Treatment of disease with n-acetyl kynurenine
a technology of n-acetyl kynurenine and n-acetyl kynurenine, which is applied in the direction of organic active ingredients, drug compositions, immunological disorders, etc., can solve the problems that the presence of n-acetyl kynurenine in low molecular weight fractions of human serum albumin has not been previously recognized, and no biological activity of n-acetyl kynurenine has
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
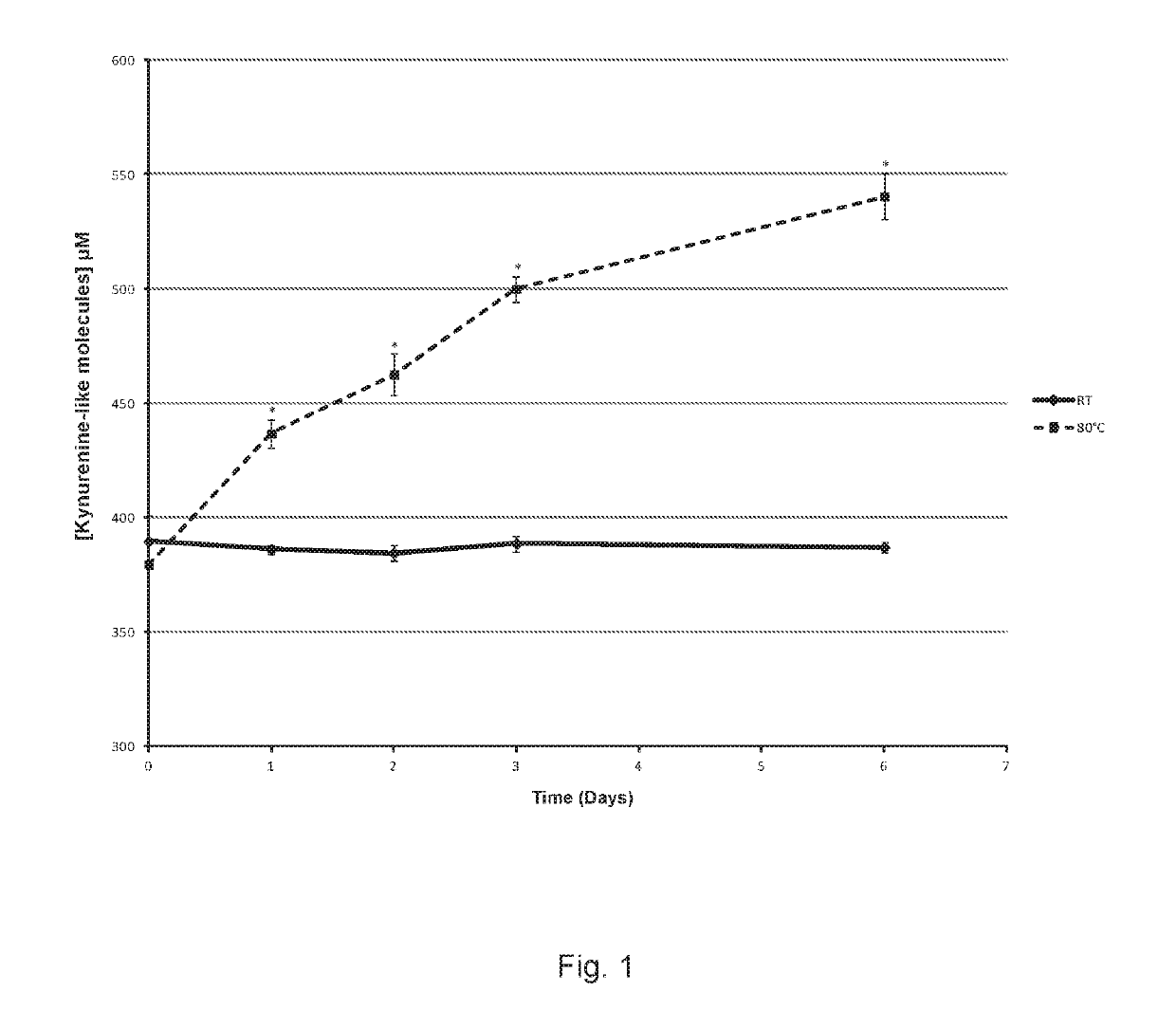
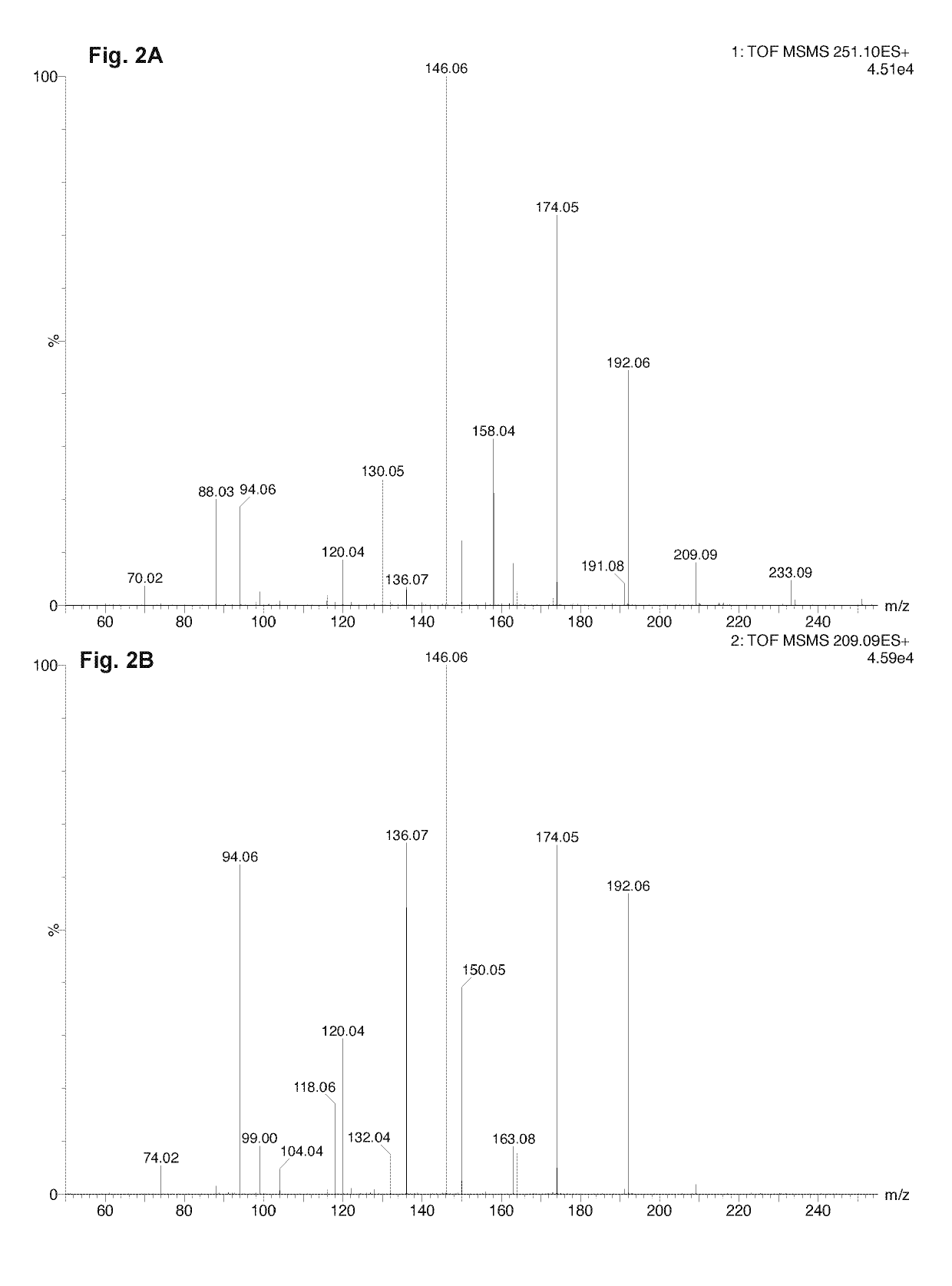
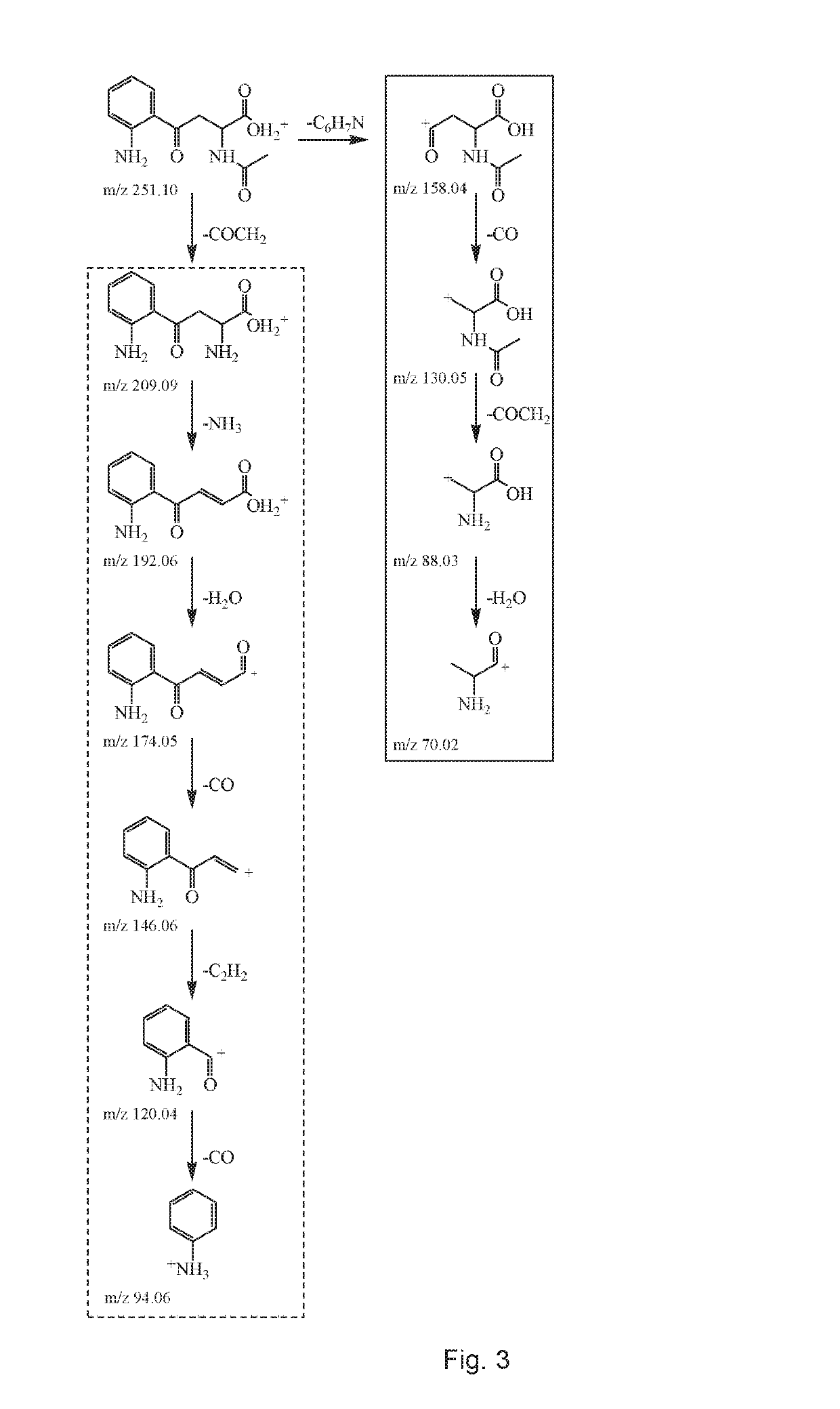
[0072]This example characterizes LMWF5A using liquid chromatography tandem-mass spectrometry (LCMS-MS) and other techniques to identify non-enzymatic breakdown products of N-acetyl tryptophan (NAT). Thermal forced degradation conditions were also applied in order to increase the amount of NAT breakdown products to improve detection and identification.
[0073]The excipient NAT is added at a concentration of 4 mM to 5% commercial solutions of human serum albumin (HSA) for the purpose of stabilizing the protein during the pasteurization process. It has been postulated that NAT provides a protective effect on the free sulfhydryl group (Cys-34) of HSA thereby diminishing protein oxidation. In the study below, the low molecular weight fraction of 5% HSA (LMWF5A) was shown to contain breakdown products of NAT. This is the first study to specifically describe the presence of N-acetyl kynurenine in a solution derived from commercially available HSA. In summary, breakdown products of NAT were d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com