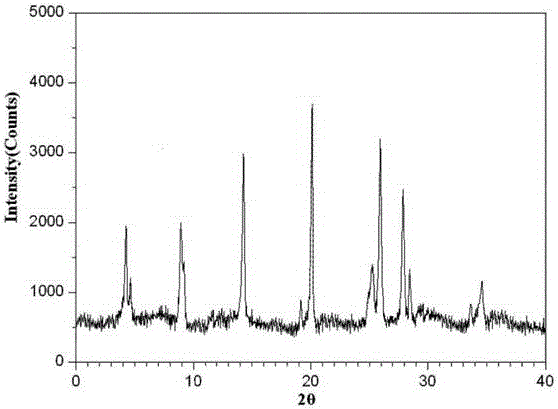
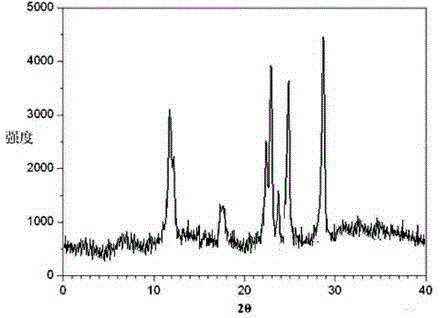
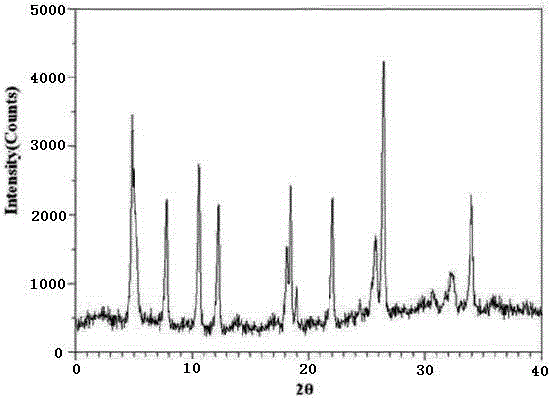
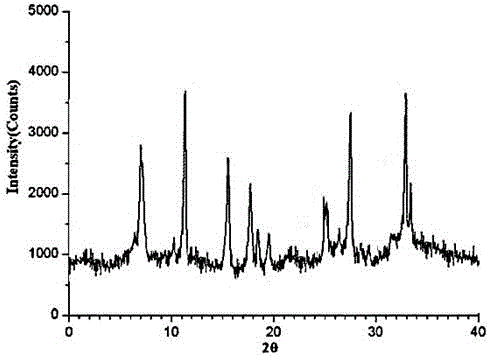
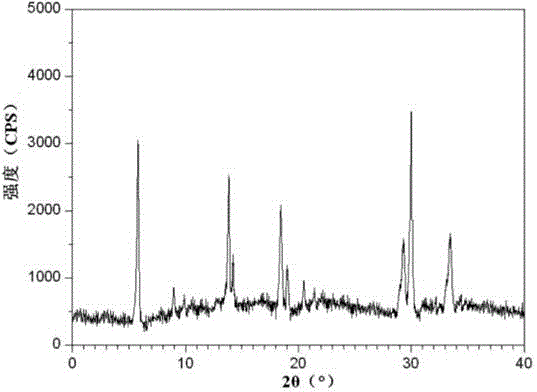
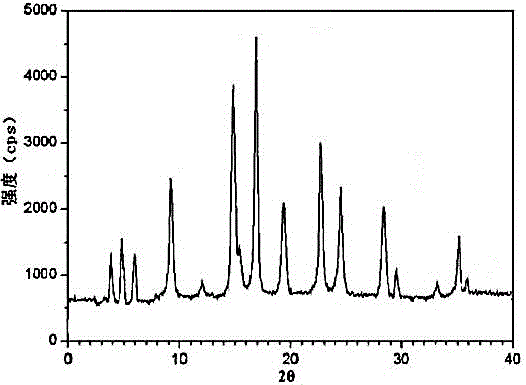
The invention relates to a ranitidine hydrochloride composition tablet medicine for treating digestive system diseases and belongs to the technical field of medicines. The ranitidine hydrochloride composition is composed of ranitidine hydrochloride, calcium hydrogen phosphate, pregelatinized starch, aluminium-magnesium silicate and aerosol, wherein ranitidine hydrochloride is a new crystal form compound, and an X-ray powder differentiation pattern obtained through Cu-Kalpha radioactive measurement is shown as a Figure 1, so that the ranitidine hydrochloride adopted in the ranitidine hydrochloride composition tablet medicine is different from the ranitidine hydrochloride reported in the prior art. Experiments show that the tablet prepared from the ranitidine hydrochloride new crystal form compound can be used for solving the problem that ranitidine hydrochloride is extremely easy to deliquesce, high in possibility of moisture adsorption and colour change and poor in stability, and the composition is simple, so that adverse reactions are greatly reduced, and the stability, pharmaceutical effect and biological availability of a tablet preparation are improved.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
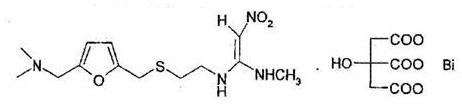
Method for preparing ranitidine carboxylic acid bismuth
InactiveCN102304107ASave resourcesProduct quality is easy to controlOrganic active ingredientsPill deliveryAlcoholFreeze-drying
The invention discloses a method for preparing a ranitidine carboxylic acid bismuth composite. The method comprises the following steps of: adding ranitidine, carboxylic acid bismuth, acid or alkali into water, controlling the pH of the reaction system at 6-12, reacting at low temperature (0-50 DEG C), stirring till the solution is basically clarified, filtering, dropping absolute ethyl alcohol into the filtrate, stirring, and separating the product from the filtrate by using an azeotropic dehydration technology or a spray drying technology or a freeze drying technology; or dissolving the crude product in water, sufficiently stirring, dropping the absolute ethyl alcohol, and obtaining the product by carrying azeotropic dehydration and refining. With pharmaceutically acceptable auxiliary materials in rational amounts, the product can be produced into troches and capsules.
Owner:王健祥
Stable pharmaceutical compositions, processes for making the same and methods of their use
The present invention is generally related to alcohol free, liquid ranitidine formulations for oral administration. In particular, the present invention is related to stable, syrup formulations having ranitidine as an active ingredient for oral administration, processes for making the same, and methods of their use. The ranitidine of the present invention is stable in non-polar media or media having a relatively low polarity such that the dielectric constant is less than about 60, and is achieved by using certain saccharides, certain relatively high molecular weight starches, and / or certain celluloses instead of alcohol.
Owner:PALEPU NAH R
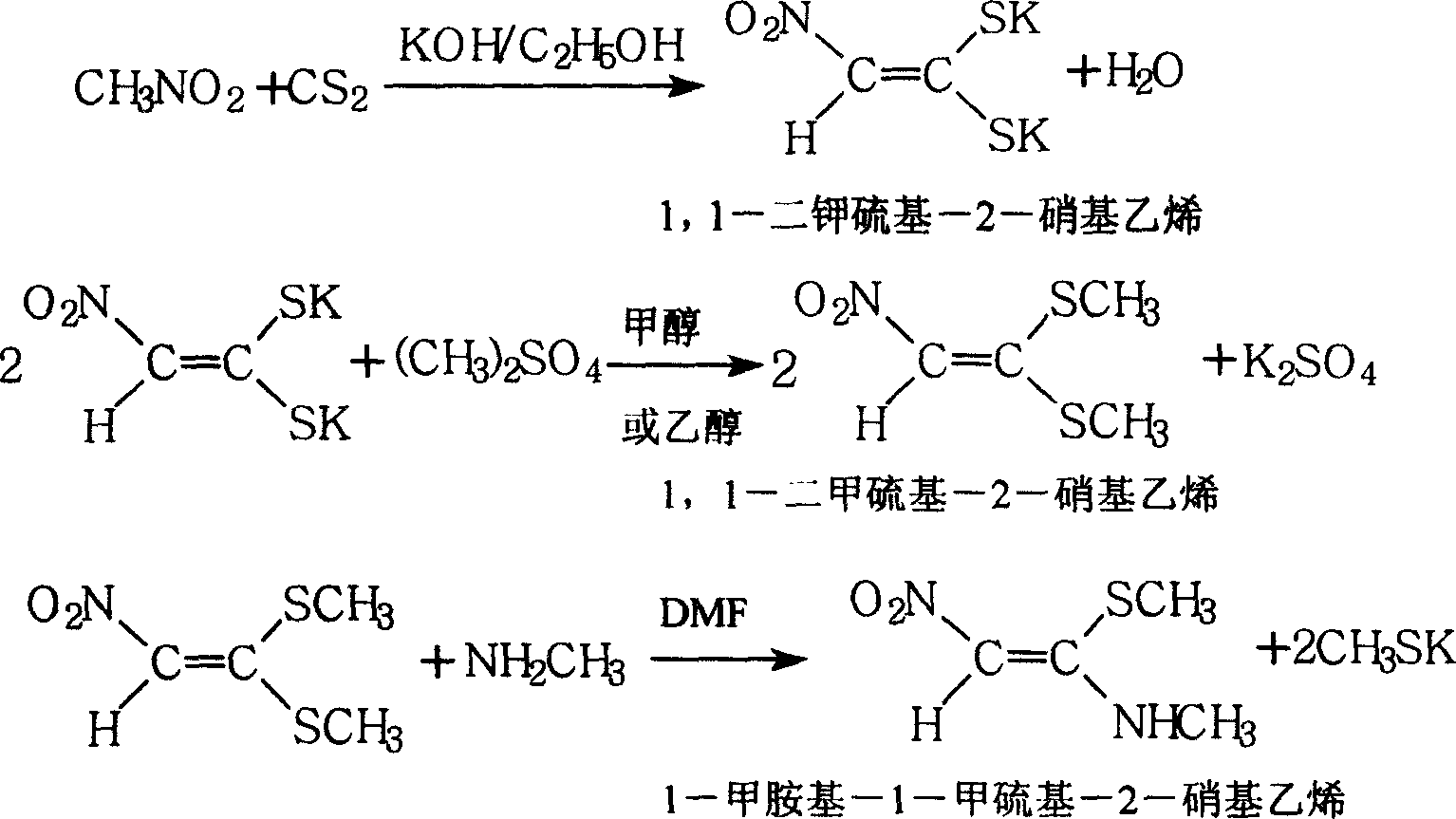
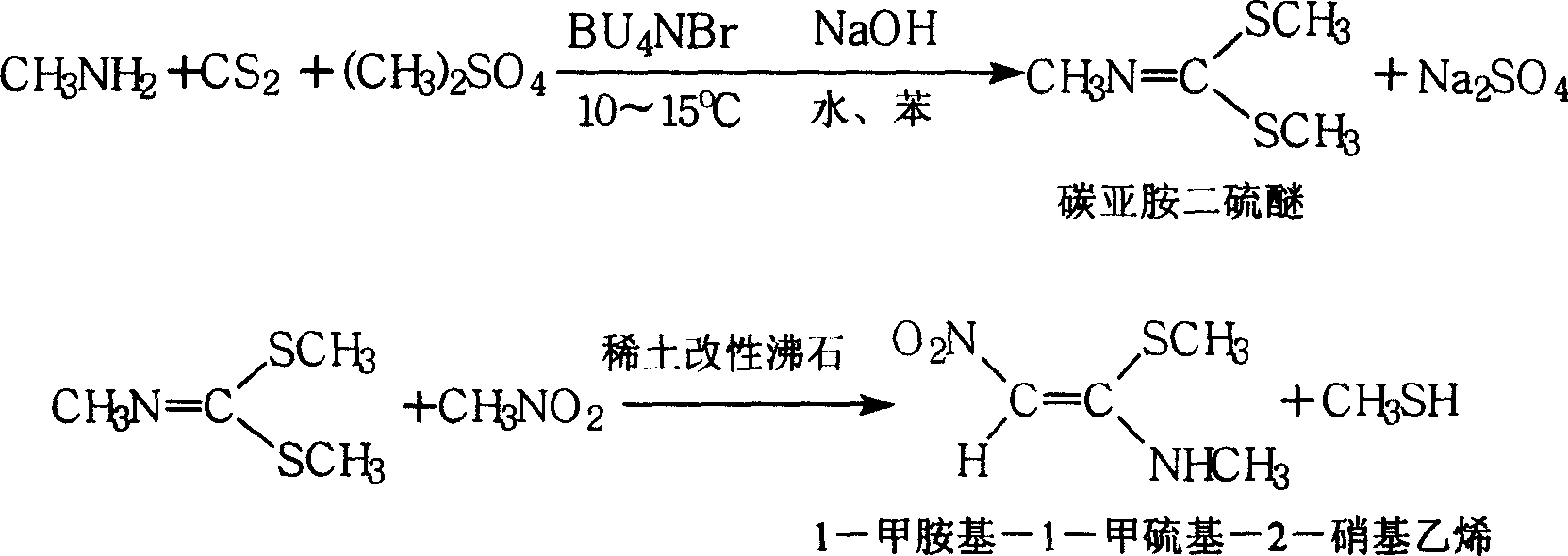
1-methylamino-1- methylthio-2-nitroethylene synthesis method
InactiveCN1962626AEmission reductionProcess safetySulfide preparationChemical synthesisSynthesis methods
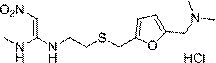
The invention discloses a new chemical synthesizing method of 1-motol-1-methylmercapto-2-nitroethylene as one intermediate of ulcer drug ranitidine, which comprises the following steps: adopting nitromethane, carbon bisulphide and potassium hydroxide as initial reacting material; synthesizing 1, 1-dimethyldisulphide-2-nitro ethylene to dissolve in the water; reacting with aminomethane to synthesize new intermediate product; filtering to obtain the product.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for refining ranitidine base
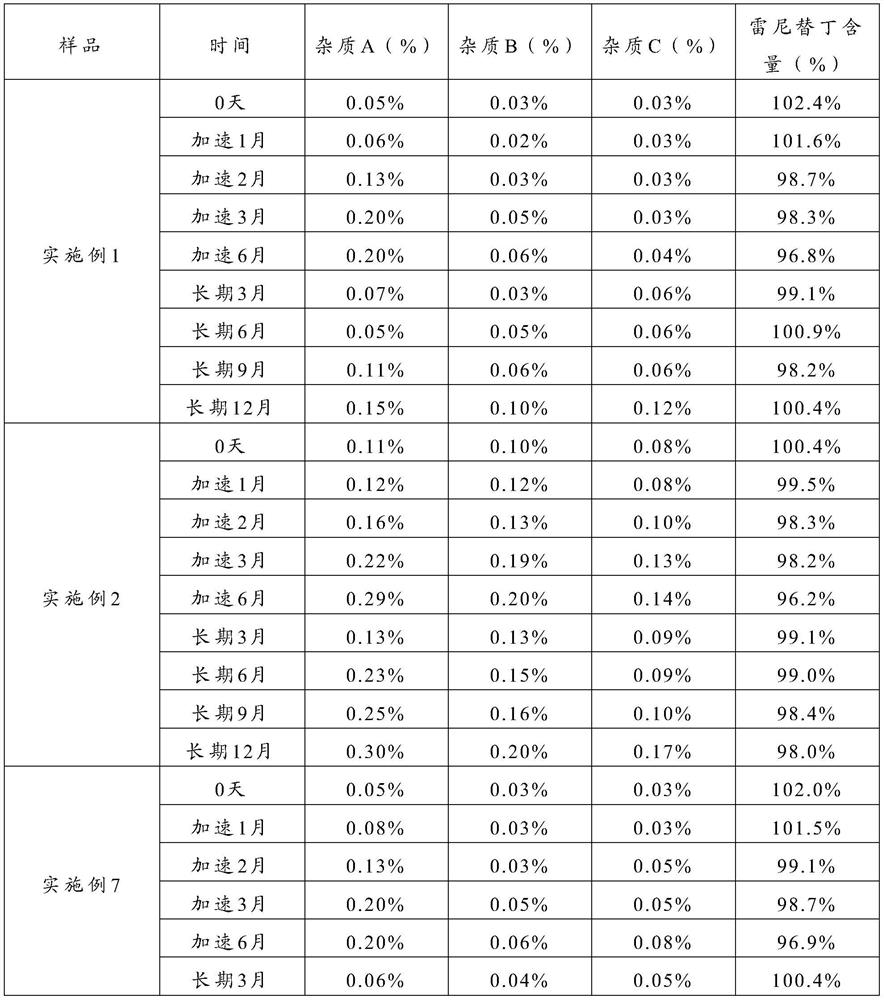
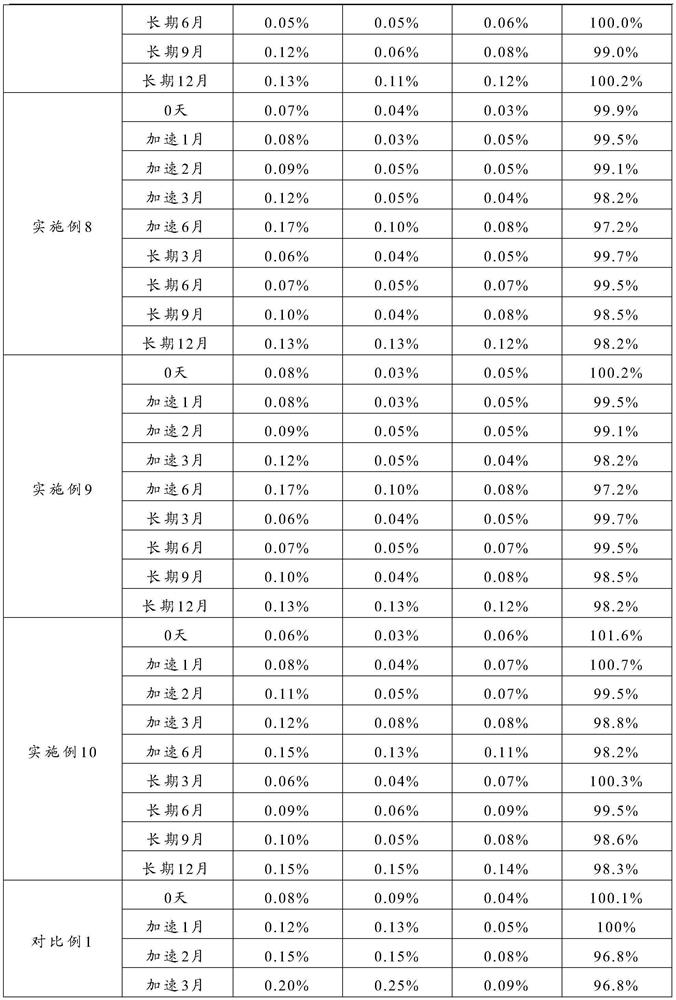
The invention relates to the technical field of separation and purification of fine chemicals, and more concretely relates to a method for refining ranitidine base. By using a mixed solvent prepared from an intensive polar solvent and a weak polar solvent, the dissolvability of ranitidine base and impurities in the solvent is different, so that the ranitidine base and the impurities can be effectively separated during a crystallization process, and an obtained refined product is high in purity and stable in property. After analyzing the ranitidine base refined through the method provided by the invention through a liquid chromatograph, the purity of the ranitidine base is not less than 99.0 percent, the total impurity is not larger than 0.15 percent, and the single impurity is not larger than 0.05 percent. A ranitidine hydrochloride product obtained through salifying the ranitidine base prepared and purified through the method provided by the invention has the purity reaching and beingsuperior to the quality level of an original drug, so that the safety of the drug during a use process is further improved.
Owner:SHANXI YUNPENG PHARMA
Method for checking total number of aerobic bacteria of ranitidine hydrochloride capsules
The invention belongs to the technical field of medical inspection, and specifically relates to a method for checking total number of aerobic bacteria of ranitidine hydrochloride capsules. The methodcomprises the following process: taking 10 g of ranitidine hydrochloride capsules in a sterile conical flask, adding a sterile sodium chloride peptone buffer of pH7.0 to dilute to 100 ml, shaking theconical flask to disperse the solution evenly, preparing a test solution of 1:10, placing the full amount of 1:10 test solution of the conical flask on a large volume side of a special bag with a homogenizer with filtering function, conducting fully tapping for 30 seconds in a tapping instrument, taking the test solution on the small volume side after filtration as the test solution of 1:10 for coarse filtration, taking 20 ml of the test solution of 1:10 for coarse filtration to be diluted to 100 ml by using the sterile sodium chloride peptone buffer of pH7.0, preparing a test solution of 1:50, adding 1 ml of the test solution of 1: 50 into a plate, injecting 15-20 ml of trypsin soybean pepton agar culture medium immediately, conducting mixing evenly, solidifying, and incubating at 30-35 DEG C for 3-5 days, and determining the number of colonies. According to the present invention, the operation steps of the method are simple, and the inspection results are stable and reliable.
Owner:GUANGZHOU MEDCAN PHARMATECH
Ranitidine hydrochloride composition for treating gastric diseases
InactiveCN105055442AHigh purityImprove liquidityOrganic active ingredientsPowder deliveryCrystal structureMoisture absorption
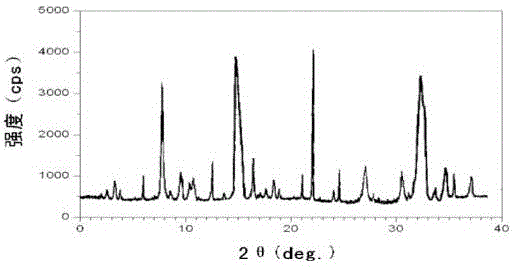
The invention relates to a ranitidine hydrochloride composition for treating gastric diseases, belonging to the technical field of medicines. The composition comprises ranitidine hydrochloride and anhydrous sodium carbonate, wherein ranitidine hydrochloride is a crystal. An X-ray powder diffraction pattern obtained through measurement by using a Cu-Kalpha ray is shown in a drawing 1 in the specification. A new crystal form of ranitidine hydrochloride provided by the invention is different from the crystal structure of the prior art. Through experimental verification, the compound in the new crystal form has high purity, good flowability and stability and low impurity content, has low possibility of moisture absorption and is safe and reliable to apply clinically. Powder injections prepared by utilizing the compound in the new crystal form have good stability after undergoing compatibility with solvents, have low content of insoluble particles and are very suitable to apply clinically.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Ranitidine hydrochloride freeze-dried powder injection compound for treatment of digestive system diseases
InactiveCN104825438ASolve deliquescenceSolve moisture absorptionOrganic active ingredientsPowder deliveryDextranPharmaceutical Substances
The invention belongs to the technical field of medicament and discloses a ranitidine hydrochloride freeze-dried powder injection compound for treatment of digestive system diseases. The ranitidine hydrochloride freeze-dried powder injection compound is composed of ranitidine hydrochloride and an excipient, wherein low-molecular-weight dextran serves as the excipient, the ranitidine hydrochloride is a novel crystal compound and different from ranitidine hydrochloride in the prior art, and an X-ray powder diffraction pattern of the ranitidine hydrochloride is obtained by Cu-K alpha ray measurement and shown as a diagram 1. According to experiment results, compared with freeze-dried powder injections prepared from the ranitidine hydrochloride in the prior art, freeze-dried powder injections prepared from the ranitidine hydrochloride which is the novel crystal compound have the advantages that the problems of proneness to deliquescence, moisture absorption and discoloration and poor stability of the ranitidine hydrochloride are solved, component simplicity is realized, insoluble particles are less after the ranitidine hydrochloride freeze-dried powder injection is compatibly used with four injections, and insoluble particle amount changes slightly in 4h after compatible application.
Owner:苗怡文
Medicinal ranitidine hydrochloride composition capsule for treating gastric ulcer
InactiveCN104906069ASolve the problems of easy oxidation, easy moisture absorption and discoloration, poor stability, etc.Improve stabilityOrganic active ingredientsOrganic chemistryCelluloseLactose
The invention discloses a medicinal ranitidine hydrochloride composition capsule for treating gastric ulcer and belongs to the technical field of medicines. The capsule is composed of ranitidine hydrochloride, lactose anhydrous, cross-linking sodium carboxymethylcellulose, silica and talcum powder. The ranitidine hydrochloride is a novel crystal compound. The X-ray powder diffraction diagram obtained through measurement of Cu-Kalpha rays is as shown in the Figure 1. The ranitidine hydrochloride is different from ranitidine hydrochloride reported in the prior art. Experiments find that compared with a ranitidine hydrochloride capsule in the prior art, the capsule prepared by the ranitidine hydrochloride as the novel crystal compound solves the problems that ranitidine hydrochloride is prone to deliquescing, absorbing moisture and changing colors and has the poor stability, and the stability, medicine effect and bioavailability of the capsule are improved.
Owner:苗怡文
Ranitidine hydrochloride composition freeze-dried powder injection for treating stomach illness
InactiveCN105055396AHigh purityImprove liquidityPowder deliveryOrganic active ingredientsMedicinePharmaceutical drug
The invention relates to a ranitidine hydrochloride composition freeze-dried powder injection for treating stomach illness and belongs to the technical field of medicine. The composition freeze-dried powder injection is prepared from ranitidine hydrochloride and an excipient. The excipient is mycose, the ranitidine hydrochloride is a new crystal form compound, and is ranitidine hydrochloride different from what is reported in the prior art by an X ray power diffraction pattern as shown in picuture 1 by using Cu-Kalpha ray measurement, through test, the new crystal form compound is high in purity, good in mobility and stability, low in impurity content and safe and reliable in clinical use, and does not easily absorb humidity, and the freeze-dried powder injection prepared by the new crystal form compound is good in stability after being matched with a solvent, extremely low in insoluble particle content and very suitable for clinical use.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
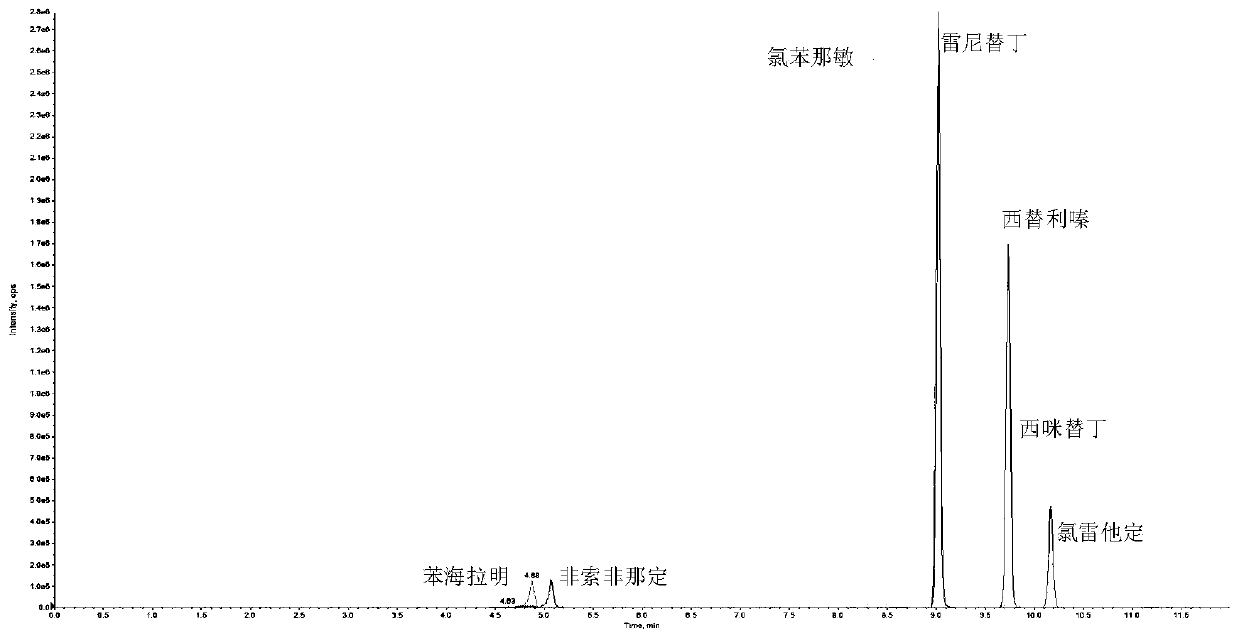
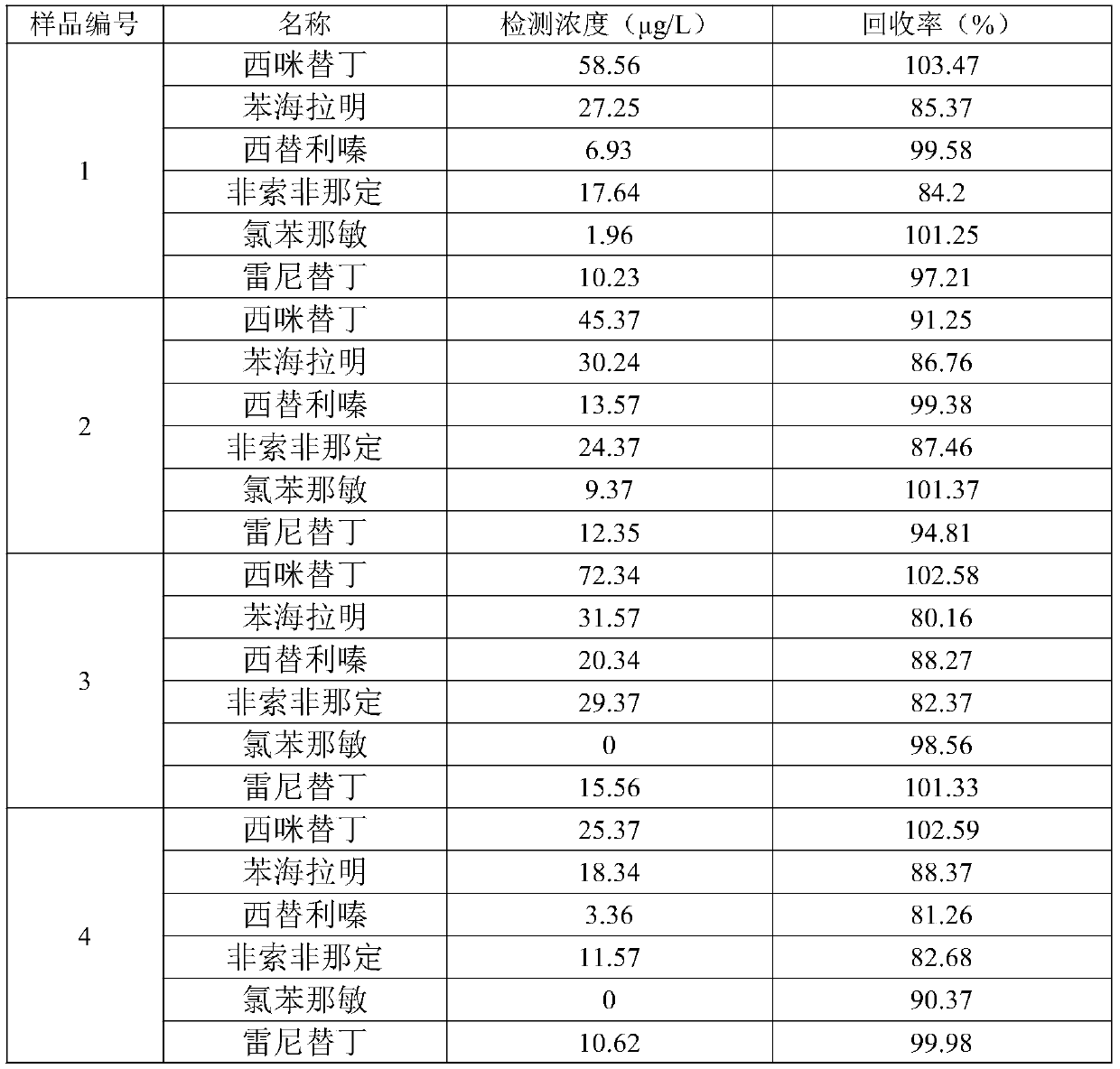
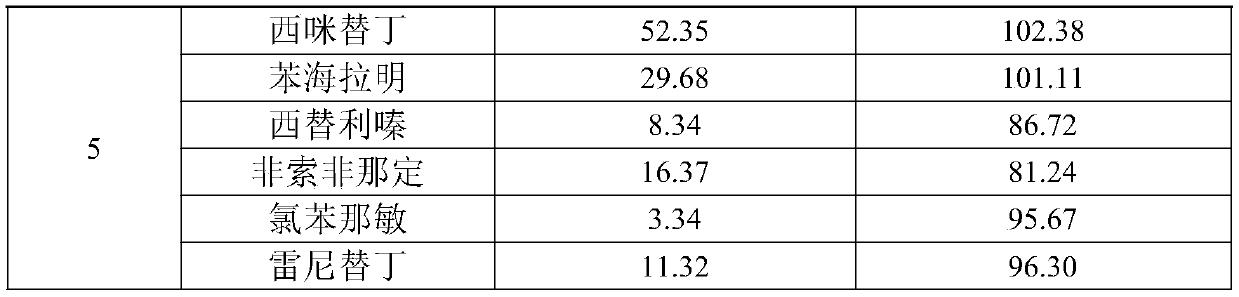
Quick detection and analysis method for antihistamine drugs in water body
The invention discloses a quick detection and analysis method for antihistamine drugs in a water body. The method can be used for detecting drugs for treating allergic diseases, and six antihistaminedrugs, including cimetidine, diphenhydramine, cetirizine, fexofenadine, chlorpheniramine and ranitidine, in a water body can be efficiently and quickly detected at the same time by combining a solid-phase extraction small column with a high performance liquid chromatography-mass spectrometry method. The method comprises the following steps: filtering a water sample, and adding an internal standardsubstance; extracting and purifying six statins in the sample by using a small solid-phase extraction column; and detecting the content of a target object in the water sample by using a high performance liquid chromatography-mass spectrometer. The method has the advantages of simple water sample treatment steps, convenient operation and good stability, and can quickly obtain the sample suitable for detection of the liquid chromatograph-mass spectrometer; the sample pretreatment cost is low, the detection can be completed by utilizing common consumables in a laboratory, the detection speed ishigh, the automation degree is high, the response is sensitive, and popularization and application are facilitated.
Owner:SHANGHAI UNIV
Preparation method of ranitidine hydrochloride tablets
InactiveCN109700775ASolve moisture absorptionPrevent drynessOrganic active ingredientsDigestive systemHydrogen phosphateMagnesium stearate
The invention belongs to the technical field of pharmaceutical preparations, and in particular relates to a preparation method of ranitidine hydrochloride tablets. The method selects auxiliary materials which are suitable for direct tabletting and have moisture-proof function, such as microcrystalline cellulose, anhydrous calcium hydrogen phosphate, magnesium stearate and silicon dioxide, adopts adirect tabletting process at the same time, solves the problem of moisture absorption of the tablet core, avoids the drying process of particles after wet granulation, and improves product quality and production efficiency. The process is simple, easy to implement, and suitable for large-scale production.
Owner:REYOUNG PHARMA
Ranitidine hydrochloride combination for inhibiting generation of gastric acid
InactiveCN105106122AHigh purityImprove liquidityOrganic active ingredientsPowder deliveryPharmaceutical drugMoisture absorption
The invention discloses a ranitidine hydrochloride combination for inhibiting generation of gastric acid and belongs to the technical field of medicine. The ranitidine hydrochloride combination is composed of ranitidine hydrochloride and sodium chloride, ranitidine hydrochloride is a crystal, and X-ray powder diffraction pattern acquired by using Cu-K alpha ray for measuring is shown as a Figure 1. New crystal form of ranitidine hydrochloride is difference from crystal form structure in the prior art; experiments verify that a compound with the new crystal form is high in purity, fluidity and stability, low in impurity content, less prone to moisture absorption and safe and reliable in clinical application; a powder injection prepared by utilizing the compound is high in stability after being combined with solvent, extremely low in insoluble particle content and quite suitable for clinical application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Medicinal ranitidine hydrochloride composition for treating peptic ulcer
InactiveCN105106125AHigh purityImprove liquidityPowder deliveryOrganic active ingredientsPeptic ulcerCrystal structure
The invention relates to a medicinal ranitidine hydrochloride composition for treating peptic ulcer. The composition comprises ranitidine hydrochloride and sodium dihydrogen phosphate. The ranitidine hydrochloride is a new crystal compound, the X-ray powder diffraction diagram, obtained by using Cu-K alpha rays for measuring, of the ranitidine hydrochloride is shown in figure 1, and the crystal structure of the pantoprazole sodium is different from that of ranitidine hydrochloride in the prior art. Experiments show that the new crystal compound is high in purity, good in flowability, good in stability, low in impurity content, less prone to moisture absorption and safe and reliable in clinical application; powder injection prepared by the new crystal compound is good in stability, good in stability after being combined with solvent, extremely low in insoluble particle content and quite suitable for clinical application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Medicine, namely, ranitidine hydrochloride composition capsule, for treating digestive system disease
InactiveCN104873484ASolve deliquescenceSolve moisture absorptionOrganic active ingredientsOrganic chemistryDiseasePharmaceutical drug
The invention discloses a medicine, namely, a ranitidine hydrochloride composition capsule, for treating a digestive system disease, and belongs to the technical field of medicines. The composition capsule is prepared from ranitidine hydrochloride and talcum powder. Ranitidine hydrochloride is a new crystal type compound, an X-ray powder diffraction diagram obtained by adopting Cu-K alpha ray measurement is as shown in the Figure1, and ranitidine hydrochloride is different from ranitidine hydrochloride reported in the prior art. Tests prove that as compared with the ranitidine hydrochloride capsule in the prior art, the capsule prepared from the new crystal type ranitidine hydrochloride compound not only solves the problems that ranitidine hydrochloride has high possibility of deliquescing, absorbing moisture and changing color as well as poor stability, but also has the advantages that the component is simple, the adverse reaction is reduced greatly, and the stability, the medicine effect and the bioavailability of the capsule preparation are improved.
Owner:苗怡文
High performance liquid chromatographic detection method for formaldehyde content in ranitidine hydrochloride
The invention discloses a high performance liquid chromatographic detection method for formaldehyde content in ranitidine hydrochloride. The high performance liquid chromatographic detection method takes an organic solvent and water as mobile phases, and adopts gradient elution to determine the formaldehyde content. During determination, a derivatization reagent is adopted for derivatization treatment of formaldehyde, a test sample is prepared into a test sample derivative solution with a diluent, a formaldehyde solution is prepared into a formaldehyde derivative control solution with a diluent, the test sample derivative solution and the formaldehyde derivative control solution are taken respectively for direct sample introduction, and chromatograms are collected; and the content of formaldehyde in ranitidine hydrochloride is calculated according to an external standard method. The high performance liquid chromatographic detection method provided by the invention has the advantages ofsimple operation, high sensitivity, quantifiable determination, high precision and good reproducibility, and meets the process production requirements.
Owner:ZENJI RES LAB
Ranitidine hydrochloride composition dry suspension for treating gastric ulcer
InactiveCN105030694AImprove stabilityImprove efficacyOrganic active ingredientsOrganic chemistryCelluloseSucrose
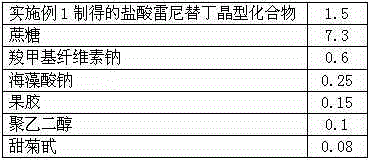
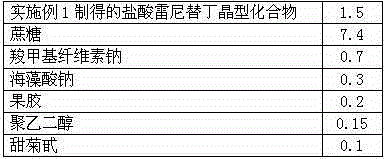
The invention discloses a ranitidine hydrochloride composition dry suspension for treating gastric ulcer, and belongs to the technical field of medicine. A composition is composed of ranitidine hydrochloride, sucrose, sodium carboxymethylcellulose, sodium alginate, pectin, polyethylene glycol and stevioside; the ranitidine hydrochloride is a new crystal form compound, is measured with a Cu-Ka ray to obtain an X-ray powder diffraction pattern and is different from ranitidine hydrochloride reported by the prior art, wherein the X-ray powder diffraction pattern is as shown in figure 1. Experiments find that the dry suspension prepared with the ranitidine hydrochloride-new crystal form compound solves the problem that the ranitidine hydrochloride is extremely prone to being deliquesced, moisture is prone to being absorbed, color is prone to being changed, and the stability is poor, the components are simple, adverse reactions are greatly prevented from occurring, and the stability, medicine effects and bioavailability of the dry suspension are improved.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
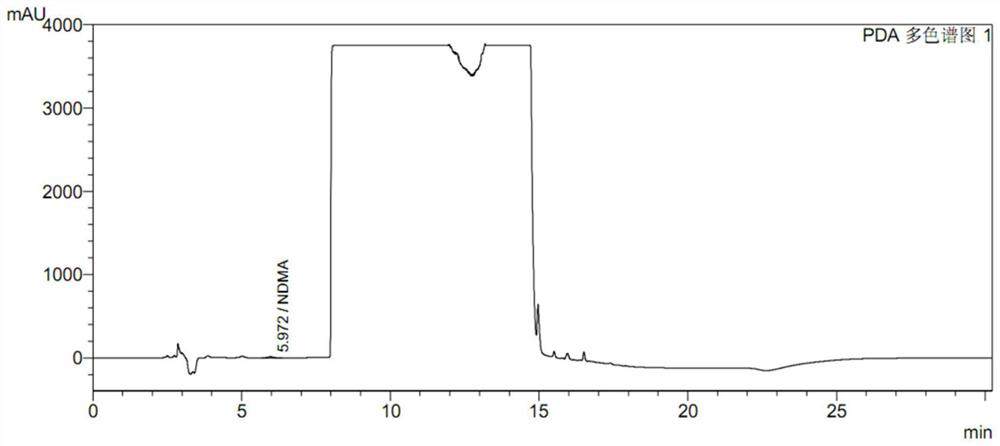
Preparation method of ranitidine hydrochloride with low NDMA content
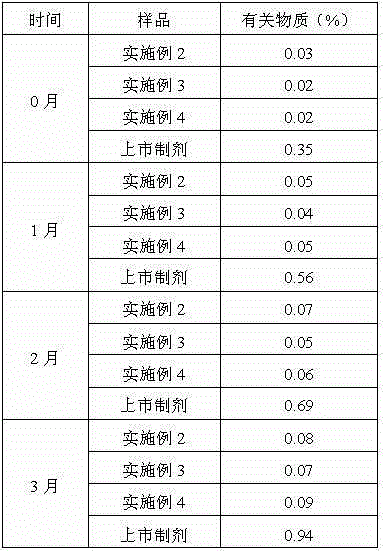
The invention relates to the technical field of ranitidine hydrochloride preparation, in particular to a preparation method of ranitidine hydrochloride with low NDMA content, which comprises the following steps: adding a ranitidine base into ethanol, and stirring until complete dissloving is achieved; cooling the solution to -5 to 5 DEG C; controlling the temperature, adding a hydrochloric acid aqueous solution, adjusting the pH value to 4.5-6.5, and uniformly stirring; adding a seed crystal, preserving heat, stirring and crystallizing for 3-5 hours; and filtering a crystal, washing, drainingand drying to obtain the off-white crystal solid ranitidine hydrochloride. The salifying method disclosed by the invention has the advantages that the steps are simple, the raw material hydrochloric acid aqueous solution is easy to obtain, the product character is good, and the impurity content of the final product NDMA (N-Nitrosodimethylamine) is low.
Owner:北京云鹏鹏程医药科技有限公司
Pharmaceutical ranitidine hydrochloride composition for treating digestive diseases
InactiveCN105232519AHigh purityImprove liquidityOrganic active ingredientsOrganic chemistryArgininePharmaceutical drug
The invention discloses a pharmaceutical ranitidine hydrochloride composition for treating digestive diseases and belongs to the technical field of medicine. The composition is composed of ranitidine hydrochloride and arginine. The ranitidine hydrochloride is crystal, and an X-ray powder diffraction pattern of the crystal obtained by measurement using Cu-KAlpha ray is shown in 1. The novel crystal form of the ranitidine hydrochloride provided by the invention is different from crystal form structures of the prior art, testing shows that this novel-crystal form compound has high purity, good fluidity, good stability and low impurity content, rarely damps and is safe and reliable to clinically apply, powder injection prepared using the novel-crystal form compound is good in stability, is highly stable after being compatible with a solvent, has very low content of dissolvable particles and is very suitable for clinical application.
Owner:杨献美
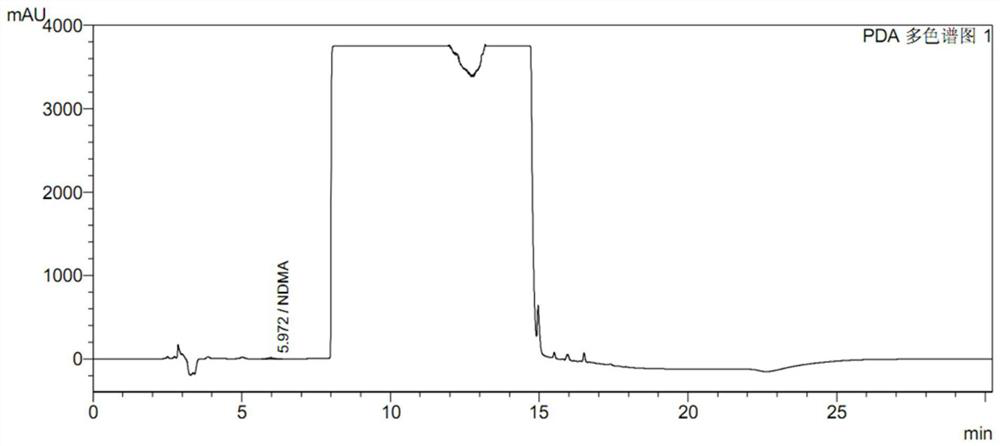
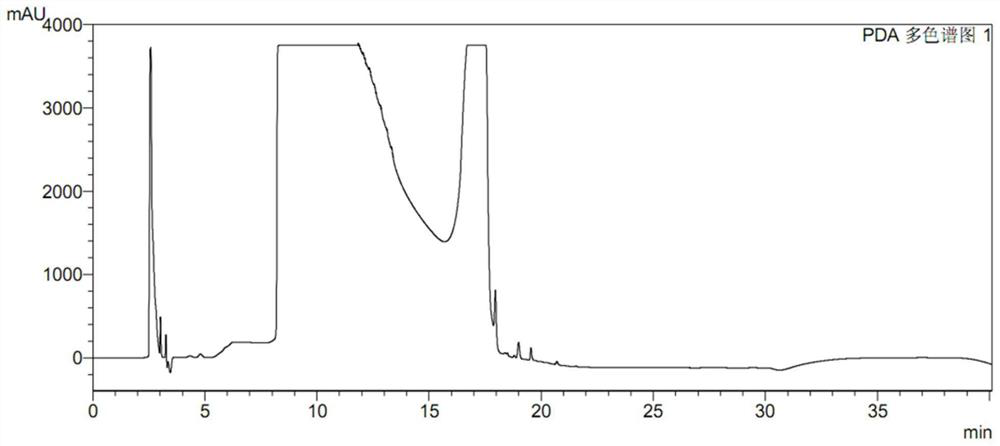
High performance liquid chromatography for determining N-nitrosodimethylamine in ranitidine and solid preparation thereof
PendingCN114878707AAvoid it happening againLower technical barriersComponent separationNitrosoHplc mass spectrometry
The invention discloses a high performance liquid chromatography method for determining N-nitrosodimethylamine in ranitidine and a solid preparation thereof, a chromatographic column is used for respectively performing chromatographic analysis on a treated test solution and a contrast solution, and the conditions of the high performance liquid chromatography are as follows: the wavelength is 235nm, the flow velocity is 1ml / min, the column temperature is 30 DEG C, and the sample introduction is 20mu l; the mobile phase of the high performance liquid chromatography comprises a mobile phase A and a mobile phase B, the mobile phase A is an alkaline aqueous mobile phase, the pH value of the alkaline aqueous mobile phase is more than 7 and less than or equal to 10.5, and the mobile phase B is an organic phase. The mobile phase system is alkaline, ranitidine is adsorbed by the stationary phase in a stable molecular state, and a better separation degree is achieved. An ammonia-ammonium bicarbonate buffer system can meet the alkalinity requirement of the system, the baseline noise is low, and the system is compatible with mass spectrum.
Owner:湖南省药品检验检测研究院
Preparation method of ranitidine hydrochloride
PendingCN114591274AHigh purityImprove solubilityOrganic chemistryMedicinal chemistryHydrochloric acid
The invention relates to the technical field of compound purification and synthesis, and provides a preparation method of ranitidine hydrochloride. According to the method, the ranitidine base crude product is adopted as a raw material, the ranitidine base crude product is purified through decoloration and first crystallization to obtain a ranitidine base refined product, then the ranitidine base refined product reacts with hydrochloric acid ethanol, high-purity ranitidine hydrochloride is obtained through second crystallization, the purity of the finally obtained ranitidine hydrochloride is 99.9% or above, the NDMA content is 0, the color is light, and the purity is high. And the yield of ranitidine hydrochloride reaches 96% or above. The method provided by the invention is simple to operate, low in cost and suitable for industrial production.
Owner:石家庄海力药业有限公司
Ranitidine hydrochloride detection method and application thereof
PendingCN111272893AEfficient separationEasy to separateComponent separationPhysical chemistryElution
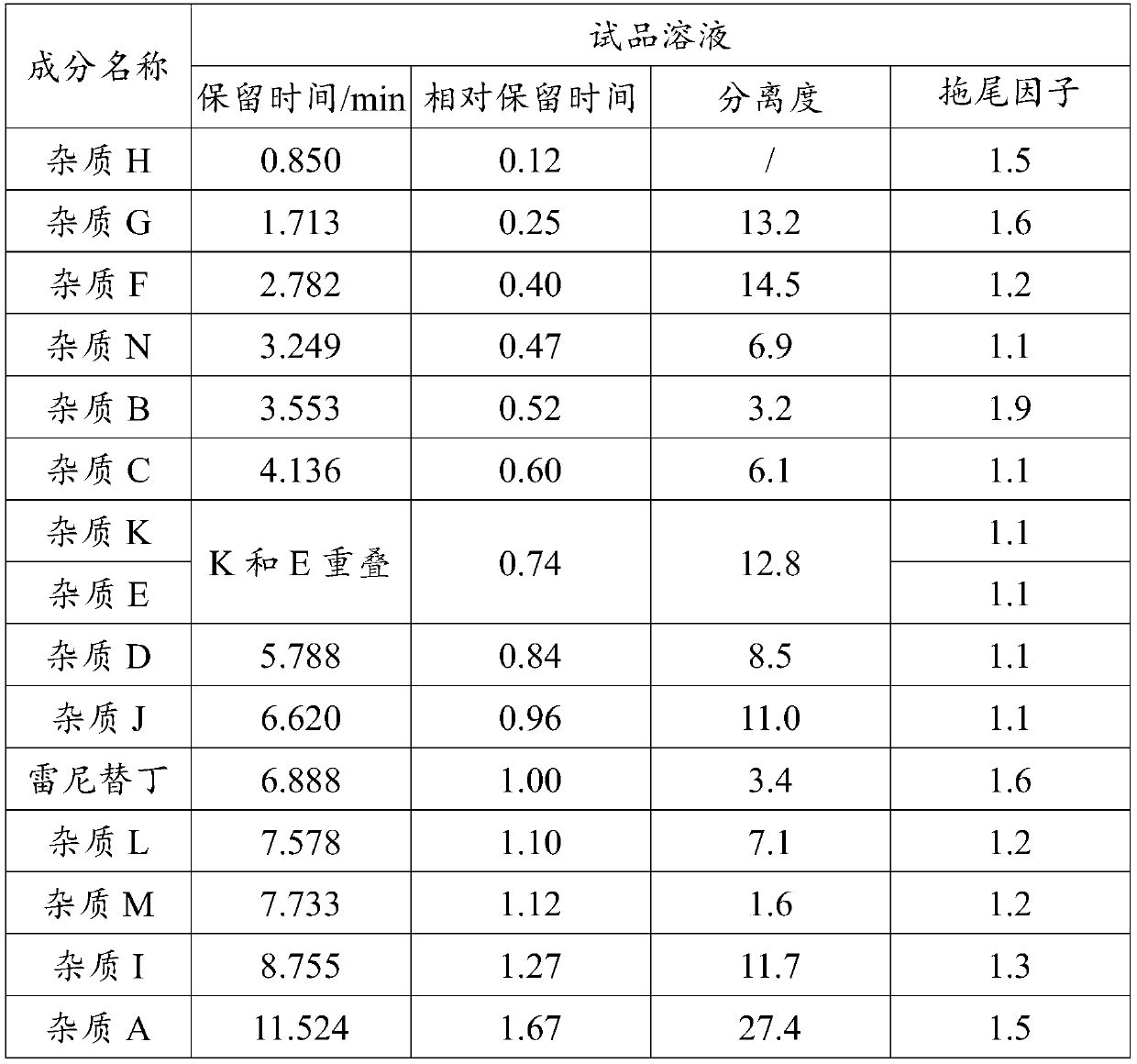
The invention discloses a ranitidine hydrochloride detection method which comprises the following steps: (1) mixing ranitidine hydrochloride with a first mobile phase to obtain a test solution; (2) injecting the test solution into a liquid chromatograph, loading to a C18 chromatographic column, and performing gradient elution by using a first mobile phase and a second mobile phase to separate ranitidine hydrochloride in the test solution from impurities; and (3) testing the separated ranitidine hydrochloride and impurities through a detector to obtain a spectrogram. The effective separation ofranitidine hydrochloride and impurities is realized by changing the variety of mobile phases and the elution gradient of the mobile phases, so that the separation degree between spectral peaks is high, and a tailing factor is realized.
Owner:FOSHAN SOIN PHARMA CO LTD
The New Indication of Oral Ranitidine in the Treatment of Erosive Esophagitis
ActiveCN108727312BHigh puritySimple stepsOrganic active ingredientsOrganic chemistryErosive esophagitisBiochemistry
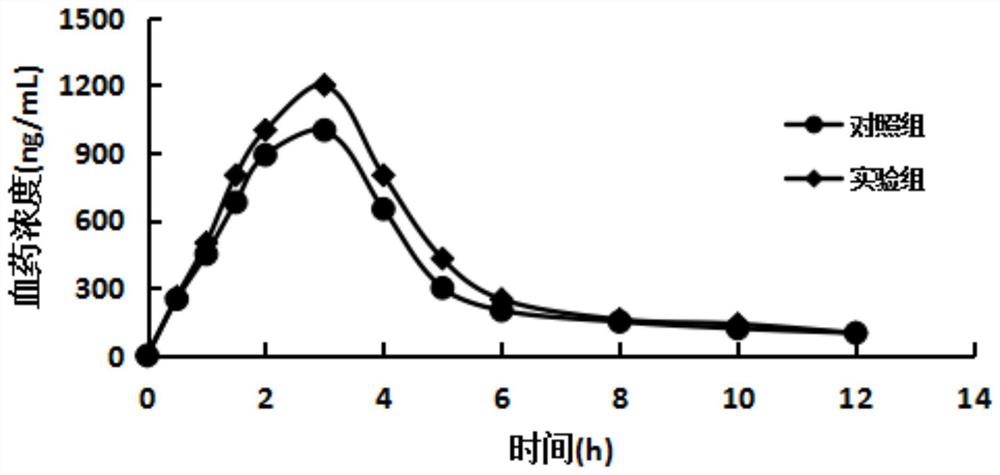
The invention discloses a ranitidine and a preparation method thereof, as well as a ranitidine preparation, a compound preparation and a preparation method thereof. Among them, ranitidine has low impurity content, high stability and simple preparation method; the ranitidine preparation and compound preparation prepared therefrom have high bioavailability and safety. The ranitidine, the ranitidine preparation and the compound preparation provided by the invention can effectively treat and maintain the treatment of erosive esophagitis.
Owner:SHANGHAI JINCHENG PHARMACEUTICAL CO LTD
New Synthesis Method of High Stability Bismuth Citrate Ranitidine
ActiveCN107501216BQuality improvementImprove stabilityCarboxylic acid salt preparationCombinatorial chemistryRanitidine Bismuth Citrate
The invention discloses a novel synthesis method for high-stability ranitidine bismuth citrate. The method includes the steps of: 1) preparing a raw material solution; 2) performing a reaction to prepare ranitidine; 3) purifying the ranitidine; 4) performing a salt forming reaction; 5) discolorizing and sterilizing a product; and 6) producing a ranitidine bismuth citrate finish product. The novel synthesis method, through reasonable process design, can increase the quality and stability of ranitidine, thereby improving the pharmacologic property and stability of the ranitidine bismuth citrate. The novel synthesis method is low in raw material cost, has gentle process conditions, is good in controllability and yield, and is suitable for industrial production.
Owner:JIANGSU HI STONE PHARMA
A method for the determination of related substances of ranitidine hydrochloride by high performance liquid chromatography
The invention relates to the field of drug detection, in particular to a method for determining related substances of ranitidine hydrochloride by high performance liquid chromatography. The method of the present invention adopts octadecylsilane bonded silica gel as a packing agent; Mobile phase comprises mobile phase A and mobile phase B, and described mobile phase A and mobile phase B are modified phosphate buffer saline and acetonitrile For the mixed solution, the pH value of the modified phosphate buffer solution is 6.70±0.05; the flow rate of the mobile phase is 1.1-1.3ml / min; gradient elution is adopted. The method of the invention can separate the related substances of ranitidine hydrochloride in the high-performance liquid chromatogram; and through optimizing the conditions, the sensitivity and accuracy of detecting each component are further improved. The method can better control the quality of ranitidine hydrochloride, and at the same time, the analysis speed is fast, the specificity is good, and the reproducibility is high. Security promotion and application.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
A kind of method of refining ranitidine base
The invention relates to the technical field of separation and purification of fine chemicals, more specifically, to a method for refining ranitidine base. In the present invention, by using a mixed solvent composed of a strong polar solvent and a weak polar solvent, there is a large difference in the solubility of ranitidine base and the impurities therein in this solvent, thereby making ranitidine base in the crystallization process Butyrine and impurities can be effectively separated, and the refined product obtained has high purity and stable properties. The ranitidine base refined by the method of the present invention is analyzed by a liquid chromatograph, and the purity of the ranitidine base is more than 99.9%, the total impurities are not more than 0.15%, and the individual impurities are not more than 0.05%. The ranitidine hydrochloride product obtained by preparing the purified ranitidine base and then salting it with the method provided by the invention has a purity reaching or better than the quality level of the original drug, thereby further improving the safety of the drug during use .
Owner:SHANXI YUNPENG PHARMA
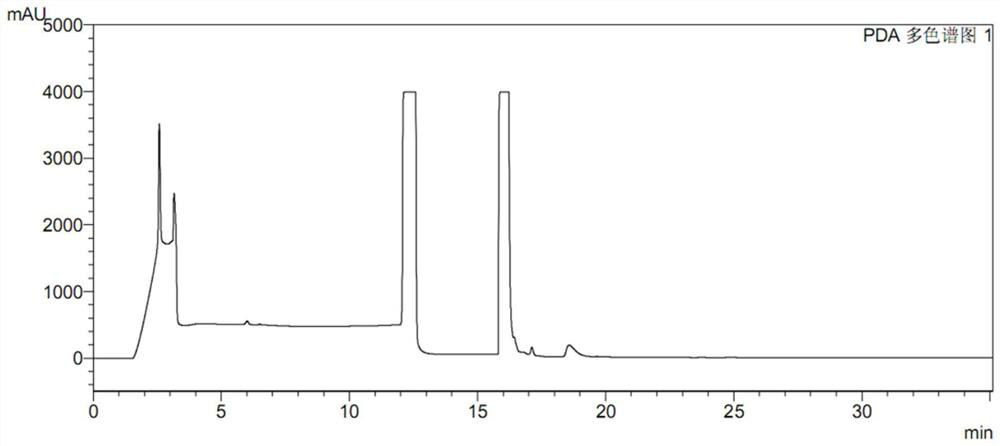
Pretreatment method of ranitidine hydrochloride sample
PendingCN114839288AEasy and quick to makeEasy to operateComponent separationSamplingNitrosoHigh concentration
The invention discloses a ranitidine hydrochloride sample pretreatment method, which comprises: S1, weighing a ranitidine hydrochloride sample, the ranitidine hydrochloride sample containing an impurity N-nitrosodimethylamine; s2, preparing a ranitidine hydrochloride test solution, and enabling the concentration of N-nitrosodimethylamine in the ranitidine hydrochloride test solution to reach the detection limit of the high performance liquid chromatography; s3, adding an alkaline solution into the ranitidine hydrochloride test solution, and enabling the alkaline solution to completely react with hydrochloric acid in ranitidine hydrochloride to obtain a ranitidine neutralization solution; s4, a silver ion solution is added into the ranitidine neutralization solution, silver ions and secondary amino groups in ranitidine are subjected to a complex reaction completely, and precipitates are generated. According to the method, the ranitidine precipitate in the high-concentration ranitidine test sample is removed, a by-product NDMA is not generated, meanwhile, the NDMA in the sample is reserved, the NDMA detection sensitivity is greatly improved, and the mass spectrum detection requirements of various models are met.
Owner:湖南省药品检验检测研究院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com