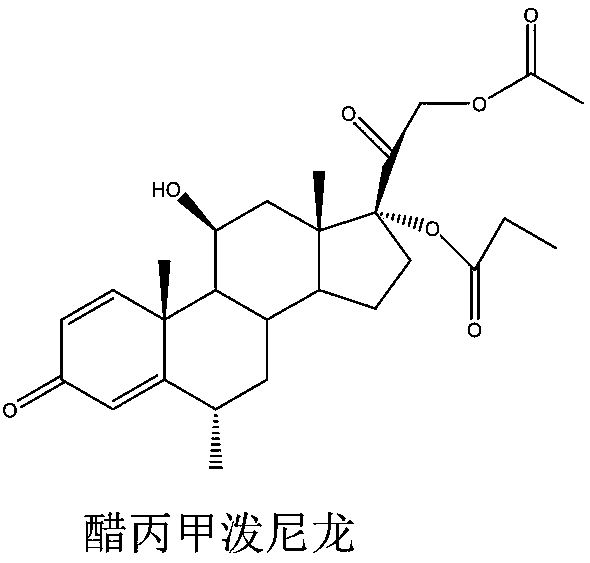
Method for detecting methylprednisolone aceponate content and related substances
A technology of methylprednisolone acetate and related substances, which is applied in the detection field of methylprednisolone acetate and related substances, and can solve problems such as instability, inability to achieve effective separation, and late peaking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
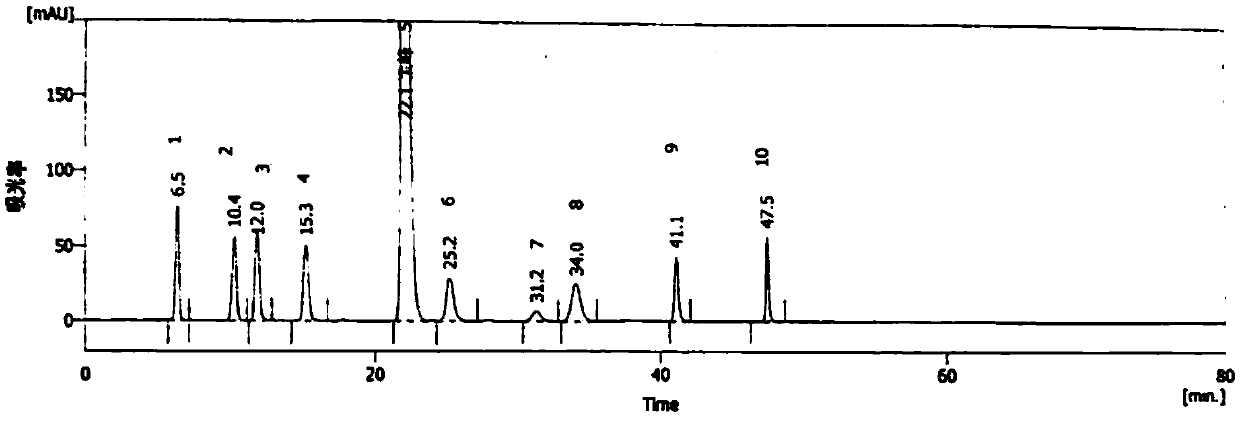
[0039] System suitability solution: Accurately weigh methylprednisolone acetate reference substance to prepare about 1.0 mg / ml of methylprednisolone acetate; take MPA-ZZ1 reference substance, MPA-ZZ2 reference substance, MPA-ZZ3 reference substance, MPA - ZZ4 reference substance, MPA-ZZ5 reference substance, MPA-ZZ6 reference substance, MPA-ZZ8 reference substance, MPA-ZZ9 reference substance and MPA-ZZ7 reference substance are prepared in appropriate amounts to contain 20.0 μg / ml, and mixed to obtain system suitability solution.
[0040] Blank solution: use acetonitrile as the blank solution.
[0041] Determine according to high-performance liquid chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0512). Use octadecylsilane bonded silica gel as filler (250mm×4.6mm, 5μm) chromatographic column; use water-methanol-acetonitrile (40:40:20) as mobile phase A and acetonitrile as mobile phase B, press Gradient elution is carried out; the detection wavelength is ...
Embodiment 2
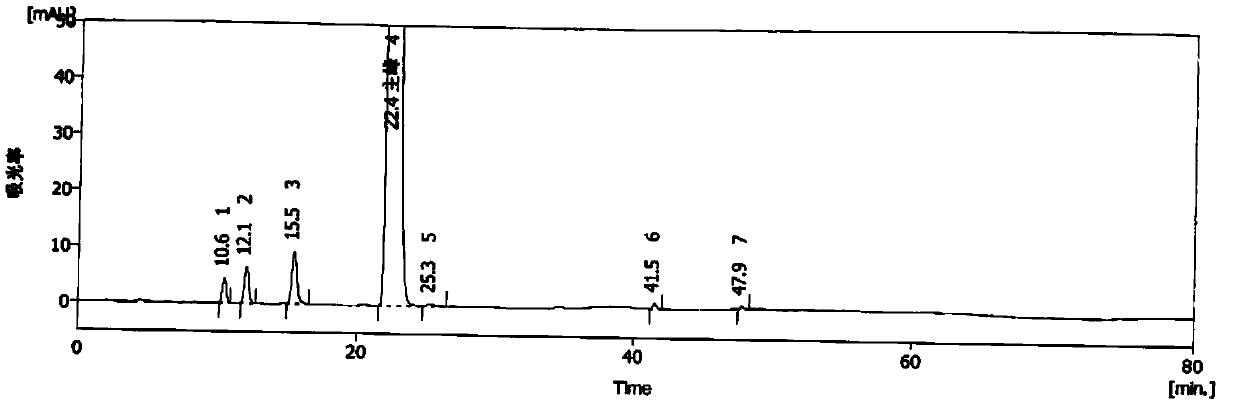
[0047] Need testing solution: take the appropriate amount of methylprednisolone acetate of MPA0810 batch, accurately weighed, add acetonitrile to dissolve and quantitatively dilute to make a solution containing about 1.0mg in every 1ml, as the need testing solution.
[0048] Reference substance solution: take 1ml of the test solution and put it in a 100ml measuring bottle, add acetonitrile to dilute to the mark, shake well, then accurately measure 1ml, put it in a 10ml measuring bottle, add acetonitrile to dilute to the mark, shake well, and use as Control solution.
[0049] Blank solution: use acetonitrile as the blank solution.
[0050] According to the following experimental conditions, take 20 μl each of the blank solution, the test solution and the reference solution, inject them into the liquid chromatograph respectively, adjust the detection sensitivity, and record the chromatograms. The chromatographic conditions are as follows:
[0051] Use octadecylsilane bonded si...
Embodiment 3
[0057] System suitability solution: Accurately weigh methylprednisolone acetate reference substance to prepare about 1.0 mg / ml of methylprednisolone acetate; take MPA-ZZ1 reference substance, MPA-ZZ2 reference substance, MPA-ZZ3 reference substance, MPA - ZZ4 reference substance, MPA-ZZ5 reference substance, MPA-ZZ6 reference substance, MPA-ZZ8 reference substance, MPA-ZZ9 reference substance and MPA-ZZ7 reference substance are prepared in appropriate amounts to contain 20.0 μg / ml, and mixed to obtain system suitability solution.
[0058] Blank solution: use acetonitrile as the blank solution.
[0059] Determine according to high-performance liquid chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0512). Use octadecylsilane bonded silica gel as filler (250mm×4.6mm, 5μm) chromatographic column; use water-methanol-acetonitrile (40:40:20) as mobile phase A and acetonitrile as mobile phase B, press Gradient elution is carried out; the detection wavelength is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com