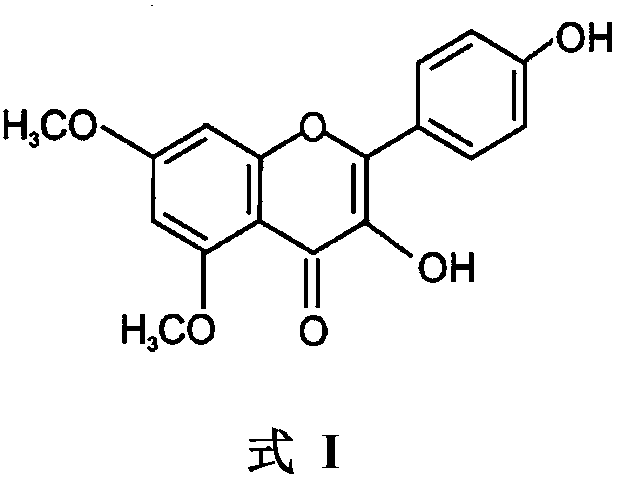
Use of 5,7-methoxy-3,4'-flavonol in resisting of respiratory tract inflammatory diseases
A technology for inflammatory diseases and hydroxyflavonoids, applied in the field of medicine, can solve the problems of unseen, low content, and low distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Rat peritoneal mast cell degranulation test
[0019] Method: Sensitized rats: Take SD rats and subcutaneously inject 0.1ml of 2.5mg / ml refined trichosanthemi powder (suspended in 4% AL(OH) 3 In the gel, after 14 days of sensitization, the femoral artery was exsanguinated to death, and 10ml of Hank's solution was injected into the abdominal cavity, and the abdominal cavity was opened gently for 2-3 minutes after the injection. Push the contents of the abdominal cavity to one side, use a dropper to absorb the peritoneal fluid into a test tube, put it in an ice bath, centrifuge at 500×g for 5 minutes, remove the supernatant, and gently shake the precipitate with 2ml of Hank solution, take 1ml of the suspension , Hank solution 1ml, antigen (10 μg / ml) 1ml, test drug 0.1ml, mix well and place in a 37°C water bath for 5 minutes, take out 1-2 drops of mast cells at the bottom of the test tube with a dropper, drop on the wave plate, and mix with l After mixing with 0....
Embodiment 2
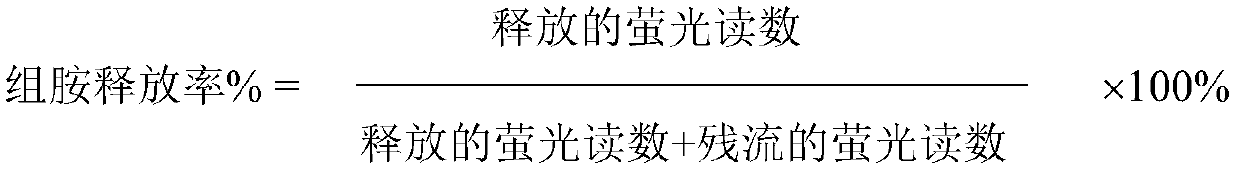
[0021] Example 2 Histamine release test of rat peritoneal mast cells:
[0022] Method: Rats were sensitized in the same way as above, and peritoneal mast cells (MC) were collected after 14-21 days. Collect MCs: Rats were bled to death from the carotid artery, and buffered at 37°C (mM) with Nac1 150, Kc1 0.7, Cacl 0.9, Na 2 HPO 4 4.3, KH 2 PO 4 3.5, Glucose 5.6, pH 7.2 flush the peritoneal cavity, 15m1×2. The peritoneal washings were collected and placed in siliconized test tubes in an ice bath (the above experimental steps were all carried out at 4°C). Centrifuge at 500×g for 5 minutes, and remove the supernatant. Shake the precipitate with an appropriate amount of buffer solution to obtain the rat peritoneal MC suspension.
[0023] Antigen induced MC to release histamine: draw 0.4ml of MC suspension of sensitized rats, add phosphatidylserine 10μg / ml, add various concentrations of test drugs (37°C, warm bath for 5min), add refined trichosanthin antigen 10μg / ml, make T...
Embodiment 3
[0030] Example 3 Rat peritoneal neutrophil β-glucuronidase release test:
[0031] Method: collection of neutrophils (NP) in rat peritoneal cavity: rats were anesthetized with ether, and 25ml of 0.7% glycogen saline was injected intraperitoneally. After 4 hours, the rats were killed by carotid artery bleeding, the abdominal cavity was opened, and the peritoneal fluid was carefully sucked (do not touch the intestinal tube), and HBSS (mM, NaCl 138.0, KC1 5.4, CaC1 21.8, HEPES 20, Clucose 5.6, 0.1% BSA, pH 7.4) at 4°C Equally dilute, centrifuge at 250×g for 5min, discard the supernatant, add 2ml of ice water to the precipitate, add 2ml of 1.8% NaCl solution after 2sec, centrifuge, remove the supernatant, resuspend in HBSS, centrifuge to remove the supernatant to prepare NP suspension . After smear, Wright staining inspection, NP purity> 95%, trypan blue inspection cell viability> 95%. fMLP induces NP release: NP suspension lml (5×10 6 ml / NP) into a siliconized glass test tube, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com