Inhalant, preparation method thereof, and application of inhalantas inhalant carrier with pullulan
A pullulan sugar and inhalant technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., can solve problems such as low lung inhalation efficiency, achieve fast dissolution speed, excellent lubricity, and increase the surface area. glossy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
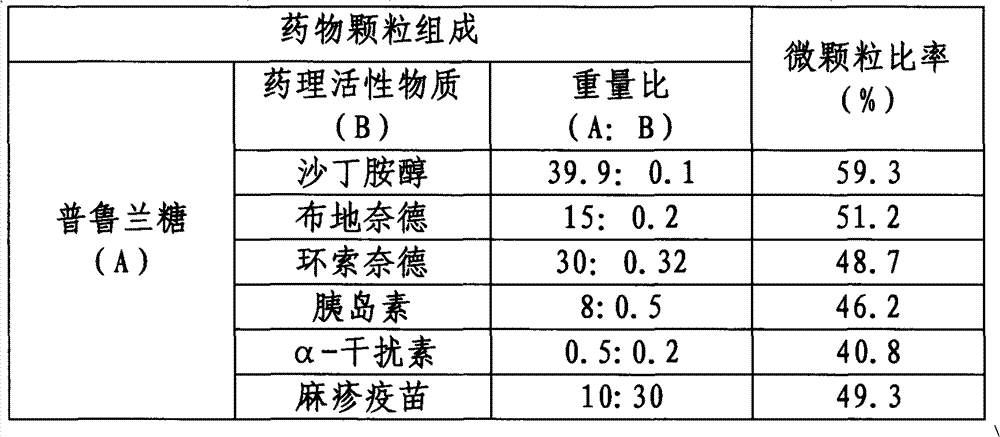
[0041] Dissolve the pullulan and pharmacologically active substances in the following Table 1 in water according to a certain weight ratio, and then spray-dry under standard working conditions to form drug granules, and the powder spray 1 can be obtained. The lung deposition efficiency was obtained under the standard operation of simulating lung deposition in vitro, expressed as the ratio of microparticles, as shown in Table 1.
[0042] Composition and microparticle ratio of drug particles in powder aerosol 1 in table 1
[0043]
Embodiment 2
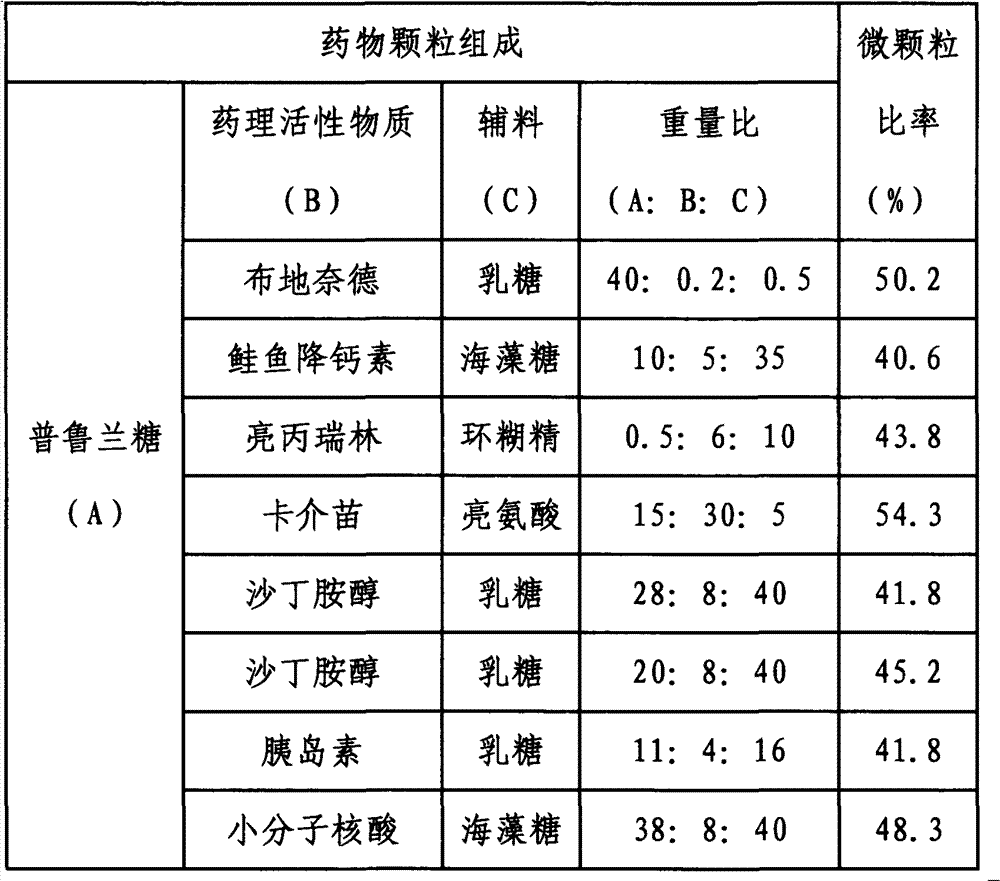
[0045] Dissolve the pharmacologically active substances in Table 2 and pullulan in the same aqueous solution at a certain weight ratio, then add adjuvants of the corresponding weight ratio to the solution as required, and spray and dry under standard working conditions to generate drug particles. Get powder spray 2. The lung deposition efficiency was obtained under the standard operation of simulating lung deposition in vitro, expressed in the ratio of microparticles, as shown in Table 2.
[0046] Composition and microparticle ratio of drug particles in powder aerosol 2 in table 2
[0047]
Embodiment 3
[0049] Dissolve the pharmacologically active substances in Table 3 in water, then add adjuvants of corresponding weight ratios as required, and spray-dry to generate drug granules under standard working conditions; the pullulan granules are spray-dried or other known methods (such as ball milling, Jet powder, spray freeze-drying) obtained. The drug granules and the pullulan granules are mixed mechanically according to a certain weight ratio to obtain the compound drug granules, and the powder aerosol 3 can be obtained. Standard detection methods were used to measure the lung deposition efficiency, expressed as the ratio of microparticles, as shown in Table 3.
[0050] Composition and microparticle ratio of drug particles in powder aerosol 3 in table 3
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com