Method for preparing inositol through enzymic catalysis and inositol
A technology for catalyzing the preparation of inositol, which is applied in the direction of fermentation, can solve the problems of complex components of cell fermentation liquid, low conversion rate of inositol, complex purification of inositol, etc., and achieve the effect of low production cost, high purity and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
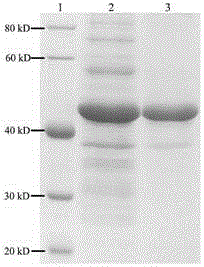
[0042] Expression of inositol-1-phosphate synthase (INO1) in Escherichia coli
[0043] Search for the inositol-1-phosphate synthase (INO1) gene or protein of thermophilic bacteria on NCBI, screen for microorganisms with an optimal growth temperature of 60-90°C, purchase strains to extract genomes and amplify related genes, or directly synthesize them Related genes, taking inositol-1-phosphate synthase (INO1) derived from Archaeoglobusfulgidus as an example, its protein sequence is numbered GeneBank on NCBI: AIG98795.1, and the sequence is handed over to Gene Synthesis Company to synthesize the corresponding DNA sequence, and Add EcoR I-Nde I and Xho I enzyme cutting sites at its two ends respectively. After the synthetic DNA and the E. coli expression vector such as pET-26b(+) were digested and recovered by Nde I and Xho I respectively, they were ligated overnight at 16°C with T4 DNA ligase, and 5 μL of the ligation product was transformed into E. coli DH5α, and the transforma...
Embodiment 2
[0069] The enzyme prepared in Example 1 was used for experiments. The starch substrate is adjusted with water to make starch milk with a dry matter content of 10%, 0.2M sodium dihydrogen phosphate is added, the pH is adjusted to 6.5, and gelatinization is carried out at 65°C. Add the enzyme powder obtained by spray drying, wherein αGP, PGM, INO1, and MIP are mixed in equal proportions according to the enzyme activity unit, and the addition amount is 1000U-10000U / L, and the four enzymes of αGP, PGM, INO1, and MIP are added per liter of reaction system Take 10000U as an example, according to the data of activity determination, at pH 7.0 and 70°C, the enzyme activities are 3U / mg, 275U / mg, 1.7U / mg, 1.7U / mg respectively. That is, the enzyme amounts of the four enzymes in the mixed enzyme are 3333 mg, 36 mg, 5882 mg, and 5882 mg, respectively. Add the mixed enzyme into the starch milk, keep the temperature constant, and react for 72 hours, and the inositol conversion rate can reach...
Embodiment 3
[0071] Adjust the starch raw material with water to make starch milk with a dry matter content of 10%, add 0.1M sodium dihydrogen phosphate, adjust the pH to 7.0, and conduct gelatinization at 75°C. Add the enzyme powder that spray-drying obtains, wherein αGP (derived from Aquifex aeolicus), PGM (derived from Thermococcus kodakaraensis), INO1 (derived from Archaeoglobusfulgidus), MIP (derived from Archaeoglobusfulgidus) add-on amount is 10000U, maintains temperature constant, After reacting for 72 hours, the inositol content was 67g / L, according to the starch molecular formula (C 6 h 10 o 5 )n, if 100g / L of starch is completely converted into inositol, 111g / L of inositol can be obtained, and the conversion rate of inositol can be calculated as 62÷111×100%=60%.
[0072] αGP and PGM were derived from Thermus thermophilus, INO1 was derived from Archaeoglobusfulgidus, MIP was derived from Thermotoga maritima, and the conversion rate was 62%. Replacing MIP with a Thermus thermop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com