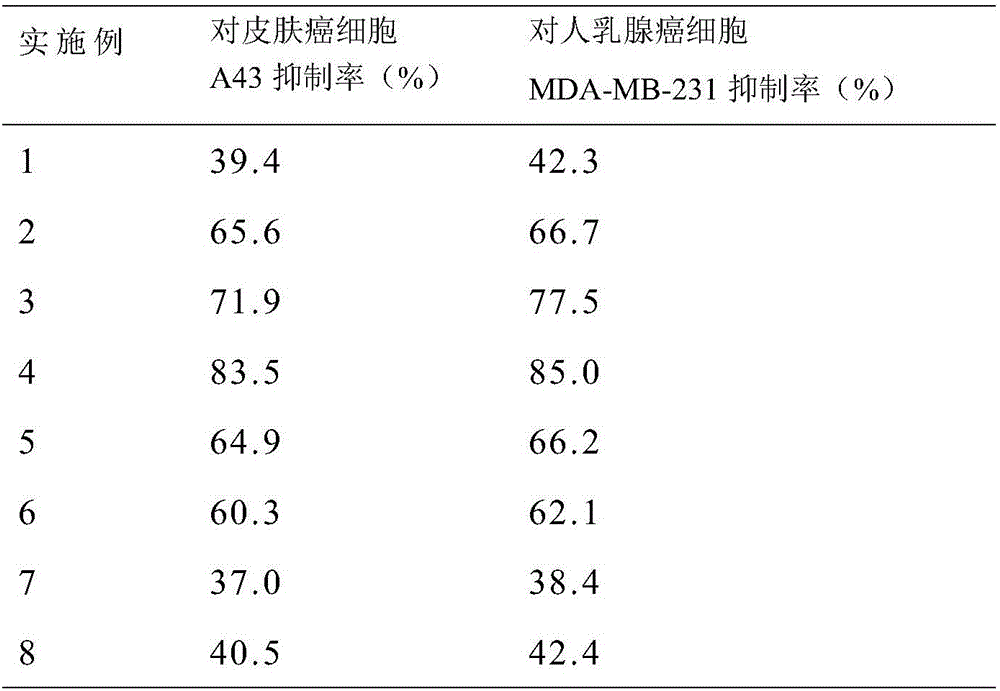
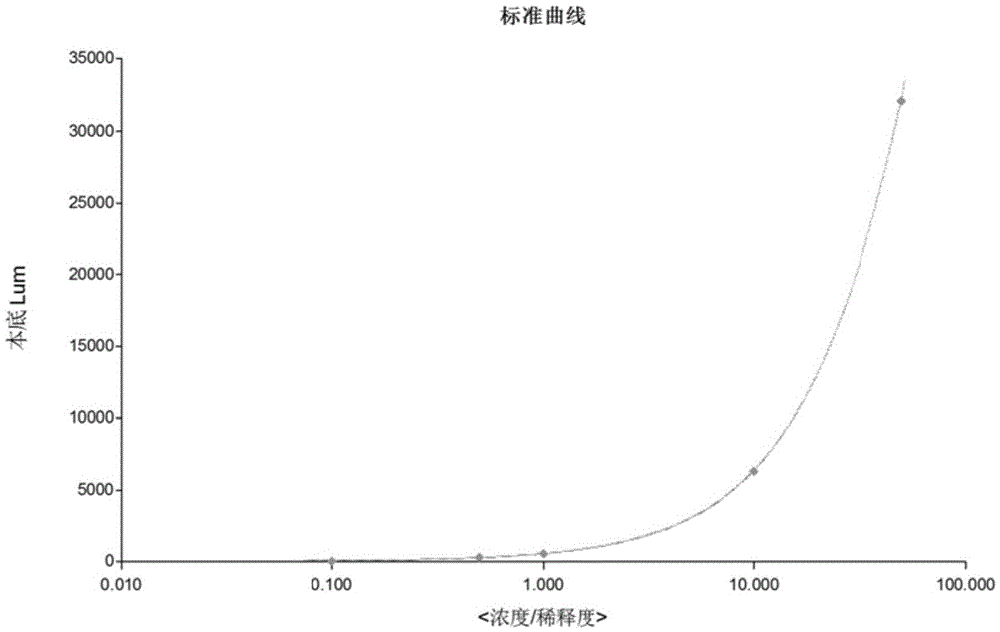
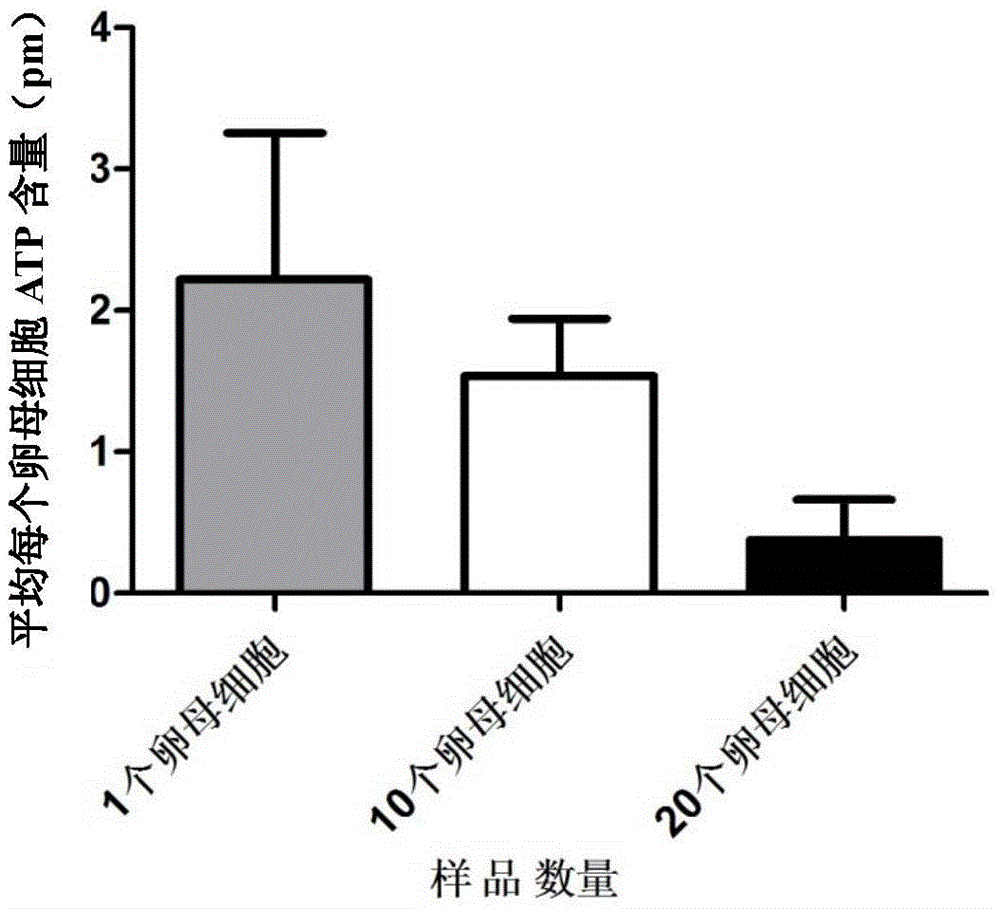
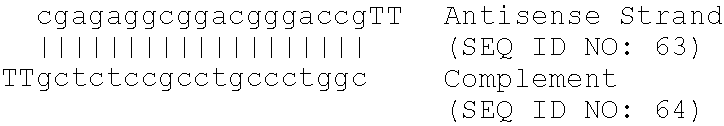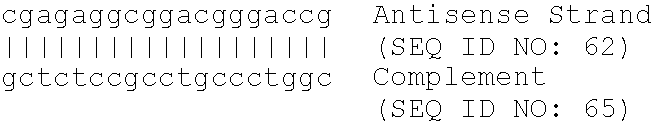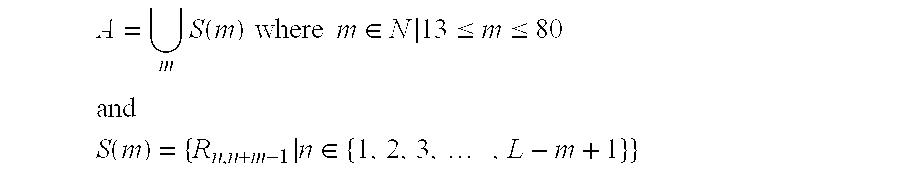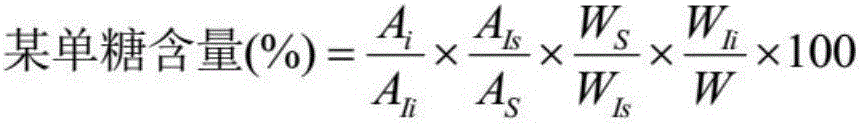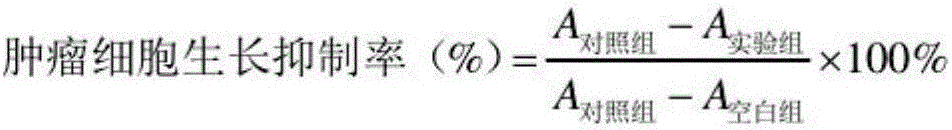Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Glucose 6-phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
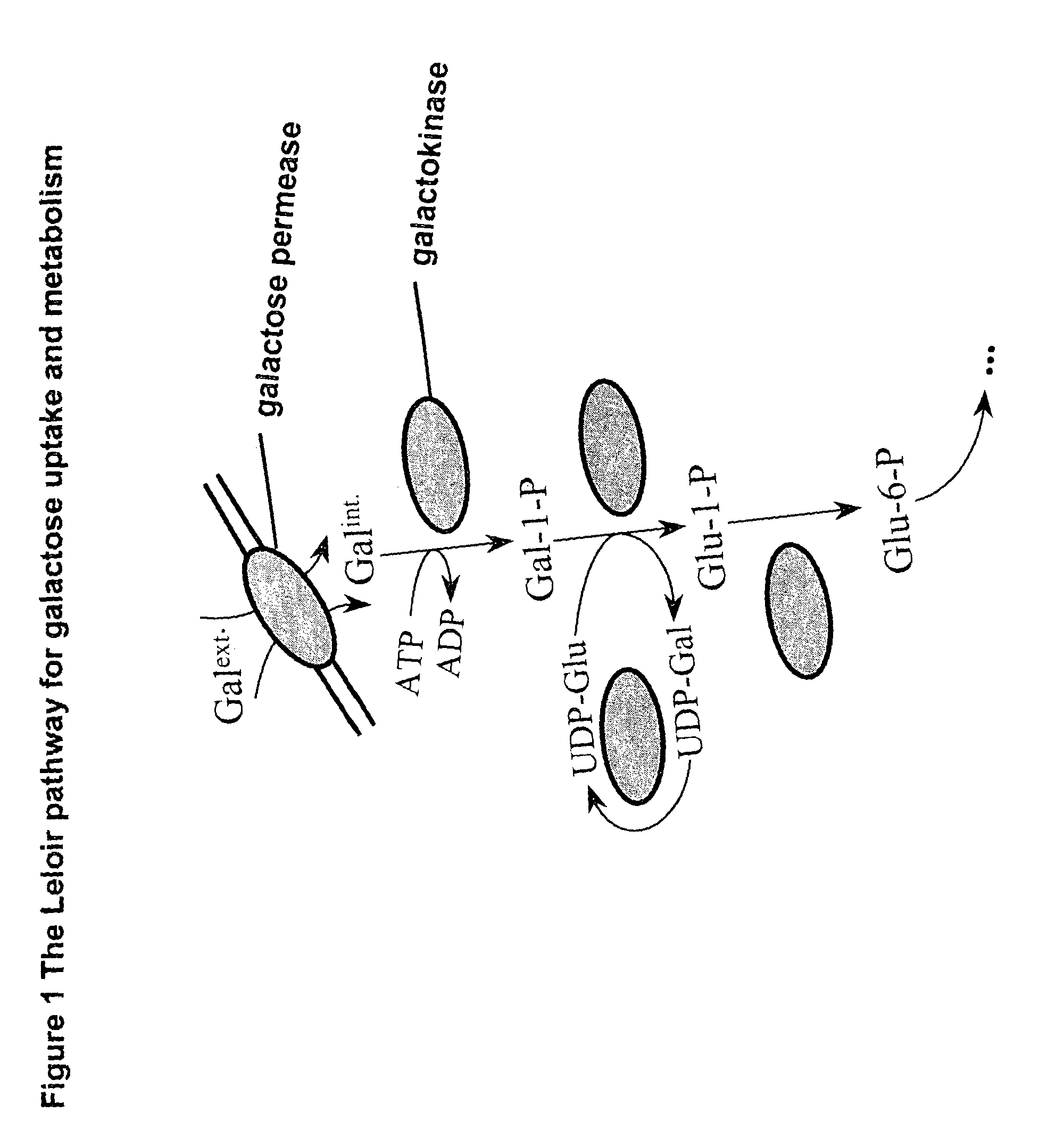
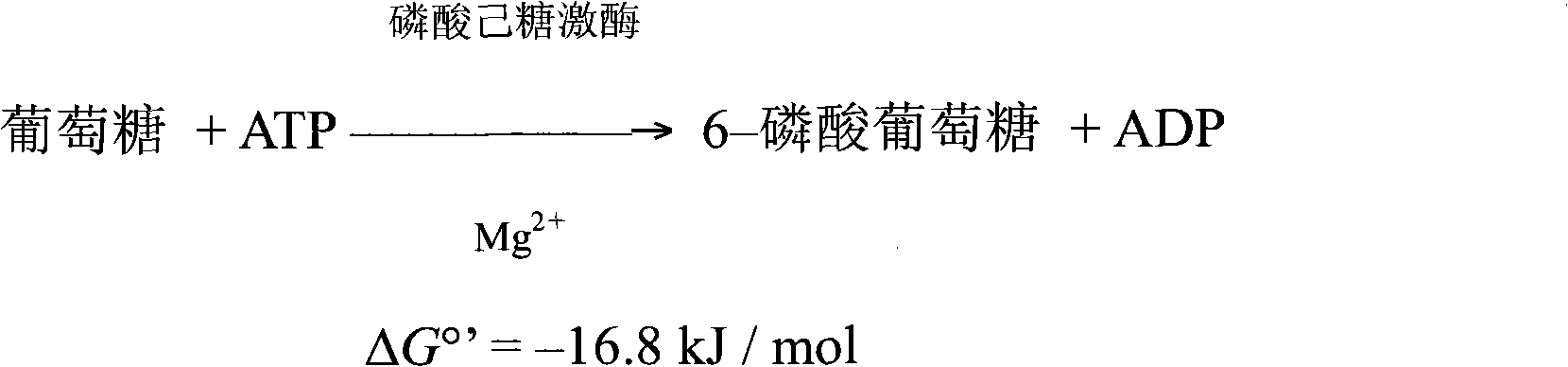
Glucose 6-phosphate (G6P, sometimes called the Robison ester) is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way.
Topically applied Glucosamine Sulfate and all its related, precursor, and derivative compounds significantly increases the skin's natural produciton of hyaluronic acid for the rejuvenation of healthier younger-looking skin; while PhosphatidylCholine is required to replace its deficiency caused by topical Dimethylaminoethanol (DMAE)
InactiveUS20070092469A1Reducing eczemaReducing psoriasisBiocideCosmetic preparationsWrinkle skinPhysiology
A topical skin rejuvenation preparation to relieve wrinkles, increase the skin's natural production of hyaluronic acid, reverse the lack of suppleness, hydrate from within, erase spider veins, reduce varicose veins, lighten aging dark blotches (“liver spots” / Lentigos, Senile Lentigines), decrease acne, and reduce under eye puffiness includes Glucosamine (2-amino-2-deoxy-alpha-D-glucose), a hexosamine (6 carbon amino sugar), including its derivative and precursor compounds: Glucosamine Sulfate, Glucosamine Hydrochloride, Glucose-6-Phosphate, Acetyl Glucosamine, Fructose-6-phosphate, Glucosamine-6-Phosphate, to increase production of Hyaluronic acid and collagen from Glucosamine Sulfate, its precursors and derivatives and to increase skin muscle tone by Dimethylaminoethanol (DMAE) while over coming deficiency it creates in each cell's production of PhosphatidylCholine, whose deficiency damages cell membranes, as well as mitochondrial and lysosome membranes.
Owner:JACOBS ERIC
Enzymatic fluorimetric assay of camp and adenylate cyclase
InactiveUS6762026B1Easy to operateReaction period can be extremely shortenedMicrobiological testing/measurementMaterial analysisRadioactive agentFluorescence
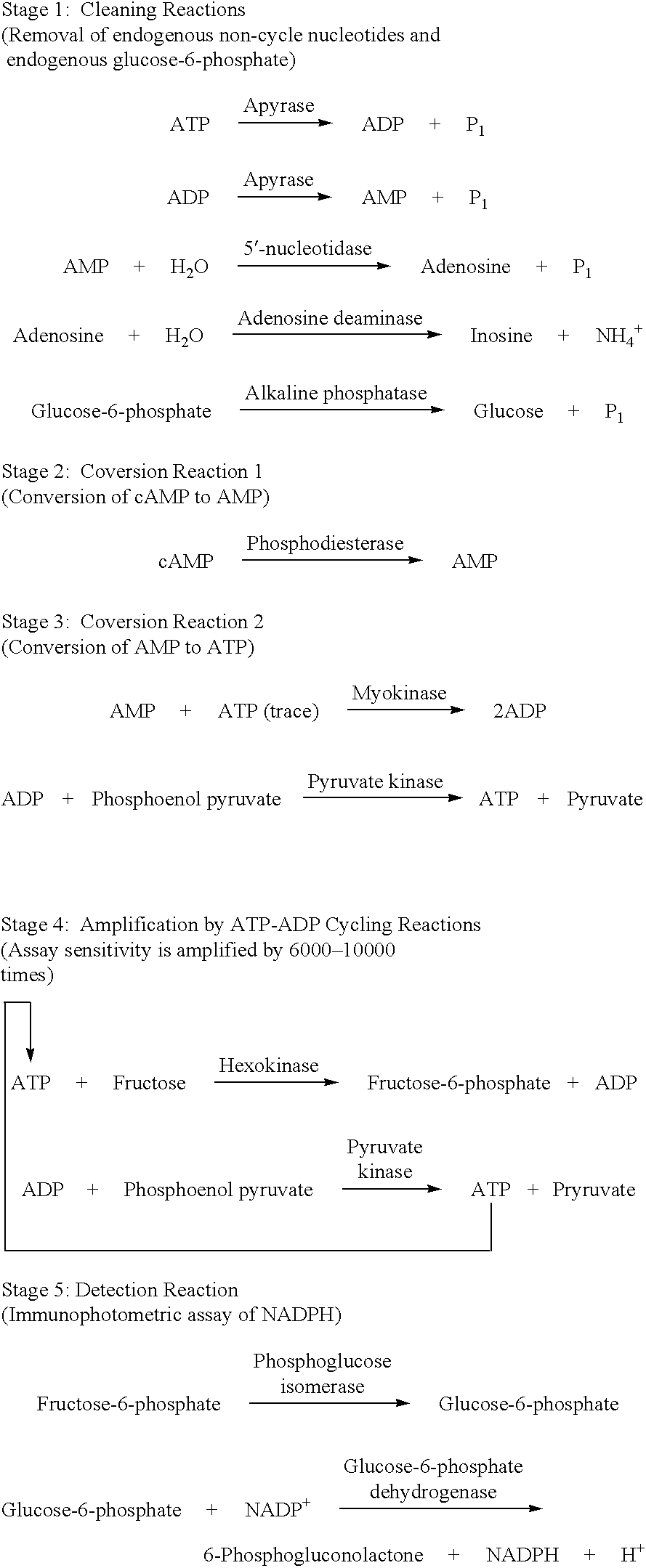
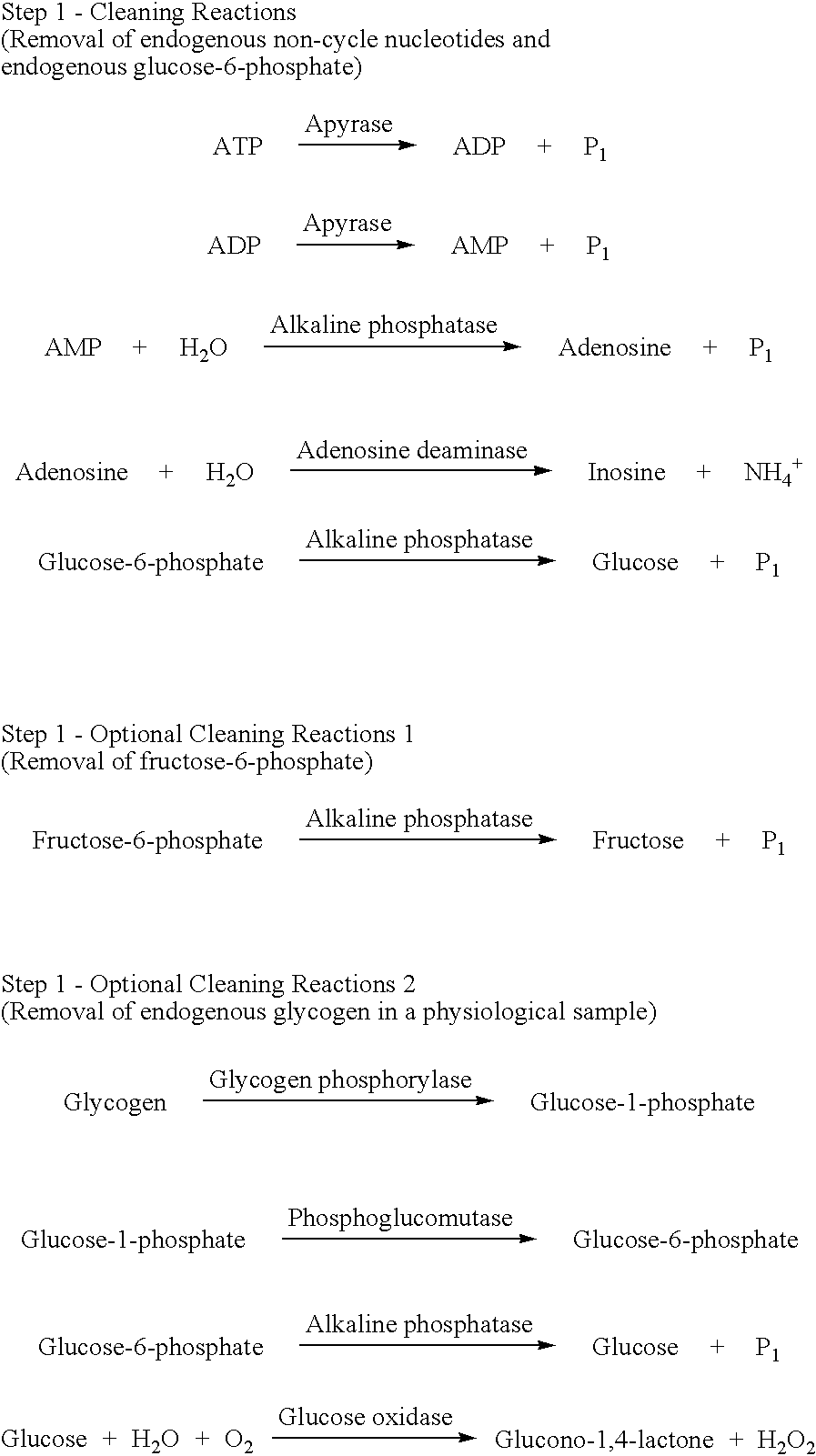
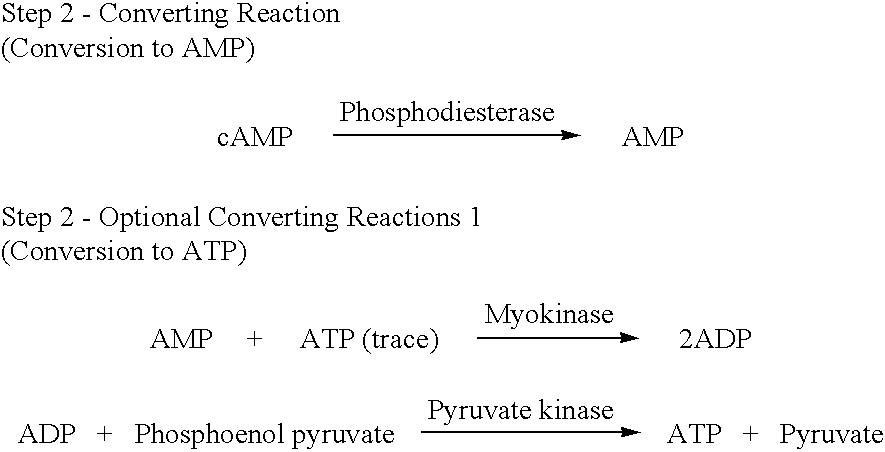
The present invention relates to a method for quickly determining cAMP content or an adenylate cyclase activity in a biological sample containing non-cyclic adenine nucleotides without the use of radioactive agents.Particularly, the present invention provides a method of determining cAMP content or an adenylate cyclase activity in a biological sample containing non-cyclic adenine nucleotides selected from the group consisting of cAMP produced by endogenous adenylate cyclase, and AMP, ATP, ADP and a mixture thereof, which comprises (1) combining a biological sample with effective amounts of apyrase, adenosine deaminase and alkaline phosphatase to enzymatically remove non-cyclic adenine nucleotides other than cAMP, and glucose-6-phosphate in the sample; (2) enzymatically converting cAMP into AMP; (3) determining an amount of AMP without the use of radioactive agents, and a kit to carry out the method.
Owner:FUSO PHARMA INDS
Recombinant yeast and substance production method using the same
ActiveUS20130295616A1Reduced activityIncrease productivityMicroorganismsTransferasesBiotechnologyAcetic acid
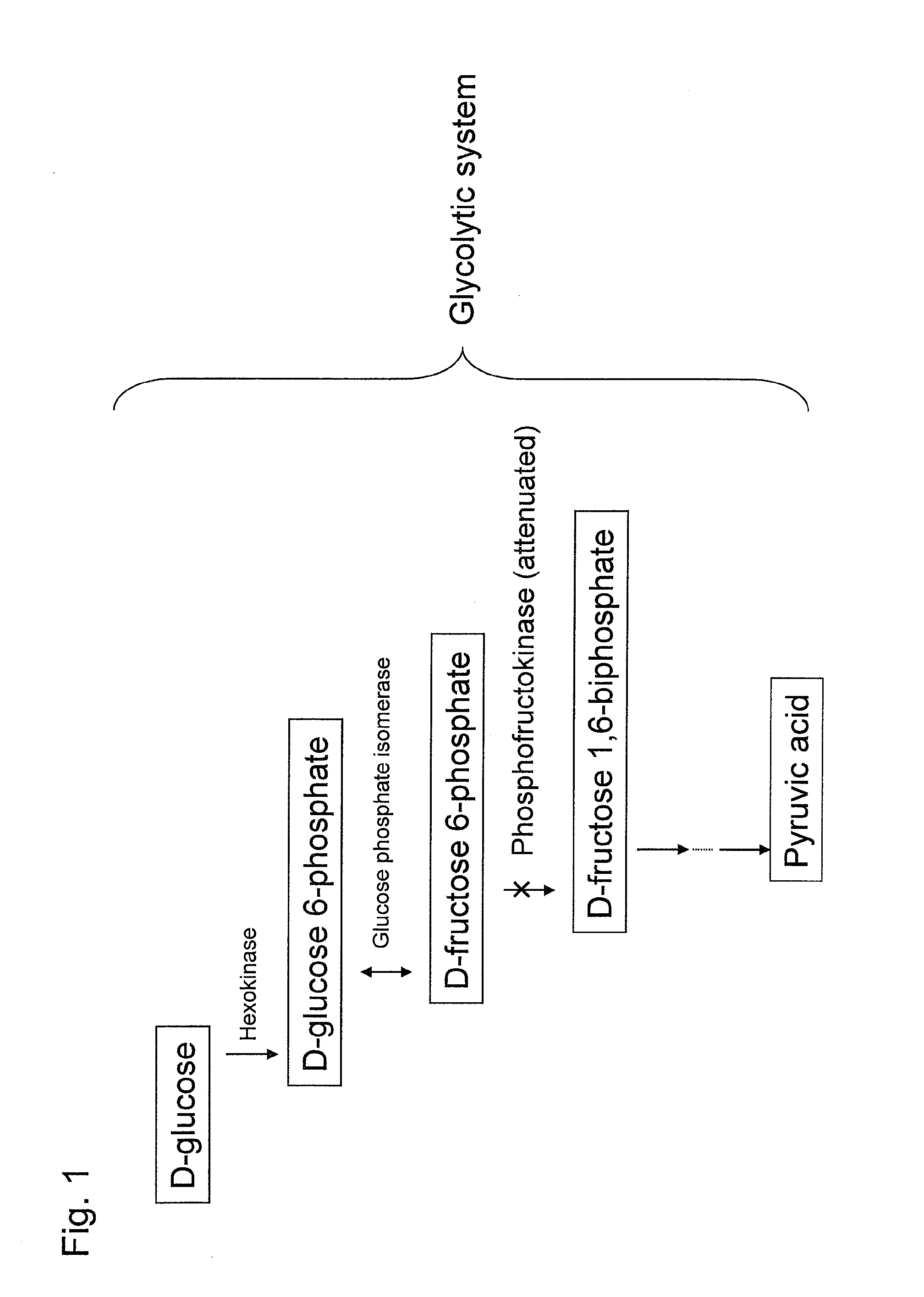
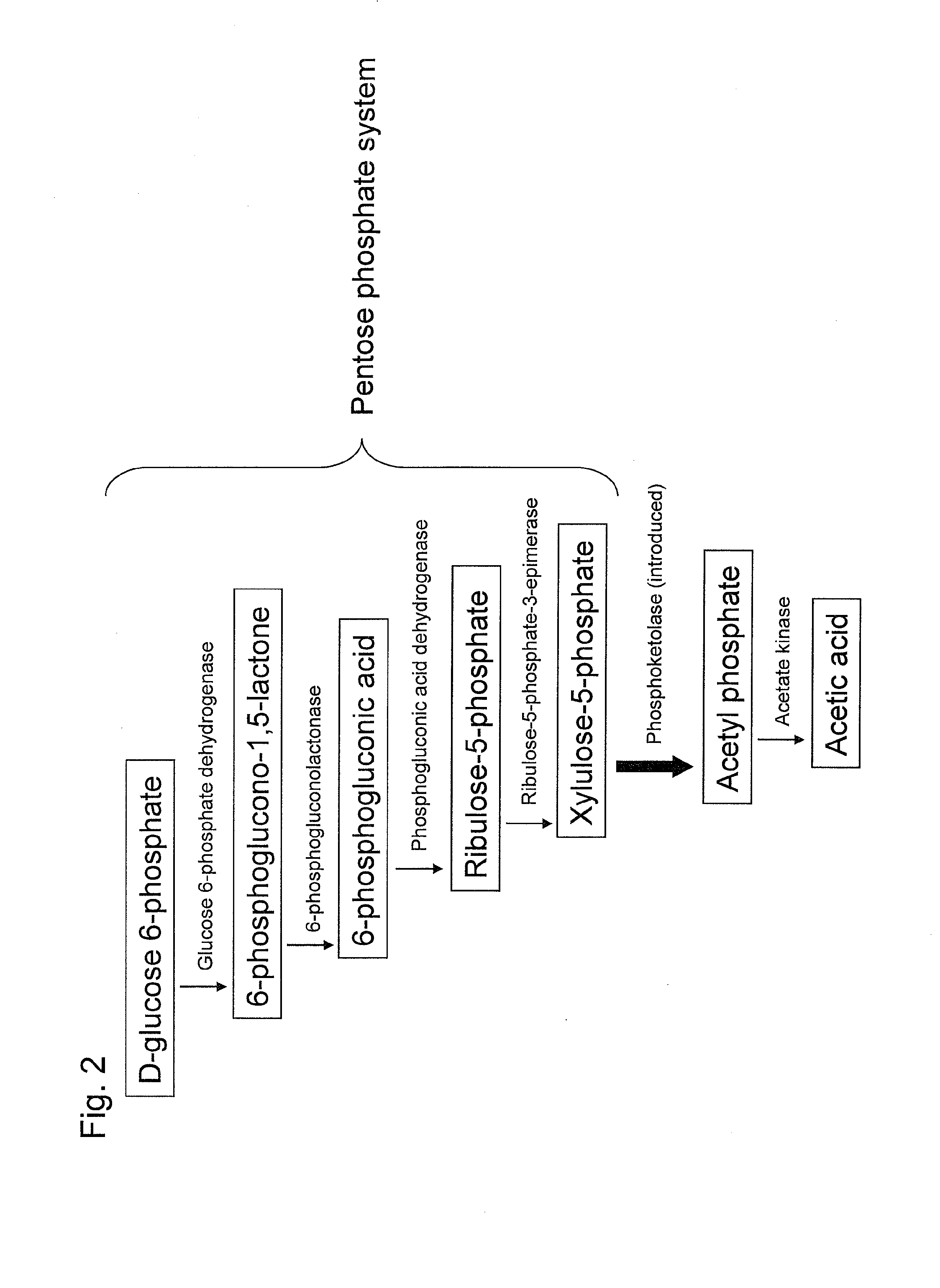
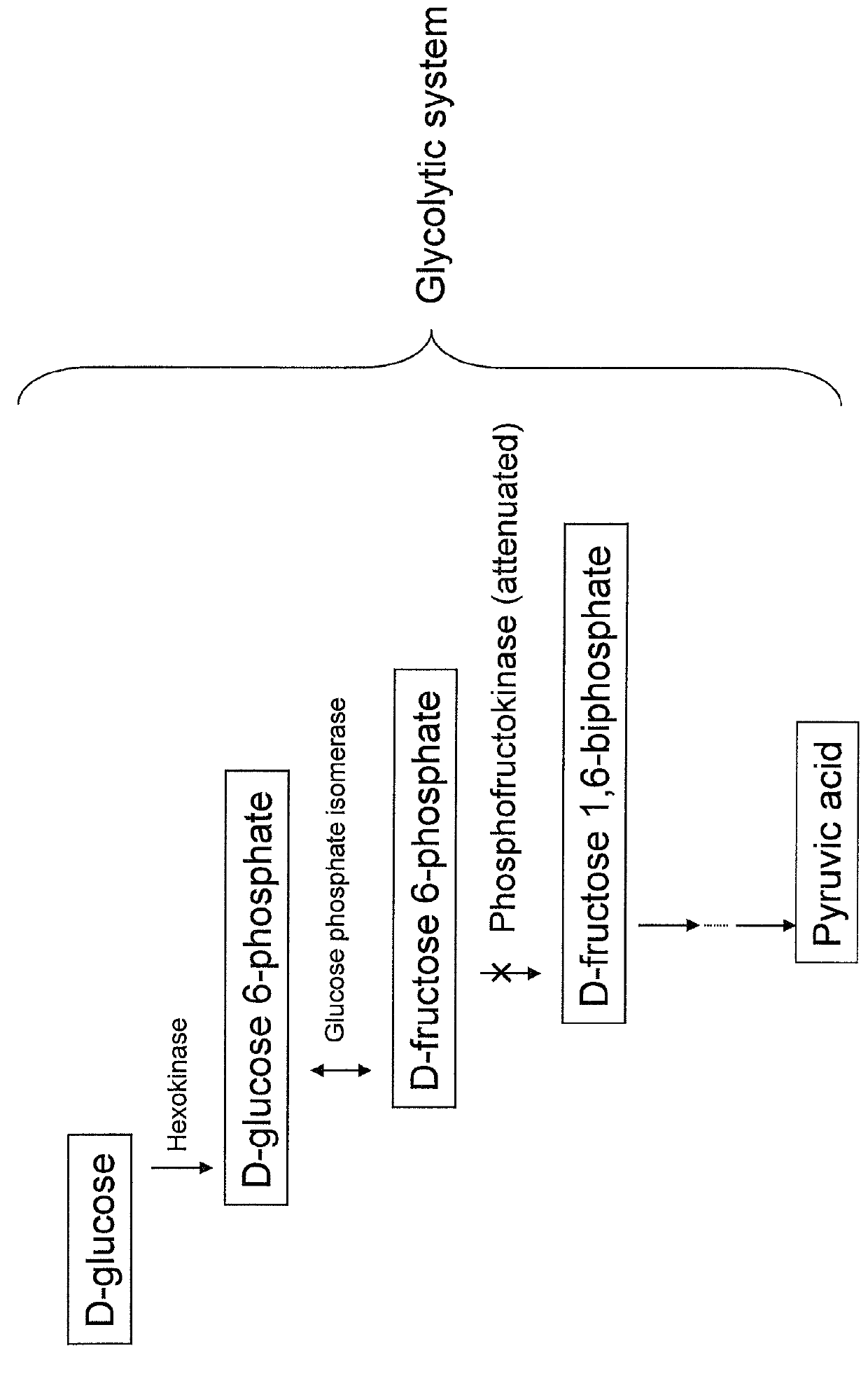
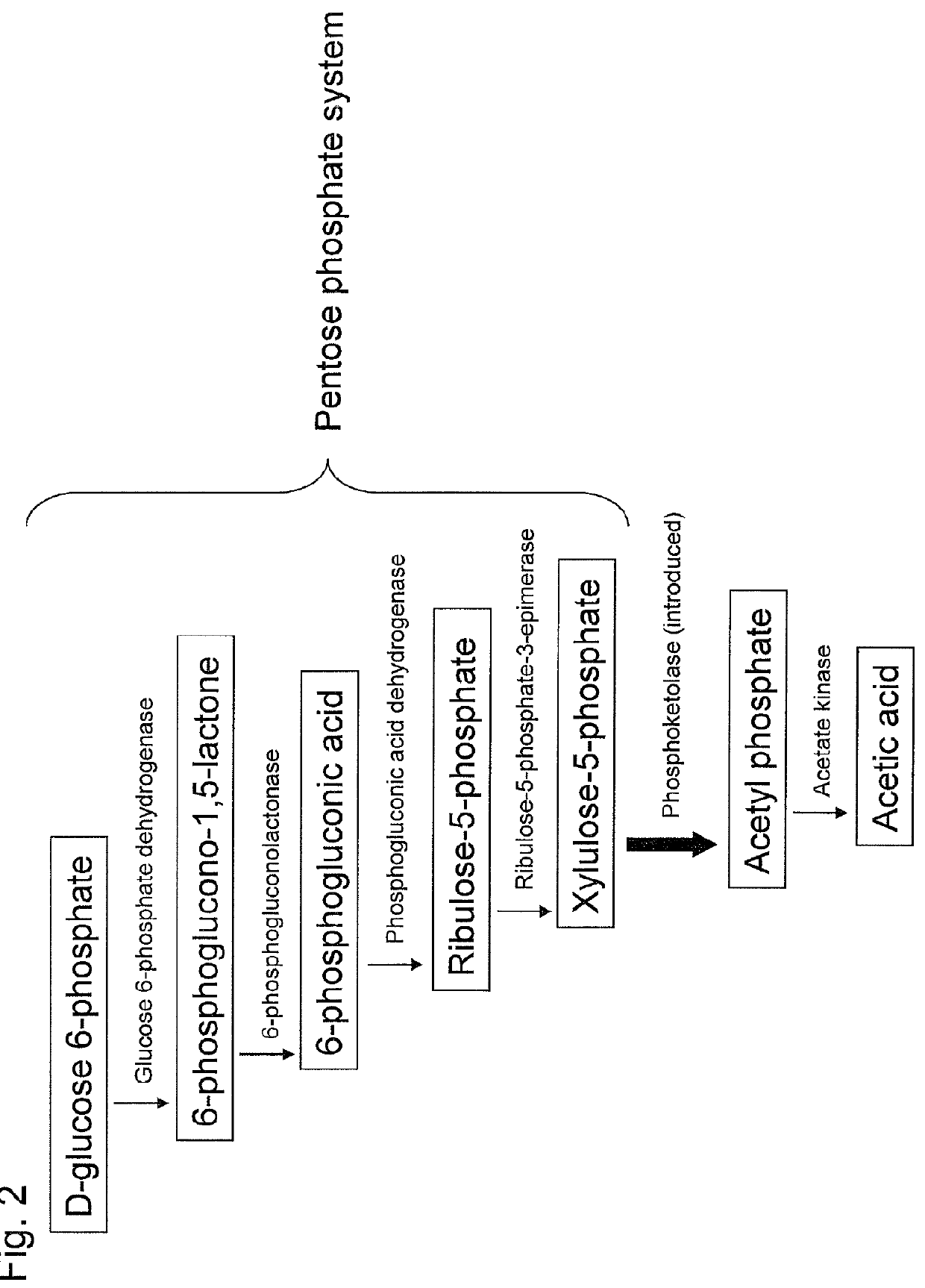
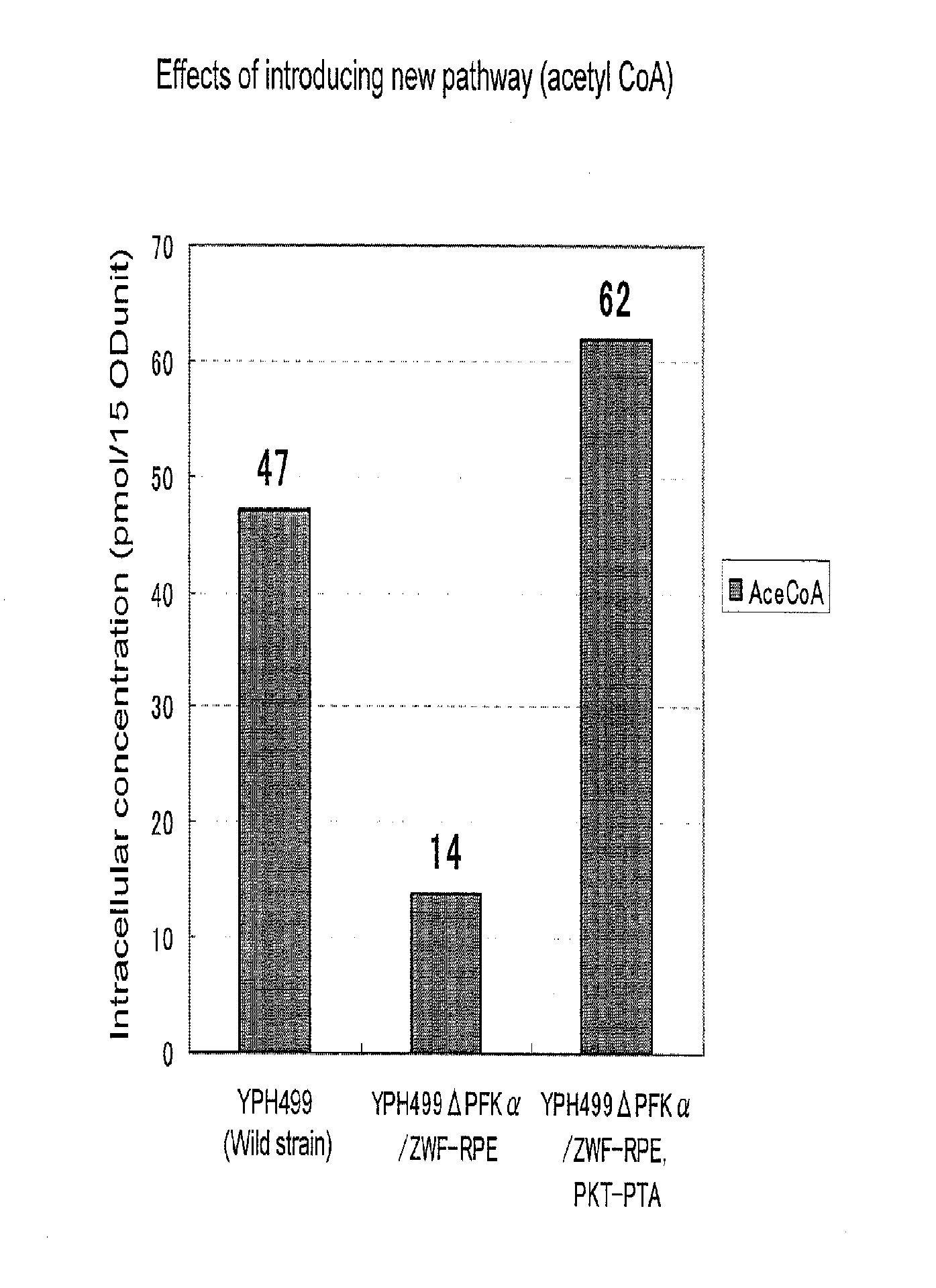
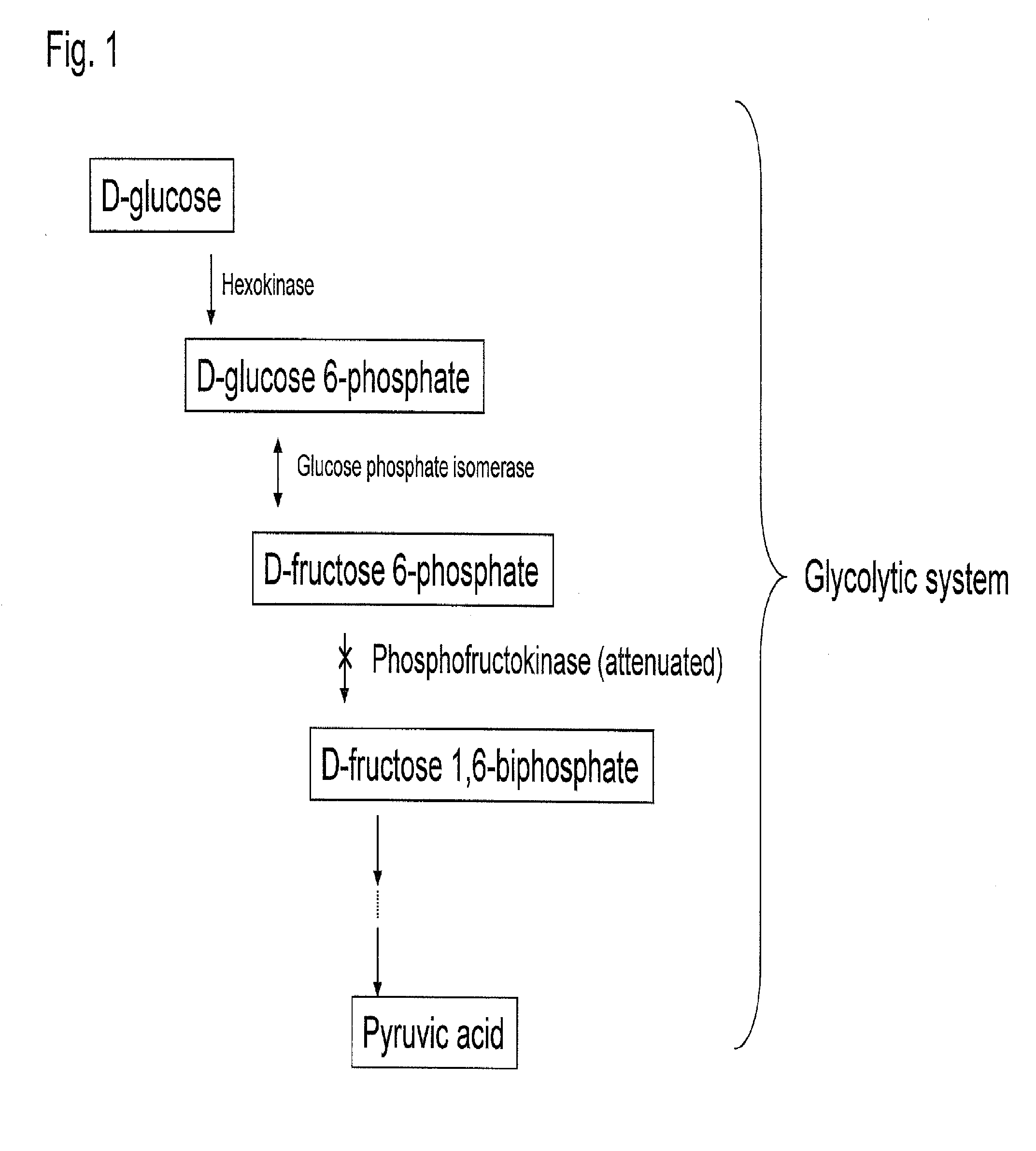
Substance productivity is improved by introducing a metabolic pathway for synthesis of acetyl-CoA or acetic acid from glucose-6-phosphate into yeast. Acetic acid productivity, acetyl-CoA productivity, and productivity of a substance made from acetyl-CoA-derived are improved by attenuating genes involved in the glycolytic system of yeast and introducing a phosphoketolase gene into the yeast.
Owner:TOYOTA JIDOSHA KK
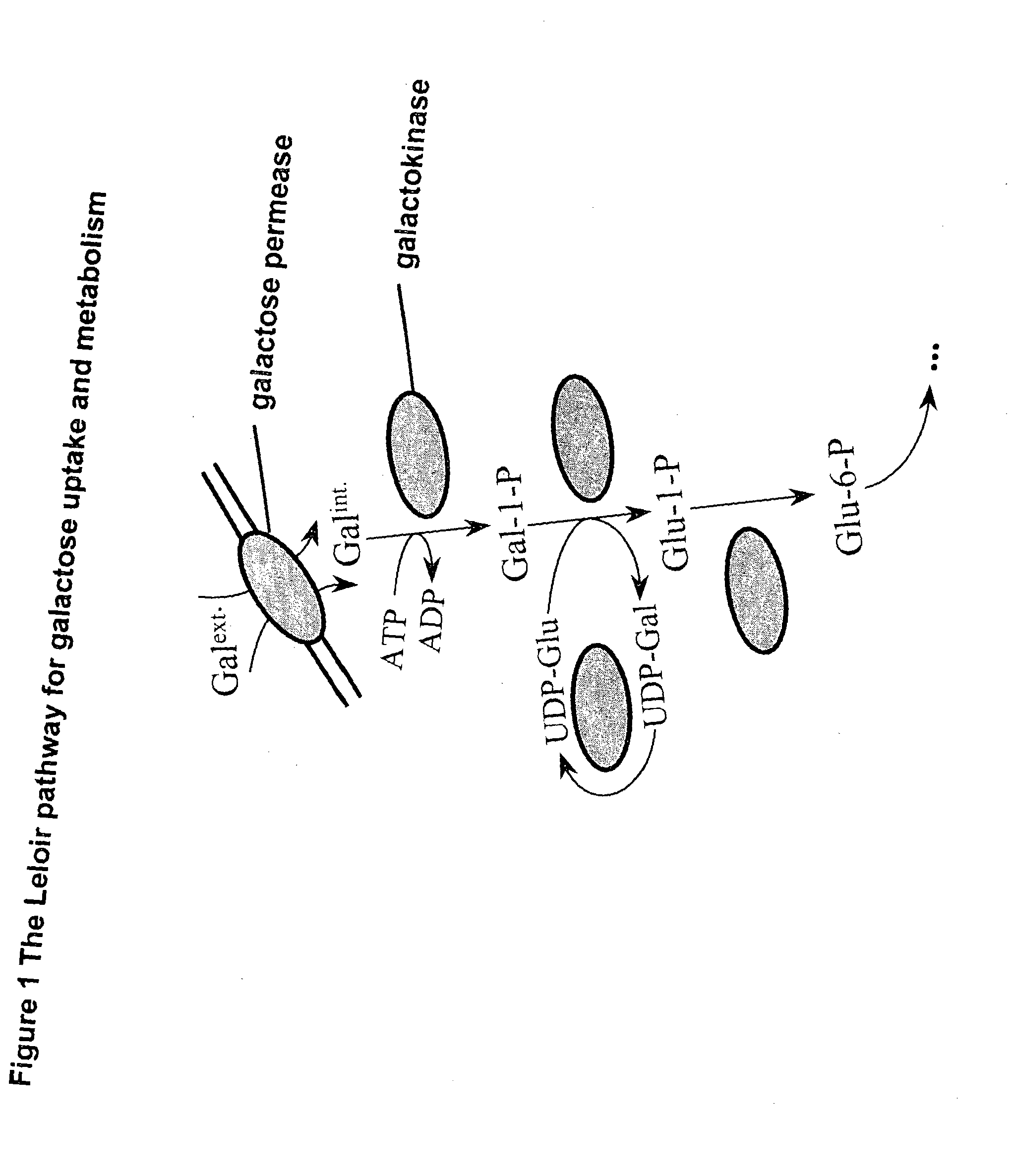
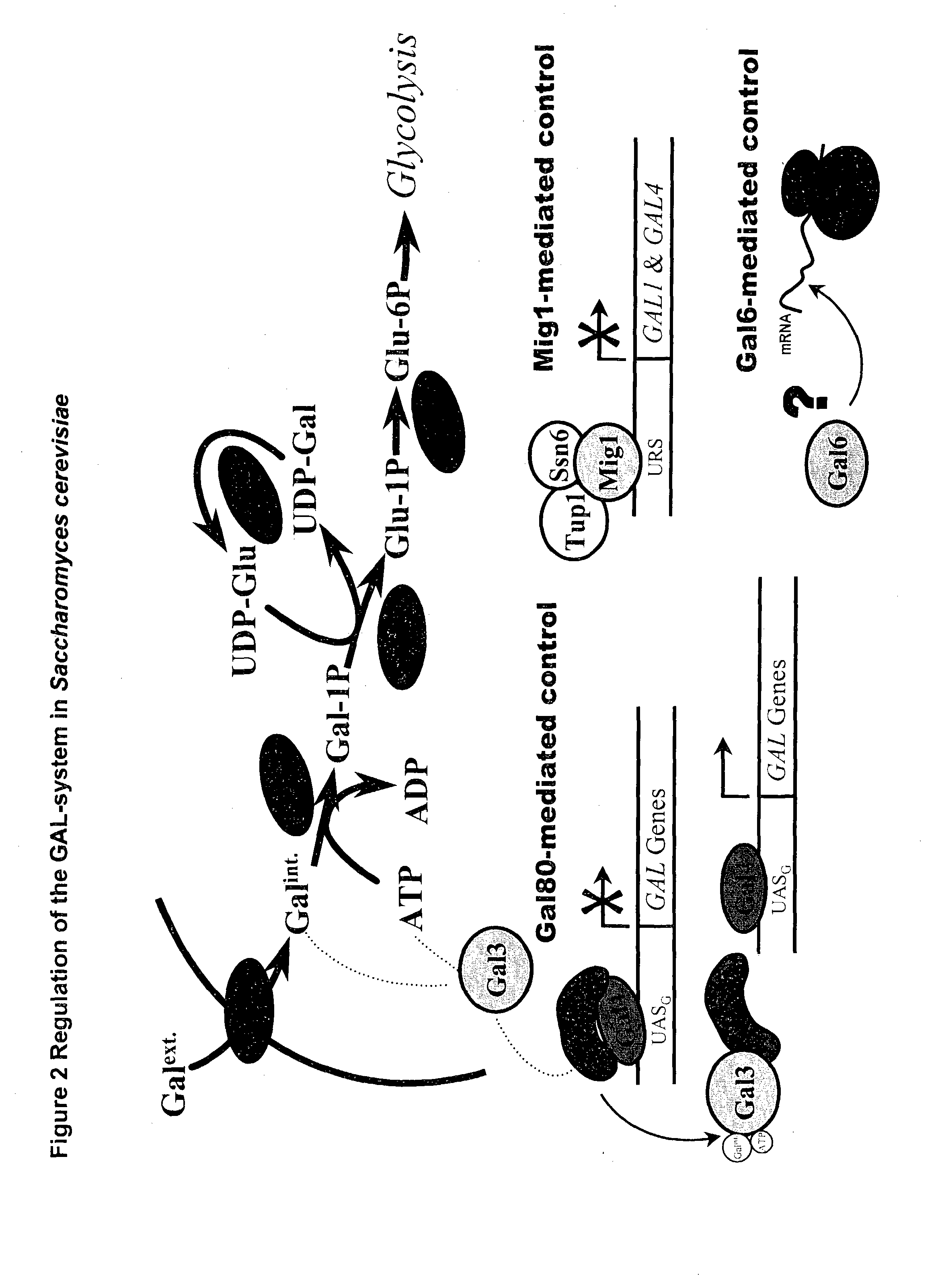
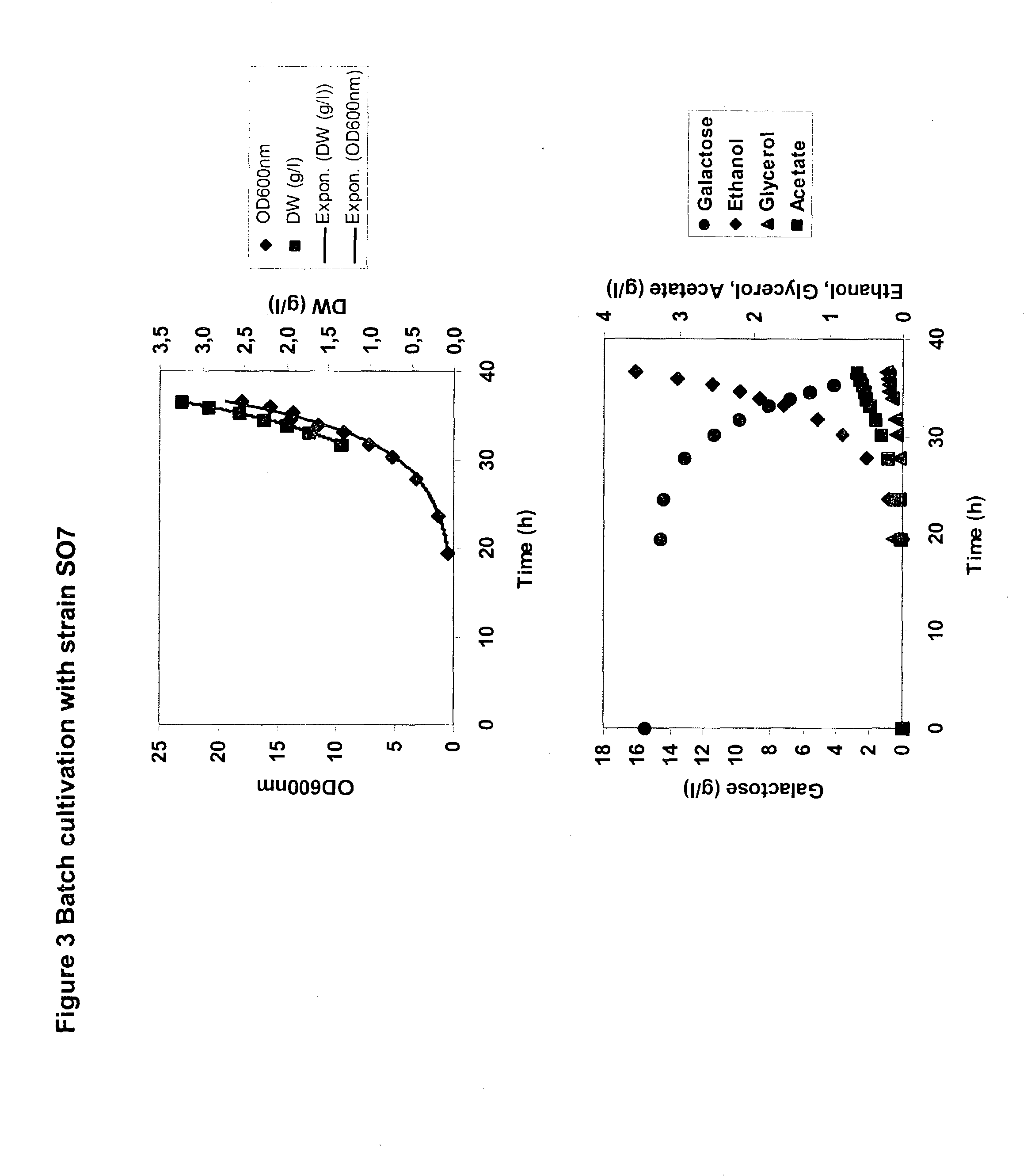
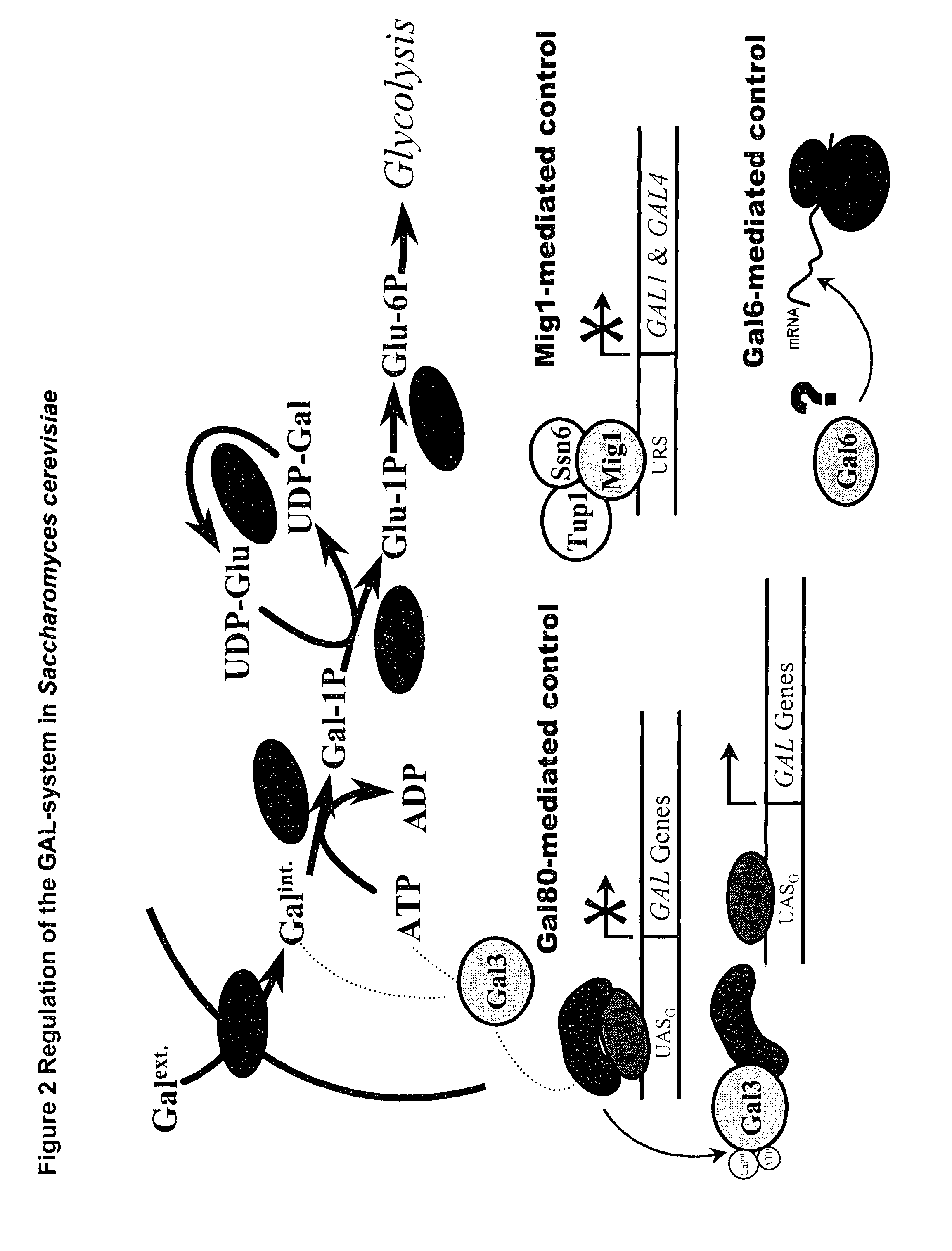
Metabolically engineered micro-organisms having improved galactose uptake
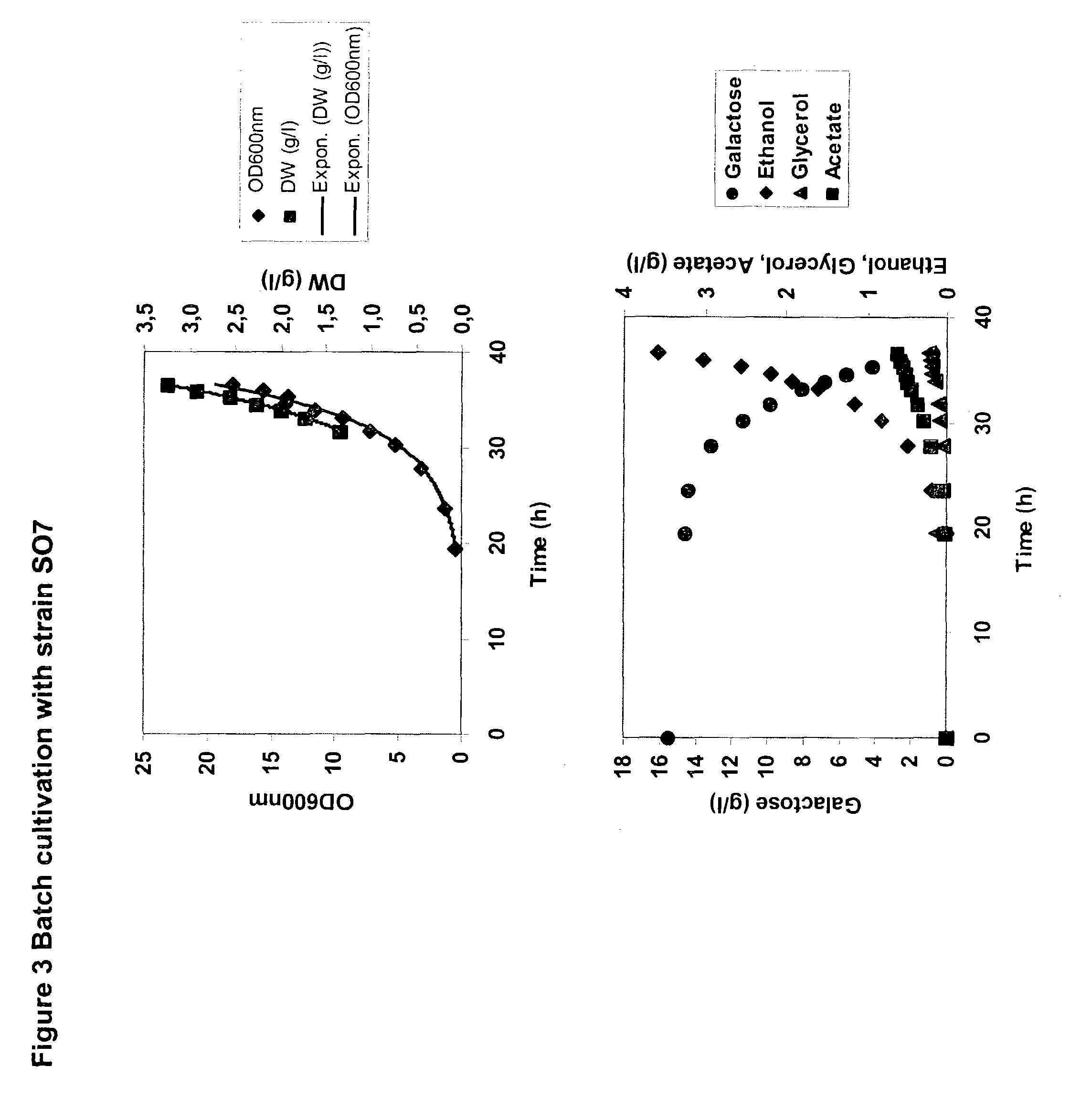
A recombinant prototrophic micro-organism such as a yeast is provided which over-expresses the activity of an enzyme catalysing the conversion of glucose-1 phosphate to glucose-6 phosphate in the galactose uptake and metabolism pathway. Suitably the increased enzyme activity is due to over-expression of the gene PGM2. This produces increased uptake of galactose and increased ethanol production when the organism is grown on a galactose containing medium.
Owner:EVOLVA SA
Method for preparing cordyceps sinensis health-care wine by using cordyceps militaris culture medium
The invention relates to a method for preparing cordyceps sinensis health-care wine by using a cordyceps militaris culture medium and belongs to the field of brewing of health-care wine. The method is characterized by comprising the following steps of: crushing the cordyceps militaris culture medium, adding rice husks, and stirring uniformly; putting in a steamer and steaming, and discharging; adding distiller's yeast into the materials, wherein the distiller's yeast is glucoamylase and yeast, dissolving the glucoamylase, adding glucose 6-phosphate, standing, adding yeast, uniformly shaking and activating, adding cool water, shaking uniformly and stirring, and uniformly scattering in the steamed materials; and feeding into a wine pool, tightly compacting, sealing, covering rice husks, naturally fermenting, discharging out of the pool, distilling and purifying to obtain raw wine, adding purified water, adjusting the alcohol degree, and putting nano cordyceps militaris powder to obtain a product. The prepared health-care wine is clear and transparent, sweet in taste and mellow, soft and tasty and refreshing; and the wine is brewed by using the cordyceps militaris culture medium, so that the wine contains a large amount of nutritional ingredients of cordyceps sinensis and has nutrient flavor of pure grain wine, and belongs to excellent health-care wine.
Owner:崔学恒
Bifidobacterium bifidum and application thereof
ActiveCN108707565ACan regulate immune responseEnhanced inhibitory effectBacteriaLactobacillusEscherichia coliMortality rate
Owner:BEIJING BOJINYUAN BIOTECH
Serum-free immune cell culture medium and preparation method thereof
InactiveCN107574150AIncrease the proportion of positive phenotypesHigh positive rateBlood/immune system cellsCell phenotypeSalidroside
The invention discloses a serum-free immune cell culture medium. The serum-free immune cell culture medium is prepared mainly from amino acids, vitamins, minerals, D-glucose, 4-hydroxyethyl piperazinyl ethanesulfonic acid, insulin, phenol red, sodium pyruvate and transferrin. The invention discloses another serum-free immune cell culture medium which further comprises cofactors including salidroside, luteolin and resveratrol. The invention discloses another serum-free immune cell culture medium which further comprises activators including an acetyl coenzyme A, a coenzyme Q10, glucose 6-phosphate and cyclic adenosine monophosphate. A part of embodiments of the invention can increase the positive ratio of cell phenotypes.
Owner:万向东方生物科技有限公司
Metabolically engineered micro-organisms having improved galactose uptake
A recombinant prototrophic micro-organism such as a yeast is provided which over-expresses the activity of an enzyme catalysing the conversion of glucose-1 phosphate to glucose-6 phosphate in the galactose uptake and metabolism pathway. Suitably the increased enzyme activity is due to over-expression of the gene PGM2. This produces increased uptake of galactose and increased ethanol production when the organism is grown on a galactose containing medium.
Owner:EVOLVA SA
Method for preparing inositol through enzymic catalysis and inositol
InactiveCN105925643AImprove conversion efficiencySimple production processFermentationGlucose polymersD-Glucose
The invention relates to a method for preparing inositol through enzymic catalysis. The method comprises the following steps: performing enzyme-catalyzed conversion on a polysaccharide or an oligosaccharide which takes glucose as a unit to obtain glucose-1-phosphate; performing enzyme-catalyzed conversion on the glucose-1-phosphate to obtain glucose-6-phosphate; performing enzyme-catalyzed conversion on the glucose-6-phosphate to obtain inositol-1-phosphate; performing enzyme-catalyzed conversion on the inositol-1-phosphate to obtain inositol. The invention also provides an inositol product which is prepared according to the method. According to the method, the conversion efficiency of the inositol is effectively improved; moreover, a production process is simple, pollution-free, and low in production cost.
Owner:浙江欣诺医药有限公司
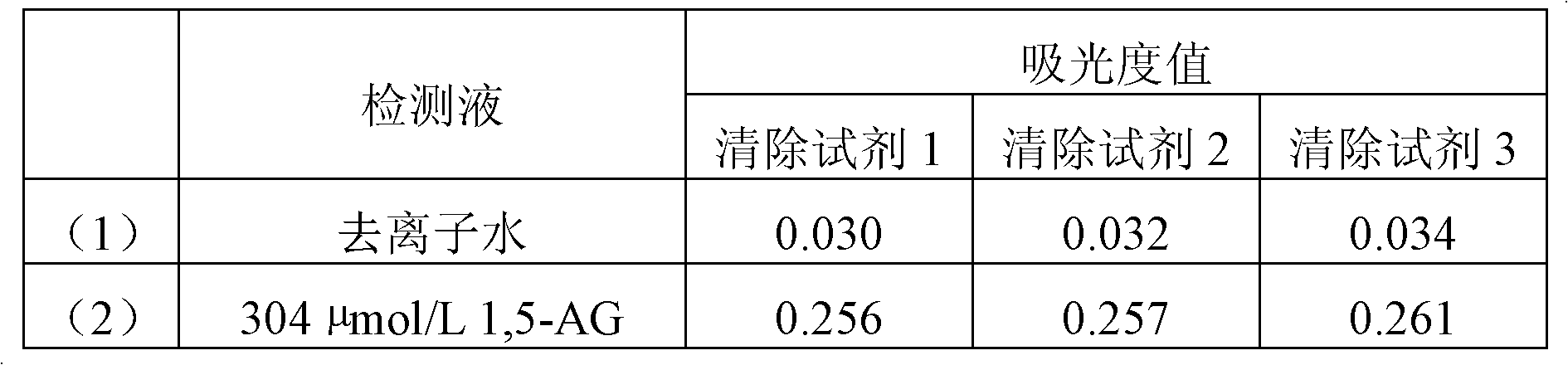
High-concentration glucose removal method and kit for determining serum 1,5 anhydro-D-glucitol based on pyranose oxidase method
The invention discloses a high-concentration glucose removal method for determining serum 1,5 anhydro-D-glucitol based on a pyranose oxidase method, which comprises the following steps: preparing a 2-morpholinoethanesulfonic acid (MES) buffer solution having a pH value of 6.0-9.0, wherein the MES buffer solution contains a certain amount of glucokinase (GK), glucose-6-phosphate dehydrogenase (G6PD), nicotinamide adenine dinucleotide phosphate (NADP), pyruvate kinase (PK), phosphoenolpyruvate (PEP) and adenosine triphosphate (ATP); and adding into a serum sample containing glucose (Glu), and reacting at 20-50 DEG C for 3-5 minutes, wherein the GK converts the Glu into glucose-6-phosphoric acid (G6P) in the presence of the ATP, and adenosine diphosphate (ADP) is generated at the same time; the G6P is further converted into glucose-6-phosphate lactone (G6PL) by the G6PD in the presence of the NADP+; and the ADP generated in the reaction is converted into ATP again under the action of thePK in the presence of the PEP. The glucose removal agent prepared by the method can be stably stored at 2-8 DEG C for 14 months, and can be used for a clinical biochemical autoanalyzer extensively used at present.
Owner:浙江夸克生物科技有限公司
Kit, preparation method of kit and method for detecting peripheral blood glycocholic acid through kit
ActiveCN106226512ALow specificityHigh detection sensitivityColor/spectral properties measurementsTaurocholic acidTaurine
The invention discloses a kit. The kit comprises a reagent R1 and a reagent R2, the reagent R1 contains a trihydroxymethyl aminomethane (Tris-HCL) buffer solution, an anti-taurine antibody, an anti-glycocholic acid antibody and glucose-6-phosphate (G6P), and the reagent R2 contains the trihydroxymethyl aminomethane (Tris-HCL) buffer solution, a glycocholic acid-G-6-PD conjugate, NADP and BSA. The invention further discloses a preparation method of the kit, particularly discloses a preparation method of the anti-taurine antibody the anti-glycocholic acid antibody and further discloses a method for detecting peripheral blood glycocholic acid through the kit. Accordingly, interference of taurocholic acid can be avoided, the detection sensitivity reaches up to 0.1 microgram per milliliter, and the advantages of high specificity and detection repeatability, good stability and the like are achieved.
Owner:杭州利安生物科技有限公司 +1
A kit, a preparation method of the kit, and a detection method of glycocholic acid in peripheral blood realized by using the kit
ActiveCN106226512BLow specificityClosed interferenceColor/spectral properties measurementsTaurocholic acidTaurine
The invention discloses a kit. The kit comprises a reagent R1 and a reagent R2, the reagent R1 contains a trihydroxymethyl aminomethane (Tris-HCL) buffer solution, an anti-taurine antibody, an anti-glycocholic acid antibody and glucose-6-phosphate (G6P), and the reagent R2 contains the trihydroxymethyl aminomethane (Tris-HCL) buffer solution, a glycocholic acid-G-6-PD conjugate, NADP and BSA. The invention further discloses a preparation method of the kit, particularly discloses a preparation method of the anti-taurine antibody the anti-glycocholic acid antibody and further discloses a method for detecting peripheral blood glycocholic acid through the kit. Accordingly, interference of taurocholic acid can be avoided, the detection sensitivity reaches up to 0.1 microgram per milliliter, and the advantages of high specificity and detection repeatability, good stability and the like are achieved.
Owner:杭州利安生物科技有限公司 +1
Recombinant strain producing l-amino acids, constructing method therefor and method for producing l-amino acids
The present application discloses recombinant bacteria producing L-amino acid(s) its construction method and the method of producing L-amino acid(s). The recombinant bacteria producing L-amino acid(s) according to the present invention has reduced expression of the glucose-6-phosphate isomerase Pgi and improved expression of the glucose-6-phosphate dehydrogenase Zwf-OpcA than the starting bacteria, wherein: said starting bacterium is a bacterial strain which can accumulate target amino acid(s). During fermenting and culturing the recombinant bacteria according to the present invention, it is observed that the effect of improving yield can be additive and the yield of L-amino acid(s) is improved obviously. The strategy of combinational modification according to the present invention develops a new method of improving the yield of L-amino acid(s) and hence it can be applied to produce L-amino acid(s) through bacterialfermentation.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Dynamic knockdown of central metabolism for redirecting glucose-6-phosphate fluxes
InactiveUS20170130210A1Reduce the amount requiredIncrease productionTransferasesFermentationHeterologousBiotechnology
Described herein are methods for dynamic redirection of metabolic flux in a cell from central metabolism towards production of heterologous products.
Owner:MASSACHUSETTS INST OF TECH
Beta-1,3-glucan manufacturing method
A method for manufacturing a linear-chain beta-1,3-glucan is disclosed that comprises polymerizing glucose-1-phosphate serving as a substrate by contacting the glucose-1-phosphate with a beta-1,3-glucan phosphorylase derived from a species in the genus Ochromonas. A laminarioligosaccharide may be added to serve as a primer. A linear-chain beta-1,3-glucan having a degree of polymerization between about 30 to 70 is also disclosed.
Owner:MIE UNIVERSITY
Antigens Targeted by Prevalent Pathogennic T Cells in Type 1 Diabetes and Uses Thereof
The present invention is based on the identification of a predominant ligand of CD8+ T cells that are responsible eq for type 1 diabetes. That ligand is islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP). Several CD8+ T cell-binding peptides from IGRP are identified, including the peptide comprising amino acids 206-214 of the IGRP sequence, which has high avidity to the most prevalent T cell receptor of pathogenic CD8+ T cells in autoimmune diabetes. The invention thus provides oligopeptide and polypeptide compositions comprising YLKTN / A / I / L / V)FL, FLWSVFWLI, (T / A)YY / G / T)FLNFM, LR(LV)(F / L)(G / N)IDLL, KWCANPDWI, and SFCKSASIP. Also provided are oligopeptide compositions 8-10 amino acids in length and completely homologous with a mammalian IGRP, where the oligopeptide is capable of binding a human MHC class I molecule. Additionally, various methods of treating a mammal using the above compositions are provided, where the mammal is at risk for or has type 1 diabetes. Also provided are methods of preventing a CD8+ T cell that is cytotoxic to pancreatic islet β-cells from destroying a mammalian β-cell, where the methods also use the above compositions. Further provided are methods for determining whether a mammal is at risk for or has type 1 diabetes, where the methods use the above compositions.
Owner:UNIV TECH INT +3
Coenzyme II NADP+ or NADPH content testing kit and its method
InactiveCN107782684AEfficient determinationEffective contentColor/spectral properties measurementsHydrogenDistilled water
The invention discloses a coenzyme II NADP+ or NADPH content testing kit and its method. The kit comprises acid extractive fluid, alkali extractive fluid, mixed solution of HEPES and EDTA, glucose 6-phosphate, PMS, MTT, glucose 6-phosphate dehydrogenase solution, NaCl solution and mixed solution of ethanol and double distilled water. The method includes steps of extracting NADP+ and NADPH in a sample by acid and alkali extracting fluids on the basis of a spectrophotometric method; performing PMS hydrogen delivering action on NADPH, and reducing MTT to be formazan; detecting light absorption value under 570 nm, and testing the NADPH content; reducing the NADP+ to be NADPH by glucose 6-phosphate dehydrogenase, thereby detecting the NADP+ content. The kit and its testing method can effectively test the content of the coenzyme II NADP+ or NADPH, and are featured by high specifity, wide application range, high stability, and others.
Owner:SUZHOU COMIN BIOTECH
Modulation of glucose-6-phosphatase translocase expression
InactiveUS20110166065A1Organic active ingredientsPeptide/protein ingredientsGlucose loweringGlycemic
Compositions and methods are provided for decreasing blood glucose levels in an animal, comprising administering to said animal an antisense inhibitor of glucose-6-phosphatase translocase expression alone or in combination with at least one glucose-lowering drug. Also provided are compositions and methods for treating diabetes and other metabolic disorders.
Owner:IONIS PHARMA INC
Recombinant yeast and substance production method using the same
Substance productivity is improved by introducing a metabolic pathway for synthesis of acetyl-CoA or acetic acid from glucose-6-phosphate into yeast. Acetic acid productivity, acetyl-CoA productivity, and productivity of a substance made from acetyl-CoA-derived are improved by attenuating genes involved in the glycolytic system of yeast and introducing a phosphoketolase gene into the yeast.
Owner:TOYOTA JIDOSHA KK
Method for preparing hypophosphorous acid (salt) and gluconic acid (salt) from glucose phosphate serving as raw material
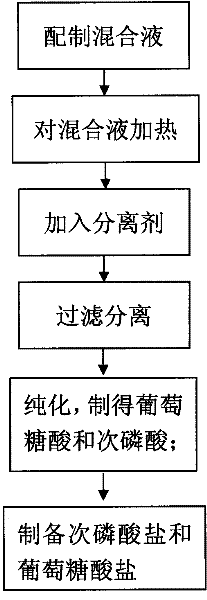
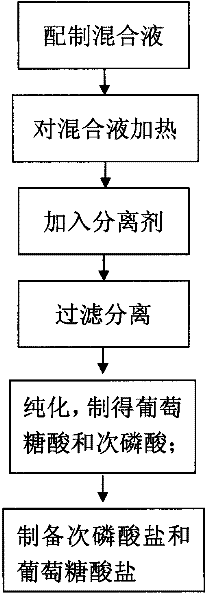
InactiveCN102190578AEasy to getLow costCarboxylic acid salt preparationHypophosphorous acidPhosphateGluconic acid
The invention discloses a method for preparing hypophosphorous acid (salt) and gluconic acid (salt) from glucose phosphate serving as a raw material, which comprises the following steps of: (1) preparing raw materials: preparing subphosphate or acid phosphate and glucose, and taking water as a solvent; (2) heating: continuously heating mixed solution at the temperature of more than or equal to 100DEG C under the air pressure of more than or equal to 0.01MPa for more than 10 minutes; (3) separating: adding a separating agent, concentrating, and separating to obtain crude products of gluconic acid salt and hypophosphorous acid salt; (4) purifying: filtering to remove precipitates to obtain gluconic acid, adding a gas or a solvent capable of reacting with the hypophosphorous acid salt into aqueous solution of the separated hypophosphorous acid salt, and filtering to remove precipitates to obtain hypophosphorous acid; (5) preparing the hypophosphorous acid salt: reacting the hypophosphorous acid with basic oxide or hydroxide to obtain the hypophosphorous acid salt; and (6) preparing the gluconic acid salt: reacting the gluconic acid with basic oxide or hydroxide to obtain the gluconicacid salt. The production process is environment-friendly and harmless, raw materials are readily available, the cost is low, the yield is high, the period is short and the safety is achieved.
Owner:石允生
Fructan consisting of 25 fructoses and 1 glucose
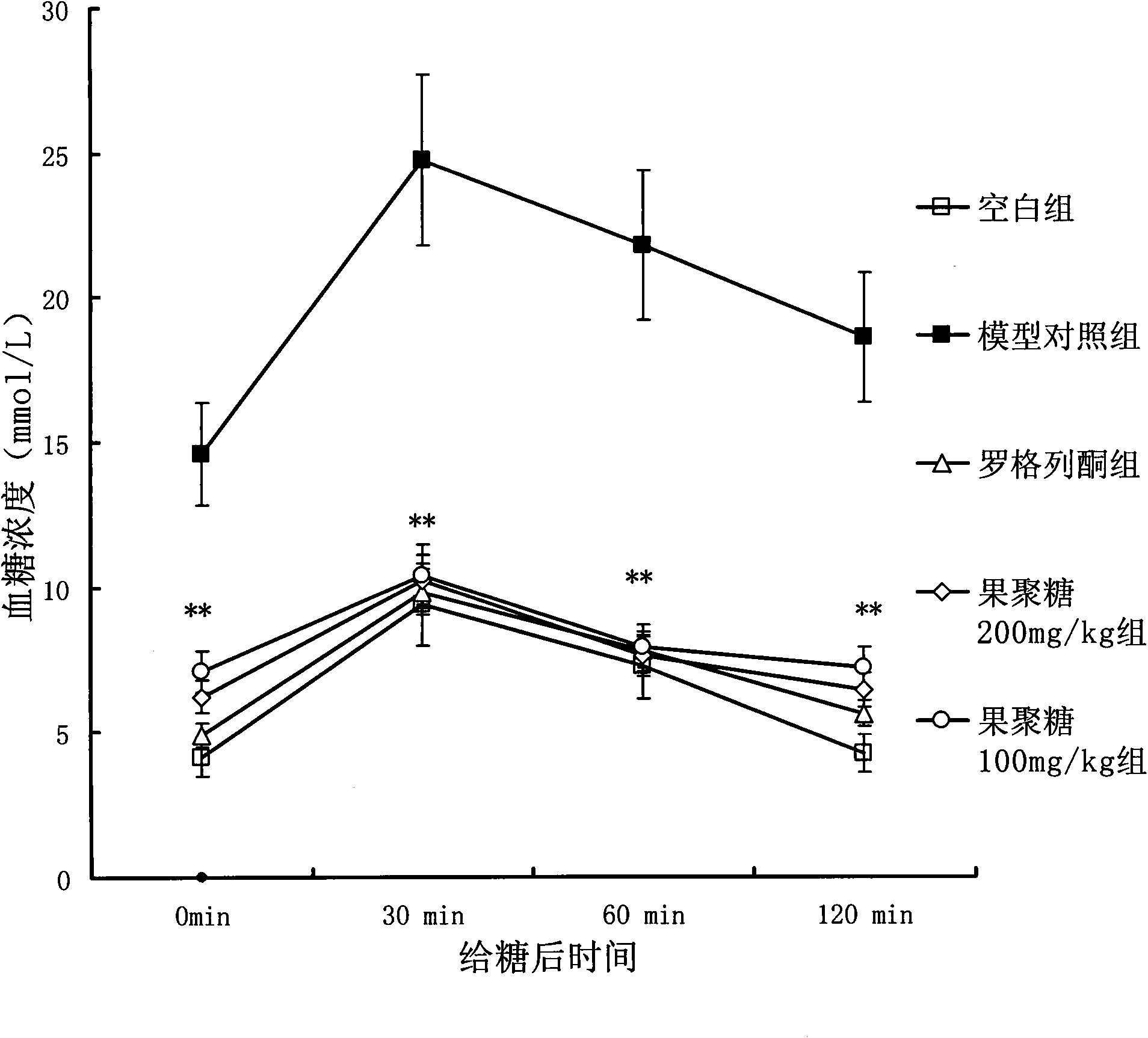
InactiveCN101838337AImprove glucose toleranceReduced activityOrganic active ingredientsMetabolism disorderPhosphoric acidFructan
The invention provides a fructan consisting of 25 beta-D-fructoses and 1 alpha-D-glucose, and a method for preparing the fructan from Hubei dwarf lilyturf tuber serving as a raw material. The fructan has obvious effect of reducing blood sugar of mice with type 2 diabetes, simultaneously improves the sugar tolerance of the mice with the type 2 diabetes, and obviously enhances the expression of insulin receptor (InsR), insulin receptor substrate (IRS-1), protein kinase and phosphatidylinositol3-kinase (PI3-K) to further accelerate the glucose to be converted into glycogen and improve the activities of glucose-6-phosphate and glucose-6-phophatase so as to obvious reduce the insulin resistance.
Owner:HUAZHONG UNIV OF SCI & TECH
Recombinant yeast and substance production method using the same
ActiveUS20160251633A1Reduced activityIncrease productivityTransferasesOxidoreductasesAcetic acidBiotechnology
Substance productivity is improved by introducing a metabolic pathway for synthesis of acetyl-CoA or acetic acid from glucose-6-phosphate into yeast. Acetic acid productivity, acetyl-CoA productivity, and productivity of a substance made from acetyl-CoA-derived are improved by attenuating genes involved in the glycolytic system of yeast and introducing a phosphoketolase gene into the yeast.
Owner:TOYOTA JIDOSHA KK
Beverage for supplementing human body energy and recovering people from fatigue as well as preparation method and application thereof
InactiveCN102058045AMaintain acid-base balanceAnti-fatigue significantly and quicklyOrganic active ingredientsAntinoxious agentsHuman bodySide effect
The invention discloses a drink for supplementing human body energy and recovering people from fatigue as well as a preparation method and application thereof. The beverage is characterized by containing glucose-6-phosphate. The preparation method comprises the following steps: mixing glucose, adenosine triphosphate (ATP) and magnesium chloride; and obtaining a mixed solution containing glucose-6-phosphate in the presence of hexokinase serving as a catalyst. Besides the glucose-6-phosphate, the beverage provided by the invention also contains a defined amount of components such as glucose, sodium ions, potassium ions, magnesium ions, and the like. The components, after being combined, can fully perform a synergistic action, and effectively perform functions of quickly supplementing human body energy, and recovering people from fatigue. Meanwhile, the beverage can supplement the potassium ions, the sodium ions and the magnesium ions, and achieve a function of maintaining acid-base balance of a human body and improving the immunity. The nutrient solution containing glucose-6-phosphate is mild, suitable for long-term consumption and free of side effects.
Owner:KAIPING GENUINE BIOCHEM PHARMA +1
Genes coding for glucose-6-phosphate-dehydrogenase proteins
Isolated nucleic acid molecule, coding for a polypeptide having the amino acid sequence referred to in each case in Table 1 / column 2, wherein the nucleic acid molecule in the amino acid position indicated in Table 1 / column 4 encodes a proteinogenic amino acid different from the particular amino acid indicated in Table 1 / column 5 in the same row.
Owner:DAESANG
A kind of g6pd deficiency gene detection kit
ActiveCN104293916BAccurate identification of genotypeReduce probabilityMicrobiological testing/measurementDiseaseFluorescence
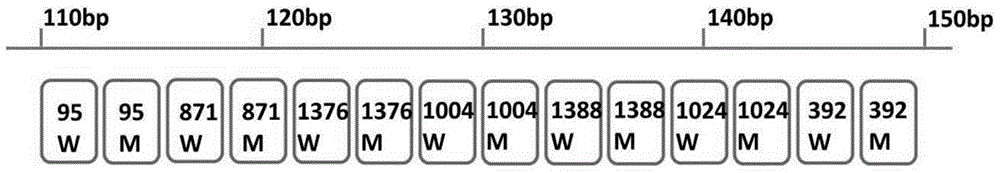
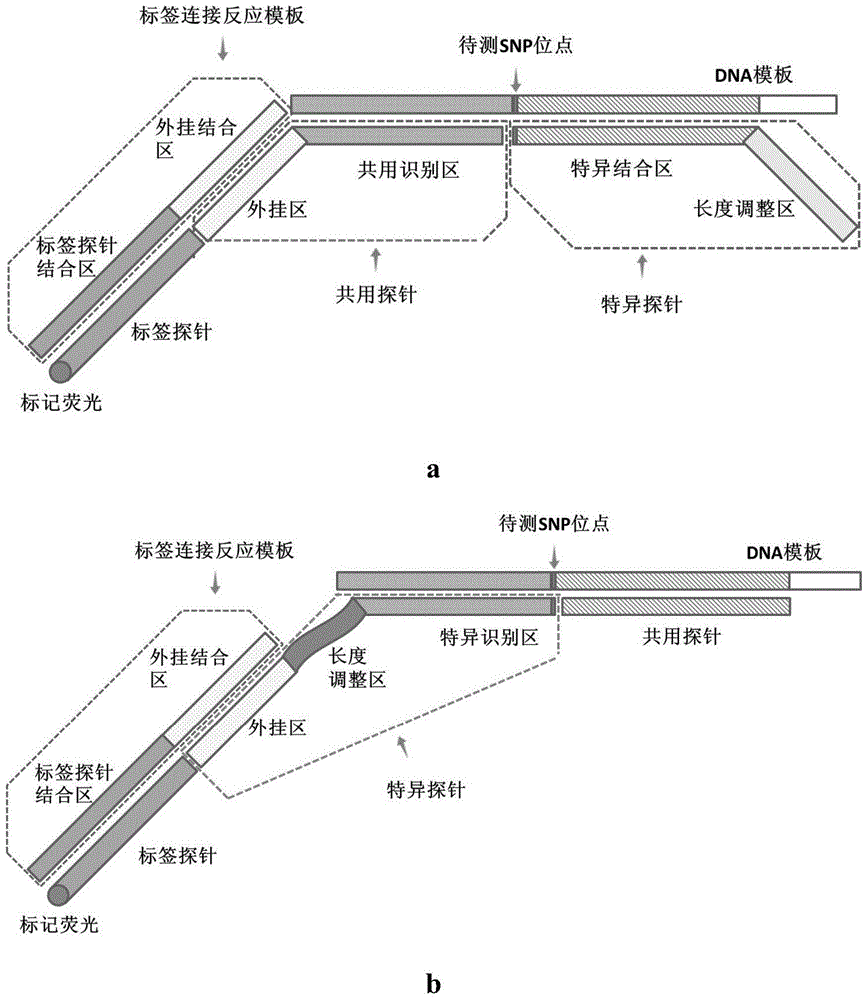
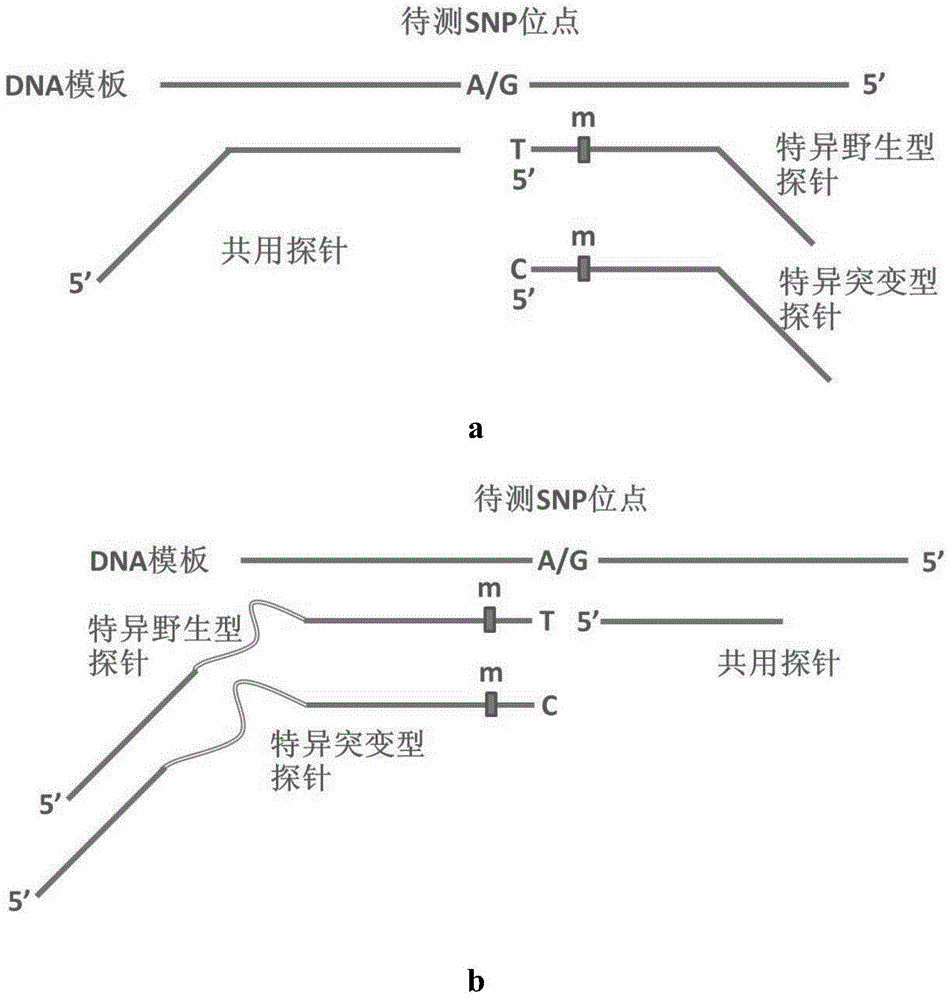
The invention discloses a gene detection kit for G6PD (glucose-6-phosphate dehydroge-nase) deficiency, which comprises fragment amplification primers and a multiplex system thereof, probes for detecting G6PD mutation SNP locus and including a specific probe and a common probe, as well as a label connection reaction template consisting of a specific add-on binding domain and a label probe binding domain, and a tag probe capable of carrying different fluorescent marks, and a connection reaction system. The genotype of a subject can be directly confirmed by selecting disease-causing gene g6pd of the G6PD deficiency disease as a detection object, the missed diagnosis rate of women heterozygote can be significantly reduced, the testing accuracy is higher than the accuracy of other conventional testing methods. In addition, the shortest testing time of the gene detection kit is only three hours, and thus the gene detection kit is a high-efficiency, accurate, and simple and convenient novel G6PD deficiency diagnosis means.
Owner:GUANGDONG HUAMEI ZHONGYUAN BIOLOGICAL SCI & TECH +1
Method for regulating composition and proportion of monosaccharide in lucid ganoderma exopolysaccharides
ActiveCN106755183AUniform structureInhibit biological activityFermentationPolysaccharide/gum food ingredientsMicroorganismMonosaccharide composition
The invention discloses a method for regulating the composition and proportion of monosaccharide in lucid ganoderma exopolysaccharides, and belongs to the field of microbial fermentation. According to the method, glucose 6-phosphate is added to regulate the proportion of glucose, mannose and galactose in the lucid ganoderma exopolysaccharides, synthesis of lucid ganoderma exopolysaccharides with specific monosaccharide composition and proportion is realized, and the lucid ganoderma exopolysaccharides prepared by applying the method has a bioactivity for effectively inhibiting tumor cells and has an inhibition rate of over 80 percent. The method lays foundation for lucid ganoderma exopolysaccharides with relatively high bioactivity, uniform structure and controllable preparation.
Owner:JIANGNAN UNIV
Kit for determining ATP content in trace amounts of sample and application thereof
InactiveCN105548110AEasy to operateAvoid the shortcoming of time-consuming and time-consumingFluorescence/phosphorescenceAtp contentSodium hydroxide
The invention relates to a kit for determining ATP content in trace amounts of sample and application thereof. The kit comprises a solution I which is a mixture of TrisHCl and bovine serum albumin; a solution II which is a sodium hydroxide solution; a solution III which is a mixture of hydrochloric acid and the TrisHCl; a reagent I comprising the TrisHCl, the bovine serum albumin, magnesium chloride, dithiothreitol, D-glucose, nicotinamide adenine dinucleotide phosphate, hexokinase and colourless glucose-6-phosphate dehydrogenase; a reagent II comprising Imidapril HCl, the bovine serum albumin, alpha-ketoglutaric acid monosodium salt, sodium hydroxide, ammonium acetate, adenosine diphosphate, glucose-6-phosphoric acid, glutamate dehydrogenase and the colourless glucose-6-phosphate dehydrogenase; a reagent III comprising the Imidapril HCl, ethylenediaminetetraacetic acid, the magnesium chloride, the ammonium acetate, NADP and glucose 6-phosphate dehydrogenase. The kit is simple to operate, and the determination result is more accurate.
Owner:NANJING MEDICAL UNIV
Kit and method for determining content of glucose 6-phosphate (G6P) based on micro method
InactiveCN109609594AAvoid decompositionQuick and effective testingMicrobiological testing/measurementBiological material analysisDecompositionTested time
The invention discloses a kit and method for determining content of glucose 6-phosphate (G6P) based on a micro method. The kit comprises the following reagents: an extract consisting of a Tris-HCl solution, a first reagent consisting of Tris, MgCl2, water and concentrated hydrochloric acid, a second reagent consisting of glucose-6-phosphate 1-dehydrogenase (G6PDH) and a third reagent consisting ofNADP. A principle of the method is as follows: the G6PDH can catalyze the G6P and NADP+ to NADPH and an increase rate of the NADPH at 340 nm is measured to reflect level of G6P content. The used micro method is rapid and effective in test and can prevent a problem of G6P decomposition possibly caused by too long test time. A sample pretreatment process is simple and easy, besides, measurement operation steps are all automatic operations of instruments, accurate data are read, each sample has a short test time, and the kit is good in repeatability and high in accuracy.
Owner:SUZHOU COMIN BIOTECH
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778956AStrong specificityThe test result is accurateMicrobiological testing/measurementUltravioletWavelength
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of hexokinase, and producing glucose-6-phosphate by reacting glucose under the existence of adenosine triphosphate, and then causing coupled reaction with phosphoglucose dehydrogenase and transferring oxidized coenzyme to reduced coenzyme. Testing the ascending range of dominant wave-length340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
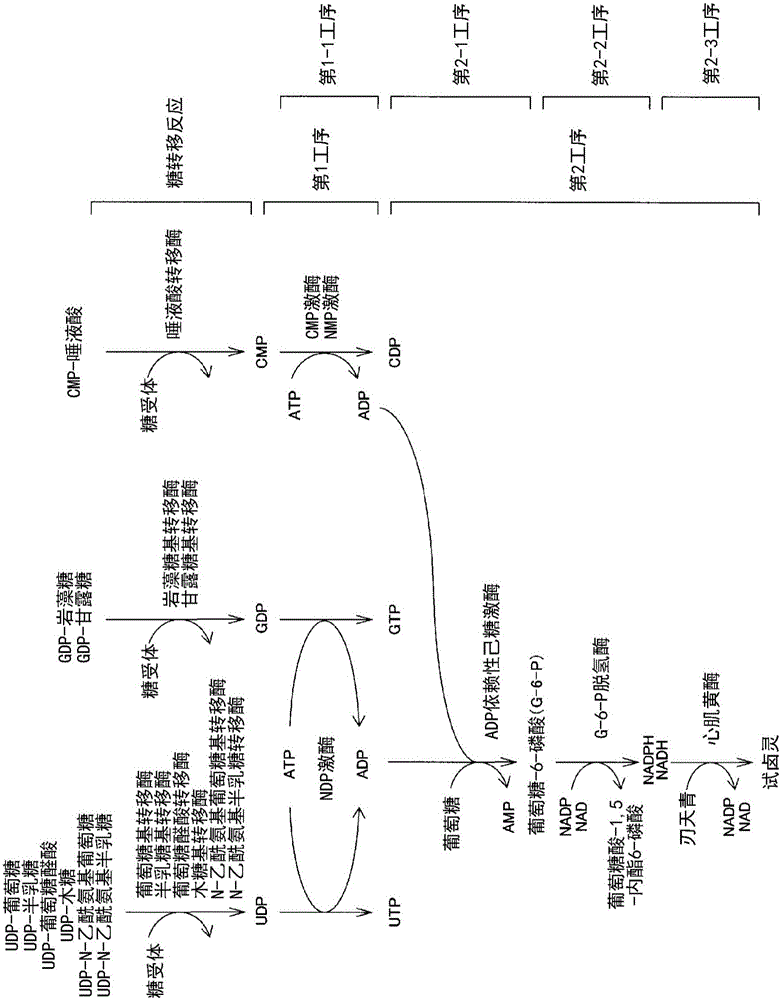
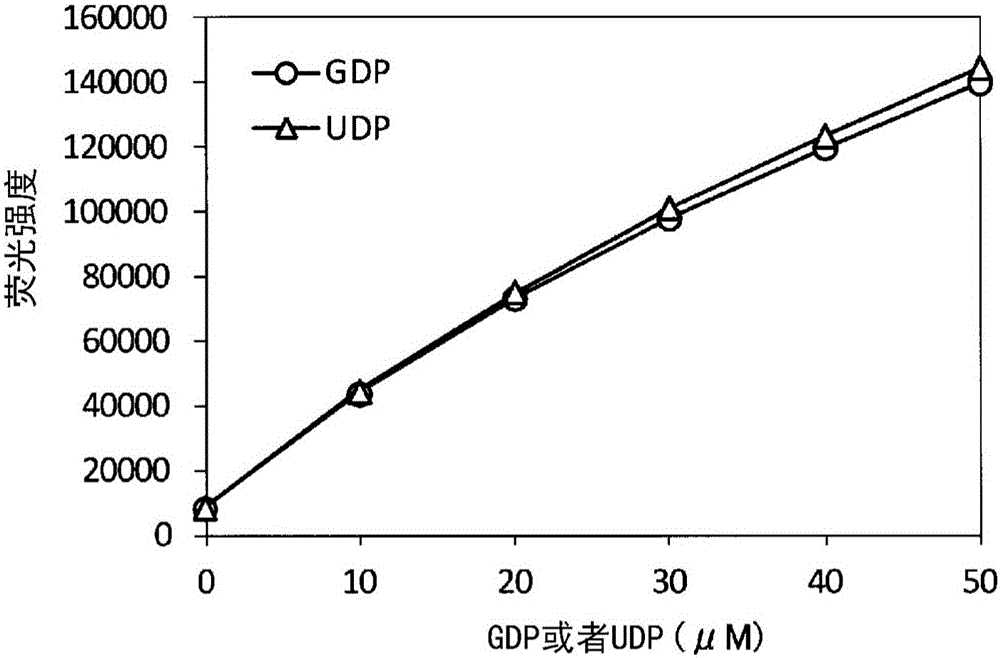
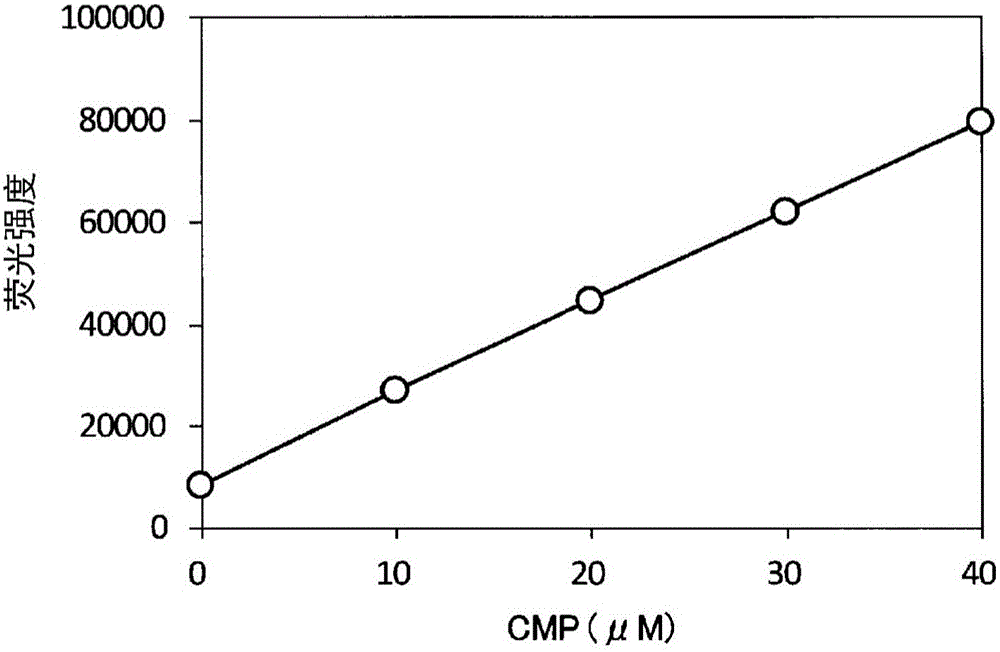
Method for detecting fluorescence or absorbance, method for suppressing background, method for measuring ADP, method for measuring activity of ADP-synthesizing enzyme, and method for measuring activity of glucosyltransferase
InactiveCN105765075AEnables high-throughput screeningMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementResazurinHexokinase
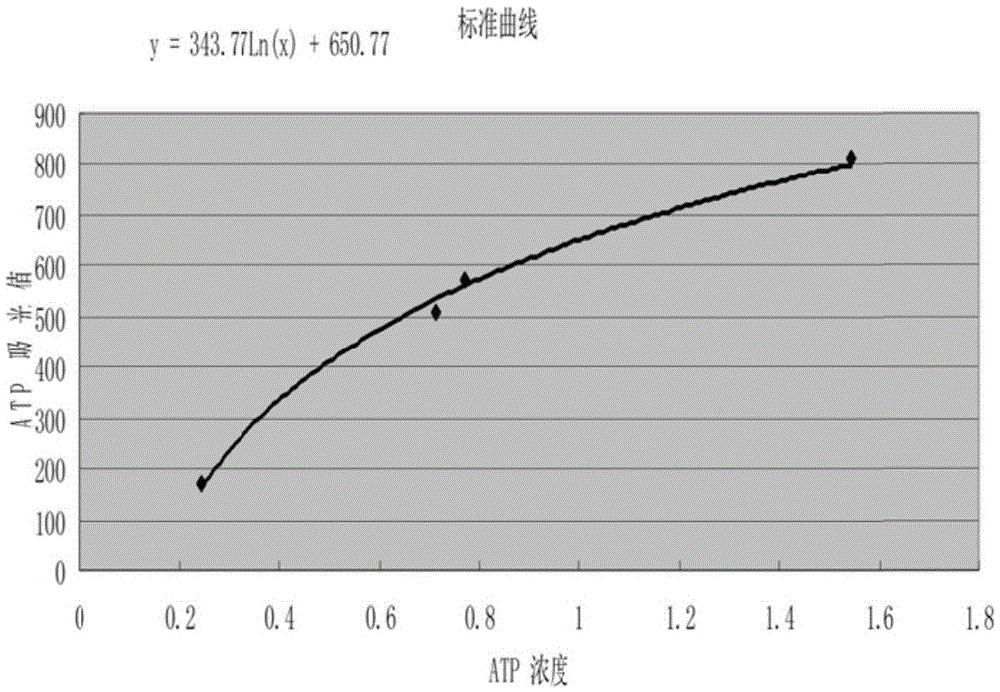
A method for detecting fluorescence or absorbance according to the present invention is characterized by comprising reducing resazurin into resorufin by a diaphorase in the presence of an SH reagent and NADH or NADPH and then measuring the resultant fluorescence intensity or absorbance. A method for measuring ADP according to the present invention is characterized by comprising: step (2-1) for treating glucose with ADP and ADP-dependent hexokinase; step (2-2) for treating glucose-6-phosphate obtained in the above step (2-1) with NAD or NADP and glucose-6-phosphate dehydrogenase; and step (2-3) for treating resazurin with NADH or NADPH obtained in the above step (2-2) and a diaphorase in the presence of an SH reagent and then measuring the resultant fluorescence intensity or absorbance.
Owner:THE UNIV OF TOKYO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com