Method for preparing hypophosphorous acid (salt) and gluconic acid (salt) from glucose phosphate serving as raw material
A technology of gluconate and gluconic acid, which is applied in the direction of carboxylate preparation, hypophosphorous acid, phosphorus oxyacid, etc., can solve the problems of long production cycle, complex process, low yield, etc., achieve low cost, easy filtration and separation, Easy to save effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1
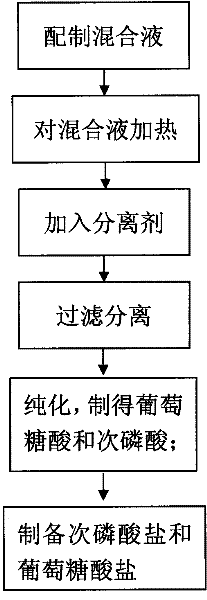
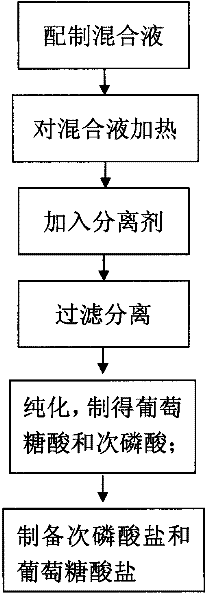
[0042] ① Add disodium hydrogen phosphate (Na 2 HPO 4 .12H 2 O) 716g, glucose (C 6 h 12 o 6 ) 180g, put in a high temperature resistant glass container. ②Place the prepared mixed solution in a high-pressure steam boiler to increase the pressure to 125°C (0.135MPa) for 30 minutes. ③Add 252g of calcium oxide ( CaO), and filtered while hot (80°C to 100°C) to remove the precipitate. ④Heat and concentrate at 100°C to obtain calcium gluconate precipitate. ⑤ Add the precipitate to 1L of pure water, heat (80°C to 100°C) to dissolve the precipitate. While it is hot (70°C~100°C), feed CO 2 Gas, filter to remove the precipitate, you can get high-purity gluconic acid. ⑥ Add various alkaline oxides (56g calcium oxide or 81g zinc oxide, etc.) and hydroxides (40g sodium hydroxide or 56g potassium hydroxide, etc.) to the obtained gluconic acid solution to obtain high-purity glucose salt.
Embodiment (2
[0044] ① Add disodium hydrogen phosphate (KH 2 PO 4 ) 136 grams, glucose (C 6 h 12 o 6 ) 180 grams, add caustic soda 40g (NaOH), make the pH of solution>8. ②Place the prepared mixed solution in a high-pressure steam boiler to increase the pressure to 125°C (0.135MPa) for 30 minutes. ③Add 252g of calcium oxide to the heated mixed solution while it is hot (80°C-100°C), Remove the precipitate by filtration while hot (80°C-100°C). ④Heat and concentrate at 100°C to obtain calcium gluconate precipitate. ⑤ Add the precipitate to 1L of pure water, heat (80°C to 100°C) to dissolve the precipitate. While it is hot (70°C~100°C), feed CO 2 Gas, filter to remove the precipitate, you can get high-purity gluconic acid. ⑥ Add various alkaline oxides (56g calcium oxide or 81g zinc oxide, etc.) and hydroxides (40g sodium hydroxide or 56g potassium hydroxide, etc.) to the obtained gluconic acid solution to obtain high-purity glucose salt.
[0045] (2) Preparation of hypophosphorous aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com