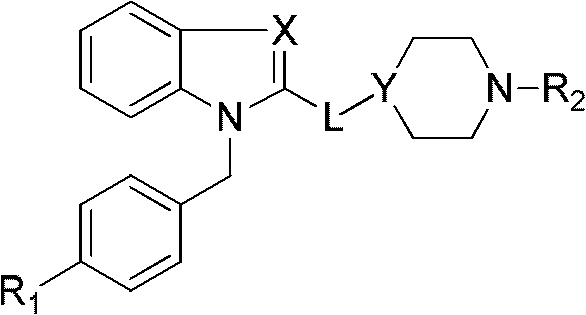
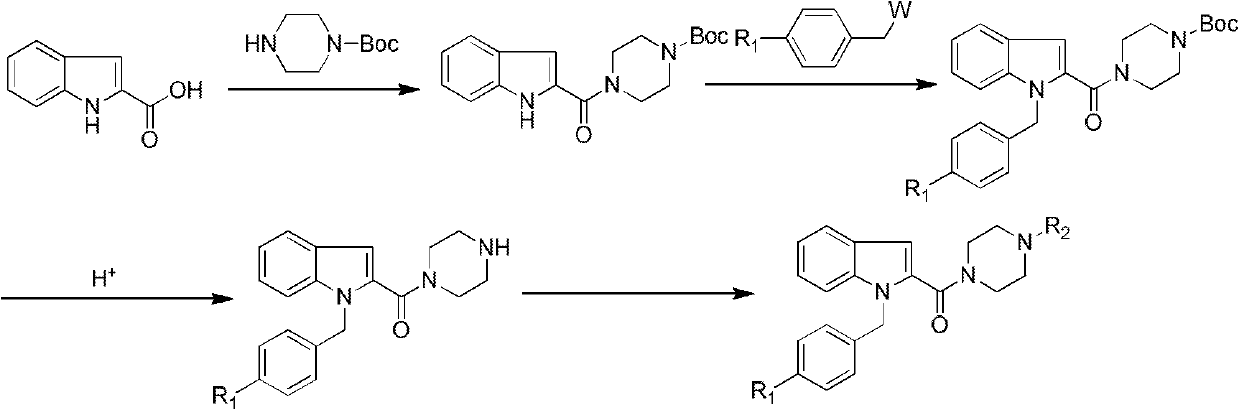
Benzoheterocycle compound and preparation method as well as medical application thereof
A compound and application technology, applied in the field of medicinal chemistry, to achieve the effects of simple operation and post-treatment, mild reaction conditions, and abundant raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1-(4-Chlorobenzyl)-2-(4-piperidine)benzimidazole (CPUYW01)
[0045]
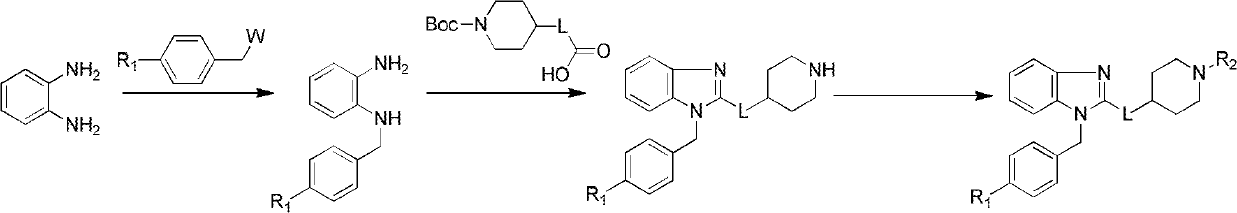
[0046] (1) Preparation of N-(4-chlorobenzyl)-o-phenylenediamine
[0047] O-phenylenediamine (1.1g, 10mmol), p-chlorobenzyl chloride (1.6g, 10mmol), K 2 CO 3 3g was put into the reaction flask in turn, 10mL of DMF was added, heated to 40°C and stirred for 3h. After the reaction was completed, the reaction solution was cooled to room temperature and poured into water, extracted twice with dichloromethane (2×20mL), washed 4 times with water, and anhydrous Na 2 SO 4 Drying and separation by column chromatography (PE:EA=8:1) gave 2.1 g of a brown solid, yield 91%, m.p.74-76°C.
[0048] (2) Preparation of N-Boc-4-piperidinecarboxylic acid
[0049] Dissolve piperidine-4-carboxylic acid (1.3g, 10mmol) in (v:v=1:2) tetrahydrofuran and 1N NaOH mixed solution 15mL, add Boc 2 O (2.4g, 11mmol), after reacting at room temperature for 2h, the liquid was separated, and the pH value was adjusted to 5-6 with 1...
Embodiment 2
[0053] 1-(4-fluorobenzyl)-2-(4-piperidine)benzimidazole (CPUYW02)
[0054]
[0055] (1) Preparation of N-(4-fluorobenzyl)-o-phenylenediamine
[0056] Prepared according to step 1 in Example 1, substituting p-fluorobenzyl bromide for p-chlorobenzyl chloride, to obtain 1.8 g of brown solid, yield 82%, m.p.78-80°C.
[0057] (2) Preparation of title compound
[0058] Prepared according to step 3 of Example 1, the target compound was obtained as a yellow solid 1.1 g, yield 32%, m.p.186-187°C. 1 H NMR (CDCl 3 , 300MHz, δppm) 1.81-2.05(m, 5H, -NH), 2.67-2.76 (dt, 2H, J=12.6Hz, J=2.7Hz, ), 2.90-3.00 (m, 1H, ), 3.21-3.25 (d, 2H, J=12.6Hz, ), 5.34(s, 2H, ), 6.99-7.02(d, 4H, J=6.3Hz, Ar-H), 7.19-7.29(m, 3H, Ar-H), 7.79-7.81(d, 1H, J=7.8Hz, Ar-H) HRMS calcd for C 19 h 20 N 3 F[M+H] + : 310.1714; found: 310.1718IR(KBr): 3382, 2935, 1652, 822
Embodiment 3
[0060] 1-(4-Chlorobenzyl)-2-(4-piperidine)benzimidazole (CPUYW03)
[0061]
[0062] (1) Ethyl N-Boc-4-piperidine ethanoate
[0063] N-Boc-4-piperidone (4.9g, 24.4mmol), triethyl phosphoroacetate (7.1g, 31.7mmol), anhydrous K 2 CO 3 (10g, 73.3mmol) was placed in 130mL of anhydrous DMF, heated to 70°C under N2 conditions, kept for 22 hours, removed the oil bath, cooled the reaction solution to 30°C, poured it into a large amount of water, a large amount of solids precipitated, pumped Filter, wash the filter cake twice with water, and dry to obtain 6.7 g of white solid, yield 97%, m.p.82-83°C.
[0064] (2) N-Boc-4-piperidine ethyl acetate
[0065] Dissolve ethyl N-Boc-4-piperidine ethanoate (2.7g, 10mmol) in ethanol and heat to 30°C, add Pd / C (270mg, 10%), ammonium formate (1.6g, 25mmol) aqueous solution. N 2 Heated to 40°C under protection. After 3.5 h, TLC monitored that the reaction was complete. Concentrate the reaction solution to a small volume, add n-hexane and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com