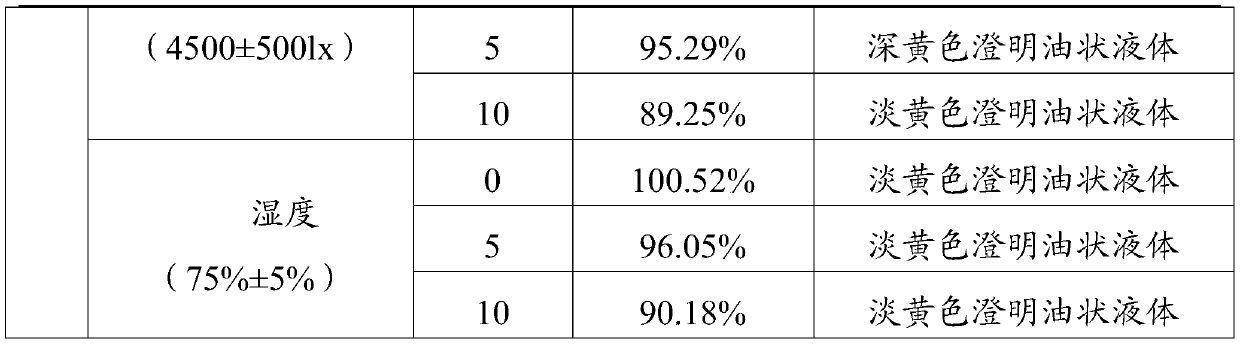
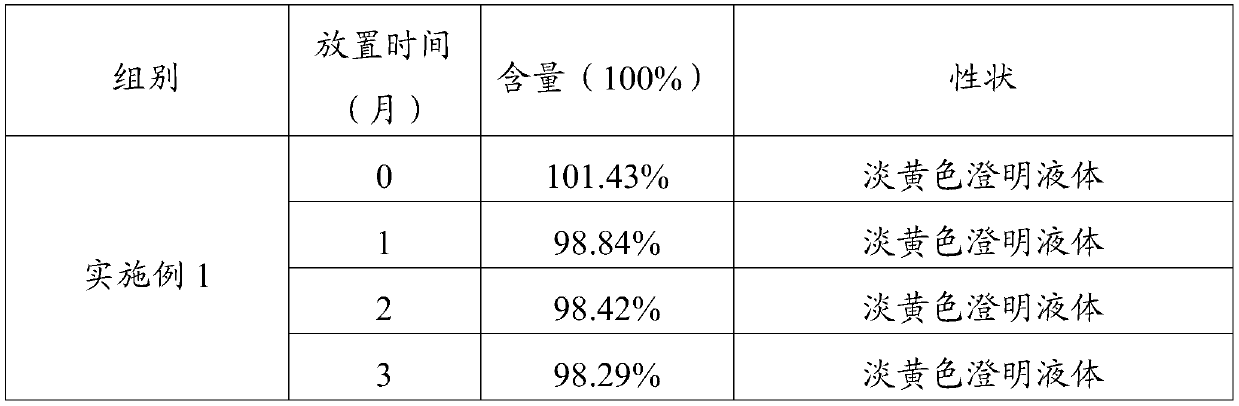
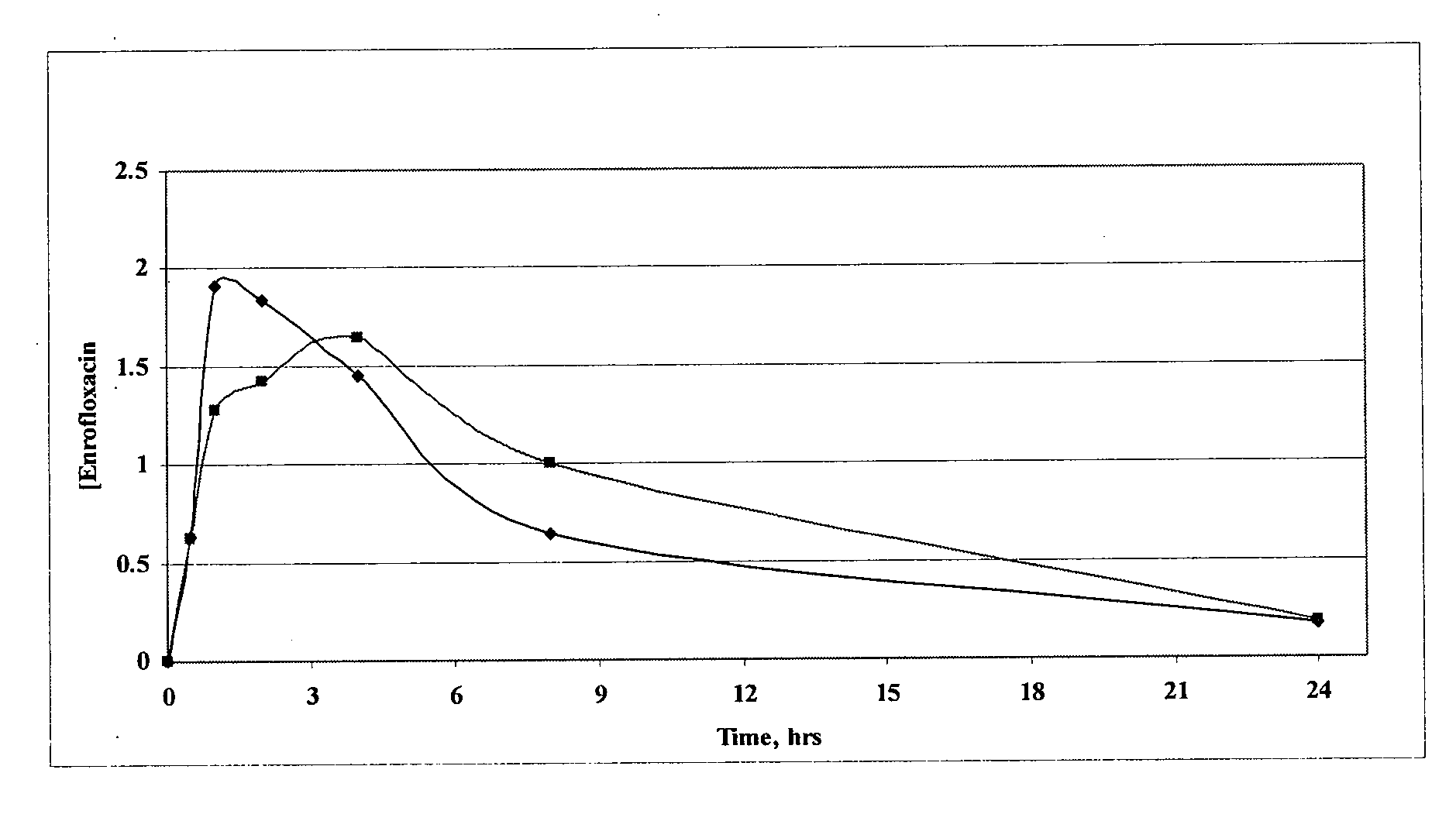
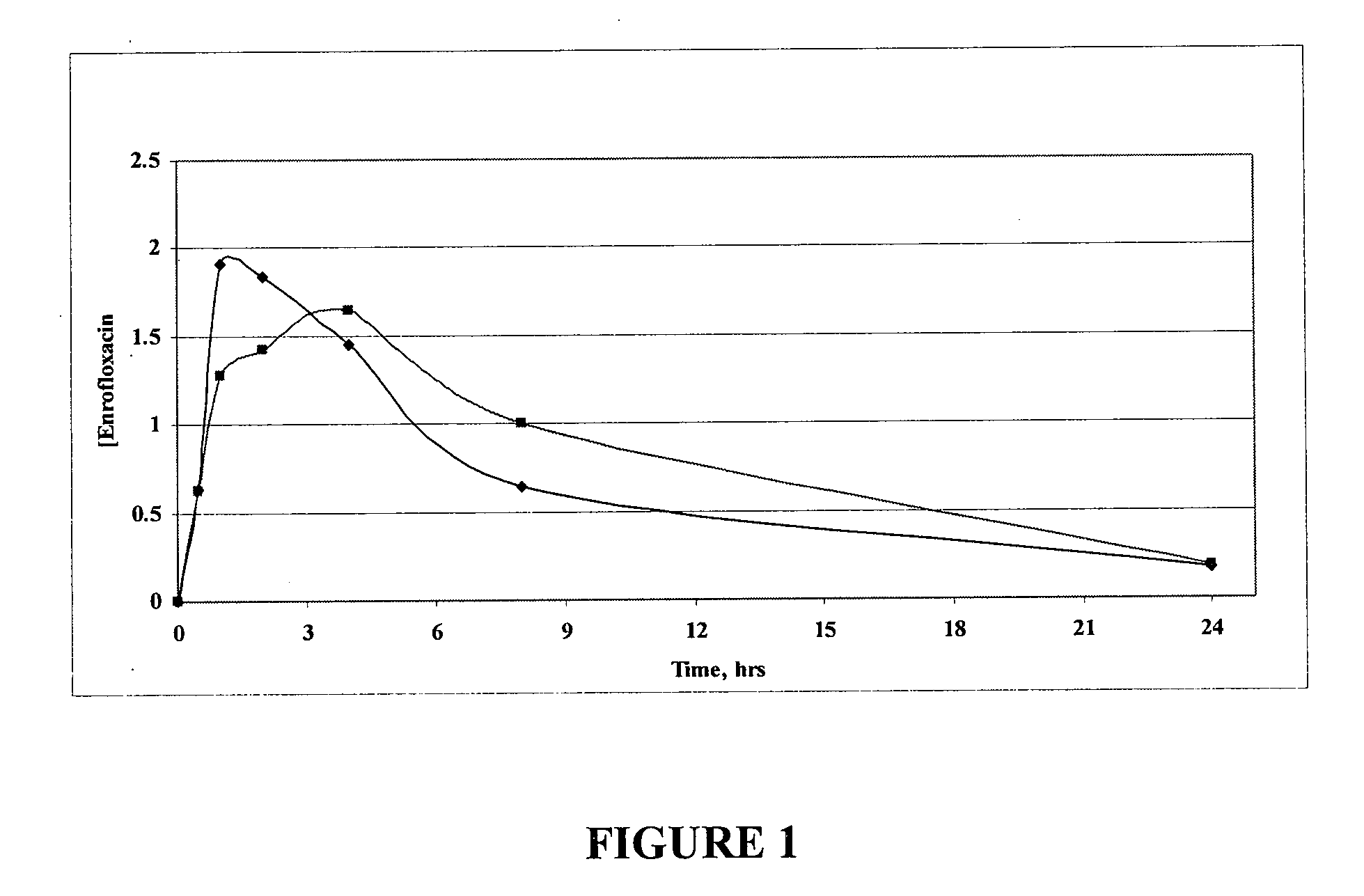
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Glycerol formal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
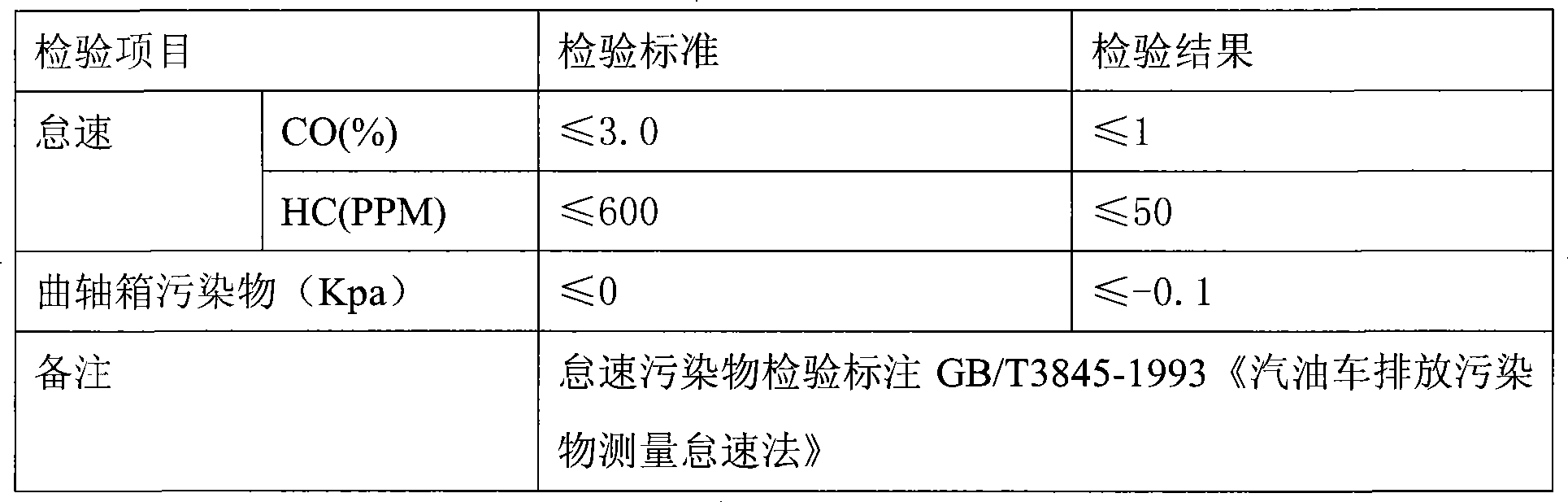
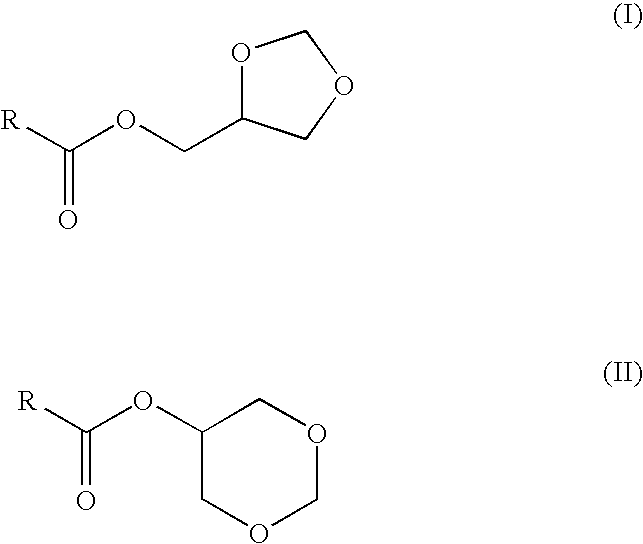
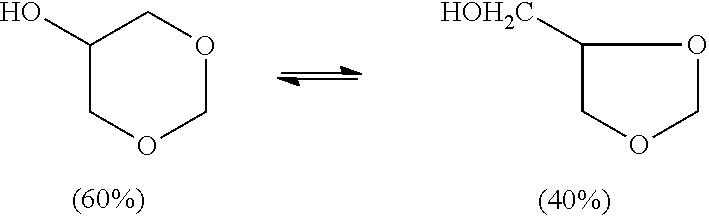
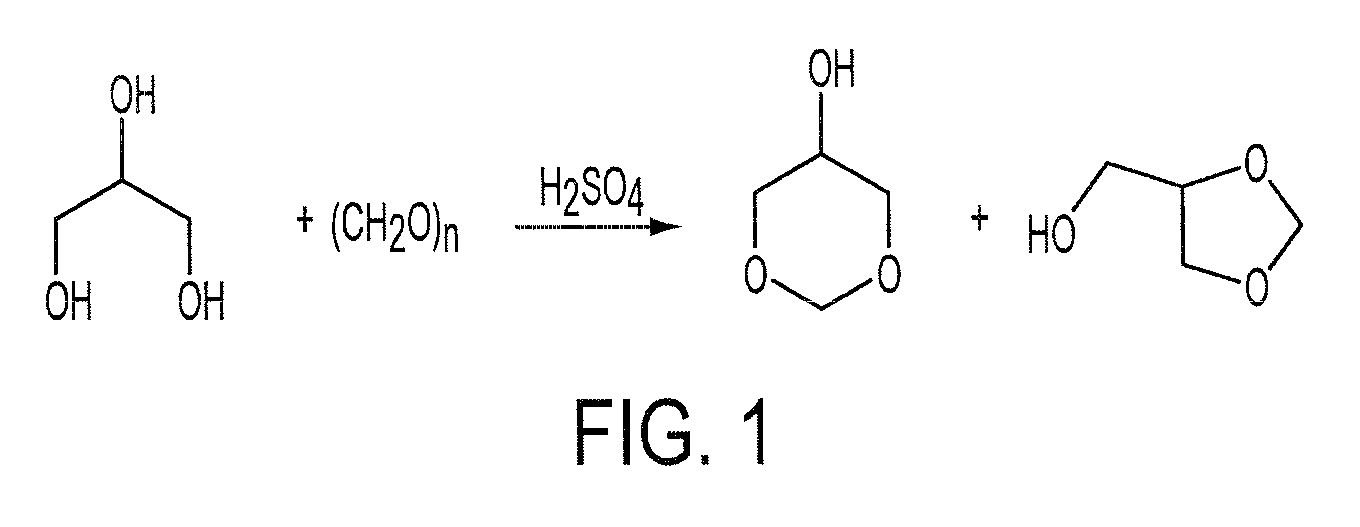
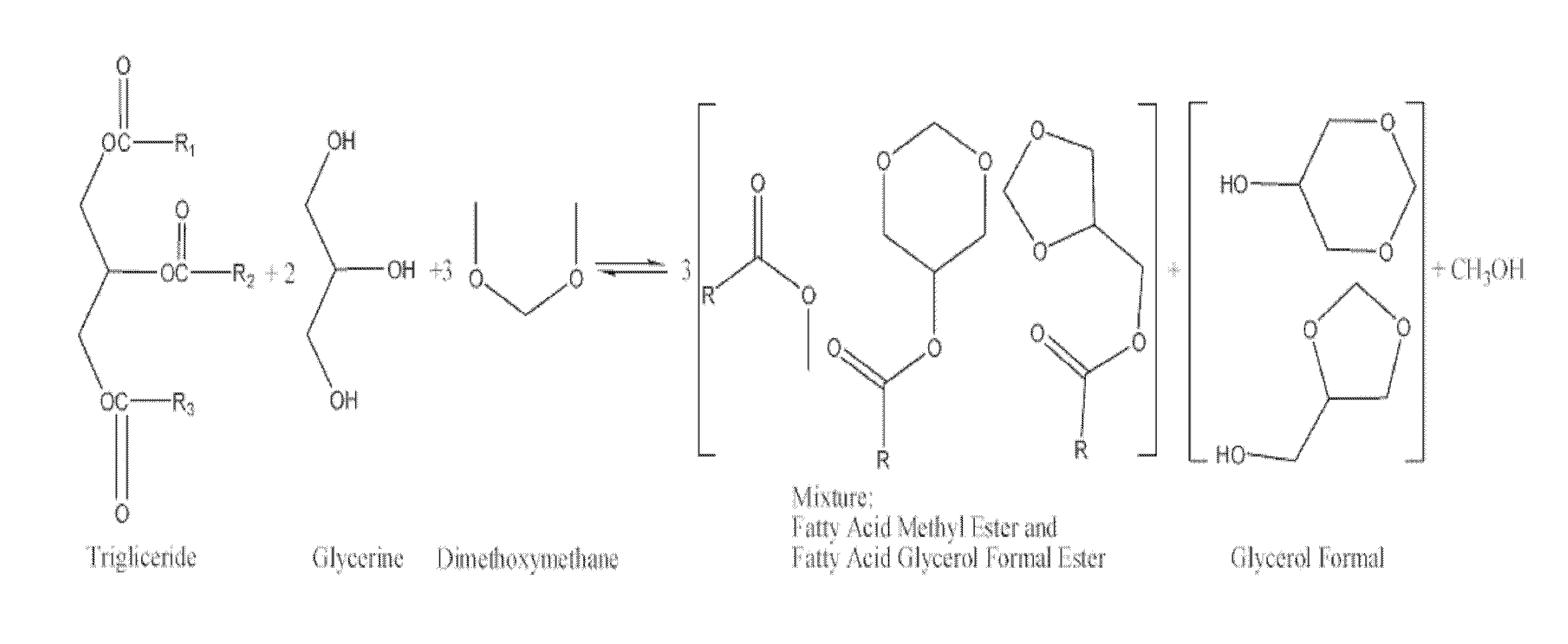
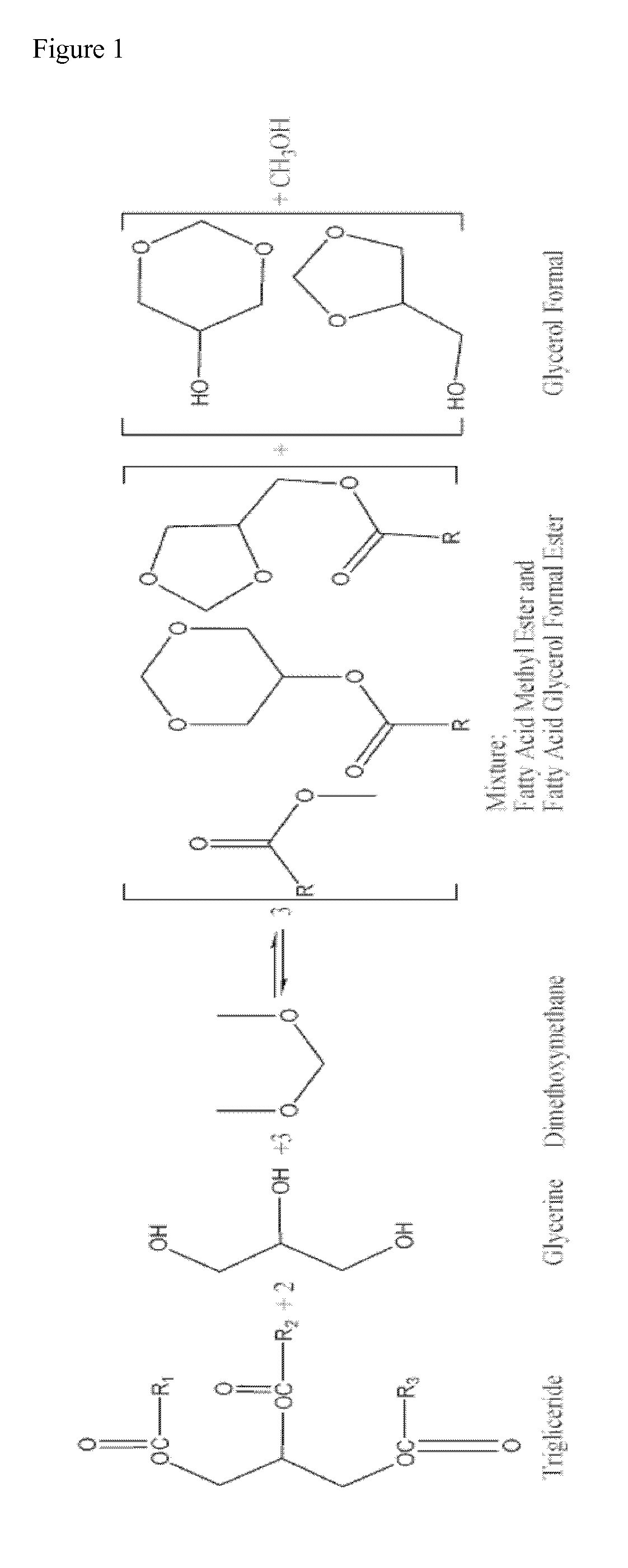
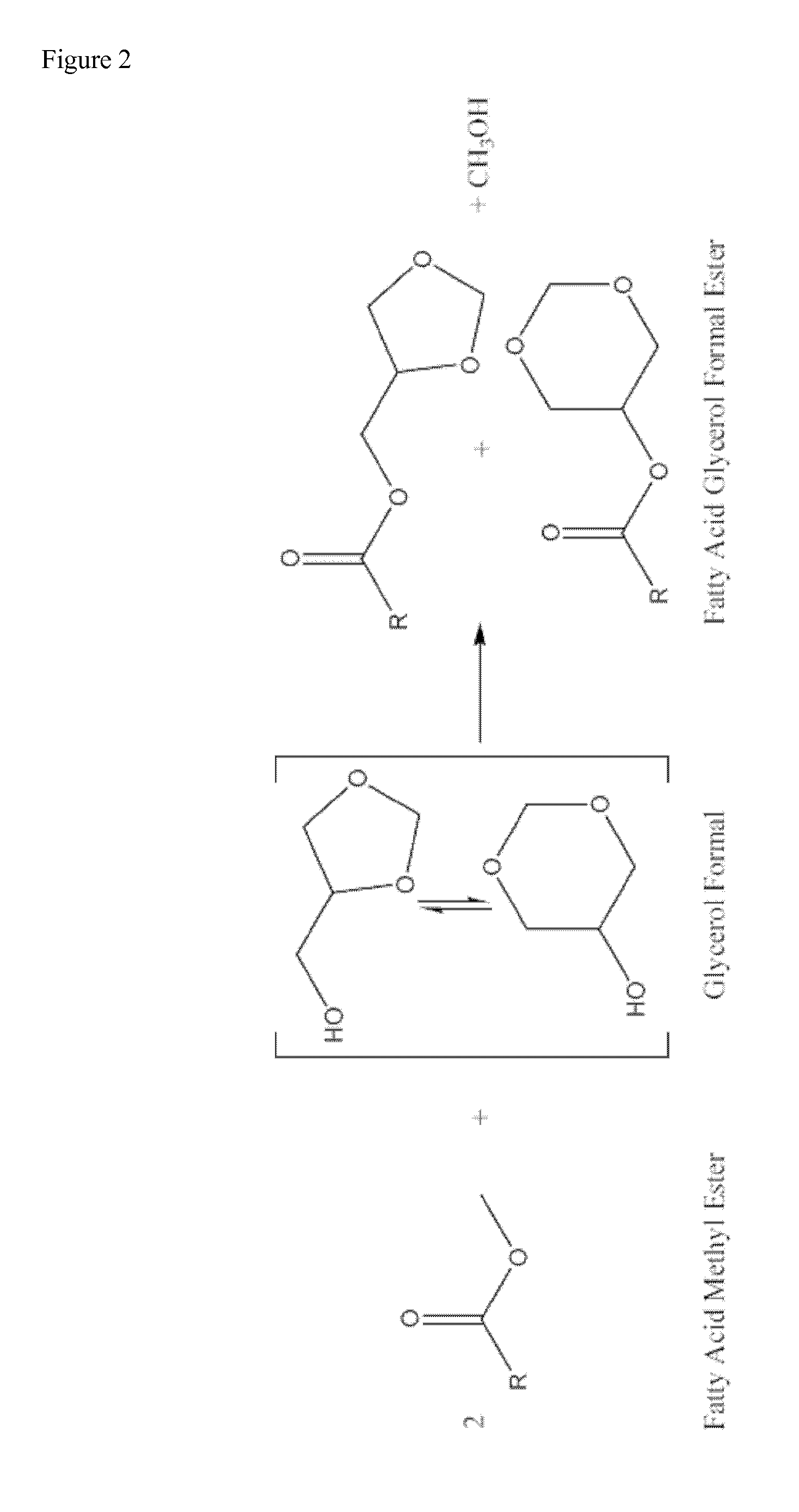
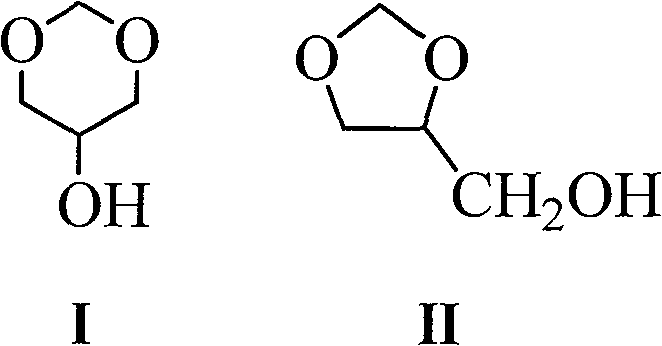
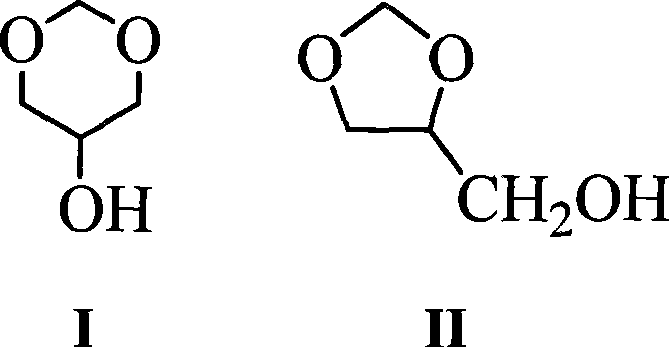
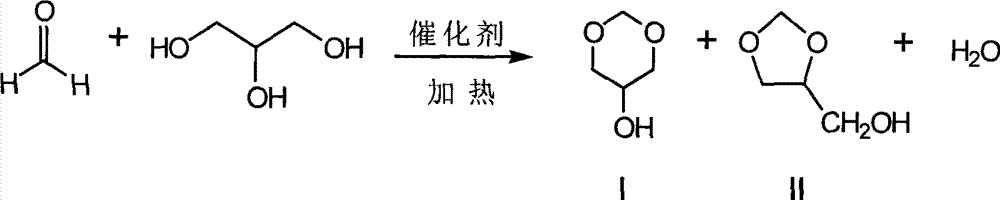
Glycerol formal (GF) is a light glycerol acetal that is composed of ~60% 5-hydroxy-1,3-dioxane and ~40% 4-hydroxymethyl-1,3-dioxo lane. It can be prepared by reacting glycerol and formaldehyde in the presence of acid catalyst.
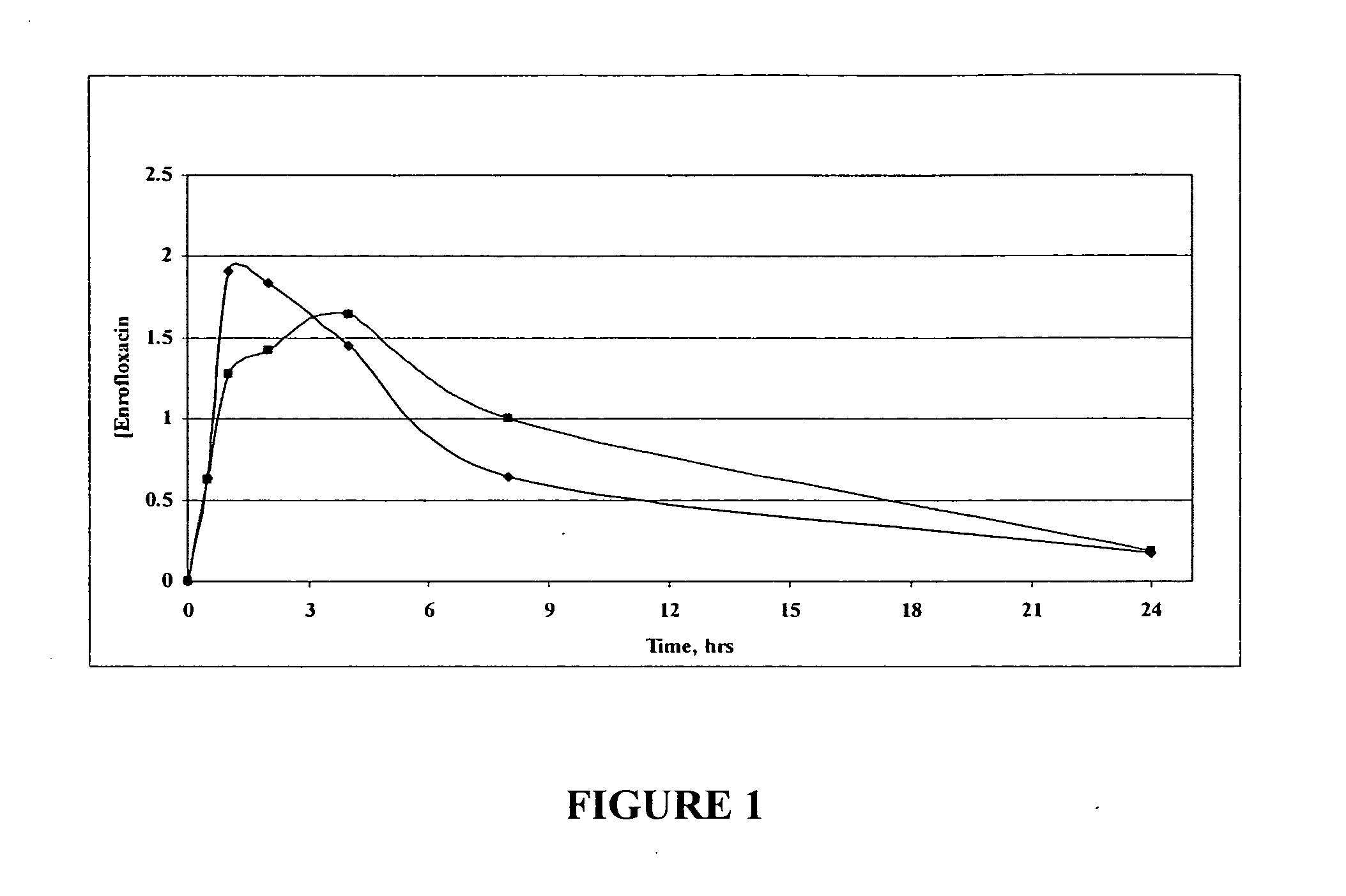
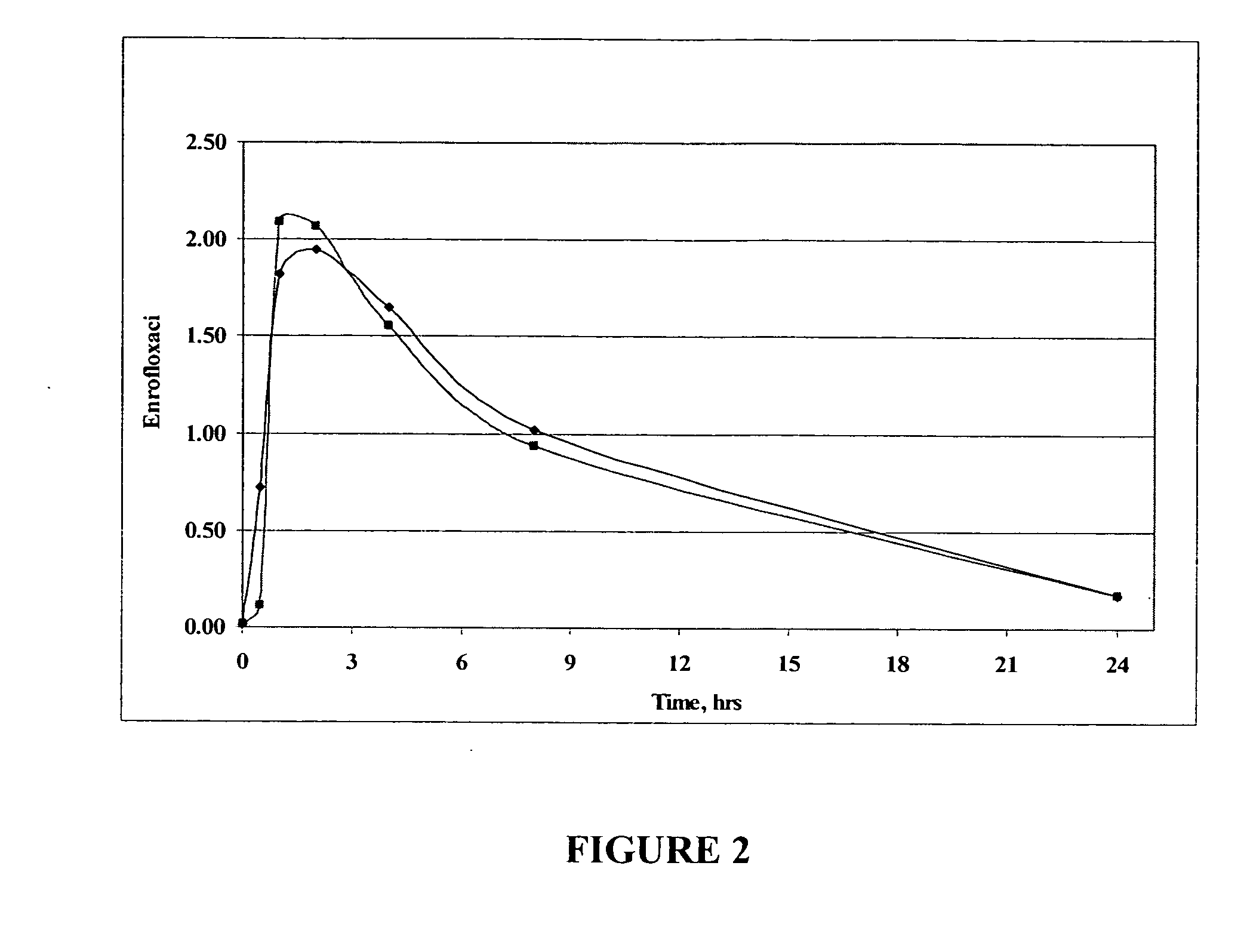
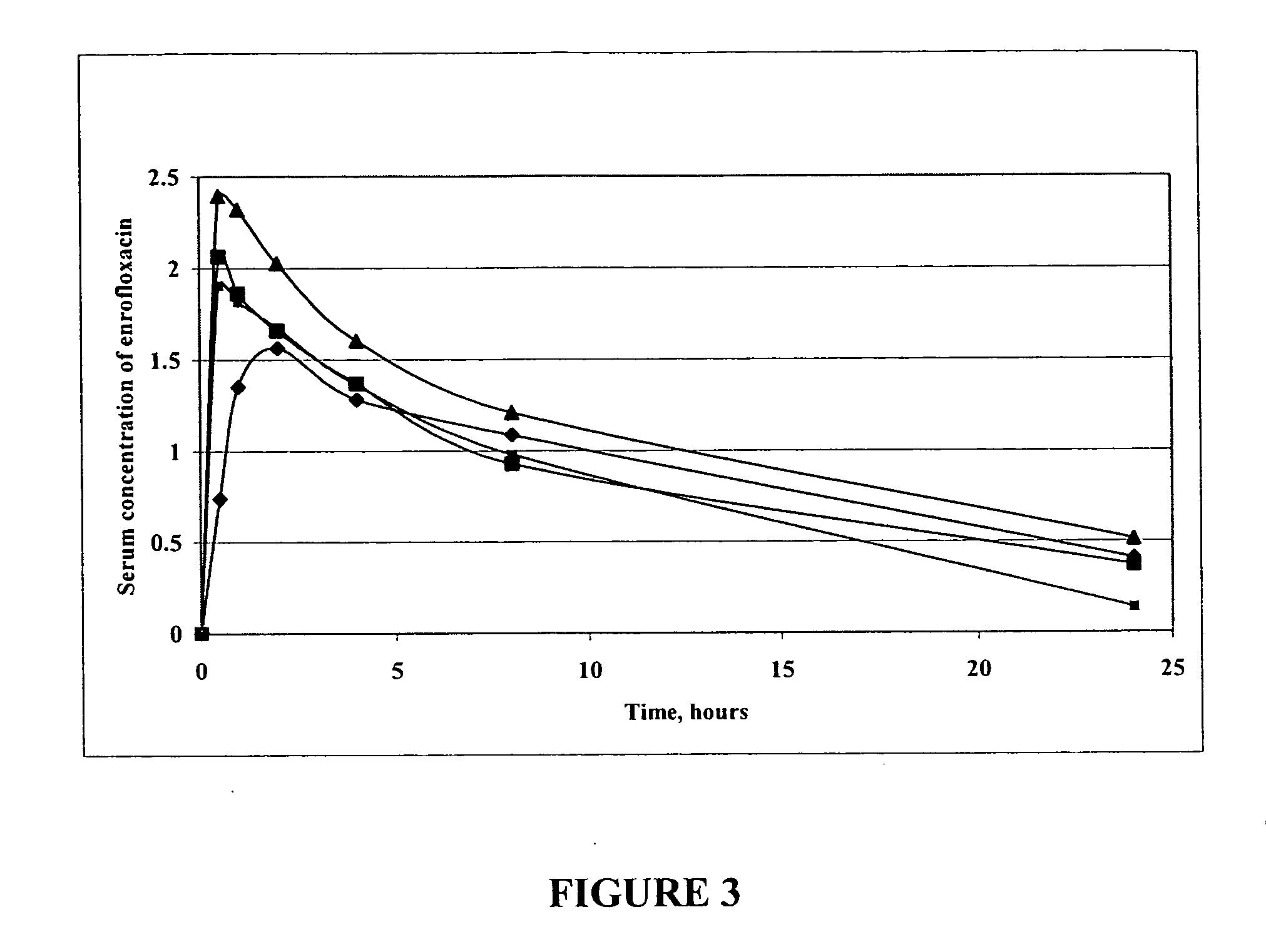
Fluoroquinolone compositions
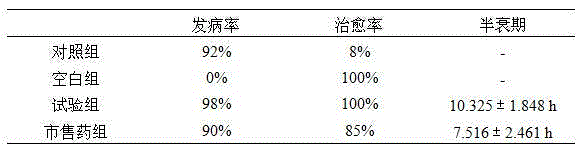
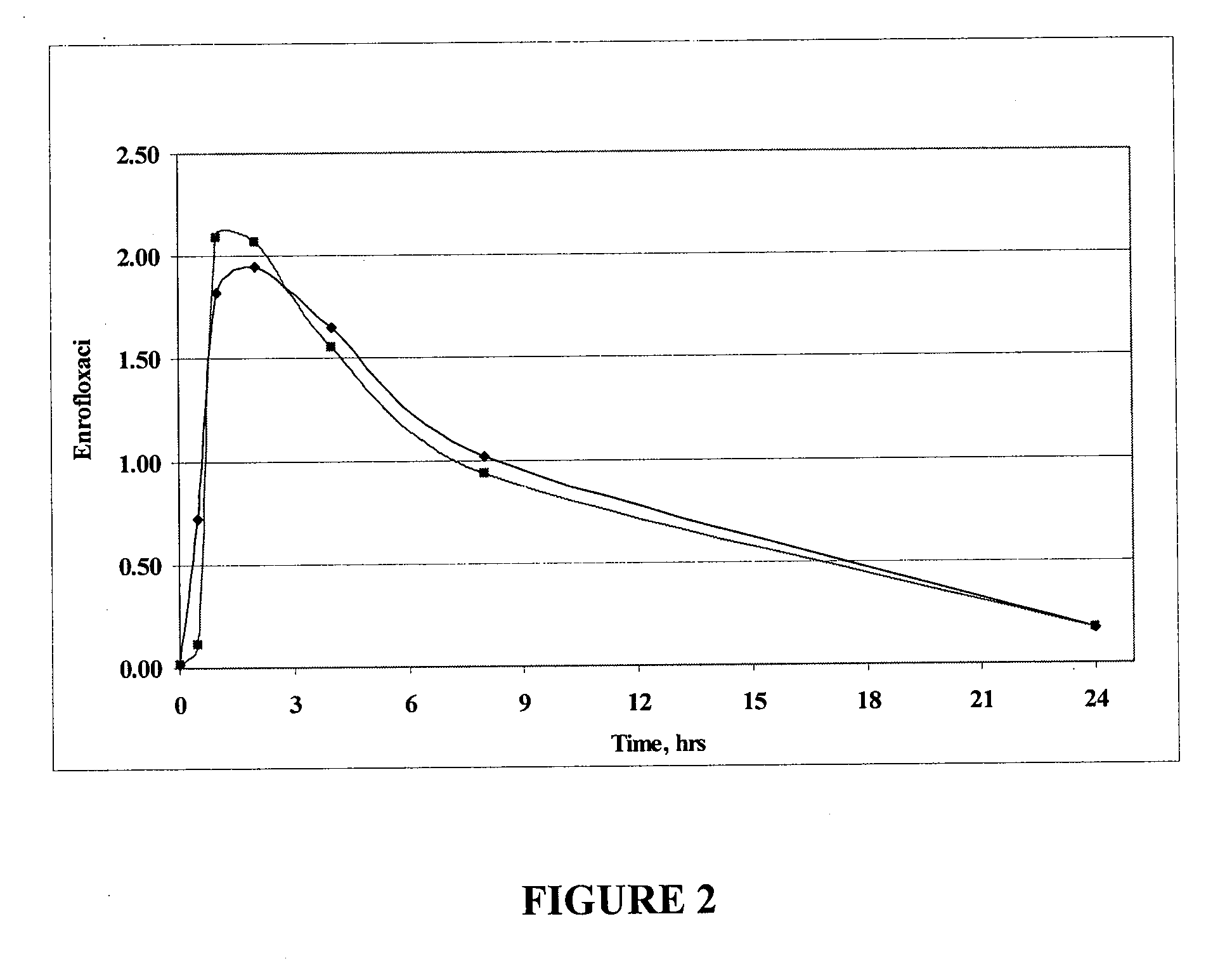
The invention relates to pharmaceutical compositions comprising (i) a fluoroquinolone, (ii) a salt formed between a carboxylate anion and a divalent metal cation, (iii) a liquid comprising an organic solvent selected from the group consisting of glycerol, propylene glycol, glycerol formal, and (iv) optionally water. The invention further relates to methods of treating or preventing a condition in an animal comprising administering to the animal in need thereof a pharmaceutical composition of the invention.
Owner:IDEXX LABORATORIES
Long acting parasiticidal composition containing a salicylanilide compound, a polymeric species and at least one other anti-parasitic compound
ActiveUS20050118221A1Improve bioavailabilityEfficient ConcentrationBiocidePharmaceutical delivery mechanismPolyethylene glycolBlood plasma
A composition prepared for treating animals suffering from parasites which parasites are known to be susceptible to at least one of the avermectins, milbemycins or salicylanilides, comprises for example ivermectin in an amount of from 0.1 to 10%(w / v), a solvent selected from the group consisting of glycerol formal, propylene glycol, polyethylene glycol and combinations thereof, and a salicylanilide such as closantel in a required dosage amount for the animal to be treated, typically about 2.5 mg / kg live weight of the animal to be treated, a polymeric species selected from the group consisting of polyvinylpyrrolidone and polyoxypropylene / polyoxyethylene block copolymers, the said polymeric species improving the bioavailability of closantel to the extend that blood plasma levels of the said compound greater than about 20 ppm over period of treatment are achievable.
Owner:NORBROOK LABORATORIES LIMITED
Low temperature resistant alcohol clean fuel oil and preparation method
InactiveCN101531930AGood miscibilitySimple preparation processLiquid carbonaceous fuelsAlcoholGasoline
The invention relates to a low temperature resistant alcohol clean fuel oil, comprising 10% to 30% of ethanol, 65% to 86% of gasoline component oil and 0.01% to 5% of glycerin and / or glycerol formal; and the percentages are weight percentages. The preparation method is as follows: at normal temperature and pressure, gasoline component oil, ethanol, glycerin and / or glycerol formal are added to a reaction kettle in turn; and the required product is obtained after stirring. The invention has very good low temperature resistant effect, and can still be normally used at the temperature of -20 DEG C. Moreover, the invention has simple preparation technology, low production cost, good alcohol oil intersolubility, and difficult lay separation. The fuel in the invention can be used at low temperature, thereby not only having broad market prospect but also having important social meaning.
Owner:WUHAN INSTITUTE OF TECHNOLOGY +1
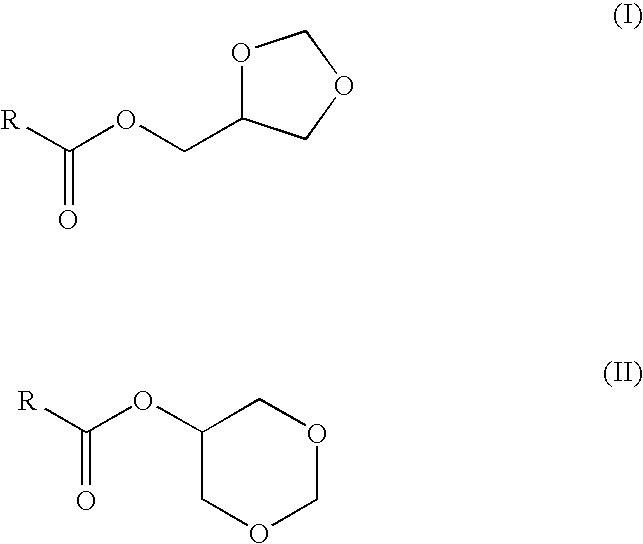
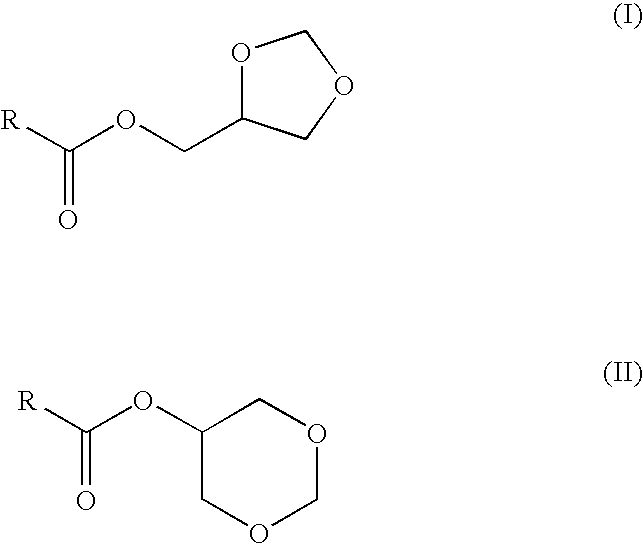
Preparation of fatty acid esters of glycerol formal and its use as biofuel
ActiveUS20100005708A1High glycerol contentIncrease cetane numberOrganic chemistryFatty acid esterificationTransesterificationTG - Triglyceride
This invention describes the preparation of fatty acid esters of glycerol formal either by a triglyceride transesterification process or, alternatively, by an esterification process of fatty acids previously obtained from the hydrolysis of triglycerides (fat splitting), with glycerol formal in the presence of an acid or basic catalyst. Also the invention describes the use of these fatty acid esters of glycerol formal prepared by said process as biofuel. In an embodiment, such biofuel is used in the preparation of other biofuels by its mixture with a product selected from a group formed by: glycerol formal, biodiesel, petrol-derived diesel, and mixtures thereof. The biofuels thus obtained are characterised to allow the complete incorporation of the glycerol obtained in the current biodiesel production process in a biodiesel fuel.
Owner:INST UNIV DE CIENCIA I TECH SA
Ursocycline injection composition
InactiveCN101185635ALong duration of actionImprove stabilityTetracycline active ingredientsPharmaceutical delivery mechanismPropylene glycolOxytetracycline
A terramycin injection composition has long pharmacodynamic action time, wherein each unit of the terramycin injection composition contains 30% terramycin free alkali, 2%-3% magnesium compound, 30%-50% 2-ketopyrrolidine, 5%-20% propylene glycol, 5%-20% glycerol formal, 1%-5% moderator and the rest is water for injection. The invention prolongs pharmacodynamic action time of terramycin, decreases possibilities of pain, swelling, tissue stimulation and tissue necrosis at the injection sites of animals and improves stability of terramycin at high temperature.
Owner:四川喜亚动物药业有限公司
Swine eperythrozoonosis-resisting compound long-acting oxytetracycline injection and preparation method thereof
InactiveCN101953841AQuick cureEffective treatmentAntipyreticTetracycline active ingredientsOxytetracycline HydrochlorideSodium bisulfate
The invention discloses swine eperythrozoonosis-resisting compound long-acting oxytetracycline injection, which comprises the following components in part by mass: oxytetracycline hydrochloride (or alkaloids thereof), roxarsone, acetaminophen, glycerol formal, polyvinylpyrrolidone K12, polyvinylpyrrolidone K17, polyethylene glycol 200, polyethylene glycol 400, magnesium chloride, sodium formaldehyde sulphoxylate, monoethanol amine and water for injection. Due to the compatibility of the oxytetracycline hydrochloride (or the alkaloids thereof), the roxarsone and the acetaminophen and synergistic action of each medicament component, the injection treats both symptoms and root causes of swine eperythrozoonosis so as to fulfill the aim of quick and effective treatment.
Owner:NORTHWEST A & F UNIV
Improved method for producing emulsifiable pesticide solutions
The current invention provides a kit of parts for producing an emulsifiable pesticide solution comprising: (a) a water miscible organic solvent selected from the list of a glycol ether, a glycerol formal, dimethylsulfoxide, gamma-butyrolactone or mixtures thereof, (b) an alkoxylated alcohol with an average of 6 to 80 moles of ethylene oxide and 2 to 60 moles of propylene oxide per mole of alcohol; and (c) a pesticidal active ingredient. The invention further provides a composition for producing an emulsifiable pesticide solution, a method for producing such a composition and a method for treating an agricultural crop. In addition the invention provides some advantageous pesticidal compositions.
Owner:ARYSTA LIFESCI BENELUX
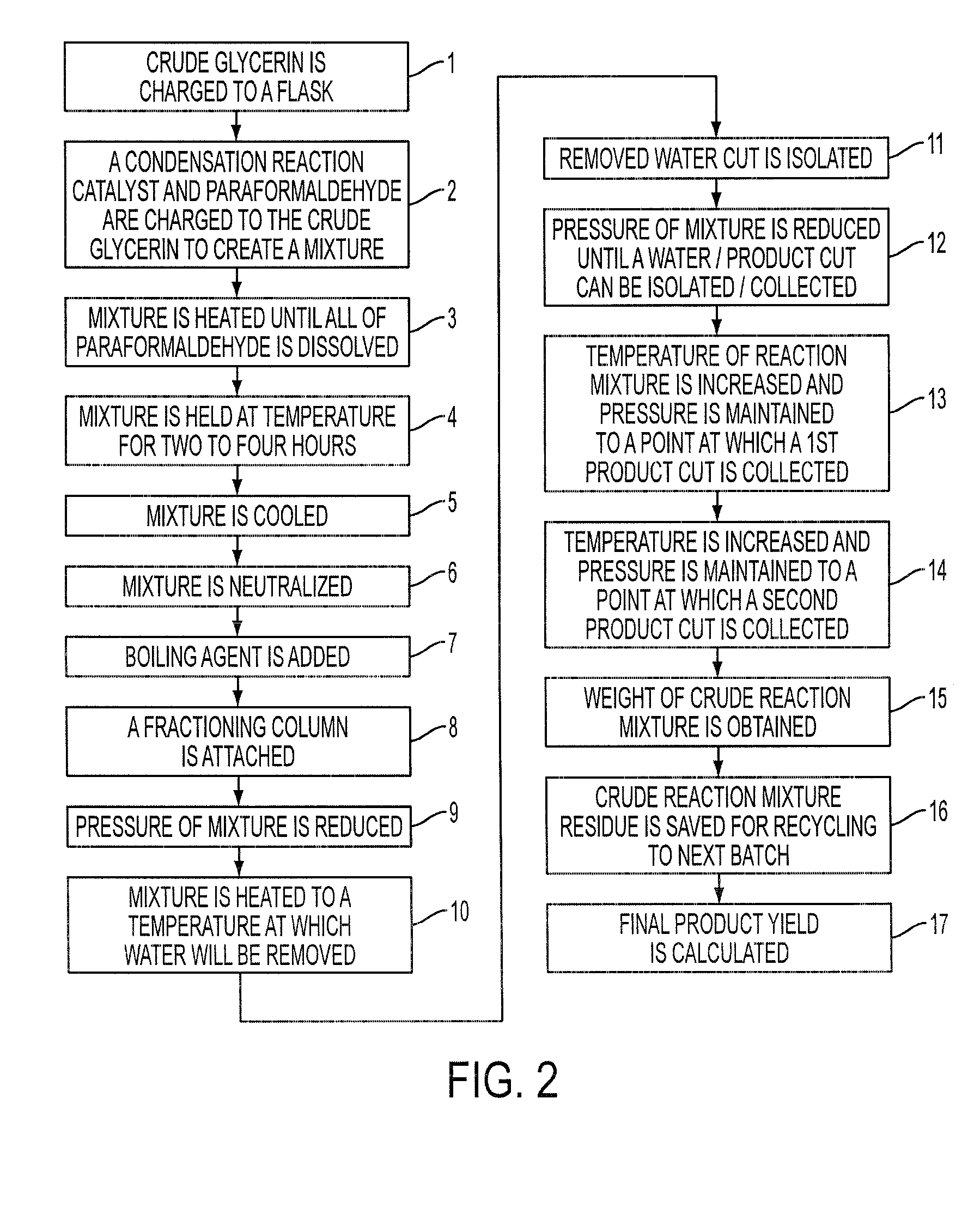
Process for the Preparation of Glycerol Formal
A process for the preparation of glycerol formal, from a paraformaldehyde and crude glycerin in a condensation reaction without the use of a secondary distilling agent for the removal of the water.
Owner:FUTUREFUEL CHEM
Preparation of fatty acid esters of glycerol formal and its use as biofuel
ActiveUS8142525B2Increase contentIncrease cetane numberOrganic chemistryFatty acid esterificationTransesterificationTriglyceride
This invention describes the preparation of fatty acid esters of glycerol formal either by a triglyceride transesterification process or, alternatively, by an esterification process of fatty acids previously obtained from the hydrolysis of triglycerides (fat splitting), with glycerol formal in the presence of an acid or basic catalyst. Also the invention describes the use of these fatty acid esters of glycerol formal prepared by said process as biofuel. In an embodiment, such biofuel is used in the preparation of other biofuels by its mixture with a product selected from a group formed by: glycerol formal, biodiesel, petrol-derived diesel, and mixtures thereof. The biofuels thus obtained are characterized to allow the complete incorporation of the glycerol obtained in the current biodiesel production process in a biodiesel fuel.
Owner:INST UNIV DE CIENCIA I TECH SA
Ivermectin slow release injection
InactiveCN106974887AQuality improvementImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismSurface-active agentsMethyl oleate
The invention discloses an ivermectin slow release injection, which is prepared from the following raw materials in parts by weight: 0.2 to 0.6 part of ivermectin, 1.5 to 4.5 parts of glycerol formal, 10 to 16 parts of propanediol, 2 to 4 parts of ethyl cellulose, 4 to 8 parts of ethyl oleate, 0.2 to 0.4 part of non-ionic surface active agents, wherein the non-ionic surface active agents are tween-80, tween-60 or tween-40. The propanediol and the glycerol formal are used as a solvent and a latent solvent; the ethyl oleate is used as a slow release agent; the product quality and the bioavailability can be well improved; the anthelminthic effect is obvious; the pharmacological activities of the ivermectin can be sufficiently achieved, so that the medicine effect is improved; a better solving scheme is provided for clinically expelling and treating parasites in bodies of livestock.
Owner:哈尔滨生物制品二厂有限责任公司
Long-acting compound doxycycline injection
InactiveCN102451184ARelief of superficial symptomsEfficient killingAntibacterial agentsTetracycline active ingredientsAntioxidantPolyethylene glycol
The invention discloses a long-acting copound doxycycline injection and a preparation process thereof. The injection comprises the following components in percentage by weight in volume: 5 to 30 percent of doxycycline, 0.2 to 1 percent of alpha-asarone, 0.5 to 10 percent of magnesium compound complexing agent, 0.1 to 1 percent of calcium copound complexing agent, 1 to 10 percent of inclusion material, 0.01 to 0.1 percent of antioxidant, and the balance of injection solvent which comprises glycerolformal, polyethylene glycol and injection water. In the composition disclosed by the invention, the alpha-asarone has effects of relieving cough and asthma and resisting infection, and can be used for effectively improving the situation of livestock bodies and reducing the death rate when being compounded with doxycycline.
Owner:TIANJIN RINGPU BIO TECH
Non-oily doramectin injection as well as preparation method and application thereof
ActiveCN107375205AImprove solubilityIncrease irritationOrganic active ingredientsPharmaceutical delivery mechanismDoramectin Injectable SolutionPropylene glycol
The invention relates to non-oily doramectin injection as well as a preparation method and an application thereof. The non-oily doramectin injection is prepared from the following components: doramectin, glycerolformal and propylene glycol, wherein doramectin accounts for 0.1%-10% of the non-oily doramectin injection by weight, a mixed solvent is prepared from glycerolformal and propylene glycol, and the mass ratio of glycerolformal to propylene glycol in the mixed solvent is (4-8):(2-6). The non-oily doramectin injection is good in stability and low in irritation.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
Compound ivermectin injection and preparation method thereof
InactiveCN104306388AGood insect resistanceBroad spectrum insect resistanceOrganic active ingredientsPharmaceutical delivery mechanismSulfite saltPyrrolidinones
The present invention discloses a composing prescription of a compound ivermectin injection and a preparation method of the compound ivermectin injection, and belongs to the veterinary medicine technology. The compound ivermectin injection comprises main drugs, a solvent and an antioxidant so as to prepare the stable high-concentration compound ivermectin injection. The injection has characteristics of advanced technology, good stability, broad spectrum, high efficiency, low irritation on the injected site, easy use, and clinical efficiency superior to the single-formula injection. According to the present invention, the raw materials comprise 0.5-5.0% of ivermectin, 2-20.0% of mebendazole, 0.5-5.0% of levamisole hydrochloride, 0.1-1% of the antioxidant, and the balance of the solvent, wherein the antioxidant is one or a mixture selected from thiourea, L-cysteamine hydrochloride, vitamin C, ethylenediamine, sodium hydrogen sulfite, anhydrous sodium sulfite, sodium formaldehydesulfoxylate dihydrate, sodium metabisulfite and the like, and the solvent is one or a mixture selected from propylene glycol, ethanol, glycerol, glycerol formal, dimethylformamide, dimethylacetamide, 2-pyrrolidone and the like.
Owner:JIANGXI NUCLEAR IND TIANDIHE PHARMA
Compound closantel sodium-ivermectin injection for animal and preparation method thereof
InactiveCN1875923AEasy to makeLow priceOrganic non-active ingredientsPharmaceutical delivery mechanismCLOSANTEL SODIUMAvermectin
The invention relates to a Closantel sodium and avermectin compound injection for animals and process for preparation, wherein the injection is prepared from Closantel sodium, avermectin, glycerol formal, benzyl carbinol and methyl glycol as raw material through mixing homogeneously under predetermined conditions.
Owner:TIANJIN SHENGJI GRP CO LTD
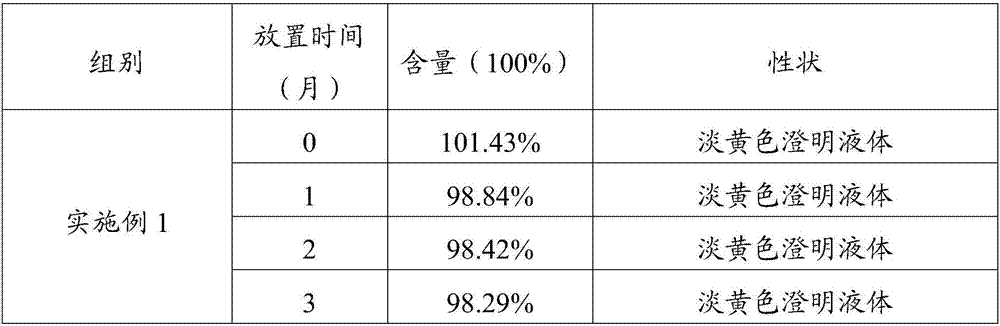
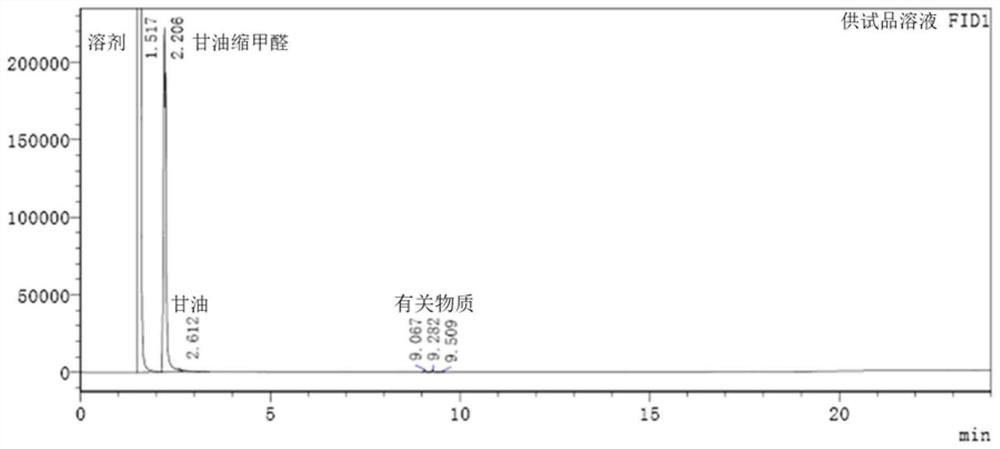
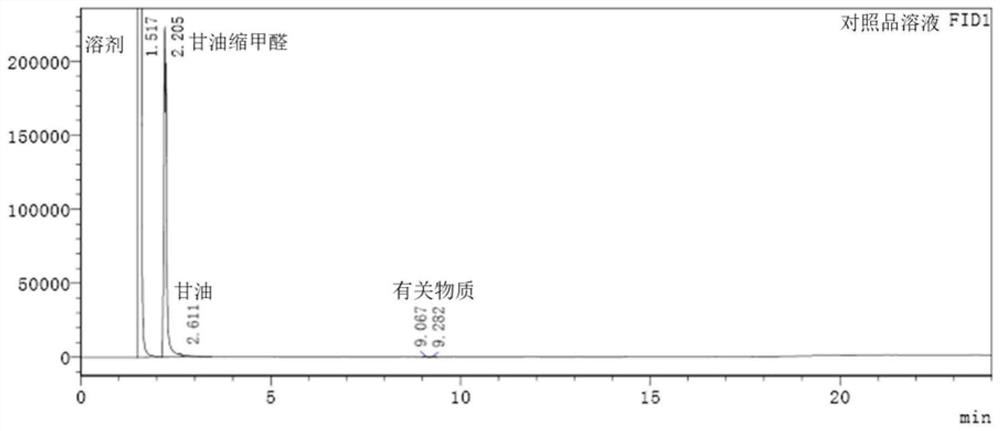
Method for determining content of glycerol formal by gas chromatography external standard method
InactiveCN113433246AAccurate measurementSensitive assayComponent separationGas liquid chromatographicQualitative analysis
The invention relates to a method for determining the content of glycerol formal by a gas chromatography external standard method, and belongs to the technical field of analysis and detection. The invention discloses a method for determining the content of glycerol formal by a gas chromatography external standard method, which adopts gas chromatography to qualitatively analyze the glycerol formal and adopts the external standard method to quantitatively analyze the glycerol formal, and can accurately, sensitively, simply, conveniently and quickly test the content of the glycerol formal. The vacancy of the content determination item in the existing glycerol formal quality standard is filled, and the glycerol formal can be conveniently used for preparing safer and more effective related medicinal preparations.
Owner:泰州骏道立同生物科技有限公司
Process for manufacturing biofuels
The present invention relates to a process for obtaining simultaneously several compositions comprising fatty acid alkyl esters (biodiesel), glycerol formal and fatty acid glycerol formal esters.
Owner:SALEBTEC SCI SL
Veterinary pyridaben preparation for killing parasites and preparation method thereof
InactiveCN108057020AImprove solubilityLow toxicityPharmaceutical non-active ingredientsAntiparasitic agentsSolubilityVeterinary Drugs
The invention discloses a veterinary pyridaben preparation for killing parasites and a preparation method thereof, and belongs to the field of veterinary drugs. The preparation comprises following components in percentage by weight: 1 to 30% of pyridaben, 5 to 60% of composite solvent, 5 to 30% of emulsifier, 1 to 25% of osmosis synergist, and the balance being diluent, wherein the composite solvent is composed of glycerol formal, propylene glycol, dimethyl acetamide, and purified water. The preparation is made of medical grade and food grade substances with low toxicity; through compounding,pyridaben can be well dissolved, and the shortages of a conventional pyridaben preparation such as large toxicity, damage to health of animals, and the like, are solved. The preparation can be directly painted or smeared on the furs and hair of animals; the active components are diffused through the furs and hair, the in-vitro parasites are killed, and moreover, the preparation does not have any side or toxic effect, does not generate any residue, and is safe, economic and environmentally friendly.
Owner:FOSHAN STANDARD BIO TECH
Ivermectin injection of anti-parasite drug for animals and preparation method of ivermectin injection
InactiveCN109966438APromote absorptionImprove insecticidal effectOrganic active ingredientsPharmaceutical delivery mechanismSide effectAdemetionine
The invention discloses ivermectin injection of an anti-parasite drug for animals and a preparation method of the ivermectin injection, and belongs to the field of preparations for animals. The ivermectin injection comprises, by weight, 1-3 parts of ivermectin B1, 2-5 parts of vitamin E, 1-4 parts of vitamin D3, 50-100 parts of absolute ethyl alcohol, 1-5 parts of glycerol formal, 0.1-2 parts of ginkgo leaf extracts, 0.5-2 parts of hairyvein agrimonia rhizome extracts, 0.1-3 parts of betelnutpalm seed extracts, 0.1-2 parts of stone-like omphalia extracts and 0.2-1 part of Japanese stemona rootextracts. The vitamins and the plant extracts are added, the ivermectin injection is conveniently absorbed by the animals, an insect-resistant spectrum is widened, drug resistance is reduced, side effects are decreased, growth of the animals is facilitated, and the preparation method is simple and suitable for popularization and use.
Owner:SHIJIAZHUANG JIUDING ANIMAL PHARMA CO LTD
Animal compound sodium closantel injection
InactiveCN101422476AIncrease weightInsect resistance range increasedPharmaceutical non-active ingredientsAntiparasitic agentsInfection rateMedical product
The invention discloses a veterinary compound sodium closantel injection which reduces poisoning probability generated when killing the parasites of cows and sheep. The invention belongs to a medical product which contains organic effective components. The preparation method thereof is as follows: 30 to 100kg of sodium closantel is added into 50 to 200L of propanediol, heated to the temperature of 50 to 60 DEG C and stirred for 10 to 25min until the mixture is clear; 1 to 20kg of ivermectin is added into 200L of glycerol formal and stirred for 15 to 20min until the liquid is clear; after the two obtained liquids are combined, the glycerol formal is added to 1000L, stirred uniformly and maintained for 15 to 20min; and after the medicine liquid is clear, the veterinary compound sodium closantel injection is obtained. The invention adopts a compound preparation, is highly effective to the parasites of cows and sheep, can be broadly applied to the parasites of cows and sheep, reduce the infection rate of the parasites and increase the weights of cows and sheep.
Owner:TIANJIN SHENGJI GRP CO LTD
Diclazuril liposome and preparation method thereof
InactiveCN104398479AGood slow releaseSolve the usage problemPowder deliveryAntiparasitic agentsSolubilityCholesterol
The invention discloses a diclazuril liposome which is prepared by the following raw materials in parts by weight: 3-4 parts of diclazuril, 10-20 parts of alpha-pyrrolidone, 30-50 parts of soybean lecithin, 1-10 parts of glycerolformal and 10-20 parts of cholesterol. Compared with the prior art, the diclazuril liposome disclosed by the invention has good stability, solubility and sustained-release property and solves the problem of use methods of the diclazuril; the drug property of the diclazuril can be effectively utilized, and the clinical usage amount can be reduced, so that the safe conversion of the drug in a human body is ensured.
Owner:ZHENGZHOU HOUYI PHARMA
Preparation method of decoquinate solution
ActiveCN104288098APromote safe productionSimple preparation processPharmaceutical delivery mechanismAntiparasitic agentsAlcoholPolyethylene glycol
The invention discloses a preparation method of a decoquinate solution. The decoquinate solution comprises a solvent, a cosolvent and a pH modifier, wherein the solvent is dimethylformamide, twain, absolute ethyl alcohol, polyethylene glycol and glycerolformal. The preparation is prepared by the following steps at normal temperature and pressure: (1) stirring dimethylformamide and the cosolvent, adding decoquinate to stir and dissolve, adjusting the pH value of the mixed solution to 3.0-4.5 by virtue of the pH modifier, and stirring evenly for later use; (2) adding absolute ethyl alcohol to polyethylene glycol, heating, stirring, and adding dioxolane-4-methanol after 30 minutes, wherein the heating temperature is 70-80 DEG C; and (3) mixing the solution prepared from the step (1) with the solution prepared from the step (2) evenly, naturally cooling to room temperature, adding twain, stirring evenly, precisely filtering and bulking. The production process of the preparation method is safe; the decoquinate solution can be prepared at normal temperature and pressure; the preparation process is simple; the effect for preventing ball insect diseases of poultry is effect; and compared with other anticoccidial drugs, the cost is relatively low.
Owner:LINZHOU SINAGRI YINGTAI BIOLOGICAL PEPTIDES CO LTD
Long-acting veterinary ivermectin injection and preparation method thereof
InactiveCN104490769AQuality improvementImprove insect repellent effectOrganic active ingredientsPharmaceutical delivery mechanismDiseaseBENZYL ALCOHOL/WATER
The invention relates to long-acting veterinary ivermectin injection. Every 1000ml long-acting veterinary ivermectin injection contains 10-15g of ivermectin, 500-600ml propylene glycol, 200-300ml of absolute ethyl alcohol, 80-120ml of glycerolformal and 80-120ml of benzyl alcohol. A preparation method of the long-acting veterinary ivermectin injection comprises the following steps: 1) adding ivermectin and propylene glycol into absolute ethyl alcohol, stirring, and dissolving; and 2) adding glycerolformal and benzyl alcohol into the solution obtained in the step 1), uniformly stirring, maintaining constant volume, carrying out sterile filtration, and encapsulating, thus the long-acting veterinary ivermectin injection is obtained. The long-acting veterinary ivermectin injection is stable in quality, effect can last for 72 hours, anthelminthic effect is remarkable, insect bodies inside and outside livestock and poultry can be dispelled, and diseases like swine, bovine and ovine horned toad can be treated by only injecting the long-acting veterinary ivermectin injection once.
Owner:CHONGQING ZONGYI PHARMA
Preparation method of slow-release ivermectin injection
InactiveCN103830167ADefinite curative effectLittle side effectsOrganic active ingredientsPharmaceutical delivery mechanismInsect repellentMethyl oleate
The invention relates to a slow-release ivermectin injection for expelling and treating parasites in livestock bodies and a preparation method thereof. The slow-release ivermectin injection mainly overcomes the disadvantages of complex production process, inconvenience in application, inaccurate dosage, poor insect repellent effects and the like of ivermectin preparation drugs in the prior art, the slow-release ivermectin injection mainly comprises ivermectin, ethyl cellulose, ethyl oleate, glycerolformal and benzyl alcohol, and the preparation method is as follows: weighing the prescription dose of the ivermectin and the ethyl cellulose to dissolving in the prescription dose of the benzyl alcohol and the ethyl oleate, using the glycerolformal to fix to a constant volume, and sterilizing with wet and hot steam at 100 DEG C for 30 minutes. The slow-release ivermectin injection has the advantages of simple production process, stable quality, simple drug application operation, accurate dosage, and significant insect repellent effects.
Owner:QINGDAO KDN BIOTECH
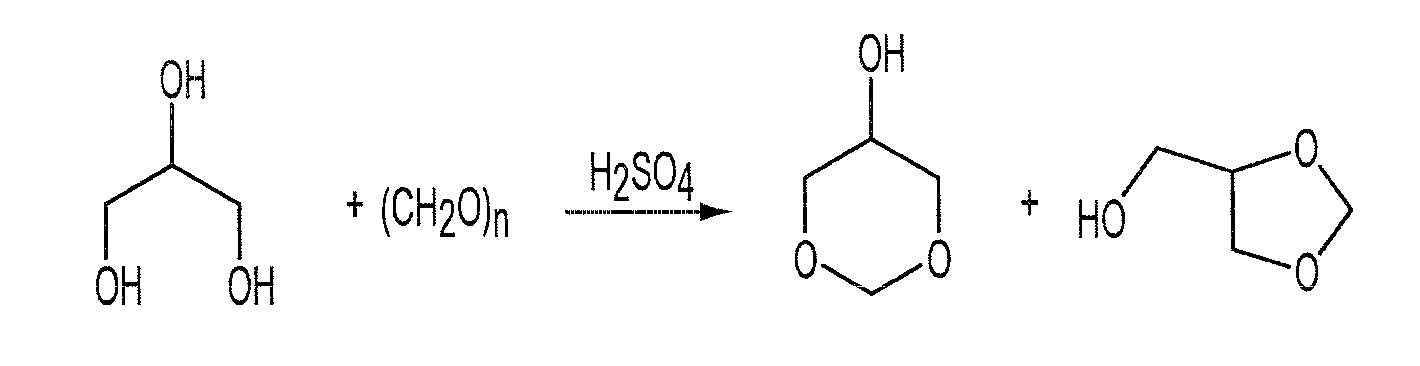
Method for synthesizing glycerol formal through reaction of glycerol and formaldehyde
The invention discloses a method for synthesizing glycerol formal through reaction of glycerol and formaldehyde. The method adopts functional ionic liquid as a catalyst and adopts formaldehyde aqueous solution and glycerol as reactants to synthesize a glycerol formal compound. The method has the advantages of mild reaction condition, high product yield, high hexatomic ring product proportion, repeatable use of the catalyst and the like.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Veterinary long-acting oxytetracycline injection liquid and preparation method thereof
InactiveCN110693826AReduce interfering impuritiesAvoid oxidation and blackeningAntibacterial agentsTetracycline active ingredientsPolythylene glycolPyrrolidinones
The invention discloses veterinary long-acting oxytetracycline injection liquid and a preparation method thereof. The veterinary long-acting oxytetracycline injection liquid contains oxytetracycline dihydrate, magnesium oxide, sodium formaldehyde sulfoxylate, glycerol formal, polyvinylpyrrolidone K12, polyethylene glycol-400, monoethanolamine and water for injection. According to the veterinary long-acting oxytetracycline injection liquid, the combined action of the oxytetracycline dihydrate with the low impurity content and the high purity, the sodium formaldehyde sulfoxylate, the magnesium oxide and the glycerol formal improves stability of the injection liquid, and the storage time of the injection liquid is prolonged; after the polyethylene glycol-400 is adopted to conduct intramuscular injection, the blood concentration peak can be reached in short time, so that intramuscular injection is easier; and by adopting the polyvinylpyrrolidone K12, drugs are slowly released, the drug action time is prolonged, the injection interval time is prolonged, the injection dosage is decreased, and animal stress response is reduced.
Owner:成都新亨药业有限公司
A kind of preparation method of decoquinate solution
ActiveCN104288098BPromote safe productionSimple preparation processPharmaceutical delivery mechanismAntiparasitic agentsPolyethylene glycolHeating temperature
The invention discloses a preparation method of a decoquinate solution. The decoquinate solution comprises a solvent, a cosolvent and a pH modifier, wherein the solvent is dimethylformamide, twain, absolute ethyl alcohol, polyethylene glycol and glycerolformal. The preparation is prepared by the following steps at normal temperature and pressure: (1) stirring dimethylformamide and the cosolvent, adding decoquinate to stir and dissolve, adjusting the pH value of the mixed solution to 3.0-4.5 by virtue of the pH modifier, and stirring evenly for later use; (2) adding absolute ethyl alcohol to polyethylene glycol, heating, stirring, and adding dioxolane-4-methanol after 30 minutes, wherein the heating temperature is 70-80 DEG C; and (3) mixing the solution prepared from the step (1) with the solution prepared from the step (2) evenly, naturally cooling to room temperature, adding twain, stirring evenly, precisely filtering and bulking. The production process of the preparation method is safe; the decoquinate solution can be prepared at normal temperature and pressure; the preparation process is simple; the effect for preventing ball insect diseases of poultry is effect; and compared with other anticoccidial drugs, the cost is relatively low.
Owner:LINZHOU SINAGRI YINGTAI BIOLOGICAL PEPTIDES CO LTD
Non-oily doramectin injection and its preparation method and application
ActiveCN107375205BOrganic active ingredientsPharmaceutical delivery mechanismDoramectin Injectable SolutionPropylene glycol
The invention relates to a non-oily doramectin injection, a preparation method and application thereof. The non-oily doramectin injection comprises the following components: doramectin, glycerol formal and propylene glycol; wherein, the weight percentage of the doramectin is 0.1%-10%, and the glycerol formal and the The propylene glycol forms a mixed solvent, and the mass ratio of the glycerin formal and the propylene glycol in the mixed solvent is (4-8):(2-6). The non-oily doramectin injection has good stability and low irritation.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
Formulation, preparation and use of a glycerol-based biofuel
ActiveUS20150353855A1Burning characteristicBiofuelsLiquid carbonaceous fuelsBiofuelFatty acid methyl ester
The present invention relates to a new biofuel formulation comprising: crude glycerol, glycerol formal, optionally at least one fatty acid glycerol formal ester and optionally at least one fatty acid methyl ester. The present invention also relates to a process for its preparation and its use for burning purposes.
Owner:INST UNIV DE CIENCIA I TECH SA
Fluoroquinolone compositions
InactiveUS20090012072A1Antibacterial agentsOrganic active ingredientsOrganic solventPropylene glycol
The invention relates to pharmaceutical compositions comprising (i) a fluoroquinolone, (ii) a salt formed between a carboxylate anion and a divalent metal cation, (iii) a liquid comprising an organic solvent selected from the group consisting of glycerol, propylene glycol, glycerol formal, and (iv) optionally water. The invention further relates to methods of treating or preventing a condition in an animal comprising administering to the animal in need thereof a pharmaceutical composition of the invention.
Owner:IDEXX LABORATORIES
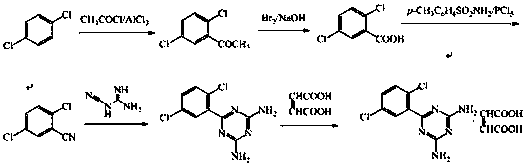
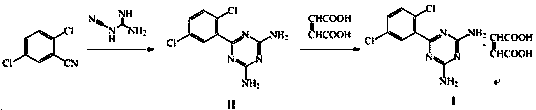
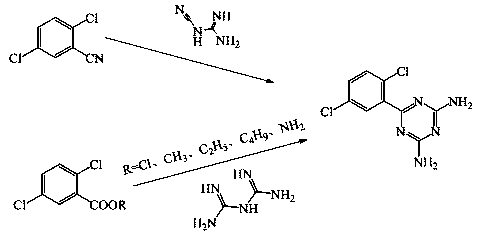
A kind of preparation method of Isoladine maleate
InactiveCN106187928BHigh yieldHigh purityCarboxylic acid salt preparationCarboxylic compound separation/purificationChemical synthesisAcetic acid
The invention provides a preparation method for irsogladine maleate, and belongs to the technical field of medicinal and chemical synthesis. The method comprises the following steps: carrying out a condensation reaction between 2,5-dichlorobenzonitrile and dicyandiamide in a non-protic strong polarity solvent with the existence of a basic catalyst to obtain an intermediate II, and enabling the intermediate II and maleic acid to salify in glyceroformol; re-crystallizing a salt in an acetic acid-acetone mixed solution to obtain the irsogladine maleate. The preparation method is easy to operate, high in yield and high in purity, can effectively shorten reaction time, and has a good industrial application value.
Owner:安徽省公众检验研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com