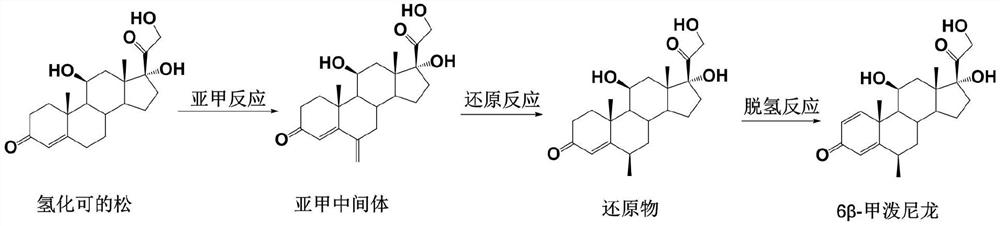
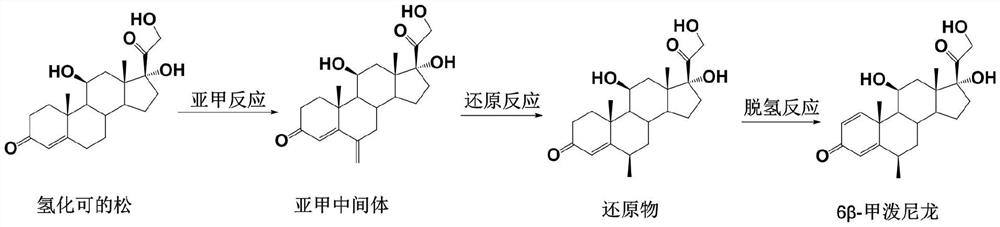
The preparation method of 6β-methylprednisolone
A technology of methylprednisolone and methylene, which is applied in the field of compound preparation, can solve the problems of long synthetic route, danger, and low yield, and achieve the effects of low cost, easy control, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 methylene intermediate
Embodiment 1-1
[0043] Dissolve 100 g of hydrocortisone in 1 L of ethanol, add 80 ml of triethyl orthoformate and 1 g of p-toluenesulfonic acid, stir at room temperature for 10 minutes, add 120 ml of 40% formaldehyde solution and 80 ml of N-methylaniline, stir at room temperature for 3 hours, add Concentrated hydrochloric acid adjusted the pH value of the reaction solution to 1-2, and kept stirring for 2 hours; the reaction solution was diluted into 3 L of water, the solid was precipitated, filtered with suction, and the filter cake was washed with water until neutral, and dried to obtain 98.5 g of a methylene intermediate with a molar yield of 98.5 g. The yield was 94.8%, and the HPLC purity was 95%.
Embodiment 1-2
[0045] Dissolve 36 g of hydrocortisone in 400 mL of methanol, add 45 ml of trimethyl orthoformate and 0.5 g of p-toluenesulfonic acid, stir at room temperature for 10 minutes, add 60 ml of 40% aqueous formaldehyde solution and 45 ml of N-methylaniline, and stir at room temperature for 3 hours. Add concentrated sulfuric acid to adjust the pH value of the reaction solution to 1-2, keep stirring for 2 hours; dilute the reaction solution into 1.5 L of water, separate out solids, filter with suction, wash the filter cake with water until neutral, and dry to obtain 35.1 g of methylene intermediate, The molar yield was 93.9%, and the HPLC purity was 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com