Methylprednisolone aceponate anhydrous crystal form and composition thereof
A methylprednisolone acetonide, crystal-free technology, applied in the field of medicine, can solve the problems of high temperature and humidity requirements, increased impurities, easy water loss, etc., and achieves the effects of high safety, good dispersion state, and stable absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Preparation of anhydrous crystal form I of methylprednisolone acetidine
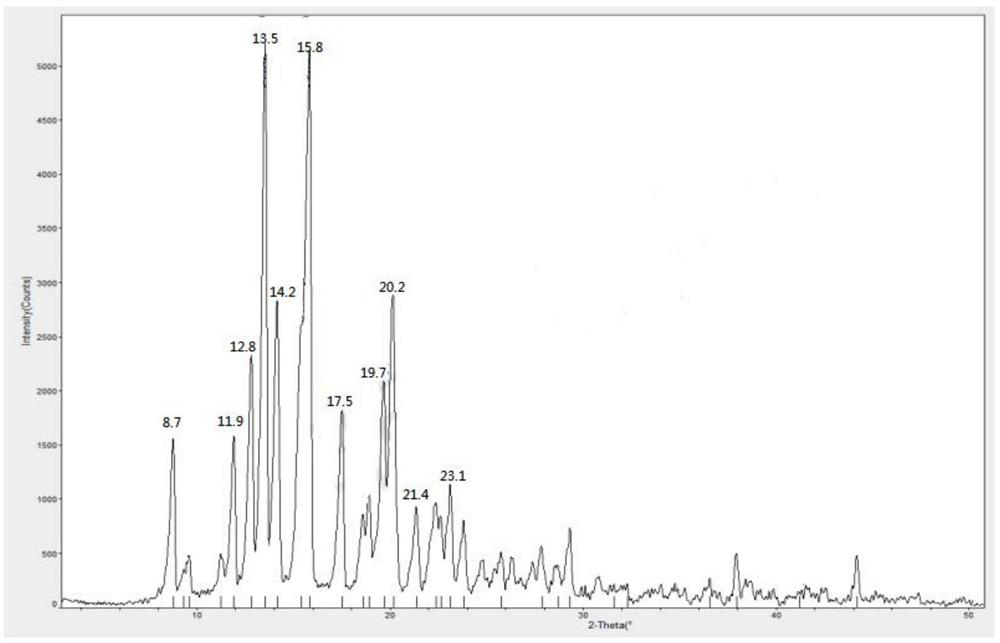
[0033] 1.1 Dissolve 10g of methylprednisolone acetate completely in 50ml of acetone, then slowly dilute it into 500ml of purified water at 0-10°C, stir for 1 hour after adding, filter to obtain methylprednisolone acetate monohydrate, and dry at 60-70°C for 14 hours. The X-ray powder diffraction measurement of the dried crystal form shows that the characteristic peak positions are 2θ=12.8°, 13.5°, 14.2°, 15.8°, 19.7°, 20.2°, such as figure 1 shown.
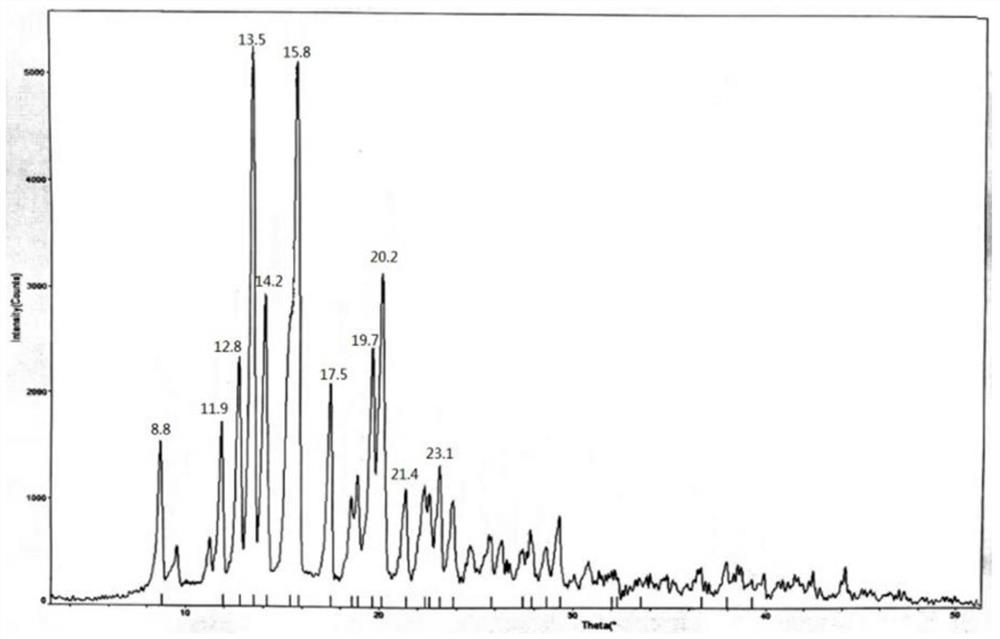
[0034] 1.2 Completely dissolve 10g of methylprednisolone acetidine in 40ml of methanol, then slowly dilute it into 500ml of purified water at 0-10°C, stir for 1 hour after adding, filter to obtain methylprednisolone acetate monohydrate, and dry at 70-80°C for 15 hours. The X-ray powder diffraction measurement of the dried crystal form shows that the characteristic peak positions are 2θ=12.8°, 13.5°, 14.1°, 15.8°, 19.6°, 20.1°, such as f...
Embodiment 2
[0044] The preparation of embodiment 2 acepromethylprednisolone emulsifiable cream
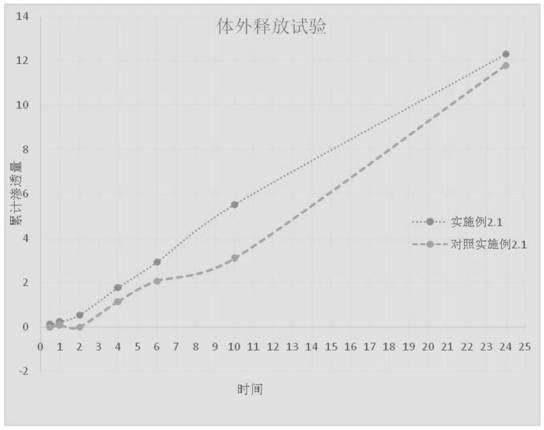
[0045] 2.1 Prepare 0.1% methylprednisolone acetidine cream with the crystal form I obtained in Example 1:
[0046] The prescription of methylprednisolone acetate cream is: methylprednisolone acetate 0.1g; white petrolatum 6g; stearyl alcohol 3g; glyceryl monostearate 5g; decyl oleate 5g; Pingpingjia A-201g; glycerin 5g; Disodium edetate 0.2g; benzyl alcohol 1g; polyvinylpyrrolidone 0.5g, the balance is water.
[0047] The methylprednisolone acetone crude drug is pulverized to a d(90) of 30 microns, and a d(50) of 10 microns. Precisely weigh the above ingredients, place each substance in a container, and heat until melted; then dissolve the water phase ingredients in water, mix the oil phase ingredients and water phase ingredients, heat to 85 degrees Celsius, add benzyl alcohol, and add vinegar and acrylic acid Methylprednisolone is stirred to obtain a suspension of the principal agent, and t...
Embodiment 3
[0057] The stability test of embodiment 3 bulk drug
[0058] Place the following APIs at room temperature (25±2°C) and in a colorless, transparent and closed watch glass, take samples at 1 month, 3 months, 6 months and 12 months respectively, and detect the content and related substances by HPLC: Octadecylsilane bonded silica gel is used as filler, detected by ultraviolet detector, detection wavelength is 245nm, mobile phase A is a mixed solvent of water, methanol and acetonitrile, mobile phase B is acetonitrile, gradient elution.
[0059] The results are shown in Table 1.
[0060] Table 1 API Stability Test
[0061]
[0062]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diffraction angle | aaaaa | aaaaa |
| diffraction angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com