Transdermal absorption medicament used for skins and comprising adjuvant-containing methylprednisolone aceponate and adjuvant-containing water
A technology of methylprednisolone acetonide and pharmaceutical excipients is applied in the directions of medical preparations containing active ingredients, skin diseases, drug combinations, etc., which can solve the problem of particle instability and inability to guarantee the Ostwald ripening of microparticle suspensions. and other problems to achieve the effect of reducing waste and ensuring suspension stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
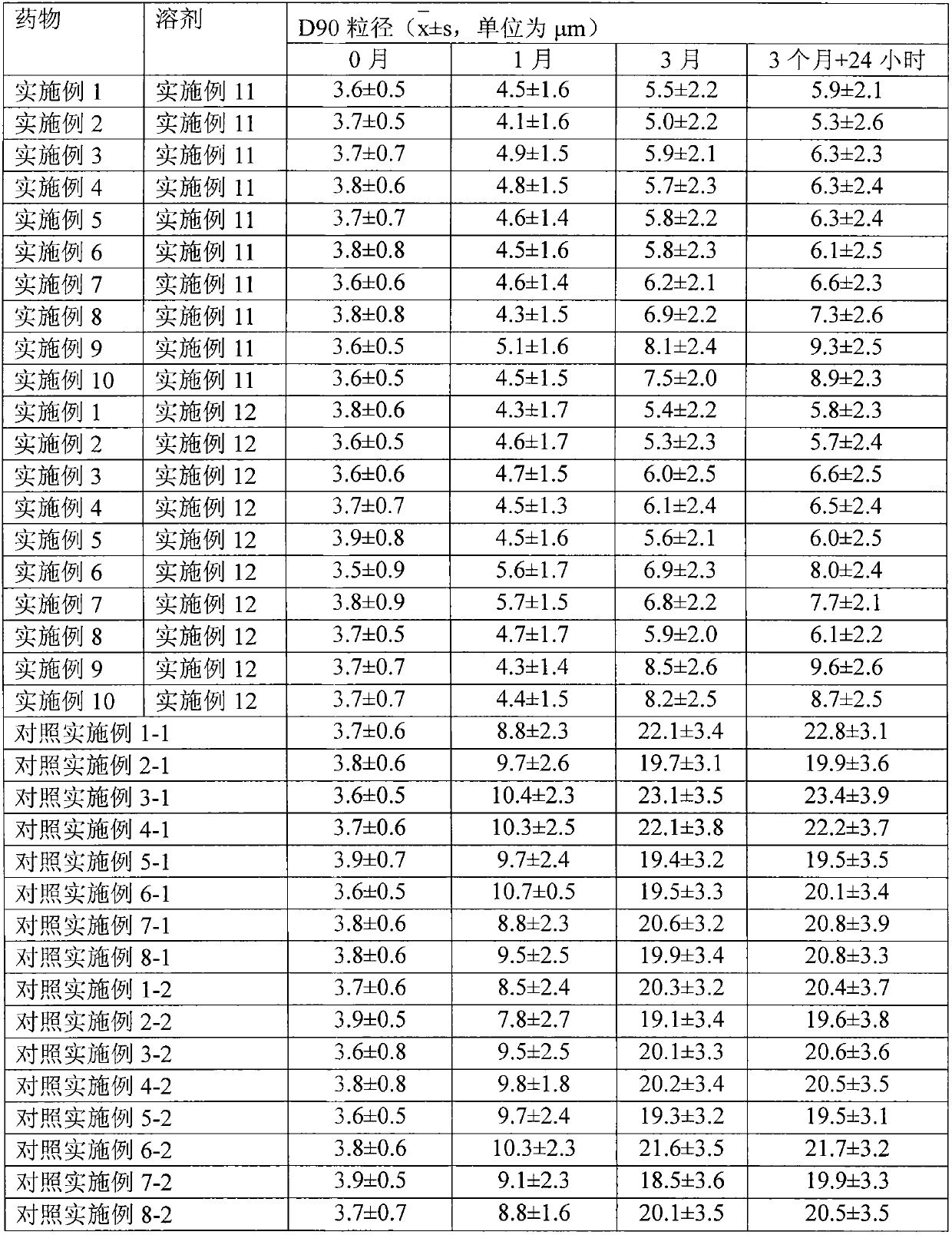
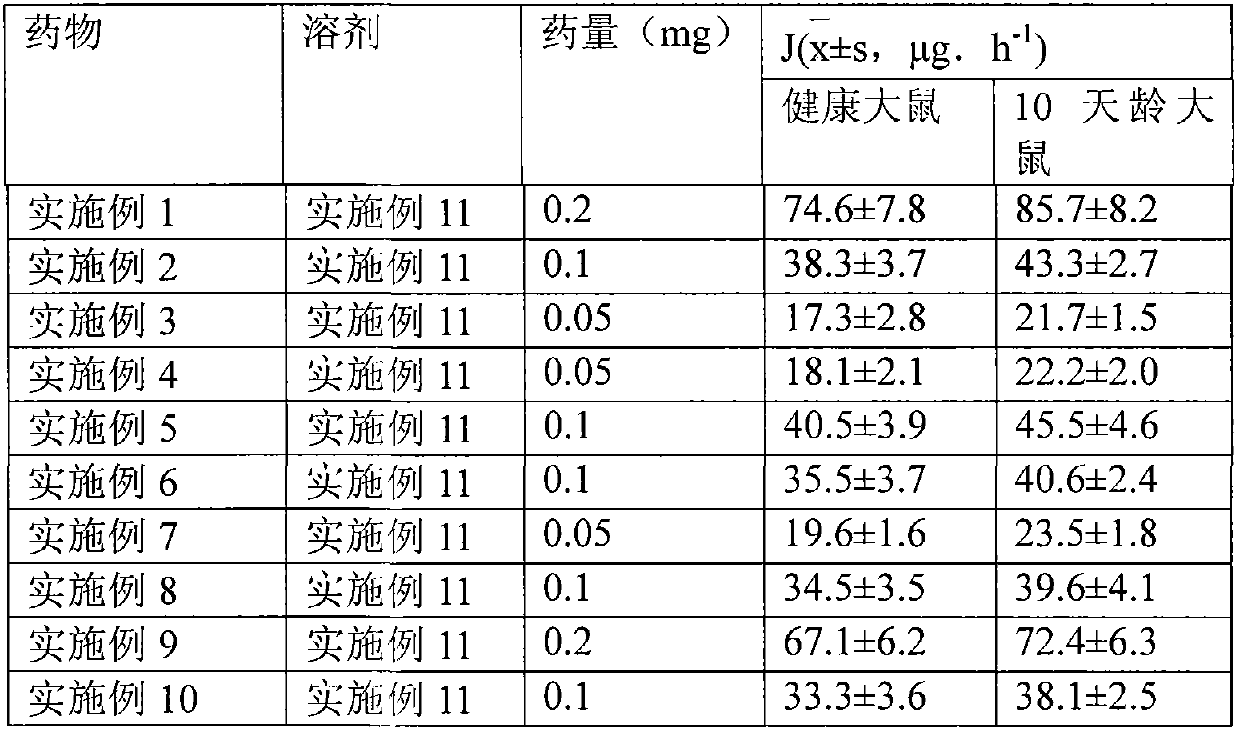
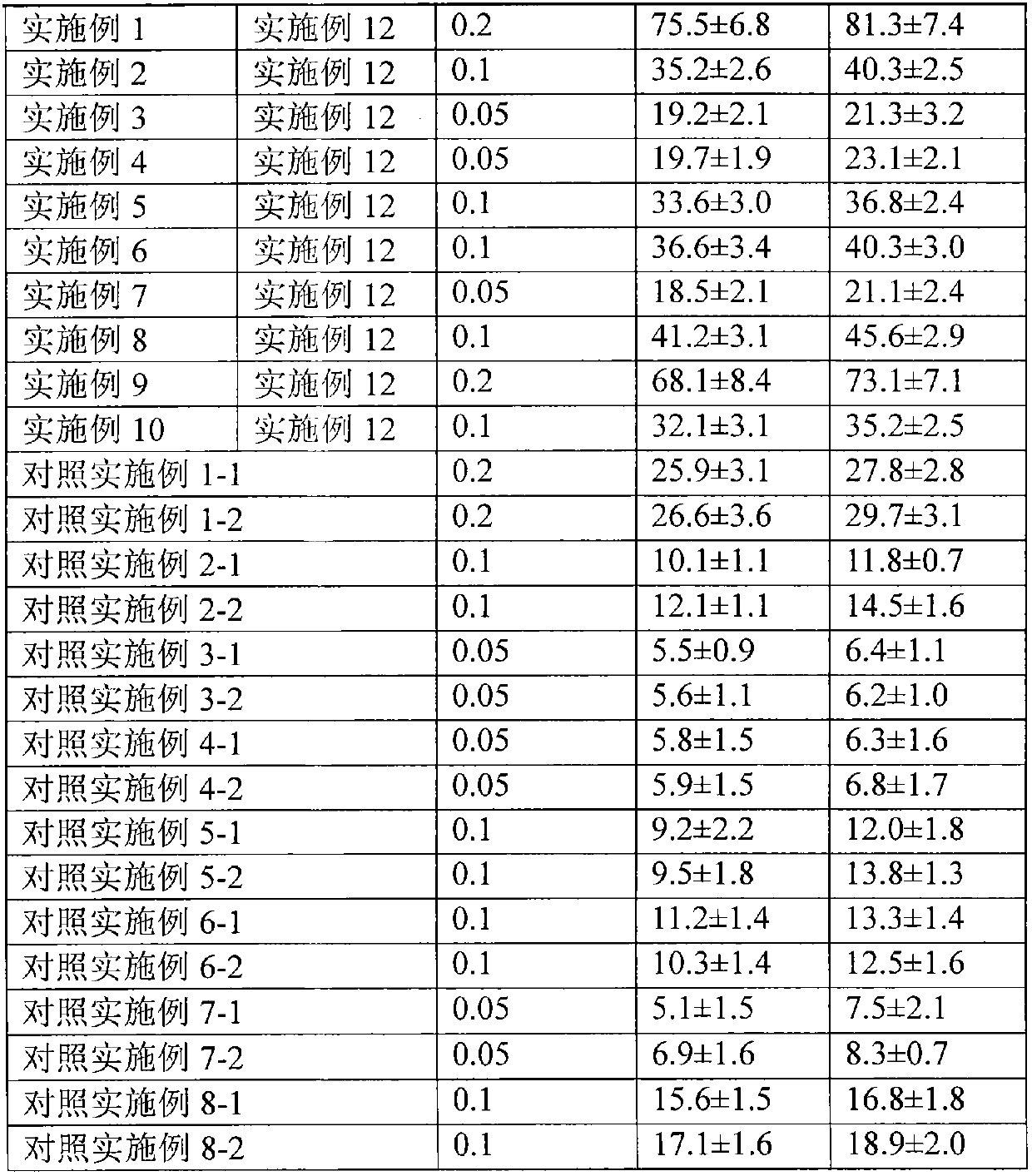
[0039] 1. Preparation of drug particles for transdermal absorption
[0040] The capsules packed with the drug microparticles obtained in Examples 1 to 10 are plant capsules, and the capsules are sealed and packaged with aluminum foil blisters after packing. The water containing auxiliary materials obtained in Examples 11 and 12 is sealed and packed in ampoules.
Embodiment 1
[0042] Dissolve 0.2g of methylprednisolone acetin in ethanol, filter, sterilize, spray-dry the filtrate, micronize the particle size to 3 μm, mix it with 1.5 g of crystalline lactose with a particle size of 30 μm, and pass it through a 200-mesh sieve for 3 times After mixing, divide into No. 2 capsules, and each capsule contains 2 mg of methylprednisolone acetone. Electron microscope observation shows that the drug particles are spherical.
[0043] The process conditions are: the inlet temperature is 100°C, the outlet temperature is 70°C, the air flow rate is 90%, the inner diameter of the nozzle outlet is 0.1cm, the air flow rate of the nozzle is 800ml / min, and the injection speed is 50mL / h
Embodiment 2
[0045] Dissolve 1 g of methylprednisolone acetin in ethanol, filter, spray dry the filtrate, micronize the particle size to 3 μm, mix it with 15 g of crystalline lactose with a particle size of 30 μm, mix it with a 200-mesh sieve for 3 times, and then sterilize , divided into No. 2 capsules, and each capsule contains 1 mg of methylprednisolone acetone. Electron microscope observation shows that the particles are spherical.
[0046] The process conditions are as follows: inlet temperature is 105°C, outlet temperature is 70°C, air flow rate is 90%, nozzle outlet inner diameter is 0.1cm, nozzle air flow rate is 800ml / min, and sample injection speed is 50mL / h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com