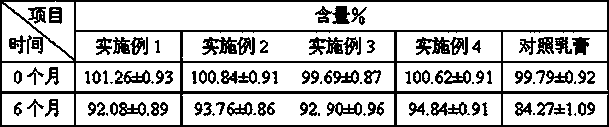
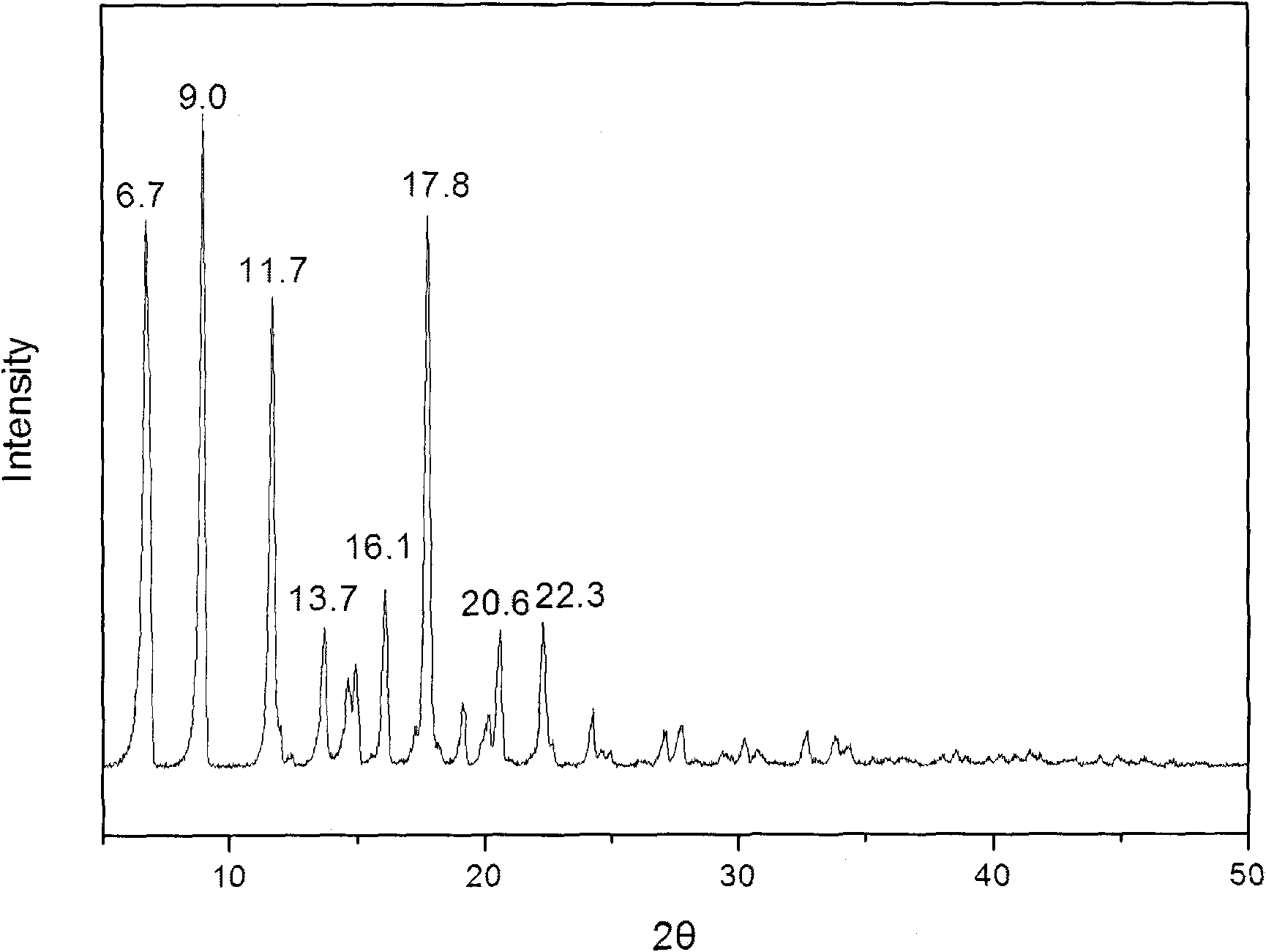
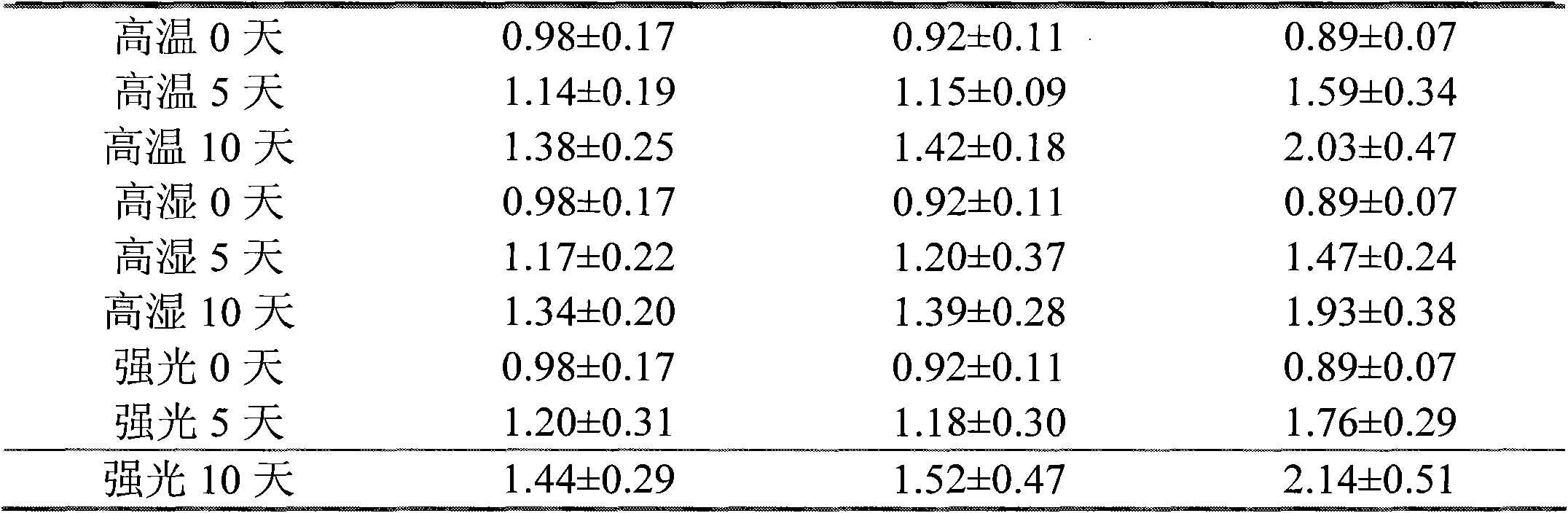
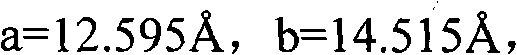Patents
Literature
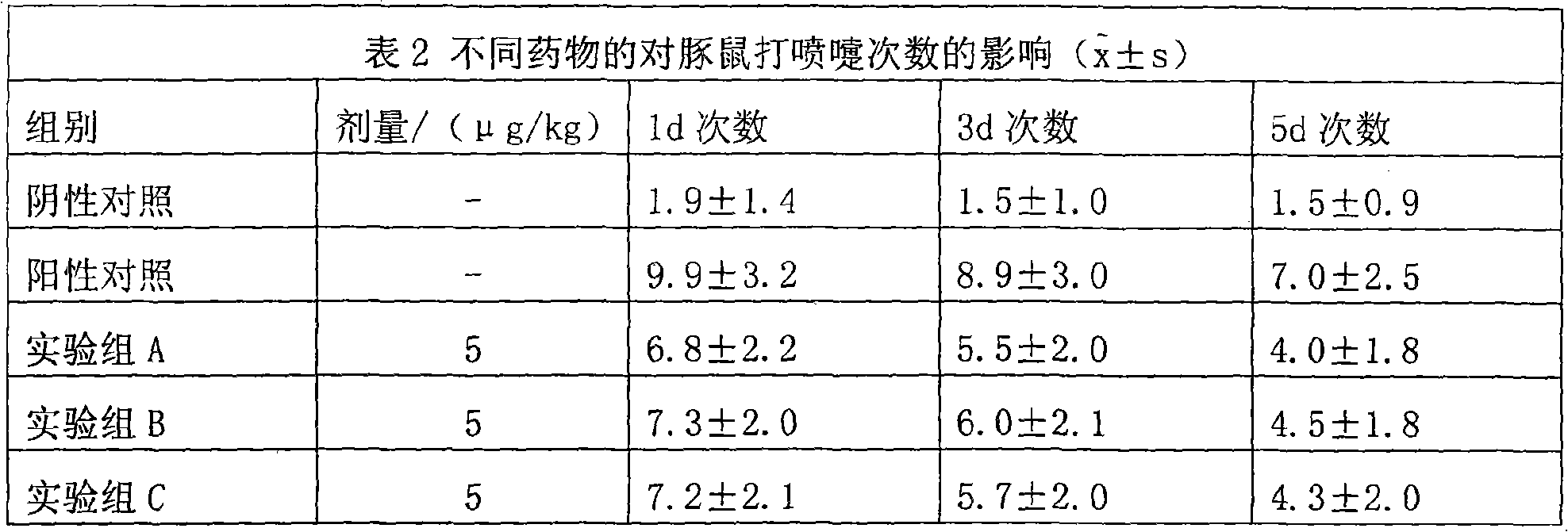
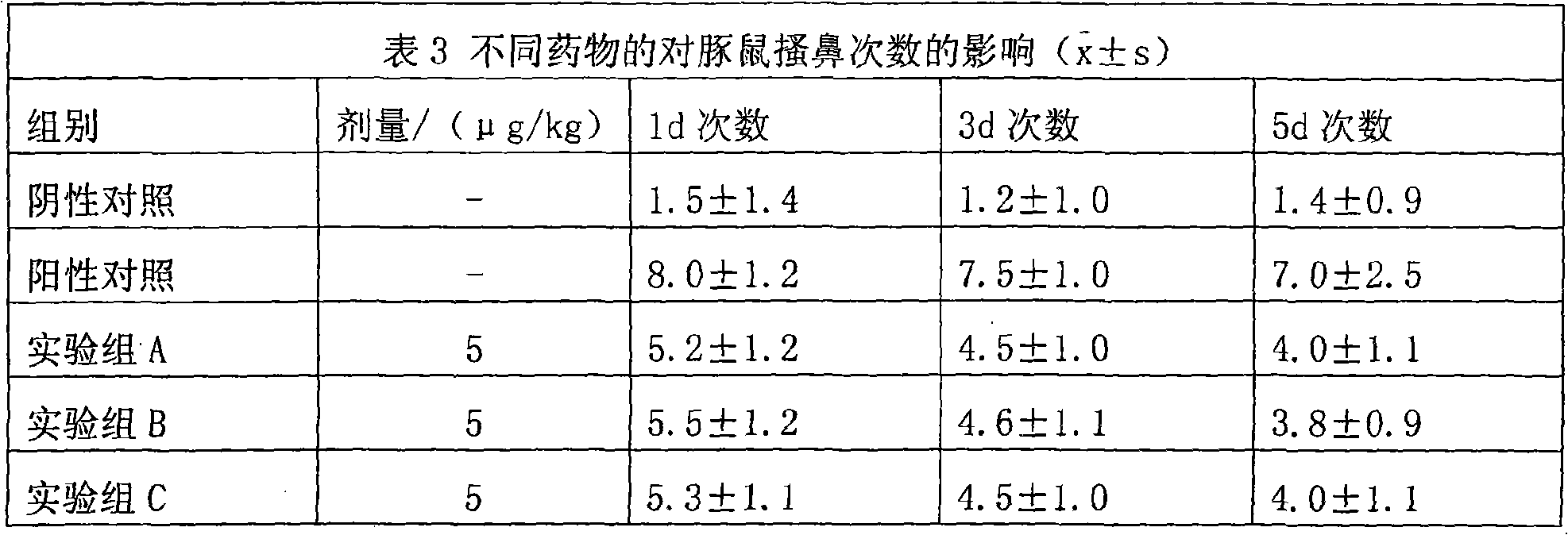
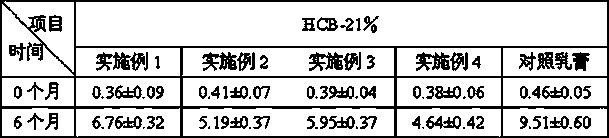
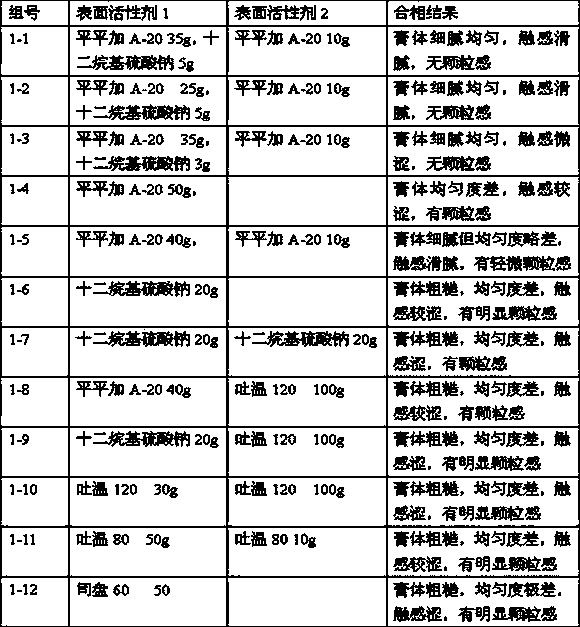
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
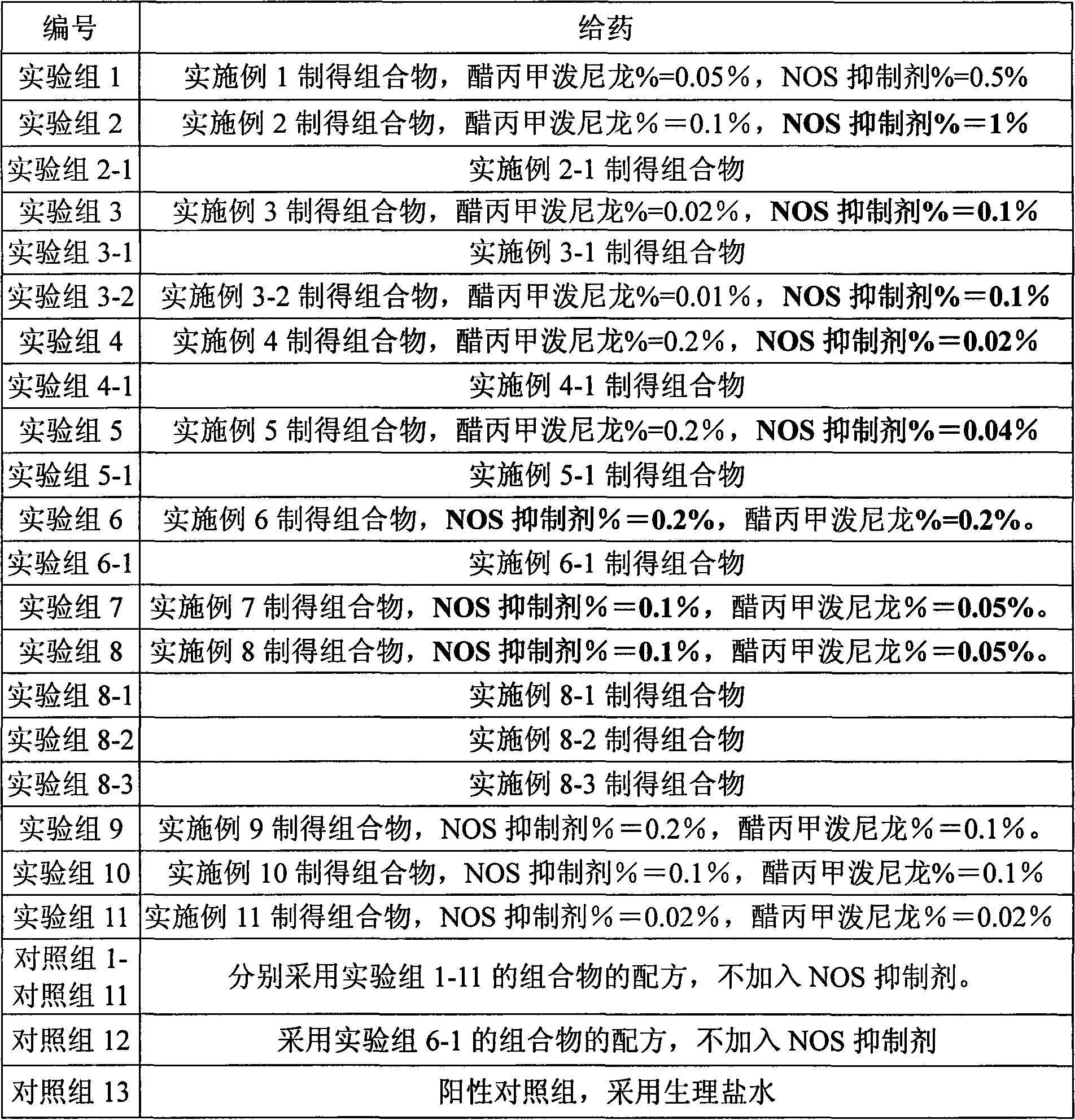
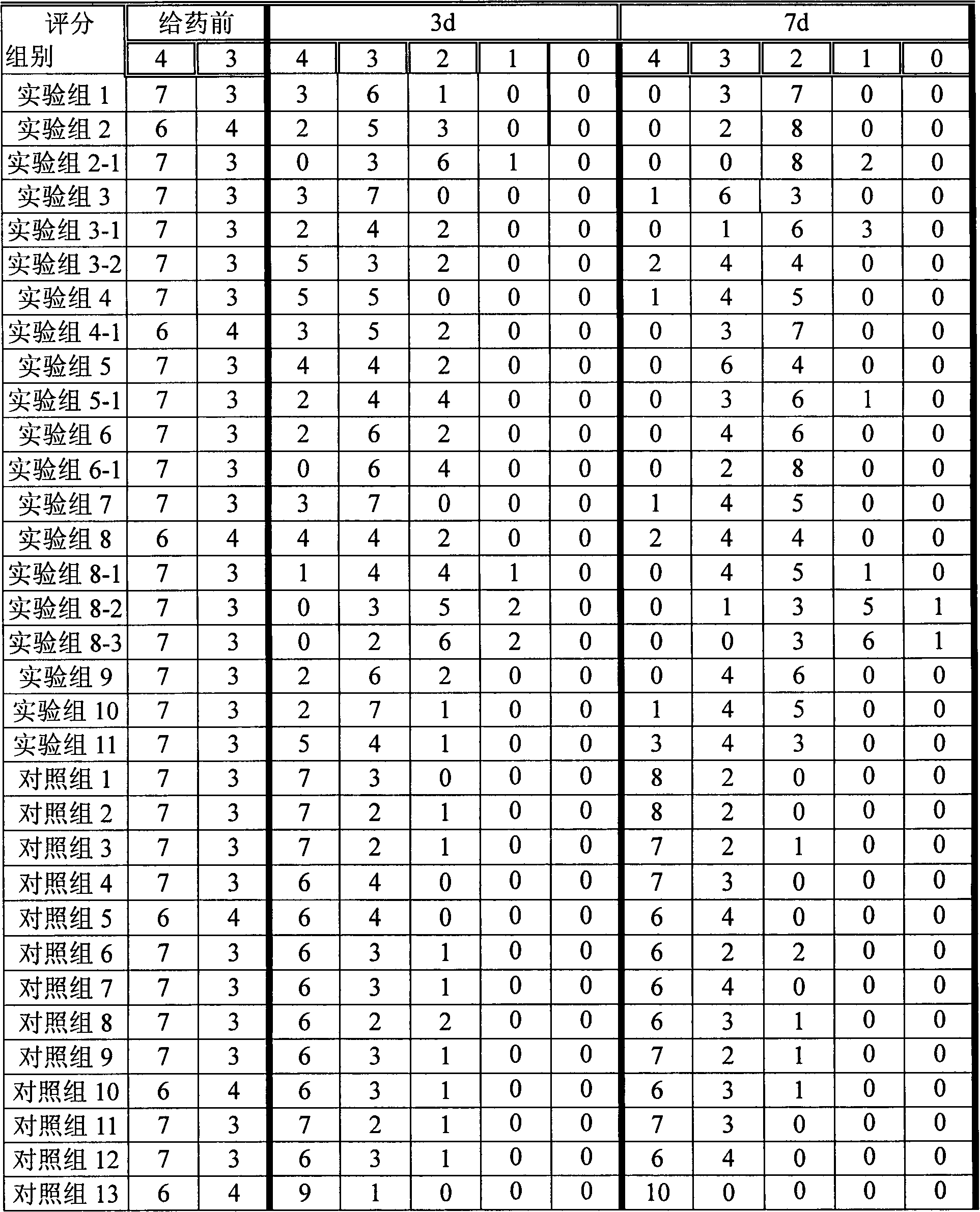
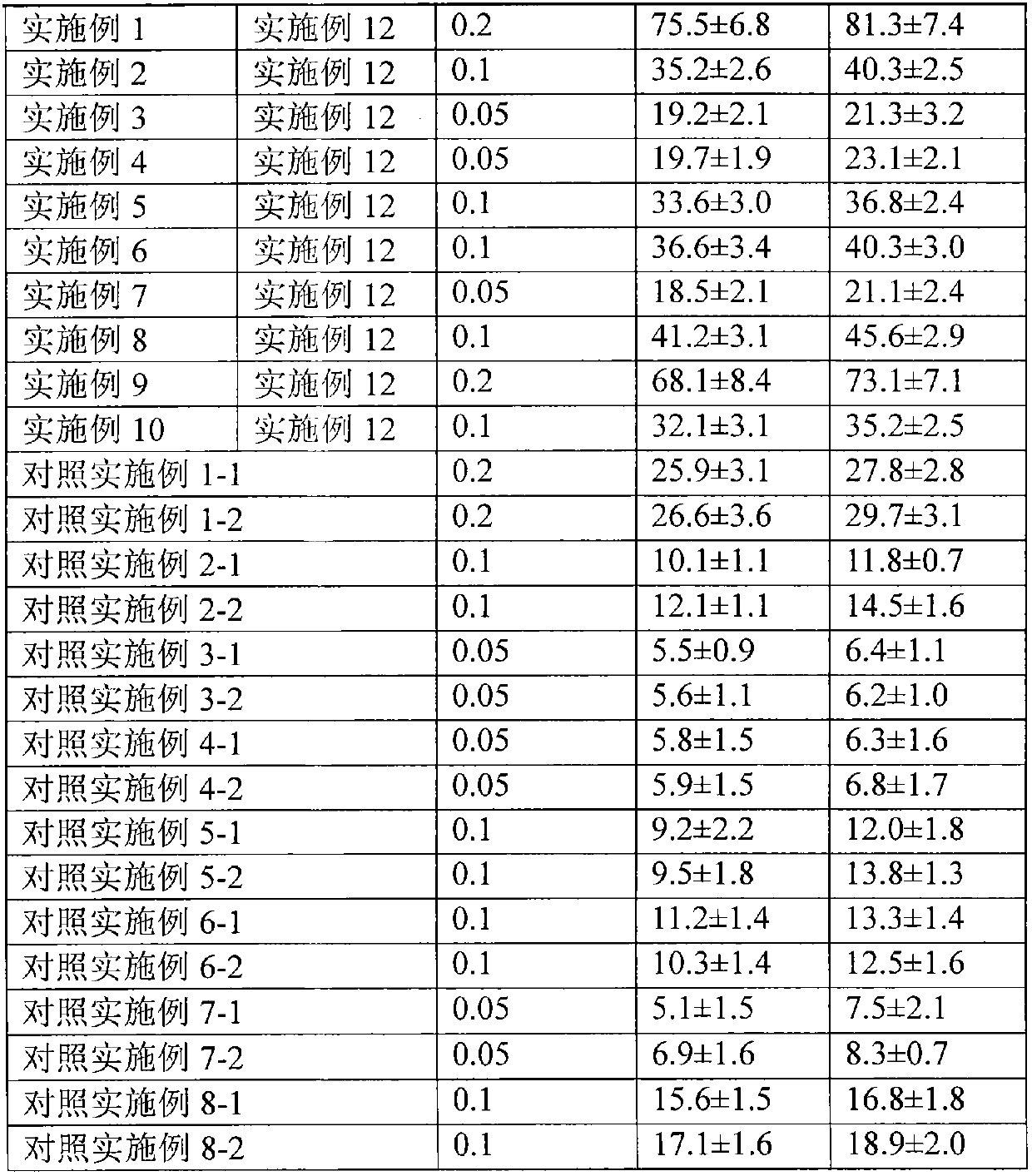
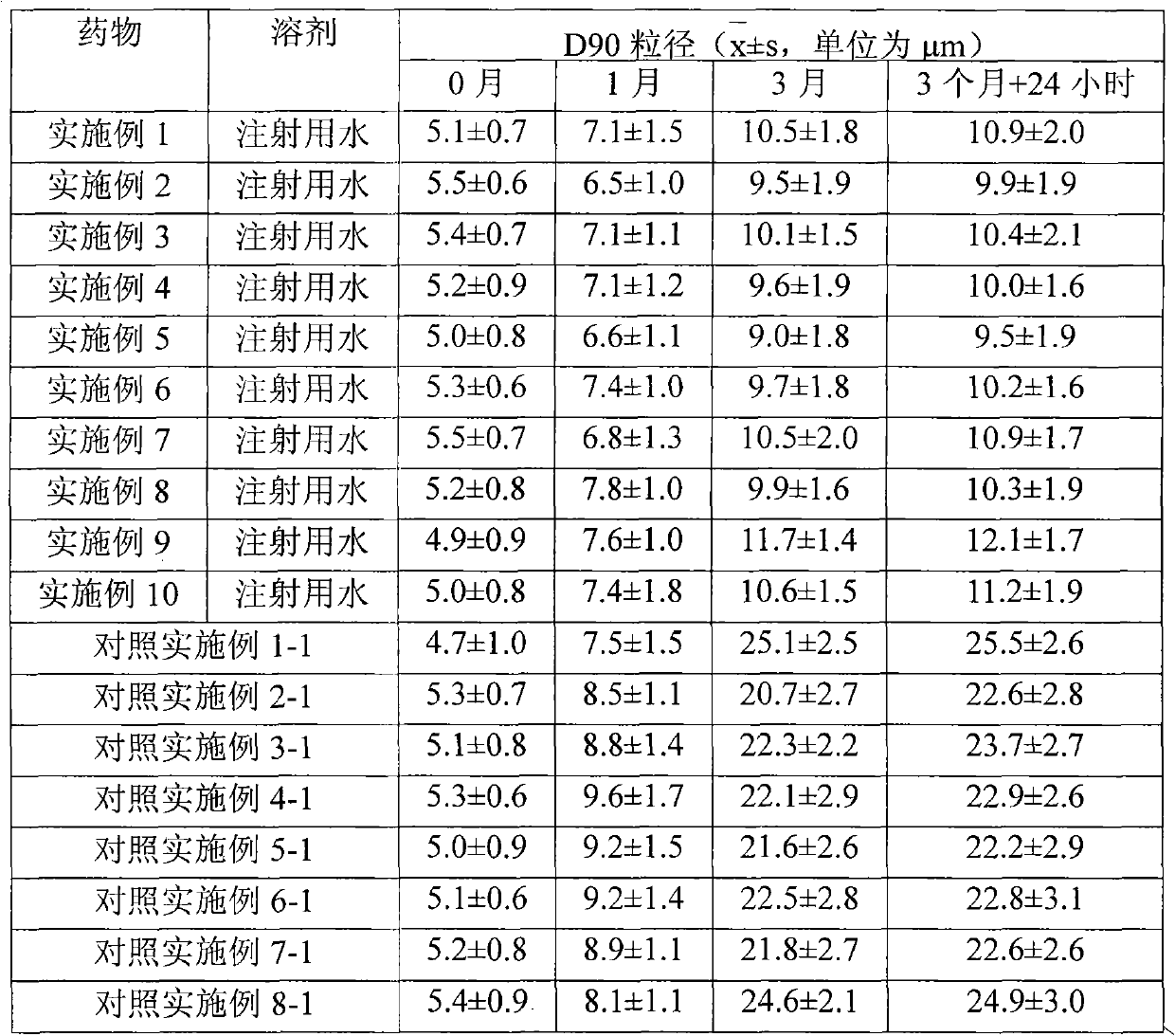
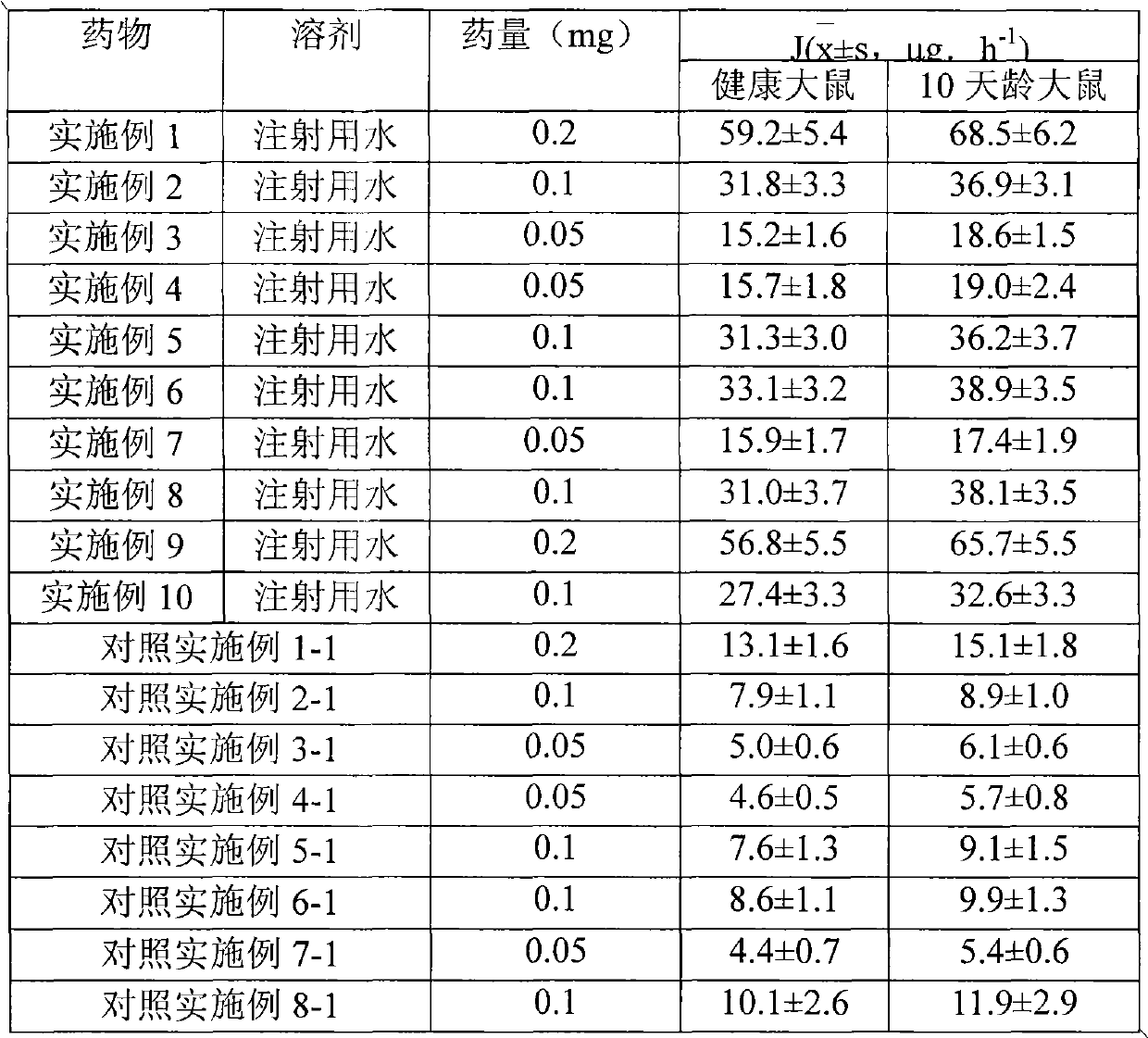
37 results about "Methylprednisolone aceponate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
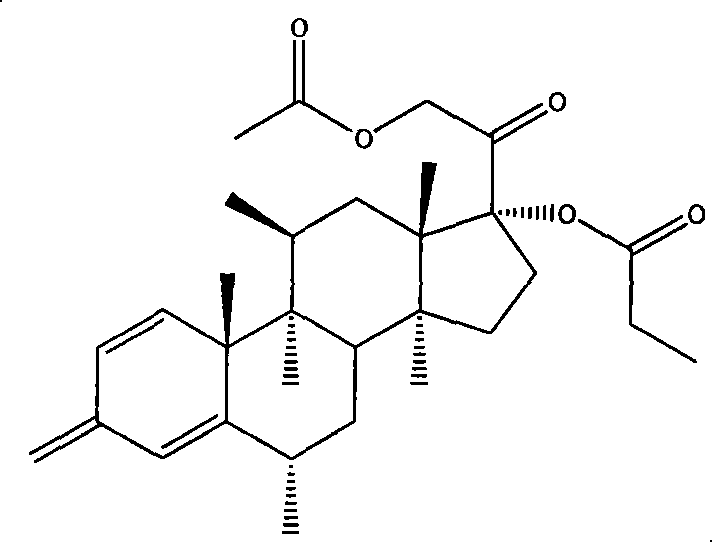
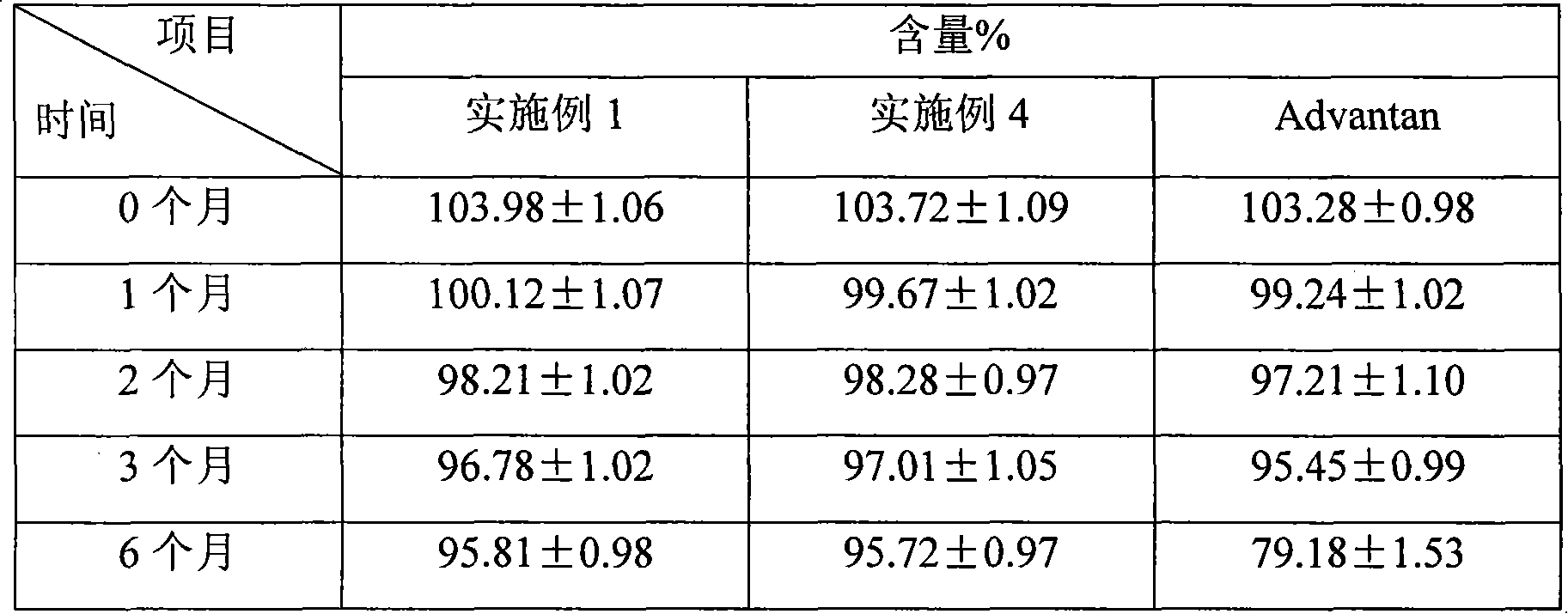
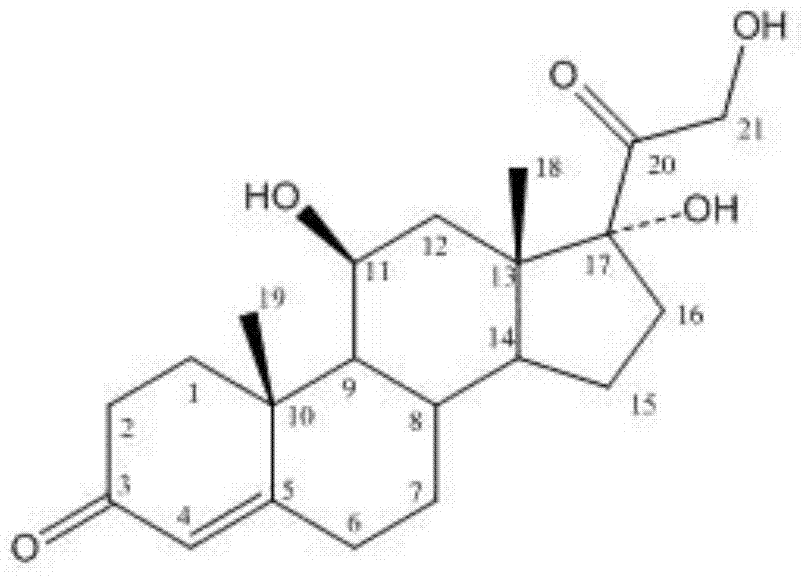
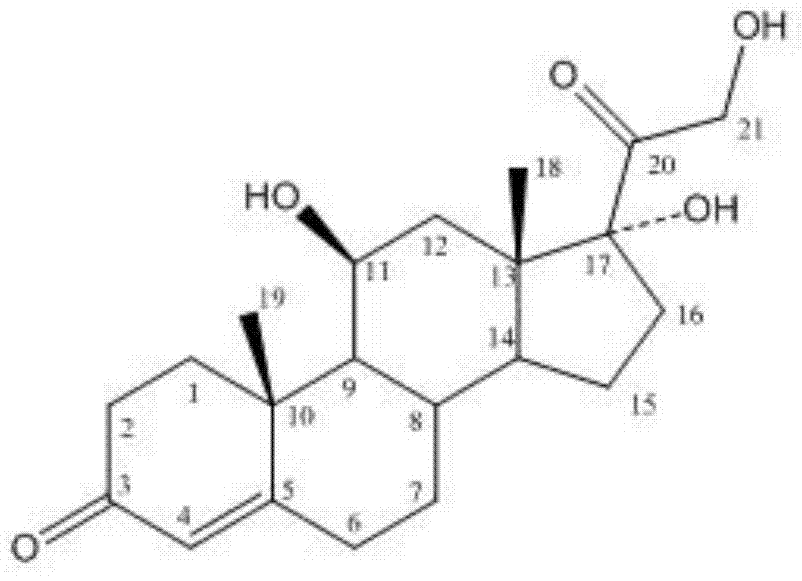
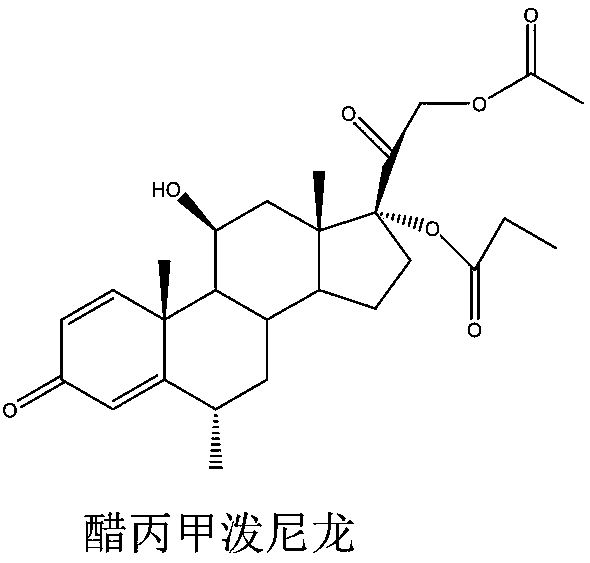
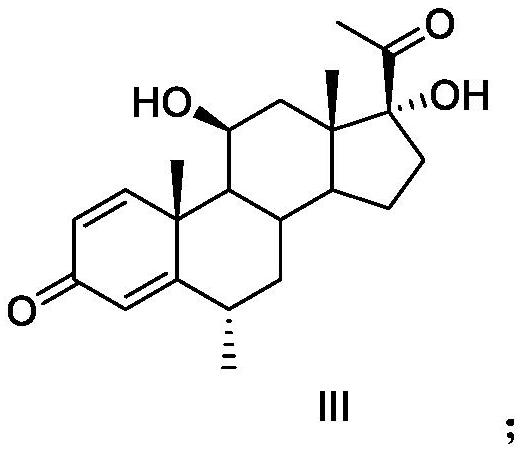
Methylprednisolone aceponate is a glucocorticosteroid.
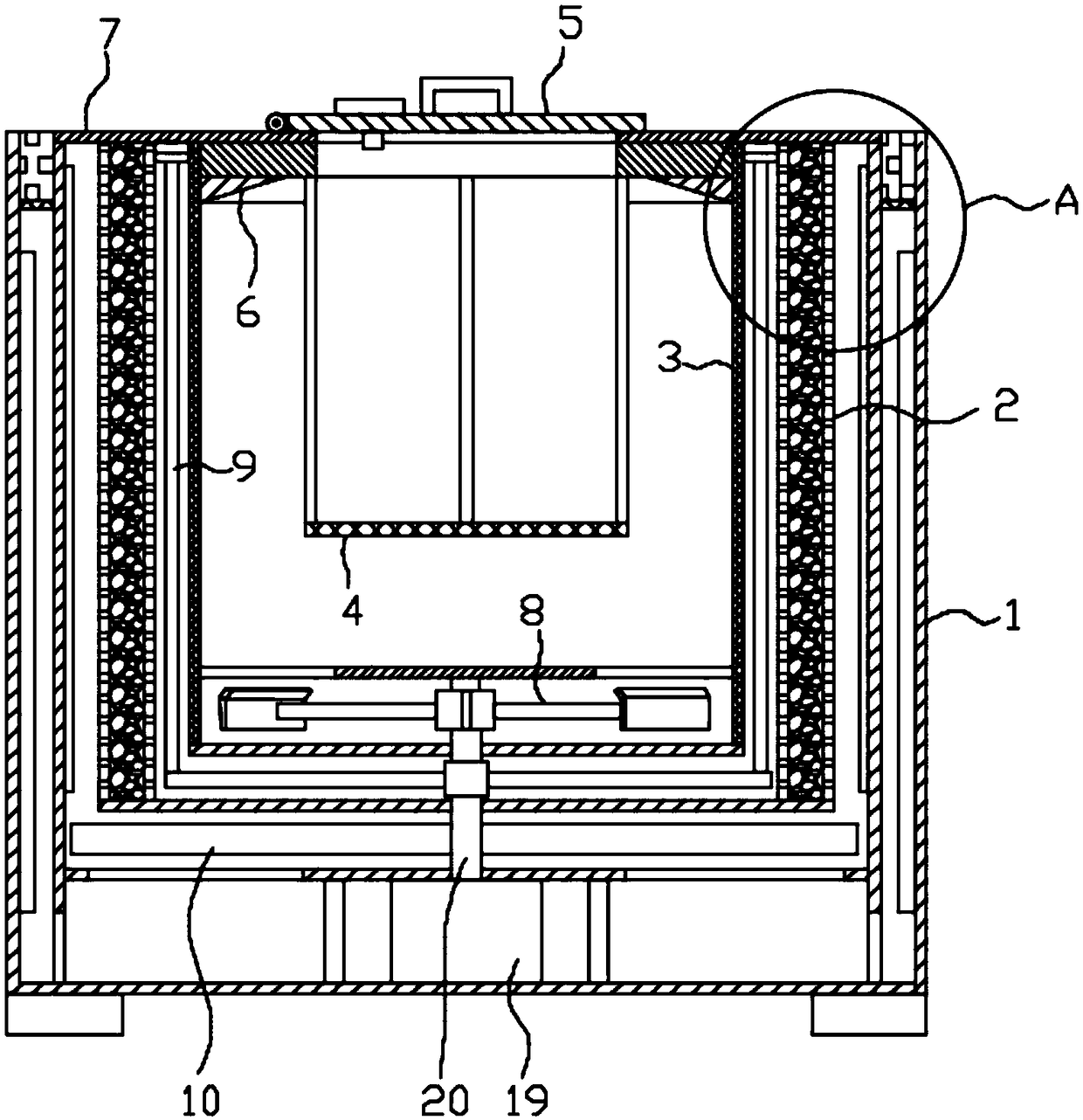
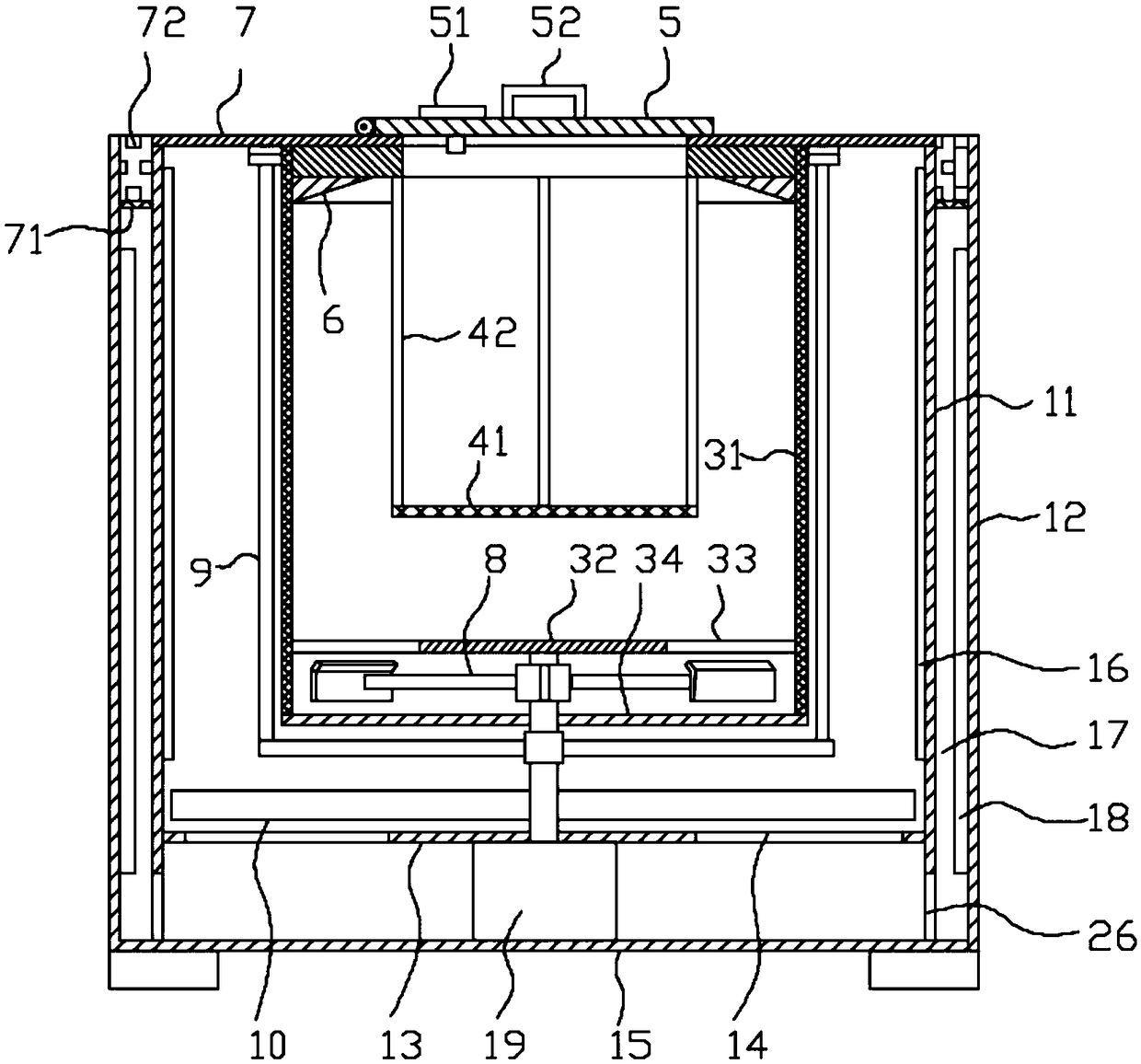
Methylprednisolone production method and production device
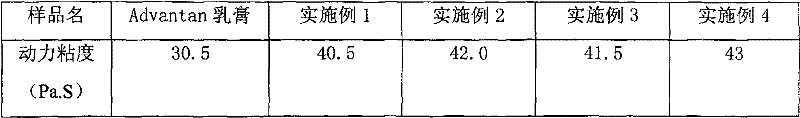
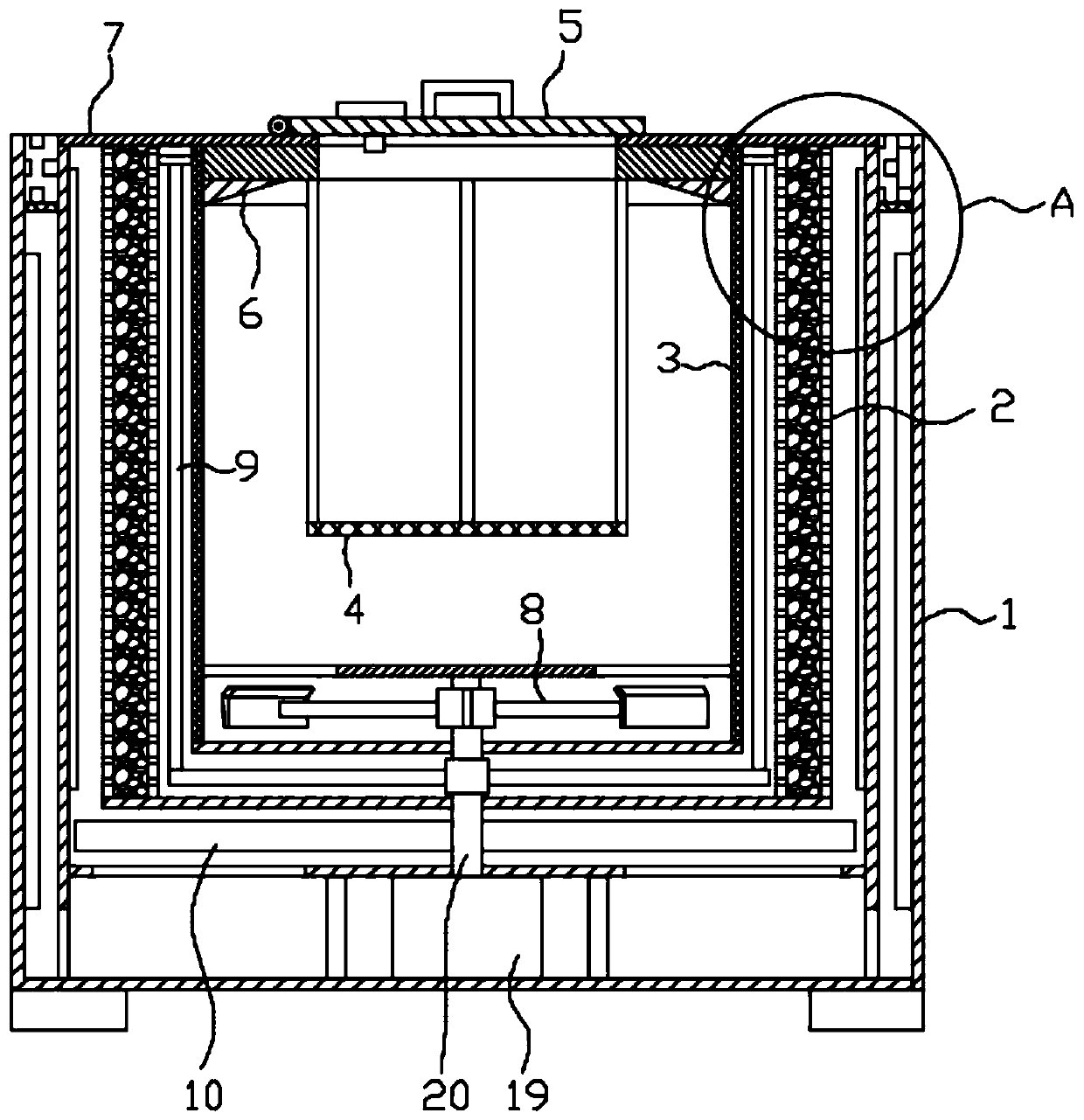
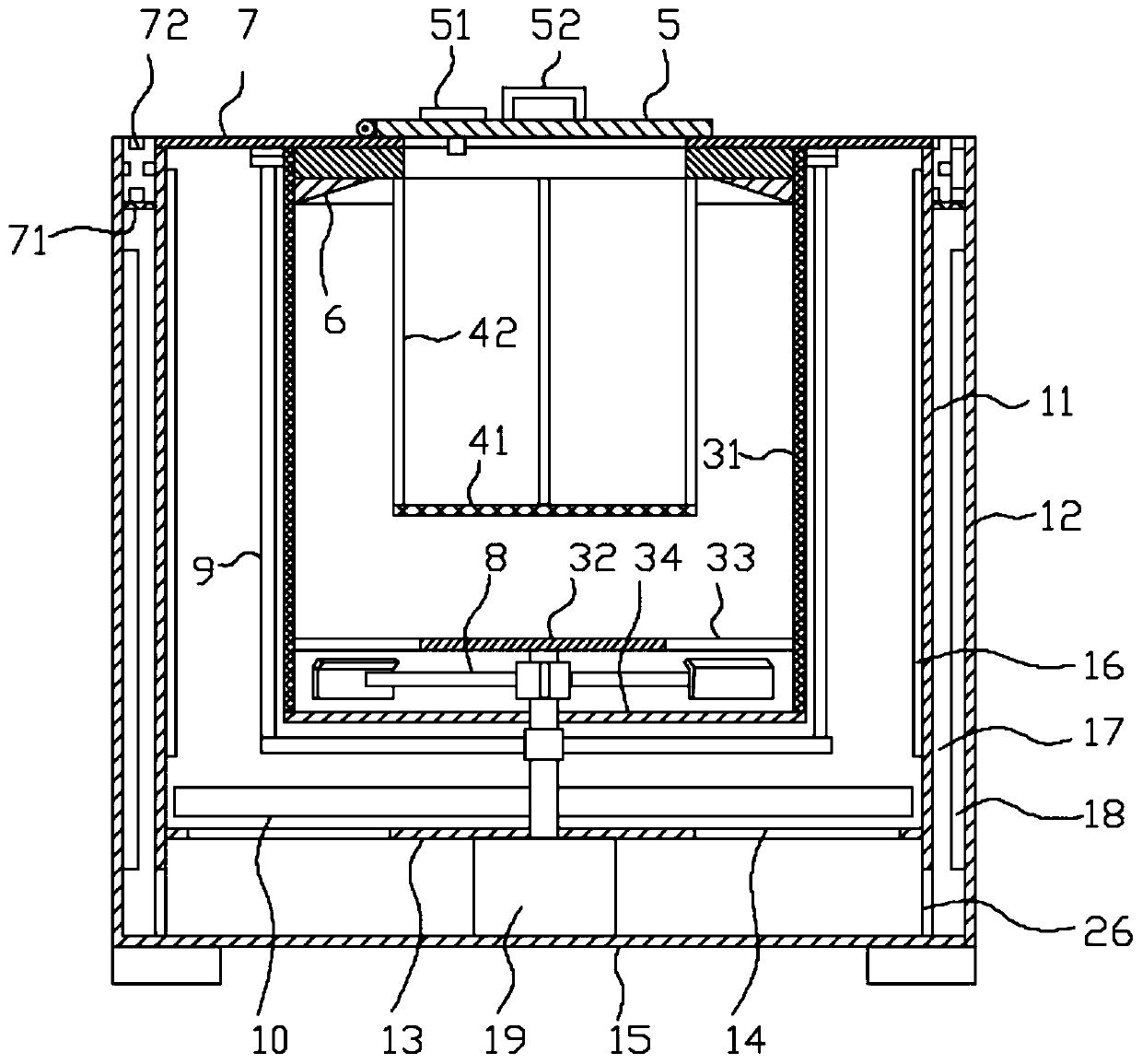
ActiveCN108912192AGuaranteed purityQuality assuranceDrying gas arrangementsSteroidsMethylprednisoloneElectric machinery
The invention discloses a methylprednisolone production device, which comprises a reaction kettle, an elutriation kettle, a centrifugal filtration device, a concentration kettle, a decoloration kettle, a biological fermentation tank and a drying device which are connected in sequence, wherein the drying device comprises an outer cylinder, a drying cylinder and an inner cylinder; a motor is arranged on the bottom surface of the outer cylinder; an exhaust fan blade, a cylinder type impeller and a vortex impeller are arranged on a motor rotating shaft; the exhaust fan blade is arranged between the drying cylinder and the bottom surface of the outer cylinder; the cylinder type impeller is arranged between the drying cylinder and the inner cylinder; the vortex impeller is arranged at the bottomof the inner cylinder; an infrared heating pipe is arranged on the top surface of the inner cylinder; a feeding port is formed in the center of the upper end of the inner cylinder; the side wall of the outer cylinder comprises an inner layer wall and an outer layer wall; an annular interlayer cavity is formed between the inner layer wall and the outer layer wall; an annular baffle is arranged atthe outlet of the upper end of the interlayer cavity, a vent is formed in the baffle, and a rotatable air purification ring is arranged at the upper end of the baffle. According to the method, the preparation time of intermediate products in the production process of the methylprednisolone can be reduced, and rapid and high-quality production of the methylprednisolone can be realized.
Owner:YUEYANG HUANYU PHARMA +6
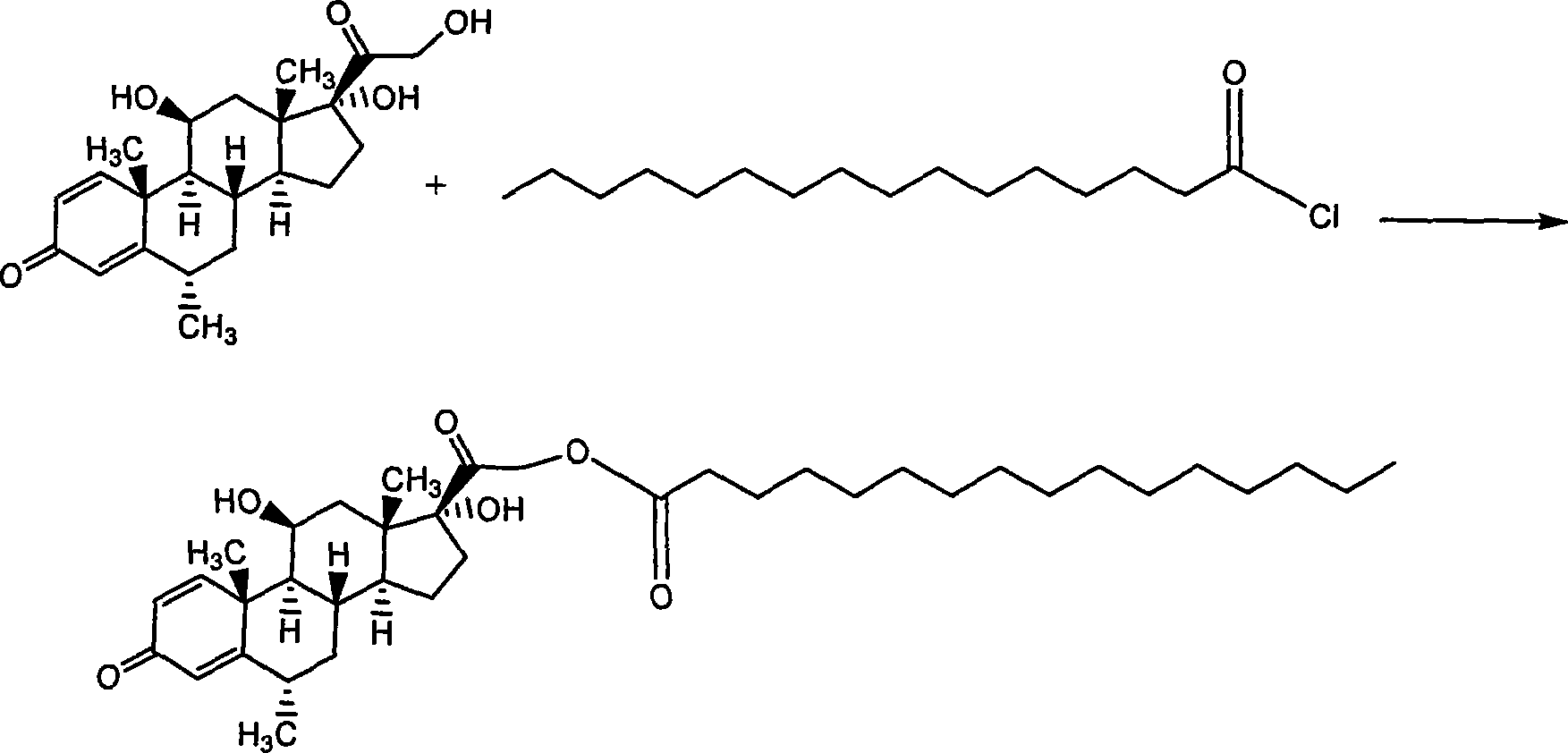
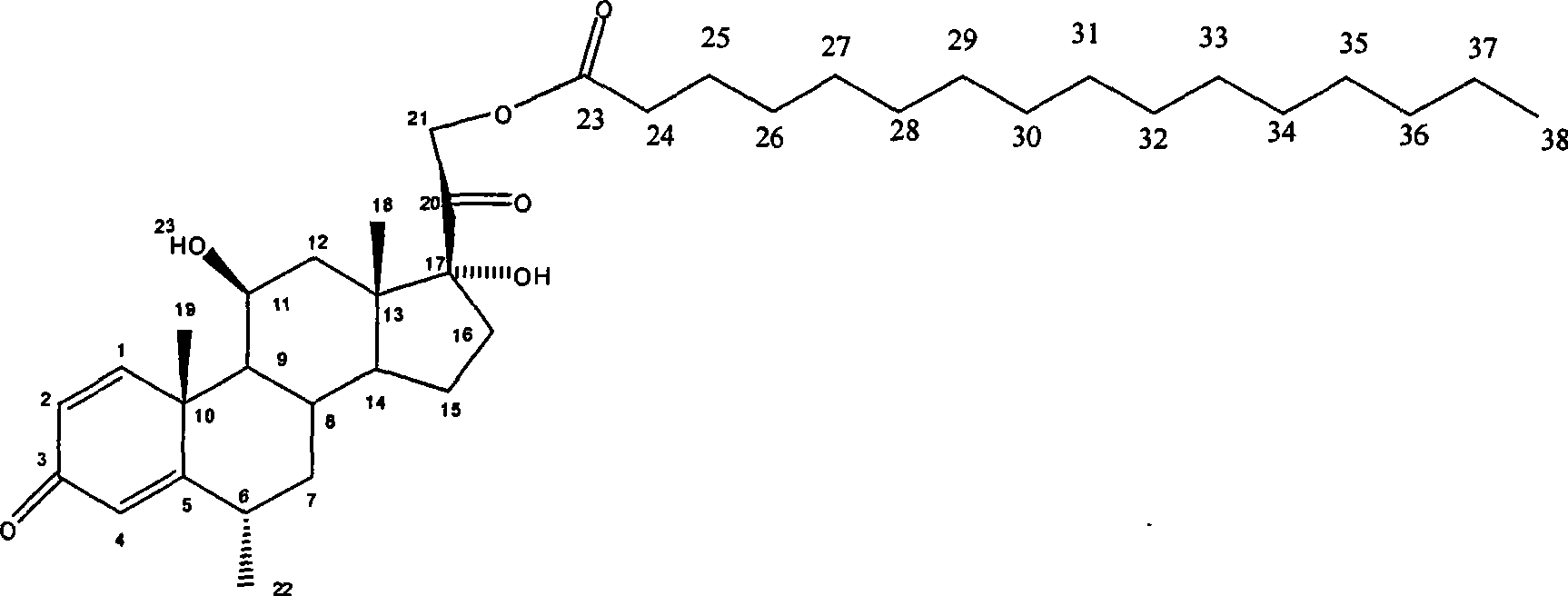
Composition with methylprednisolone palmitate as active component for treating local inflammation
InactiveCN101412741AEnhanced inhibitory effectOrganic active ingredientsAntipyreticActive componentMethylprednisolone
The invention relates to a drug composition for treating local inflammation, which uses methylprednisolone palmitate as an active composition. The drug composition consists of the methylprednisolone palmitate as the active composition and an inactive composition suitable for local injection, and is used for treating local inflammation of people or mammal through local injection.
Owner:TIANJIN JINYAO GRP
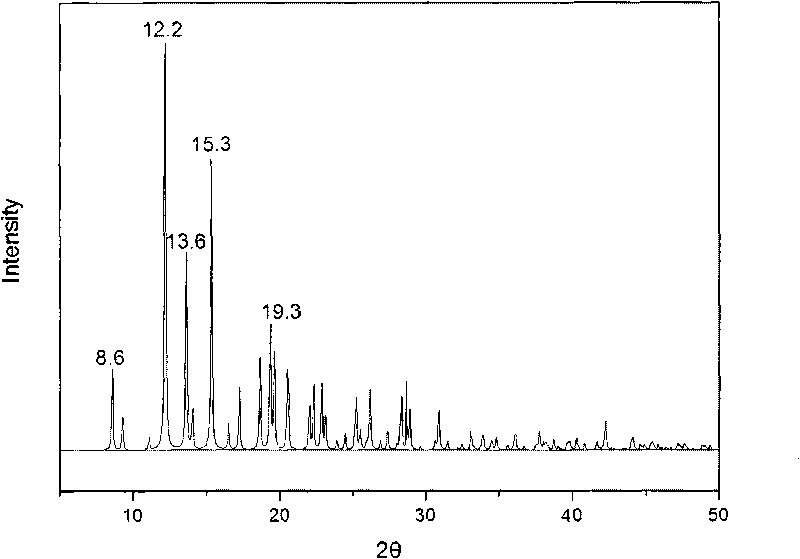
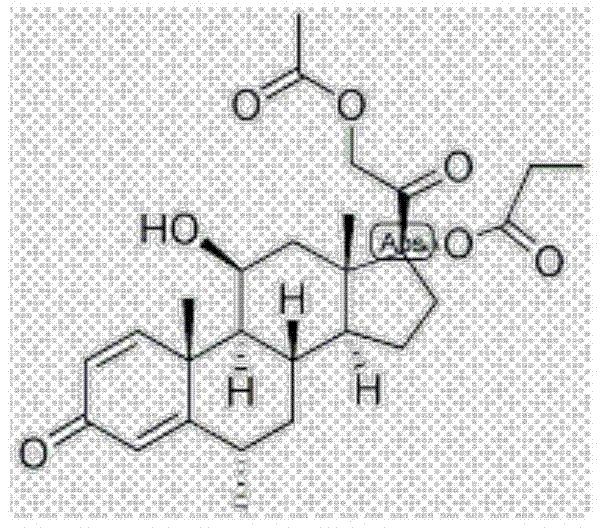
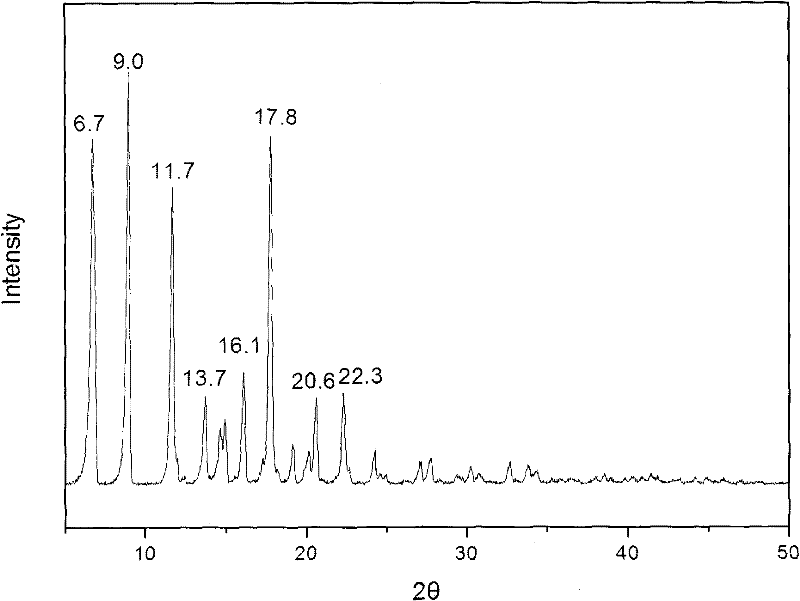
Methylprednisolone aceponate monohydrate, crystal form and preparation method thereof
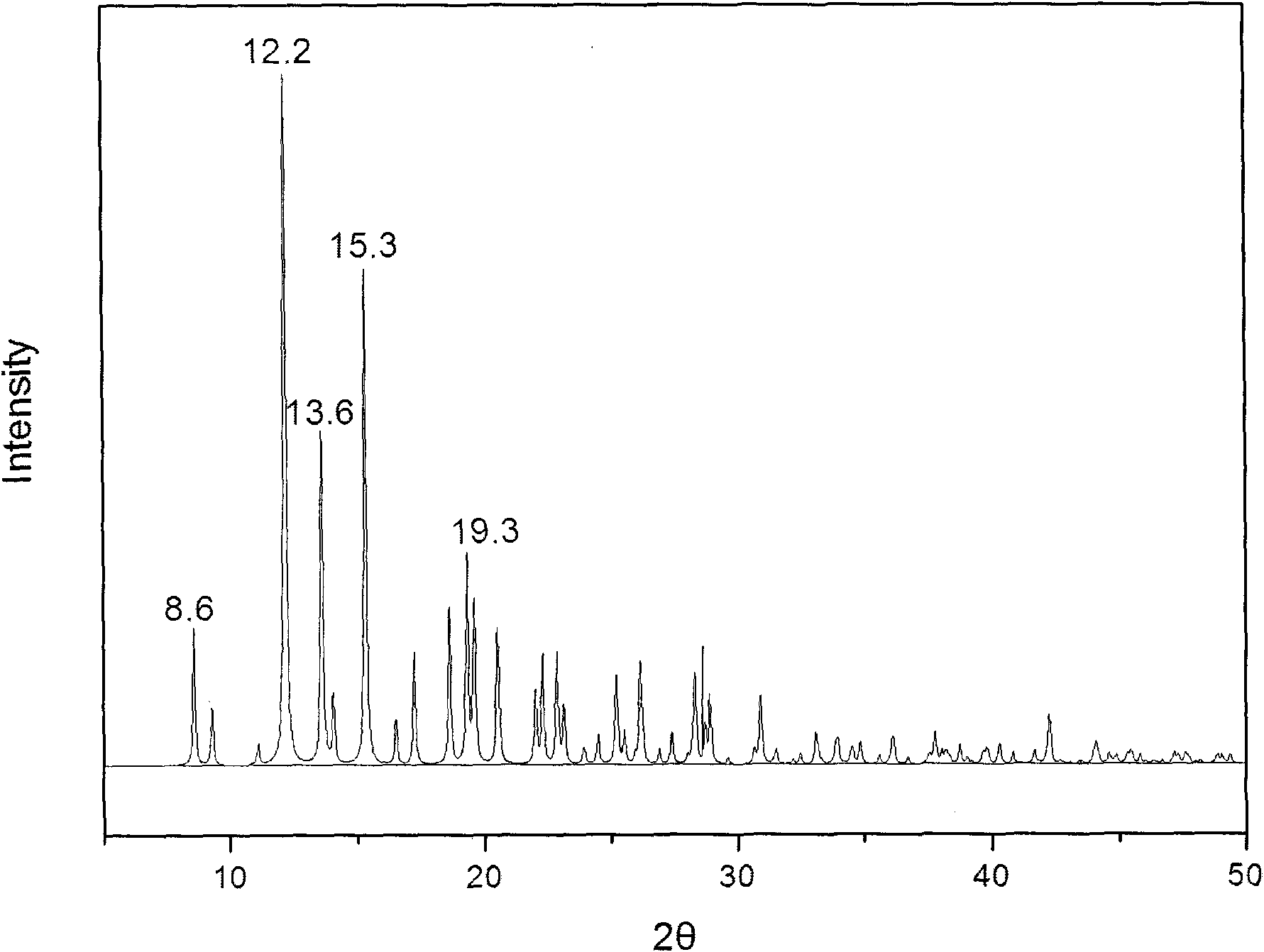
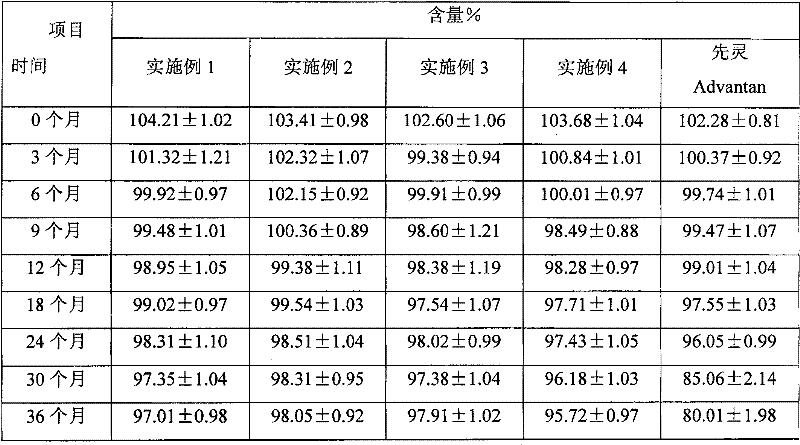
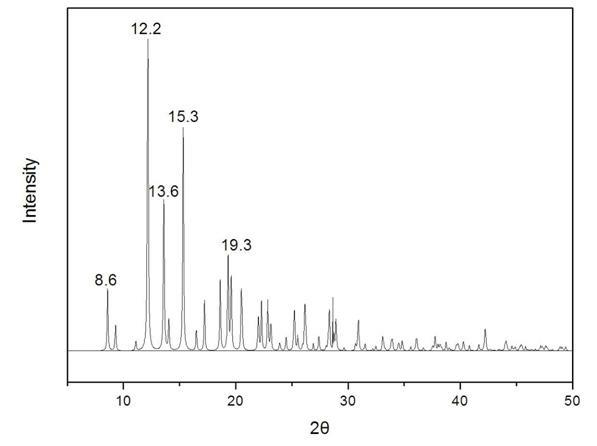
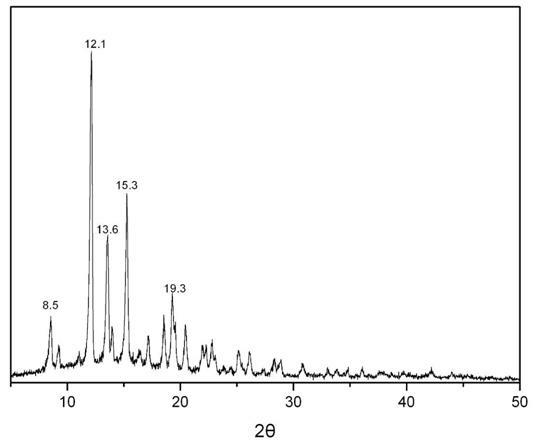
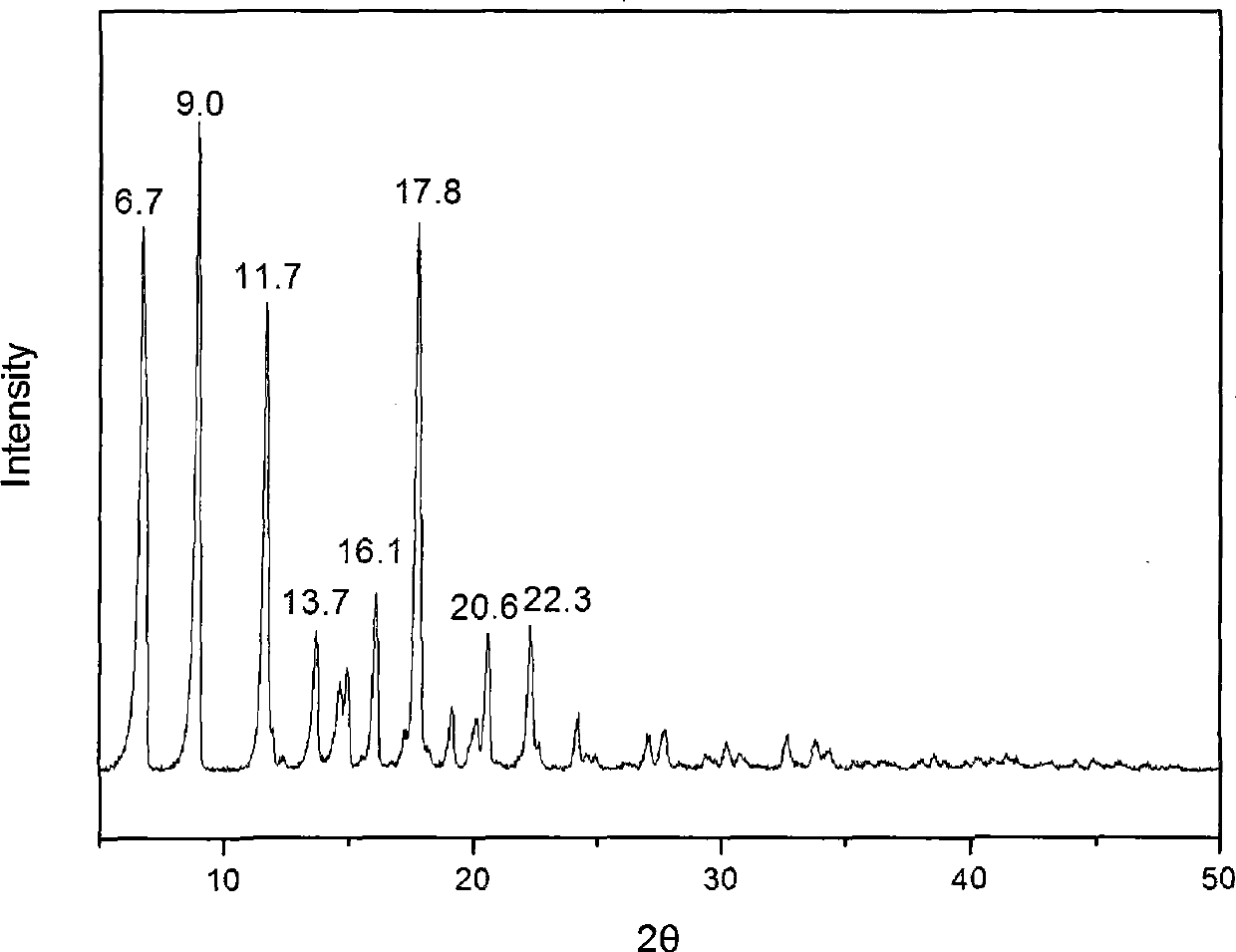
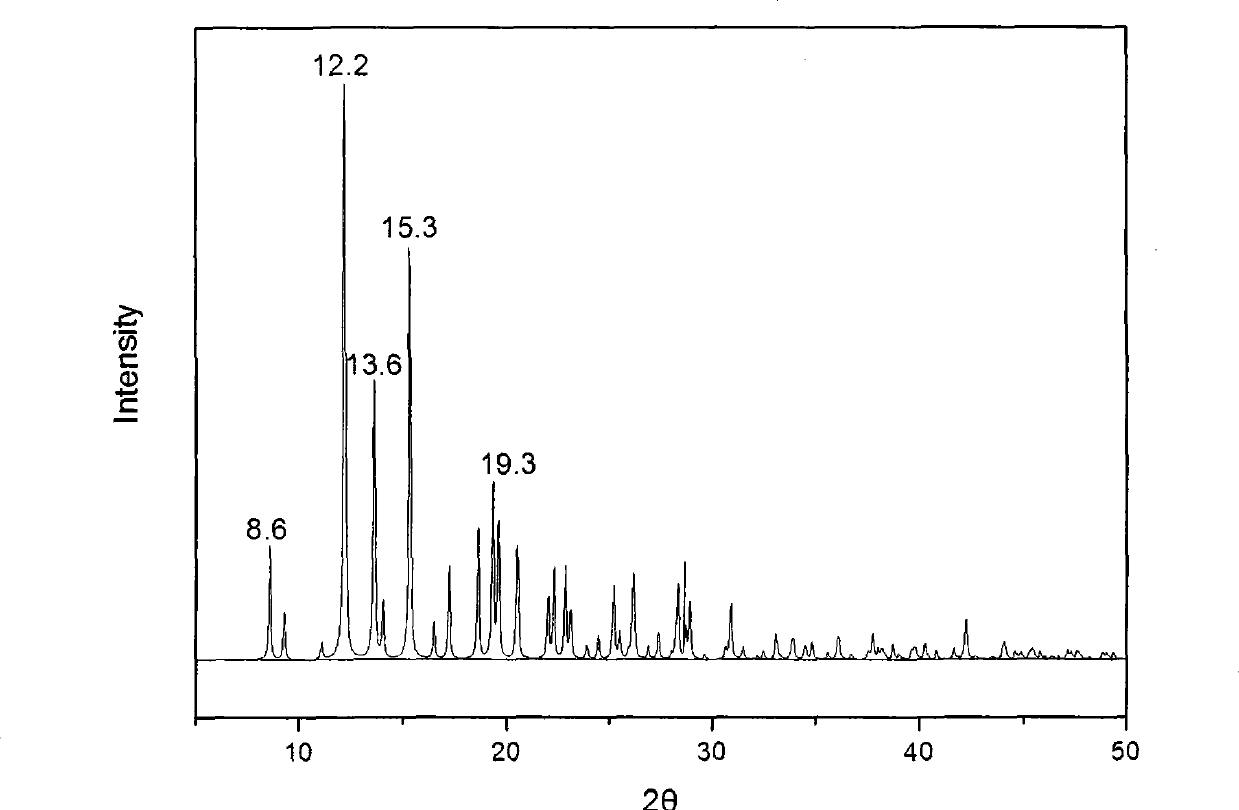
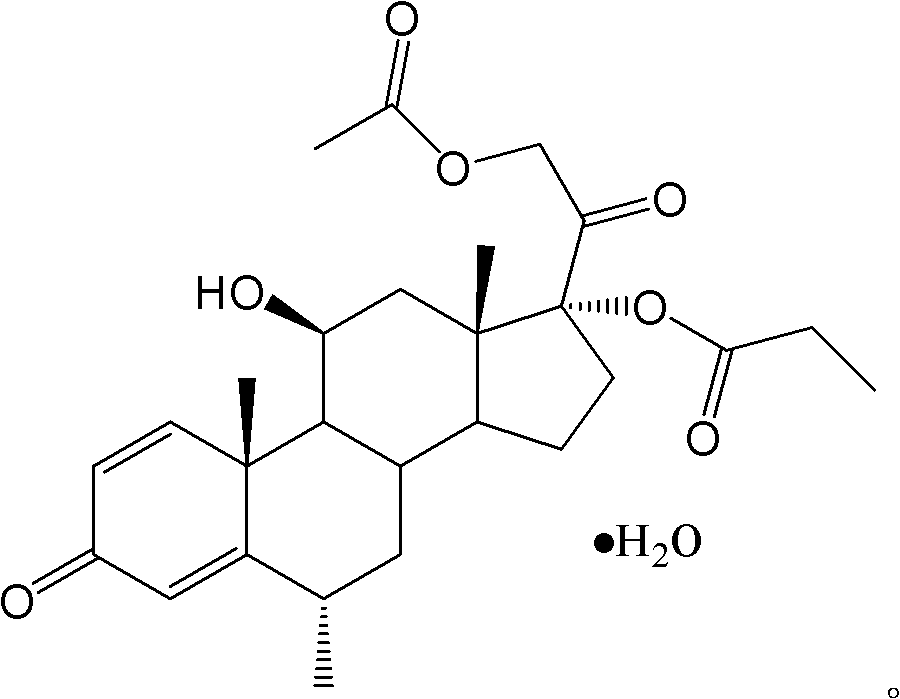
The invention provides a methylprednisolone aceponate monohydrate, a crystal form and a preparation method thereof. The crystal parameters of the methylprednisolone aceponate monohydrate are alpha 90.00, beta 90.00, gamma 90.00, crystal cell volume is that the crystal belongs to a rhombic system, and the space group is P212121. X-ray powder diffraction is used for measurement, the X-ray powder diffraction of the methylprednisolone aceponate monohydrate has characteristic peaks when the diffraction angle 2theta is equal to 8.6 degrees, 12.2 degrees, 13.6 degrees, 15.3 degrees, 18.6 degrees, 19.3 degrees and 20.5 degrees, and the relative diffraction intensity of the methylprednisolone aceponate monohydrate is substantially represented by the detailed spectrogram as figure 2. The methylprednisolone aceponate monohydrate can be prepared by a method of completely dissolving methylprednisolone aceponate in the mixed solution of water and an organic solvent and finally evaporating the organic solvent.
Owner:TIANJIN JINYAO GRP
Uses of methylprednisolone and derivatives thereof in preparing medicament for treating allergic rhinitis
ActiveCN101347436ADecreased systemic effectsLow therapeutic doseOrganic active ingredientsAerosol deliveryNasal cavityAdditive ingredient
A pharmaceutical composition for treating allergic rhinitis comprises one or a plurality of kinds from methylprednisolone taken as active ingredient or pharmaceutically acceptable salt thereof or esterified matters thereof, and a pharmaceutical composition consisting of one or a plurality of inactive ingredients applicable to local action inside nasal cavity.
Owner:天津药业集团有限公司
Medicine composition consisting of methylprednisolone aceponate and zinc oxide
InactiveCN103877118AOrganic active ingredientsInorganic active ingredientsHormone drugBULK ACTIVE INGREDIENT
Owner:TIANJIN JINYAO GRP
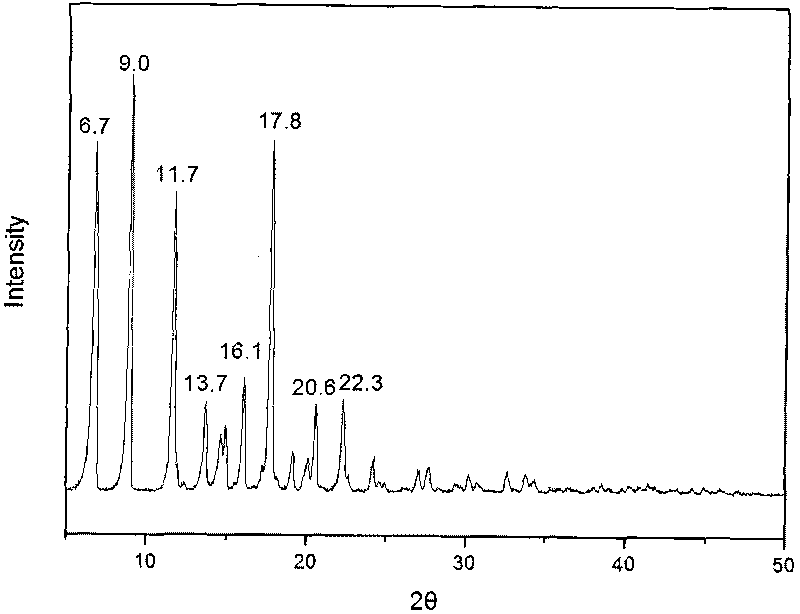
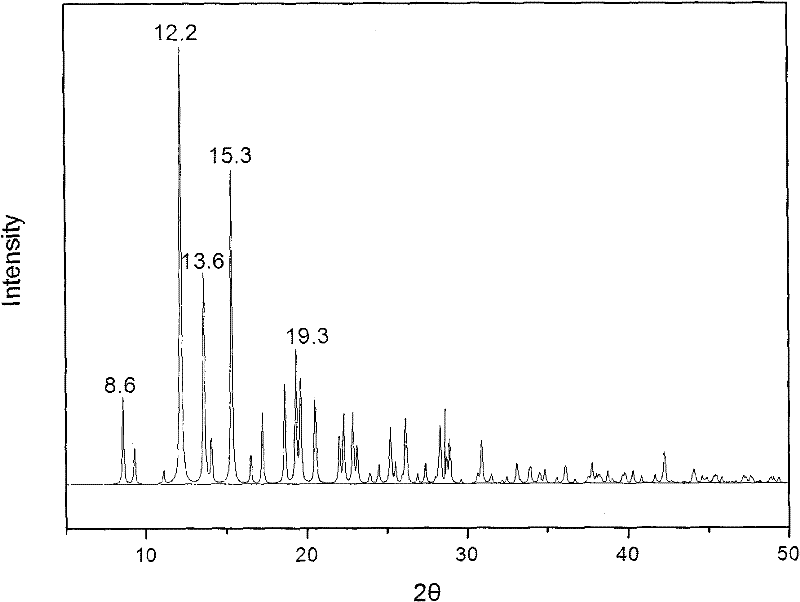
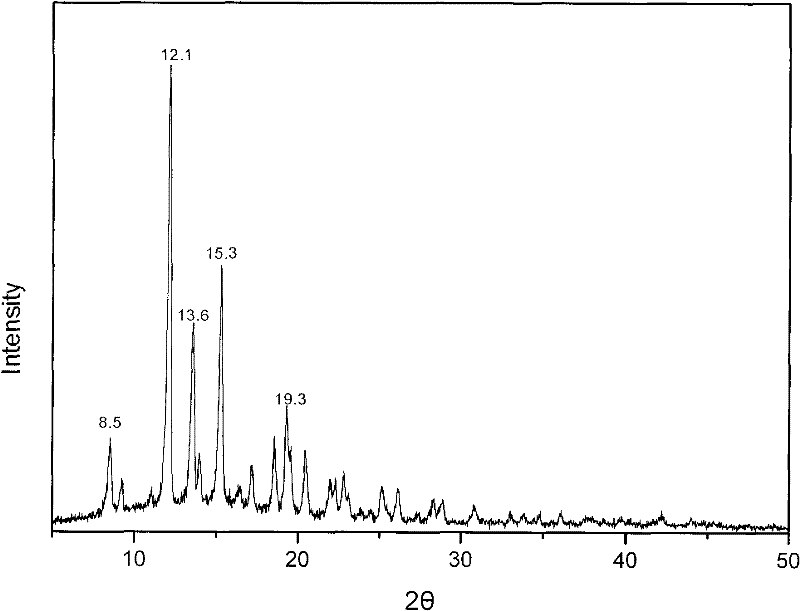
Methylprednisolone aceponate new crystal form and preparation method thereof
InactiveCN101805387ASmall particle sizeGuaranteed stabilityOrganic active ingredientsAntipyreticOrganic solventX-ray
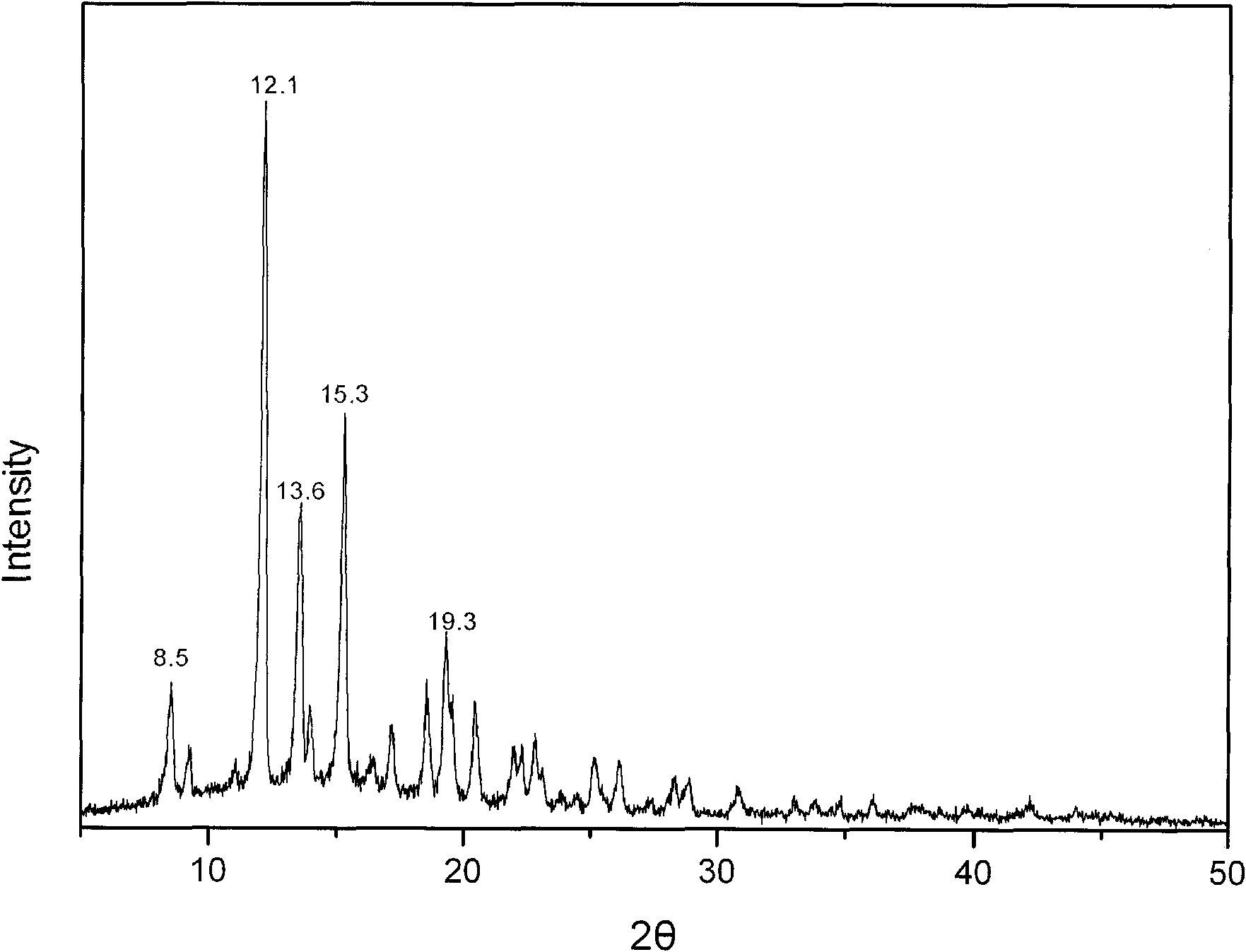
The invention relates to a methylprednisolone aceponate new crystal form and preparation method thereof. X-ray powder diffraction has characteristic peak at diffraction angle 2theta of 8.6 degrees, 12.2 degrees, 13.6 degrees, 15.3 degrees, 18.6 degrees and 19.3 degrees, and the method that methylprednisolone aceponate is fully dissolved into mixed solution of water and organic solvent, and then the organic solvent is evaporated can be adopted to prepare the new crystal form.
Owner:TIANJIN JINYAO GRP
Oral cavity pasting tablets using Methylprednisolone and derivatives thereof as active components
ActiveCN101371824AAvoid potential hazardsAvoid harmOrganic active ingredientsDigestive systemAdditive ingredientWater insoluble
The invention discloses an oral sticking tablet used for treating canker sore of humans or mammals. The oral sticking tablet comprises a sticking layer containing an active ingredient and a water insoluble protective layer, wherein, a non-active ingredient contained in the sticking layer consists of a filling agent, an adhesive sustained-release agent and a lubricant which are applicable to tablets; the water insoluble protective layer is composed of polyacrylic acid resin and / or ethyl cellulose which can be used as protective coating, and a plasticizer; in addition, the active ingredient is methylprednisolone, or one or a combination of pharmaceutically acceptable esters.
Owner:TIANJIN PHARMA GROUP CORP
Hormone emulsifiable paste
ActiveCN101468024BImprove stabilityDisadvantages against prone to degradationOrganic active ingredientsAerosol deliveryHormones regulationOil phase
The invention relates to hormone cream with methylprednisolone aceponate as active constituent. The invention consists of following materials: solid matter as oil phase substrate, consistency adjusting agent, moisturizer, emulsifier, stabilizing agent and the balance water. The active constituent is dispersed in the cream as water suspension, and suspension assistant should be added in the water suspension.
Owner:TIANJIN JINYAO GRP
New crystal form of methylprednisolone aceponate and preparation method
InactiveCN102659886ASmall particle sizeGuaranteed stabilitySteroidsMethylprednisolone aceponatePowder diffraction
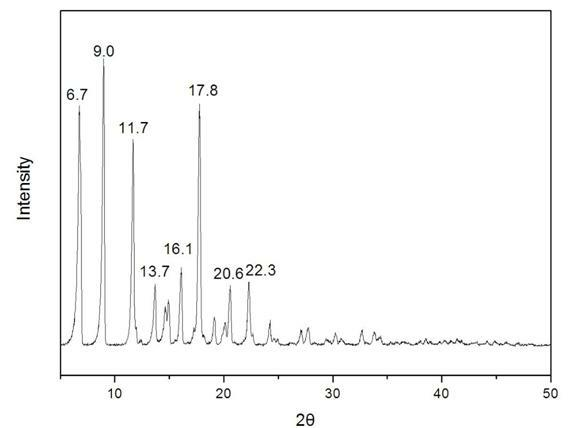
The invention relates to a new crystal form of methylprednisolone aceponate and a preparation method. According to the new crystal form, X-ray powder diffraction has characteristic peaks at diffraction angles 2 theta of 8.6 degrees, 12.2 degrees, 13.6 degrees, 15.3 degrees, 18.6 degrees and 19.3 degrees. According to the method, the methylprednisolone aceponate is dissolved in a mixed solution of water and an organic solvent completely, and the organic solvent is evaporated so as to prepare a methylprednisolone aceponate crystal.
Owner:TIANJIN JINYAO GRP
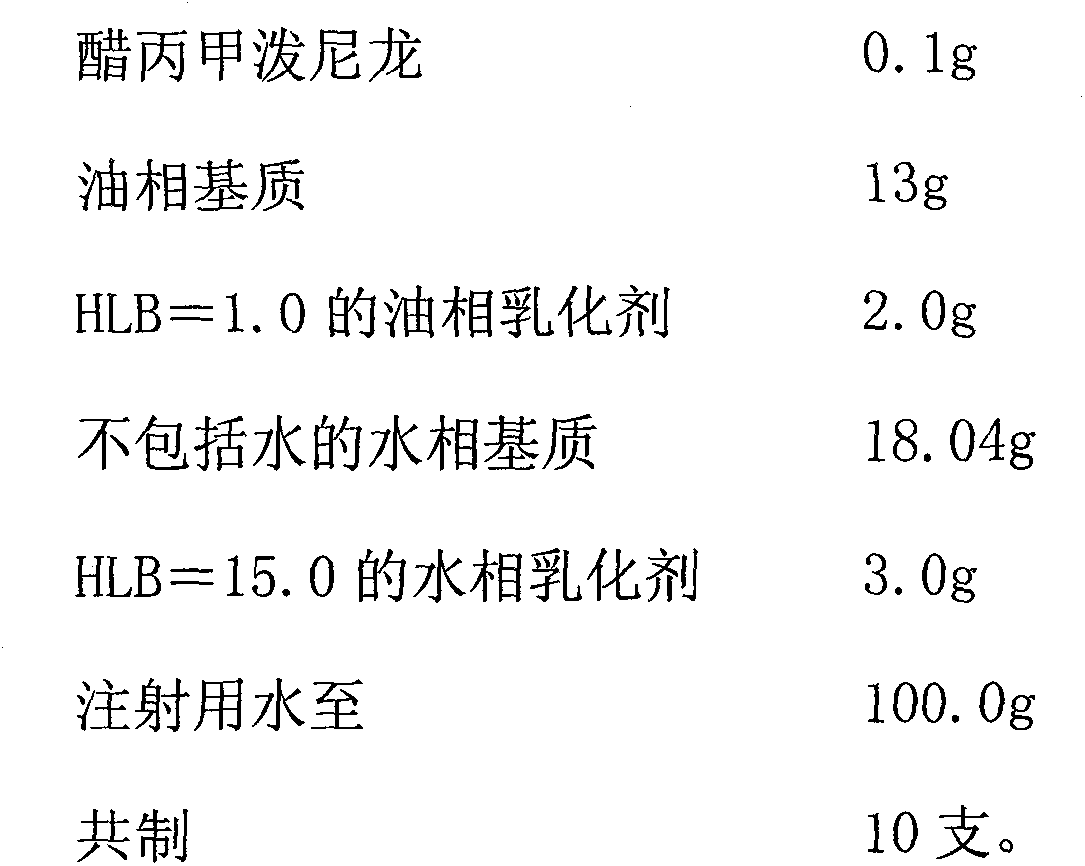
Methylprednisolone aceponate cream preparation
The invention discloses a methylprednisolone aceponate cream preparation, which is characterized by consisting of methylprednisolone aceponate, oil phase matrix, oil phase emulsifier, aqueous phase matrix and aqueous phase emulsifier, wherein the HLB (hydrophile-lipophile balance) value of the oil phase emulsifier is 1.0 to 4.0, the oil phase emulsifier accounts for 2 to 8 percent of the weight of the cream, the HLB value of the aqueous phase emulsifier is 15 to 18, and the aqueous phase emulsifier accounts for 3 to 9 percent of the weight of the cream. The cream preparation has good stability and release degree.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Glucocorticoid drug tablet and preparation method thereof
InactiveCN109925288AControl impurity growthAvoid contactOrganic active ingredientsAntipyreticMedicineGlucocorticoid
The invention belongs to the field of pharmaceutical preparations, and relates to a glucocorticoid drug tablet and a preparation method thereof. The raw material medicine of the tablet is methylprednisolone having a particle size of D90 30-40 [mu]m, and the preparation method comprises the following steps: the raw material medicine is pulverized by air flow, and then uniformly mixed with a filler,a disintegrating agent and a lubricant, and then a mixture compressed to obtain a finished product. The glucocorticoid drug tablet prepared by the method has the advantages of high dissolution rate and high stability, and the preparation process improves preparation efficiency, reduces equipment energy consumption, and is suitable for industrial large-scale production.
Owner:JIANGSU SKYRUN PHARMA CO LTD
Methylprednisolone sodium succinate powder for injection and preparation method thereof
ActiveCN111249240AQuality improvementImprove stabilityOrganic active ingredientsPowder deliveryPenicillinFreeze-drying
Owner:武汉人福药业有限责任公司
Skin drug composition containing methylprednisolone aceponate and amino acid
InactiveCN103127136AEliminate side effectsPromote absorptionOrganic active ingredientsAntipyreticAcid derivativeBULK ACTIVE INGREDIENT
A skin drug composition containing methylprednisolone aceponate and an amino acid comprises the methylprednisolone aceponate which serves as an active ingredient, an amino acid derivative which serves as a nitric oxide synthase (NOS) inhibitor, one or more types of drug auxiliary materials applicable to skin, and the balance water.
Owner:TIANJIN JINYAO GRP
Methylprednisolone aceponate lipidosome cream
InactiveCN101601651AImprove stabilityPromote absorptionOrganic active ingredientsOrganic non-active ingredientsLiposome VesiclePh buffering
The invention relates to a lipidosome medical composition which uses methylprednisolone aceponate as an active component, and a cyst diameter of lipidosome is smaller than 800 nm. The composition comprises 0.025 percent to 0.2 percent of methylprednisolone aceponate as the active component, 0.5 percent to 6 percent of phospholipid, 0 percent to 1 percent of lipophilic additive, 0.01 percent to 1 percent of antioxidant which is used for preserving the medical composition, a pH buffering agent which is used for retaining the pH value from 5 to 7.5, 3 percent to 15 percent of humectant, 20 percent to 30 percent of oil phrase component, 0.01 percent to 0.1 percent of antimicrobial preservative and the balanced of water. The methylprednisolone aceponate lipidosome cream is used for treating skin diseases of human beings or animals.
Owner:TIANJIN JINYAO GRP
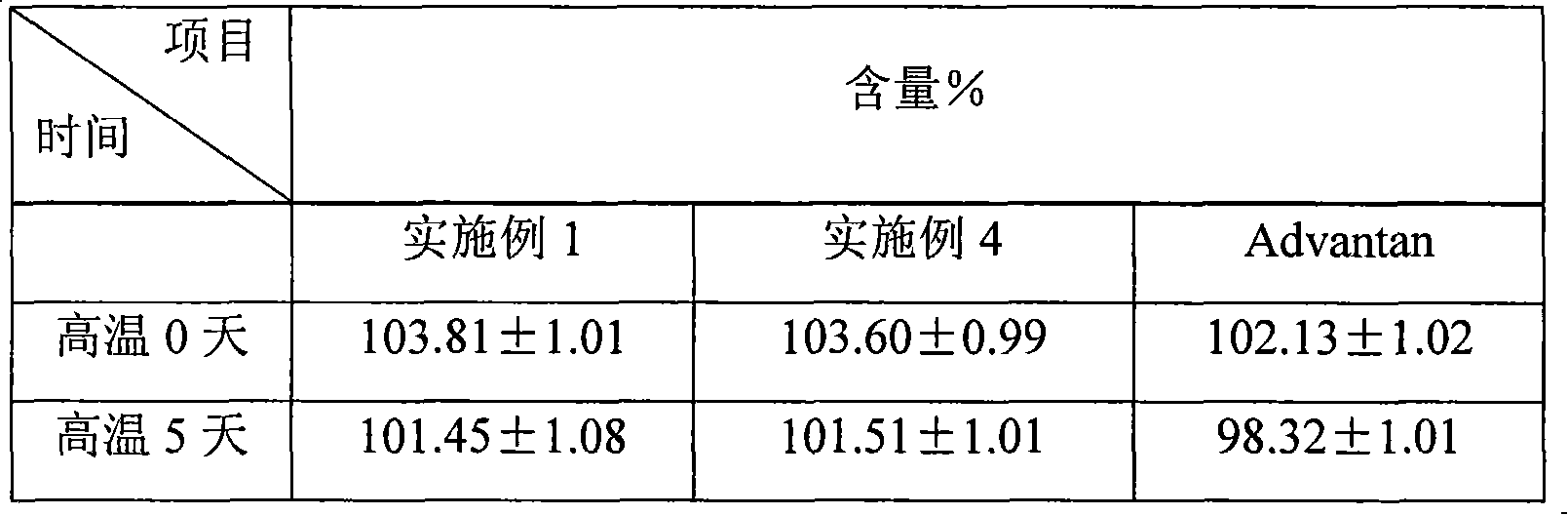
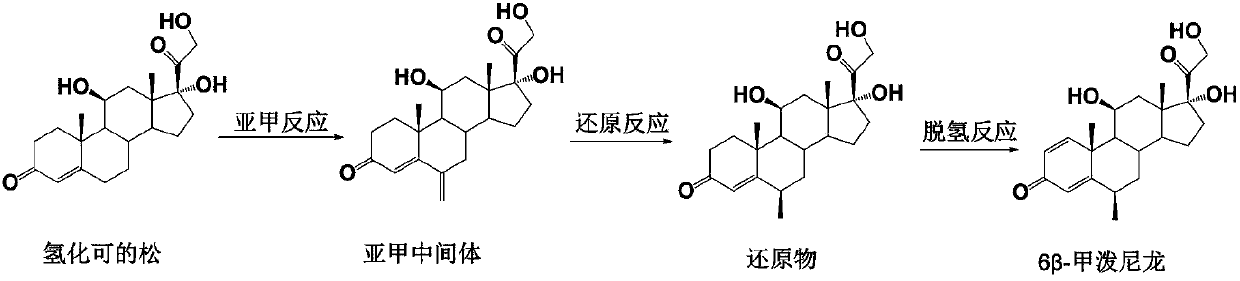
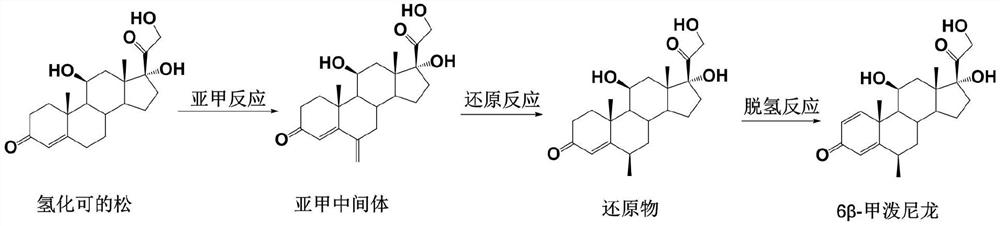
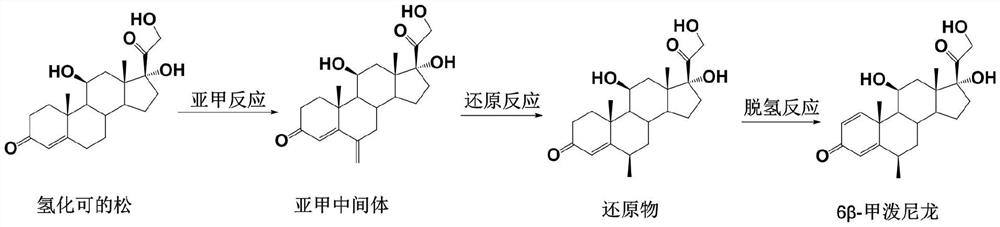
Preparation method of 6 beta-methylprednisolone
The invention provides a preparation method of 6 beta-methylprednisolone. Hydrocortisone is used as a reaction initiator, and 6 beta-methylprednisolone is prepared through methylene reaction, reduction reaction, and dehydrogenation reaction. The preparation method comprises following steps: (1) methylene reaction: reacting hydrocortisone with an alkylation reagent at the temperature of 0-50 DEG Cin the presence of a catalyst, then reacting with formaldehyde and N-methylaniline, adjusting the pH value to 1-2 to achieve acidic conditions, and obtaining a methylene intermediate after the reaction is finished; (2) reduction reaction: heating the methylene intermediate and cyclohexene to react under the catalysis of palladium on carbon and a monophosphite ester ligand to prepare a reduction product; and (3) dehydrogenation reaction: carrying out heating reflux reaction on the reduction product and a dehydrogenation reagent to obtain the 6 beta methylprednisolone. The preparation method hasthe beneficial effects that the catalyst is added in the reduction reaction, so that the 6 beta-methylprednisolone can be prepared through three-step chemical reaction.
Owner:TIANJIN PHARMA GROUP CORP
Methylprednisolone sodium succinate for injection and preparation method thereof
ActiveCN111150709AUniform appearanceGood resolubilityOrganic active ingredientsPowder deliverySuccinic acidLactose
The invention discloses a methylprednisolone sodium succinate preparation for injection and a preparation method thereof. The methylprednisolone sodium succinate preparation has a prescription composed of methylprednisolone succinate, sodium dihydrogen phosphate, disodium hydrogen phosphate, sodium hydroxide, mannitol, lactose and water for injection, which are different in usage amounts accordingto the specification of methylprednisolone sodium succinate for injection. According to the invention, the control of the dispersion rotating speed of methylprednisolone succinate, particularly the control of related parameters like the rotating speed and time of an alkali liquor adding program is emphasized in the preparation process of a liquid medicine; freeze-drying is carried out at a top speed; a process of gradient staged drying is adopted; the prepared methylprednisolone sodium succinate for injection is loose and powdery in appearance, good in re-solubility, free of opalescence through light inspection after re-dissolution, qualified in insoluble particle inspection, small in content of free methylprednisolone and related substances and capable of meeting the quality standard requirements; meanwhile, the freeze-drying time is greatly shortened; and the production efficiency is improved.
Owner:天津梅花生物医药科技有限公司
Transdermal absorption medicament used for skins and comprising adjuvant-containing methylprednisolone aceponate and adjuvant-containing water
InactiveCN102552282AGuaranteed stabilityReduce wasteOrganic active ingredientsSolution deliveryAdjuvantPharmaceutic Adjuvant
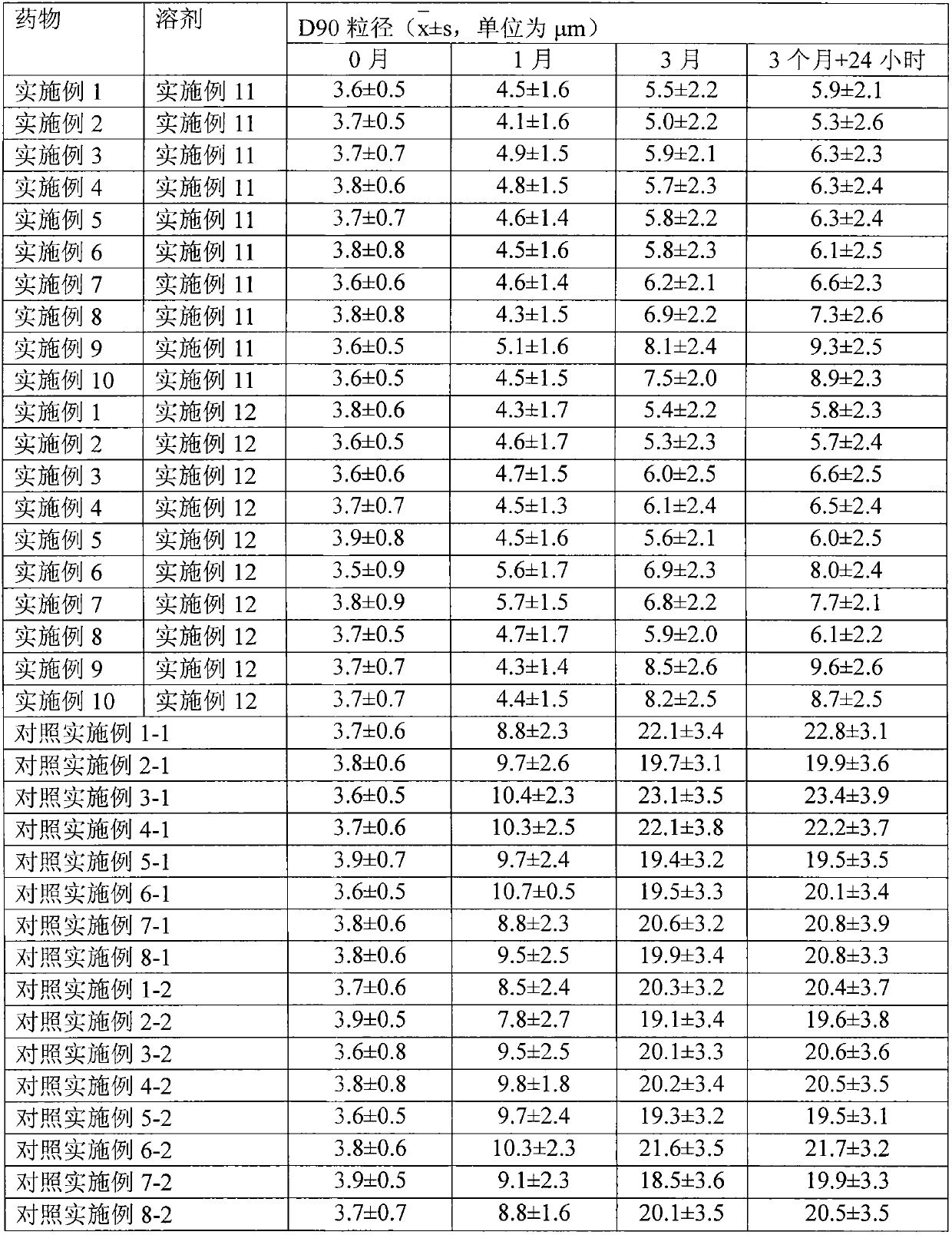
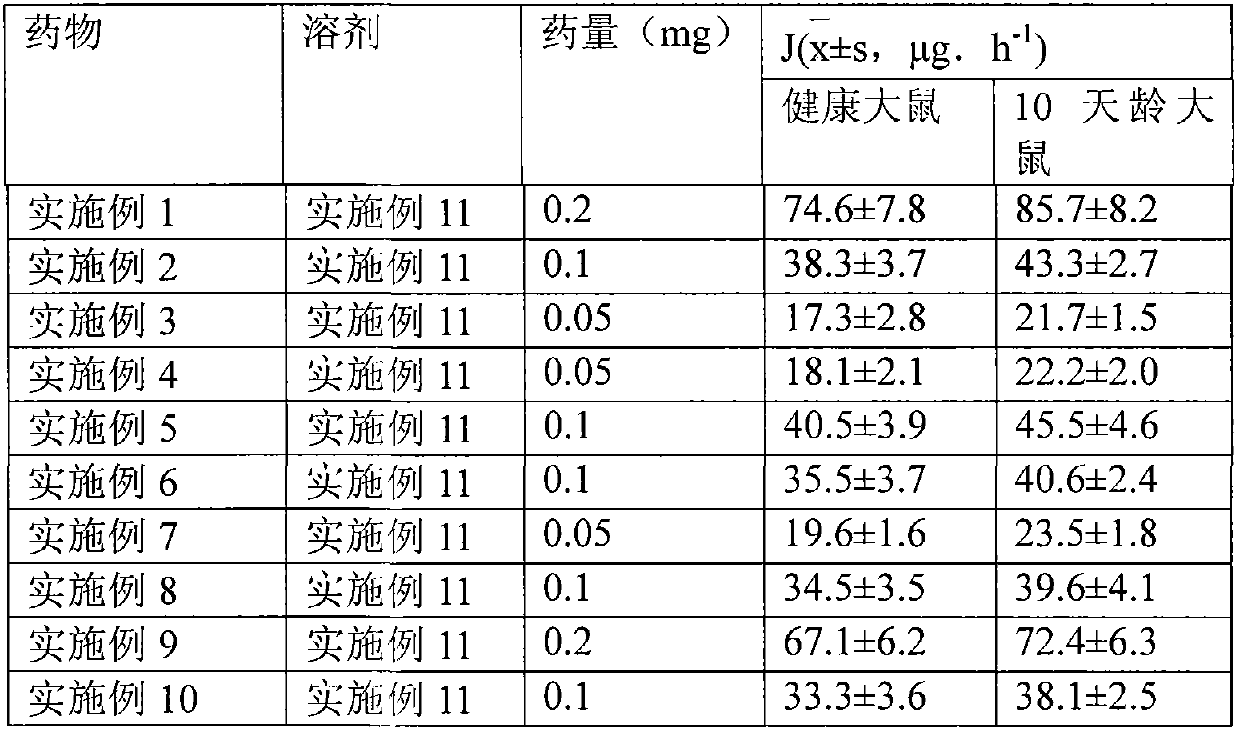
The invention relates to a transdermal absorption medicament used for skins and comprising adjuvant-containing methylprednisolone aceponate and adjuvant-containing water. The medicament comprises individually packaged methylprednisolone aceponate which contains one or several solid pharmaceutic adjuvants, is insoluble in water and has D90 particle sizes of 0.1-10mum, and individually packaged water containing one or several pharmaceutic adjuvants soluble in water.
Owner:TIANJIN JINYAO GRP
Adjuvant-containing methylprednisolone aceponate separation type water suspension agent drug for treating skin diseases
InactiveCN102552130AReduce wasteEasy to useOrganic active ingredientsSolution deliveryDiseaseAdjuvant
The invention relates to an adjuvant-containing methylprednisolone aceponate separation type water suspension agent drug for treating skin diseases. The drug comprises methylprednisolone aceponate and water, wherein the packing manner of the methylprednisolone aceponate adopts the individual packing, the methylprednisolone aceponate contains one or a plurality of skin medicine adjuvants, and is insoluble, the D90 particle size is 0.1-10 mum, and the packing manner of the water adopts the individual packing.
Owner:TIANJIN JINYAO GRP
A kind of microemulsion preparation of methylprednisolone acetate and preparation method thereof
InactiveCN104758246BImprove stabilityGood storage stabilityOrganic active ingredientsEmulsion deliveryPropionateSolubility
Belonging to the technical field of pharmaceutical preparations, the invention relates to a microemulsion preparation of methylprednisolone aceponate and a preparation method thereof. The microemulsion preparation of methylprednisolone aceponate is composed of the following components by mass: 0.01-1% of methylprednisolone aceponate, 10%-30% of a surfactant, 15-40% of a cosurfactant, 10-20% of an oil phase, and the balance water. The preparation method includes: mixing the oil phase, the surfactant and the co-surfactant evenly, adding methylprednisolone aceponate, conducting stirring for dissolving, then adding water, and performing stirring to obtain the microemulsion. According to the invention, methylprednisolone aceponate is made into a microemulsion state, and the dissolvability of methylprednisolone aceponate in the matrix is increased so as to make methylprednisolone aceponate highly diffusive, thus improving bioavailability. Also, the microemulsion has smaller particle size, is isotropic and more uniform, belongs to the homodisperse system, and has more excellent thermodynamic stability.
Owner:JIANGSU YUNYANG PHARMA GRP
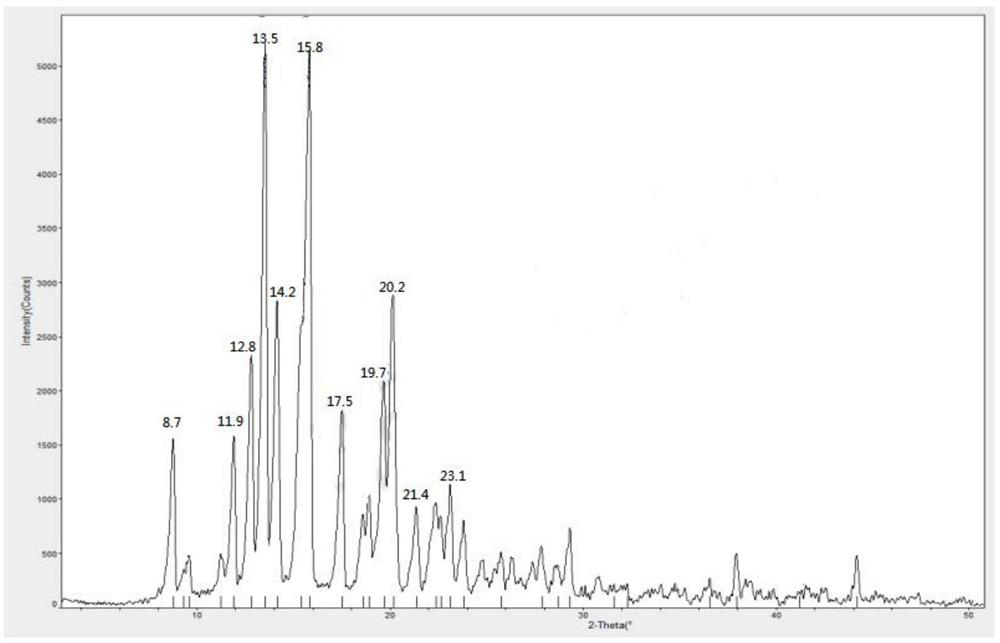
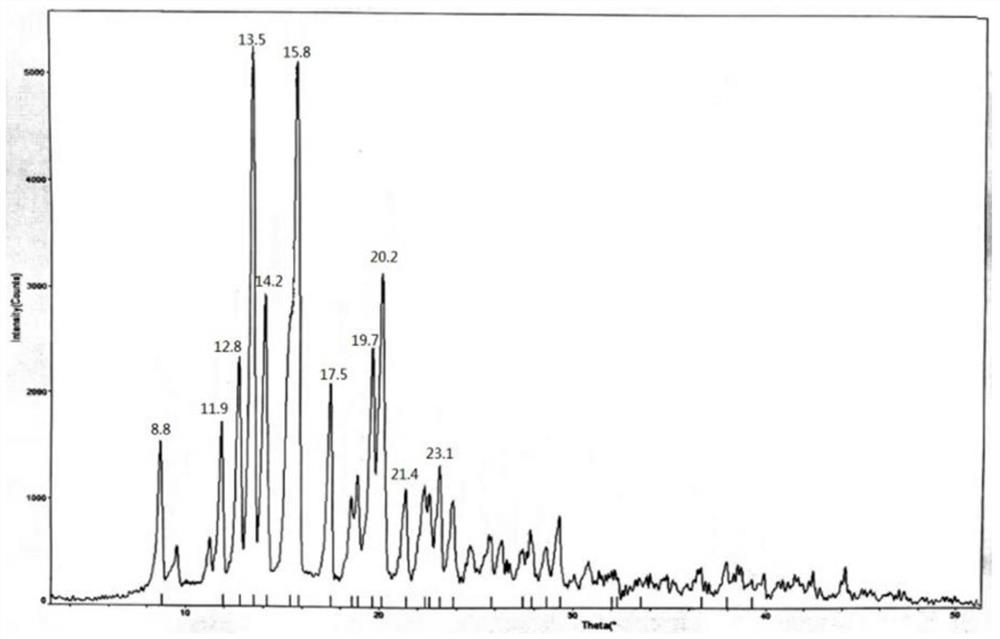
Methylprednisolone aceponate anhydrous crystal form and composition thereof
PendingCN111748010AEasy to storeImprove stabilityOrganic active ingredientsAntipyreticPhysical chemistryActive ingredient
The invention provides a methylprednisolone aceponate anhydrous crystal form I. Characteristic peaks exist when the X-ray powder diffraction angle 2theta of the crystal form I is equal to 12.8 degrees, 14.2 degrees, 15.8 degrees, 19.7 degrees and 20.2 degrees. The preparation method of the crystal form I comprises drying methylprednisolone aceponate monohydrate to obtain the methylprednisolone aceponate crystal form I. According to the invention, the methylprednisolone aceponate in the anhydrous crystal form I is prepared as a raw material medicine, the raw material medicine of the crystal form is more stable, and after the anhydrous crystal form I of the methylprednisolone aceponate is used for preparing cream, the cream is more uniform, good in dispersion state, high in safety and stablein absorption.
Owner:TIANJIN PHARMA GROUP CORP
Microemulsion preparation of methylprednisolone aceponate and preparation method thereof
InactiveCN104758246AImprove stabilityGood storage stabilityOrganic active ingredientsEmulsion deliverySolubilityOil phase
Belonging to the technical field of pharmaceutical preparations, the invention relates to a microemulsion preparation of methylprednisolone aceponate and a preparation method thereof. The microemulsion preparation of methylprednisolone aceponate is composed of the following components by mass: 0.01-1% of methylprednisolone aceponate, 10%-30% of a surfactant, 15-40% of a cosurfactant, 10-20% of an oil phase, and the balance water. The preparation method includes: mixing the oil phase, the surfactant and the co-surfactant evenly, adding methylprednisolone aceponate, conducting stirring for dissolving, then adding water, and performing stirring to obtain the microemulsion. According to the invention, methylprednisolone aceponate is made into a microemulsion state, and the dissolvability of methylprednisolone aceponate in the matrix is increased so as to make methylprednisolone aceponate highly diffusive, thus improving bioavailability. Also, the microemulsion has smaller particle size, is isotropic and more uniform, belongs to the homodisperse system, and has more excellent thermodynamic stability.
Owner:JIANGSU YUNYANG PHARMA GRP
The preparation method of 6β-methylprednisolone
The invention provides a preparation method for 6β-methylprednisolone, using hydrocortisone as a reaction starting material, through methylene reaction, reduction reaction, and dehydrogenation reaction to prepare 6β-methylprednisolone, comprising the following steps (1) methylene Reaction: Hydrocortisone reacts with an alkylating agent at a temperature of 0-50°C under the condition of a catalyst, then reacts with formaldehyde and N-methylaniline, and adjusts the pH value of the acidic condition to 1-2, and obtains methylene intermediate; (2) reduction reaction: under the catalysis of palladium carbon and monophosphite ligand, methylene intermediate reacts with cyclohexene heating to prepare the reduced product; (3) dehydrogenation reaction: the Reduction and dehydrogenation reagent, heat reflux reaction, 6β methylprednisolone. The beneficial effect of the present invention is that by adding a catalyst in the reduction reaction, 6β-methylprednisolone can be prepared through three steps of chemical reactions.
Owner:TIANJIN PHARMA GROUP CORP
Methylprednisolone aceponate monohydrate, crystal form and preparation method thereof
The invention provides a methylprednisolone aceponate monohydrate, a crystal form and a preparation method thereof. The crystal parameters of the methylprednisolone aceponate monohydrate are alpha 90.00, beta 90.00, gamma 90.00, crystal cell volume is that the crystal belongs to a rhombic system, and the space group is P212121. X-ray powder diffraction is used for measurement, the X-ray powder diffraction of the methylprednisolone aceponate monohydrate has characteristic peaks when the diffraction angle 2theta is equal to 8.6 degrees, 12.2 degrees, 13.6 degrees, 15.3 degrees, 18.6 degrees, 19.3 degrees and 20.5 degrees, and the relative diffraction intensity of the methylprednisolone aceponate monohydrate is substantially represented by the detailed spectrogram as figure 2. The methylprednisolone aceponate monohydrate can be prepared by a method of completely dissolving methylprednisolone aceponate in the mixed solution of water and an organic solvent and finally evaporating the organic solvent.
Owner:TIANJIN JINYAO GRP
Methylprednisolone aceponate cream preparation
The invention discloses a methylprednisolone aceponate cream preparation, which is characterized by consisting of methylprednisolone aceponate, oil phase matrix, oil phase emulsifier, aqueous phase matrix and aqueous phase emulsifier, wherein the HLB (hydrophile-lipophile balance) value of the oil phase emulsifier is 1.0 to 4.0, the oil phase emulsifier accounts for 2 to 8 percent of the weight of the cream, the HLB value of the aqueous phase emulsifier is 15 to 18, and the aqueous phase emulsifier accounts for 3 to 9 percent of the weight of the cream. The cream preparation has good stability and release degree.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
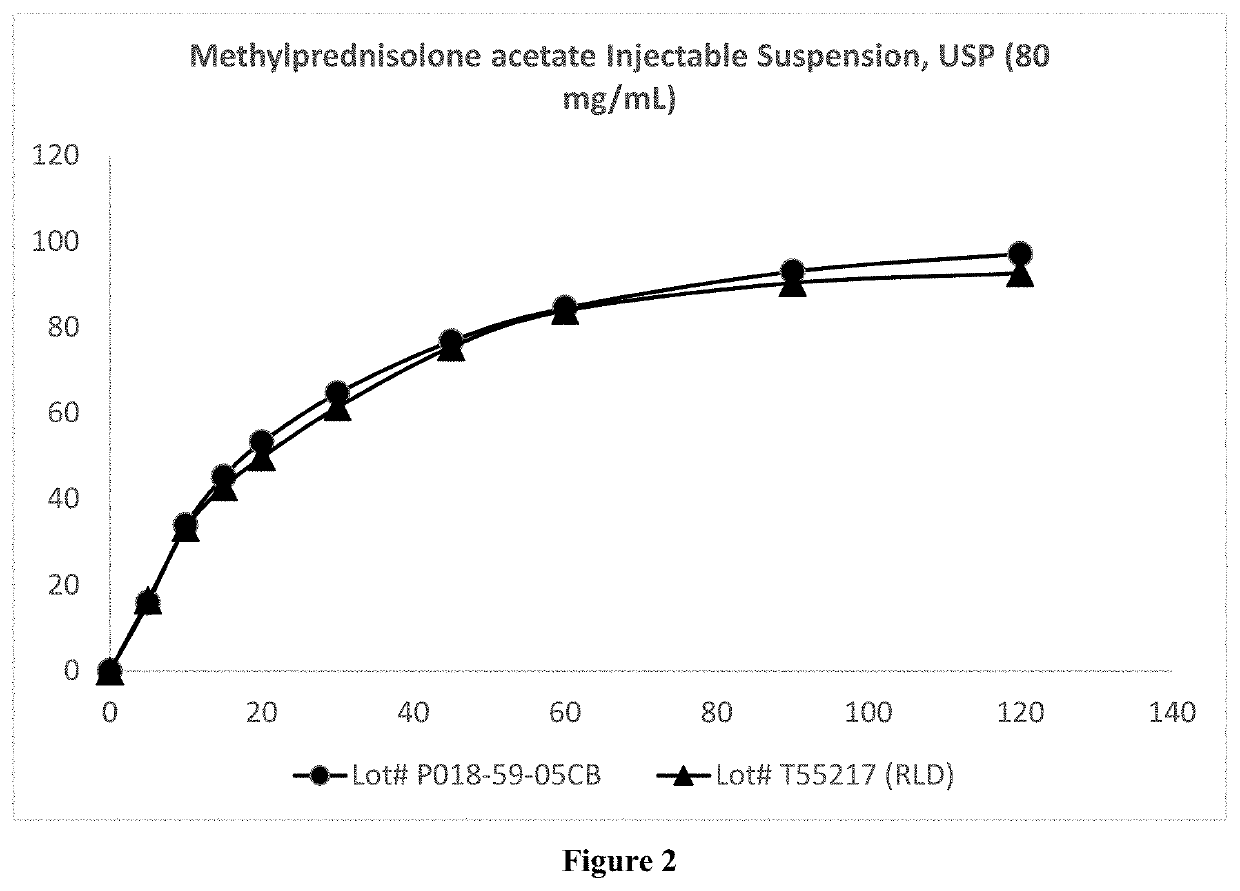
Preparation of Microparticulate Methylprednisolone Acetate Suspension
ActiveUS20210252019A1Easily resuspendedImprove stabilityOrganic active ingredientsSolution deliveryEthylic acidMETHYLPREDNISOLONE ACETATE INJECTION
The present invention relates to a process for preparation of a water-insoluble steroid composition by moist heat sterilization. The invention particularly relates to a process for preparation of a water-insoluble steroid composition comprising moist heat sterilization or autoclaving of an aqueous slurry of methylprednisolone acetate in the presence of a specified quantity of polysorbate. The suspensions prepared by using the current invention exhibited good physical and chemical stability. Compositions related thereto are also disclosed.
Owner:SOMERSET THERAPEUTICS LLC
Methylprednisolone sodium succinate powder for injection and preparation method thereof
ActiveCN111249240BQuality improvementImprove stabilityPowder deliveryOrganic active ingredientsFreeze-dryingBuffering agent
The invention relates to a methylprednisolone sodium succinate powder for injection and a preparation method thereof. The preparation method comprises the following steps: taking methylprednisolone sodium succinate and buffer (or weighing methylprednisolone sodium succinate, buffer and lactose) according to specifications, stirring and dissolving in a water bath less than 50°C with water for injection, After clarification, adjust the pH to 7.4-7.8 with sodium hydroxide solution, and constant volume with water for injection; (2) filter the solution obtained in step (1) with a microporous membrane and pack into vials; (3) divide the step ( 2) Freeze-dry the solution after subpackaging to obtain the methylprednisolone succinic acid freeze-dried powder for injection; the freeze-drying includes pre-freezing, primary drying and secondary drying, and the pre-freezing stage includes annealing operate. The methylprednisolone sodium succinate powder injection prepared by the method has less free methylprednisolone and total impurities, can greatly improve the quality of the medicine, and simultaneously reduce the freeze-drying time and improve the production efficiency.
Owner:武汉人福药业有限责任公司
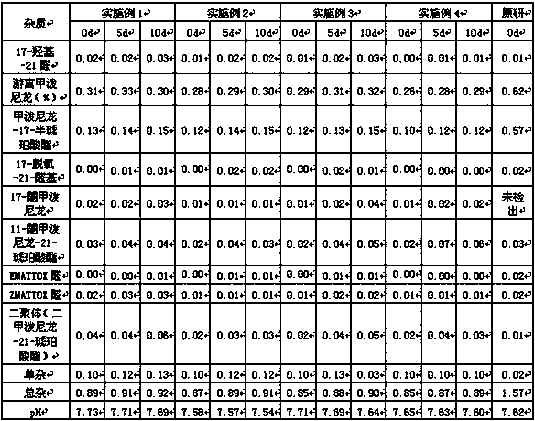
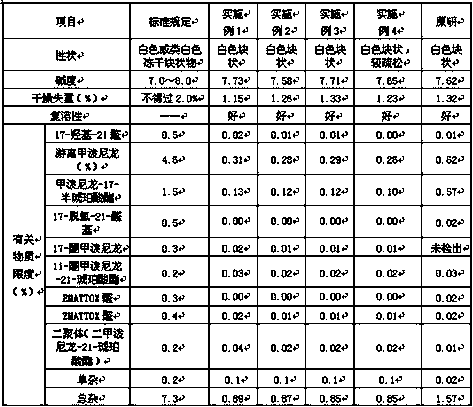
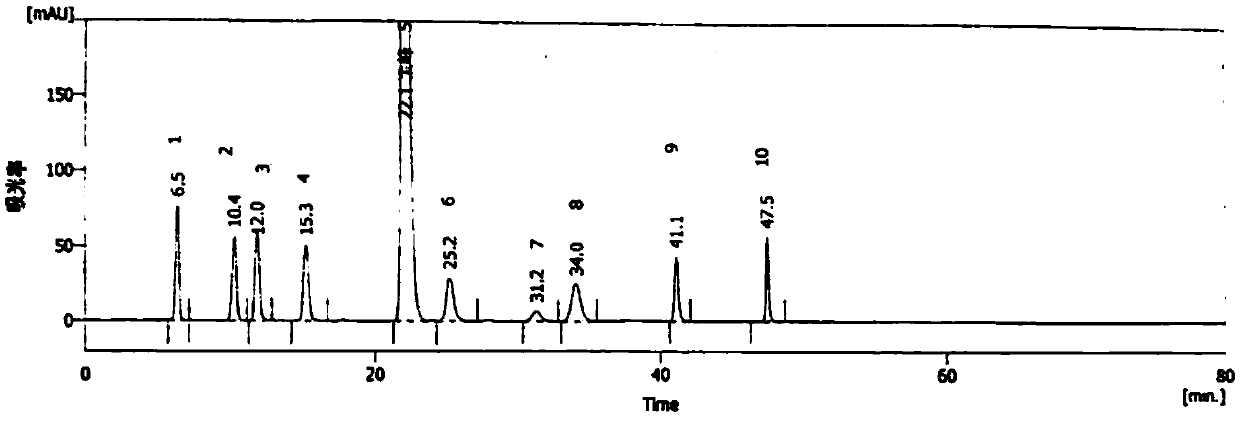
Method for detecting methylprednisolone aceponate content and related substances
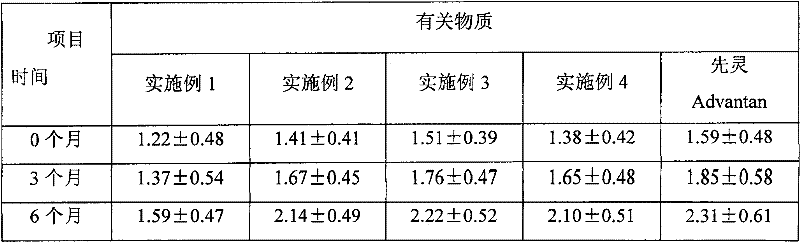
The invention provides a method for detecting the methylprednisolone aceponate content and related substances. Column chromatography is employed, octadecylsilane chemically bonded silica is used as afiller, an ultraviolet detector is used for detecting, the detection wavelength is 235-255 nm, the mobile phase A is a mixed solvent of water, methanol and acetonitrile, wherein the ratio of water is38%-42%, the ratio of methanol is 38%-42%, the ratio of acetonitrile is 16%-24%, and the mobile phase B is acetonitrile, and gradient elution is performed. The method has the beneficial effects that the content of methylprednisolone aceponate and nine contained related substances can be accurately and effectively detected and separated at the same time, and the method has good specificity and accuracy and is suitable for detecting the content of methylprednisolone aceponate and the related substances.
Owner:TIANJIN PHARMA GROUP CORP
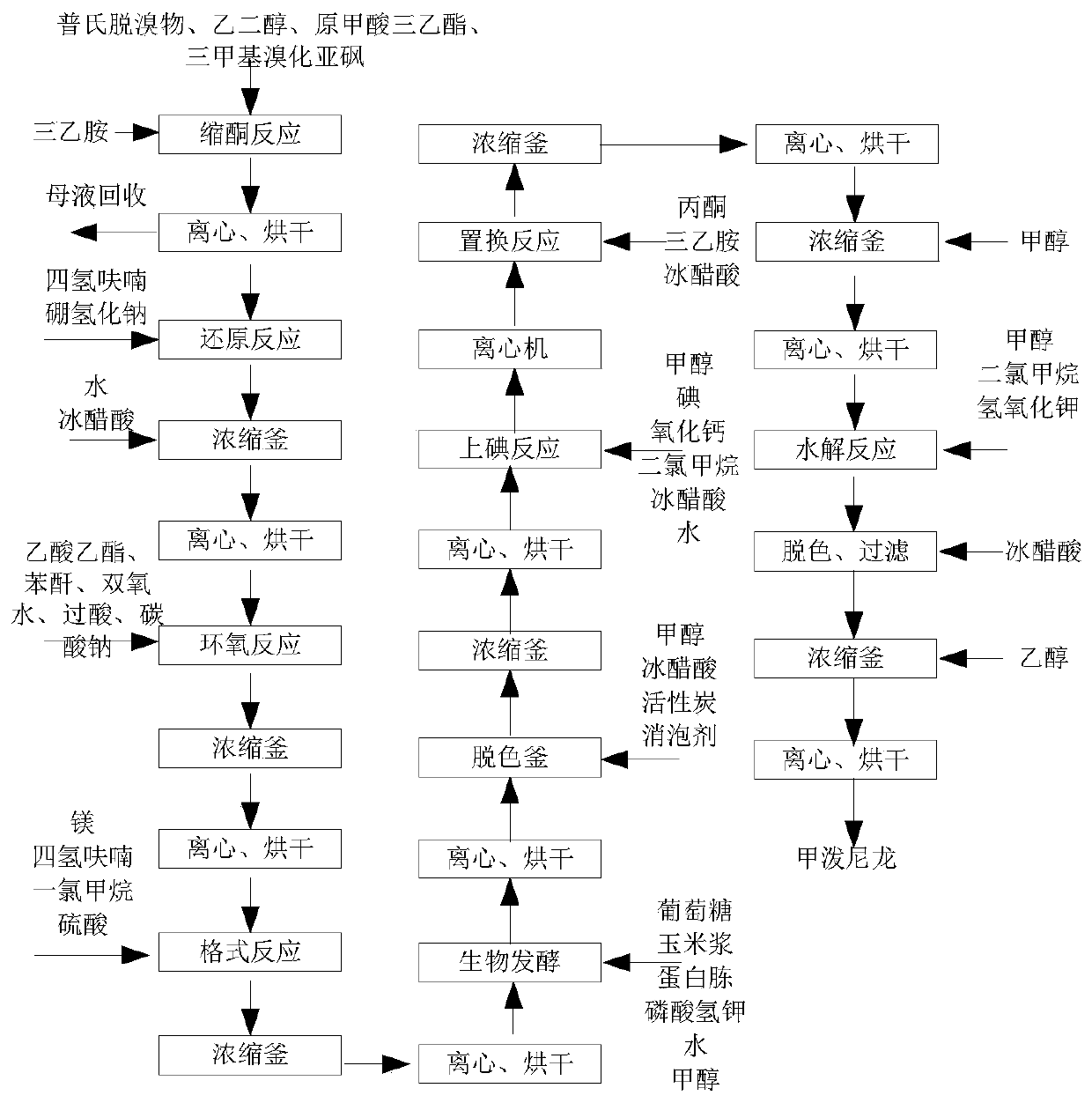
A kind of methylprednisolone production process and production device
ActiveCN108912192BGuaranteed purityQuality assuranceDrying gas arrangementsSteroidsMethylprednisoloneElectric machinery
The invention discloses a methylprednisolone production device, which comprises a reaction kettle, an elutriation kettle, a centrifugal filtration device, a concentration kettle, a decoloration kettle, a biological fermentation tank and a drying device which are connected in sequence, wherein the drying device comprises an outer cylinder, a drying cylinder and an inner cylinder; a motor is arranged on the bottom surface of the outer cylinder; an exhaust fan blade, a cylinder type impeller and a vortex impeller are arranged on a motor rotating shaft; the exhaust fan blade is arranged between the drying cylinder and the bottom surface of the outer cylinder; the cylinder type impeller is arranged between the drying cylinder and the inner cylinder; the vortex impeller is arranged at the bottomof the inner cylinder; an infrared heating pipe is arranged on the top surface of the inner cylinder; a feeding port is formed in the center of the upper end of the inner cylinder; the side wall of the outer cylinder comprises an inner layer wall and an outer layer wall; an annular interlayer cavity is formed between the inner layer wall and the outer layer wall; an annular baffle is arranged atthe outlet of the upper end of the interlayer cavity, a vent is formed in the baffle, and a rotatable air purification ring is arranged at the upper end of the baffle. According to the method, the preparation time of intermediate products in the production process of the methylprednisolone can be reduced, and rapid and high-quality production of the methylprednisolone can be realized.
Owner:YUEYANG HUANYU PHARMA +6
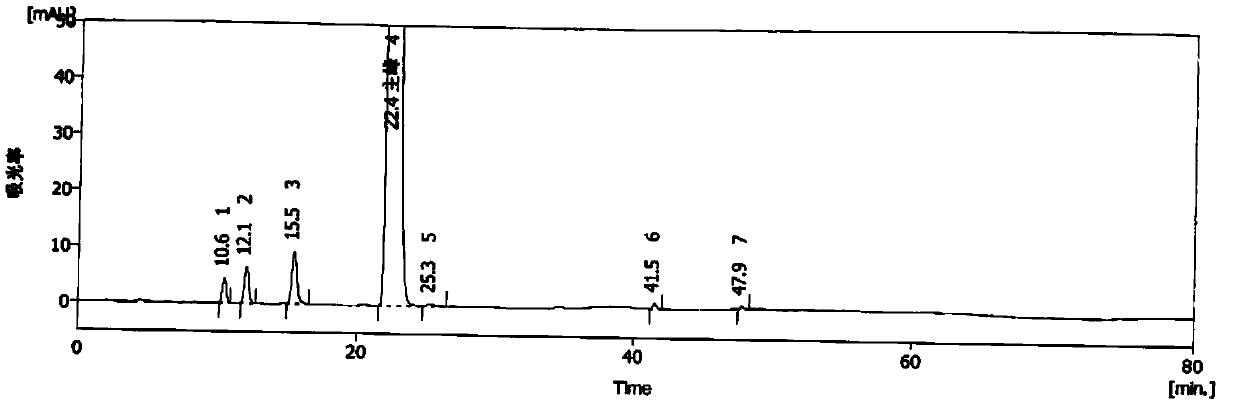
Analysis method of methylprednisolone intermediate
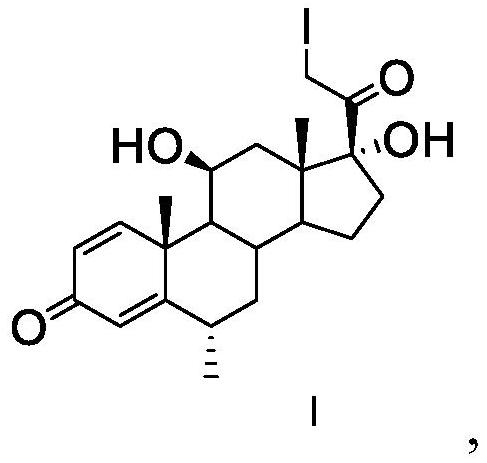
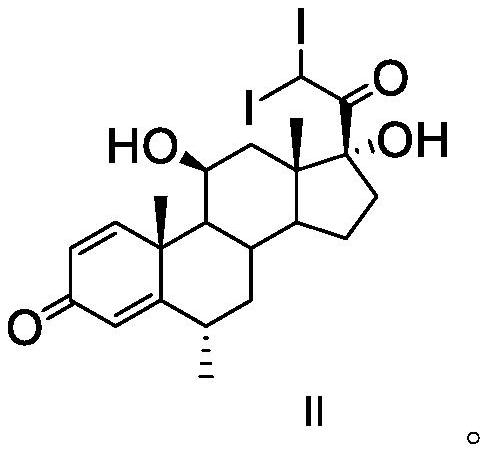
The invention discloses an analysis method of a methylprednisolone intermediate, which is characterized in that a high performance liquid chromatography method is adopted, and a substance to be detected is eluted. Chromatographic conditions are as follows: a stationary phase is a chromatographic column of octadecylsilane chemically bonded silica, in mobile phases, a mobile phase A is water, and a mobile phase B is methanol; the volume ratio of the water to the methanol is (50: 50)-(67: 33); the substance to be detected comprises methylprednisolone-iodide and / or methylprednisolone-diiodide. The analysis method provided by the invention has the characteristics of good separation degree, simplicity, rapidness, strong specificity, high sensitivity and the like.
Owner:武汉九珑人福药业有限责任公司
Methylprednisolone aceponate new crystal form and preparation method thereof
InactiveCN101805387BSmall particle sizeGuaranteed stabilityOrganic active ingredientsAntipyreticOrganic solventX-ray
Owner:TIANJIN JINYAO GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com