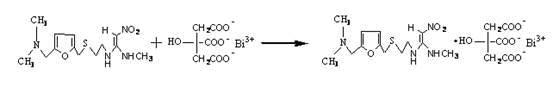
Method for preparing ranitidine carboxylic acid bismuth
A technology of bismuth ranitidine carboxylate and ranitidine carboxylate, which is applied in the field of preparation of bismuth ranitidine carboxylate, can solve problems such as difficult post-processing, long reaction time, and affecting product purity, and achieve product The quality is easy to control, the price is low, and the effect of saving resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 38g of ranitidine, 35g of bismuth citrate, and 80ml of sodium hydroxide into water, control the pH of the reaction system to 6-12, and the reaction temperature to 0-50°C, stir until the solution is basically clear, filter, and add the filtrate dropwise to anhydrous Ethanol, stirring, the filtrate adopts azeotropic dehydration technology or spray drying technology or freeze drying technology to separate out the product.
Embodiment 2
[0025] All the other are identical with embodiment 1 or 2, and difference is to replace the bismuth citrate in embodiment 1 with bismuth tartrate 35g.
Embodiment 3
[0027] All the other are identical with embodiment 1 or 2, and difference is to obtain crude product with spray-drying method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com