Analysis method of methylprednisolone intermediate
A technology of methylprednisolone and an analytical method is applied in the field of detection and analysis of methylprednisolone intermediates and achieves the effects of strong specificity, good separation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0039] Preparation example 1: preparation of the sample to be tested
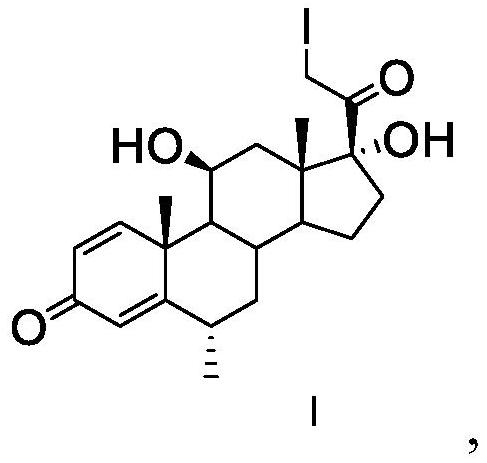
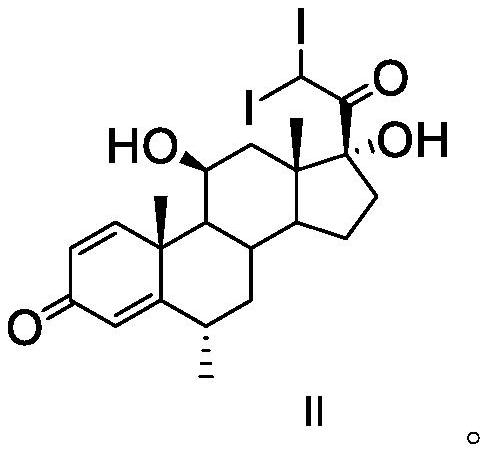
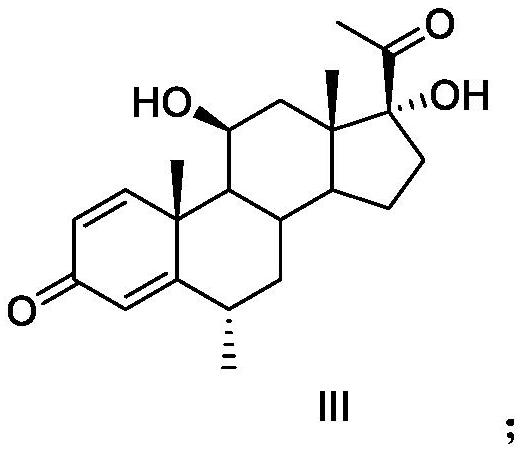
[0040] Add 0.1g of calcium chloride and 10mL of methanol into a 25ml single-necked bottle, dissolve and add 1.7g of iodine, and use it as iodine solution for later use. Add 2g of ammonium chloride and 10mL of water into a 25ml single-necked bottle to prepare an ammonium chloride solution, which will be used as an ammonium chloride solution for later use. Add 20mL of dichloromethane, 0.2g of calcium chloride, 0.3g of calcium oxide, 2g of methylprednisolone dehydrogenate and 10mL of methanol into a 100ml three-neck flask, add iodine solution dropwise, and react for about 1 hour. Pump the prepared ammonium chloride solution into the three-necked flask, and react for about 2 hours; add 30 mL of water to the reaction solution under stirring, filter, wash with a small amount of water, and collect the filter cake to obtain methylprednisolone iodide.
Embodiment 1
[0042] Chromatographic conditions:
[0043] Injection volume: 20μl, detection wavelength: 230nm, chromatographic column: octadecylsilane bonded silica gel as filler (YMC, ODS-A, 15cm*4.6mm, 5μm), flow rate: 1ml / min, column Temperature: 20°C, mobile phase: methanol, water and trifluoroacetic acid, the volume ratio is 62:38:0.01, sample configuration: take an appropriate amount of the sample to be tested, weigh it accurately, dissolve it with an appropriate amount of methanol, and dilute it with the mobile phase to 0.4mg / ml concentration of the solution, shake well, accurately measure 20μl into the liquid chromatograph, record the chromatogram to 1.5 times the retention time of the main peak, the results are shown below.
[0044]
[0045] Remarks: Impurity A is methylprednisolone, impurity B is methylprednisolone dehydrogenate, impurity C is an unknown impurity with RRT ≈ 0.94, and impurity D is an unknown impurity with RRT ≈ 1.59.
Embodiment 2
[0047] Chromatographic conditions:
[0048] Injection volume: 20μl, detection wavelength: 240nm, chromatographic column: octadecylsilane bonded silica gel as filler (YMC, ODS-A, 15cm*4.6mm, 5μm), flow rate: 1ml / min, column Temperature: 30°C, mobile phase: methanol, water and trifluoroacetic acid, the volume ratio is 62:38:0.01, sample configuration: take an appropriate amount of the sample to be tested, weigh it accurately, dissolve it with an appropriate amount of methanol, and dilute it with the mobile phase to 0.5mg / ml concentration of the solution, shake well, accurately measure 20μl into the liquid chromatograph, record the chromatogram to twice the retention time of the main peak, the results are shown below.
[0049]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com