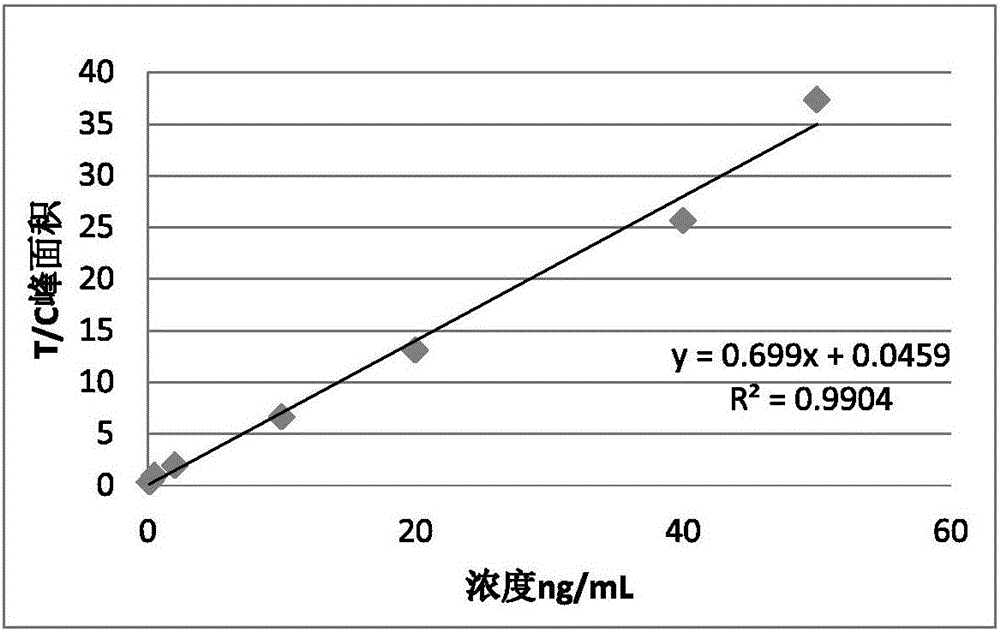
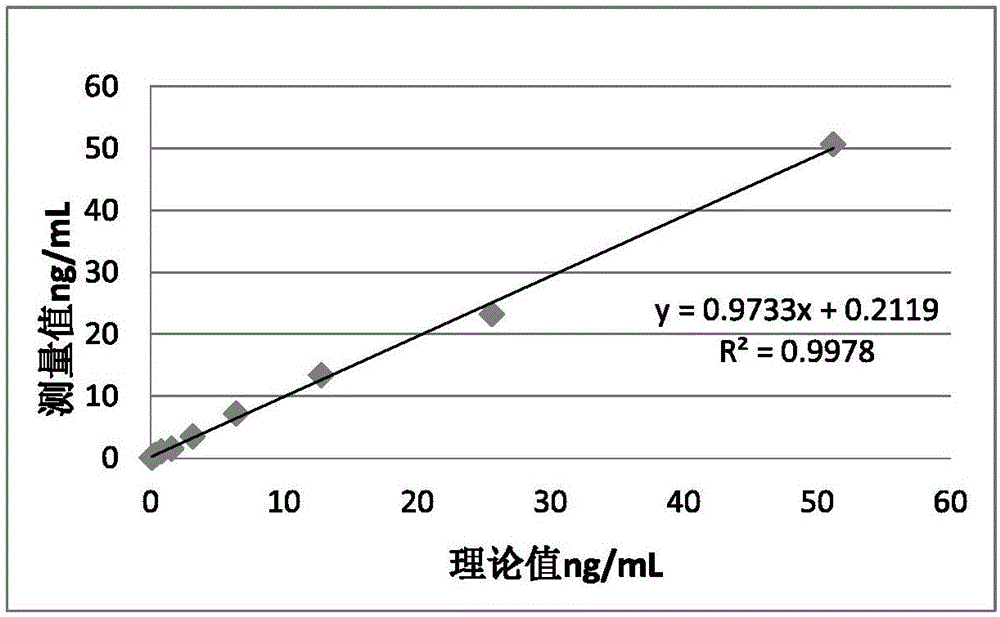
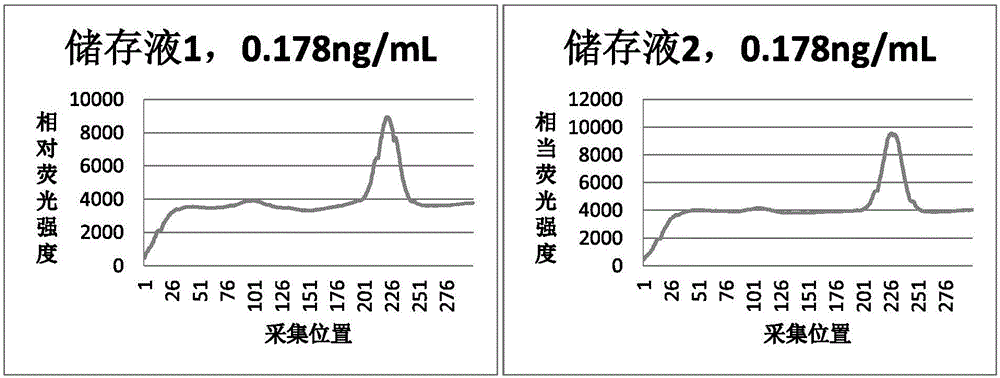
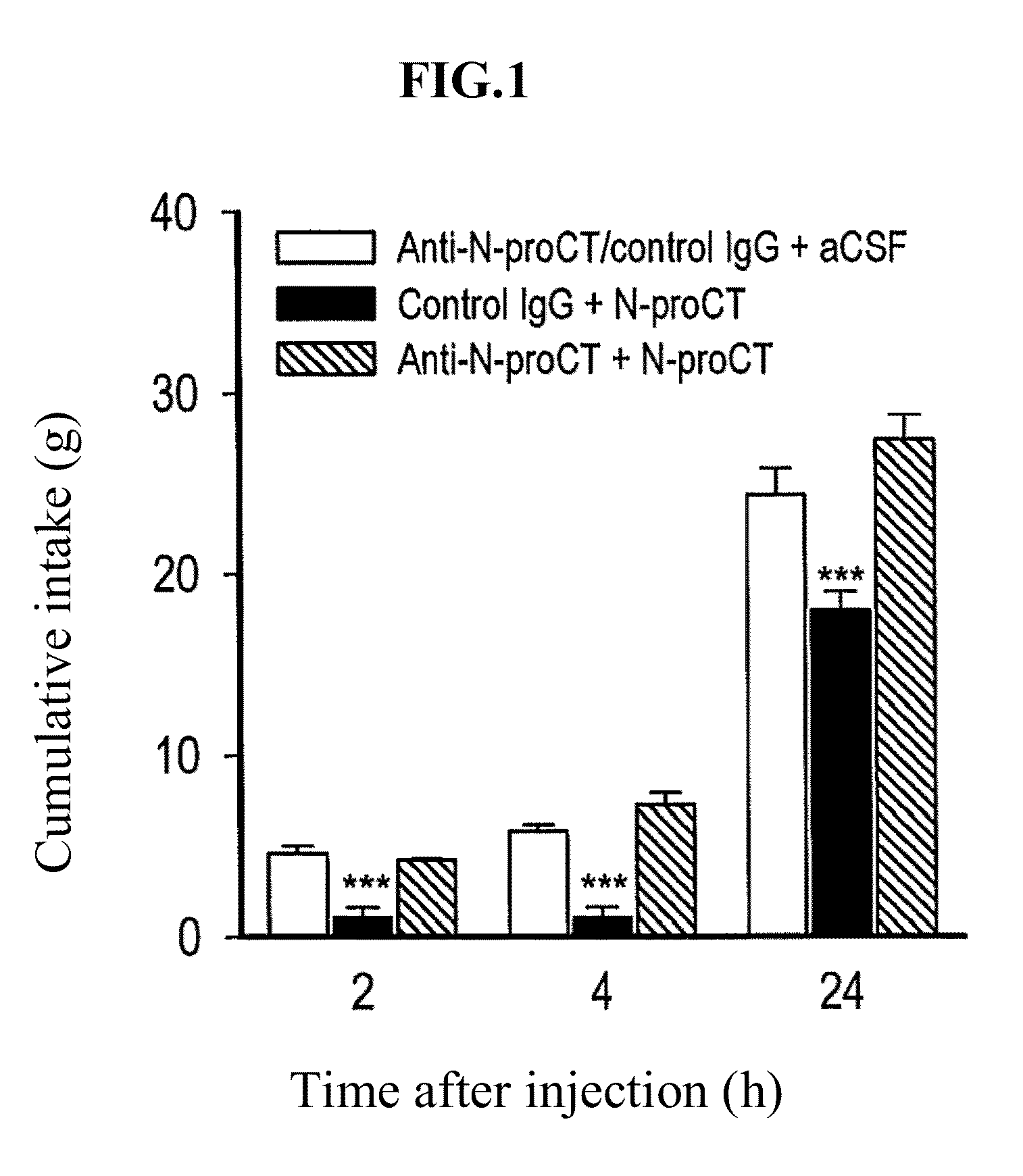
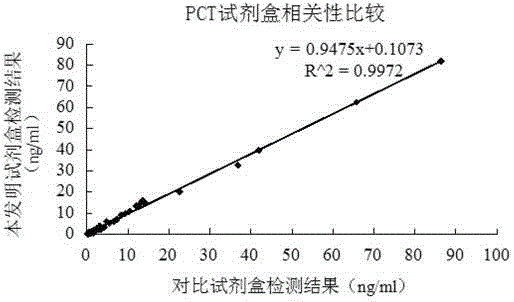
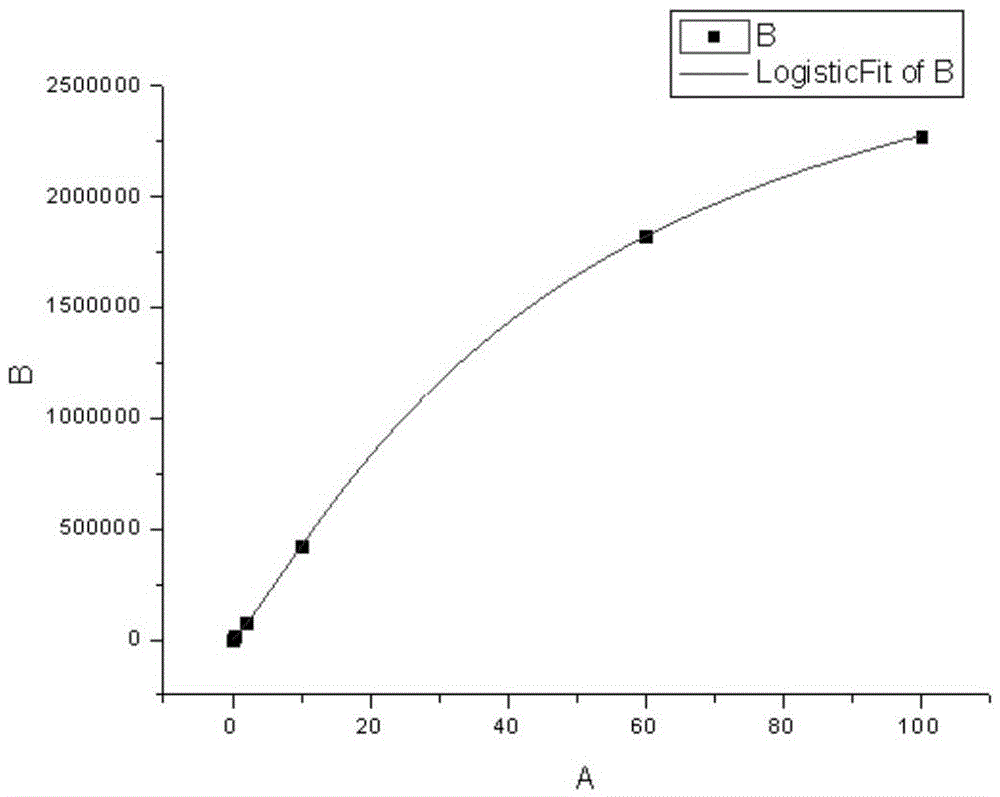
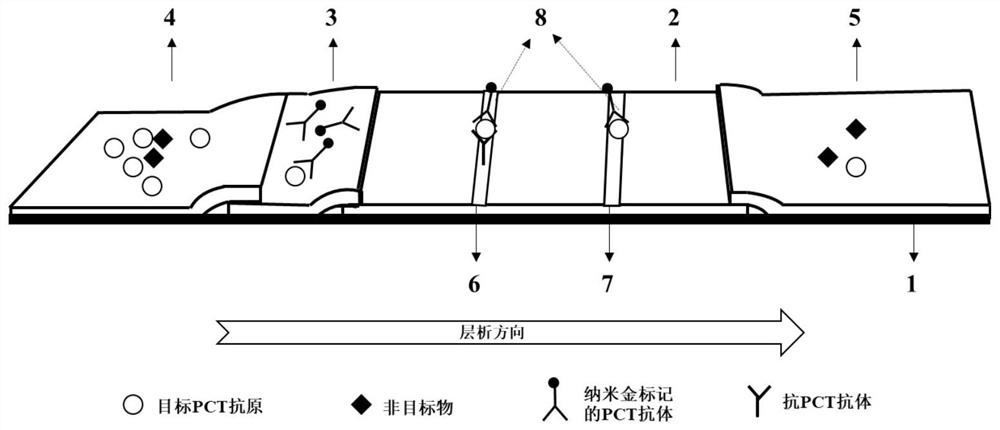
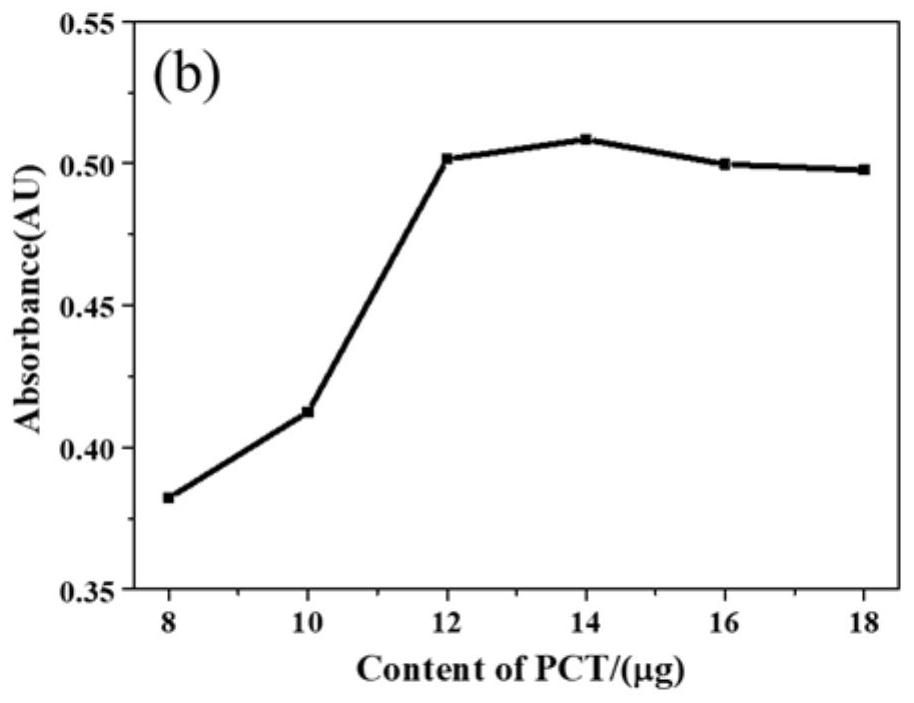
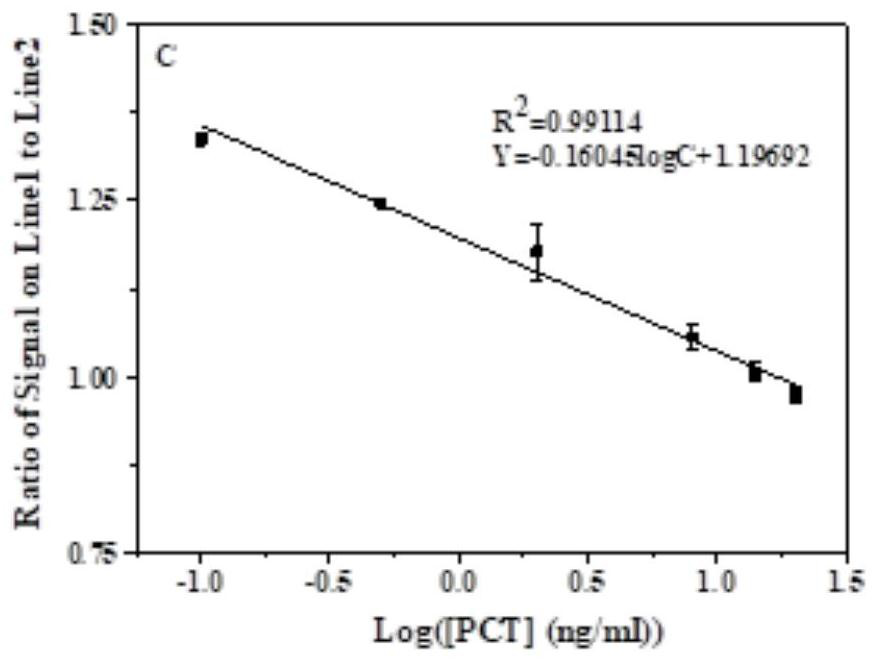
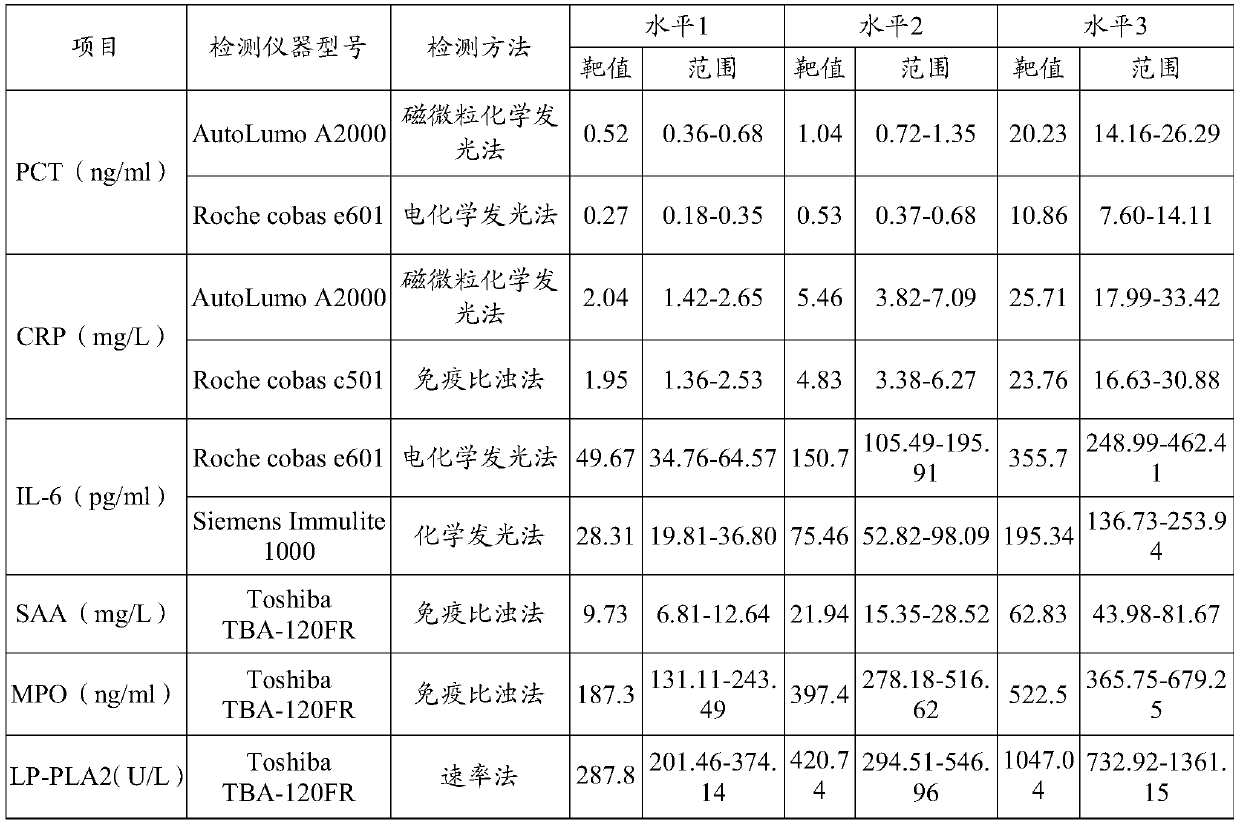
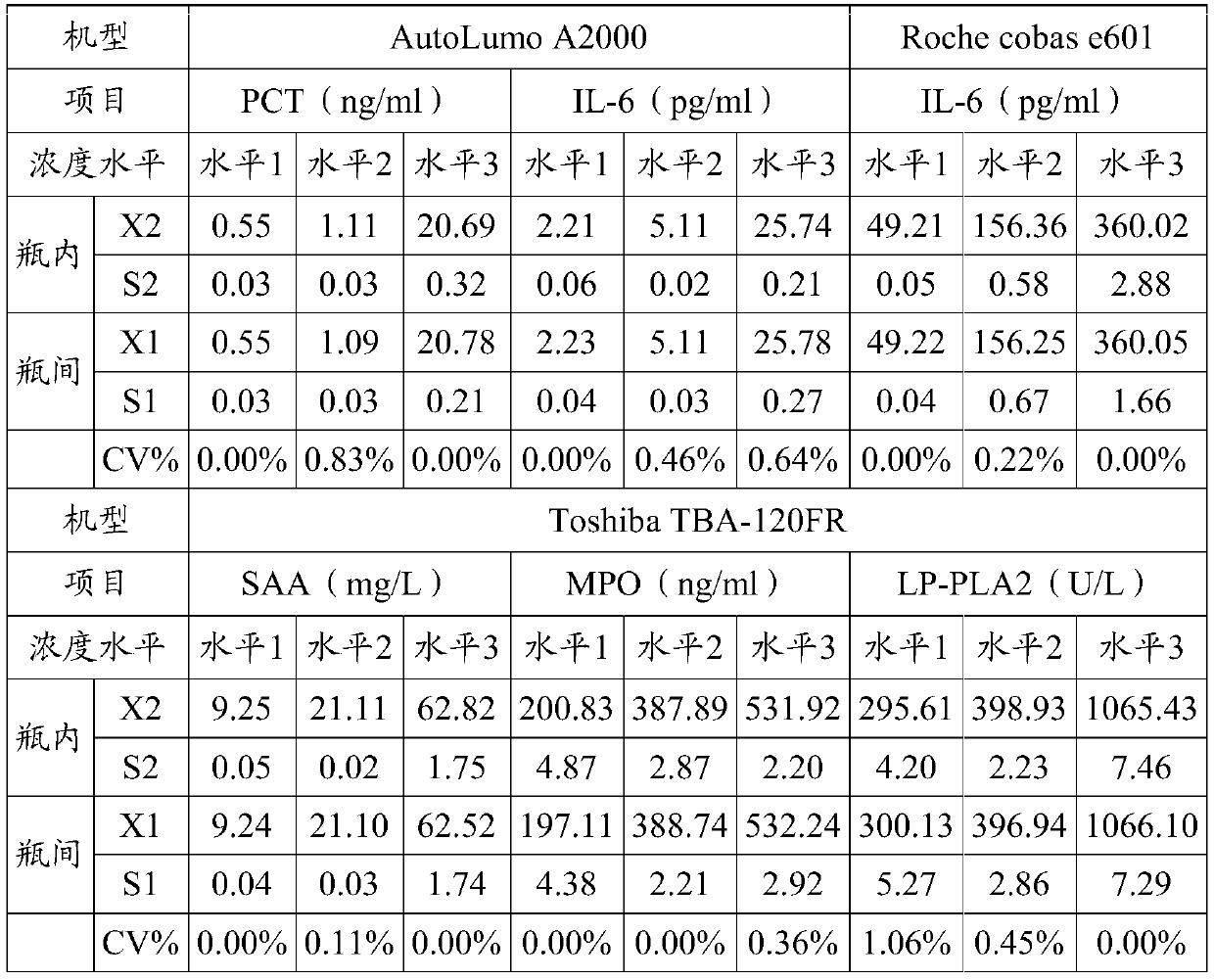
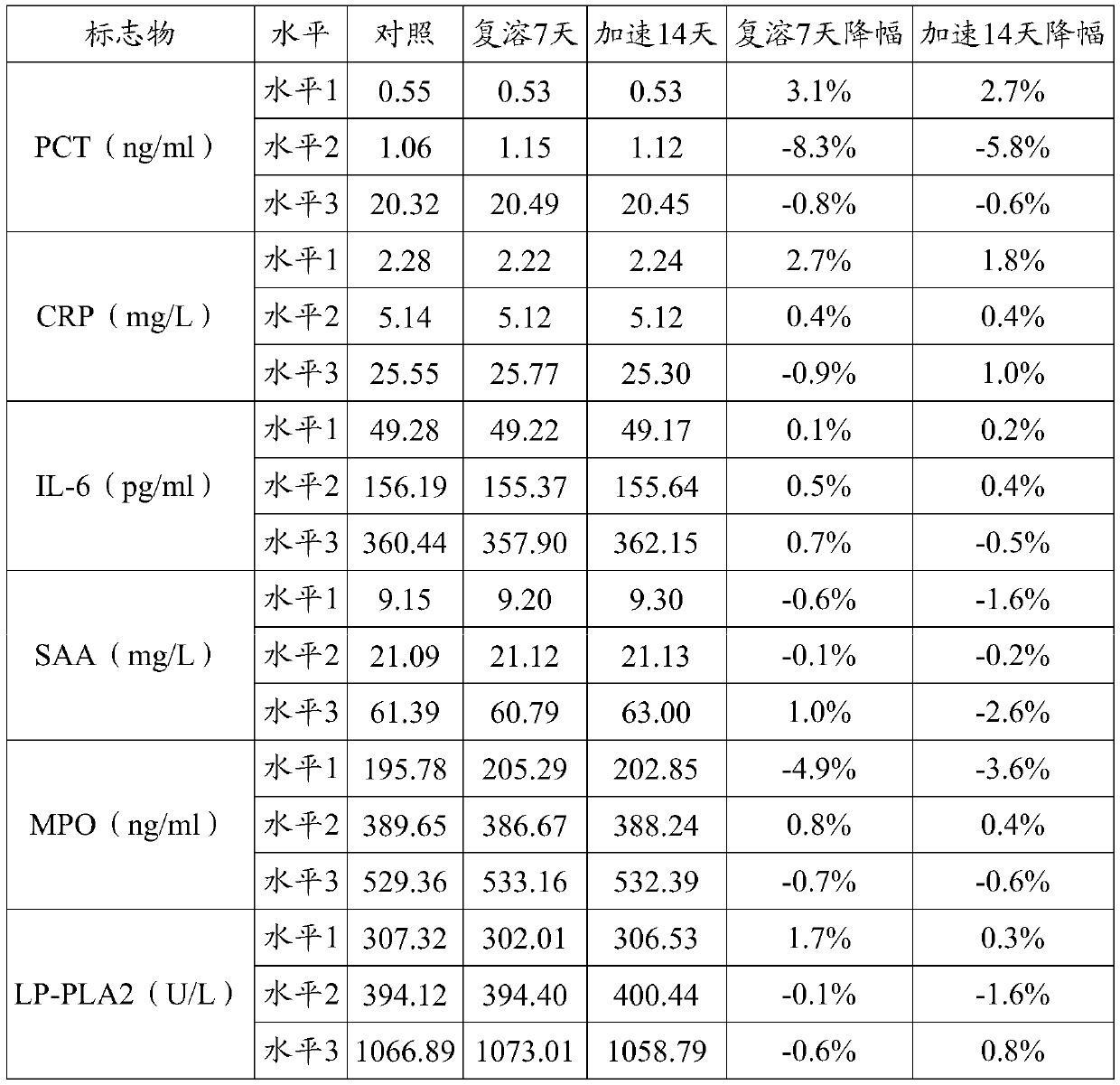
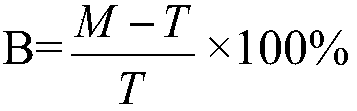Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
85 results about "Pro calcitonin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Procalcitonin (PCT) is a peptide precursor of the hormone calcitonin, the latter being involved with calcium homeostasis. It arises once preprocalcitonin is cleaved by endopeptidase. It was first identified by Leonard J. Deftos and Bernard A. Roos in the 1970s.
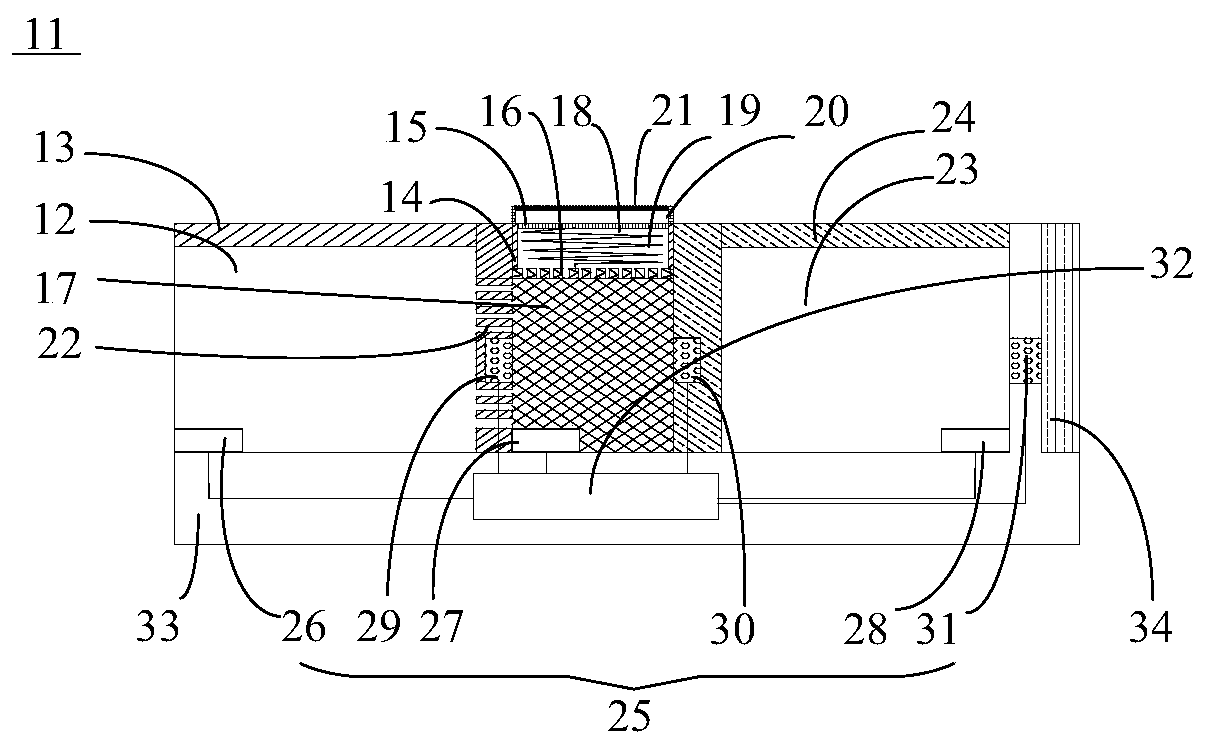
Chemiluminescence quantitative detection kit for procalcitonin, and preparation method and detection method thereof
ActiveCN103901203ALow cross-reactivityImprove accuracyChemiluminescene/bioluminescenceBiotin-streptavidin complexN-Hydroxysuccinimide
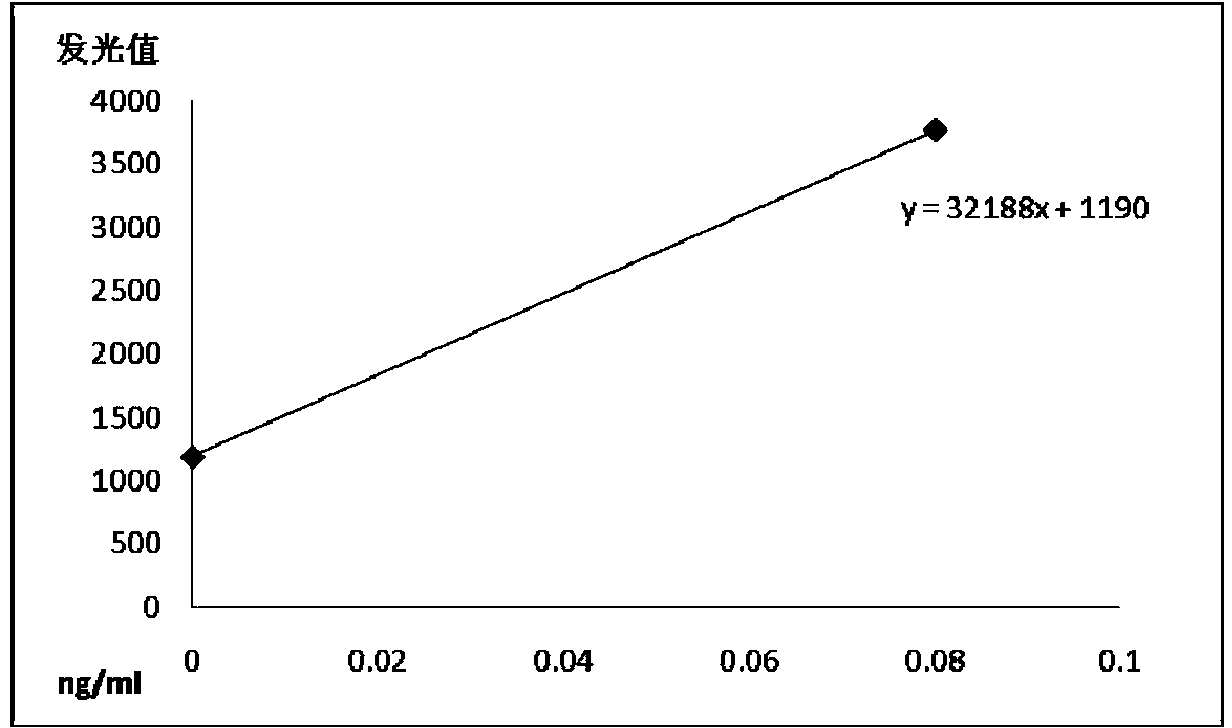
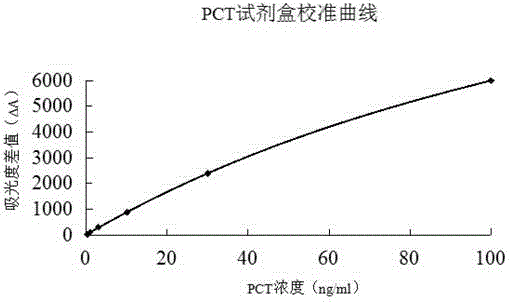
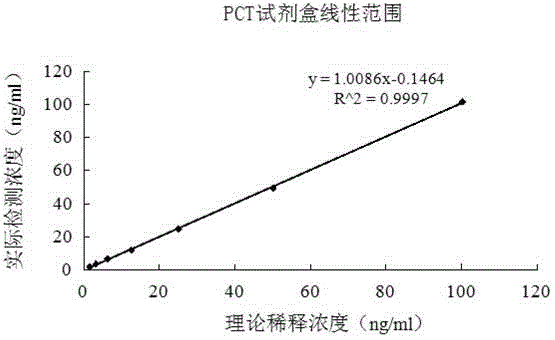
The invention relates to a chemiluminescence quantitative detection kit for procalcitonin, and a preparation method and detection method thereof. The kit comprises a procalcitonin series standard substance, a magnetic separation reagent (magnetic particle suspension coupled with streptavidin), a first reagent (anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label) and a second reagent (anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label). The sensitivity of the kit prepared from the magnetic particle suspension coupled with streptavidin, anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label and anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label is up to 0.008ng / ml; and the kit has the advantages of high accuracy, high precision, no need of prediluting the sample, and wide detection range, and is simple and time-saving to operate.
Owner:SUZHOU HAOOUBO BIOPHARML
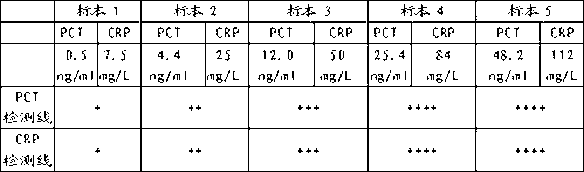
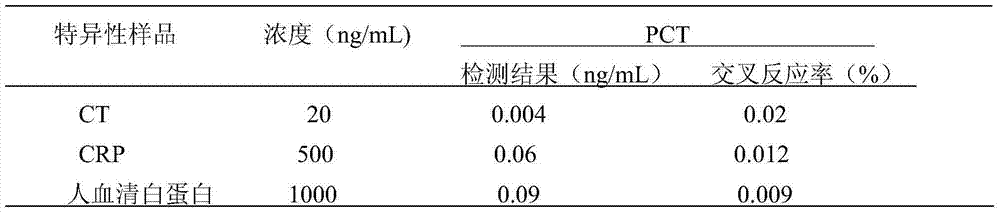
Colloidal gold test strip for combined detection of procalcitonin (PCT)/C-reactive protein (CRP) and preparation method thereof
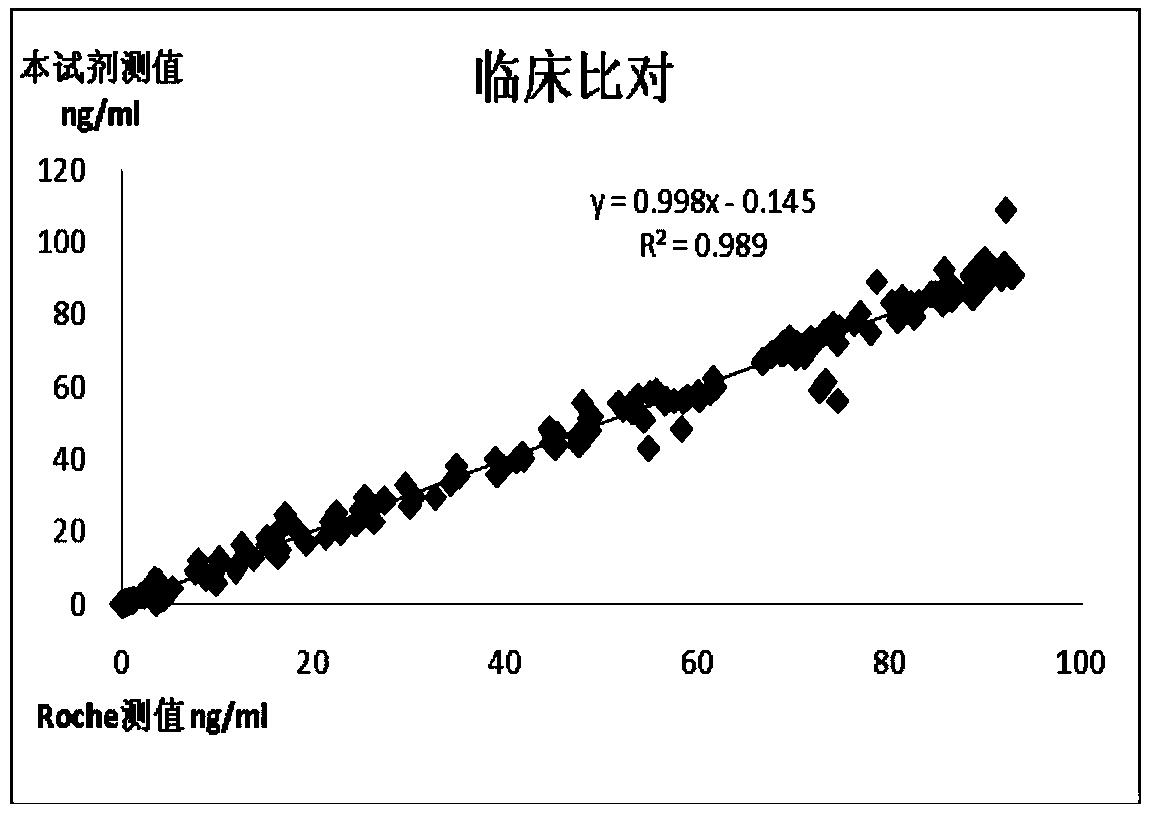
The invention belongs to the field of clinical medical examination, particularly relates to a colloidal gold test strip for the combined detection of procalcitonin (PCT) / C-reactive protein (CRP). The colloidal gold test strip comprises a test strip bottom lining, and a sample pad, a polyester film on which gold-labeled antibodies are coated, a coating film and absorbent paper which are sequentially overlapped with and adhered to each other on the test strip bottom lining, wherein the coating film is provided with a control line which coats a rabbit antimouse immunoglobulin G (IgG) antibody, and two detection lines which are parallel to the control line and coat an antibody which can be in specific binding with a to-be-detected antigen PCT and an antibody which can be in the specific binding with a to-be-detected antigen CRP respectively; and two kinds of gold-labeled antibodies are provided, namely the antibody which is labeled by colloidal gold and can be in the specific binding with the to-be-detected antigen PCT and the antibody which is labeled by the colloidal gold and can be in the specific binding with the to-be-detected antigen CRP. By the colloidal gold test strip, the PCT / CRP can be detected simultaneously, the accuracy of diagnosing an inflammatory reaction is improved, and the gold test strip is easy to operate.
Owner:GETEIN BIOTECH
Procalcitonin light-initiated chemiluminescence immunoassay kit and preparation method thereof
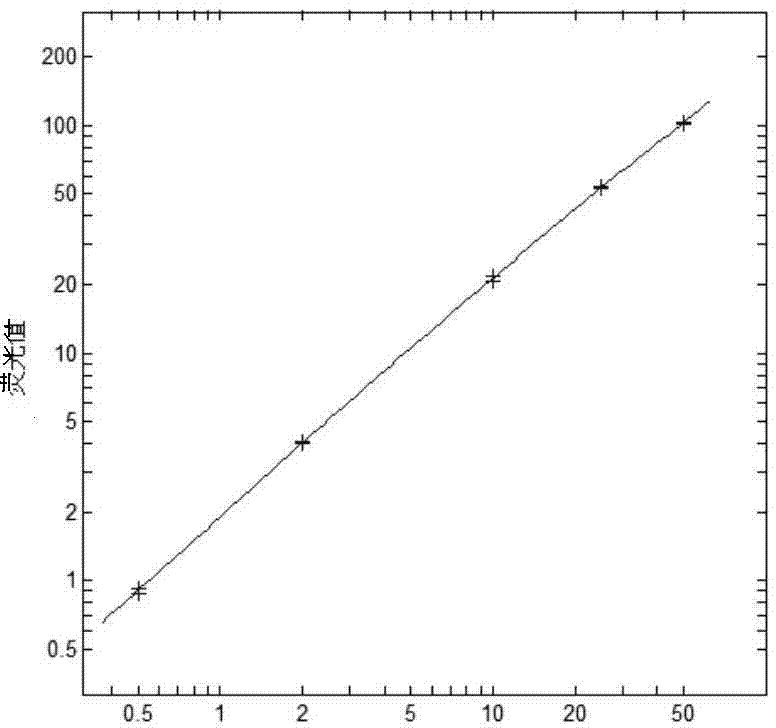
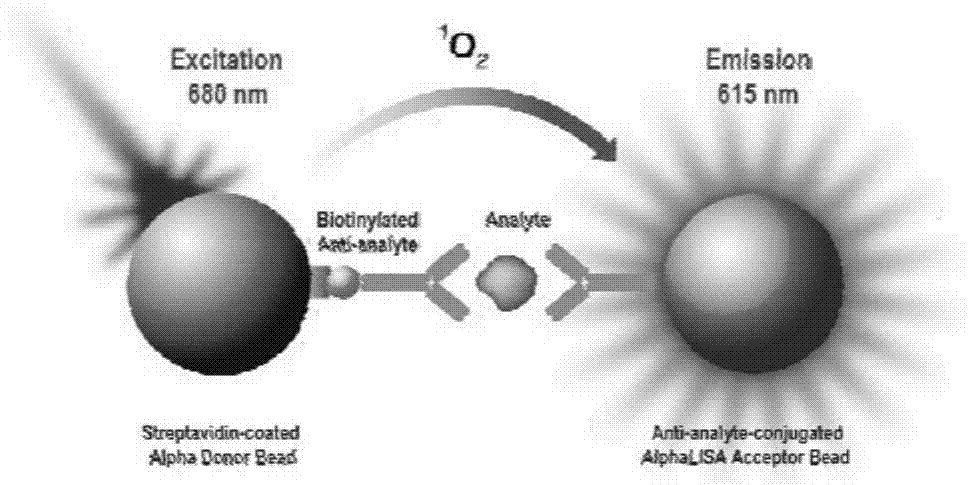
The invention discloses a procalcitonin light-initiated chemiluminescence immunoassay kit and a preparation method thereof. The procalcitonin light-initiated chemiluminescence immunoassay kit disclosed by the invention is composed of a white opaque 96-pore plate, a procalcitonin calibrating product, a receptor microsphere coated with an anti-procalcitonin monoclonal antibody, a biotinylated anti-procalcitonin monoclonal antibody, and a streptavidin biotinylated donor microsphere. The procalcitonin light-initiated chemiluminescence immunoassay kit disclosed by the invention has the advantages of rapidness, high sensitivity, wide measuring range, simplicity in operation, and the like, and has higher sensitivity and wider detectability in comparison with an enzyme immunoassay, can be used for diagnosing and identifying individual infectious diseases, and has an application value.
Owner:GUANGZHOU DARUI BIOTECH
PCT/SAA combined test paper strip for rapid detection and preparation method thereof
InactiveCN104569414AAccurate diagnosisHigh clinical reference valueMaterial analysisAntiendomysial antibodiesSerum amyloid protein
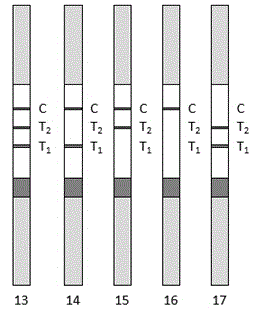
The invention relates to a test paper strip for (PCT / SAA) combined rapid detection of human procalcitonin / serum amyloid protein A. The test paper strip comprises a detecting card shell, a test strip, a sample pad, a gold conjugate pad coated with two colloidal gold labeled antibodies, a nitrocellulose membrane and water absorbing paper, wherein the nitrocellulose membrane is provided with a T1 detection line coated with a solid-phase matched anti-SAA monoclonal antibody, a T2 detection line coated with a solid-phase matched anti-PCT monoclonal antibody, and a control line C which is parallel to the detection line and coated with a goat anti mouse IgG monoclonal antibody. Two kinds of colloidal gold labeled antibodies are provided, which are respectively a colloidal gold labeled antibody which can be specifically combined with a to-be-detected antigen PCT and an antibody which can be specifically combined with a to-be-detected antigen SAA. The test paper strip disclosed by the invention can be used for simultaneously and rapidly detecting the PCT / SAA in a patient sample in a combined manner and has the advantages of improving the diagnosis accuracy of early inflammatory reaction of infectious diseases and being simple to operate, rapid and convenient.
Owner:MAANSHAN GUOSHENG BIO TECH
Kit for detecting procalcitonin in blood and method for detecting procalcitonin
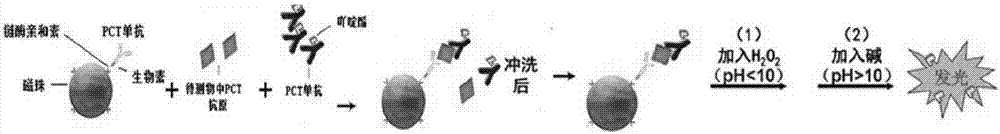
The invention discloses a kit for detecting procalcitonin in the blood and a method for detecting the procalcitonin. The method includes the steps of 1), coating magnetic beads with streptavidin; 2), marking a first monoclonal antibody of the procalcitonin with biotin; 3), combining the magnetic beads coated with the streptavidin with the first monoclonal antibody of the procalcitonin, marked with the biotin for reaction to obtain a magnetic bead-SA-biotin-PCT monoclonal antibody solvent; 4), marking acridinium ester with a second monoclonal antibody of the procalcitonin to obtain an acridinium ester-PCT monoclonal antibody solvent; 5), allowing the procalcitonin in a sample or a calibration product to react with the acridinium ester-PCT monoclonal antibody solvent and the magnetic bead-SA-biotin-PCT monoclonal antibody solvent; 6), after cleaning, calculating content of the procalcitonin in the sample. By the arrangement, the problem that coming out of results takes a long time is solved, the kit and the method can be applied to a peripheral whole blood sample, and detection range is widened without reducing flexibility.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Procalcitonin immunofluorescence quantitation test strip and preparation method thereof
ActiveCN106370840AGood storage stabilityGood precisionDisease diagnosisBiological testingImmunofluorescenceMedicine
The invention discloses a procalciton inimmunofluorescence quantitation test strip and a preparation method thereof. Compared with a test strip prepared by a common stock solution, the procalcitonin immunofluorescence quantitation test strip prepared by the invention is excellent in storage stability and better in precision, the peaking of a detected sample has a higher signal and a smoother base line, and the accuracy is higher.
Owner:ZHONGSHAN CHUANGYI BIOCHEM ENG
Antibodies against n-procalcitonin
Specific antibodies against N-procalcitonin, peptides, genetic constructions and methods for the obtainment of the peptides used in the obtainment of the antibodies. These antibodies can be used for preparing drugs, or diagnostic kits for diseases that develop with systemic inflammatory response or metabolic stress.
Owner:UNIV DE SEVILLA +1
Fabrication method of PbS/Co3O4 compound signal reduction-type photoelectric chemical immunosensor
ActiveCN110346438AIncrease roughnessPromote growthMaterial analysis by electric/magnetic meansOxygenLead sulfide
The invention relates to fabrication method and application of a lead sulfide-cobalt oxide compound (PbS / Co3O4)-based photoelectric chemical procalcitonin sensor. Black titanium dioxide nanoparticle (B-TiO2 NPs), iodine oxygen bismuth nanosheet (BiOI NSs) and gold nanoparticle (Au NPs) are combined on indium tin oxide (ITO) conductive glass and are used as a substrate material, the light absorption range can be expanded by a formed sensitization structure, the photoproduction electron hole separation efficiency is facilitated, and meanwhile, the sensor is used for loading an antibody. The fabricated PbS / Co3O4 compound competes for a light source and a hole sacrifice agent with the substrate material, the signal reduction-type photoelectric chemical immunosensor is built, sensitive detection of the procalcitonin is achieved, and the method is of important significance to early diagnosis and monitoring of bacteria inflammatory disease infection.
Owner:UNIV OF JINAN
High-stability recombinant procalcitonin and preparation method and application thereof
ActiveCN104610443AAvoid hydrolysisImprove stabilityAntibody mimetics/scaffoldsCalcitoninsEscherichia coliWild type
The invention provides a high-stability recombinant procalcitonin. The structure of protein is PCT amino polypeptide-joint arm 1-calcitonin-joint arm 2-katacalein, wherein an amino acid sequence is as shown in SEQ ID NO:1; two parts of degradable peptide chains, namely 58-59aa and 92-95aa in PCT are replaced with flexible peptide chain joint arms; an amino acid sequence of the antigen is reversely translated by virtue of optimal codons of escherichia coli so as to obtain a high-stability PCT antigen gene composed of the optimal codons of escherichia coli; and finally cloning expression is carried out to obtain a high-stability PCT antigen. Compared with a wild PCT, the antigen also keeps the antigenicity of the wild PCT except for higher stability. The high-stability PCT protein gene provided by the invention is composed of the optimal codons of escherichia coli, has relatively high expression quantity in escherichia coli, and is suitable for large-scale preparation. Meanwhile, a recombinant PCT can be applied to preparation of standard substances in immunodetection and drawing of standard curves, so that accurate quantification of the PCT content is achieved.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Preparation method and application of electroluminescent immune sensor based on dual-co-reaction reagent amplifying signal
InactiveCN106323952ALarge specific surface areaStrong film formingChemiluminescene/bioluminescenceMaterial analysis by electric/magnetic meansAntigenPotassium persulfate
The invention relates to the technical field of electrochemiluminescence immune sensors, and in particular relates to a preparation method and application of an immune sensor taking a cadmium sulfide and molybdenum disulfide nano compound (CdS / MoS2) as a luminescent material and a substrate material and taking potassium persulfate and hydrogen peroxide as a dual-co-reaction reagent for enhancing luminescent intensity. Semiconductor nano materials, namely CdS and MoS2, with similar band gaps are compounded, so that the electric conductivity and electron-hole separation efficiency can be increased; and K2S2O8 and H2O2, serving as co-reaction reagents, have synergistic effects, so that the luminescent intensity of the sensor can be increased, and the stability can be enhanced. Based on specific binding between antigen antibodies, the sensor is used for detecting procalcitonin (PCT); and according to the difference of PCT with different concentration to the obstruction degree of electron transfer, the sensor has different electrochemiluminescence intensity, and detection of PCT is realized.
Owner:UNIV OF JINAN
Detection kit for procalcitonin (PCT)
The invention relates to the technical field of procalcitonin (PCT) detection, in particular to a detection kit for procalcitonin (PCT) and a preparation and application method thereof. A reagent R1 contains a buffer solution, potassium chloride, ethyl carbamate, 2-hydroxypropyl-Beta-cyclodextrin, sorbitol, PEG-20000, N, N-diethyl-p-octadecylsulfonimidopropylammonium propanesulfonate, and a preservative. A reagent R2 comprise a buffer solution, ethyl carbamate, 2-hydroxypropyl-Beta-cyclodextrin, sorbitol, rabbit anti-human calcitonin (PCT) antibody-coated latex particles (65nm), rabbit anti-human calcitonin (PCT) antibody-coated latex particles (145nm), rabbit anti-human calcitonin (PCT) antibody-coated latex particles (280nm), N, N-diethyl-p-octadecylsulfopropylammonium propanesulfonate, and a preservative. According to the detection kit for procalcitonin (PCT), the stability and the linear range of the reagent are remarkably improved and the sensitivity and accuracy of the reagentare remarkably enhanced.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Detecting sepsis
InactiveUS20190154704A1Improve patient outcomesSignificant comprehensive benefitsDisease diagnosisBiological testingWhole bodyDesmosine
A method for predicting sepsis or diagnosing systemic inflammatory response syndrome (SIRS) and / or sepsis in a subject comprises determining levels of at least three markers selected from CCL23, A1AT, CRP, sICAM, PLA2, IL-6, procalcitonin, MMP8, TNFalpha, AcPGP, enzymatic MMP activity, TIMP1, sRAGE and desmosine in a sample taken from the subject. The combined levels of the at least three markers are used to predict or diagnose SIRS and / or sepsis. The methods may be performed on a subject with SIRS and which is used to identify an infection in the subject. A preferred panel of markers includes CCL23, A1AT, sICAM, sICAM / VCAM-1 and CRP. Corresponding products, methods of treatment and medical uses are provided.
Owner:MOLOGIC LTD
Preparation and application of electrochemical luminescence sensor for detecting procalcitonin based on energy transfer between g-C3N4 and CuO
ActiveCN110687177AGood biocompatibilityHigh sensitivityMaterial electrochemical variablesAntigenCarbon nanotube
The invention discloses preparation and an application of an electrochemical luminescence sensor for detecting procalcitonin based on energy transfer between g-C3N4 and CuO, and belongs to the technical field of construction of novel sensors. Based on the good specificity between antigen and antibody, the sensor utilizes Au and carbon nanotube modified g-C3N4 composite material g-C3N4-CNT@Au as asubstrate luminescent material and polydopamine coated CuO as a quenching agent, and the electrochemical luminescence sensor is constructed through layer-by-layer self-assembly. The electrochemical luminescence sensor constructed by the invention has a wider detection range, higher sensitivity and lower detection limit, and has important significance for detection of procalcitonin.
Owner:UNIV OF JINAN
Kit for detecting procalcitonin with spatial proximity chemiluminescence method and a detection method of kit
InactiveCN109142335AEliminate glow interferenceFew componentsChemiluminescene/bioluminescenceBiological material analysisAntibodyAdjuvant
The invention relates to a kit for detecting procalcitonin with a spatial proximity chemiluminescence method and a detection method of the kit. The kit comprises an enzyme marker, a luminescent marker, an adjuvant, a trigger and a calibrator, wherein the calibrator contains calibration products of PCT antigens with different concentrations and a 0.1 M phosphate diluent; the enzyme marker containscomponents of a peroxidase labeled PCT detection antibody and a 0.05 M phosphate buffer; the luminescent marker contains components of a 9,10-dihydracridine labeled PCT capture antibody and 0.05 M Tris buffer; the adjuvant contains components of a luminescent adjuvant and a citrate buffer; the trigger is a 0.05 M Tris buffer. The detection method as a true homogeneous chemiluminescence technologydoes not need a carrier, coating and washing processes are omitted, and the kit comprises few components, has high sensitivity and good repeatability and is easy to operate.
Owner:无锡壹闪生物科技有限公司
Preparation method and application of electrochemical catalysis assisted self-enhanced photoelectrochemical immunosensor for detecting procalcitonin
ActiveCN110297023AIncrease loading capacityImprove signal stabilityMaterial electrochemical variablesMicrosphereElectrochemistry
The invention relates to a preparation method and application of an electrochemical catalysis assisted self-enhanced photoelectrochemical immunosensor for detecting procalcitonin. According to the preparation method provided by the invention, a porous nano-array BiVO4 / CuS is used as a substrate material, and electrochemical catalysis assisted self-enhanced photocurrent is obtained under visible light irradiation and an anode bias voltage. The two components of the substrate material have good energy band matching, which is beneficial to the separation of electron hole pairs; the photoexcited holes can oxidize water to generate H2O2 under the anode bias voltage, the hole-excited H2O2 can be catalytically reduced by CuS, thereby further effectively suppressing the separation of the electronhole pairs, and improving the intensity of photocurrent. Polystyrene microspheres are used as secondary antibody markers to significantly improve the sensitivity of the sensor, the amounts of combinedsecondary antibody markers are different due to different amounts of procalcitonin to be detected, such that the photocurrent signal response degrees are different. The constructed sensor achieves sensitive detection of the procalcitonin at a detection limit of 17.8 fg / mL.
Owner:UNIV OF JINAN
A latex-enhanced immune turbidimetric kit for quantitative detection of procalcitonin pct
ActiveCN102759631BHigh detection sensitivityMeet the needs of clinical applicationsMaterial analysis by observing effect on chemical indicatorLatex particlePolystyrene
The invention relates to a latex-enhanced immune turbidimetric kit for quantitatively detecting procalcitonin PCT. The kit includes R1 reagent: containing a protective agent, a reaction enhancer, a preservative and a buffer; R2 reagent: containing a protective agent, a preservative reagent, buffer and sensitized polystyrene latex particles coated with anti-human PCT antibody; and calibrator: containing protective agent, preservative, buffer and PCT recombinant protein; The ethylene latex particles are linked by streptavidin-biotin; the particle size of the latex particles in the R2 reagent is 40-500nm. The kit can be used for quantitative detection of PCT content in human blood on a biochemical analyzer and a scattering turbidimetric analyzer. The invention provides a convenient and fast PCT detection kit with high sensitivity, strong specificity and accurate quantification, which has strong instrument compatibility and low detection cost, and makes up for the clinical demand for PCT turbidimetric products.
Owner:NANJING NORMAN BIOLOGICAL TECH
Kit for detecting procalcitonin in blood and preparation method
ActiveCN110441531AIncrease ionic strengthImprove signal-to-noise ratioBiological testingBiocompatibility TestingTriton x100
The invention discloses a kit for detecting procalcitonin in blood and a preparation method. The kit comprises a solid-phase carrier coated with a captured antibody, a biotin-labeled detection antibody, fluorescein labeled streptavidin, a washing buffer solution and the like. The surface of a quartz needle is coated with a polysaccharide compound with biocompatibility, wherein the polysaccharide compound can effectively enhance the specific immune signal, and the amplification of a detection signal is realized by forming a plurality of fluorescein-streptavidin molecular layers on the surface of the polysaccharide compound; and according to the kit, SDS with relatively high washing capability and Triton-X100 are added in the washing buffer solution, and the ionic strength of the washing solution is improved, so that the non-specific interaction between the molecules is effectively controlled, the low background and the high signal-to-noise ratio of the detection result are further realized, and the sensitivity and accuracy of detection are improved.
Owner:NINGBO AUCHEER BIOTECHNOLOGY CO LTD
A chemiluminescence quantitative detection kit for procalcitonin and its preparation method and detection method
ActiveCN103901203BLow cross-reactivityImprove accuracyChemiluminescene/bioluminescenceBiotin-streptavidin complexMonoclonal antibody
The invention relates to a chemiluminescence quantitative detection kit for procalcitonin, and a preparation method and detection method thereof. The kit comprises a procalcitonin series standard substance, a magnetic separation reagent (magnetic particle suspension coupled with streptavidin), a first reagent (anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label) and a second reagent (anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label). The sensitivity of the kit prepared from the magnetic particle suspension coupled with streptavidin, anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label and anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label is up to 0.008ng / ml; and the kit has the advantages of high accuracy, high precision, no need of prediluting the sample, and wide detection range, and is simple and time-saving to operate.
Owner:SUZHOU HAOOUBO BIOPHARML
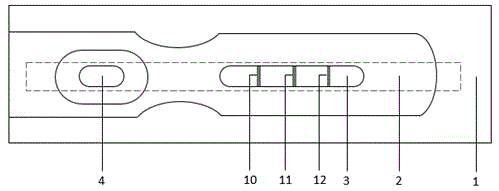
Immunochromatographic test strip for quantitatively detecting procalcitonin and quantitative detection method thereof
InactiveCN112305230ARealize quantitative detectionImprove linear rangeDisease diagnosisBiological testingAntigenNitrocellulose
The invention discloses an immunochromatographic test strip for quantitatively detecting procalcitonin and a quantitative detection method thereof. The test strip comprises a PVC lining plate, a nitrocellulose membrane (NC membrane), a combination pad, a sample pad and a water absorption pad; the PVC lining plate is sequentially overlapped and pasted with the sample pad, the combination pad, the NC membrane and the water absorption pad from left to right; the binding pad contains a nanogold labeled first antibody for resisting procalcitonin; the nitrocellulose membrane is provided with a detection area and a control area, fluorescent strips are respectively arranged in the detection area and the control area, a detection line is arranged on the fluorescent strip of the detection area, an anti-procalcitonin coated antibody is used as the detection line, a control line is arranged on the fluorescent strip of the control area, and a procalcitonin antigen is coated on the control line. Compared with a common method for detecting procalcitonin at present, the method has the advantages of simplicity and convenience in operation, quickness, convenience in carrying, small sample consumption and the like, and meanwhile, the linear range and the stability of detection are also improved.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Quality control product of inflammation marker and preparation method
PendingCN110967494AEasy to prepareReduce manufacturing costDisease diagnosisBiological testingPhospholipasePeroxidase
The invention belongs to the field of clinical medicine detection, and discloses a quality control product of an inflammatory marker and a preparation method of the quality control product. The quality control product of the inflammation marker is composed of a matrix liquid and an inflammation marker component, wherein the matrix liquid is composed of a matrix, a 5-100 mmol / L buffer agent, a 0.01-5 g / L protein protective agent, a 5-100 g / L saccharide and a 0.05-5 g / L preservative; the inflammation marker comprises the following components: procalcitonin, C reactive protein, serum amyloid protein A, myeloperoxidase, interleukin-6 and lipoprotein related phospholipase. The quality control product of the inflammatory marker contains a plurality of inflammatory markers, so that the joint detection of different inflammatory items is realized. Meanwhile, commercial human serum or plasma is adopted as a matrix, the channel source is stable, large-batch production can be achieved, the clinical sample conformity degree is high, the universality is high, the matrix effect can be reduced to the maximum extent, and the uniformity, stability and other performance are good.
Owner:郑州标源生物科技有限公司
Detection reagent for procalcitonin colloidal gold by immunoturbidimetry
ActiveCN108362892ANo pollution in the processComponent scienceBiological material analysisBiological testingPolyethylene glycolBovine serum albumin
The invention relates to a detection reagent for procalcitonin colloidal gold by immunoturbidimetry, which can effectively solve the detection problem of procalcitonin. A technical scheme is characterized that the detection reagent comprises a reagent 1 and a reagent 2, the reagent 1 is prepared by the following steps: water is added in tromethamine and sodium azide, the materials are uniformly mixed, and hydrochloric acid is used for adjusting a pH value; and the reagent 2 is prepared by the following steps: water is added in tromethamine, bovine serum albumin, polyvinylpyrrolidone, polyethylene glycol 20000, tween-20, cane sugar, sodium azide, and casein, the materials are uniformly mixed, and hydrochloric acid is used for adjusting the pH value to obtain a buffer solution, the buffer solution is used for dissolving anti-human procalcitonin antibody-labeled colloidal gold particles, concentration is adjusted, and the reagent 2 is obtained. The detection reagent has the advantages ofscientific component, novel and unique characteristics, easy preparation, low cost, stable performance, long shelf-life, accurate testing, no environmental pollution, and obvious economic and social benefits, and is an innovation for the procalcitonin detection reagent.
Owner:HENAN ACAD OF SCI INST OF BIOLOGY LIABILITY
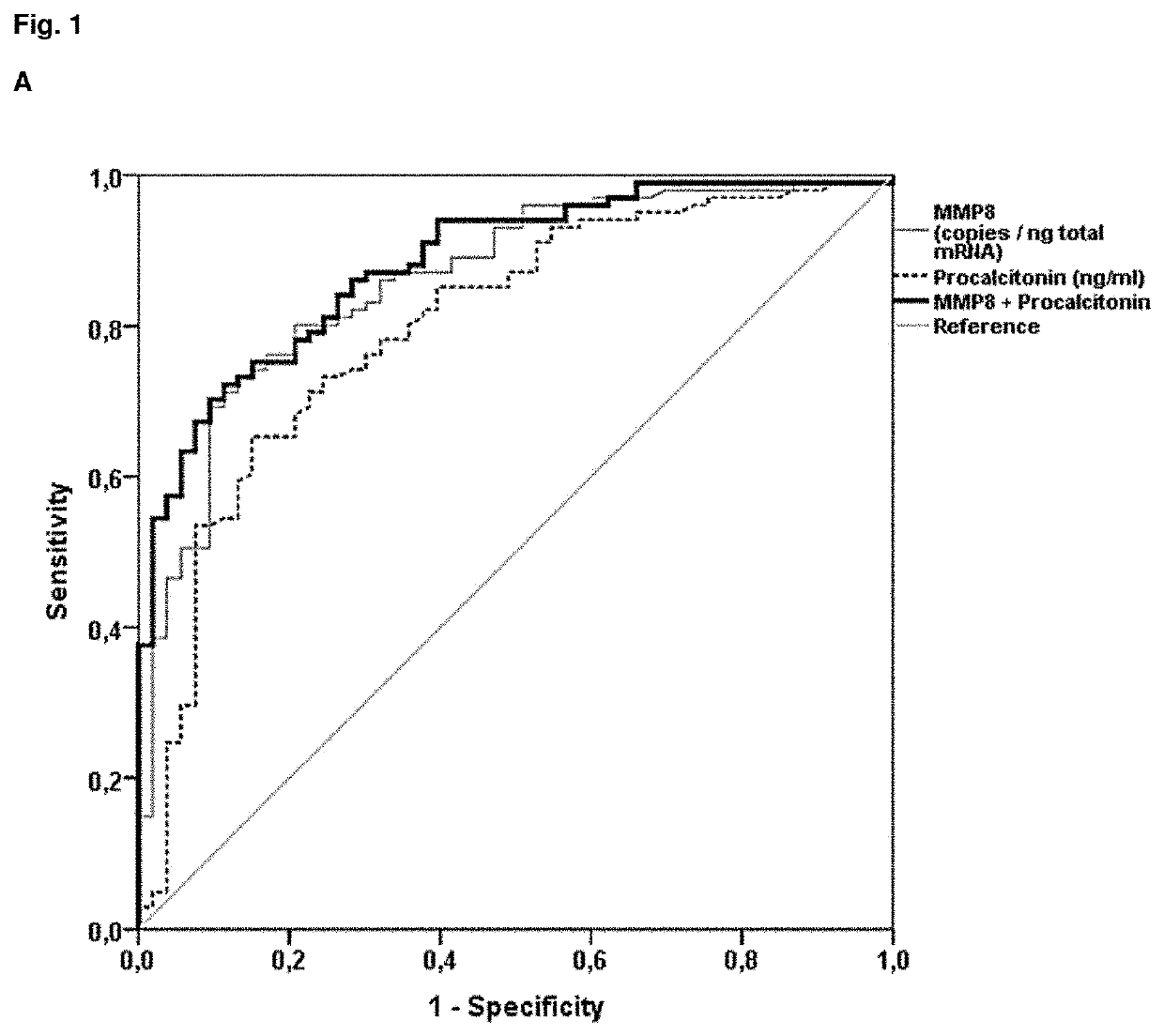
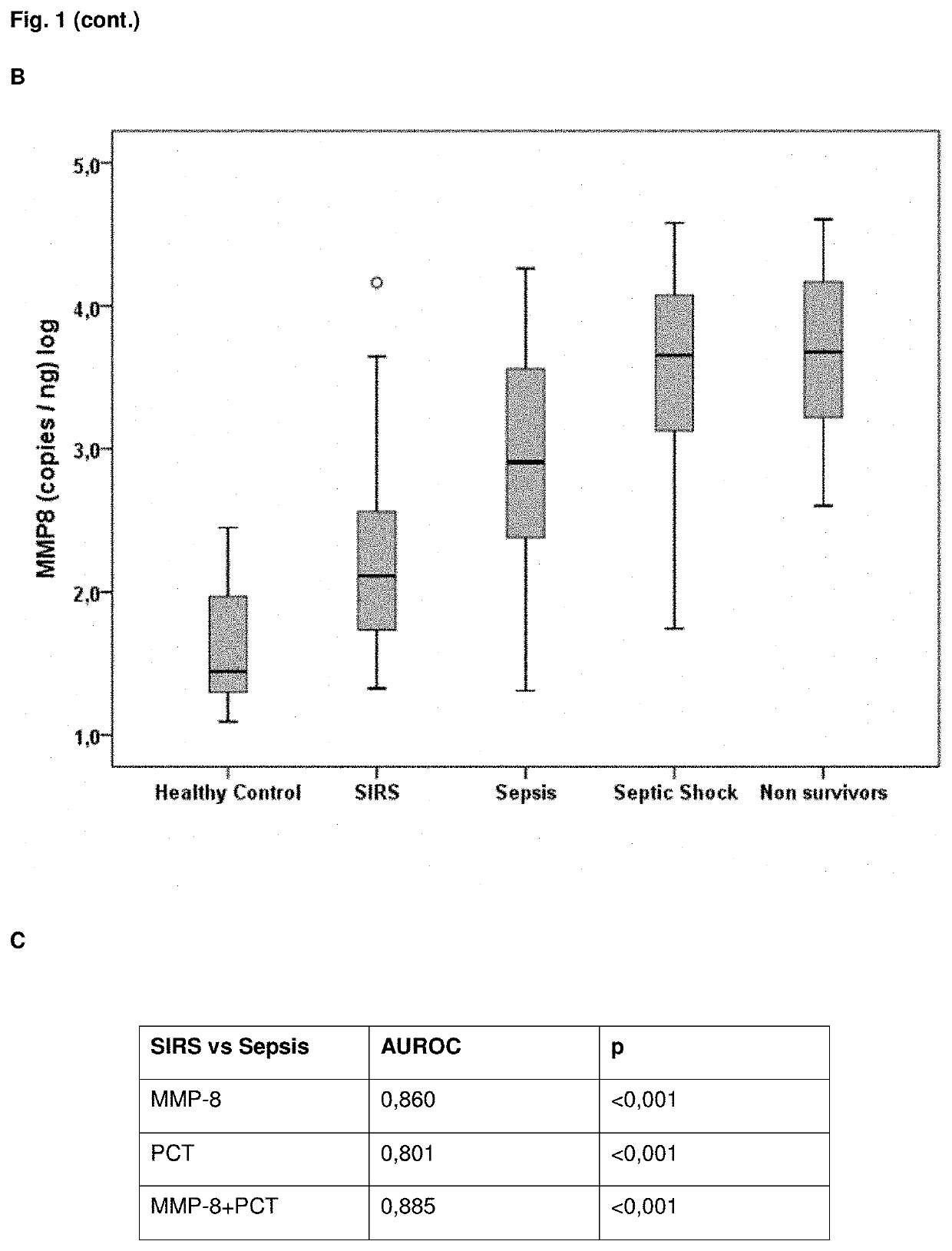
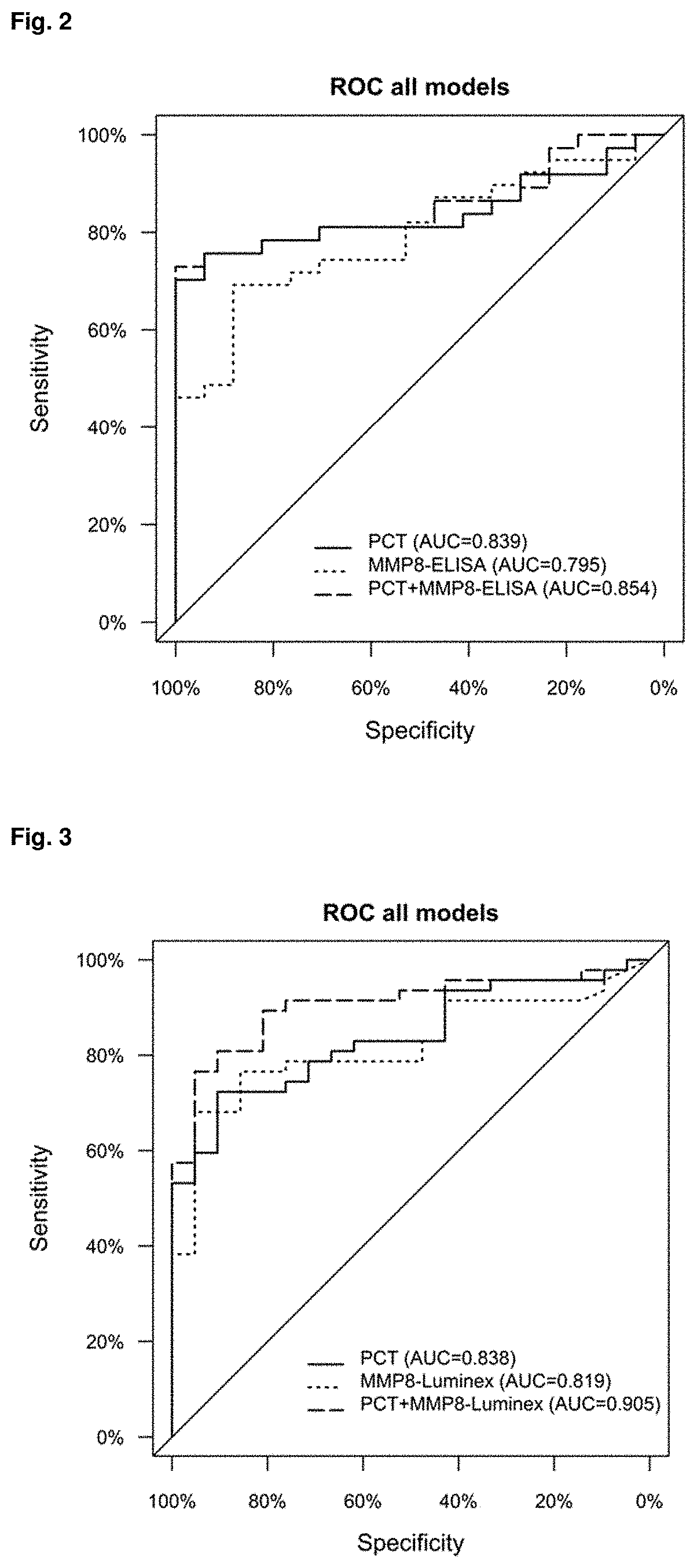
Mmp-8 as a marker for identifying infectious disease
PendingUS20200225248A1Reliable diagnosisHigh statistical certaintyMicrobiological testing/measurementDisease diagnosisCalcitoninMatrix metalloproteases
The invention relates to a method for the diagnosis, prognosis, risk assessment, risk stratification, monitoring, therapy guidance and / or therapy control of an infectious disease in a subject, wherein said method comprises providing a sample of said subject; determining a level of matrix metalloprotease-8 (MMP-8) or fragment(s) thereof in a sample of said subject, wherein said level of MMP-8 or fragment(s) thereof distinguishes between the presence and absence of an infectious disease in a patient with symptoms of a systemic inflammatory condition. In a preferred embodiment the invention relates to the determination of procalcitonin (PCT) and MMP-8 and their combined use to distinguish between the presence and absence of infectious disease in patients with symptoms of systemic inflammatory condition. The invention also relates to a computer-implemented method and a kit for conducting the method of the invention.
Owner:BRAHMS GMBH +1
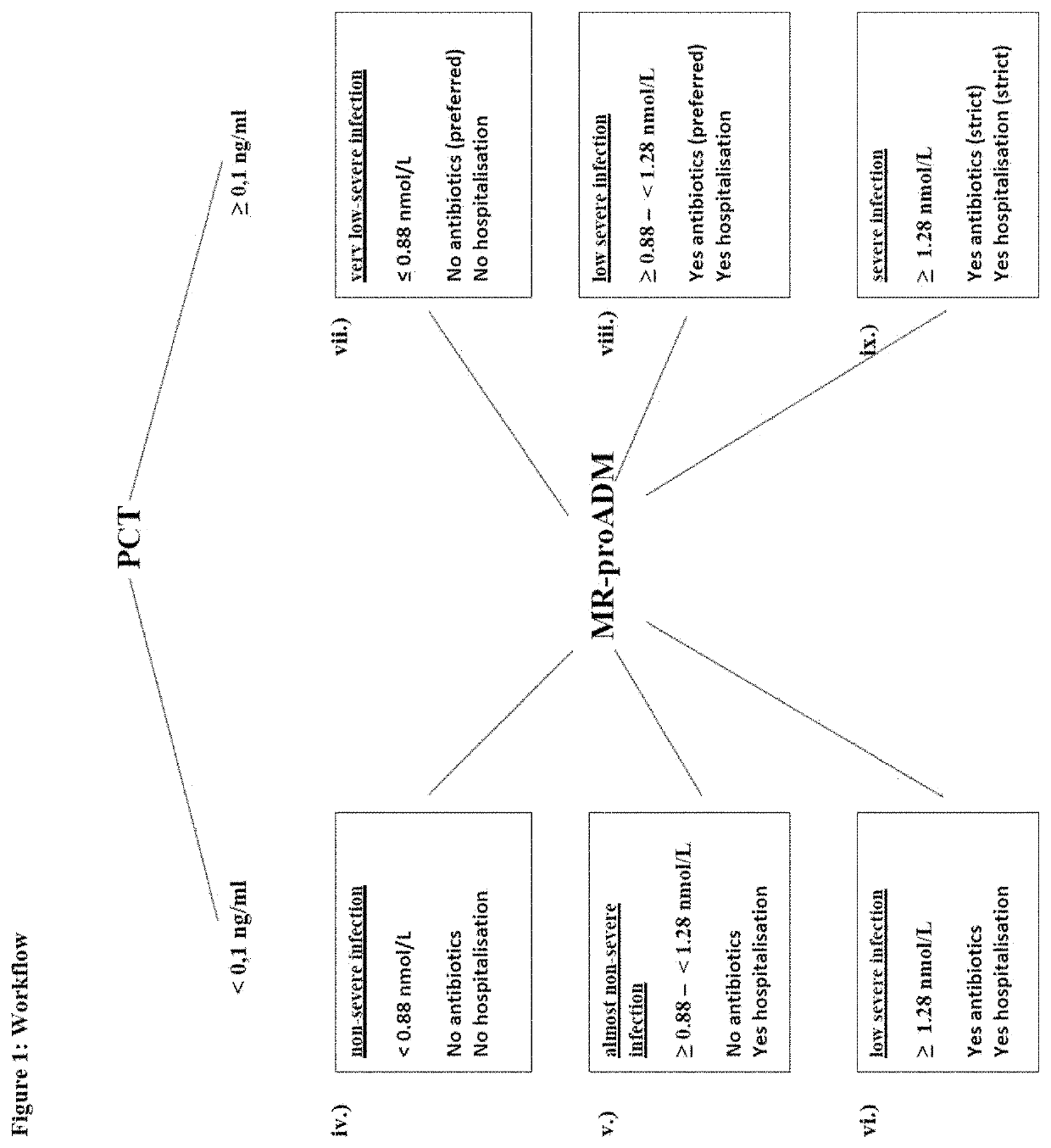
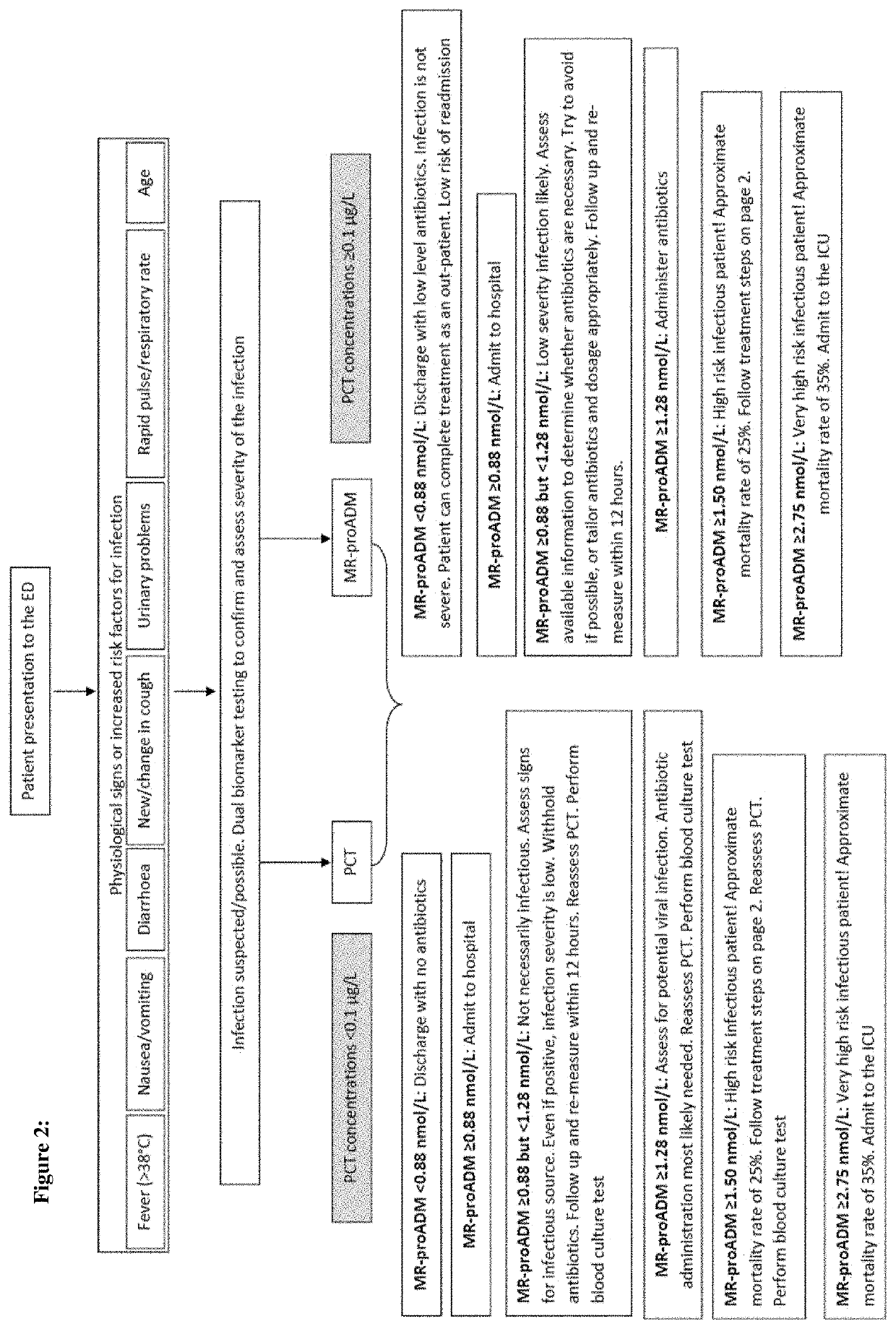
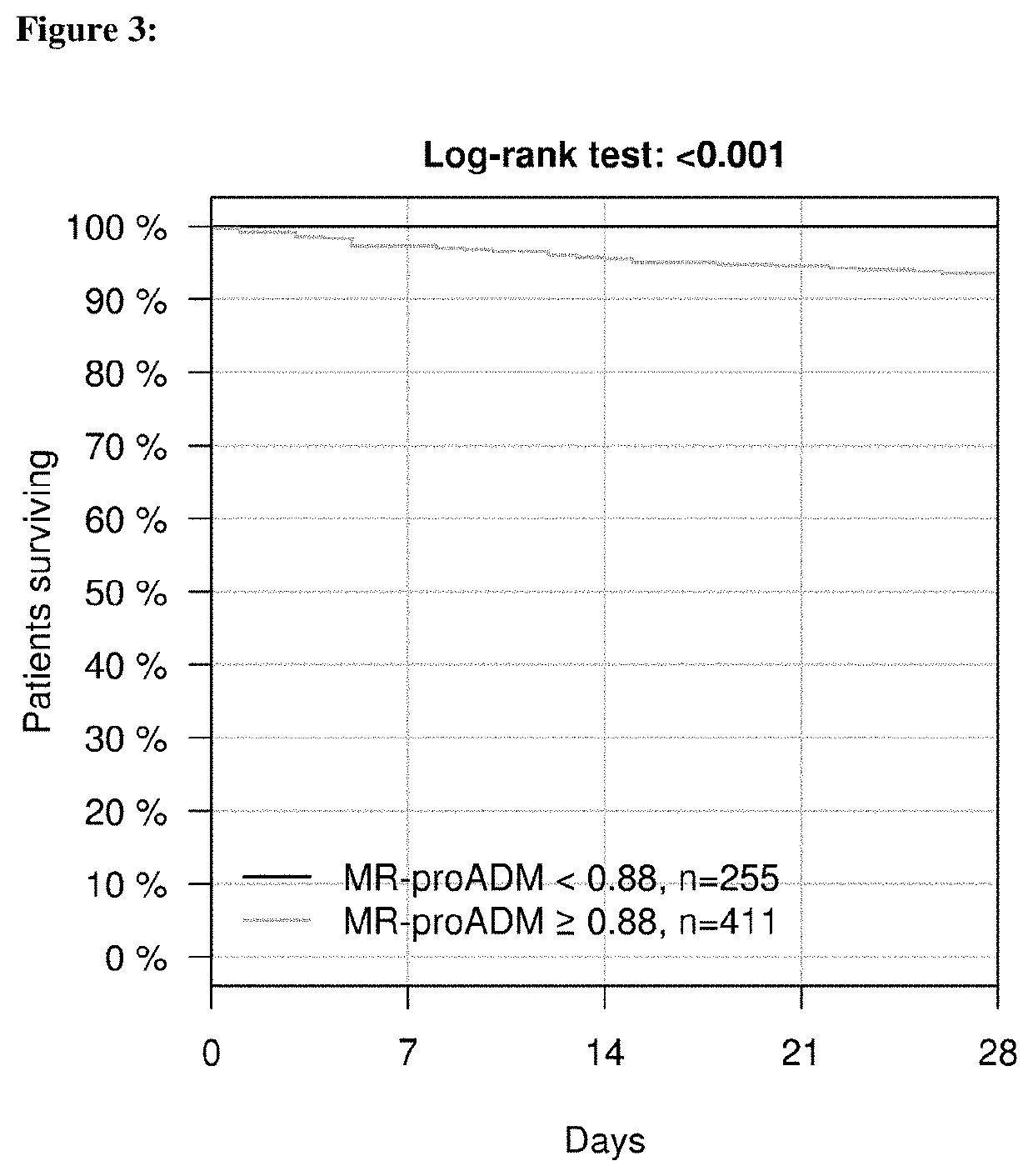
Workflow for risk assessment and patient management using procalcitonin and midregional-proadrenomedullin
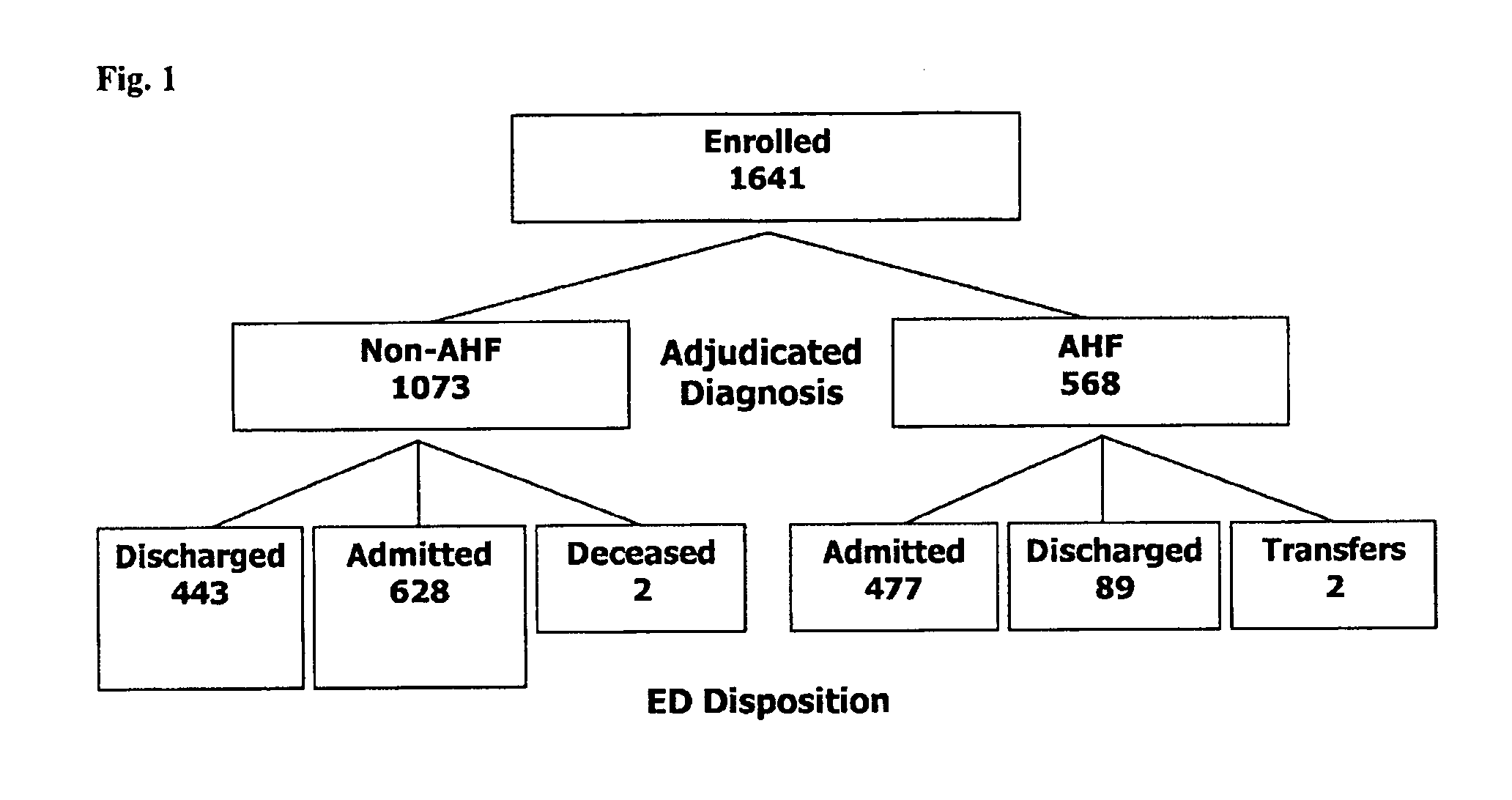
InactiveUS20210109117A1Easy to manageEasy to monitorDisease diagnosisBiological testingPatient managementOut of hospital
The present invention is in the field of clinical diagnostics. Particularly, the present invention relates to the assessment of severity of a subject being suspected of an infection or having an infection, who may have physiological signs or increased risk factors for infection, in particular from an infectious disease by determination of the levels of Procalcitonin (hereinafter: PCT) (SEQ ID No: 1 and / or proadrenomedullin (hereinafter: proADM)) (SEQ ID No: 3) or a partial peptide or fragment thereof, in particular midregional proadrenomedullin (MR-proADM) (SEQ ID No: 2), in a sample of a patient and the invention is related to a workflow hereto. Moreover, the invention refers to the assessment related to an infection like ruling out / in a patient and stratification, risk assessment, in particular to avoid rehospitalisation and hospital and post-discharge mortality.
Owner:BRAHMS GMBH
Activated fluorescent latex microsphere for procalcitonin fluorescent immunochromatographic test card
ActiveCN106405070ANot easy to reuniteEasy to useDisease diagnosisBiological testingMicrosphereFluorescence
The invention discloses an activated fluorescent latex microsphere for a procalcitonin fluorescent immunochromatographic test card, and an application thereof. The preparation method of the activated fluorescent latex microsphere for the procalcitonin fluorescent immunochromatographic test card comprises the steps of 1) adding a surfactant into a buffer solution that is 8-10 in pH value, and then adding dimethylformamide, adding N, N'-dicyclohexylcarbodiimide and N-hydroxysuccinimide; 2) adjusting the dispersion liquid of a fluorescent latex microsphere on the amino surface to be 8-10 in pH value, adding the dispersion liquid into a mixture obtained in the step 1), stirring for reaction, centrifuging to remove a supernatant after the completion of the reaction, and obtaining a surface-activated fluorescent latex microsphere. The surfactant is a mixture of polyethylene glycol fatty acid ester and stearate, wherein the weight ratio of polyethylene glycol fatty acid ester to stearate is 0.5-5:1.
Owner:BEIJING ELCOTEQ BIO TECH
Fluorescent immunochromatographic test paper for detecting procalcitonin, and preparation method thereof
PendingCN110780067AImprove labeling efficiencyImprove stabilityBiological material analysisBiological testingNitrocelluloseImmunofluorescence
The invention discloses fluorescent immunochromatographic test paper for detecting procalcitonin. The fluorescent immunochromatographic test paper comprises a PVC bottom plate, wherein a sample pad, acombination pad, a nitrocellulose membrane and a water absorption pad are sequentially arranged on the PVC bottom plate from left to right; one end of the sample pad is fixed on the PVC bottom plate,the other end of the sample pad is lapped on the combination pad, and a sample dripping hole is formed in the center of the sample pad; one end of the combination pad is fixed on the PVC bottom plate, and the other end of the combination pad is lapped on the nitrocellulose membrane; a detection line and a quality control line are sequentially arranged on the nitrocellulose membrane from left to right; and the left end of the water absorption pad is lapped on the nitrocellulose membrane. The fluorescent immunochromatographic test paper labels antibody proteins by means of time-resolved immunofluorescent microspheres, and carries out capture by using polyclonal antibodies, so that the detection sensitivity is higher; and the fluorescent immunochromatographic test paper greatly shortens thedetection time, is high in efficiency, and has good stability and repeatability.
Owner:南京欧凯生物科技有限公司
Carbon nano-tube procalcitonin detection kit and preparation method thereof
The invention discloses a carbon nano-tube procalcitonin detection kit and a preparation method thereof. A test paper base plate is sequentially adhered with a sample pad, a carbon nano-tube colloidal gold pad, a nitrocellulose membrane and a water absorbent pad. The carbon nano-tube procalcitonin detection kit is characterized in that the carbon nano-tube colloidal gold pad is adhered with a carbon nano-tube colloidal gold compound labeled procalcitonin monoclonal antibody 1, and the nitrocellulose membrane is coated with a detection line and a quality control line of a procalcitonin monoclonal antibody 2. According to the kit disclosed by the invention, procalcitonin in a sample is detected by adopting a carbon nano-tube colloidal gold labelling technology so as to judge whether bacterial infection occurs. The carbon nano-tube procalcitonin detection kit disclosed by the invention has the characteristics of simplicity in operation, rapid reaction, high sensitivity, strong specificity and the like, and is suitable for field detection and self-detection.
Owner:BENXI TAISITEJIE BIOTECH
Molecular markers for urinary tract infections
ActiveUS8748195B2Simple methodDiagnose and/or monitorDisease diagnosisImmunoglobulinsBacteriuriaUpper urinary tract infection
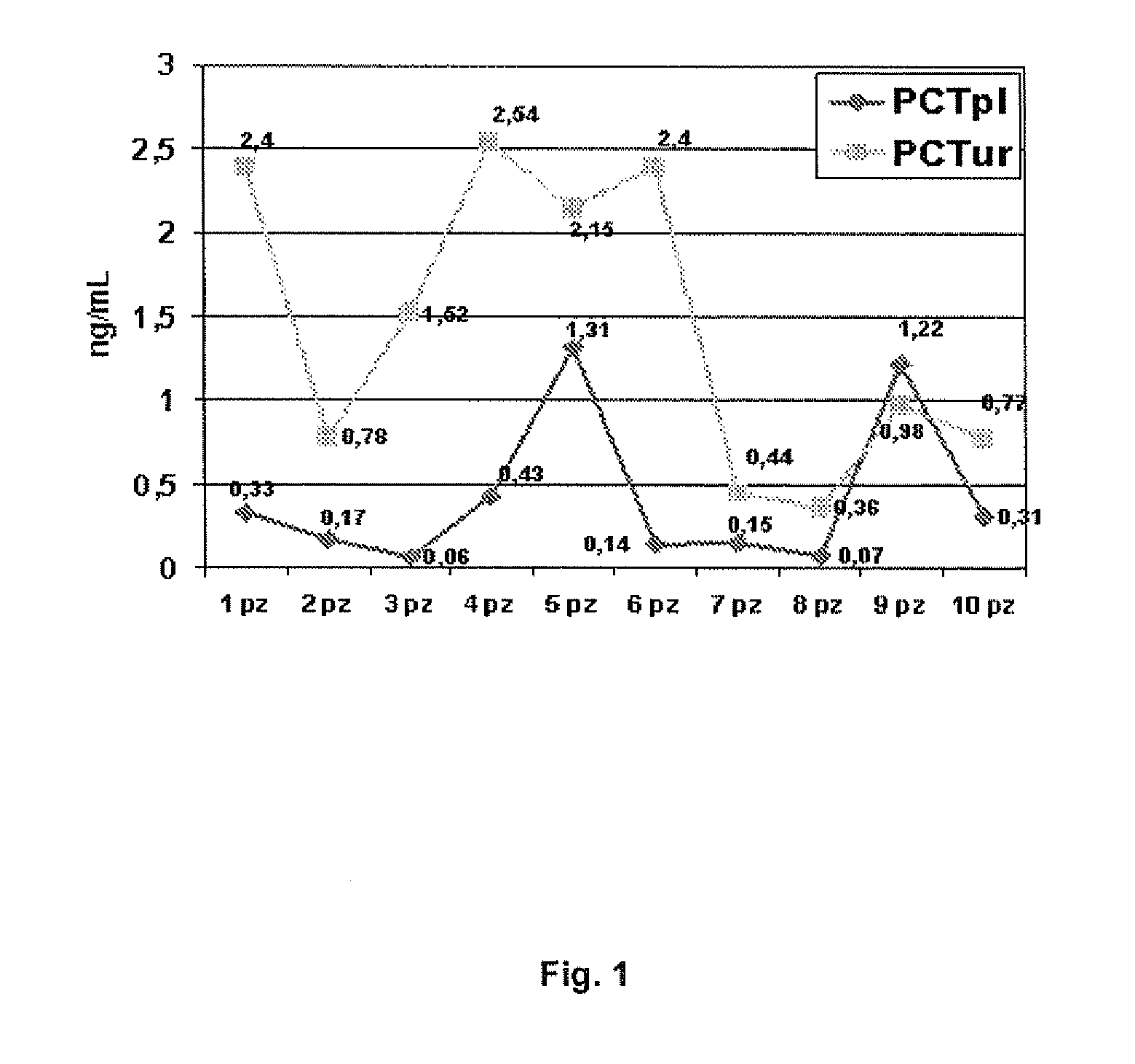
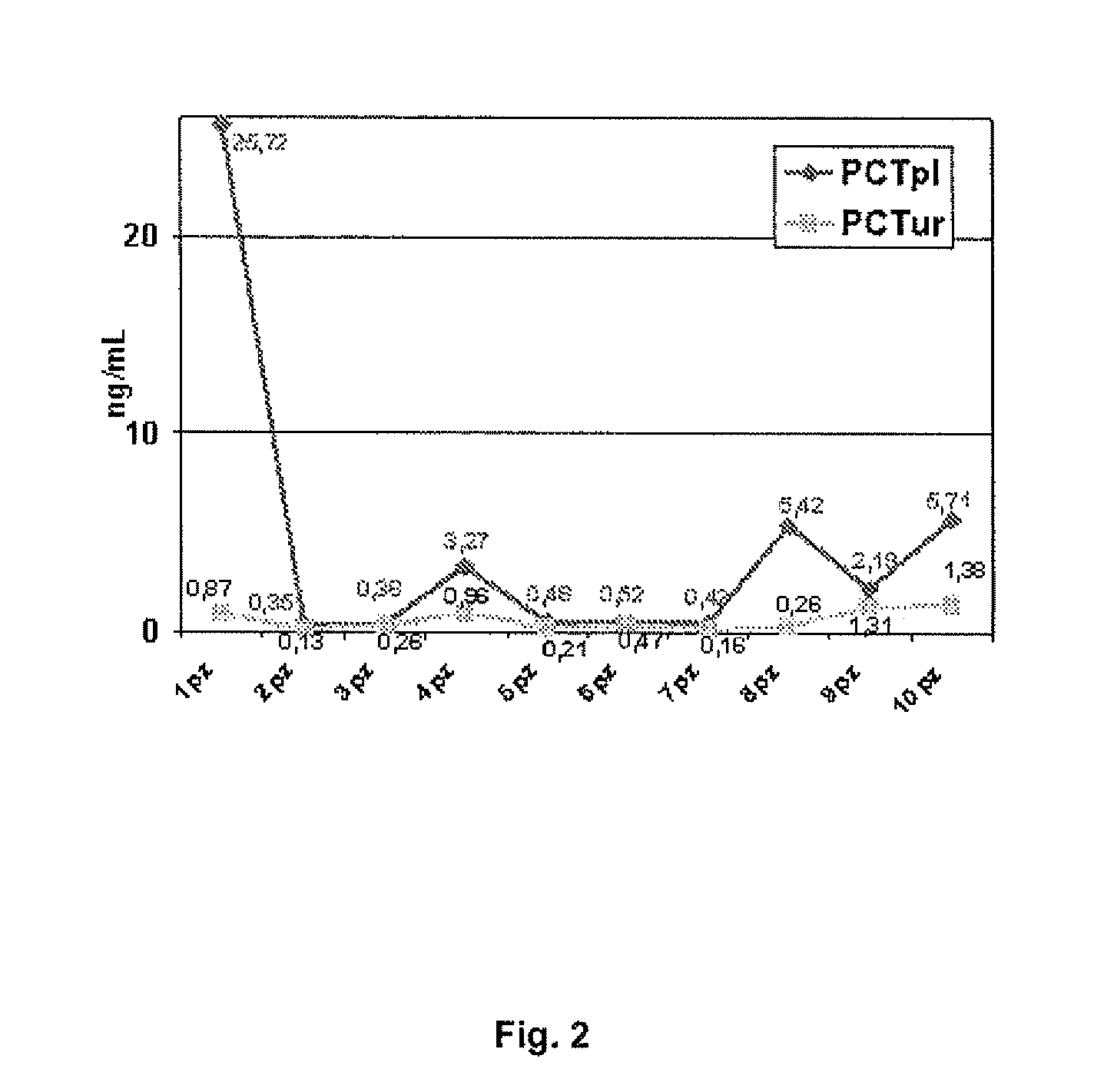
The invention relates to an in vitro method for determining procalcitonin levels in plasma and urine as a diagnostic marker to identify patients with urinary tract infections, in vitro methods to perform said determination, a kit for the diagnosis of patients with urinary tract infections, and the usefulness of procalcitonin in the diagnosis urinary tract infections.
Owner:BRAHMS GMBH
Risk assessment for antibotics treatment in patients suffering from primary non-infectious disease by determining the level of procalcitonin
ActiveUS20120100635A1Increased riskImmunoglobulins against animals/humansDisease diagnosisIncreased riskAntibiotic Y
The present invention relates to a diagnostic method for the identification of a subject suffering from a primary non-infectious disease having an increased risk of an adverse outcome potentially being induced by the administration of an antibiotic to said subject comprising the determination of the level of Procalcitonin (PCT) or a fragment thereof or a precursor or fragment thereof having a length of at least 12 amino acid residues in a sample of a bodily fluid from said subject and the correlation of the determined level to a potential risk induced by the administration of an antibiotic.
Owner:BRAHMS GMBH
Procalcitonin and C-reactive protein combined detection kit
ActiveCN111537737AHigh precisionSave materialDisease diagnosisBiological testingReagent stripCalcitonin
The invention discloses a procalcitonin and C-reactive protein combined detection kit. The kit comprises a kit body, a refrigeration mechanism, a control system, a reagent strip, a first detection object, a second detection object and a reaction buffer solution, the box body is divided into a first box body, a second box body and a third box body which are sequentially arranged in the length direction, the reagent strip, the first detection object and the second detection object are all stored in the first box body, the reaction buffer solution is stored in the third box body, the first detection object contains a biotin labeled PCT antibody complex and a fluorescently labeled Anti-chicken IgY antibody complex, the second detection object contains a fluorescently-labeled PCT antibody complex, and the reaction buffer solution contains a fluorescently-labeled CRP antibody complex and the fluorescently-labeled Anti-chicken IgY antibody complex. The precision of simultaneous detection of PCT and CRP is improved, and the stability of the detection reagent product is improved.
Owner:巴迪泰(广西)生物科技有限公司
Immunoturbidimetry kit for determining procalcitonin in whole blood
PendingCN111856039AImprove anti-interference abilityLow costBiological material analysisBiological testingActive agentMicrosphere
Owner:SUZHOU DIAGVITA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com