Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Intoxicative inhalant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inhalants are a broad range of household and industrial chemicals whose volatile vapors or pressurized gases can be concentrated and breathed in via the nose or mouth to produce intoxication (called "getting high" in slang), in a manner not intended by the manufacturer. They are inhaled at room temperature through volatilization (in the case of gasoline or acetone) or from a pressurized container (e.g., nitrous oxide or butane), and do not include drugs that are sniffed after burning or heating. For example, amyl nitrite (poppers), nitrous oxide and toluene – a solvent widely used in contact cement, permanent markers, and certain types of glue – are considered inhalants, but smoking tobacco, cannabis, and crack are not, even though these drugs are inhaled as smoke.
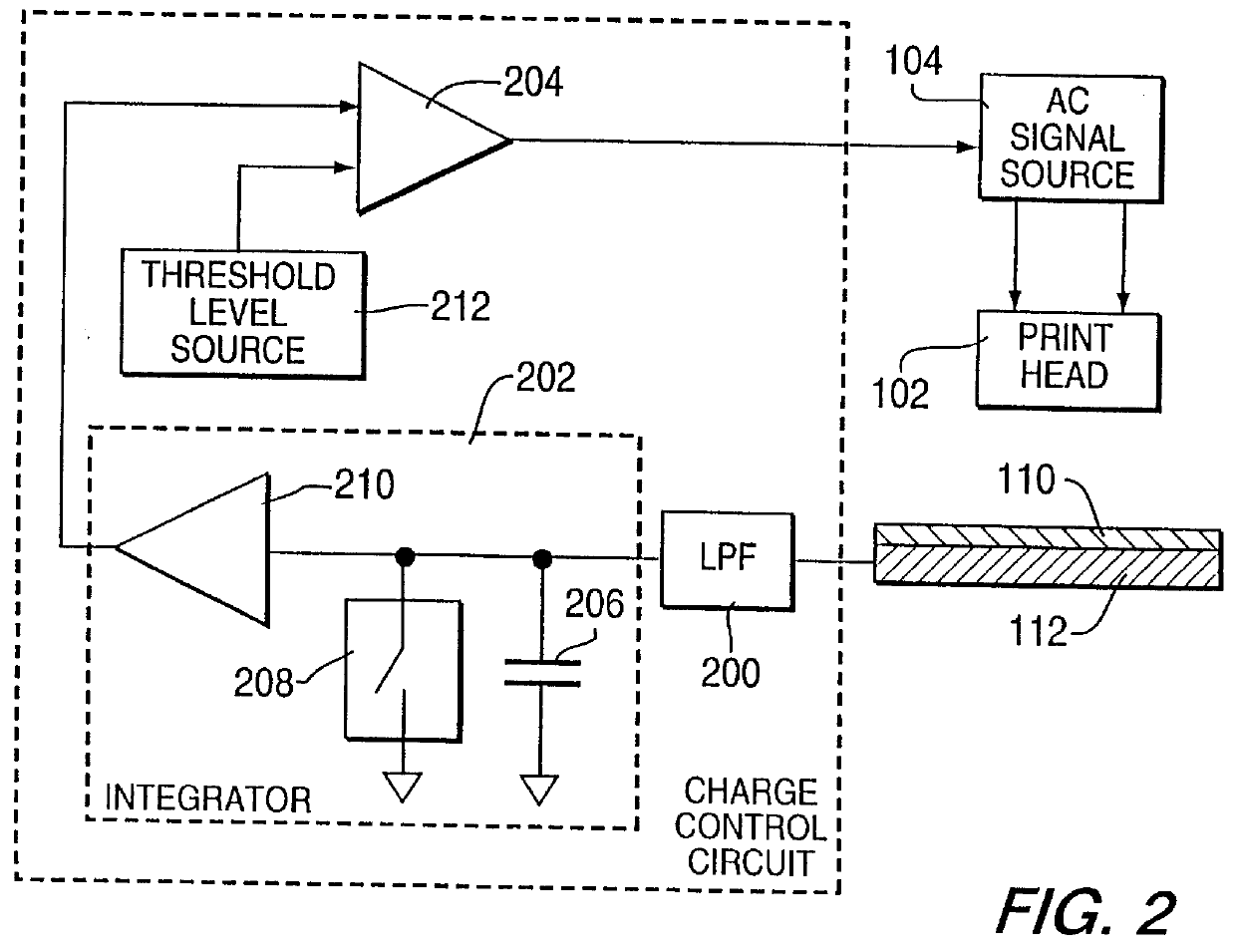
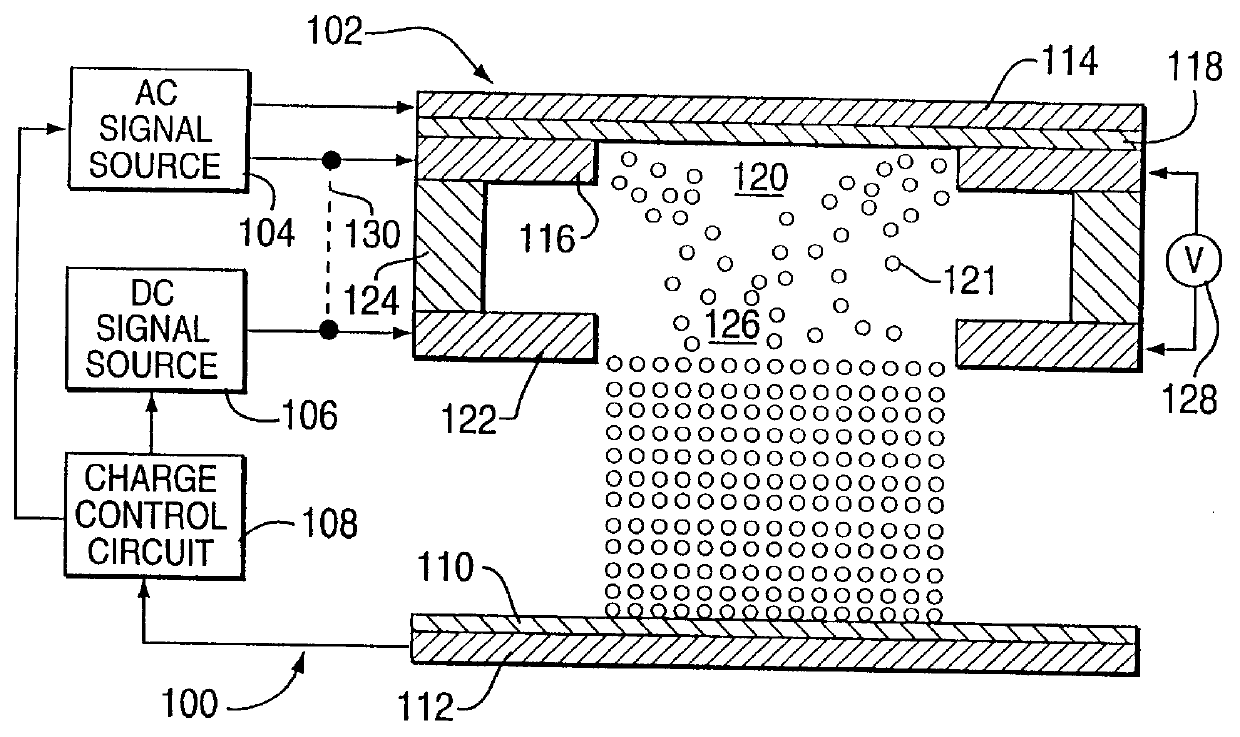
Method for electrostatically depositing a medicament powder upon predefined regions of a substrate
Method for electrostatically depositing select doses of medicament powder at select locations on a substrate. Specifically, the apparatus contains a charged particle emitter for generating charged particles that charge a predefined region of a substrate and a charge accumulation control circuit for computing the amount of charge accumulated upon the substrate and deactivating the emitter when a selected quantity of charge has accumulated. Additionally, a triboelectric charging apparatus charges the medicament powder and forms a charged medicament cloud proximate the charged region of the substrate. The medicament particles within the medicament cloud electrostatically adhere to the charged region. The quantity of charge accumulated on the substrate at the predefined region and the charge-to-mass ratio of the medicament powder in the cloud control the amount (dose) of medicament deposited and retained by the substrate. Consequently, this apparatus accurately controls both medicament dosage and deposition location. Furthermore, since the substrate can be of any dielectric material that retains an electrostatic charge, the apparatus can be used to deposit medicament on substrates that are presently used in oral medicament consumption, e.g., substrates that are used to fabricate suppositories, inhalants, tablets, capsules and the like.
Owner:DELSYS PHARMA
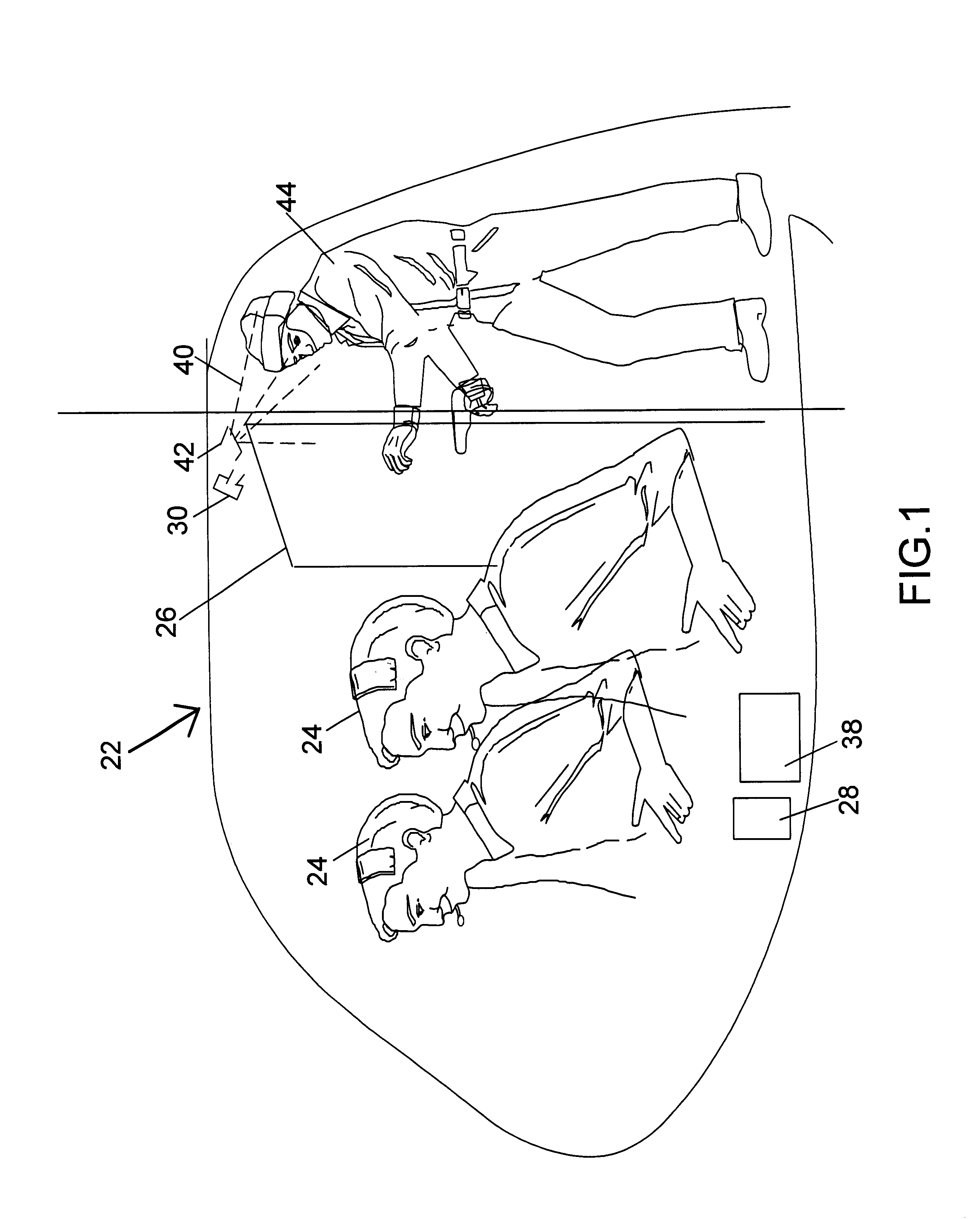
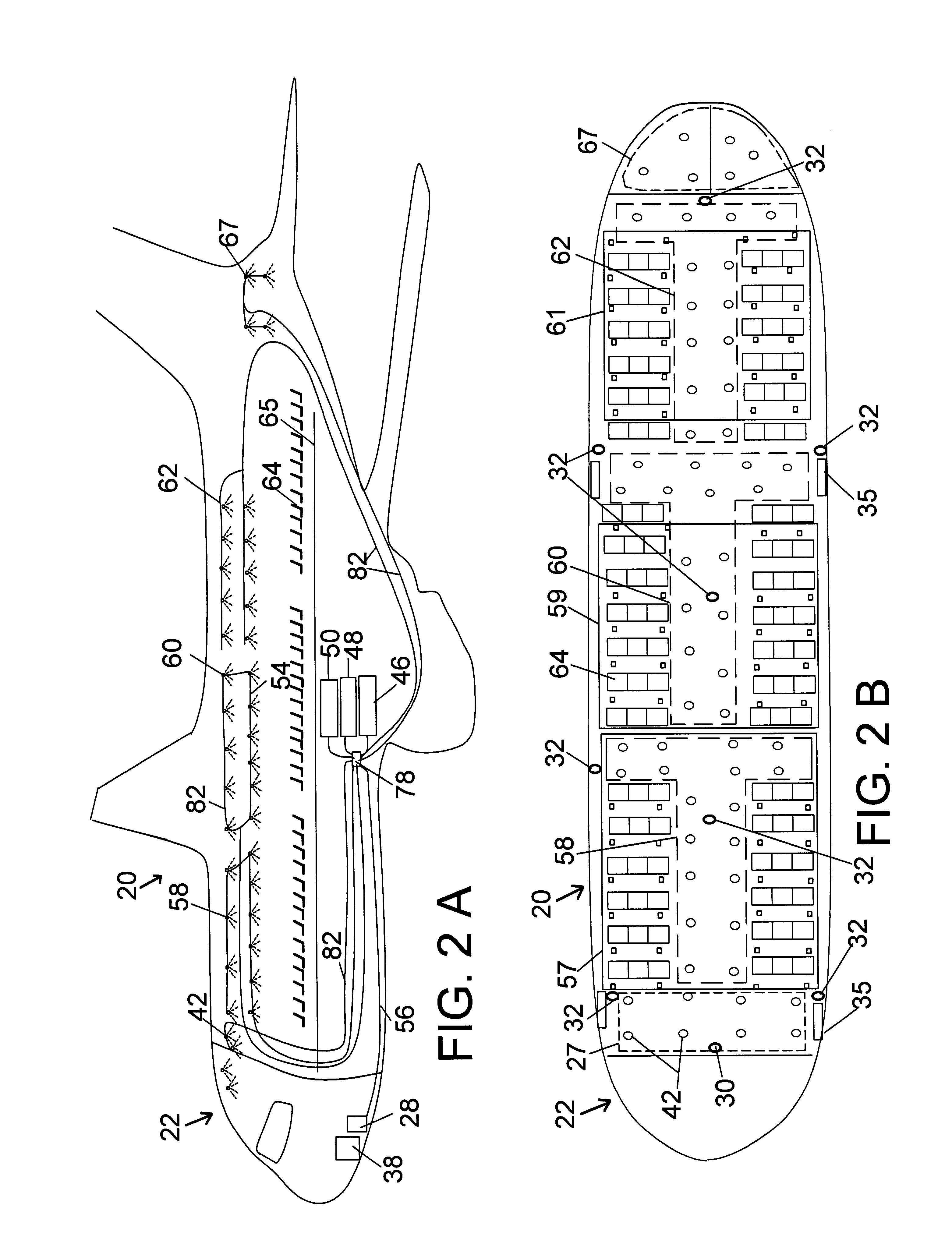
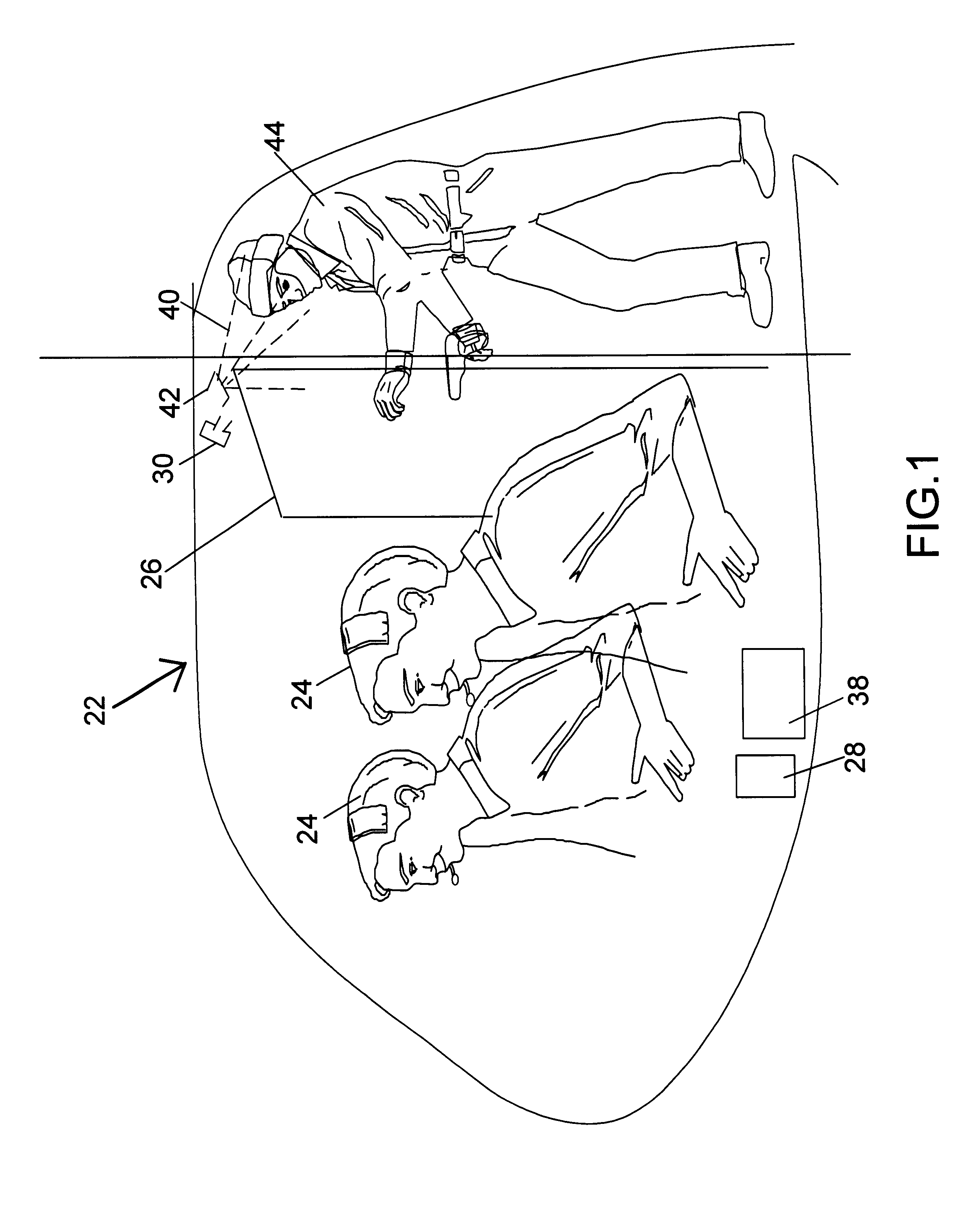
Method and system for countering hostile activity aboard an airplane
InactiveUS6696928B1Not limitedElectric signal transmission systemsDigital data processing detailsJet aeroplaneWhole body
A method of countering terrorism or hostile activity in an airplane by using a built-in defense system within the aircraft. The defense system includes chemical sprays, laser guns, and pre-programmed sound alarm systems. The aerosol chemicals range from benign fogging agents to non-lethal incapacitating agents from the categories of inhalants, general anesthetics, and irritants. Any of the systems can be used singly or in any combination. These systems can be activated manually from the control panel in the cockpit, or via a remote wireless system by the flight crew from anywhere within the plane. Such activation being password and code protected.
Owner:BOVEJA BIRINDER R +1
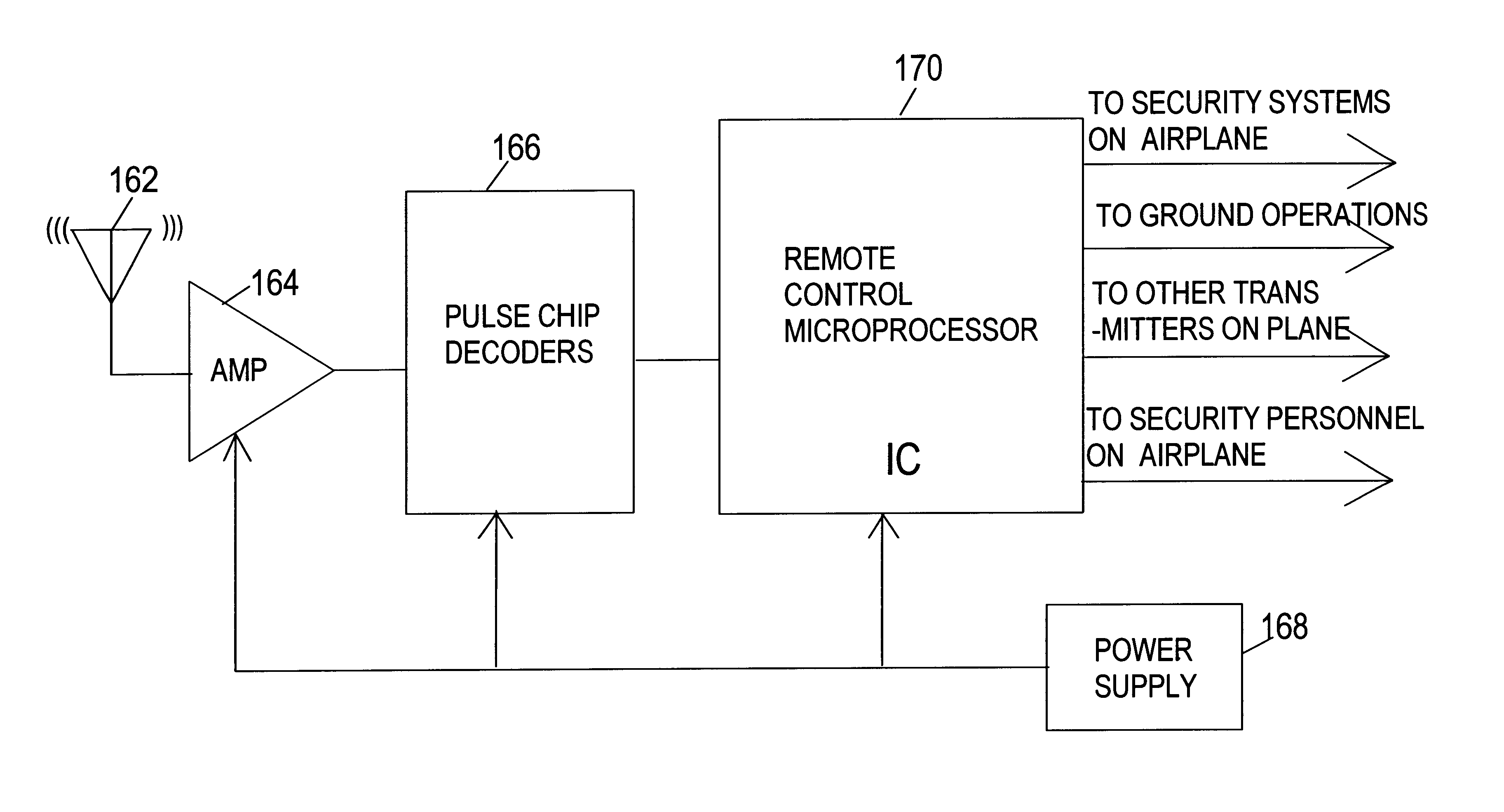
Wireless remote control of systems for countering hostile activity aboard an airplane
InactiveUS6771186B1Digital data processing detailsAir-treatment apparatus arrangementsWhole bodyIncapacitating agent
A method of countering terrorism or hostile activity in an airplane by using a multitude of built-in systems within the aircraft. The built-in systems includes chemical sprays, laser guns, and pre-programmed sound alarm systems. The aerosol chemicals range from benign fogging agents to non-lethal incapacitating agents from the categories of inhalants, general anesthetics, and irritants. Any of the systems can be used singly or in any combination. These systems can be activated manually from the control panel in the cockpit, or via a remote wireless activation system by the flight crew from anywhere within the plane. Such activation being password and code protected.
Owner:BOVEJA BIRINDER R +1
Stabilized Bacteriophage Formulations
InactiveUS20080038322A1Easy to prepareLittle and no loss in titerAntibacterial agentsBiocideDrug carrierBacteriophage
Stabilized bacteriophage compositions, and methods for preparing stabilized bacteriophage compositions are provided. The method for producing an antibacterial composition involves adsorbing an aqueous solution of one or more bacteriophages, or one or more phage components, onto a matrix to produce a composition, and drying the composition to produce the antibacterial composition. An antibacterial composition comprising one or more strain of bacteriophage, or one or more phage component, adsorbed onto a matrix is also provided. The antibacterial composition may also be encapsulated. The antibacterial composition, or the encapsulated antibacterial composition, may be used within a cream, lotion or gel, be admixed with a pharmaceutical carrier and administered topically, orally, nasally, used as a powdered inhalant, or the antibacterial composition or encapsulated antibacterial composition, may be added to a feed for animal, aquatic or avian uses.
Owner:CHR HANSEN AS
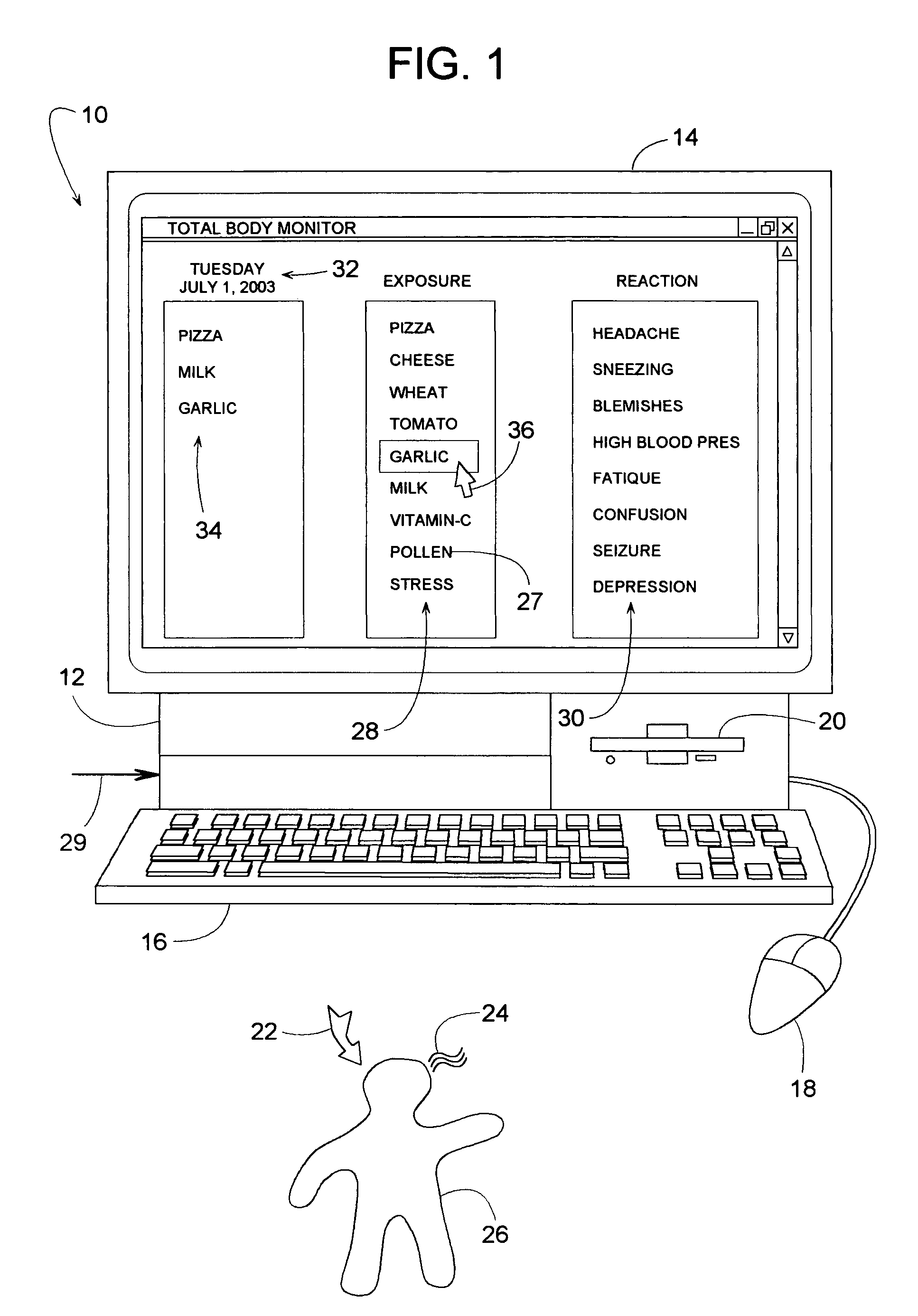
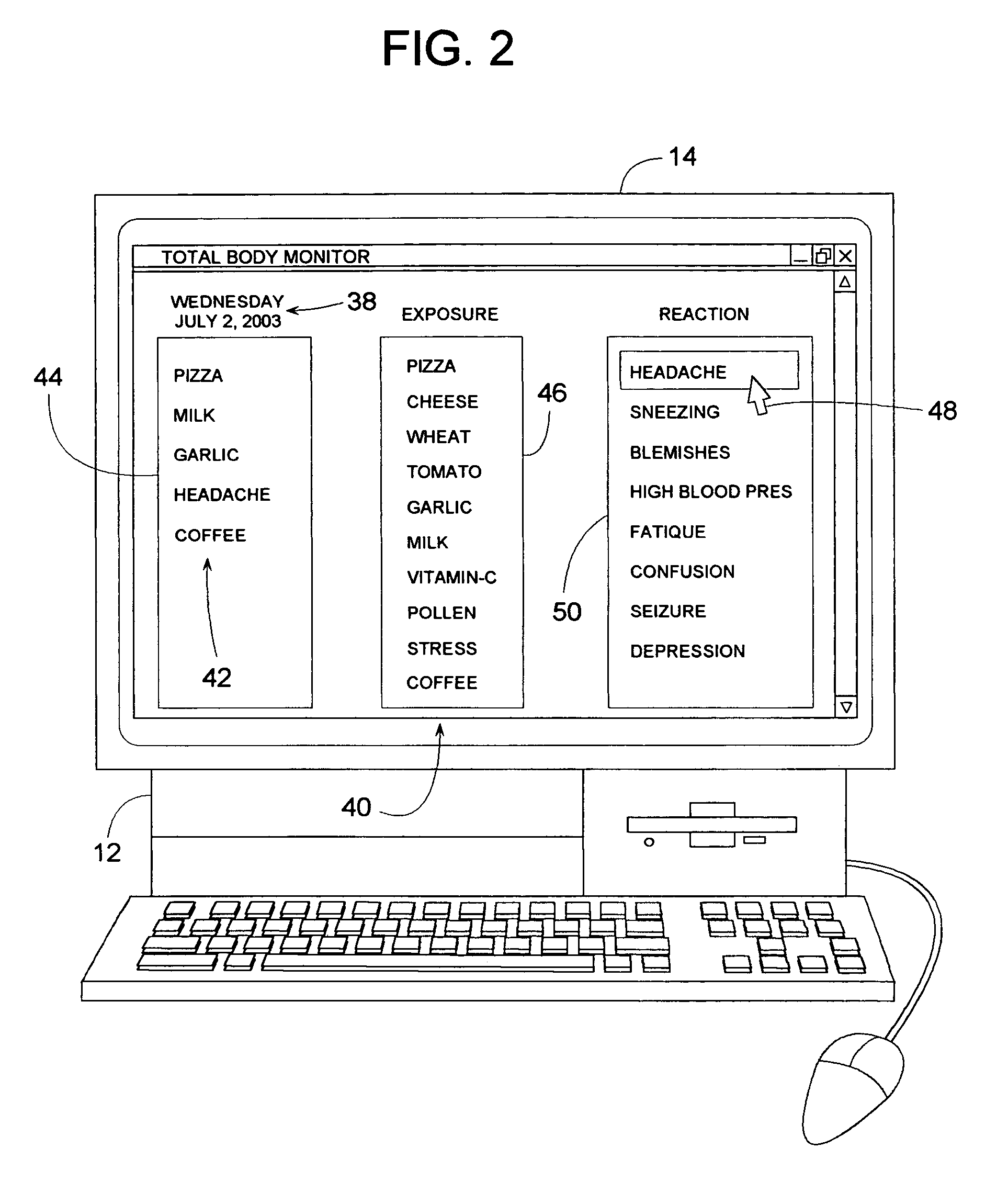
Method for identifying allergens and other influencing agents that may cause a reaction
InactiveUS7613619B1Easy to identifyEasy data collectionData processing applicationsHealth-index calculationAnalysis dataHeadaches
A test method that helps identify foods that may be causing a reaction in an individual involves the individual entering into a computer a daily log of all the foods they routinely eat and any reactions that they may experience. The reactions may be headaches, fatigue, physical pain, depression, etc. The test method can be done without the individual having to follow any prescribed diet. After recording several weeks or months of data, the computer analyzes the data to determine if any significant mathematical correlations exist between a reaction and any of the foods, whereby a high positive correlation may suggest that the food is perhaps related to the reaction. In addition to food items, the method can analyze the correlation of other influencing agents such as environmental exposures, inhalants, menses, and stress.
Owner:HARTER MICHAEL R +2
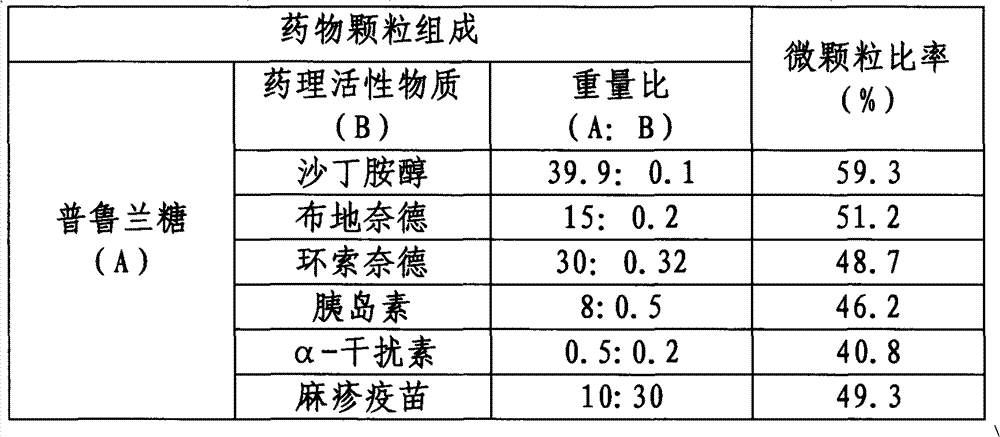
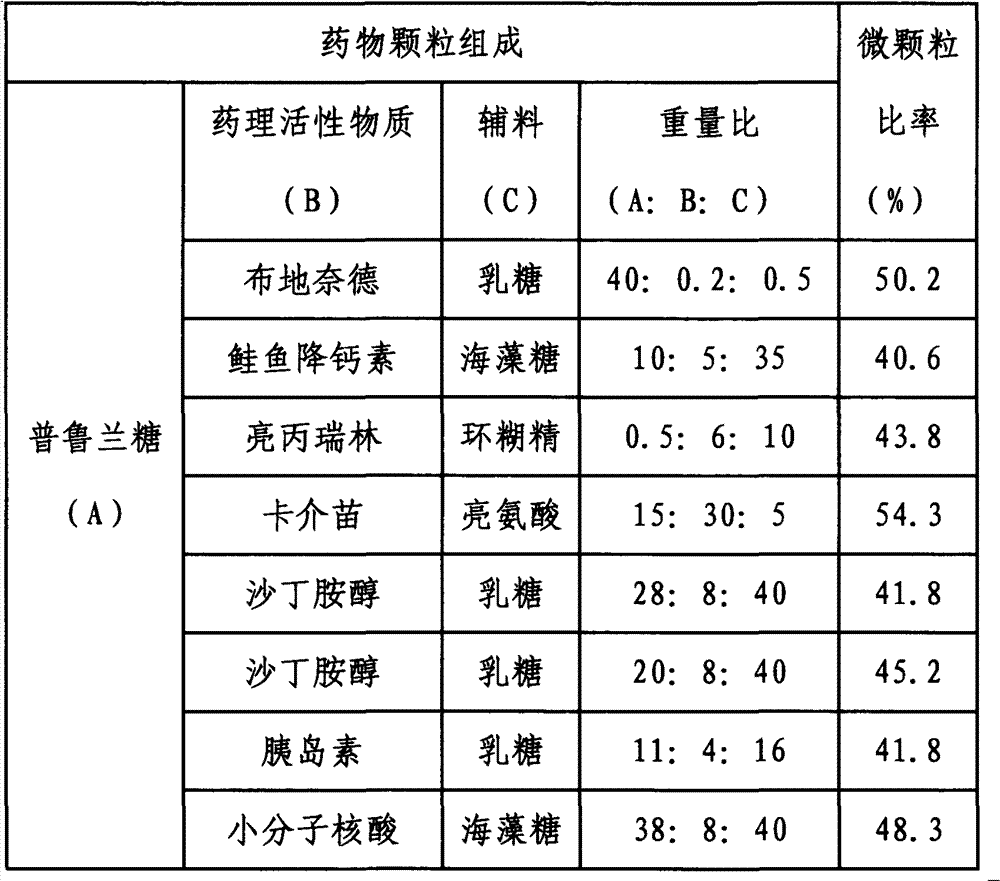
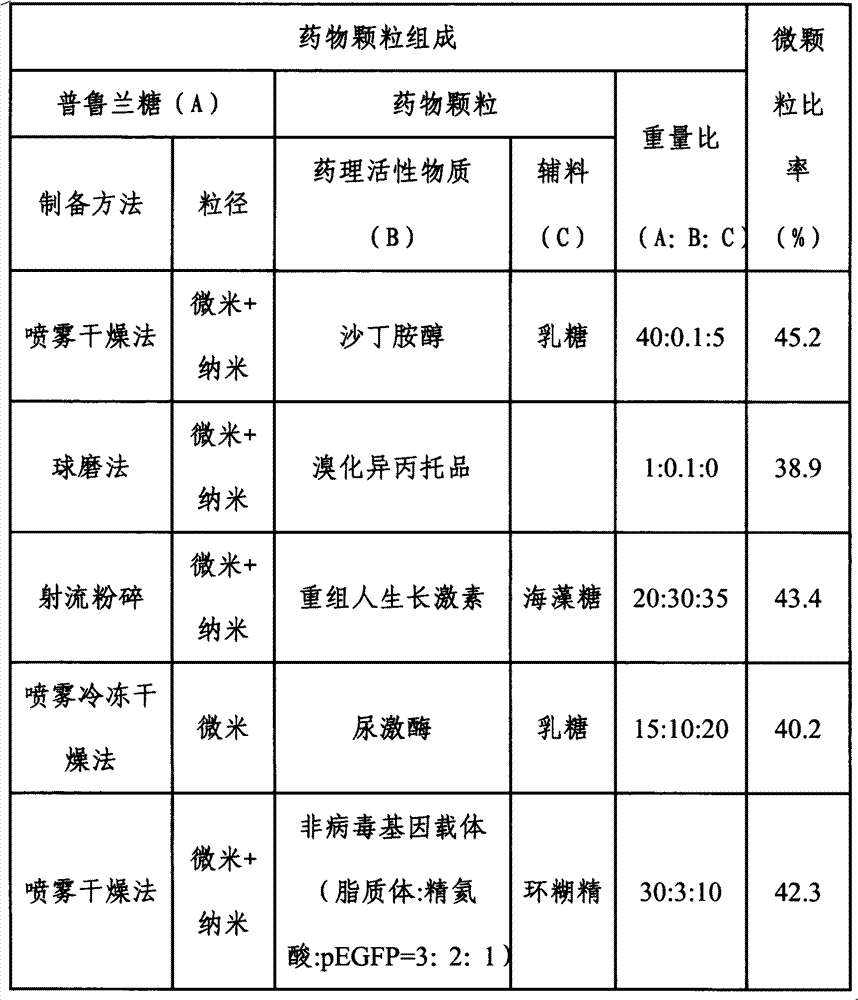
Inhalant, preparation method thereof, and application of inhalantas inhalant carrier with pullulan
InactiveCN103110611ANeutral lowFast dissolutionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsPullulanInhalant Product
The invention discloses inhalant, a preparation method thereof, and application of the inhalant as an inhalant carrier with pullulan. The particle diameter of drug particles for lung inhalation in the dry-powder inhalant disclosed by the invention is 0.1-50 mu m, preferably 0.3-15 mu m, more preferably 0.5-5 mu m. Generally, the dosage of drugs inhaled for each time is 0.1 mu g to 500 mg. The effective deposition rate of the inhalant in the lung is increased by 16-400%. The inhalant has high bioavailability and can be used for treating local or systemic diseases. The inhalant disclosed by the invention has important value for increasing the pulmonary delivery efficiency of drugs.
Owner:SUZHOU HUIREN BIOLOGICAL SCI & TECH
Inhalation system and method
A system includes but is not limited to at least one manifold; an inhalant dissemination device coupled to the at least one manifold; an inhalant characterization device coupled to the at least one manifold; and a control module operably coupled to the inhalant dissemination device and the inhalant characterization device, said control module configured to (a) determine an inhalant concentration in a manifold, and (b) calculate at least one of a retrospective and a prospective inhaled dose in response to the inhalant concentration, and (c) start and stop a flow through the manifold until the at least one of the retrospective and the prospective inhaled dose is greater than or equal to a specified dose. A method includes but is not limited to starting a flow of an inhalant through a manifold; determining an inhalant concentration of the inhalant in the manifold; and stopping the flow of the inhalant through the manifold when the inhalant concentration is in a first specified inhalant-concentration range.
Owner:US SEC THE ARMY ON BEHALF OF USAMRMC
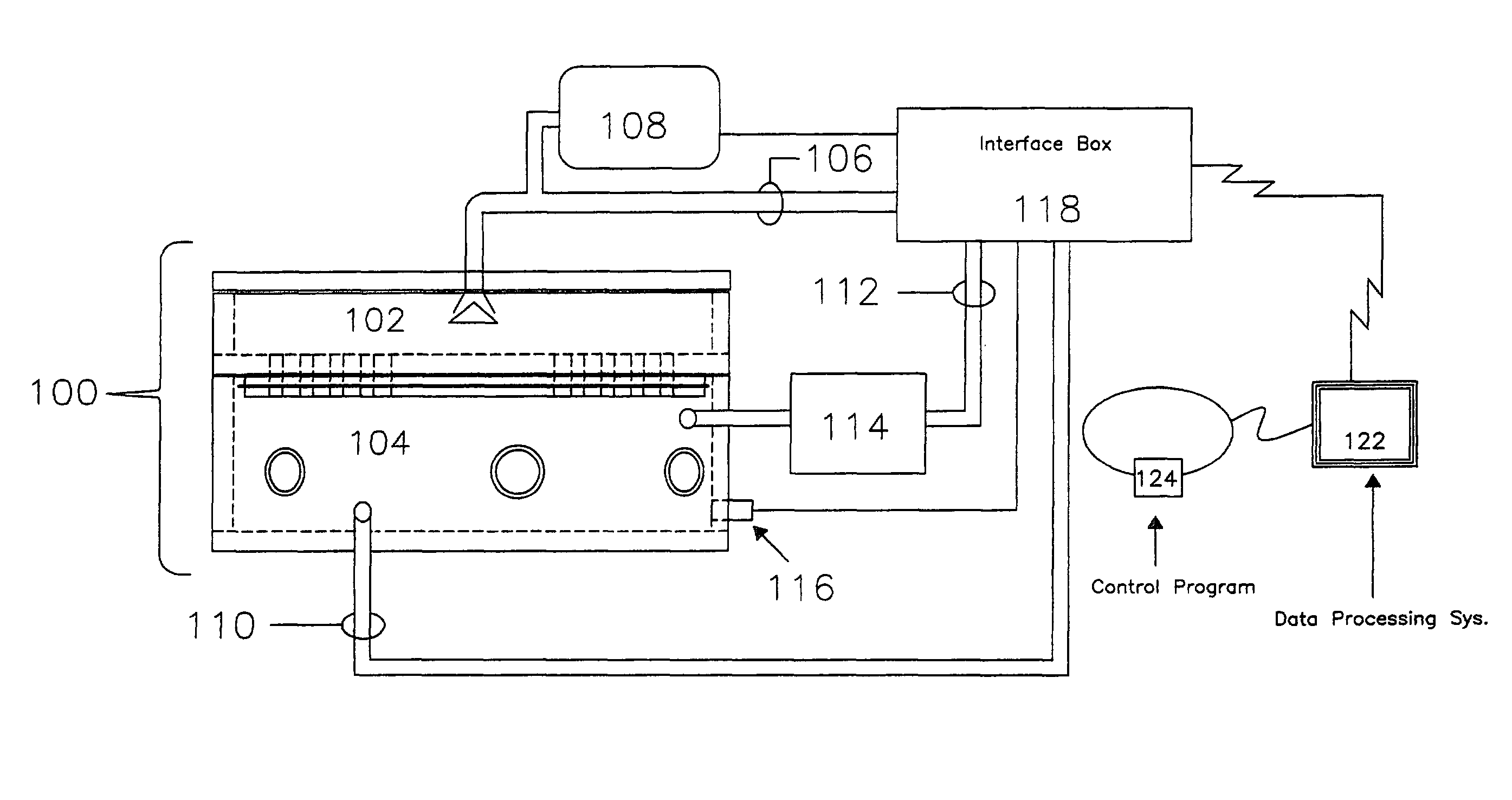
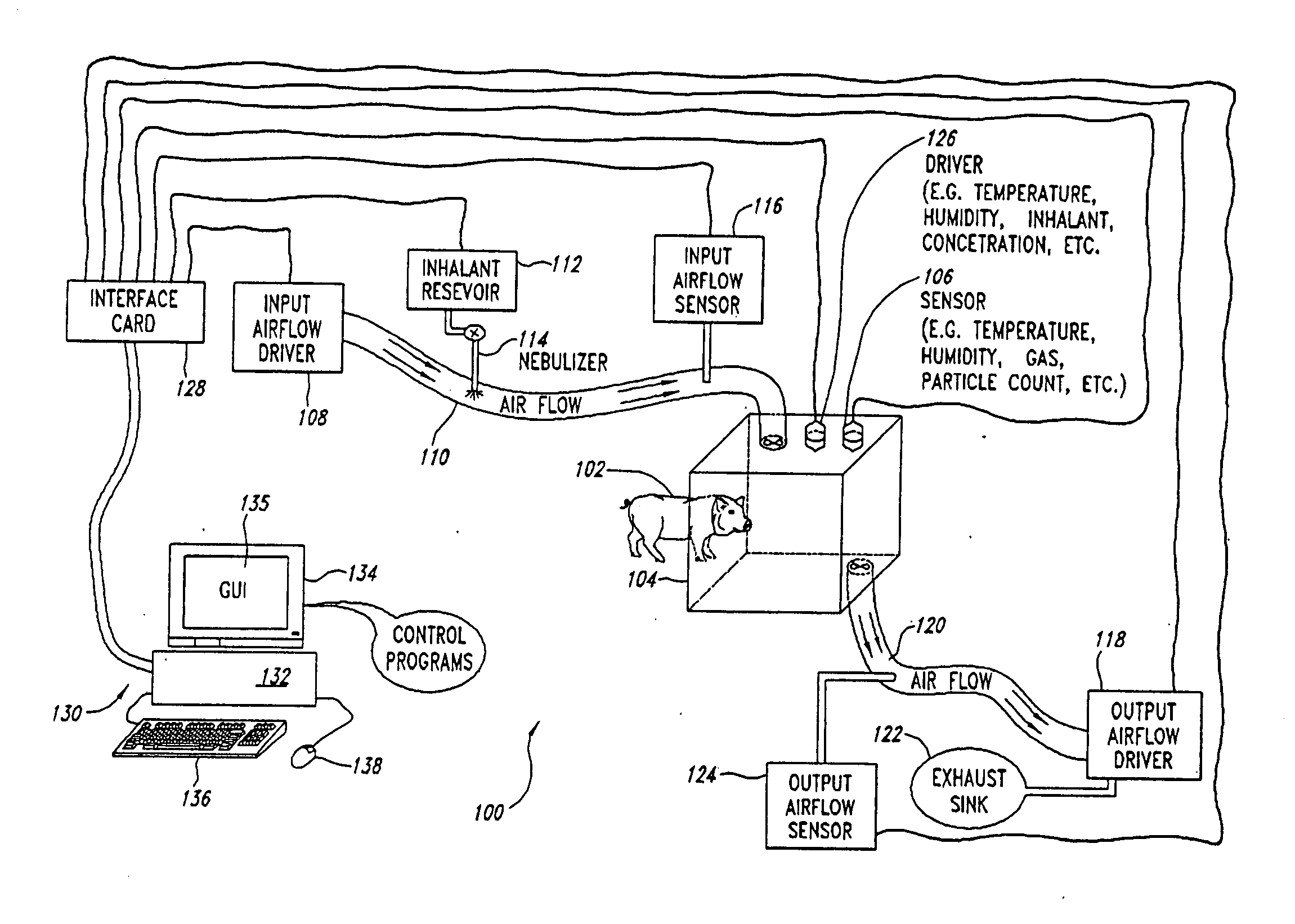
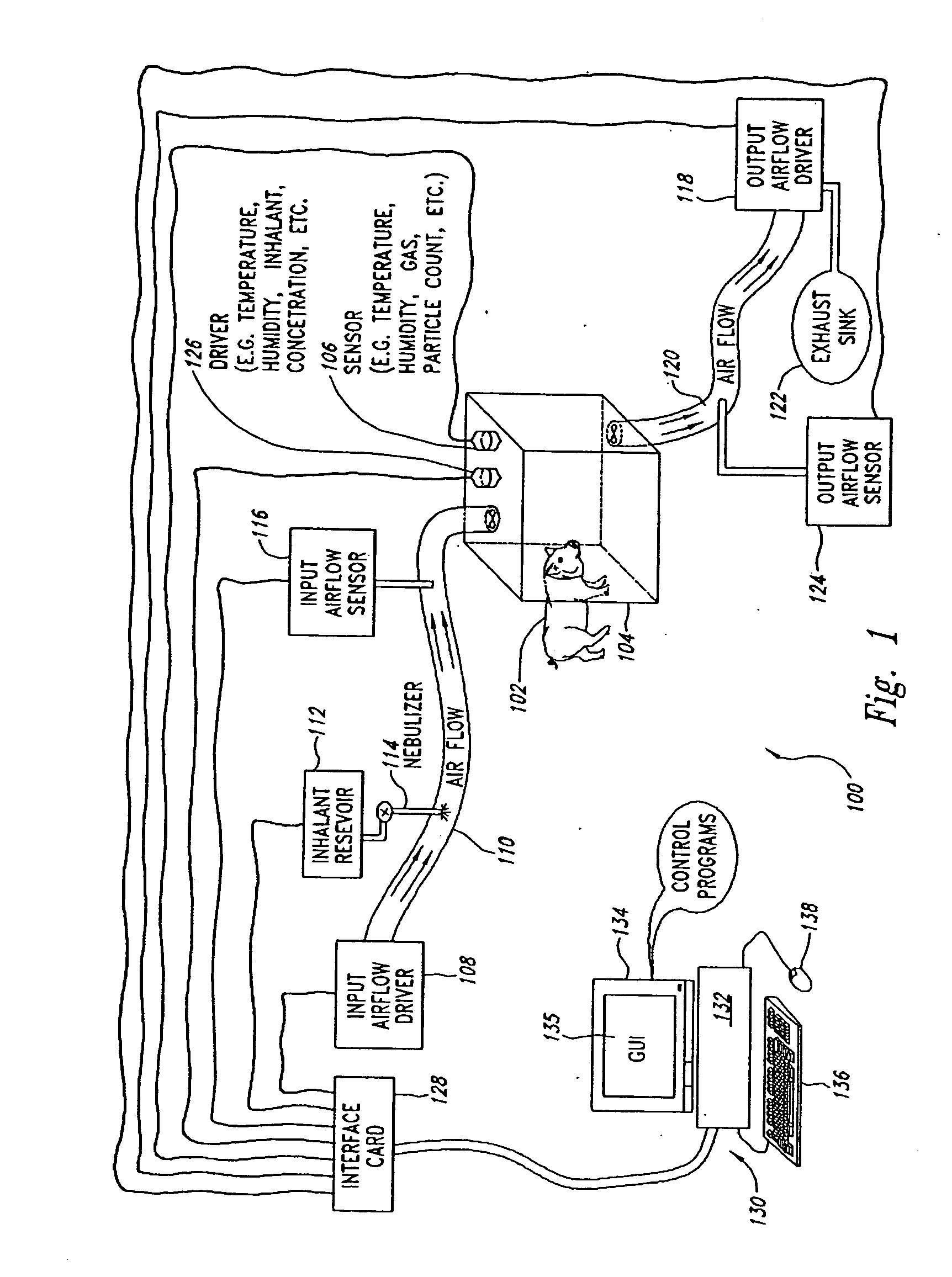
Automated inhalation toxicology exposure system and method
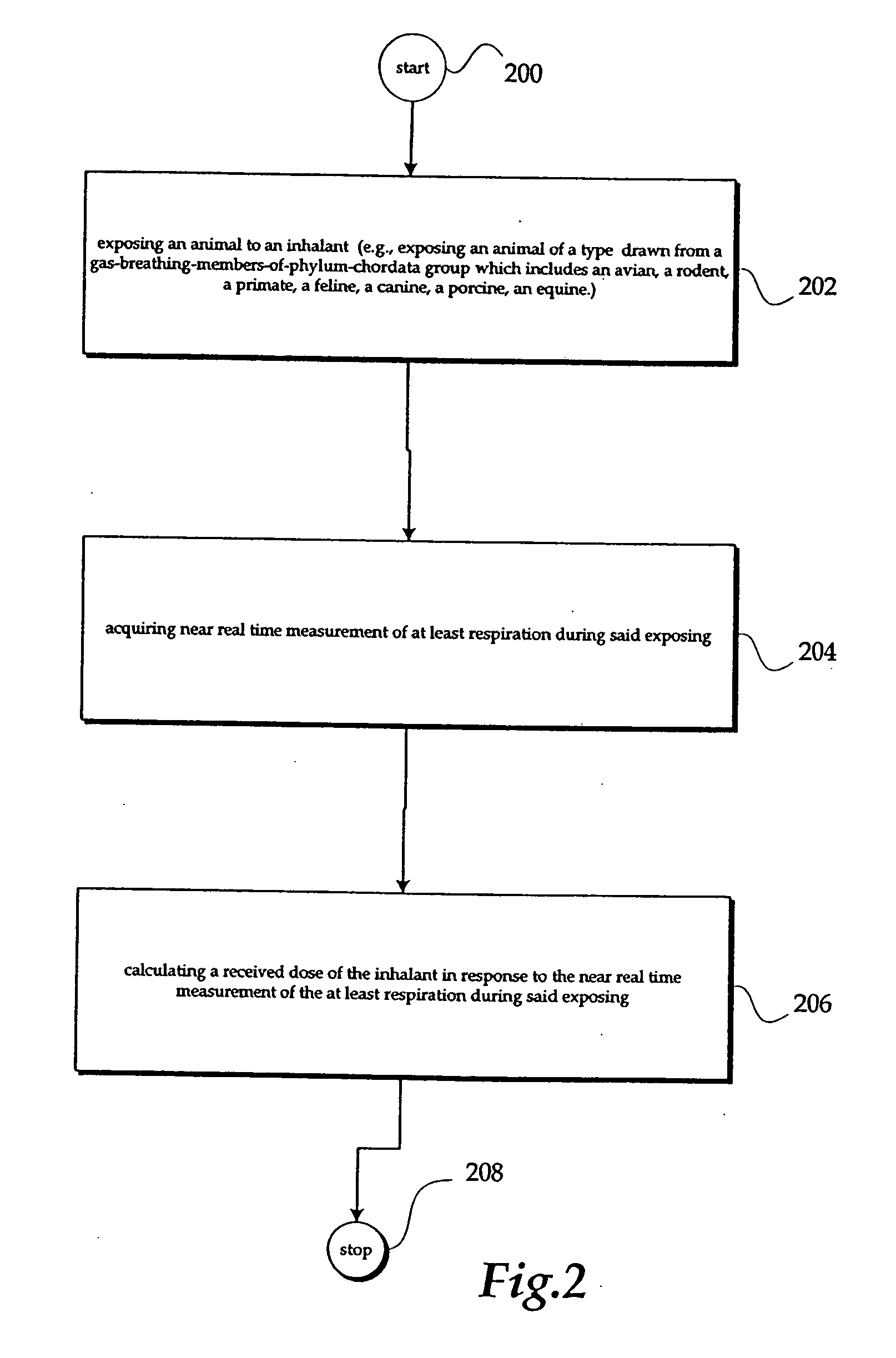
In one embodiment, a method includes but is not limited to exposing an animal to an inhalant; acquiring near real time measurement of at least respiration during said exposing; and calculating a received dose of the inhalant in response to the near real time measurement of the at least respiration during said exposing. In one embodiment, a method includes but is not limited to automatically controlling an environment of an inhalant chamber; and automatically controlling a concentration of an inhalant in the inhalant chamber. In one embodiment, a method includes but is not limited to displaying near real time measurement data related to an animal in an inhalant chamber. In addition to the foregoing, other method embodiments are described in the claims, drawings, and text forming a part of the present application. In one or more various embodiments, related systems include but are not limited to circuitry and / or programming for effecting the foregoing-described method embodiments; the circuitry and / or programming can be virtually any combination of hardware, software, and / or firmware configured to effect the foregoing-described method embodiments depending upon the design choices of the system designer. In one embodiment, a system includes but is not limited to at least one inhalant chamber; and at least one animal respiration sensor integral with the at least one inhalant chamber.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Inhalant exposure system
InactiveUS20090013997A1Avoid lostHigh trafficRespiratorsLiquid surface applicatorsMedicineExhaled air
Owner:BATTELLE MEMORIAL INST
Carbon dioxide delivery apparatus and method for using same
InactiveUS7299802B2Increase carbon dioxide levelsEasy to manageRespiratorsMedical devicesAtmospheric airPhysiology
The present invention relates to a method of increasing the carbon dioxide level in a patient in by administering to the patient an inhalant that comprises a mixture of carbon dioxide and atmospheric air. The method can be used to treat a patient suffering from asthma, allergies, muscle tension, pain, insomnia, and / or mental stress. The present invention also relates to a device that may be used in a method of an inhalant that comprises a mixture of carbon dioxide and atmospheric air to a patient.
Owner:FELDMAN SPENCER
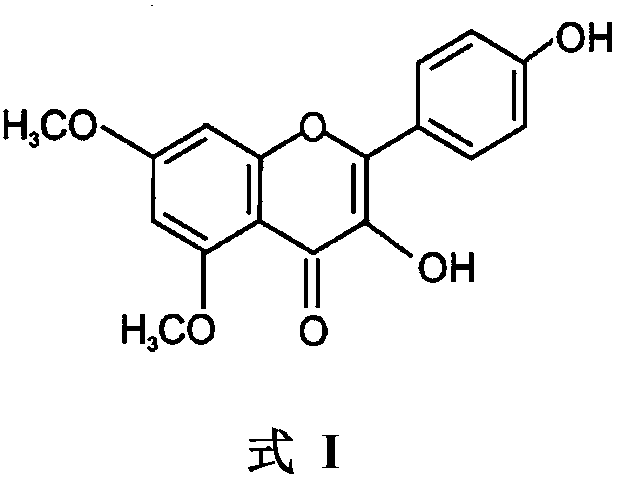
Use of 5,7-methoxy-3,4'-flavonol in resisting of respiratory tract inflammatory diseases
InactiveCN108904489AEnhance anti-inflammatorySignificant anti-bronchial smooth muscle contractionPowder deliveryOrganic active ingredientsDiseaseAcute bronchitis
The invention provides use of 5,7-methoxy-3,4'-flavonol in resisting of respiratory tract inflammatory diseases and relates to application of the 5,7-methoxy-3,4'-flavonol in preparation of drugs forresisting the respiratory tract inflammatory diseases. According to the use, proven by a series of experiments, 7-methoxy-3,4'-flavonol has obvious inflammation resisting, oxidation resisting, tracheacontraction reaction resisting activity; absorption can be achieved through oral drug administration, a relatively high drug effect is displayed, the bioavailability is 10% to 25%, and the 7-methoxy-3,4'-flavonol can be prepared into oral application preparations, injections and inhalants. The 5,7-methoxy-3,4'-flavonol can replace glucocorticoid preparations and is applicable to various respiratory tract inflammatory diseases such as bronchial asthma, acute bronchitis, chronic obstructive pulmonary disease, acute / chronic lung injury and respiratory distress and pulmonary fibrosis.
Owner:杭州勃锐思莫生物医药科技有限责任公司
Use of radish chitin-binding protein in preparing dental health product
InactiveCN101406435ANo side effectsEasy to cleanCosmetic preparationsToilet preparationsToothpasteConjugated protein
The invention relates to application of radish chitin conjugated protein in preparing oral hygiene products such as toothpaste, dental floss, mouth wash, oral spray or inhalant, and chewing gum and so on The oral hygiene products are used for removing and / or reducing dental plagues and / or bacteria in the oral cavity, thereby reducing the incidence of odontopathies (decayed tooth, periodontal disease, etc.) and halitosis.
Owner:宋秋兰
Fudosteine inhalant composition
InactiveCN108078964AUniform contentIncrease the amount of deposition in the effective partPowder deliveryOrganic active ingredientsFUDOSTEINEMedicine
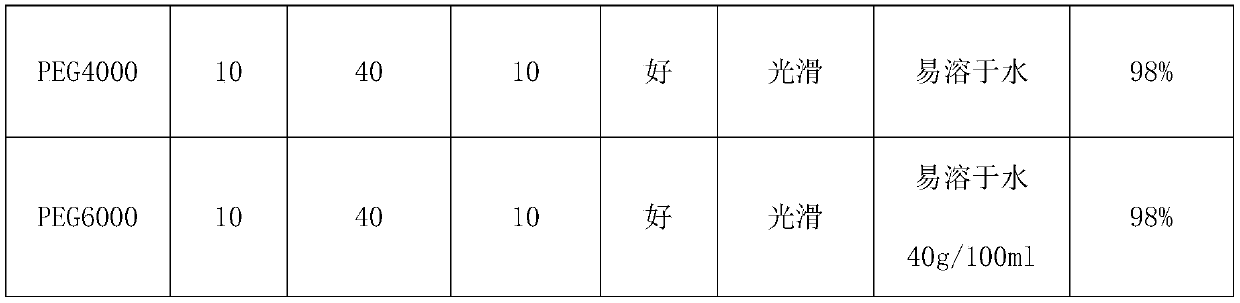
The invention belongs to the technical field of medicine, and relates to a fudosteine inhalant and a preparation method thereof. The fudosteine inhalant provided by the invention is prepared from 60 to 90 percent of fudosteine and 10 to 40 percent of lactose, wherein the percentage is the mass percentage; the particle diameter D90 of the fudosteine is preferably 2 mu m to 5 mu m. The invention provides a fudosteine dry powder inhalant with high bioavailability.
Owner:DISHA PHARMA GRP +1
Preparation for treating allergic rhinitis and asthma
InactiveCN1615842AIndicator qualifiedPowder deliveryOrganic active ingredientsIrritationNonallergic rhinitis
The present invention provides a kind of preparation for treating allergic rhinitis and asthma, and features that each ml of the preparation contains isoforskolin 0.005-0.4 mg except medicine supplementary material and is prepared into acceptable inhalant. The preparation has high stability, no irritation to skin including damaged skin and relatively high antiphlogistic effect. It has curative effect on allergic rhinitis and bronchial asthma, and is practical.
Owner:KUNMING ZIJIAN BIOTECH
Traditional Chinese medicine effective part for treating influenza virus and its preparation process
The invention discloses an effective portion of traditional Chinese medicament for resisting influenza virus, which is prepared from selfheal 5-200 parts, mulberry leaf 5-1-00 parts, wild chrysanthemum flower 5-100 parts as raw material through disintegrating, and supercritical CO2 extraction. The effective portion can be prepared into tablets, capsules, granules, oral liquids, injections, inhalants, sprays or drop pills.
Owner:GUANGZHOU XINGQUN PHARMA
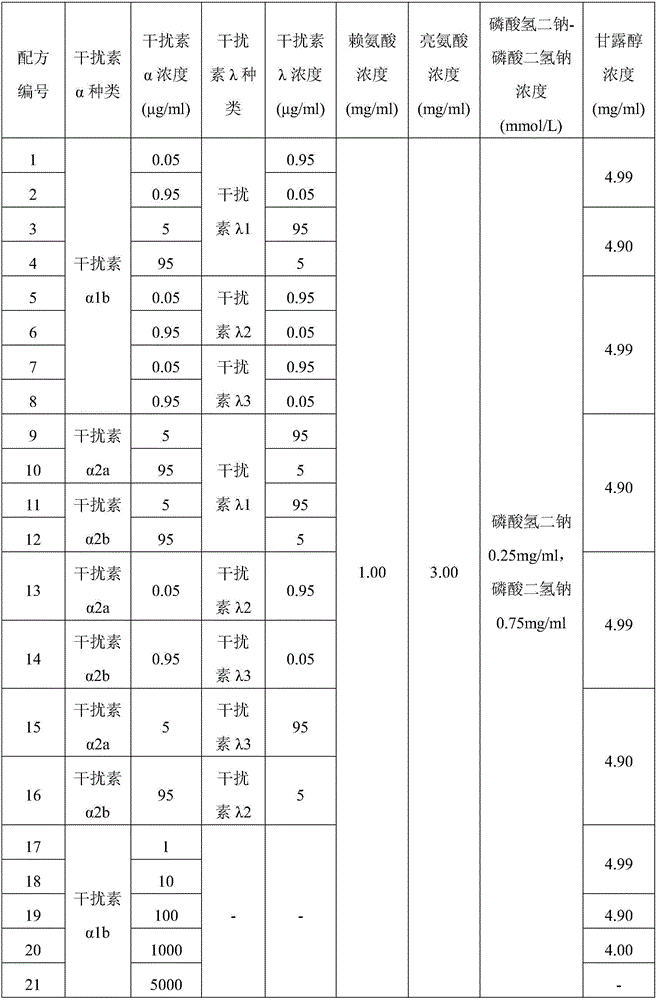
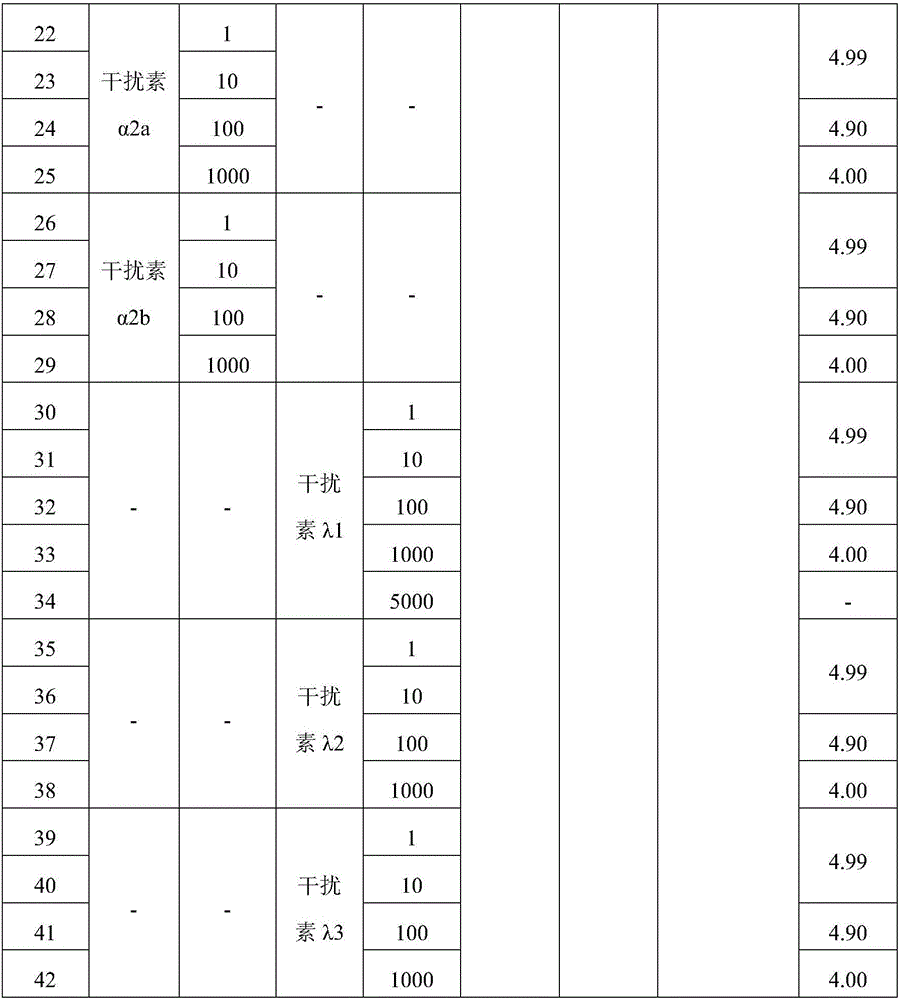
Dry powder inhaler of interferon
The invention belongs to the field of biotechnology pharmaceutical preparations, and relates to a dry powder inhaler of an interferon. The dry powder inhaler, having single dose of 1 mg, 2 mg, 5 mg, 10 mg, 20 mg, 40 mg or 60 mg, contains 1-100 [mu]g of interferon and an appropriate amount of a pharmaceutically acceptable adjuvant for the dry powder inhaler; the interferon is composed of an interferon alpha and an interferon lambda in a weight ratio of (1:19)-(19:1). The dry powder inhaler of interferon provided by the invention is used for treating viral pneumonia and can significantly improve the therapeutic effect in comparison with the dry powder inhaler of the interferon alpha or the dry powder inhaler of the interferon lambda used alone.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
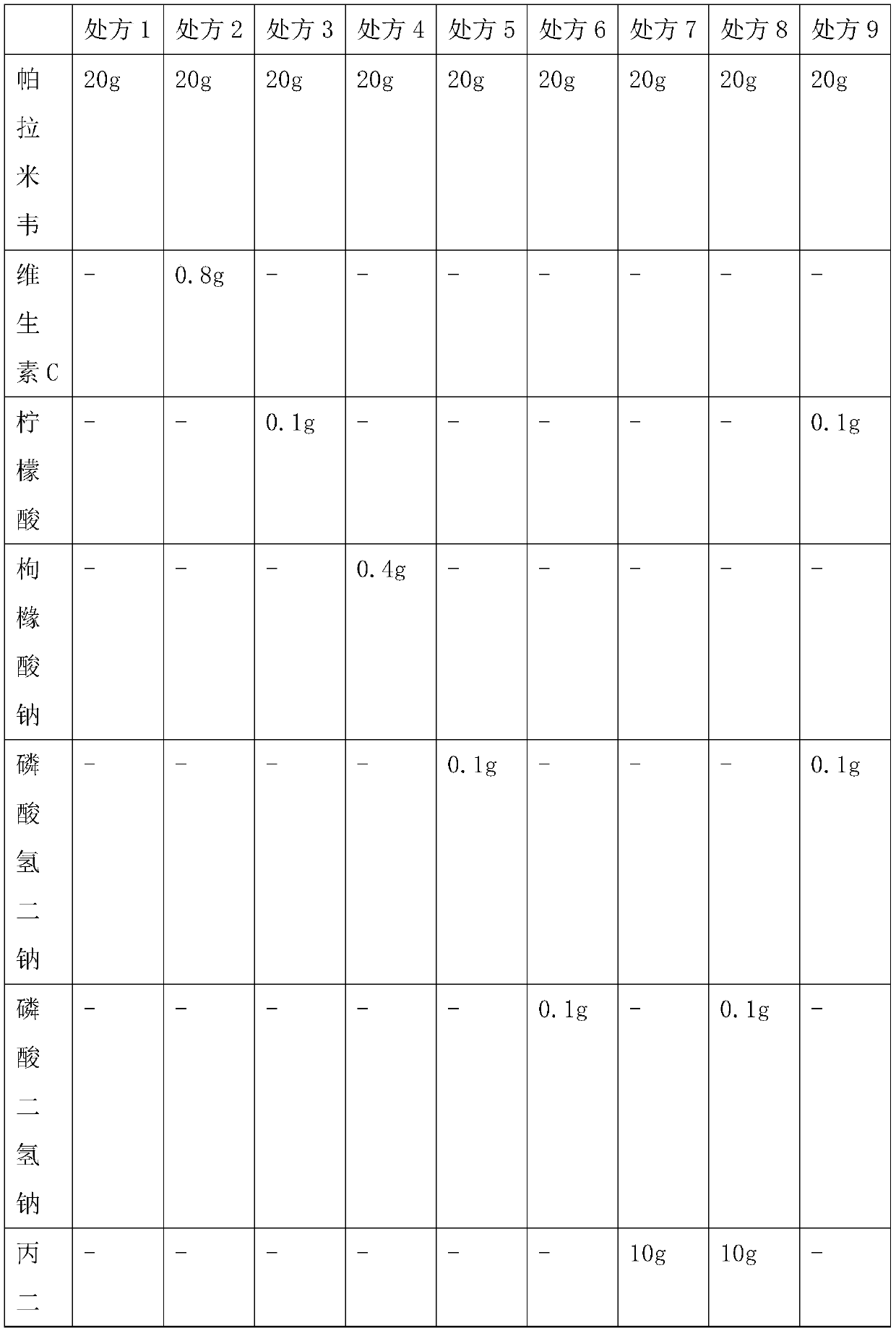
Peramivir solution type inhalant and preparation method thereof
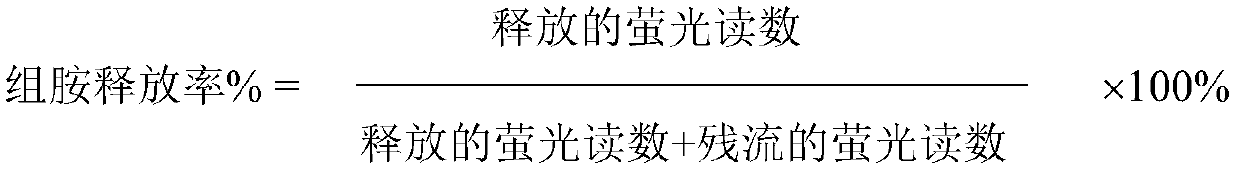
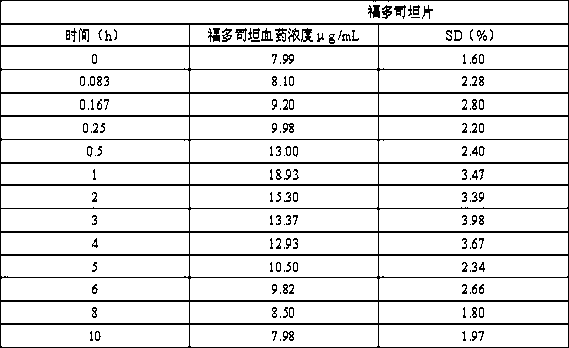
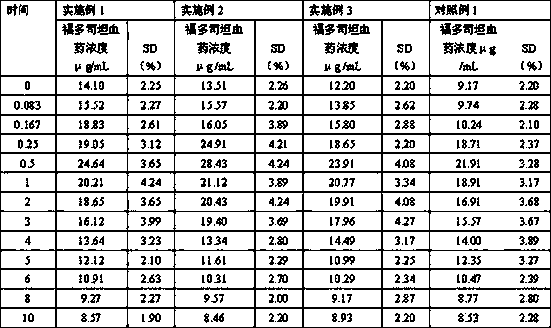
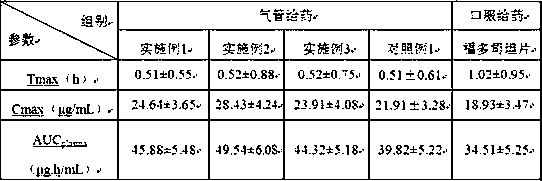
ActiveCN109771398AImprove thermal stabilityImprove stabilityOrganic active ingredientsDispersion deliveryIntoxicative inhalantChemistry
The invention relates to a peramivir solution type inhalant and a preparation method thereof. The solution type inhalant is prepared from peramivir, an osmotic pressure adjusting agent, a pH adjustingagent and water, the concentration of the peramivir is more than 15 mg / ml, the concentration of the osmotic pressure adjusting agent is less than 8 mg / ml, the pH of the solution type inhalant is 5.0-6.0, heating sterilizing is not adopted, and the osmotic pressure adjusting agent is selected from chlorine, sodium or glucose. The solution type inhalant has better stability and excellent lung targeting, which significantly improves the effectiveness and safety of medicines.
Owner:GUANGZHOU NUCIEN PHARM CO LTD
Simvastatin dry powder inhalant and preparation method thereof
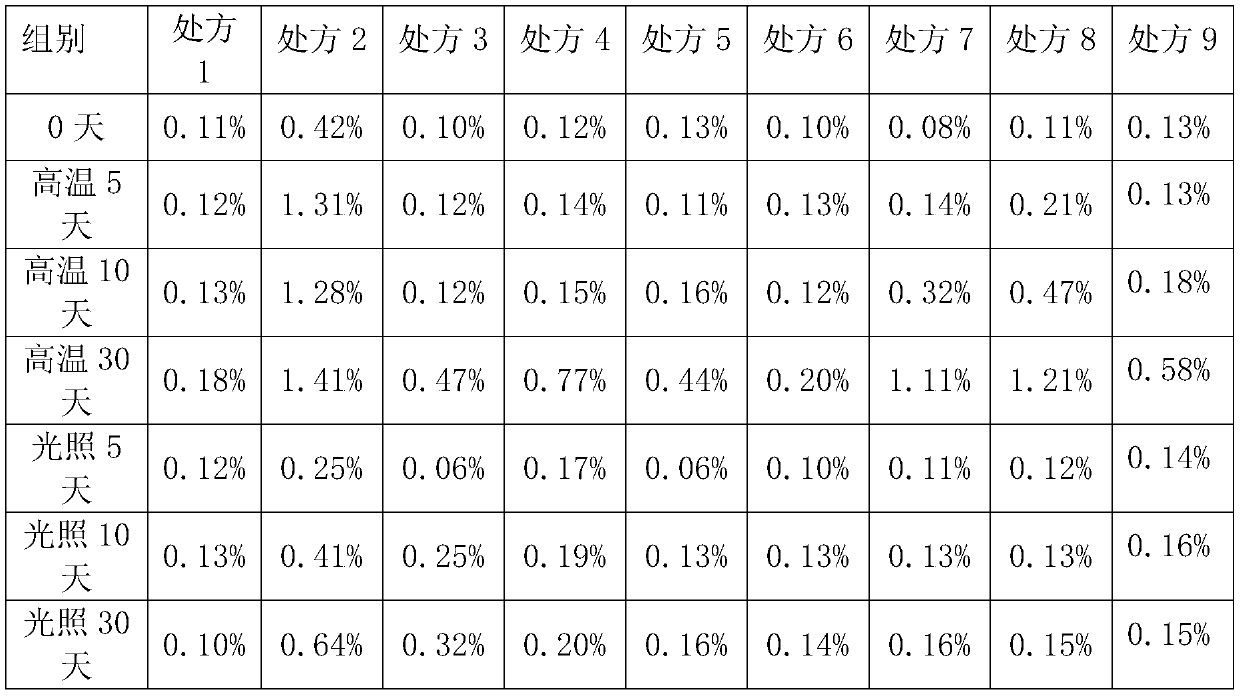
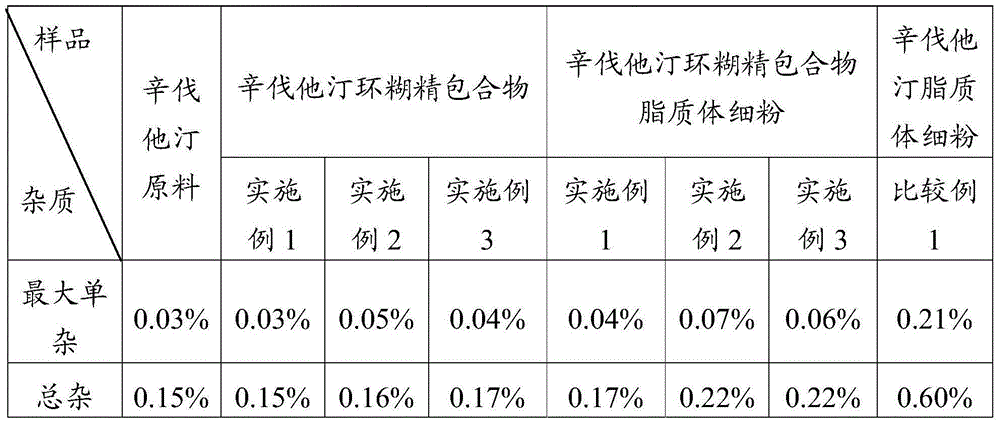
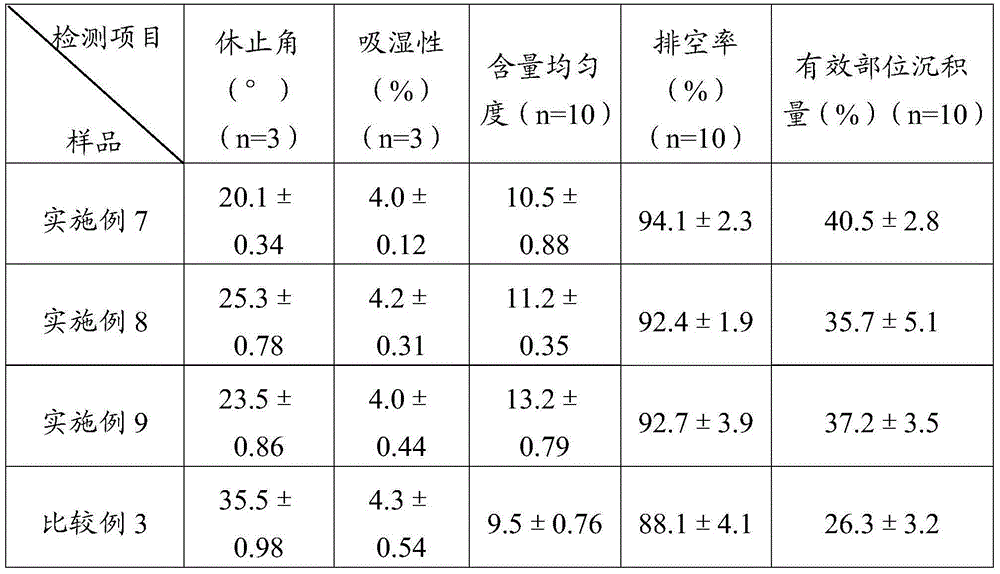
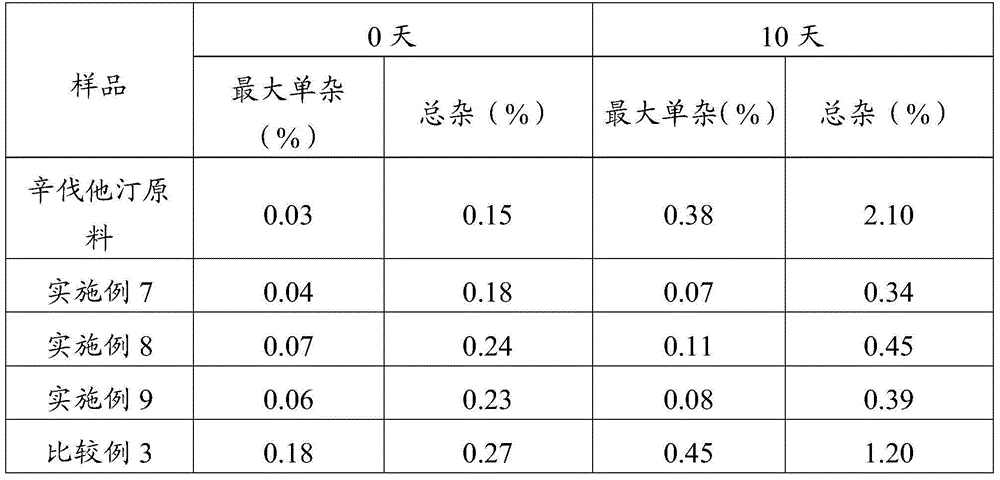
ActiveCN105078931AIncrease the amount of deposition in the effective partImprove efficacyOrganic active ingredientsPharmaceutical delivery mechanismCyclodextrinCurative effect
The invention provides a simvastatin dry powder inhalant. The simvastatin dry powder inhalant comprises a simvastatin liposome compound and pharmaceutically acceptable auxiliary materials. The simvastatin liposome compound is obtained by compounding cyclodextrin-coated simvastatin with phospholipid. The invention further provides a preparation method of the simvastatin dry powder inhalant. According to the simvastatin dry powder inhalant, the cyclodextrin-coated simvastatin and the phospholipid are compounded into the simvastatin liposome compound which is then compounded with the pharmaceutically acceptable auxiliary materials to obtain the dry powder inhalant; after pulmonary administration of the simvastatin dry powder inhalant, effective part deposition amount of simvastatin can be increased remarkably, so that the efficacy of pulmonary simvastatin administration on treatment of pulmonary diseases such as asthma and chronic pulmonary obstruction is improved. Experimental results show that, by the simvastatin dry powder inhalant, the effective part deposition amount is increased remarkably and curative effect on the pulmonary diseases is improved.
Owner:GUANGZHOU GONGHE MEDICINE TECH
Body health benefits environment-friendly type substituted cigarette inhalant and preparation and application
The present invention relates to a substitute smoking agent for cigarette. Its raw material composition includes 0.3-1.5mg of nicotine, 0.3-2g of fluorotrichloromethane, 0.1-0.8g of aerosol made up by mixing dry ice and ice according to the ratio of 1:2-5, 0.005-0.05g of tobacco flavouring agent and 0-0.5g of nutrients necessary for human body. Said invention also provides its preparation method and concrete steps.
Owner:BEIJING CHUANGYE HENGJI INT TRADE
New powdered inhalants based on modified lactose mixtures as excipient
The invention relates to a new process for preparing modified lactose mixtures, these lactose mixtures per se and compositions of medicaments for powder inhalation consisting of one or more micronised active substances and the modified lactose according to the invention.
Owner:BOEHRINGER INGELHEIM INT GMBH
Externally applied compound composition consisting of tadines and leukotriene antagonist
InactiveCN102068432AImmunological disordersHeterocyclic compound active ingredientsDiseaseMontelukast
The invention relates to an externally applied compound composition consisting of tadines and a leukotriene antagonist, wherein the tadines are preferably loratadine and salt thereof; and the leukotriene antagonist is preferably montelukast and salt thereof. The externally applied compound composition can be prepared into externally applied preparations such as nasal drops, eye drops, eye ointments, ointments, gel, inhalants, patches and the like. The externally applied compound composition can be used for local treatment for allergic diseases, can quickly improve allergic symptoms, and has the characteristics of high efficiency, safety, convenient use and the like.
Owner:李仲昆
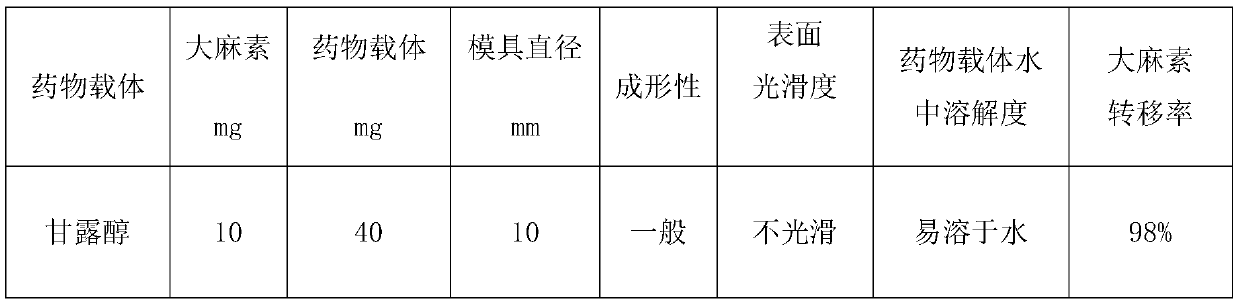
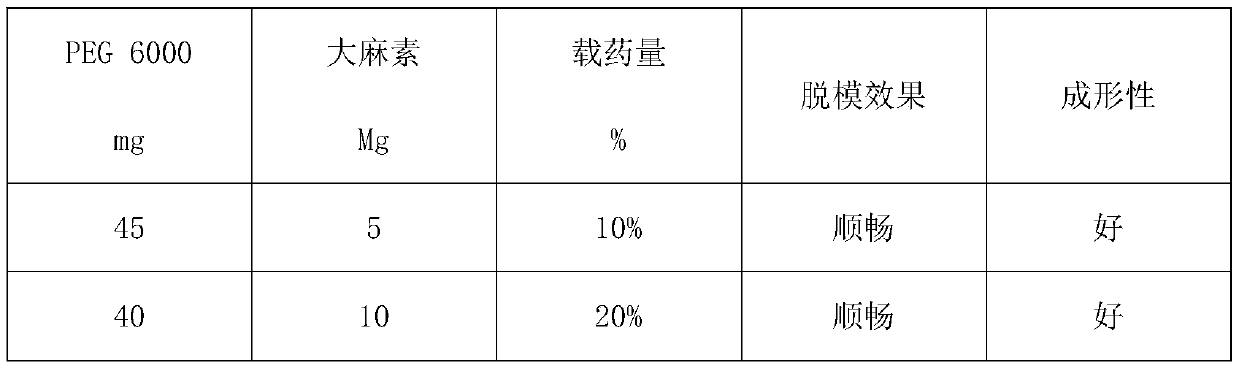
Application of cannabinoids in preparation of inhalants
ActiveCN110200953AShort inhalation timeImprove bioavailabilitySenses disorderNervous disorderBlood concentrationCannabinoid
The invention discloses application of cannabinoids in the preparation of inhalants. Inhaling the inhalants takes short time which is shorter than 1 min for a patient; the inhalants act fast; peakingtime of blood concentration is short; the inhalants are highly bioavailable.
Owner:HANYI BIO TECH CO LTD
Preparation for treating allergic rhinitis and asthma
Owner:KUNMING ZIJIAN BIOTECH
Medicine for treating rhinitis and preparation method thereof
InactiveCN109674910ASignificant clinical effectRelieve symptomsRespiratory disorderGnetophyta medical ingredientsModern medicineRaw material
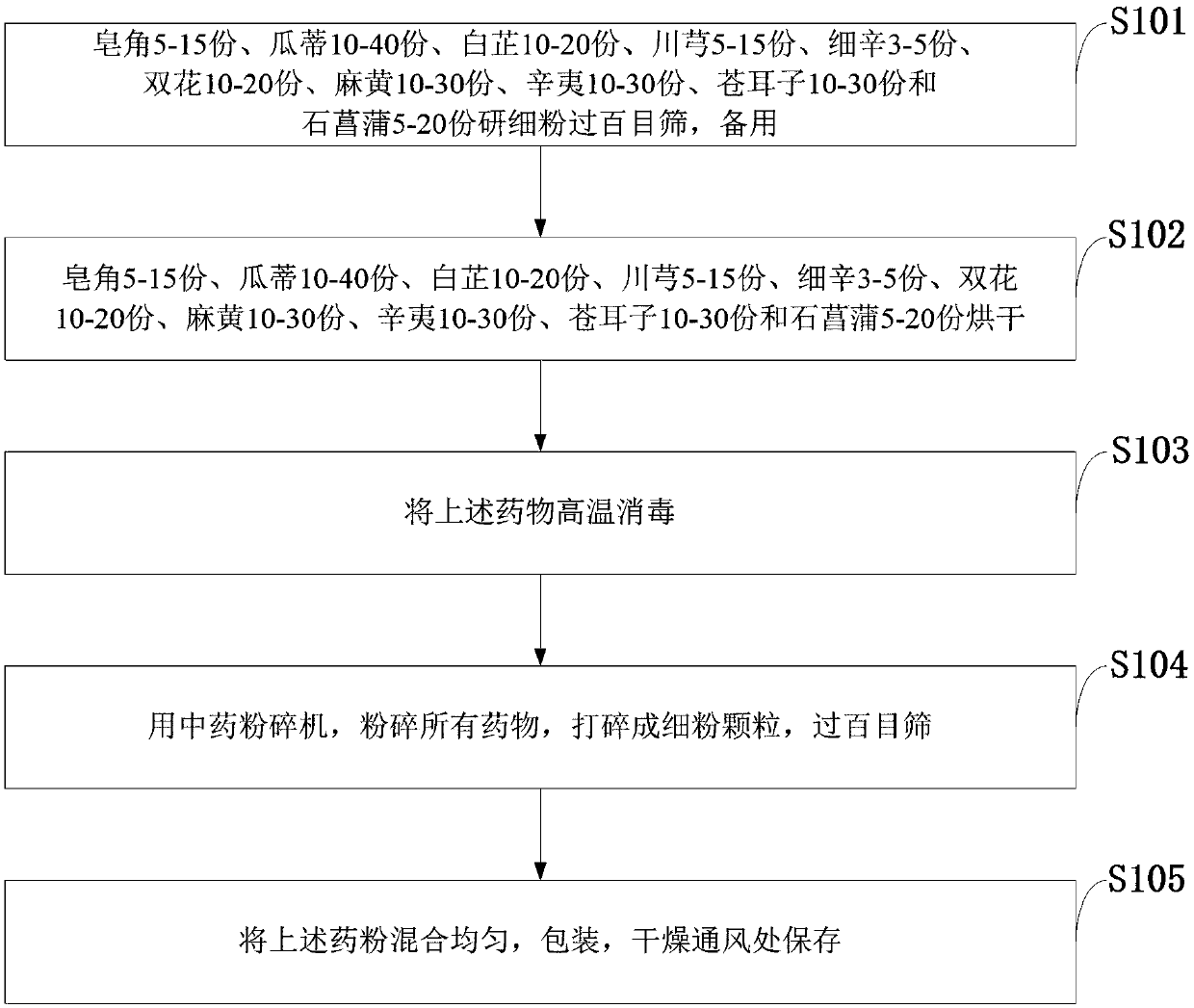
The invention belongs to the technical field of traditional Chinese medicine, and discloses medicine for treating rhinitis and a preparation method thereof. The medicine for treating rhinitis is prepared from the following raw materials in parts by mass: 5 to 15 parts of Chinese honeylocust fruits, 10 to 40 parts of muskmelon pedicel, 10 to 20 parts of radix angelicae, 5 to 15 parts of rhizoma chuanxiong, 3 to 5 parts of herba asari, 10 to 20 parts of lonicera japonica, 10 to 30 parts of herba ephedrae, 10 to 30 parts of flos magnoliae, 10 to 30 parts of fructus xanthii and 5 to 20 parts of rhizoma acori tatarinowii. An inhalant can directly reach the affected part to sufficiently achieve the maximum effects of the medicine; the discomfort is instantly relieved, so that a patient feels that uncomfortable symptoms of stuffy and running noise and the like are relieved; the use is convenient; the time limitation is avoided. The medicine invented by combining the ancient external use medicine spraying method with the modern medicine pharmacological research; the clinic effects are obvious. The clinic evaluation is performed according to the following curative effect standards: ineffectiveness: symptoms of stuffy noise, itching nose, paroxysmal sneezing and runny nose and are like are not changed; effectiveness: the symptoms are relieved; excellent effectiveness: the symptoms are obviously relieved.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Chinese ephedra and semen armeniacae amarae lung-freeing traditional Chinese medicine composition and preparation method thereof
InactiveCN109381595AEnhance immune functionEasy to buyAnthropod material medical ingredientsPill deliveryWarm waterLiquorices
The invention discloses a Chinese ephedra and semen armeniacae amarae lung-freeing traditional Chinese medicine composition and a preparation method thereof, and the Chinese ephedra and semen armeniacae amarae lung-freeing traditional Chinese medicine composition comprises the following raw materials in parts by weight: 2-8 parts of Chinese ephedra, 2-8 parts of mint, 2-8 parts of periostracum cicadae, 2-18 parts of honeysuckle, 4-18 parts of radix scutellariae, 4-16 parts of semen armeniacae amarae, 2-9 parts of thunberg fritillary bulb, 2-8 parts of platycodon grandiflorum, 2-8 parts of liquorice, 2-8 parts of jasmine, 10-20 parts of radix aucklandiae and 80-170 parts of refined honey. A Chinese ephedra and semen armeniacae amarae lung-freeing granule or drink disclosed by the inventionhas the effects of tonifying qi and tonifying deficiency, freeing lung, moistening dryness, reducing phlegm and relieving cough, relieving pain and resolving masses, and has the function of enhancingthe immunity of the organism, the traditional Chinese medicine composition is beneficial to emission of harmful inhalants, has the effects of freeing lung and moistening dryness, relieving cough and relieving sore throat, has a good effect on symptoms such as cough and excessive phlegm, and can be prepared into honeyed pills, can be taken with warm water and is convenient to use; the problem thatin the prior art the Chinese ephedra and semen armeniacae amarae lung-freeing traditional Chinese medicine composition is particles and needed to be taken by mixing with boiled water is solved.
Owner:KANGSHOU PHARMACY CO LTD HUNAN
Medicine for preventing and treating coronary heart disease and preparation method and application thereof
InactiveCN106727933AImprove systolic functionReasonable prescriptionCardiovascular disorderPlant ingredientsCoronary artery diseaseAdditive ingredient
The invention discloses a medicine for preventing and treating coronary heart disease and a preparation method and application thereof. The medicine is extract of ginseng and root of red-rooted salvia, the extract mainly comprises ginsenoside, saccharide, salvianolic acid, amino acid and protein ingredient, and the medicine can be prepared into tablets, capsules, granules, pills and inhalants. The medicine is especially suitable for application in treating coronary heart disease due to qi deficiency and blood stasis or coronary heart disease combined with lower cardiac function and can obviously improve lower cardiac function symptoms caused by the coronary heart disease; syndrome differentiation and treatment rules in theories of traditional Chinese medicine are adopted, nature of symptoms of the coronary heart disease is analyzed deeply, defects that medicine on the current market has are avoided, a novel treatment option is provided for the coronary heart disease, especially that combined with lower cardiac function, and market space is made up well.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
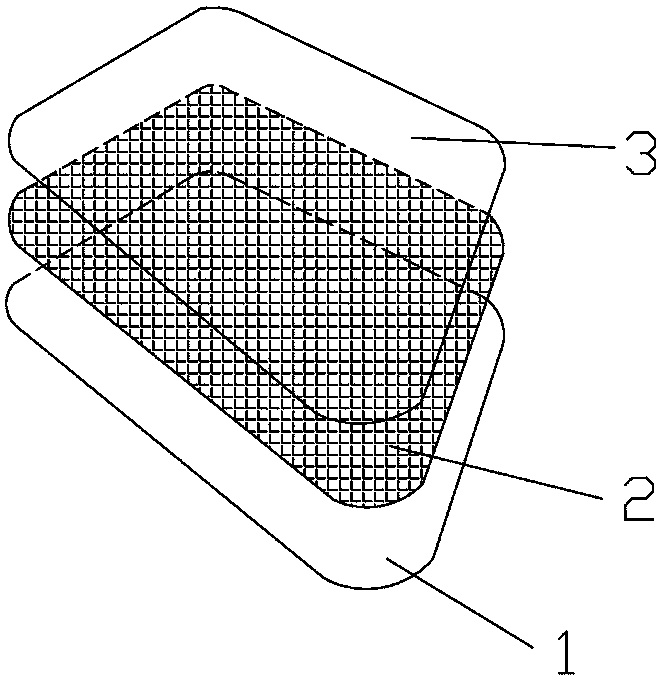
Transdermal patch capable of relieving nasal obstruction
InactiveCN108143772AStrong stickinessPaste firmlyHydroxy compound active ingredientsPharmaceutical non-active ingredientsTransdermal patchMenthol
The invention relates to a transdermal patch capable of relieving nasal obstruction and aims to effectively solve the problems that U-shaped inhalants cause discomfort in use and externally-applied patches are short in acting time, excessively low in adhesion and easy to fall off. The technical scheme adopted by the invention is as follows: the transdermal patch capable of relieving nasal obstruction is composed of an anti-adhesion layer 1, an adhesion layer 2 and a backing layer 3, wherein the adhesion layer 2 is an externally-applied product prepared by taking menthol, eucalyptus oil and ethanol as raw materials, taking an acrylate adhesive as a substrate, mixing, coating and die cutting; the adhesion layer is prepared from the following raw materials in percentage by weight: 4-6% of eucalyptus oil, 1.5-2.5% of menthol, 70-76% of acrylate adhesive and 18-22% of ethanol. The transdermal patch is safe and non-toxic and is pleasant in fragrance, convenient to use and free of discomfort;the acrylate adhesive layer is high in viscidity, steady to stick, difficult to fall off and long in acting time, is capable of rapidly relieving the nasal obstruction symptom, and is a great innovation in nasal patch products.
Owner:HENAN LINGRUI PHARMA
Sevoflurane inhalant
ActiveCN105106182AReduce degradationImprove stabilityEther/acetal active ingredientsPharmaceutical delivery mechanismPeppermintsBottle
The invention belongs to the technical field of medicine and particularly relates to a sevoflurane inhalant which contains sevoflurane, peppermint oil and ethanol and is prepared through the following method: dissolving peppermint oil in ethanol to form a solution, adding the solution in sevoflurane to form a mixture, and filling the mixture in a glass bottle. Compared with the prior art, the technology is simple, and the stability is excellent.
Owner:SHANDONG NEWTIME PHARMA
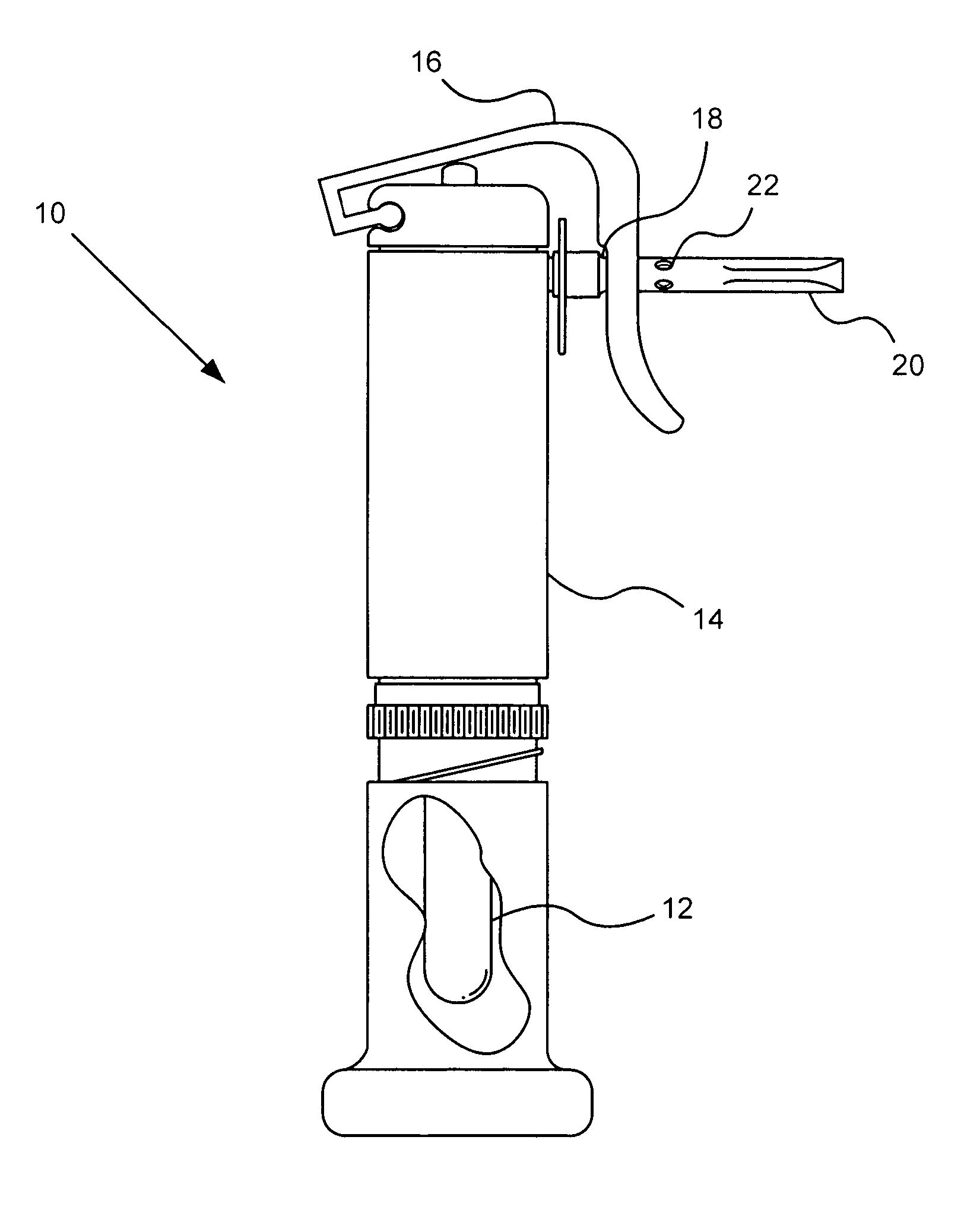
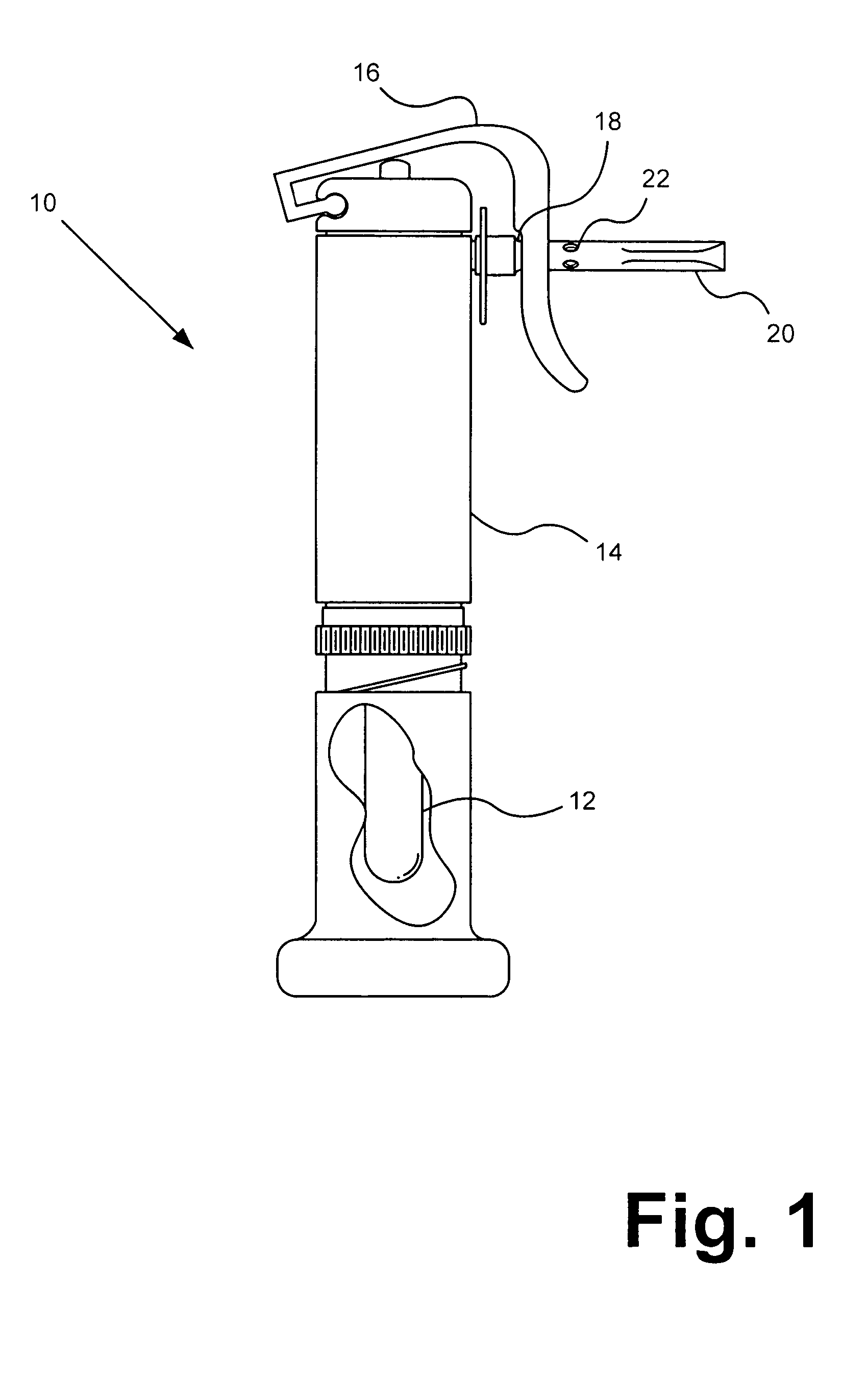
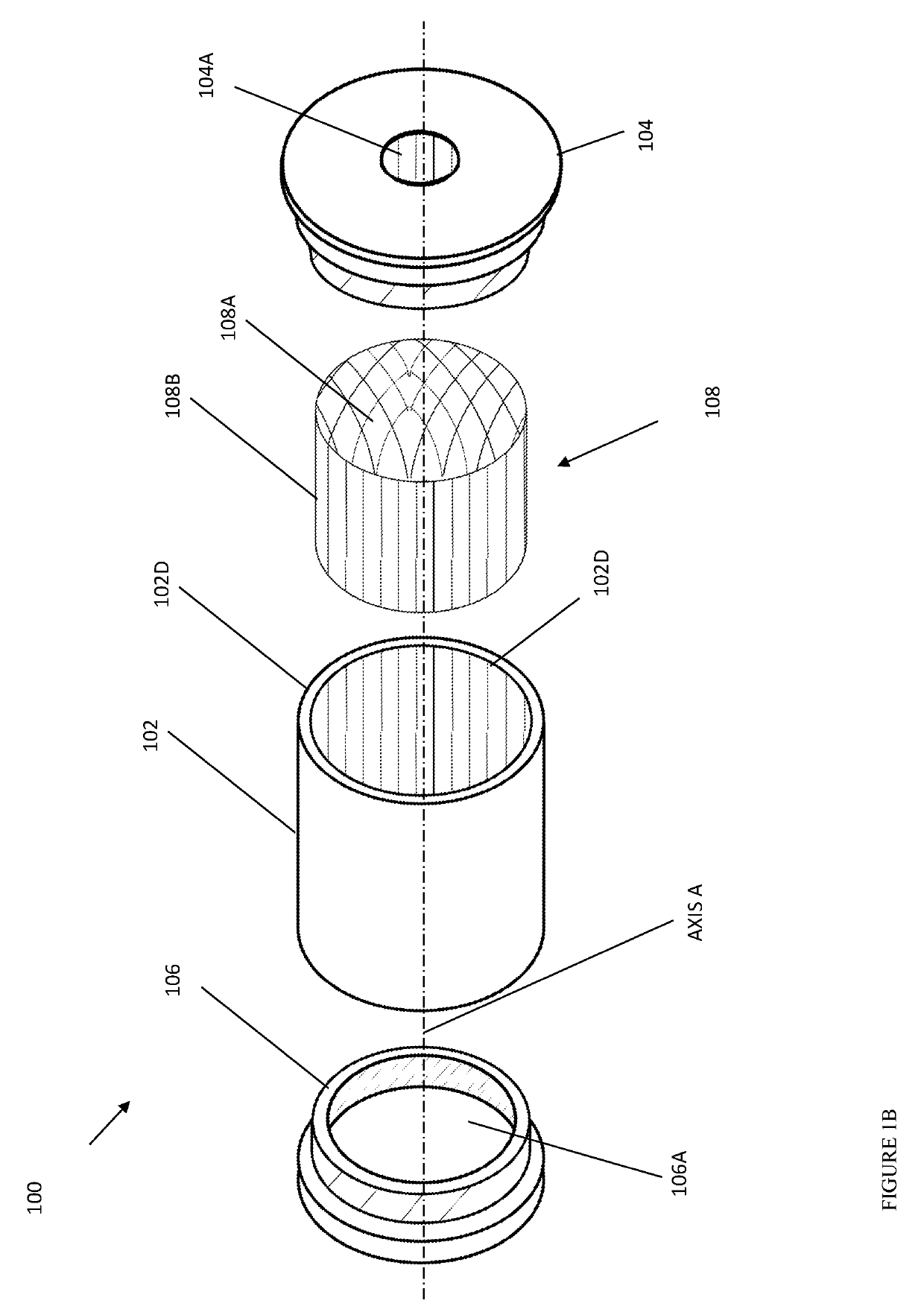
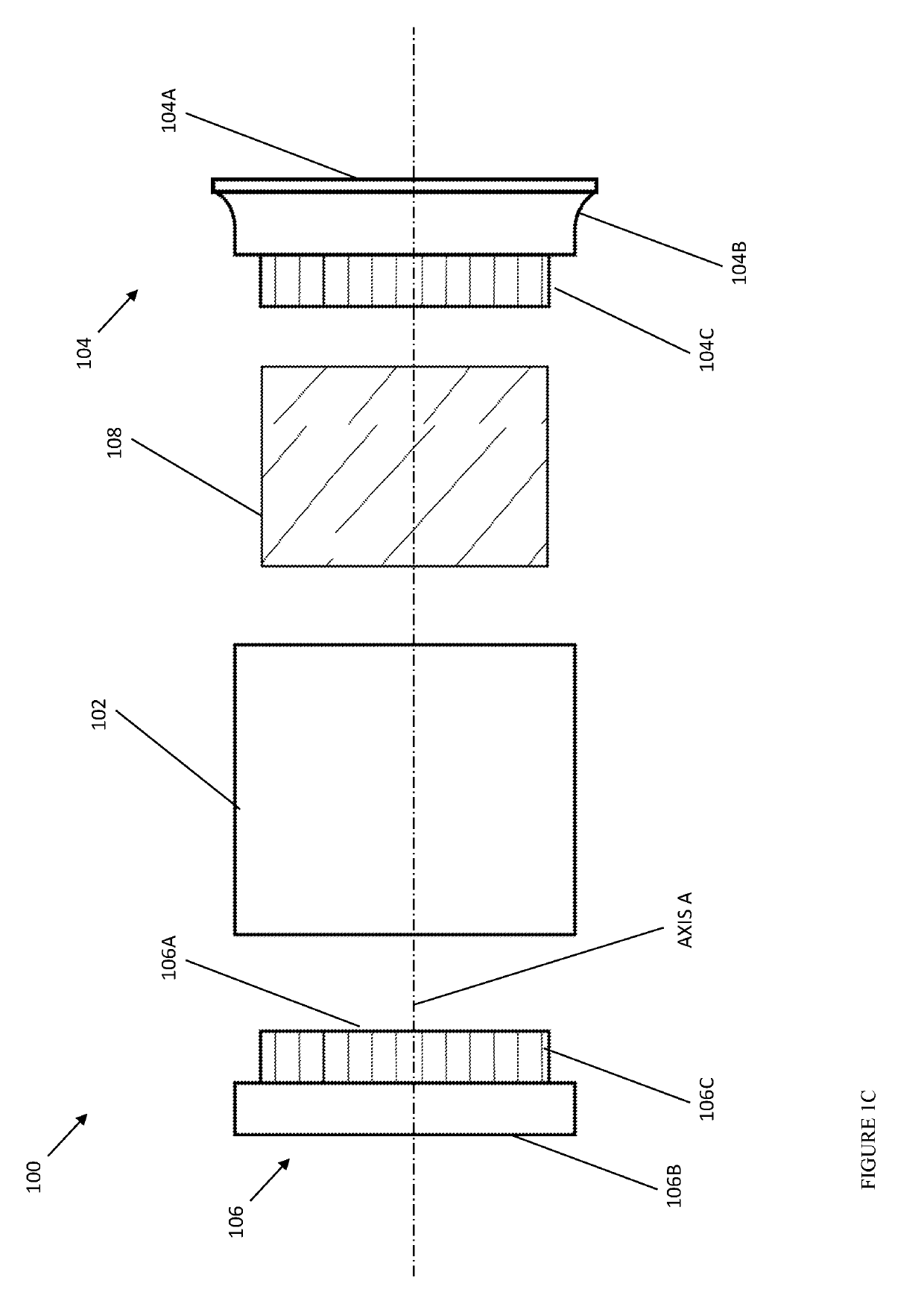
Portable inhalant filter device
A device and method of use thereof enable a portable device to be moveably disposed between a smoker and an output access point of a smoking pipe. The device temporarily forms a channel for inhalants to pass from a smoking pipe to a user's mouth. The smoking pipe may be selected from at least two different pipe structures. A cross section of the channel may be circular, orthogonal or elliptical. The device may include a particulate filter positioned between an inhalant input location and inhalant output location of the device. The filter may be insertable and removable from the tube. A source ledge may extend outward from the inhalant input location and / or a mouth end ledge may extend beyond a cross-section of the channel. A plurality of removable mouthpieces may be alternatively coupled and removed from the inhalant output location. The mouthpiece or a source piece may be elastic.
Owner:FONG JASON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com