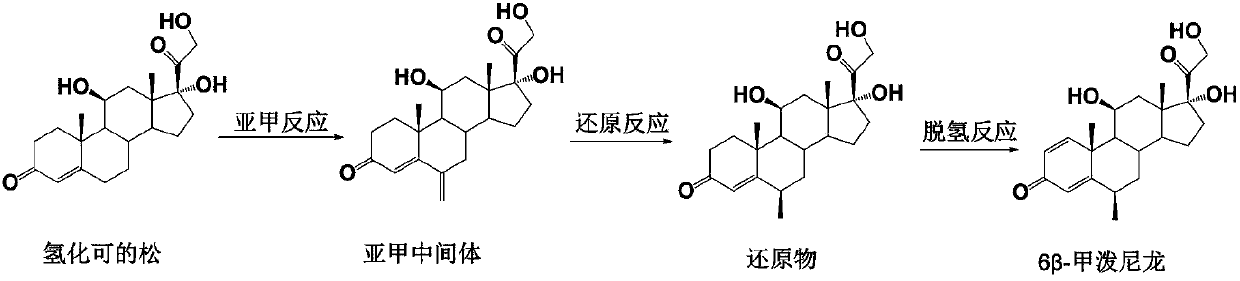
Preparation method of 6 beta-methylprednisolone
A technology for methylprednisolone and methylene, which is applied in the field of compound preparation, can solve the problems of long synthesis route, danger, low yield and the like, and achieves the effects of easy control, low cost and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 methylene intermediate
Embodiment 1-1
[0043] Dissolve 100g of hydrocortisone in 1L of ethanol, add 80ml of triethyl orthoformate and 1g of p-toluenesulfonic acid, stir at room temperature for 10 minutes, add 120ml of 40% formaldehyde aqueous solution and 80ml of N-methylaniline, stir at room temperature for 3 hours, add Concentrated hydrochloric acid adjusted the pH value of the reaction solution to 1-2, and kept stirring for 2 hours; the reaction solution was diluted into 3 L of water, and solids were precipitated, filtered with suction, and the filter cake was washed with water until neutral, and dried to obtain 98.5 g of methylene intermediate, molar yield Yield 94.8%, HPLC purity 95%.
Embodiment 1-2
[0045] Dissolve 36g of hydrocortisone in 400mL of methanol, add 45ml of trimethyl orthoformate and 0.5g of p-toluenesulfonic acid, stir at room temperature for 10 minutes, add 60ml of 40% aqueous formaldehyde and 45ml of N-methylaniline, and stir at room temperature for 3 hours. Add concentrated sulfuric acid to adjust the pH value of the reaction solution to 1-2, keep stirring for 2 hours; dilute the reaction solution into 1.5L water, precipitate solids, filter with suction, wash the filter cake with water until neutral, and dry to obtain 35.1 g of methylene intermediate. The molar yield is 93.9%, and the HPLC purity is 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com