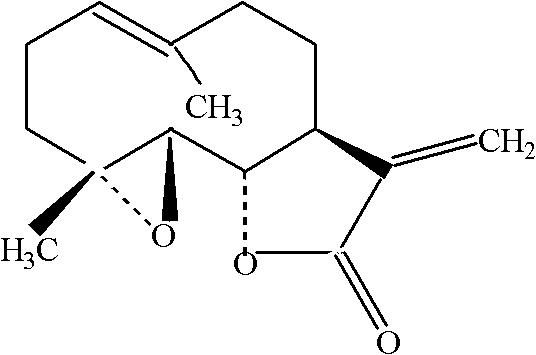
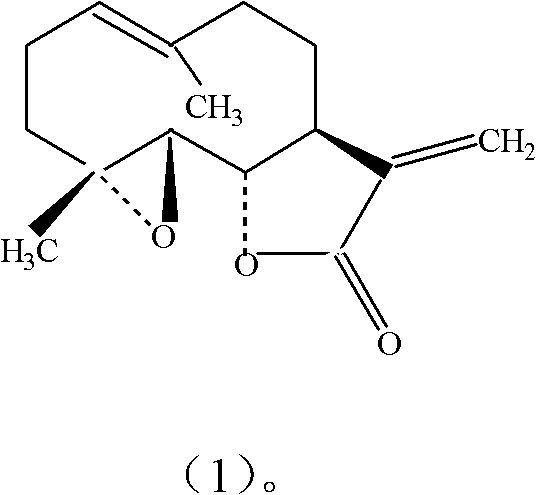
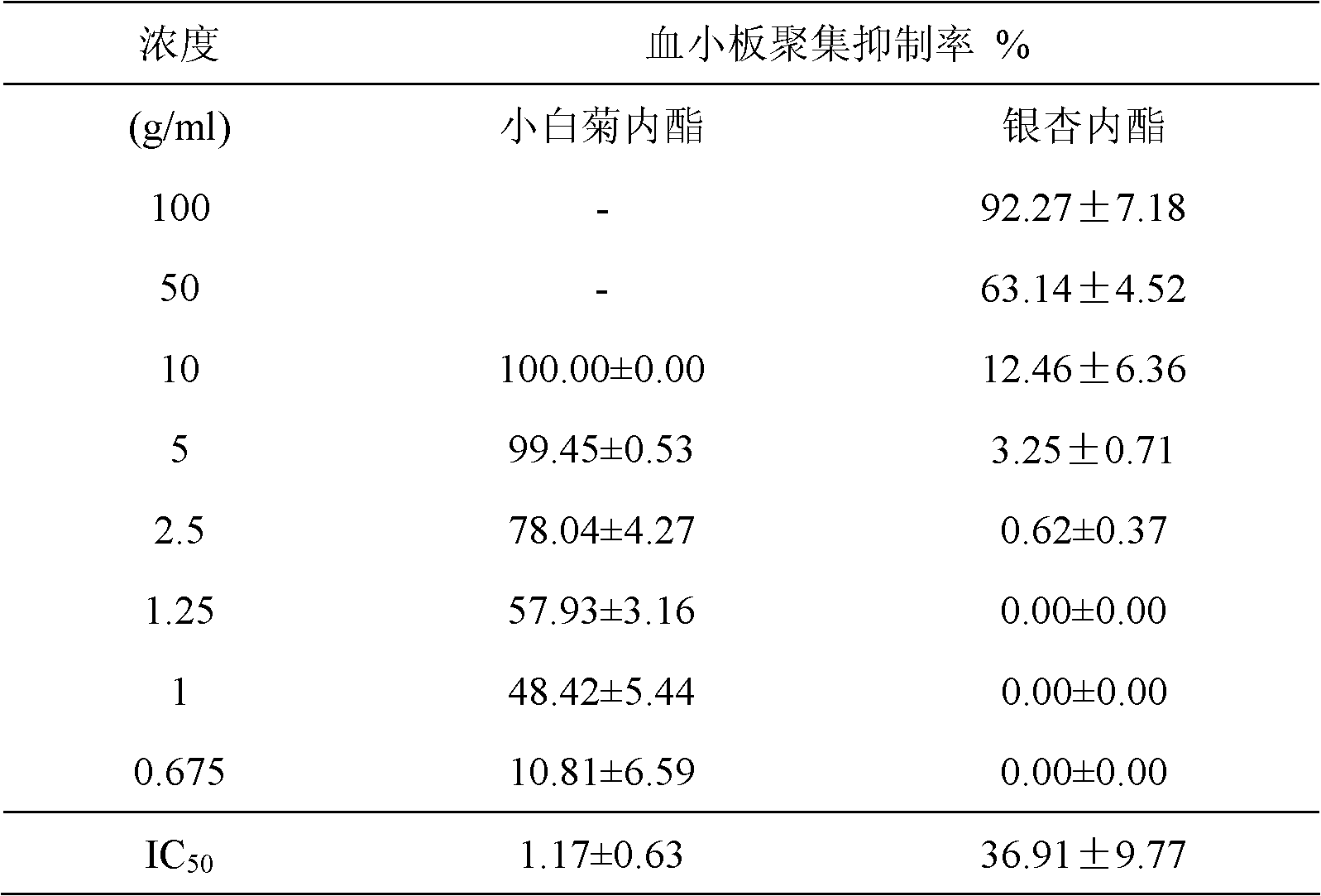
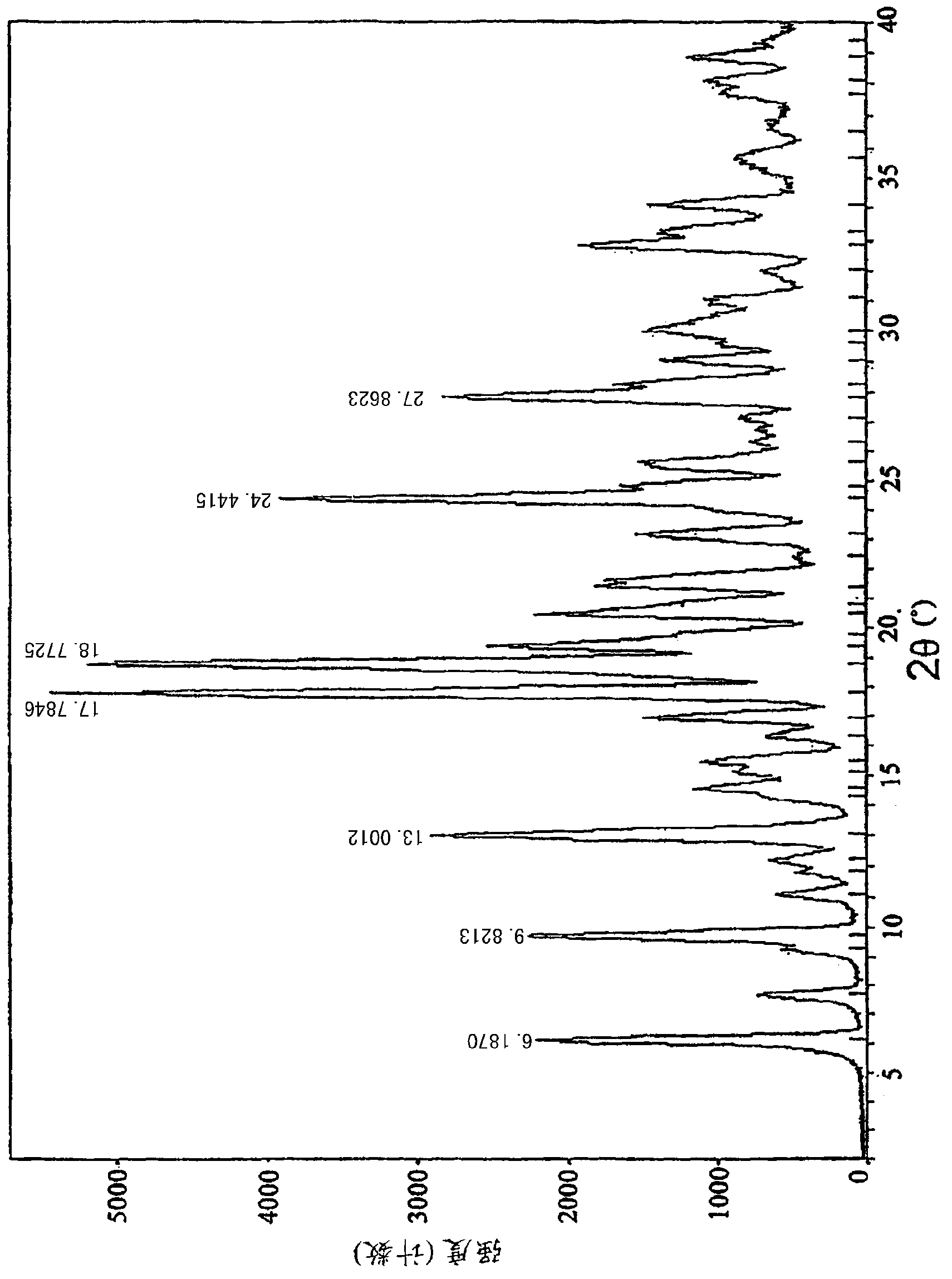
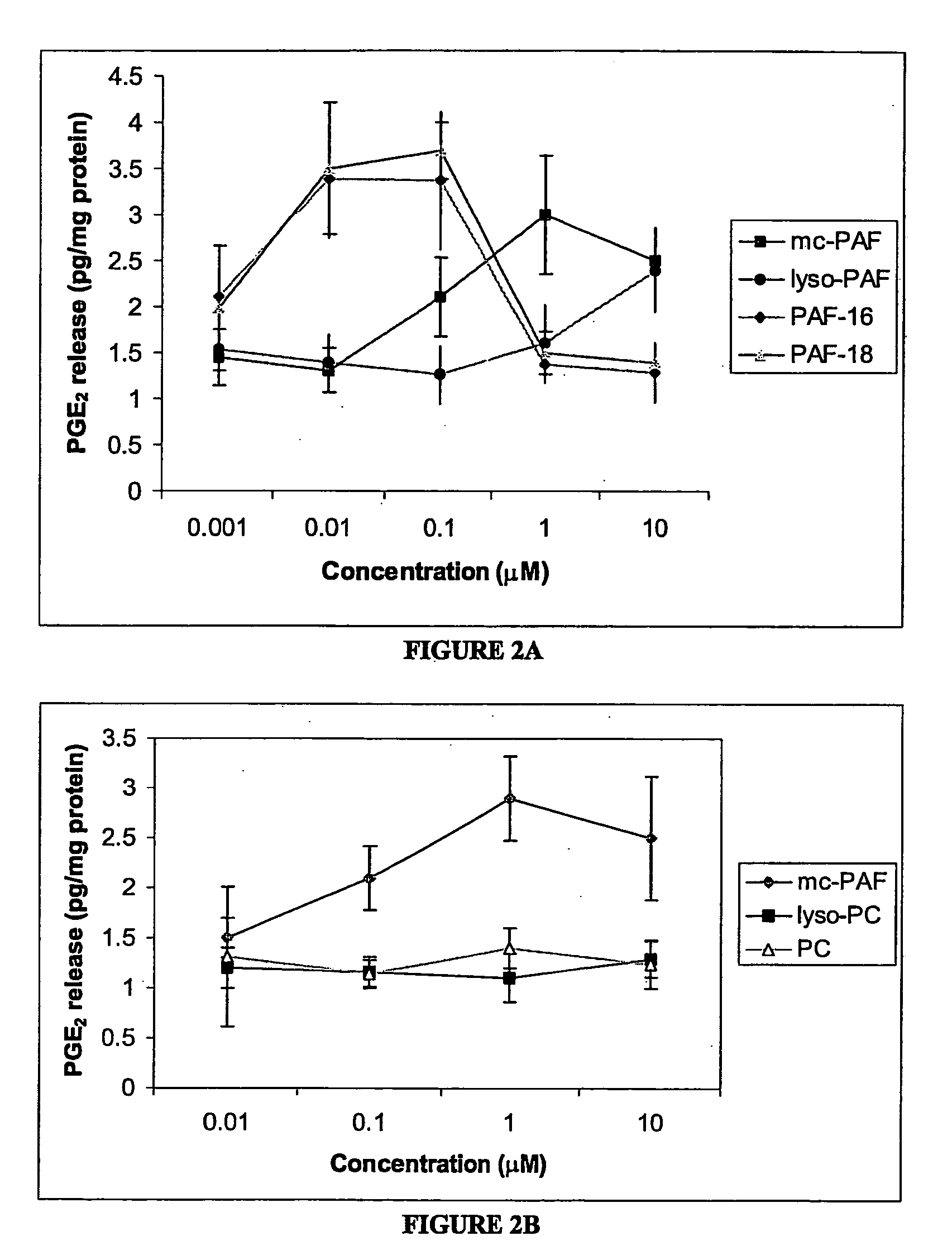
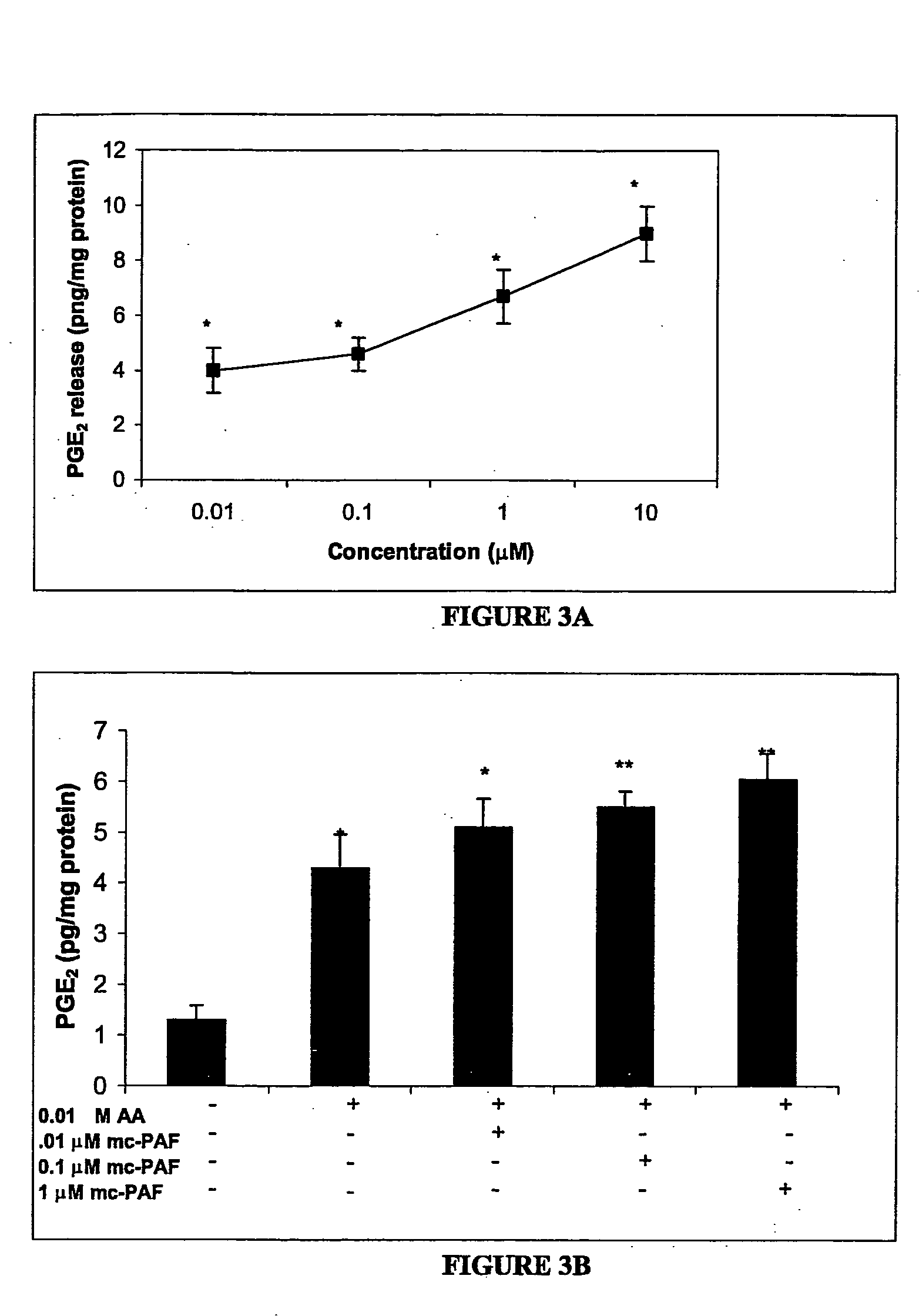
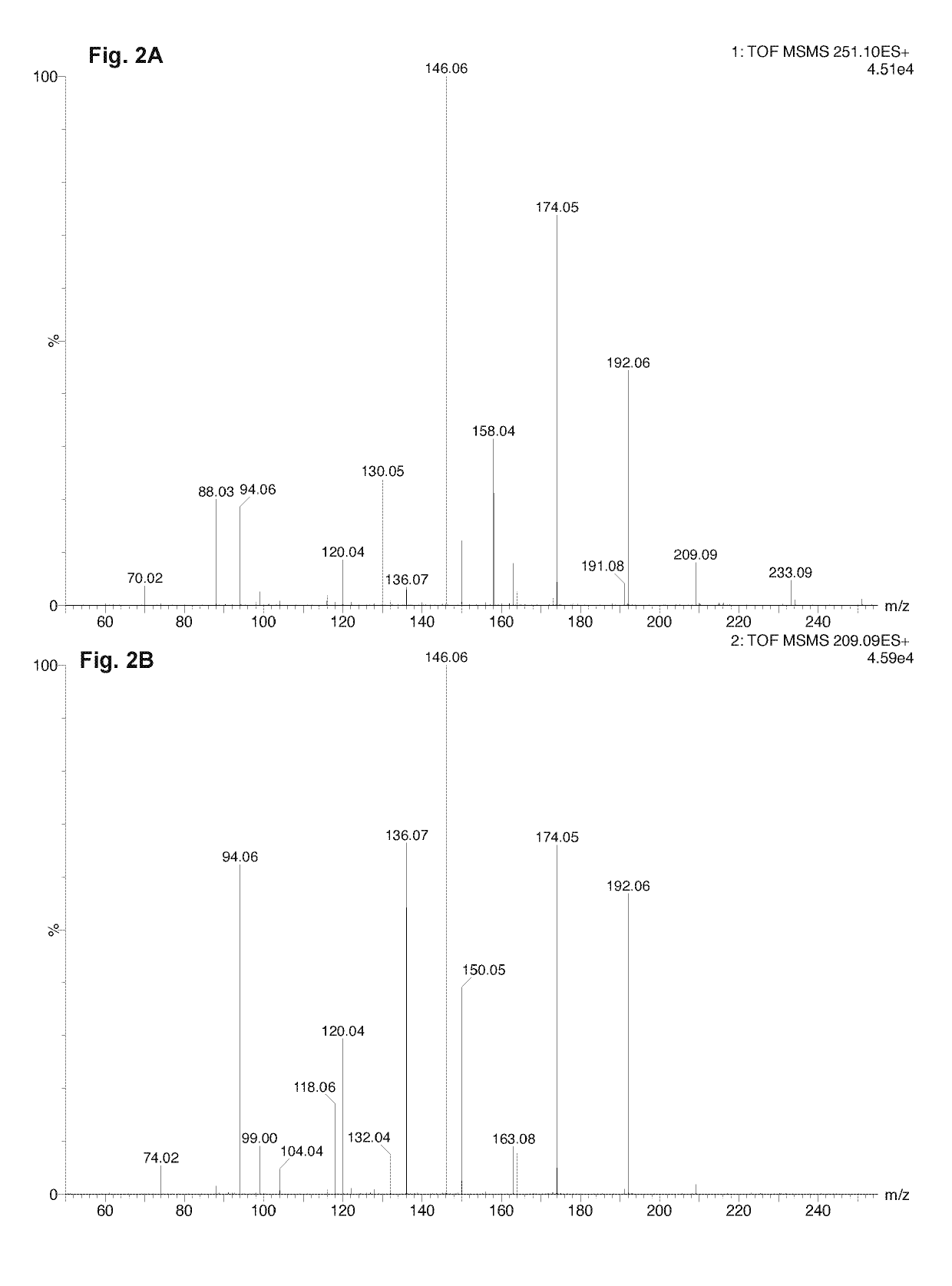
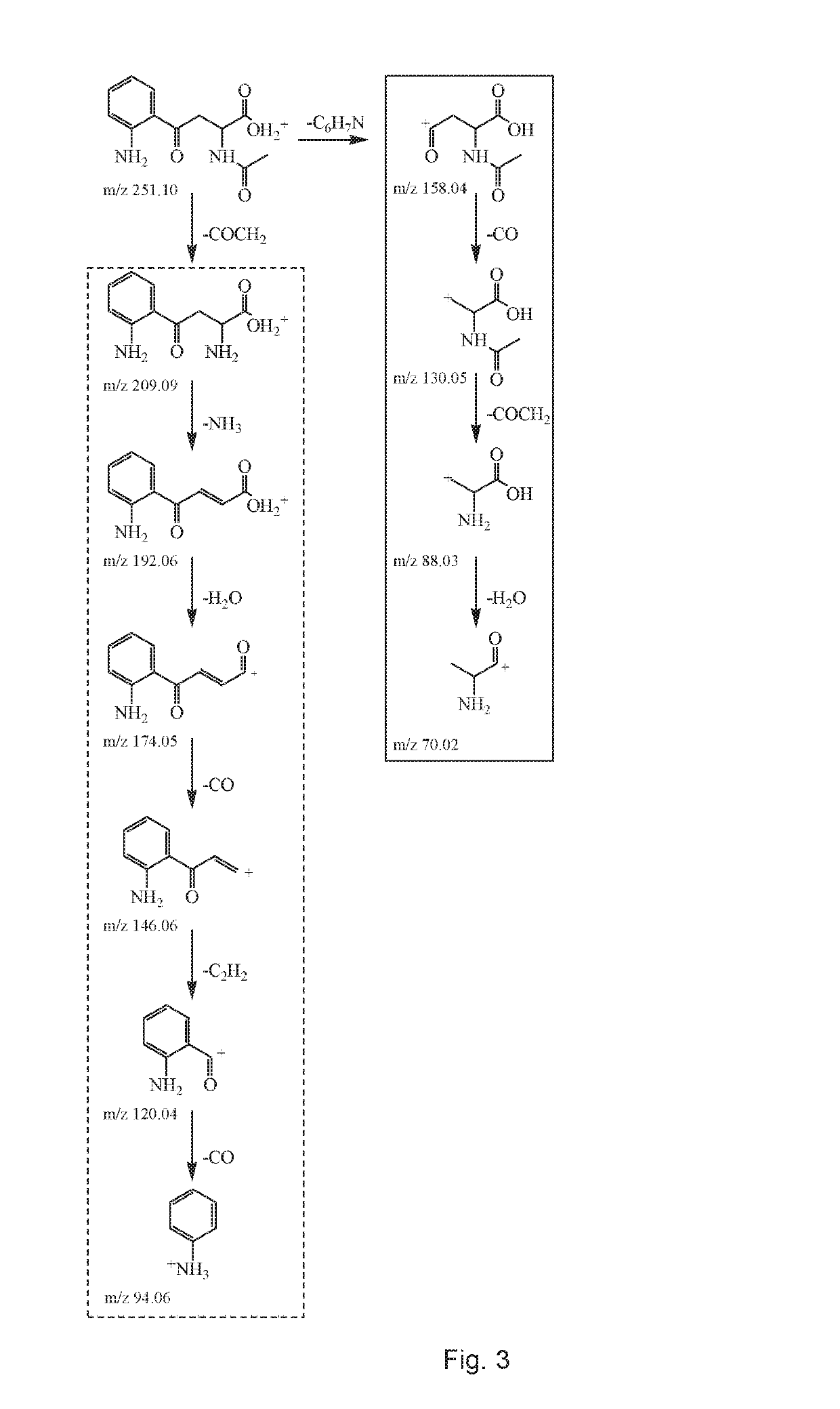
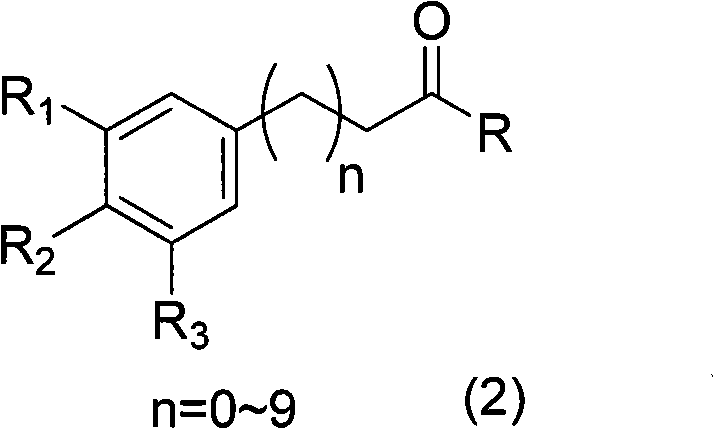
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Platelet-activating factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Platelet-activating factor, also known as PAF, PAF-acether or AGEPC (acetyl-glyceryl-ether-phosphorylcholine), is a potent phospholipid activator and mediator of many leukocyte functions, platelet aggregation and degranulation, inflammation, and anaphylaxis. It is also involved in changes to vascular permeability, the oxidative burst, chemotaxis of leukocytes, as well as augmentation of arachidonic acid metabolism in phagocytes.
Degradable wound repair material and preparation method thereof
InactiveCN103830768AGood biocompatibilityGood for degradation and absorptionAbsorbent padsBandagesWhite blood cellPancreatic hormone
The invention relates to a degradable wound repair material and a preparation method thereof. The degradable wound repair material includes a matrix component and an auxiliary component, wherein the matrix component includes a protein ingredient, and the auxiliary component includes at least one of an antibacterial agent and an active factor. Specifically, the antibacterial agent is a synthetic antibacterial drug, an inorganic antibacterial agent, an organic antibacterial agent, or a natural antibacterial agent, and the active factor is at least one of the following active factors: an epidermal growth factor, an FGF vascular endothelial growth factor, a platelet-derived growth factor, a platelet activating factor, an insulin-like growth factor, a tumor necrosis factor, interleukin, colony stimulating factor-1, various bone morphogenetic proteins or transforming growth factors. By loading the antibacterial agent and active factor component on the basis of the matrix component, the degradable wound repair material provided by the invention can have biological activity on the basis of meeting degradability, can better promote wound repair, and has good anti-infection properties during use.
Owner:SHENZHEN LANDO BIOMATERIALS
Method of enhancing transmucosal delivery of therapeutic compounds
InactiveUS20070077283A1Improve breathabilityPeptide/protein ingredientsPharmaceutical delivery mechanismActive agentPharmacology
A composition comprising a biologically active agent and a permeation enhancing lipid wherein the permeation enhancing lipid is a platelet activating factor antagonist or a biologically inactive a platelet activating factor, and increases permeability of the biologically active agent across a tissue layer. Also disclosed is a process of increasing the permeability of a biological agent across a layer tissue comprising contacting the tissue layer with a composition comprising the biological agent and a permeation enhancing lipid wherein the permeation enhancing lipid is a platelet activating factor antagonist or a biologically inactive platelet activating factor.
Owner:NASTECH PHARMA
Preparation method of ginkgolide K
InactiveCN101824041AHigh yieldSimple reaction conditionsOrganic chemistryCardiovascular disorderNatural productGinkgolide
Owner:广东省中药研究所
Application of Piperlongumine compounds for resisting platelet aggregation
The invention relates to application of Piperlongumine compounds for resisting platelet aggregation, in particular to application of the Piperlongumine compounds which are used for preparing medicines and health care products for controlling thrombus or cardiovascular and cerebrovascular diseases. Experimental results indicate that the Piperlongumine compounds have remarkable effects on the platelet aggregation initiated by collagen, arachidonic acid and platelet activating factors, and the inhibition efficiency of the Piperlongumine compounds is superior to aspirin and also in a linear relationship with the compound concentration; thereby indicating that the Piperlongumine compounds have very wide prospects as a category of medicines or health care products for resisting the thrombus or controlling the cardiovascular and cerebrovascular diseases.
Owner:合肥华纳生物医药科技有限公司
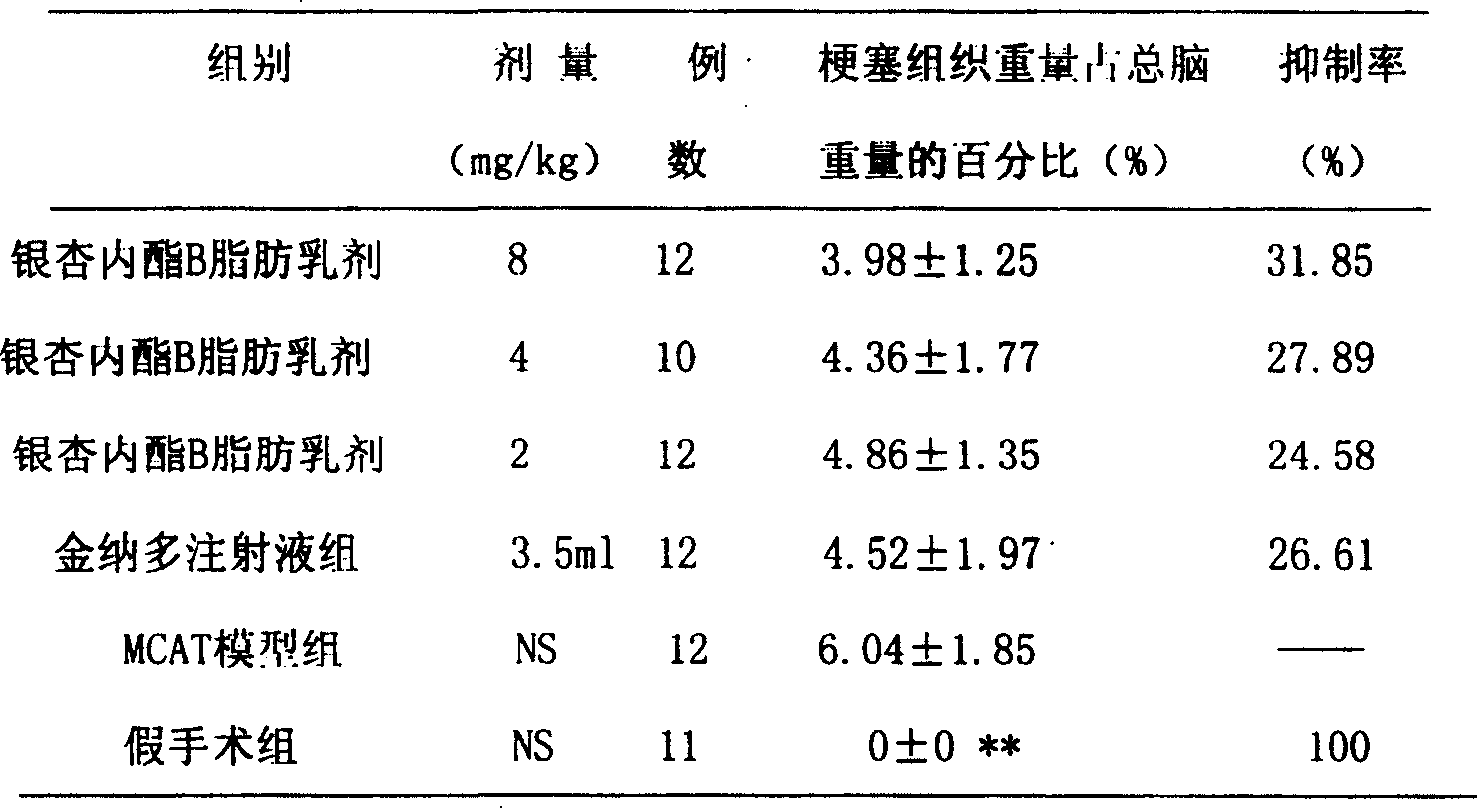
Fat emulsion of biobalide B and preparation method
InactiveCN1759828AExcellent pharmaceutical characteristicsSignificant effectOrganic active ingredientsEmulsion deliveryEmulsionFat emulsion
A fatty emulsion of ginkalide B and its preparing process are disclosed. It has the strong antagonism to platelet activating factor.
Owner:刘晓东 +1
Application of parthenolide as platelet-activating factor (PAF) antagonist
InactiveCN102579426AReduce contentAntibacterial agentsOrganic active ingredientsThrombusHepatic fibrosis
The invention discloses application of parthenolide as a platelet-activating factor (PAF) inhibitor. The parthenolide can be used as a safe PAF antagonist to be applied to treatment, auxiliary treatment and prevention of diseases such as thrombus, atherosclerosis, ischemic cardio-vascular disease, cerebral ischemia, acute pancreatitis, endotoxic shock, asthma, hepatic fibrosis and hepatocirrhosis, injury of nerve, gastrointestinal ulcer and necrosis, psoriasis, systemic lupus erythematosus and acquired immune deficiency syndrome related to platelet-activating factor.
Owner:韩颖
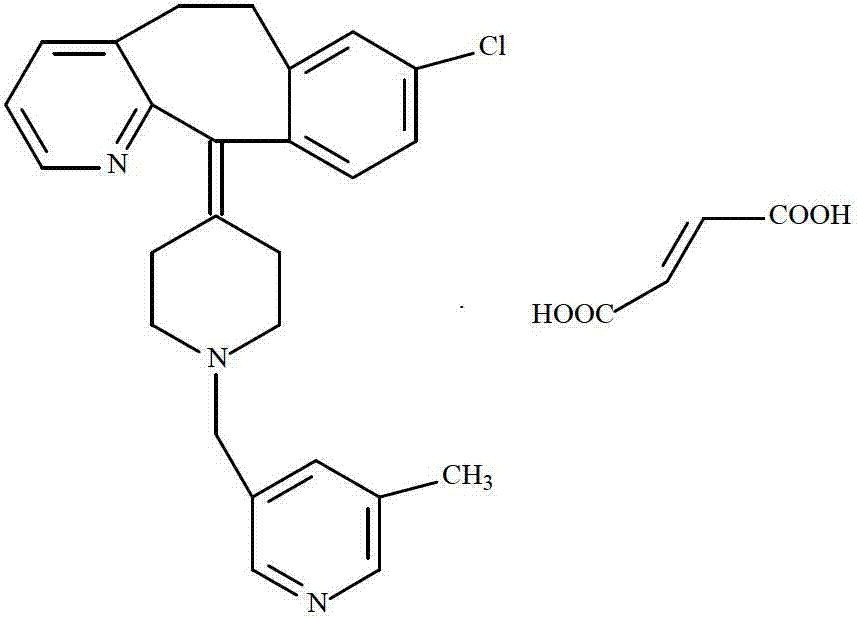
Rupatadine fumarate compound
The invention provides a rupatadine fumarate compound which has dual effects of antihistamine and antagonistic platelet activating factor (PAF). According to the research, allergy and inflammatory diseases are multi-factor complex processes caused by generation and release of various different media; histamine is the most inflammatory medium appearing in allergy early symptom, and the disease symptoms including sneezing, rhinocnesmus, tearing, running nose, skin itch, wheal and the like are mostly caused by histamine H1 receptor. And PAF also can cause bronchial constriction and increased permeability of the vessel, so that running nose, nasal congestion, wheal and itch are caused; meanwhile, the PAF is also a main cause of asthma. The antiallergic drug which is clinically used only has an antihistamine activity effect, but does not have a PAF antagonistic effect.
Owner:海思科制药(眉山)有限公司
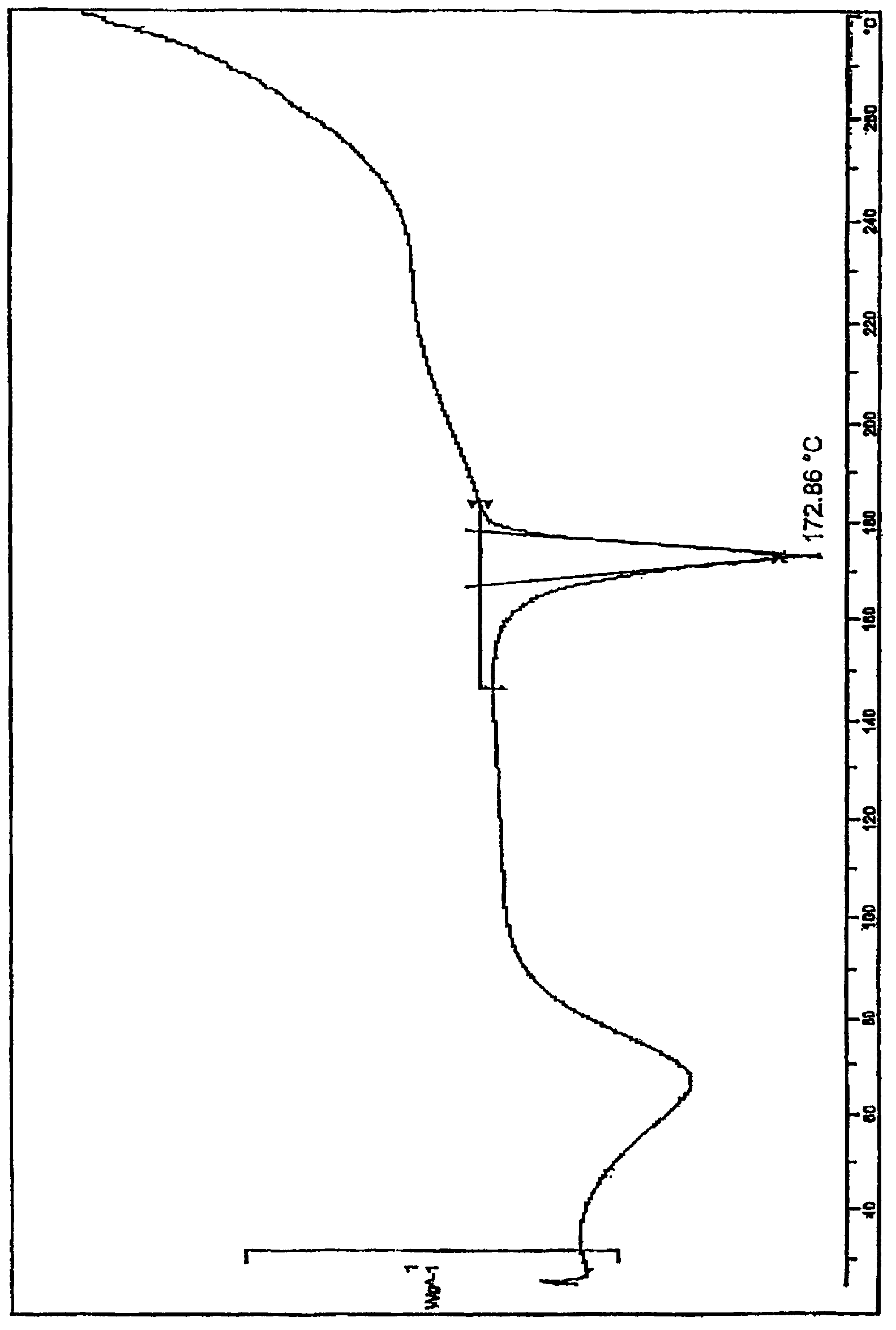
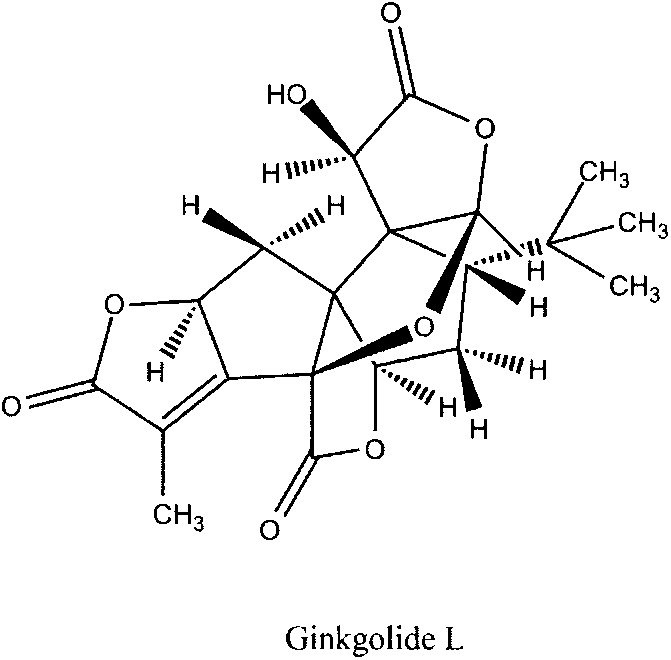
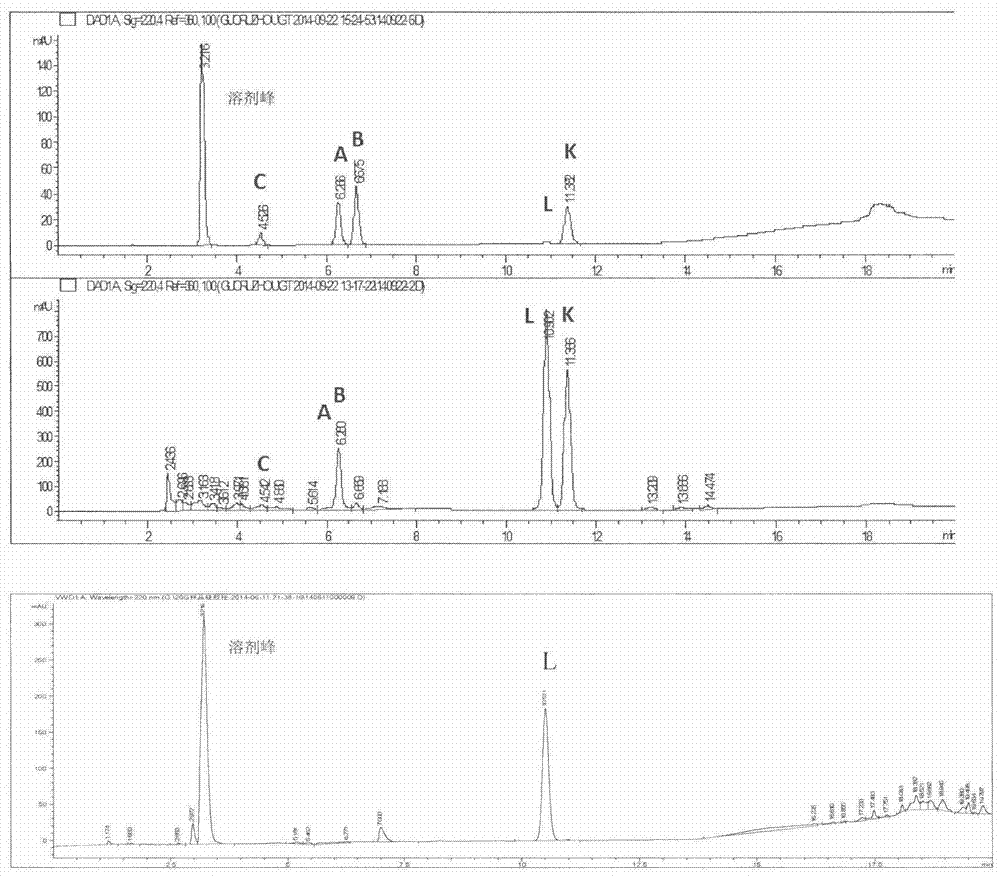
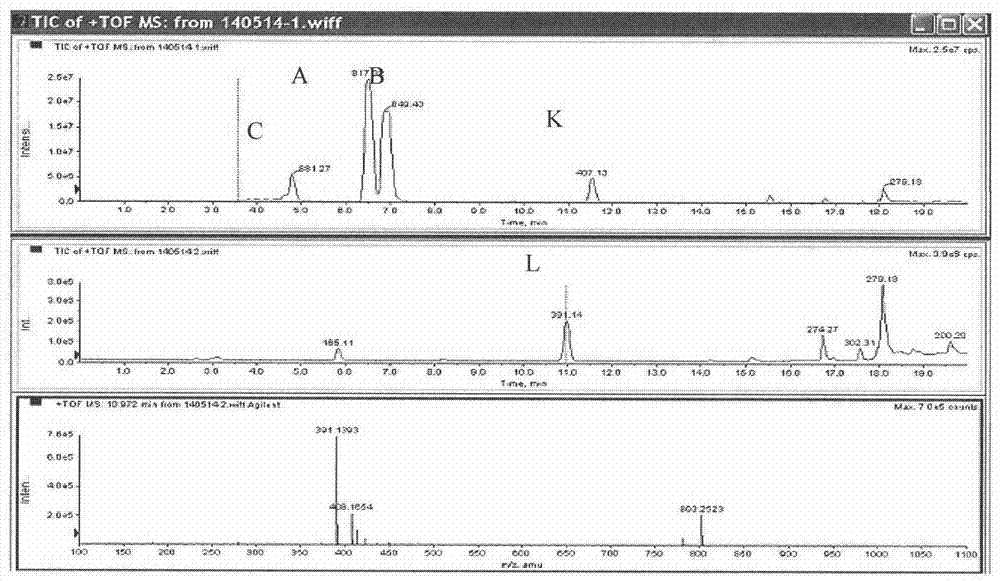
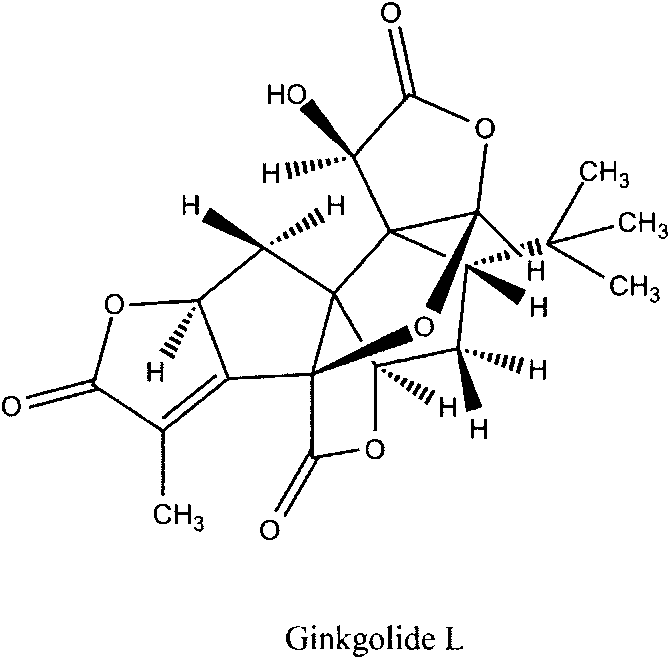
Preparation method of ginkgolide L
A ginkgolide type compound which serves as a powerful antagonist of platelet activating factors (PAF) has very good protective effects on focal cerebral ischemia and diffuse total cerebral ischemia. Ginkgolide is divided into ginkgolide A, ginkgolide B, ginkgolide C, bilobalide, ginkgolide K, ginkgolide L and the like. The content of ginkgolide L in ginkgo leaves is extremely low, so that ginkgolide L is difficult to obtain, and research report on ginkgolide L is nearly zero. The invention relates to a preparation method of ginkgolide L. Ginkgolide L is extracted from commercially available ginkgo total lactone or ginkgo total lactone obtained based on a known method. The preparation method is simple to operate, the raw materials are low in cost, a plenty of ginkgolide L can be obtained rapidly, and basis is provided for research on ginkgolide L.
Owner:CHINA PHARM UNIV
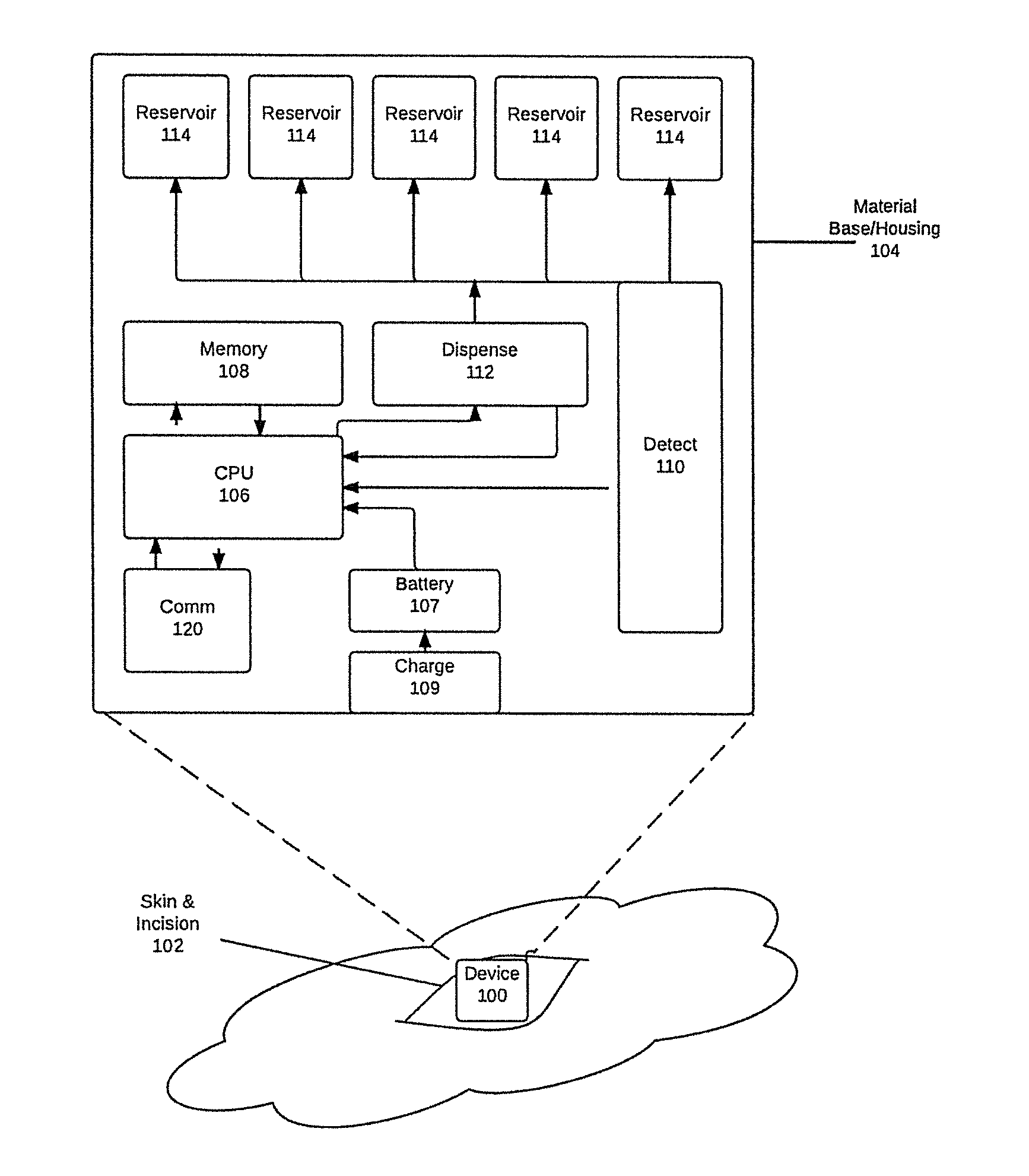
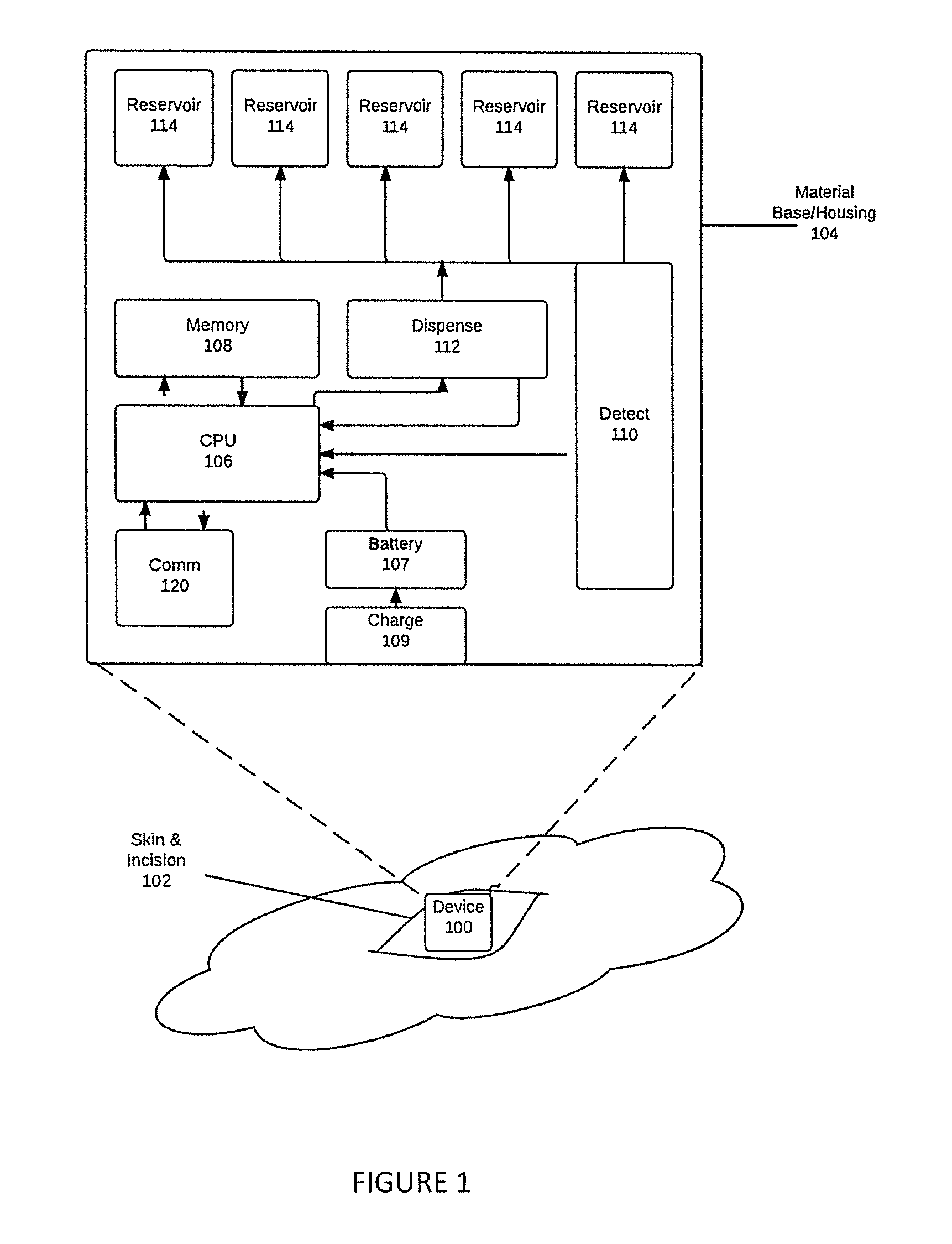
Implantable device for automatic delivery of medication for allergic reactions
InactiveUS20170035968A1Save many lives worldwideReduce riskOrganic active ingredientsMedical devicesCytokineBiomarker (petroleum)
Apparatus, implanted subcutaneously or in muscle, fat, joint spaces, or body cavities of any type, detects and responds to an allergic and / or anaphylactic reaction. Detection is carried out by monitoring the levels of biomarker molecules that indicate the occurrence of an allergic and / or anaphylactic reaction, such as: histamine, leukotrienes, prostaglandins, cytokines, tryptase, Fc-ε-RI complexes, anaphylatoxin C3a, chymase, carboxypeptidase A, platelet-activating factor8, other mast cell degranulation byproducts, or other basophil activation compounds. An appropriate dosage of medication (such as epinephrine, antihistamines, or steroids) is automatically dispensed to mitigate the allergic / anaphylactic response. The simplicity and ease of use of the invention has the potential to save many lives worldwide, while dramatically mitigating the risks of the current methods of handling severe allergic reactions using external auto-injectors. 8 Sala Cunill, A., Cardona, V. Biomarkers of anaphylaxis, beyond tryptase. Current Opinion in Allergy and Clinical Immunology. Vol 15(4), August 2015, p 329-336. Doi: 10.1097 / ACI.0000000000000184.
Owner:HASSAN ALEXANDER +1
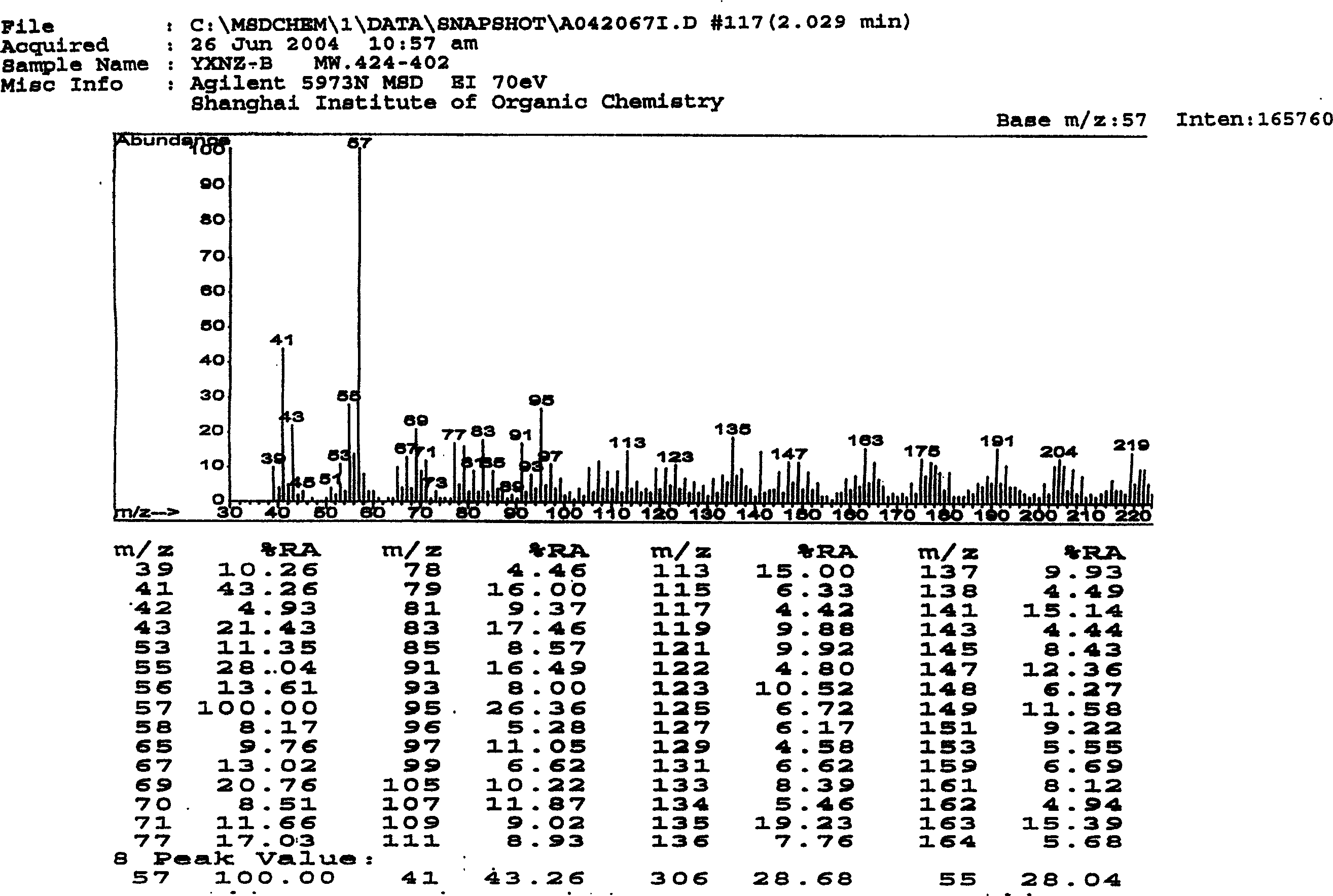
Assay method for platelet-activating factor
InactiveUS20040175776A1Efficient removalComponent separationOther chemical processesTreatment effectGas chromatography–mass spectrometry
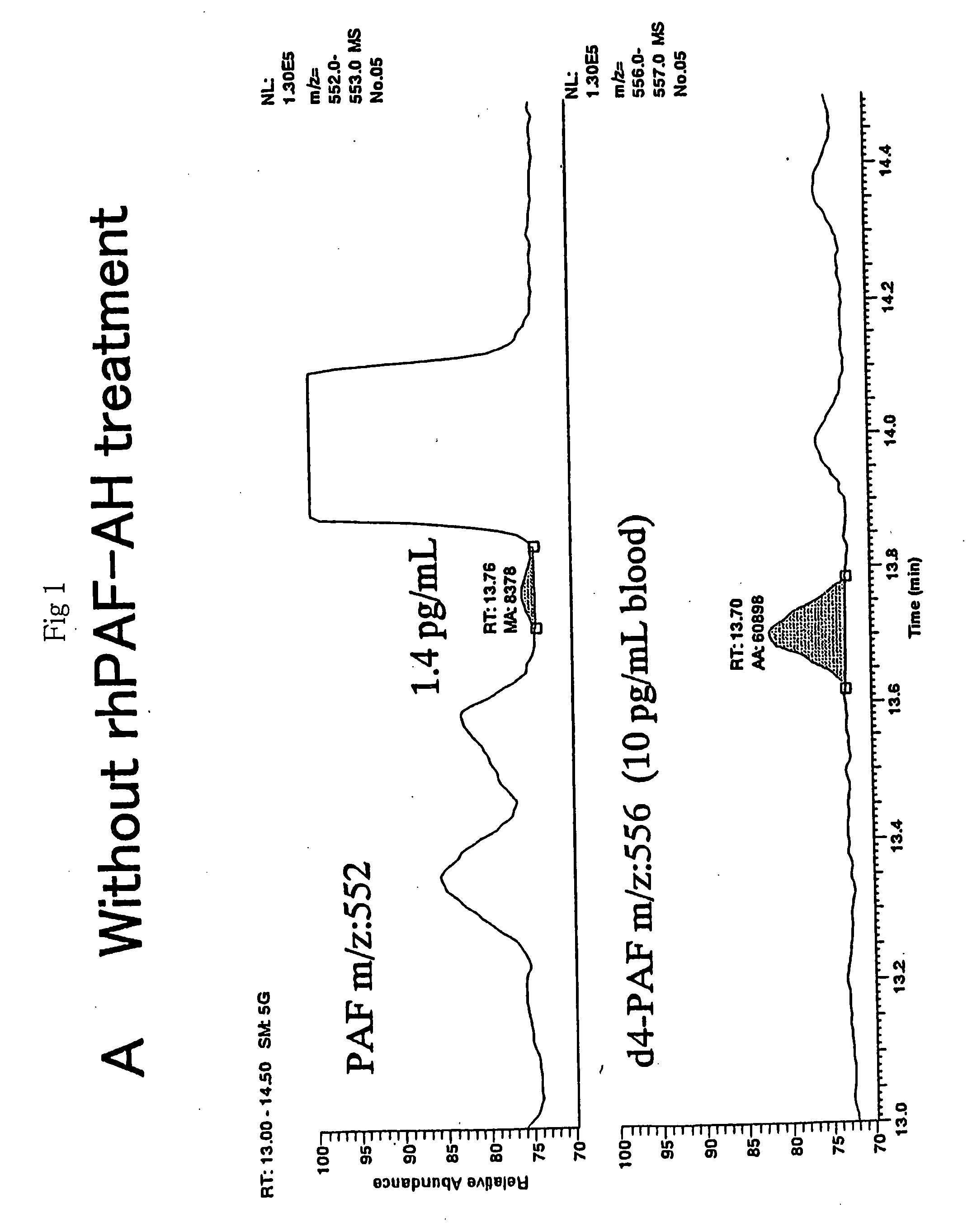
There is provided a highly specific and highly sensitive assay method for platelet-activating factor (PAF). The invention may be employed to measure PAF levels in biological samples for elucidating the role of PAF in various pathological conditions. In addition, it is expected to facilitate diagnosis of various diseases associated with PAF fluctuation, and also contributing to evaluation of therapeutic effects on such diseases. The invention is a method whereby PAF is selectively extracted and purified from a biological sample, and then the PAF is measured by highly sensitive and specific gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry.
Owner:ASUBIO PHARMA
Platelet-activating factor antagonists as analgesic, anti-inflammatory, uterine contraction inhibiting, and anti-tumor agents
InactiveUS20050032713A1Prevent proliferationBlock cellBiocideCarbohydrate active ingredientsAbnormal tissue growthAnalgesics effects
Antagonists to platelet-activating factor provide analgesic effects as well as limit the release of inflammatory mediators. Use of these antagonists in the form of pharmaceutical compositions or nutritionals is beneficial (1) in the treatment of acute and / or chronic pain; (2) in the inhibition of inappropriate or excessive contraction of the uterus; (3) in the treatment of septic shock; and (4) in the inhibition of angiogenesis and / or tumor cell proliferation.
Owner:MASSACHUSETTS INST OF TECH
Medicinal compound for resisting platelet activating factor
InactiveCN1900082AGood water solubilityImprove bioavailabilityOrganic chemistryAntipyreticSolubilityMedicine
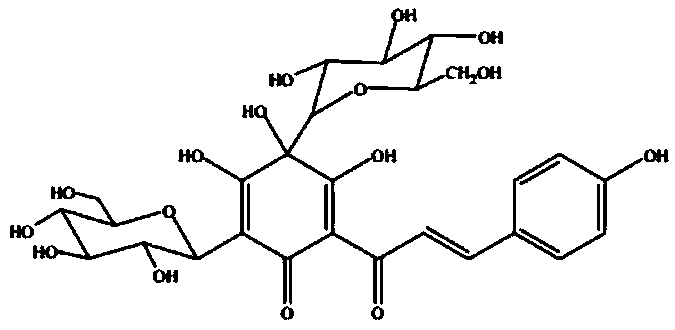
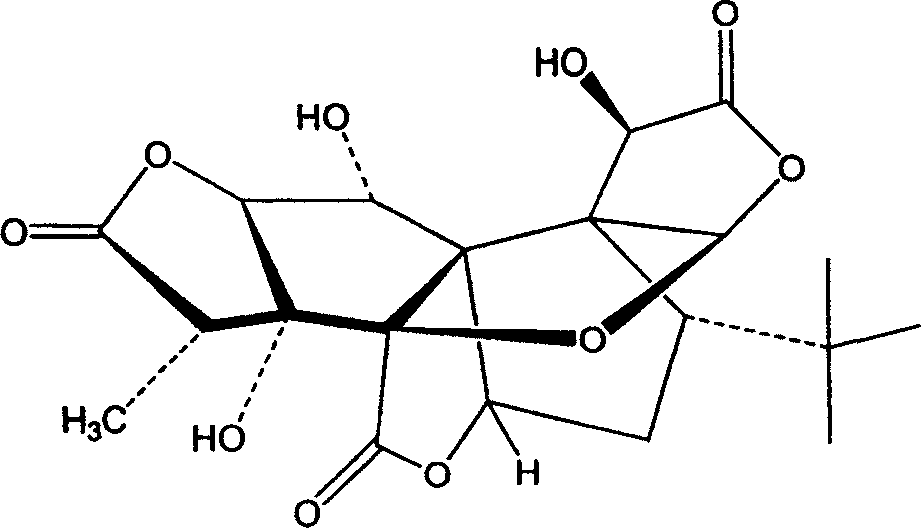
The present invention discloses a kind of medicine compound antaonizing platelet activating factor. The medicine compound is bilobalide B with nitrogen-containing radical connected to the hydroxyl radial in the site 10 and has the structure as shown. The medicine compound of the present invention has high water solubility, high bioavailability and high curative effect, and its salt has greatly raised water solubility, bioavailability and curative effect.
Owner:秦引林
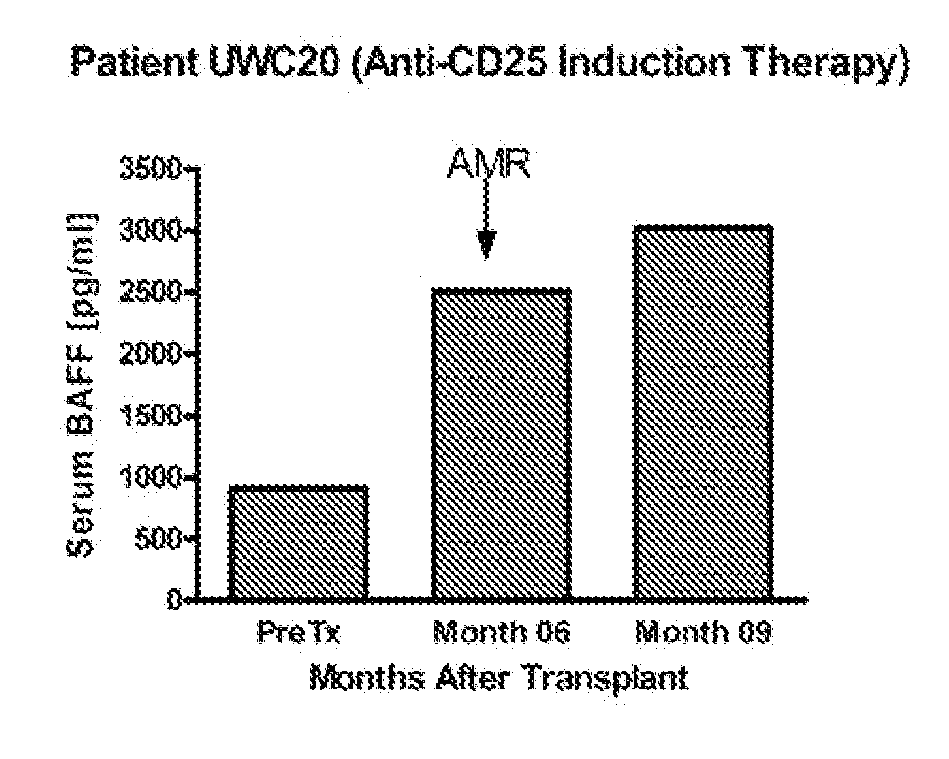
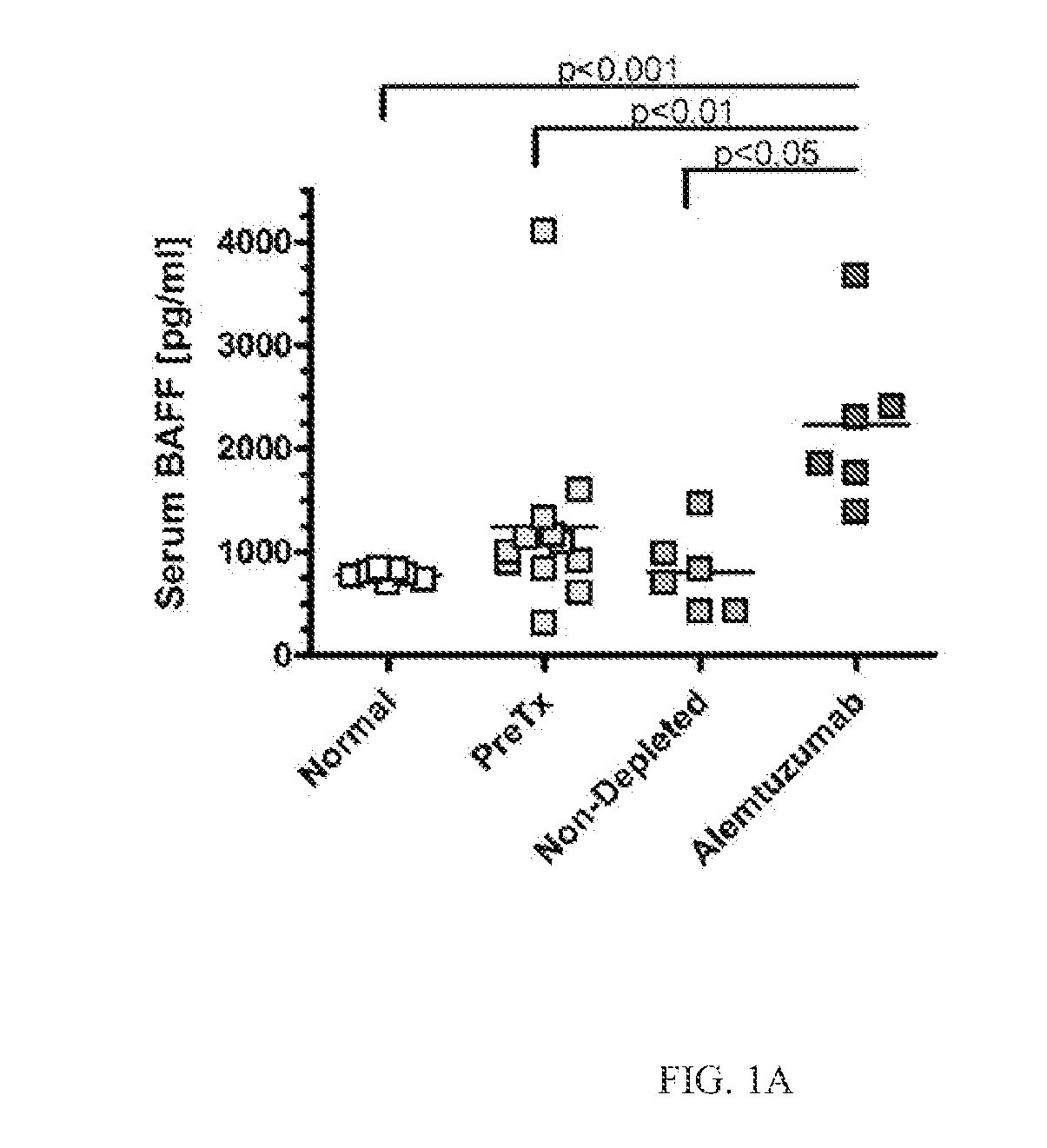
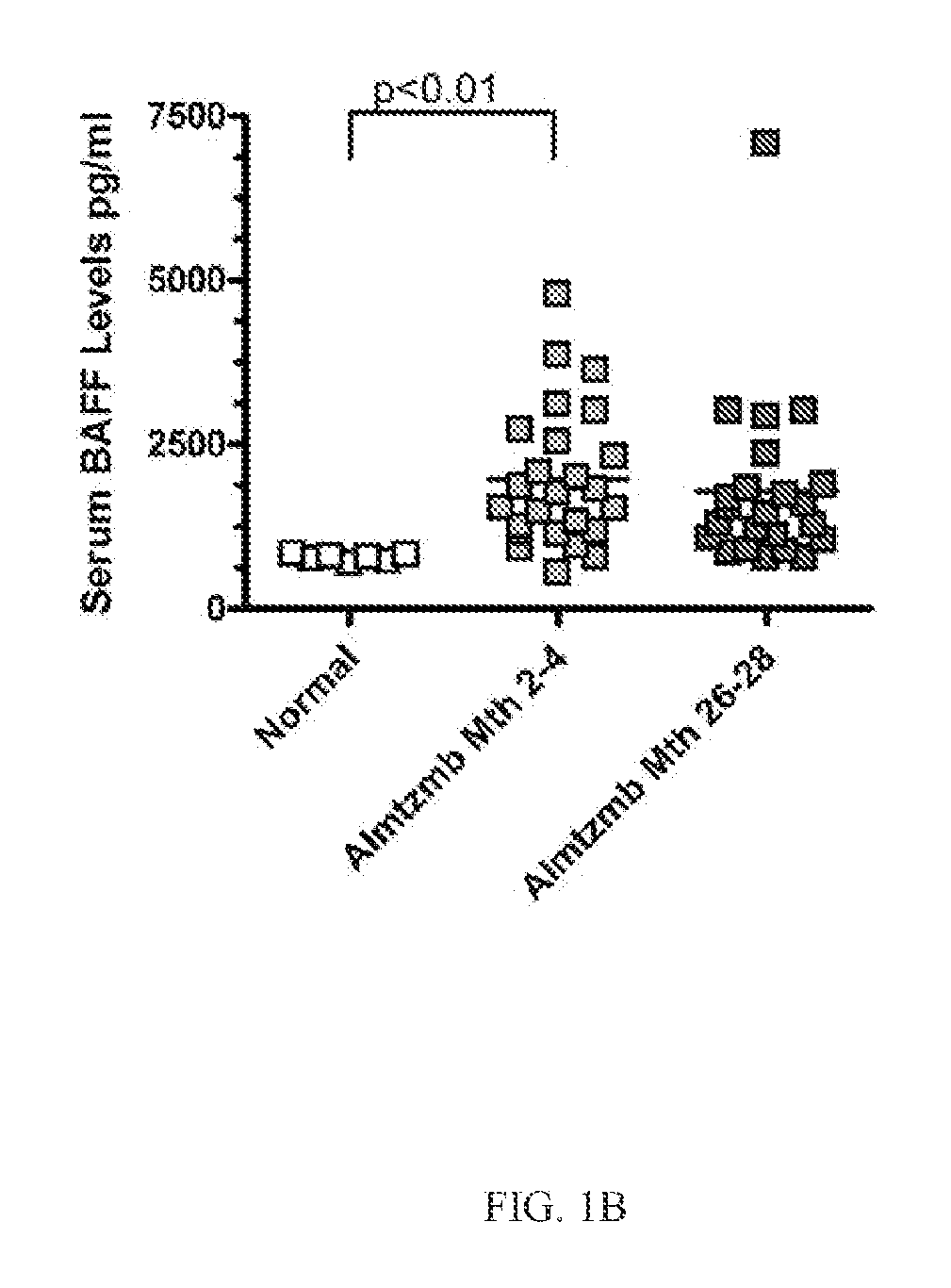
Detection of b-cell activating factor as a biomarker for antibody mediated rejection in transplant recipients
ActiveUS20120264142A1Reduce the possibilityDisease diagnosisImmunological disordersAntibody mediated rejectionBiomarker (petroleum)
The invention relate to methods, compositions, and kits for detection of biomarkers. hi one embodiment, the invention relates to a method for detecting AMR biomarkers in a biological sample. In another embodiment, the invention relates to a method for detecting, monitoring, diagnosing and predicting antibody mediated rejection. In yet another embodiment, the invention relates to a method for monitoring a subject for antibody mediated rejection comprising detecting BAFF or a BAFF variant in a biological sample. In still another embodiment, the invention relates to a kit for detecting BAFF in a urine sample.
Owner:WISCONSIN ALUMNI RES FOUND
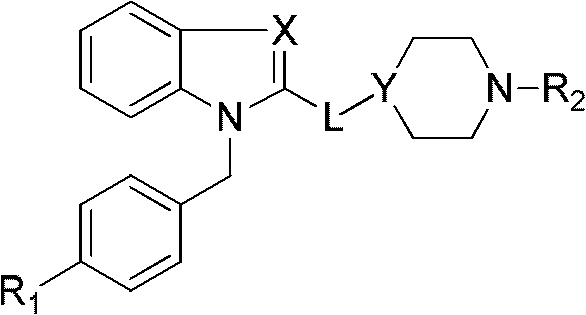
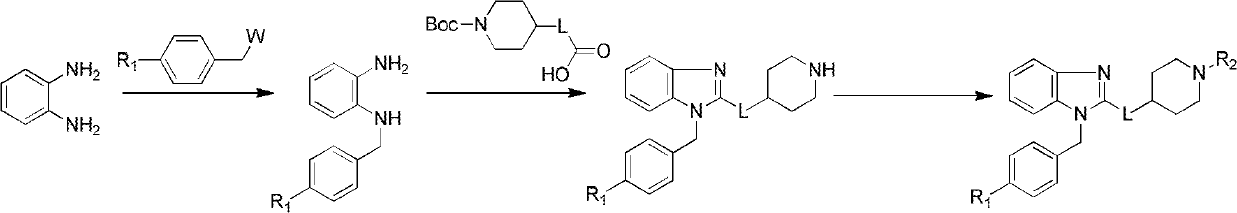
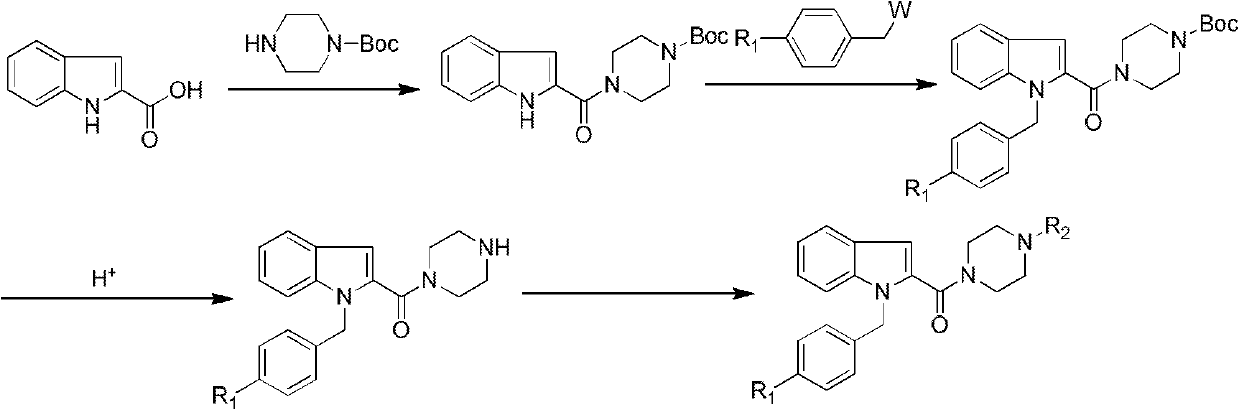
Benzoheterocycle compound and preparation method as well as medical application thereof
ActiveCN102627631AAntagonistic activityStrong resistanceOrganic active ingredientsOrganic chemistryAllergic pharyngitisAllergic asthma
The invention relates to the field of pharmaceutical chemistry, in particular to a benzoheterocycle compound and a preparation method, a pharmaceutical preparation comprising same as well as medical application thereof. Pharmacological experiments prove that the compound has a strong antagonistic effect on H1 receptors; and a part of compounds have certain antagonistic activity to the PAF (Platelet Activating Factor) receptors. The type of compounds and the medical preparations thereof can be used for preparing medicaments for treating a series of diseases caused by allergy, such as allergic asthma, allergic rhinitis, allergic pharyngitis, urticaria or eczema.
Owner:CHINA PHARM UNIV +1
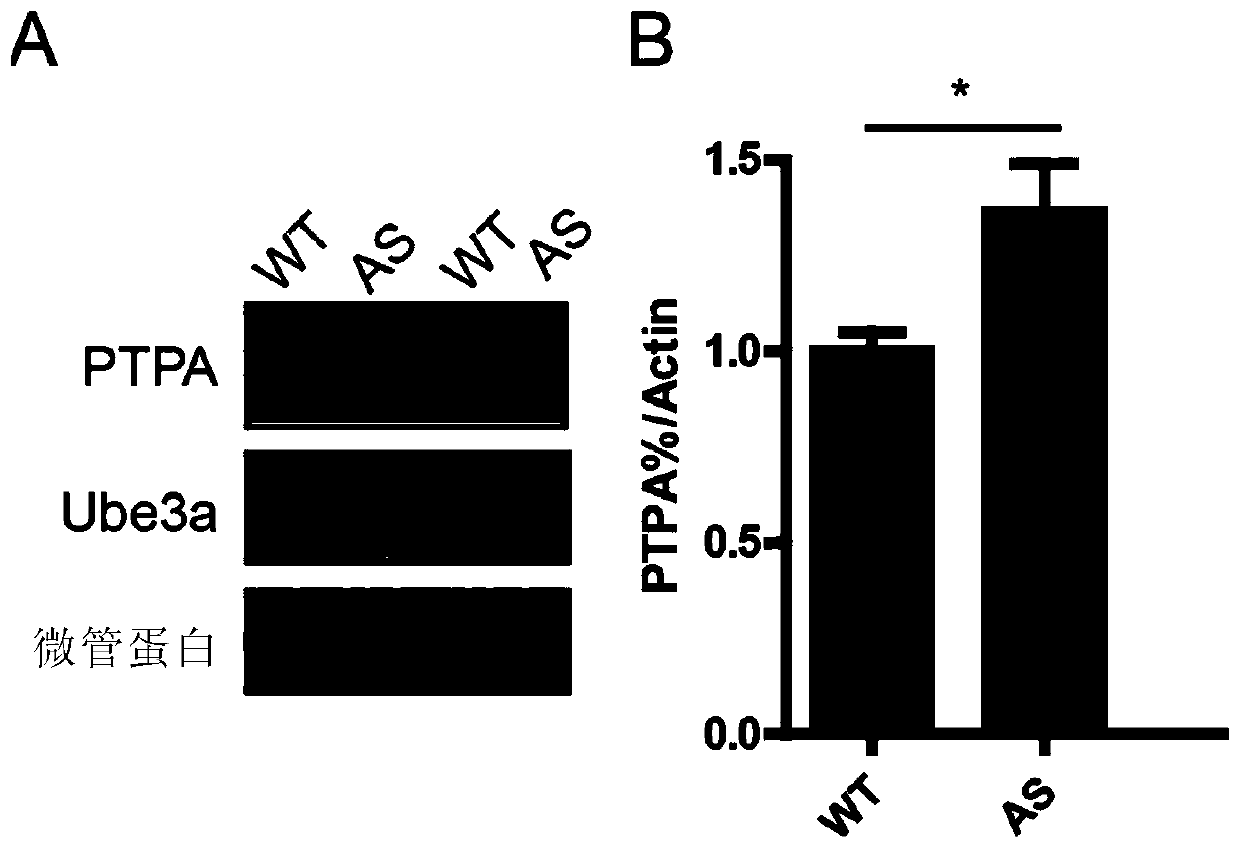
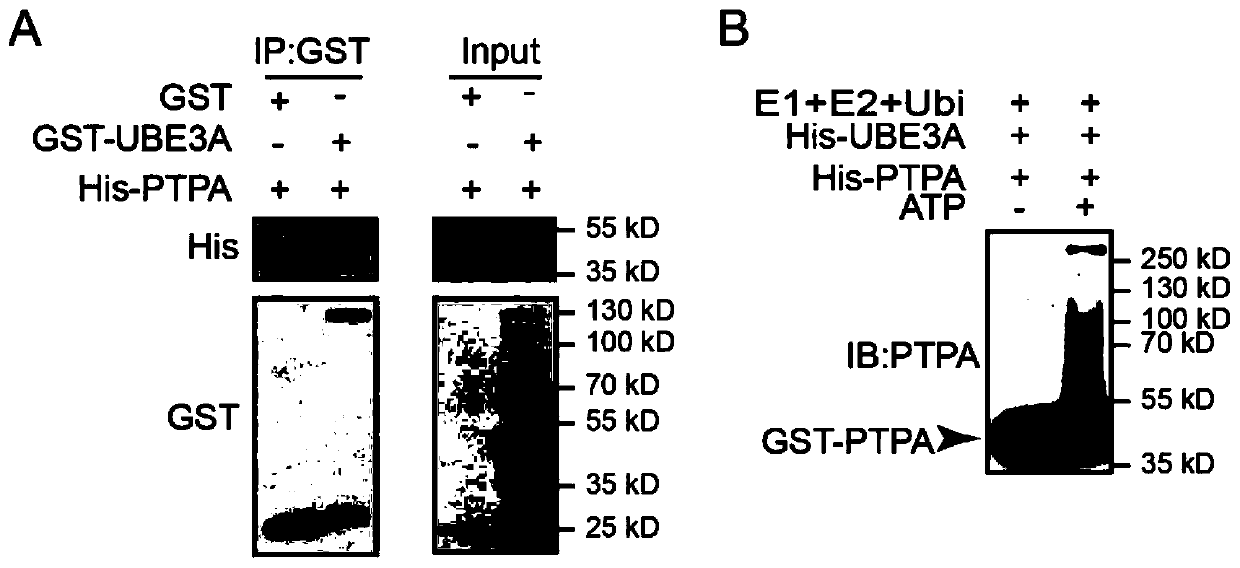
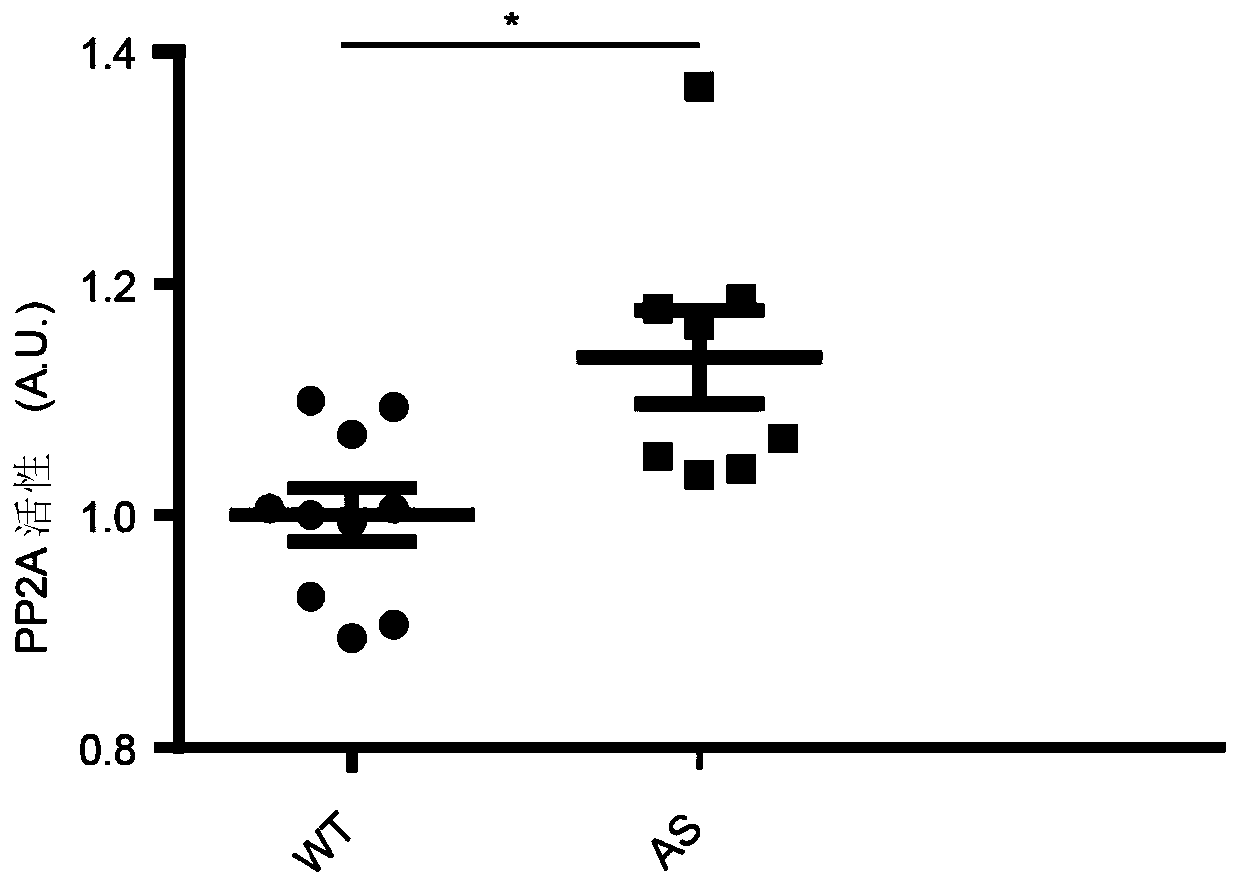
Application of Ube3a-ubiquitinated protein phosphatase 2A (PP2A) activating factor, namely tyrosine phosphatase activating factor (PTPA), for treating Angelman syndrome and autism
The invention relates to application of an Ube3a-ubiquitinated protein phosphatase 2A (PP2A) activating factor, namely a tyrosine phosphatase activating factor (PTPA), for treating Angelman syndrome and autism. The invention discloses new pathogenesis of Angelman syndrome and / or autism or other related diseases; that is to say, lack or over-expression of Ube3a results in abnormal activity of the tyrosine phosphatase activating factor (PTPA) on the protein phosphatase 2A (PP2A). Thus, the factors can be used as targets for developing drugs to alleviate or treat Angelman syndrome and / or autism or other related diseases. The factors can also be used as markers for diagnosing and assessing Angelman syndrome and / or autism or other related diseases.
Owner:CENT FOR EXCELLENCE IN BRAIN SCI & INTELLIGENCE TECH CHINESE ACAD OF SCI
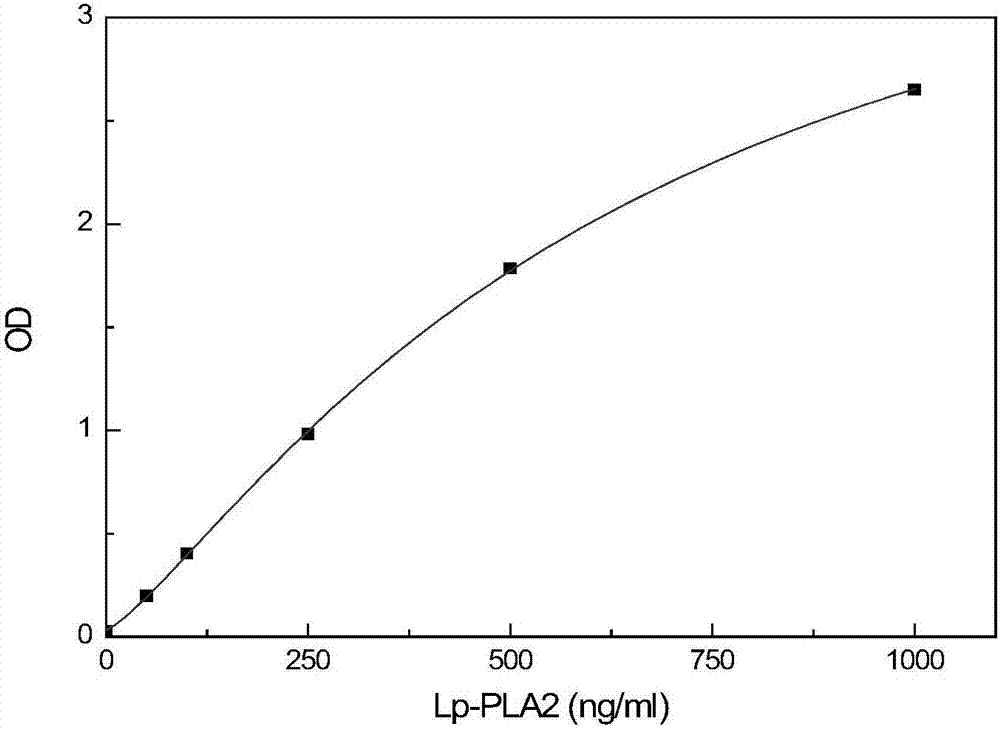
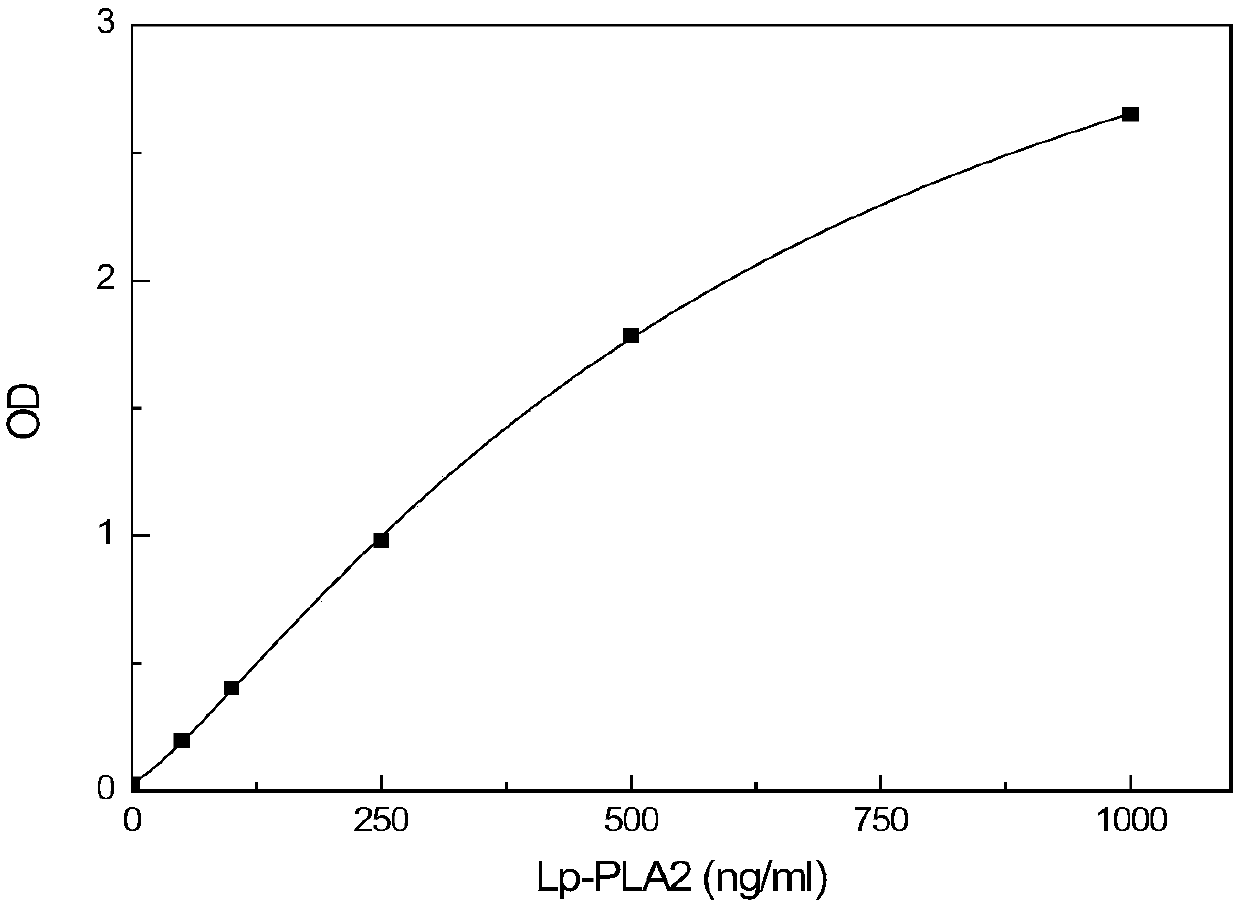
Detection kit for activity and total amount of lipoprotein-associated phospholipase Lp-PLA2 and preparation method thereof
ActiveCN106872687AAccurate judgmentModified Enzyme AssayColor/spectral properties measurementsBiological testingAntigenPhospholipase
The invention discloses a detection kit and a detection method for the activity and the total amount of lipoprotein-associated phospholipase Lp-PLA2. The detection kit is prepared from a solid-phase carrier, a zymolytic substrate, a buffer solution, an enzyme labeled antibody reagent, a cleaning solution, a chromogenic substrate, a stop solution, an enzymatic activity calibration substance and an enzymatic calibration-free substance, wherein the enzyme-linked immunosorbent assay plate is coated with an anti-Lp-PLA2 specific N-terminal non-inhibiting antibody; the zymolytic substrate is a platelet activating factor, a platelet activating factor analogue or phosphatidylcholine modified by the platelet activating factor analogue in an oxidative manner; the enzyme labeled antibody reagent is horseradish peroxidase or alkaline phosphatase or a biotin labeled anti-Lp-PLA2 inhibiting antibody; the enzymatic activity calibration substance comprises multiple concentrations of paranitrophenol standard substances; the enzymatic calibration-free substance comprises multiple reference calibration substances containing Lp-PLA2 antigens. By using the detection kit, the inference of other matter in a sample can be avoided; the in-vivo condition of the Lp-PLA2 can be more accurately reflected; the more accurate judgment basis is provided for clinic.
Owner:海格德生物科技(深圳)有限公司
Maturation of mammalian hepatocytes
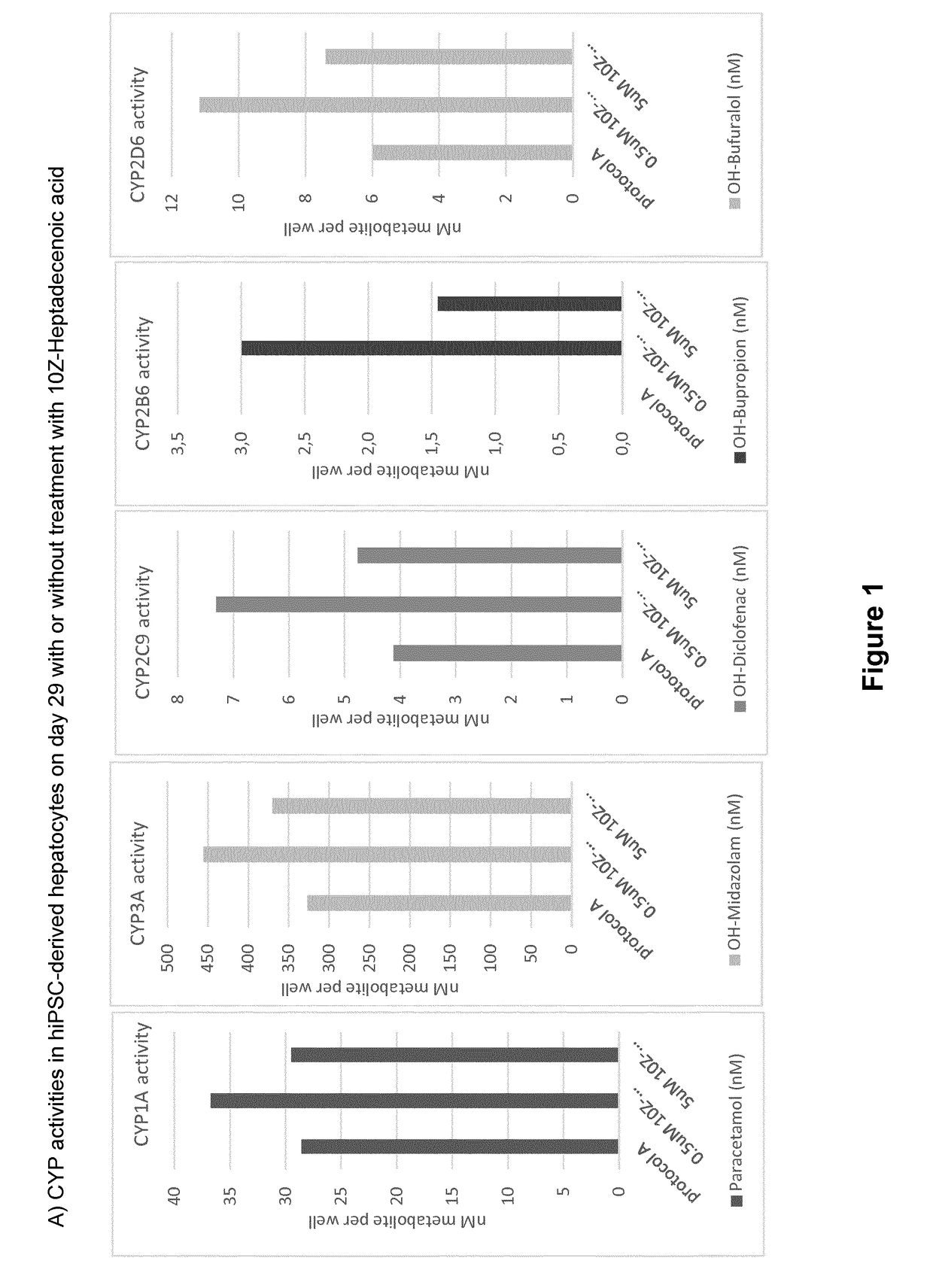
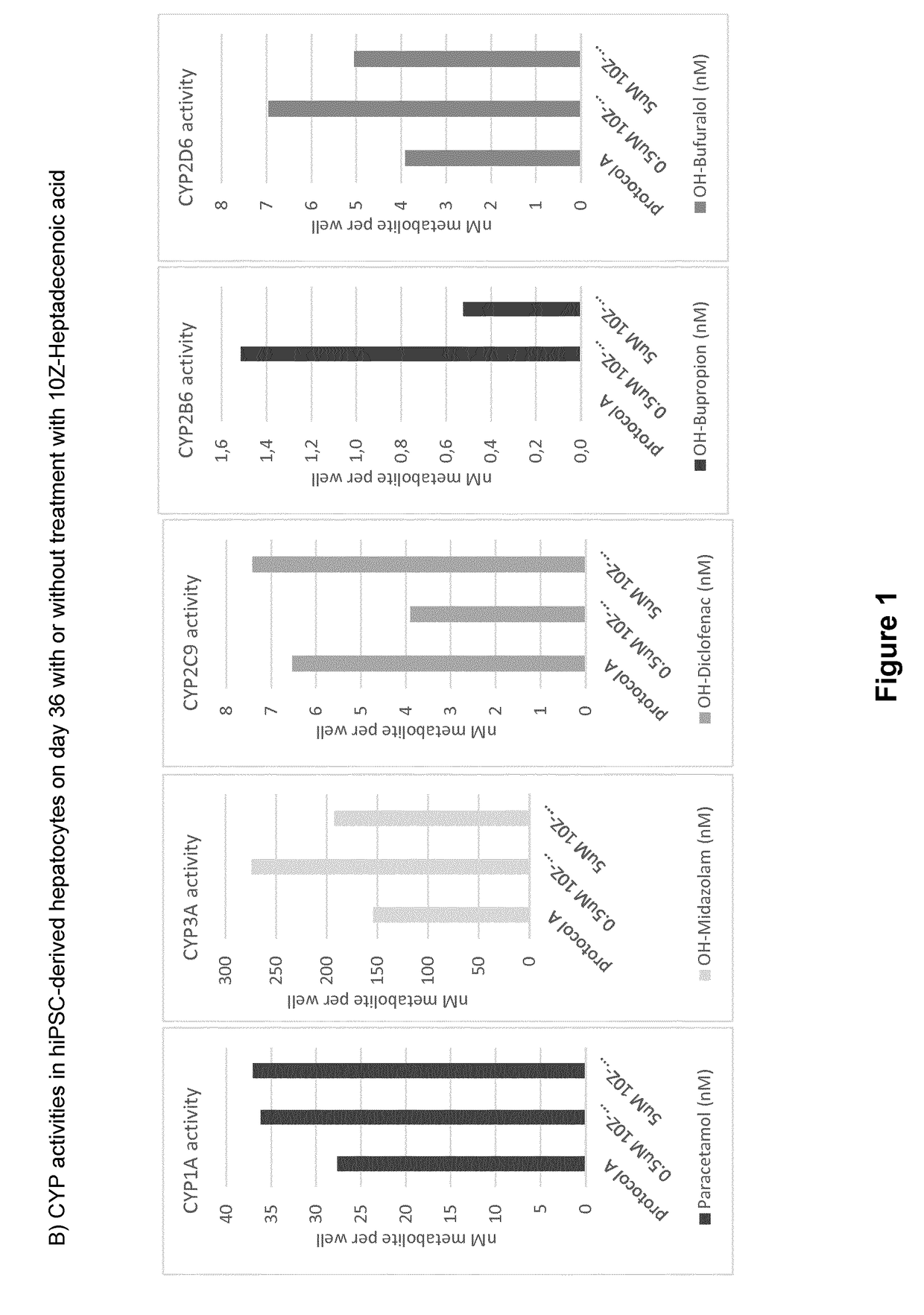
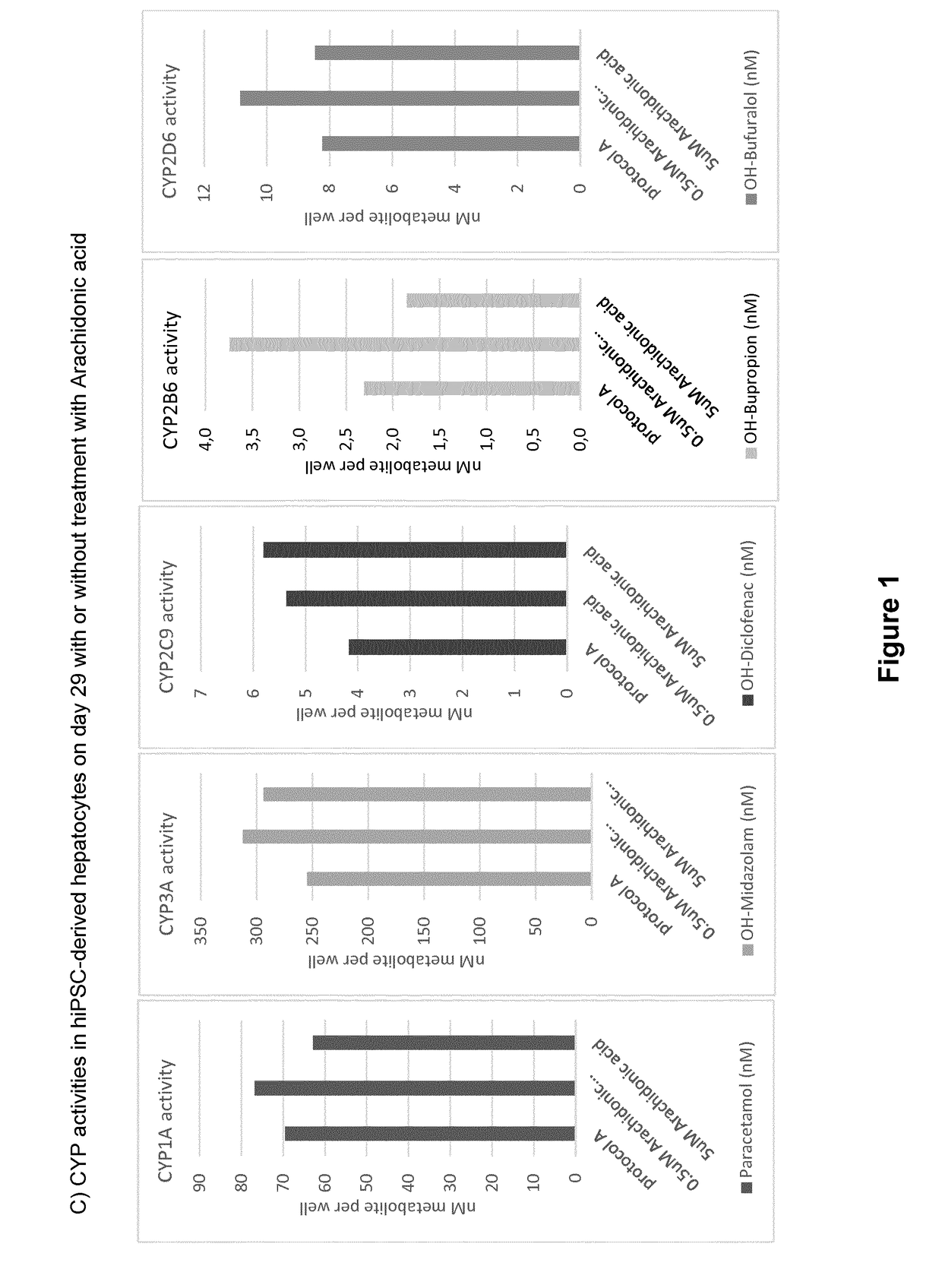
ActiveUS20190024044A1Improve the level ofRegulating trafficCulture processArtificial cell constructsMetaboliteDirected differentiation
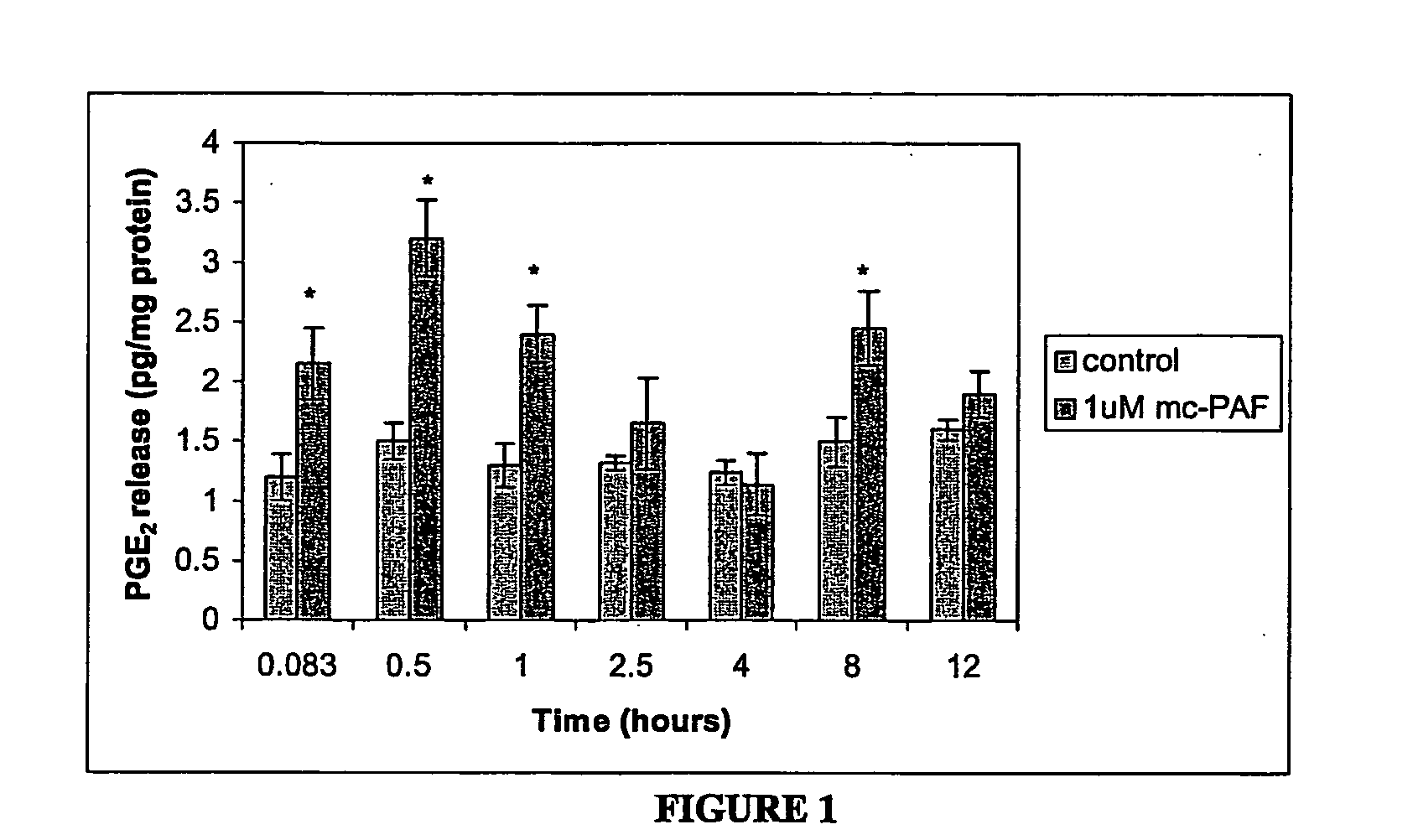
The present invention relates to directed differentiation and maturation of mammalian hepatocytes, such as human hepatocytes. The hepatocyte obtained in accordance with the present invention show a phenotype which is more similar to that of primary hepatocytes than previously shown. In particular, the present invention relates to exposure of mammalian hepatocytes, such as human hepatocytes, to at least one maturation factor selected from the group consisting of Src kinase inhibitors, vitamin D including precursors, metabolites and analogs thereof, hypoxia inducing compounds, sphingosine and sphingosine derivatives, activators of peroxisome proliferator-activated receptors (PPARs), platelet-activating factor (PAF), PKC inhibitors, and combinations thereof.
Owner:TAKARA BIO EURO
Rupatadine fumarate compound
The invention provides a rupatadine fumarate compound which has dual effects of antihistamine and antagonistic platelet activating factor (PAF). According to the research, allergy and inflammatory diseases are multi-factor complex processes caused by generation and release of various different media; histamine is the most inflammatory medium appearing in allergy early symptom, and the disease symptoms including sneezing, rhinocnesmus, tearing, running nose, skin itch, wheal and the like are mostly caused by histamine H1 receptor. And PAF also can cause bronchial constriction and increased permeability of the vessel, so that running nose, nasal congestion, wheal and itch are caused; meanwhile, the PAF is also a main cause of asthma. The antiallergic drug which is clinically used only has an antihistamine activity effect, but does not have a PAF antagonistic effect.
Owner:海思科制药(眉山)有限公司
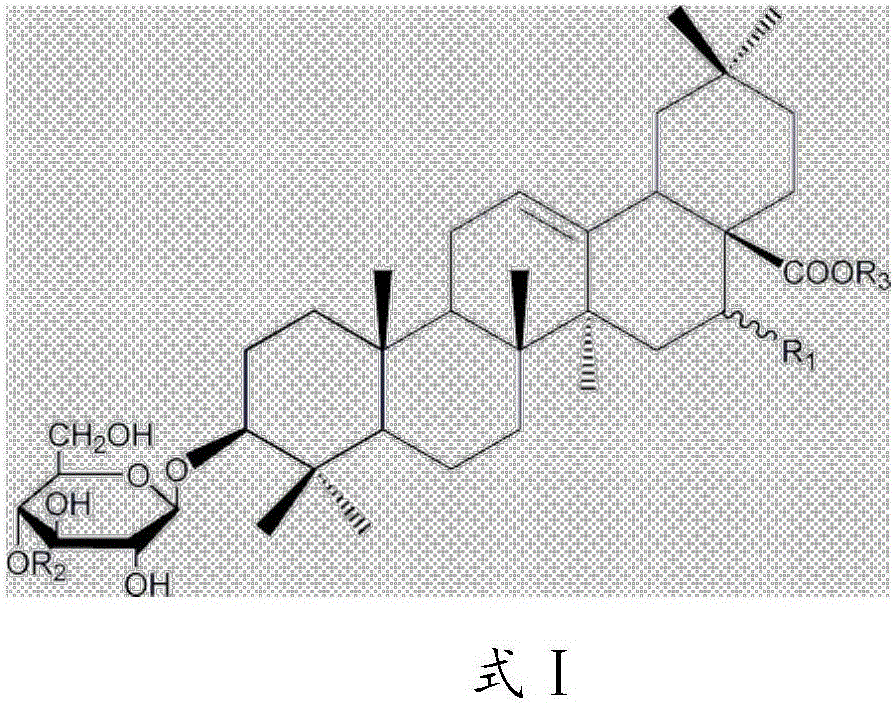
Application of triterpenoid saponins compound
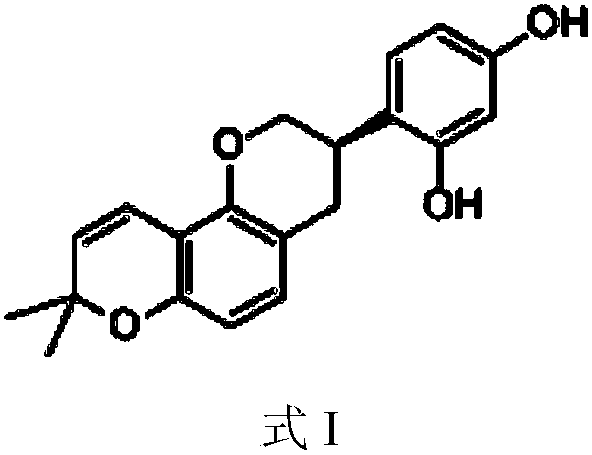
ActiveCN106344599AReduce infiltrationInhibition of activationOrganic active ingredientsRespiratory disorderPlatelet-activating factor receptorCSF secretion
The invention relates to the technical field of medicines and in particular relates to application of a triterpenoid saponins compound. The invention provides application of the triterpenoid saponins compound with a structure formula I to preparation of a medicine for treating asthma. An experiment testifies that the triterpenoid saponins compound with the structure formula I has an obvious inhibition effect on a platelet activating factor receptor and can be used for alleviating tachypnea and accelerated frequency, caused by the asthma, and remarkably reducing the amount of Eos (Eosinophile granulocyte) in rat blood and reducing infiltration on trachea by the Eos; meanwhile, the secretion of GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor) in BALF (Bronchoalveolar Lavage Fluid) of guinea pigs can be inhibited and a PAF (Platelet-Activating Factor) level of blood serum of the guinea pigs is remarkably reduced, and the activation of inflammatory cells and the releasing of inflammatory transmitters are stopped, so that inflammation of the trachea is alleviated and smooth muscles of bronchus are relaxed; the high responsiveness of the trachea is reduced to treat the asthma, so that the triterpenoid saponins compound has the effect of treating the asthma and application value.
Owner:JIANGSU KANION PHARMA CO LTD
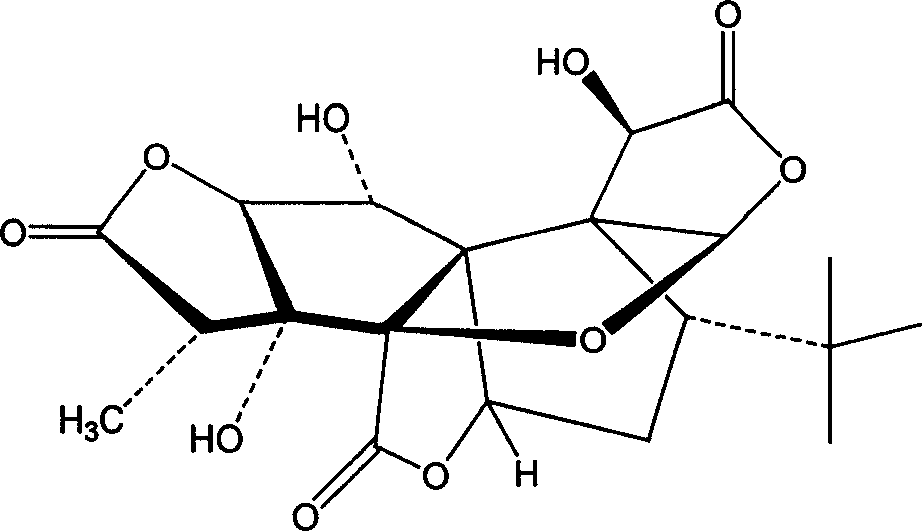
Ginkgolide B derivative and its salt, and their preparation methods and uses
The invention belongs to the technical field of medicine and relates to a derivative of ginkgolide B structure, shown as the formula 1 and formula 2, having a carboxylic group introduced to a hydroxylgroup 10, an ester derivative with the carboxylic group, and an organic or inorganic salt pharmaceutically acceptable. By applying chemical structural modifications to ginkgolide B as a matrix, so that solubility is improved, bioavailability is improved, and therapeutic effect is enhanced; the prepared compound and its carboxylate have evident antagonistic action upon the platelet activating factor, evident anticoagulant action and evident resistance to acute cerebral ischemia, and are applicable to the preparation of drugs to prevent and treat ischemic stroke, thrombosis, angina, cardiopulmonary infarct, inflammations, asthma, and other diseases associated with the platelet activating factor.
Owner:FUDAN UNIV
Application of hydroxyl carthamin yellow A in preparation of drugs for treating pyaemia
The invention belongs to the field of medicines and relates to new use of hydroxyl carthamin yellow A in treatment of pyaemia through inhibiting occurrence and development of the pyaemia. The invention particularly relates to application of the hydroxyl carthamin yellow A in treatment of the pyaemia through lowering expression of high-mobility group protein B1 (HMGB1) of late stage inflammatory factor, lowering release of tissue factor (TF) and platelet activating factor (PAF) and lowering expression levels of cytotoxin T lymphocyte related antigen 4 (CTLA-4) and fork head box protein transcription factor P3 (Foxp3) of regulatory T cells (Treg).
Owner:TIANJIN CHASE SUN PHARM CO LTD
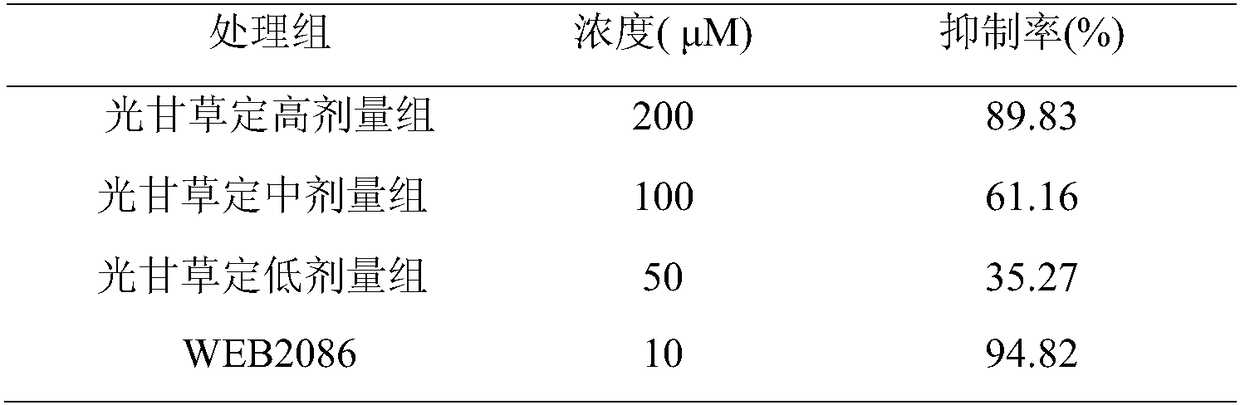
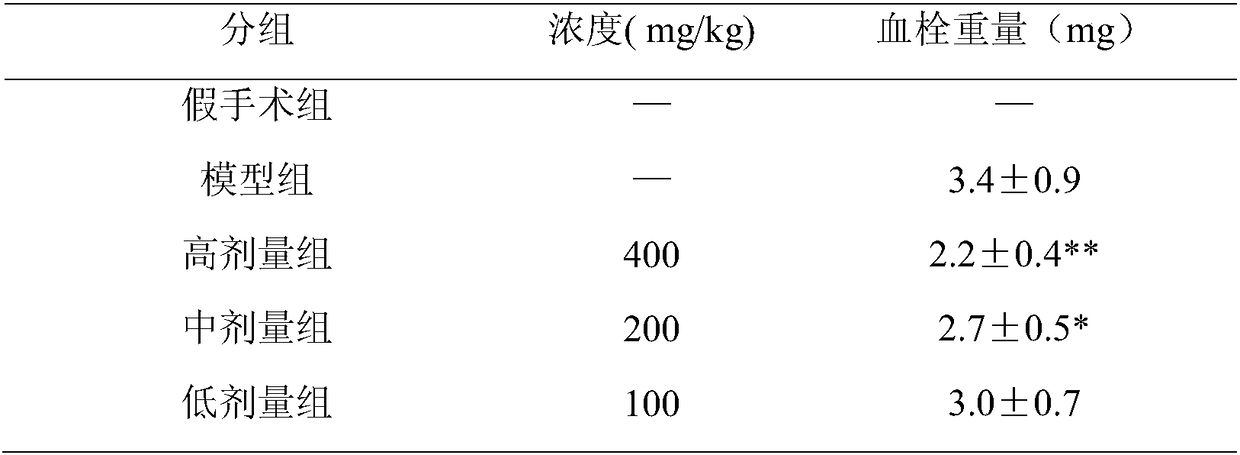
Application of glabridin in preparation of drugs to treat thrombotic diseases
InactiveCN108785299AOrganic active ingredientsBlood disorderP-selectinPlatelet-activating factor receptor
The invention discloses application of glabridin in preparation of drugs to treat thrombotic diseases and belongs to the technical field of medicine. Experiments show that glabridin can significantlyinhibit platelet activating factor receptors, and can significantly reduce, in vivo, p-selectin, vWF, GPIIb / IIIa and TXB2 levels in serum of a thrombosis model rat due to external application of FeCl3solution. venous injection of glabridin can significantly inhibit the rise of whole blood PAF (platelet activating factor) concentration induced by artery thrombosis and can significantly reduce IP3content in platelets and the expression of platelet IP3 receptors; it is indicated that glabridin can inhibit platelet PAF-IP3-IP3R-[Ca2+]i signal transduction pathway, and the inhibition is one of mechanisms of glabridin to inhibit platelet activating and gathering. Glabridin can inhibit thrombosis via multiple targets and multiple pathways, and is functional in and worthy of treating thromboticdiseases.
Owner:JIANGSU KANION PHARMA CO LTD
Treatment of disease with n-acetyl kynurenine
The invention provides a method, composition and kit for treating T-cell mediated diseases, degenerative joint diseases or diseases mediated by platelet activating factor (PAF) comprising administering to an animal in need thereof, an effective amount a pharmaceutical composition containing N-acetyl-kynurenine (NAK) or pharmaceutically acceptable salts thereof as the active ingredient.
Owner:AMPIO PHARMA
Method of diagnosing cardiovascular disease
InactiveUS7662577B2Improve accuracyBiocidePhosphorous compound active ingredientsVascular diseasePlatelet
A method of and kit for diagnosing cardiovascular disease in a human involves assessing the presence and / or concentration of antibodies to platelet activating factor (PAF) in a sample of body fluid of the human.
Owner:ATHERA BIOTECH
Application of Piperlongumine compounds for resisting platelet aggregation
The invention relates to application of Piperlongumine compounds for resisting platelet aggregation, in particular to application of the Piperlongumine compounds which are used for preparing medicines and health care products for controlling thrombus or cardiovascular and cerebrovascular diseases. Experimental results indicate that the Piperlongumine compounds have remarkable effects on the platelet aggregation initiated by collagen, arachidonic acid and platelet activating factors, and the inhibition efficiency of the Piperlongumine compounds is superior to aspirin and also in a linear relationship with the compound concentration; thereby indicating that the Piperlongumine compounds have very wide prospects as a category of medicines or health care products for resisting the thrombus or controlling the cardiovascular and cerebrovascular diseases.
Owner:合肥华纳生物医药科技有限公司
Application of FGFR2b inhibiting molecule in preparation of drug for treating PAF-mediated disease
PendingCN111760026AImprove fibrosisInhibit the inflammatory responseAntibacterial agentsNervous disorderDiseasePhosphorylation
The invention discloses application of a FGFR2b inhibiting molecule in preparation of a drug for treating a PAF-mediated disease. The application is a research result obtained on the basis of that aninventor of the invention discovers that FGF-7 can induce generation of a platelet activating factor PAF. The inventor of the invention discovers that the rising of a level of a proinflammatory factorcan be induced after the PAF is generated, then, an inflammatory reaction is initiated, a molecule inhibiting bonding between the FGF-7 and FGFR2serves as a competitive antagonist of FGF7 and is bonded to a specific receptor FGFR2b of the FGF7 so as to prevent the FGFR2b from being activated into phosphorylated FGFR2b, and an excessive inflammatory reaction can be effectively inhibited, so that ARDS symptoms are treated, and lung fibrosis can be improved.
Owner:汪炬
Lipoprotein-associated phospholipase lp-pla2 activity and total amount detection kit and preparation method thereof
ActiveCN106872687BAccurate judgmentMicrobiological testing/measurementColor/spectral properties measurementsAntigenPhospholipase
The invention discloses a detection kit and a detection method for the activity and the total amount of lipoprotein-associated phospholipase Lp-PLA2. The detection kit is prepared from a solid-phase carrier, a zymolytic substrate, a buffer solution, an enzyme labeled antibody reagent, a cleaning solution, a chromogenic substrate, a stop solution, an enzymatic activity calibration substance and an enzymatic calibration-free substance, wherein the enzyme-linked immunosorbent assay plate is coated with an anti-Lp-PLA2 specific N-terminal non-inhibiting antibody; the zymolytic substrate is a platelet activating factor, a platelet activating factor analogue or phosphatidylcholine modified by the platelet activating factor analogue in an oxidative manner; the enzyme labeled antibody reagent is horseradish peroxidase or alkaline phosphatase or a biotin labeled anti-Lp-PLA2 inhibiting antibody; the enzymatic activity calibration substance comprises multiple concentrations of paranitrophenol standard substances; the enzymatic calibration-free substance comprises multiple reference calibration substances containing Lp-PLA2 antigens. By using the detection kit, the inference of other matter in a sample can be avoided; the in-vivo condition of the Lp-PLA2 can be more accurately reflected; the more accurate judgment basis is provided for clinic.
Owner:海格德生物科技(深圳)有限公司
A kind of preparation method of Ginkgolide L
A ginkgolide type compound which serves as a powerful antagonist of platelet activating factors (PAF) has very good protective effects on focal cerebral ischemia and diffuse total cerebral ischemia. Ginkgolide is divided into ginkgolide A, ginkgolide B, ginkgolide C, bilobalide, ginkgolide K, ginkgolide L and the like. The content of ginkgolide L in ginkgo leaves is extremely low, so that ginkgolide L is difficult to obtain, and research report on ginkgolide L is nearly zero. The invention relates to a preparation method of ginkgolide L. Ginkgolide L is extracted from commercially available ginkgo total lactone or ginkgo total lactone obtained based on a known method. The preparation method is simple to operate, the raw materials are low in cost, a plenty of ginkgolide L can be obtained rapidly, and basis is provided for research on ginkgolide L.
Owner:CHINA PHARM UNIV
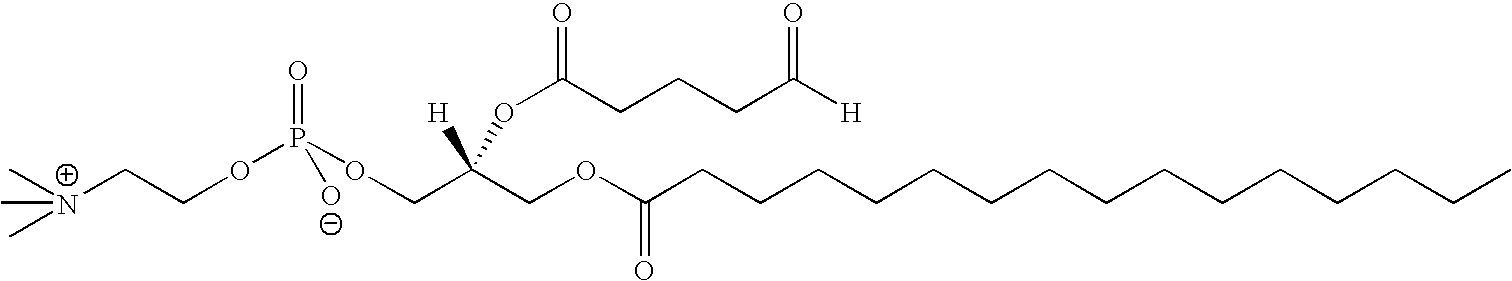
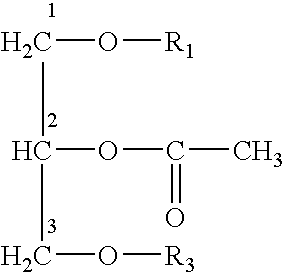
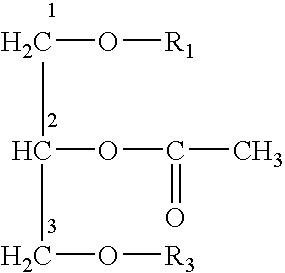
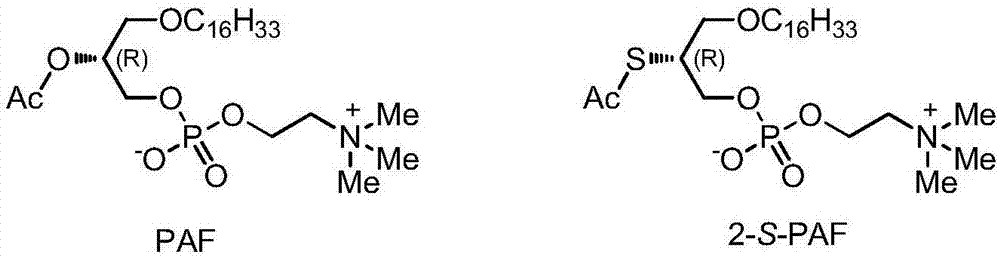
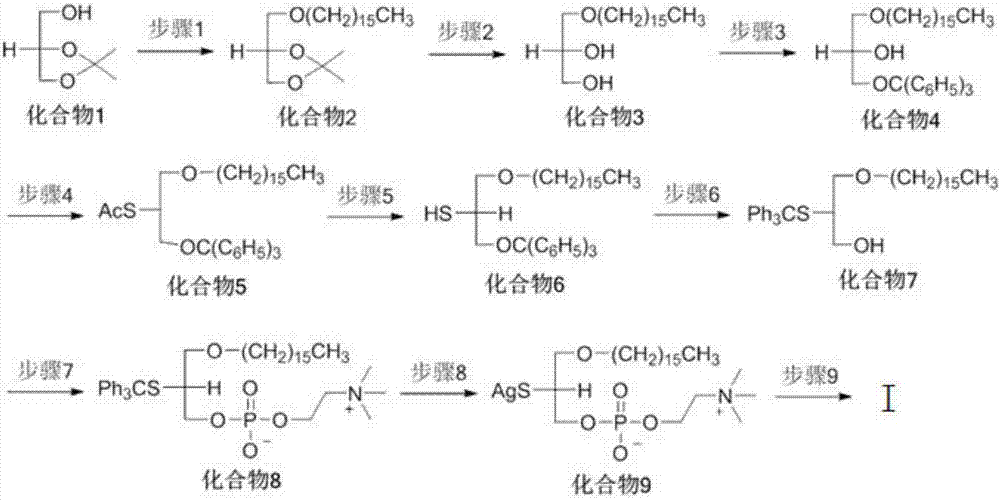
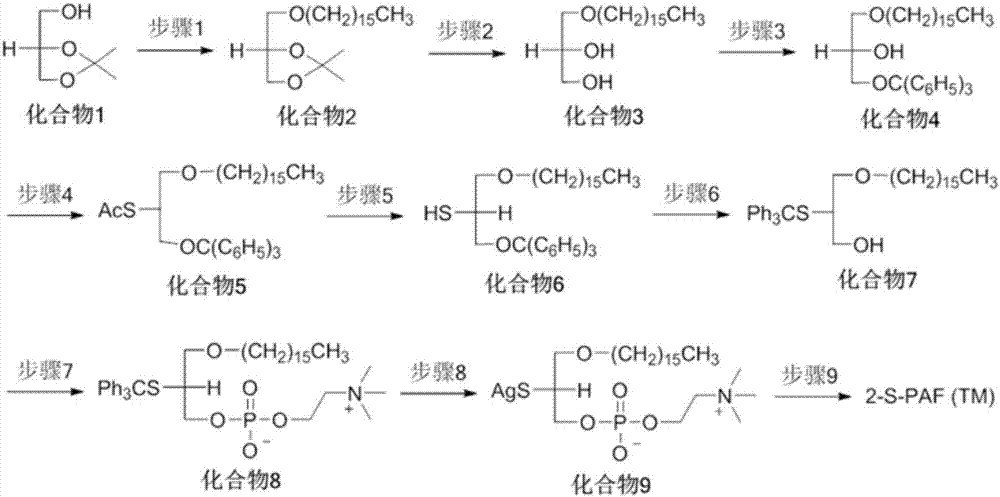
Platelet activating factor derivative and synthesis method thereof
InactiveCN106967113ASolve yieldSettle the priceGroup 5/15 element organic compoundsPhosphatide foodstuff compositionsChemical synthesisSynthesis methods
The invention belongs to the technical field of chemical synthesis and discloses platelet activating factor derivatives with the structure of formula (I), wherein the substituent R represents an acyl group. Its synthetic route uses the acetonide of the chiral source S configuration as the initial reactant. After 9 steps of reaction, the present invention adopts this method for the first time to synthesize 2-thioplatelet activating factor and its derivatives, with high yield and easy advantages of separation. The invention realizes the low-cost and large-scale synthesis of 2-S-PAF and its derivatives, and is of great significance to the activity research, biological experiment, clinical application and disease treatment of 2-S-PAF and its derivatives.
Owner:NORTHWEST A & F UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com