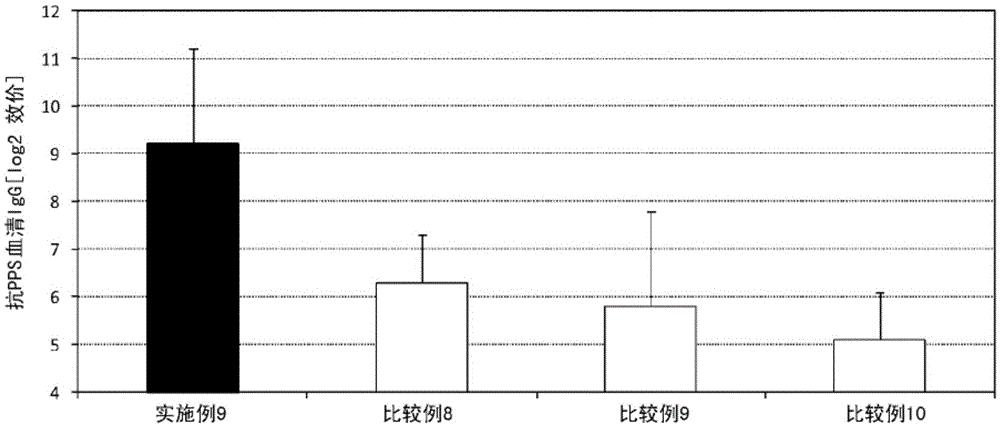
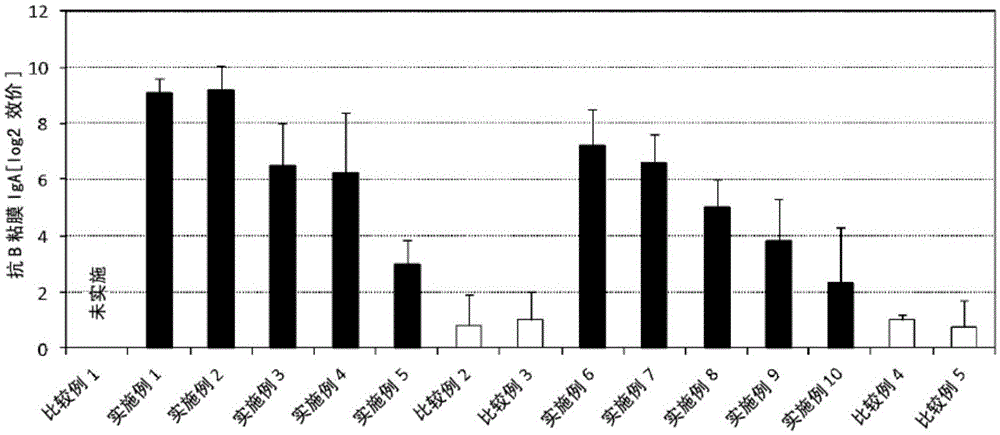
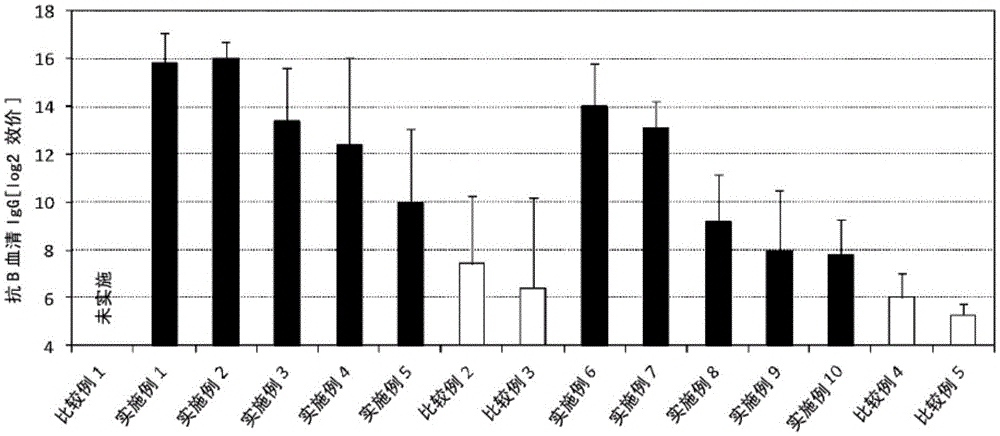
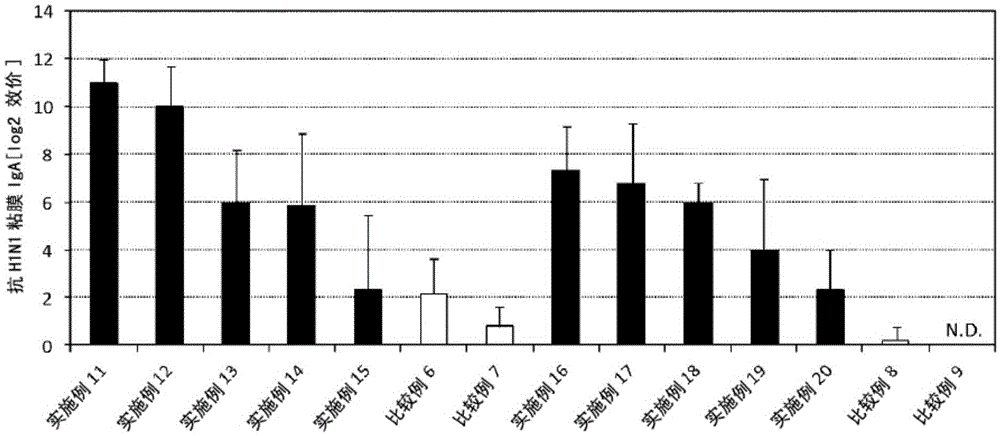
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Genus Methanosarcina" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methanosarcina is a genus of euryarchaeote archaea that produce methane. These single-celled organisms are known as anaerobic methanogens that produce methane using all three metabolic pathways for methanogenesis.
Methane-producing composite microbial inoculum and preparation method thereof
InactiveCN101705199AReasonable designBreed fastBacteriaMicroorganism based processesBiogas productionLiquid methane
The invention relates to a methane-producing composite microbial inoculum and a preparation method thereof, which can solve the long-standing difficult problems that the existing biogas digester is lack of high-efficient methane-producing bacteria, thereby resulting in long initial start-up time of biogas production and poor stability of the biogas production. Methanosarcina acetoacidophilum DSM-N0.2834, methanobacterium formicicum DSM-No.1535, methanobrevibacter arboriphilicus DSM-No.1125, methanolobus tindarius DSM-No.2278 and methanothrix concilii DSM-No.6752 collected in German collection of microorganisms and cell cultures are adopted and inoculated in a liquid methane bacterial culture medium under the anaerobic condition, the independent enlarged culture is carried out grade by grade respectively, and bacterial liquid after the cultured methane bacteria is mixed together according to the volume ratio, thereby preparing a liquid methane-producing composite microbial inoculum; and the liquid methane-producing composite microbial inoculum can also be prepared into a solid methane-producing composite microbial inoculum. The method has reasonable design and adopts the high-efficient strains with the optimal combination and the rapid propagation technology, and the developed high-efficient methane-producing composite microbial inoculum can significantly accelerate the start-up time of the biogas production when in new construction of the biogas digester and large material change, and improve the efficiency and the stability of the biogas production of the biogas digester.
Owner:北京合百意可再生能源技术有限公司 +1
Fast culture process in anaerobic grain sludge
InactiveCN1887737AReduce startup timePromote growthBacteriaWaste based fuelGenus MethanosarcinaMicroorganism
Owner:TAIYUAN UNIV OF TECH
Microbial agent for sewage treatment, attachment carrier of microbial agent and preparation method of microbial agent
The invention discloses a microbial agent for sewage treatment. The microbial agent comprises, by weight, 8-10 parts of clostridium acetobutylicum, 12-16 parts of bacillus licheniformis, 16-20 parts of bacillus subtilis, 5-7 parts of lactobacillus acidophilus, 5-7 parts of methanosarcina, 3-5 parts of methanosaeta, 6-8 parts of rhodopseudomonas palustris, 4-6 parts of cellulomonas flavigena, 8-10parts of streptococcus thermophilus, 8-10 parts of alcaligenes faecalis, 3-5 parts of bacillus amyloliquefaciens, 2-4 parts of bacillus nitrosomonas, 1-3 parts of issatchenkia orientalis and 3-5 partsof streptomyces bullis. Besides, the invention further discloses an attachment carrier of the microbial agent and a preparation method of the microbial agent. The microbial agent can be effectively attached to the carrier and accordingly has better activity. The microbial agent is reasonable in strain preparation and can be effectively attached to the attachment carrier to treat sewage, and the method for sewage treatment is simple, good in treatment effect and worthy of popularization.
Owner:ANJIEYU BEIJING OILFIELD TECHNICAL SERVICES CO LTD
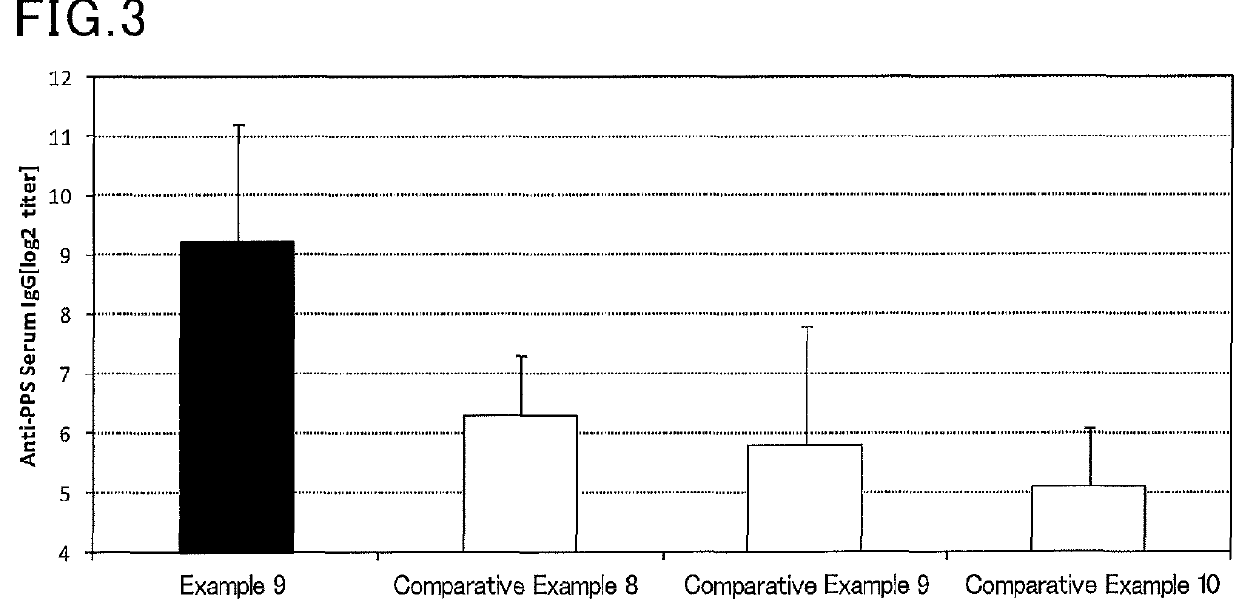
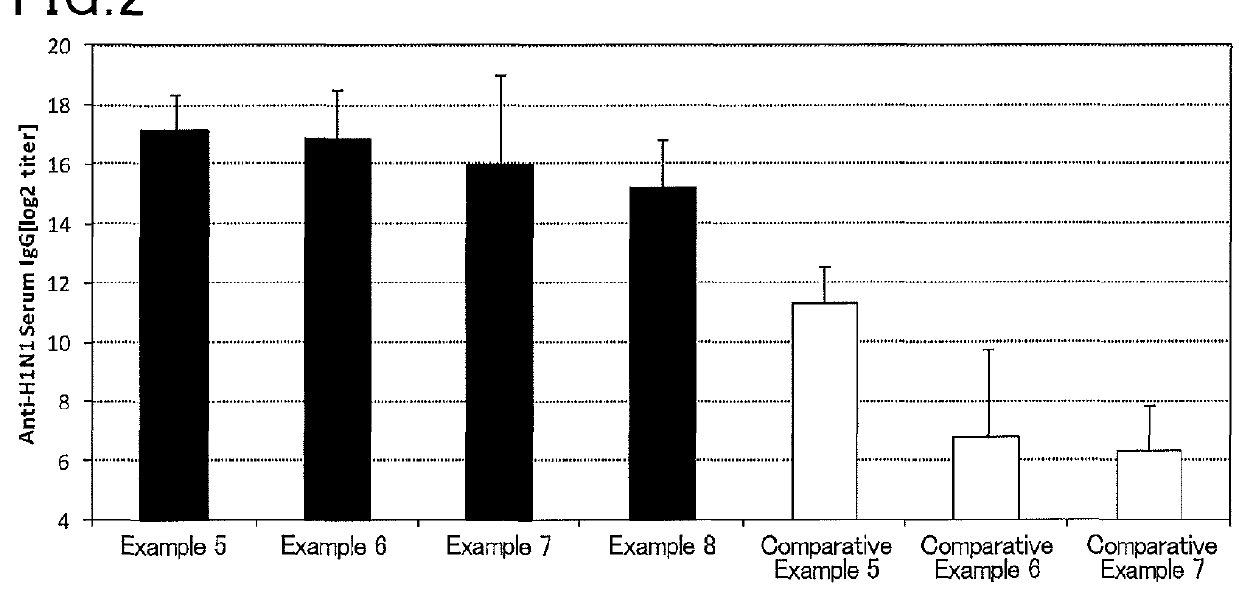
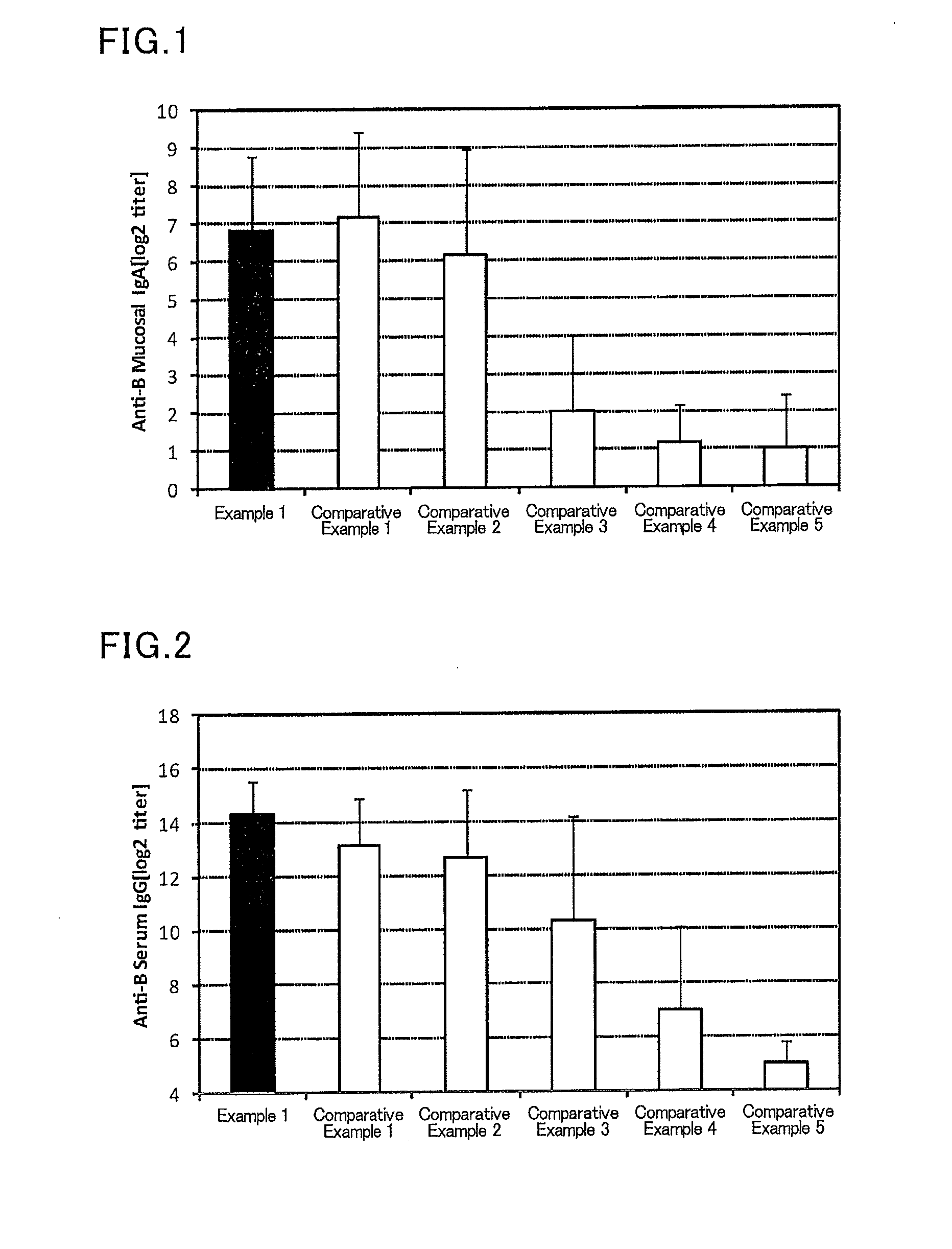
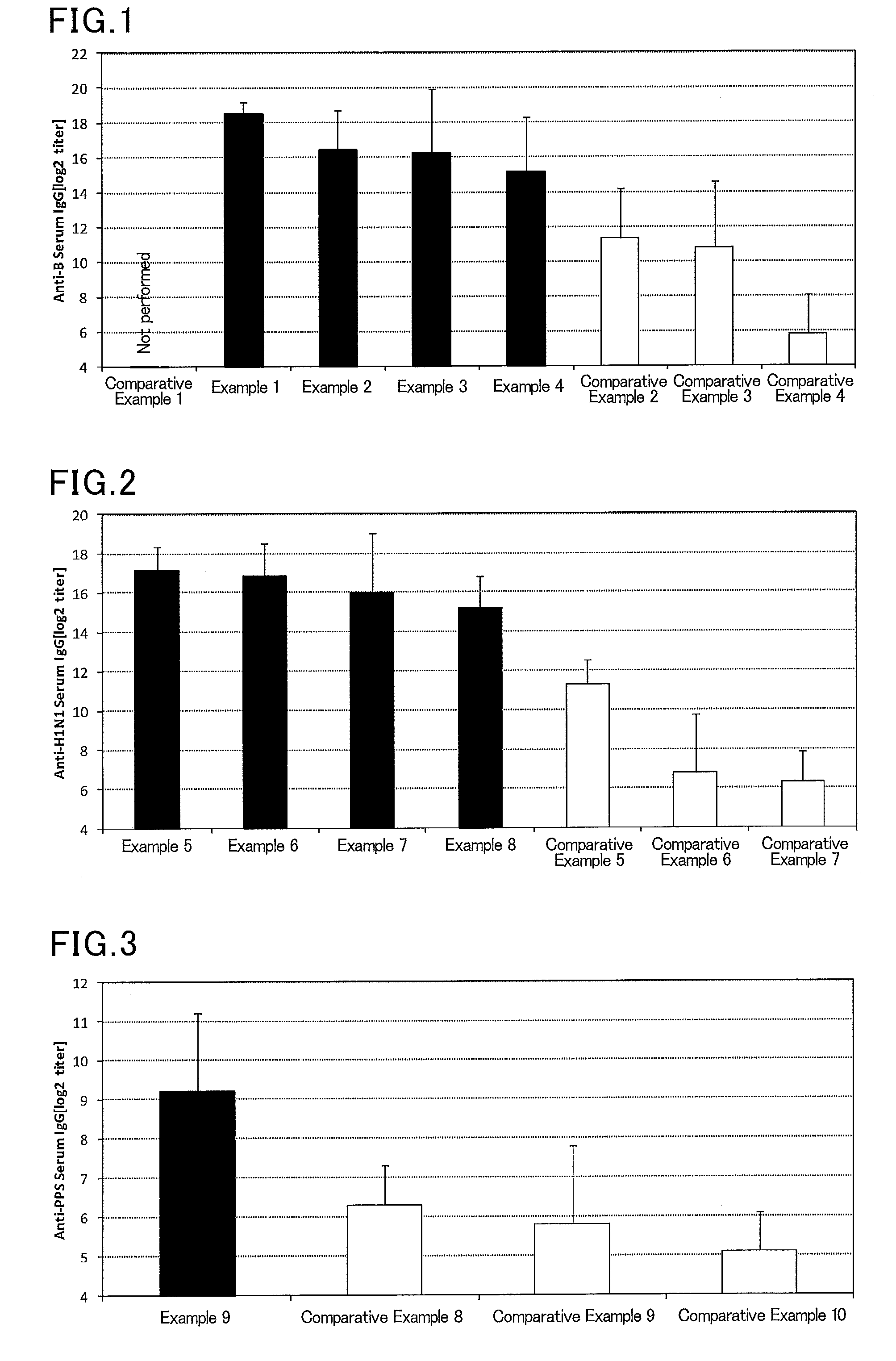
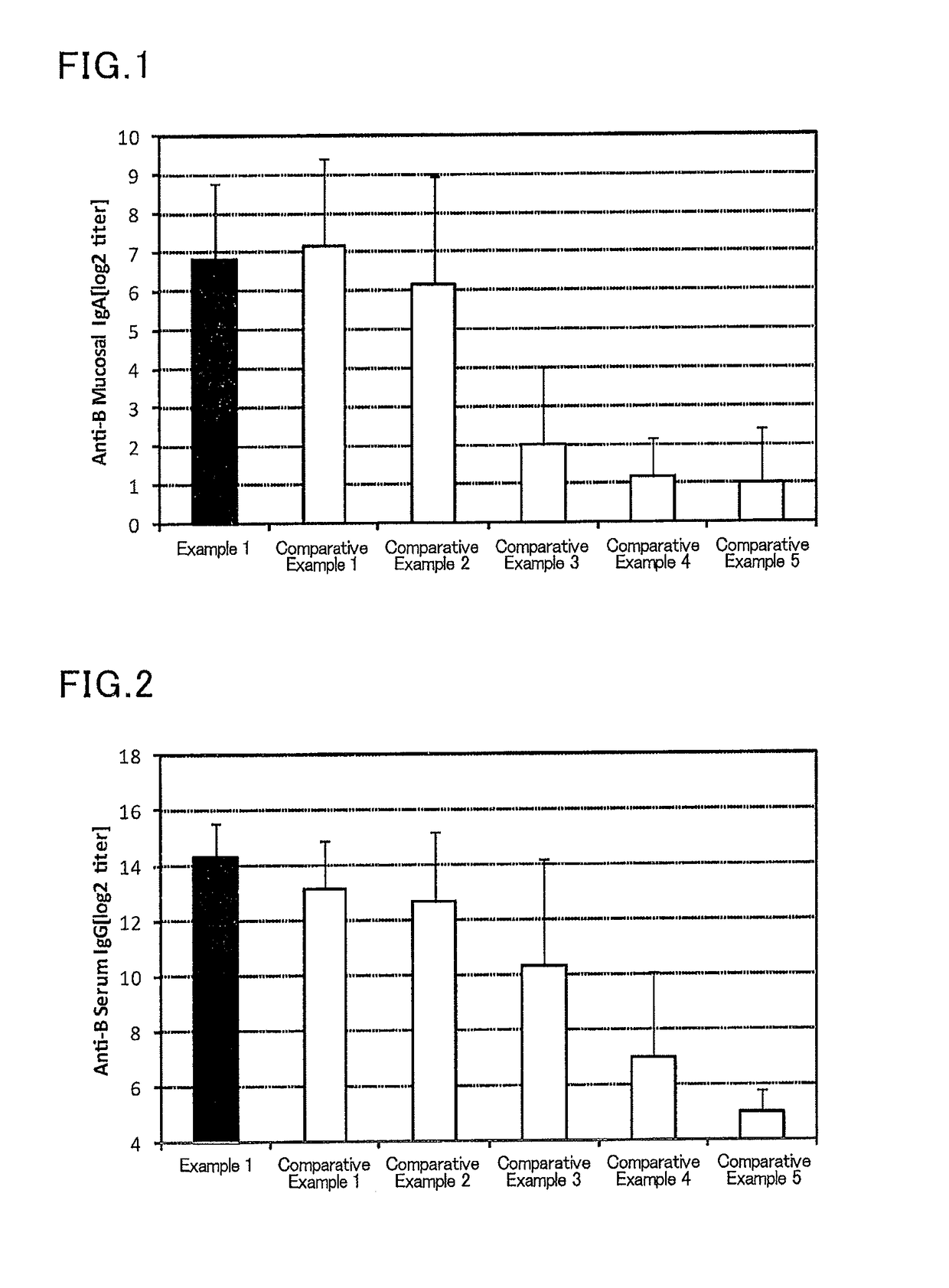
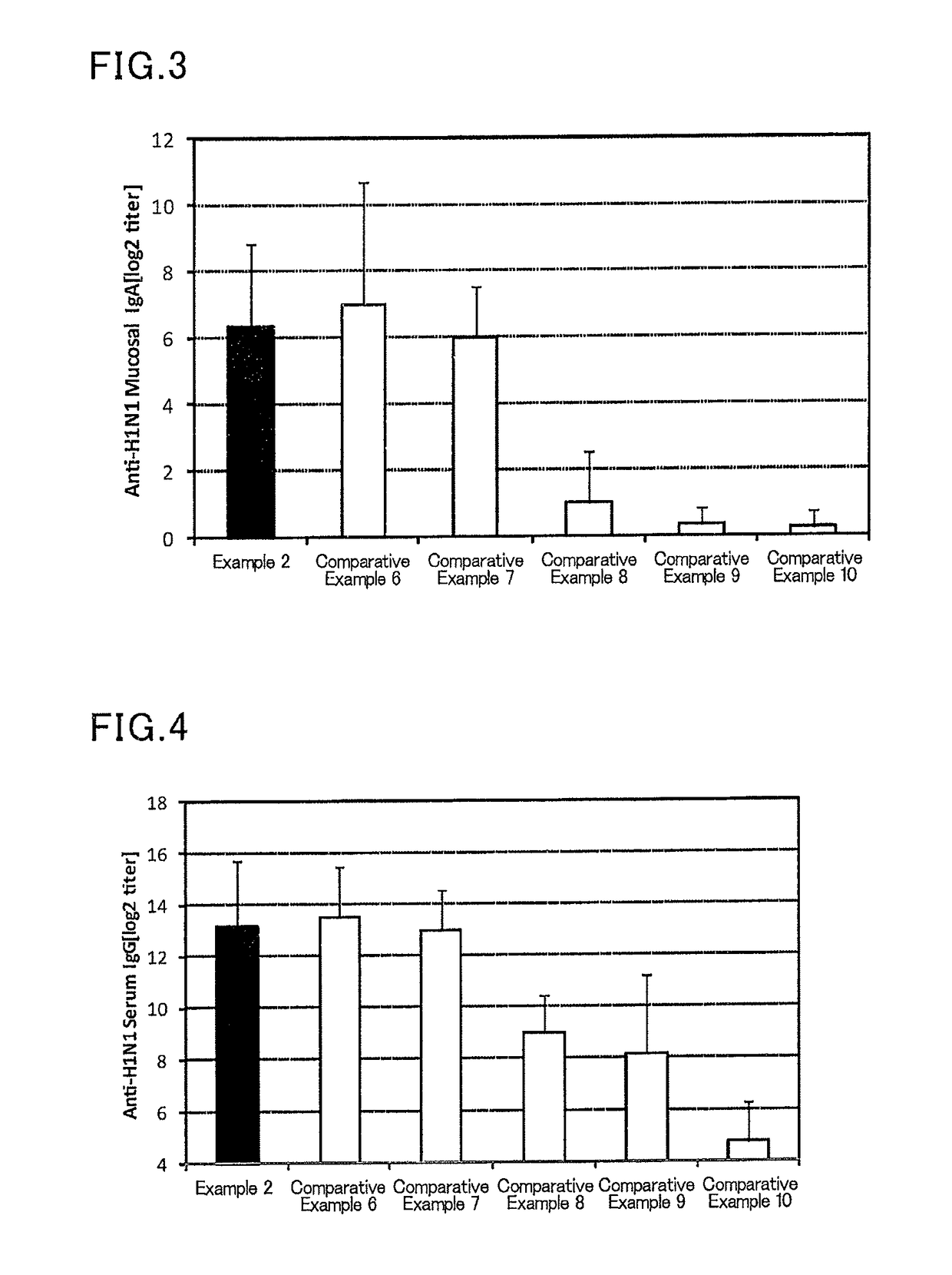
Mucosal vaccine composition
InactiveUS20160213773A1Safe and effectiveSsRNA viruses negative-senseBacterial antigen ingredientsMucosal Immune ResponsesXanthomonas
The present invention aims at providing a vaccine composition capable of being administered to an intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, or rectal mucous membrane, that is safe, useful as a prophylactic or therapeutic agent for infectious diseases or cancers, and capable of effectively inducing the systemic immune response and mucosal immune response. The present invention provides a mucosal vaccine composition to be administered to at least one mucous membrane selected from the group consisting of a human or animal intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, and rectal mucous membrane, the mucosal vaccine composition containing: at least one antigen; and as an adjuvant, a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof, wherein a mass ratio between the adjuvant and the antigen (total mass of the adjuvant / total mass of the antigen) is 0.002 to 500.
Owner:NITTO DENKO CORP
Marsh gas fermentation material and preparation method thereof
InactiveCN104232726ASolve unhandled problemsReduce startup timeMicroorganism based processesWaste based fuelStart timeSludge
The invention discloses a marsh gas fermentation material and a preparation method thereof, belonging to the technical field of a biological fermentation method for preparing marsh gas. The marsh gas fermentation material comprises a fermentation main material and a fermentation initiator, wherein the fermentation main material is prepared from Flammulina velutipes residue, Enteromorpha, corn straw, fish pond sludge, wheat straw, animal manure and wine stillage; the fermentation initiator is prepared from fish pond sludge, wheat straw, animal manure and strains; the strains comprise Bacteroides succinogenes, Butyrivibrio fibrisolvens, Bacillus subtilis, microzyme, sarcina methanica and Methanosaeta in a ratio of 1:5:3:3:2:2; and the weight ratio of the fermentation main material to the fermentation initiator is (15-19):1. The marsh gas fermentation material fully utilizes the wastes Flammulina velutipes residue and Enteromorpha, and can greatly shorten the start time and increase the gas production quantity and gas production speed in the marsh gas fermentation process.
Owner:INST OF AGRI RESOURCES & ENVIRONMENT SHANDONG ACADEMY OF AGRI SCI
Injectable vaccine composition
InactiveUS9962439B2Induce systemic immune responsePharmaceutical delivery mechanismAntiviralsBacteroidesAntigen
The present invention aims at providing an injectable vaccine composition that is safe, useful as a prophylactic or therapeutic agent for cancers or infectious diseases, and capable of inducing the systemic immune response safely and effectively. The present invention is an injectable vaccine composition to be administered by injection to a human being or an animal, containing: at least one antigen, and as an adjuvant, a lipopolysaccharide derived from at least one gram negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
Mucosal vaccine composition
ActiveUS20160193327A1Safe and effectiveSsRNA viruses negative-senseAntibacterial agentsMucosal Immune ResponsesXanthomonas
The present invention aims at providing a mucosal vaccine composition that can be administered to an intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, or rectal mucous membrane, that is useful as a prophylactic or therapeutic agent for infectious diseases or cancers, and is capable of safely and effectively inducing the systemic immune response and mucosal immune response. The present invention provides a mucosal vaccine composition to be administered to at least one mucous membrane selected from the group consisting of a human or animal intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, and rectal mucous membrane, containing: at least one antigen; and as an adjuvant, a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
Injectable vaccine composition
InactiveUS20160287697A1Effectively induce systemic immune responseInduce systemic immune responsePharmaceutical delivery mechanismAntiviralsAntigenBacteroides
The present invention aims at providing an injectable vaccine composition that is safe, useful as a prophylactic or therapeutic agent for cancers or infectious diseases, and capable of inducing the systemic immune response safely and effectively. The present invention is an injectable vaccine composition to be administered by injection to a human being or an animal, containing: at least one antigen, and as an adjuvant, a lipopolysaccharide derived from at least one gram negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
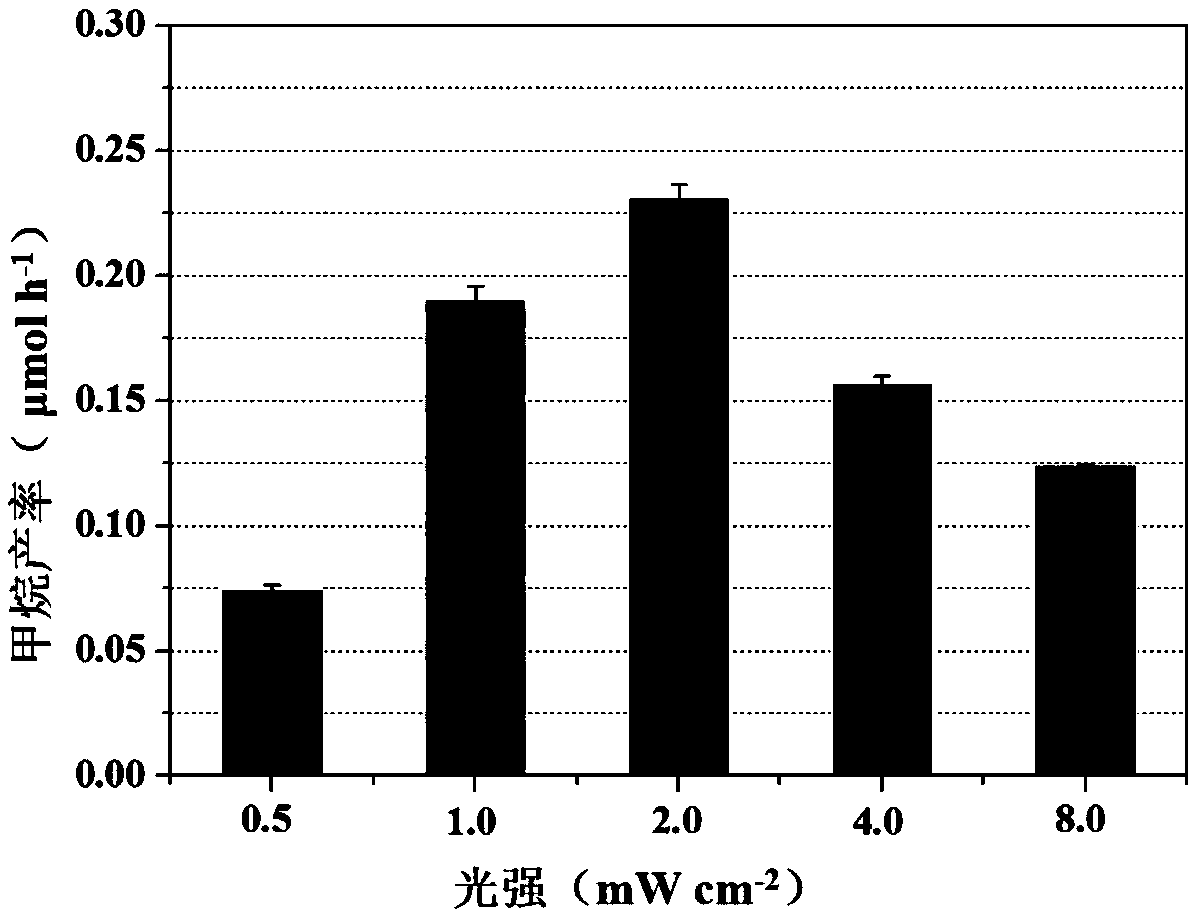
Method for reducing carbon dioxide to produce methane by optically driving methane octococcus
ActiveCN109666703AReduce processing costsHigh selectivityMicroorganism based processesWaste based fuelGenus MethanosarcinaLight energy
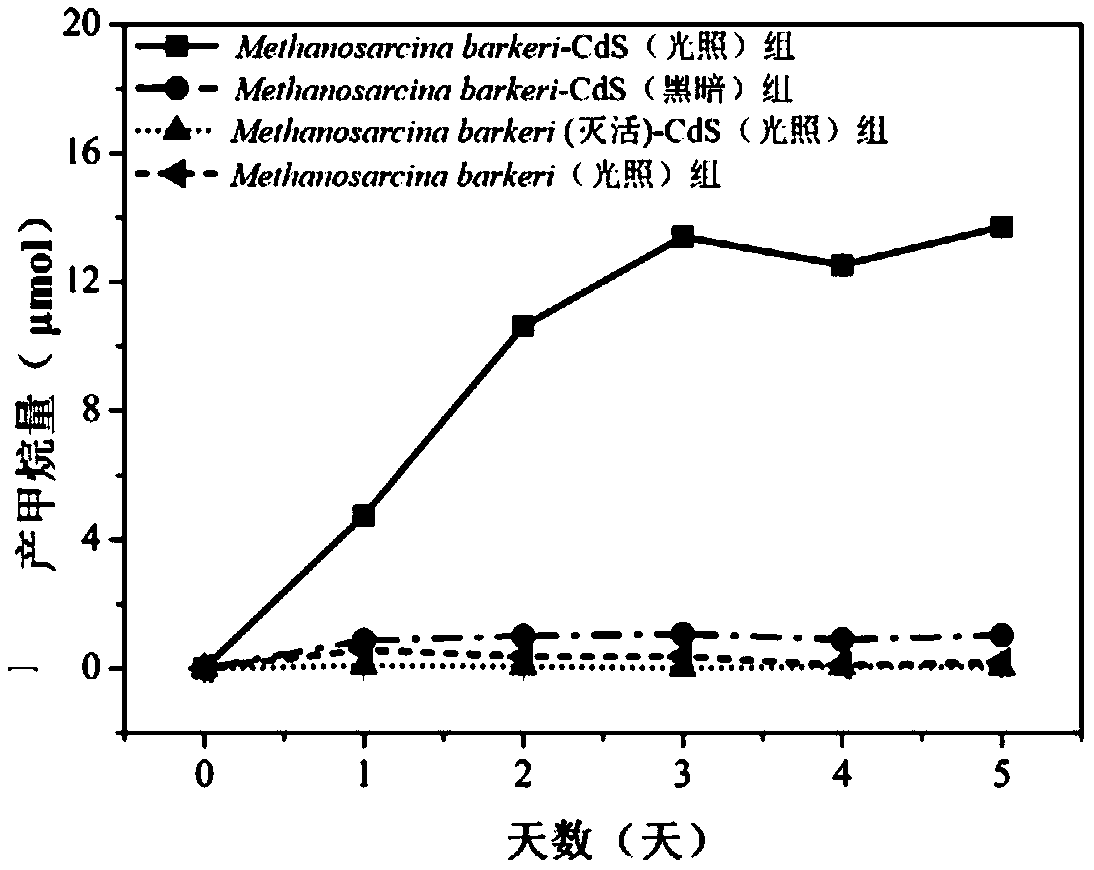
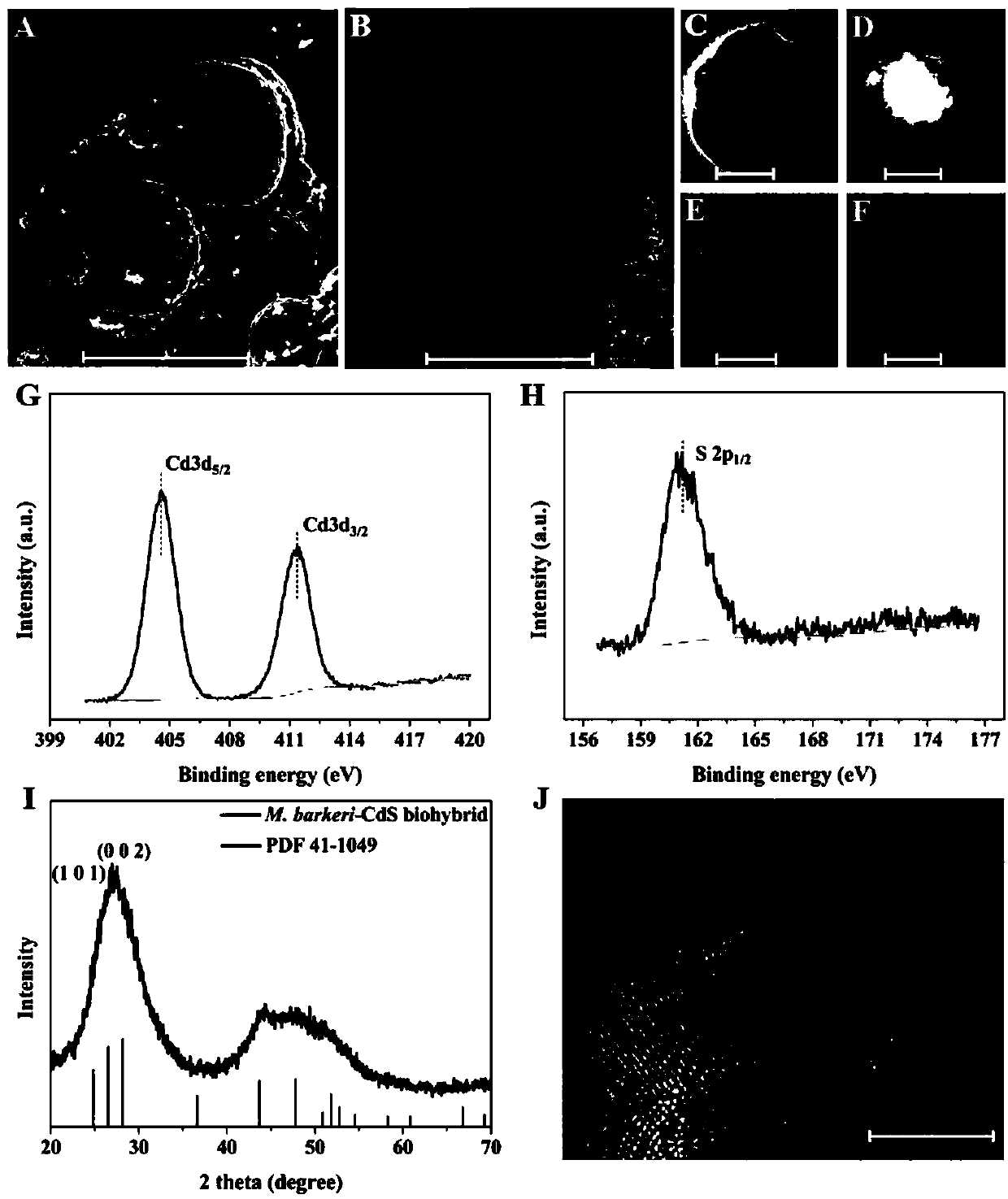
The invention discloses a method for reducing carbon dioxide to produce methane by light-driven methane octococcus, which comprises the following steps: a nano semiconductor is deposited on the surface of Methanosarcina, and CO2 is introduced; Methanosarcina reduces CO2 to produce methane by using photogenerated electrons under the condition of light. According to the method, selective CO2 reduction to generate methane can be achieved by taking rich and cheap light energy as a driving force, and the process cost is obviously reduced. The method overcomes the defects of complex process, large energy consumption, poor product selectivity and the like in the traditional CO2 reduction methane production process; the method has the advantages of simple process, low energy consumption and strongproduct selectivity, so that large-scale popularization and application can be carried out.
Owner:FUJIAN AGRI & FORESTRY UNIV
Anaerobic active sludge process
InactiveCN1887738AHigh removal rateImprove utilization rateTreatment with aerobic and anaerobic processesHigh concentrationSequencing batch reactor
The anaerobic sequencing batch active sludge process belongs to the field of environment protecting and waste water treating technology. The present invention features the two-stage serially contained anaerobic sequencing batch reactor (ASBR) for optimizing methane bacteria colony, the separate adsorption in the reactor A and biodegradation in the reactor B, and the application of the technological process for treating medium and high concentration insoluble organic waste water. Compared with traditional ASBR, the present invention has 10-30 % raised organic load, 10-20 % reduced power consumption and 10 % raised organic matter eliminating rate.
Owner:TAIYUAN UNIV OF TECH
Bactericide for generating methane and preparation technology thereof
InactiveCN101921707AQuick conversionWide range of temperature adaptationBacteriaMicroorganism based processesAgricultural scienceMass ratio
The invention relates to a microbial agent and a preparation technology thereof, belonging to the technical filed of agricultural production. In the preparation technology, Clostridium thermocellum, Clostridium beijerinckii, Methanocaldococcus jannaschii, Methanothermobacter wolfeii, Methanosarcina thermophila and Methanosarcina barkeri Schnellen are coordinated in mass ratio of 1-2:1-3:1:1:1:1-4, the total number of effective viable bacteria, the operation is carried out in an asepsis condition, and the product is kept is a dark place. To a house hold fermenting tank, a bactericide for generating methane is required to be added, the additive amount accounts for 0.1-0.2% of the feeding amount; to a semi-batch (semi-continuous) fermentation mode and a continuous fermentation mode, the additive amount accounts for 0.05-0.1% of the feeding amount. The bactericide for generating methane has wide adaptability to temperature, can generate methane continuously from 30 DEG C to 85 DEG C, and does not have strict requirements for organic matter materials. The technology can be used for household small-size methane tanks and large-scale industrial methane apparatuses to use.
Owner:刘秀丽
Biogas fermentation initiator and preparation method thereof
InactiveCN104152494AReduce startup timeIncrease gas productionMicroorganism based processesWaste based fuelGenus MethanosarcinaStart time
The invention discloses a biogas fermentation initiator and a preparation method thereof, and belongs to the technical field of biogas preparation through a biological fermentation method. The present problems of slow start, low gas yield efficiency, and no gas yield in the fermentation process are solved. The initiator is composed of the following raw materials: sludge in fishpond, wheat straw, animal manure, ammonium bicarbonate, and bacterium strains; wherein the bacterium strains are bacteroides succinoge-nes, butyrivibrio fibrisolvens, bacillus subtilis, saccharomycetes, methanosarcina, and methanosaeta. The biogas fermentation initiator is applied to biogas fermentation, can greatly shorten the start time (biogas can be produced after one day), and is capable of increasing the biogas yield and biogas generation speed.
Owner:INST OF AGRI RESOURCES & ENVIRONMENT SHANDONG ACADEMY OF AGRI SCI
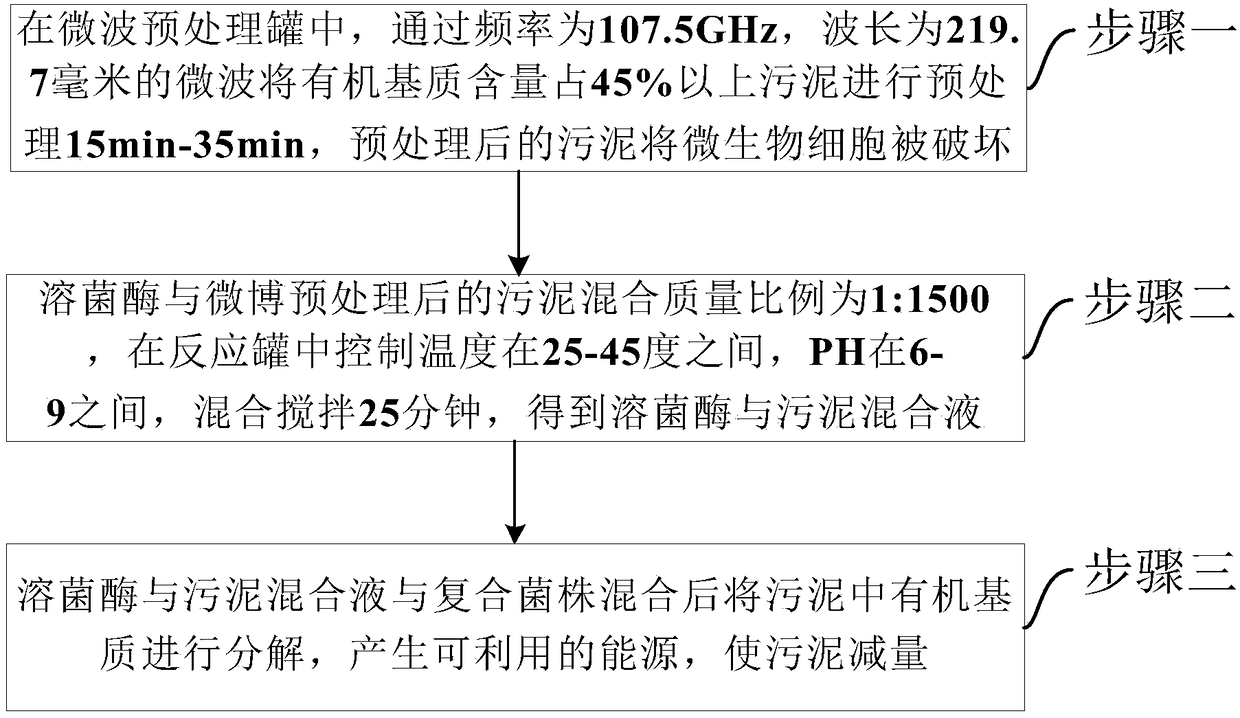
Sludge decrement method by combining microwaves, biological enzyme and microorganisms
ActiveCN108439744ASolve efficiency problemsSolve the investment costSludge treatmentSpecific water treatment objectivesPeptostreptococcusMethanosarcina barkeri
The invention provides a sludge decrement method by combining microwaves, biological enzyme and microorganisms. The method comprises the steps that sludge of which the content of an organic medium is45% or above is pretreated for 15-35 min in a microwave pretreatment tank; the mixing mass ratio of lysozyme to the sludge after microwave pretreatment is 1:1,500, and a mixed solution of lysozyme andthe sludge is obtained in a reaction tank; after the mixed solution of lysozyme and the sludge is mixed with composite bacterial strains, the organic medium in the sludge is decomposed, available energy is generated, the sludge amount is decreased, and the composite bacterial strains are peptostreptococcus, veillonella, propionibacteria, fire source methanococcus lactobacillus, methanosarcina barkeri, extreme thermophilic archaea and anaerobic ammonium oxidation bacteria. According to the sludge decrement method, the microwaves, special-effect enzyme and microorganisms are adopted for completely decreasing the amount of organic medium sludge generated by a biochemical system, and the sludge is converted into the available energy through a technology; the method has the ideal decrement effect on biochemical sludge generated in the process of treating municipal wastewater and industrial wastewater.
Owner:北京赛富威环境工程技术有限公司
Nasal mucosal vaccine composition
InactiveUS10071155B2Safely and effectively induce humoral immunitySsRNA viruses negative-senseAntibacterial agentsMucosal Immune ResponsesInfectious illness
The present invention provides a nasal mucosal vaccine composition which is safe, useful as a preventive or therapeutic agent for infectious diseases or cancers, and capable of inducing systemic immune responses and mucosal immune responses effectively. The present invention provides a nasal mucosal vaccine composition to be administered to a human or animal nasal mucous membrane, the nasal mucosal vaccine composition containing at least one antigen excluding antigens derived from influenza viruses; and as an adjuvant, a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
Allergy vaccine composition
ActiveUS20180147235A1Induce immune toleranceImprove the quality of lifeOrganic active ingredientsBacterial antigen ingredientsBacteroidesAcidocella
The present invention provides an allergy vaccine composition that is useful as a prophylactic or therapeutic agent for an allergic disease and can safely and effectively induce immune tolerance. The vaccine composition is to be administered to a human or animal with an allergic disease for the prevention or treatment of the allergic disease. The vaccine composition contains: an immunomodulator which is a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt of the lipopolysaccharide; and at least one allergen.
Owner:NITTO DENKO CORP
Injectable vaccine composition
InactiveCN105530953AInduce systemic immune responsePharmaceutical delivery mechanismAntiviralsAntigenEnterobacter
The purpose of the present invention is to provide an injectable vaccine composition that is safe and useful as a prophylactic or therapeutic agent for cancer or infectious diseases and can safely and effectively induce a systemic immune response. An injectable vaccine composition that is to be administered by injection to human or animals, characterized by comprising at least one kind of antigen and a lipopolysaccharide or a salt thereof as an immunostimulator, said lipopolysaccharide being derived from at least one kind of gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas and Enterobacter.
Owner:NITTO DENKO CORP
Mucosal vaccine composition
InactiveCN105555308AImmune inductionSsRNA viruses negative-senseSsRNA viruses positive-senseMucosal Immune ResponsesGenus Serratia
The purpose of the present invention is to provide a vaccine composition which is safe and effective as a preventive medicine or a treatment medicine for infections and / or cancer, can effectively induce a systemic immune response and a mucosal immune response, and which is capable of being administered to the oral mucosa, ocular mucosa, ear mucosa, genital mucosa, pharyngeal mucosa, respiratory mucosa, bronchial mucosa, pulmonary mucosa, gastric mucosa, intestinal mucosa, or rectal mucosa. The present invention is a mucosal vaccine composition administered to at least one type of mucous membrane of a person or animal, the mucous membrane being selected from a group comprising the oral mucosa, ocular mucosa, ear mucosa, genital mucosa, pharyngeal mucosa, respiratory mucosa, bronchial mucosa, pulmonary mucosa, gastric mucosa, intestinal mucosa, and rectal mucosa. The mucosal vaccine composition is characterized by containing at least one type of antigen, and, as an immunostimulant agent, a lipopolysaccharide or a salt thereof derived from at least one type of a gram-negative bacterium selected from a group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter. Moreover, the mucosal vaccine composition is characterized in that the mass ratio of the abovementioned immunostimulant agent and antigen (the total mass of the immunostimulant agent / the total mass of the antigen) is between 0.002 and 500.
Owner:NITTO DENKO CORP
Nasal mucosal vaccine composition
InactiveCN105530954AAntibacterial agentsSsRNA viruses negative-senseMucosal Immune ResponsesImmune activation
The objective of the present invention is to provide a nasal mucosal vaccine composition that is safe, useful as a prevention or treatment agent for cancer or infectious disease, and can effectively induce a systemic immune response and a mucosal immune response. The nasal mucosal vaccine composition, which is administered to the nasal mucosa of humans or animals, is characterized by containing at least one type of antigen (but excluding influenza virus-derived antigens) and, as an immune activation agent, a lipopolysaccharide derived from at least one gram-negative bacteria selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
Microbial agent for reducing sludge, carrier thereof and preparation method for carrier thereof
The invention discloses a microbial agent for reducing sludge. The microbial agent comprises the following components in parts by weight: 8-10 parts of clostridium acetobutylicum, 12-16 parts of bacillus licheniformis, 16-20 parts of bacillus subtilis, 5-7 parts of lactobacillus acidophilus, 5-7 parts of methanosarcina, 3-5 parts of methanosaeta, 6-8 parts of rhodopseudomonas palustris, 4-6 partsof cellulomonas flavigena, 8-10 parts of streptococcus thermophilus, 8-10 parts of alcaligenes faecalis, 3-5 parts of bacillus amyloliquefaciens, 2-4 parts of bacillus nitrosomonas, 1-3 parts of issatchenkia orientalis and 3-5 parts of streptomyces oxalis. The invention also discloses a carrier of the microbial agent for reducing sludge and a preparation method thereof. The carrier is capable of effectively attaching microorganisms and endowing microorganisms with higher activity. Strains are reasonably prepared in the microbial agent disclosed by the invention; the microbial agent is attachedto the carrier for treating sewage; when the method is used for treating sewage, the operation is simple, the treatment effect is excellent and sludge deposition is less; the method is worthy of popularization.
Owner:ANJIEYU BEIJING OILFIELD TECHNICAL SERVICES CO LTD
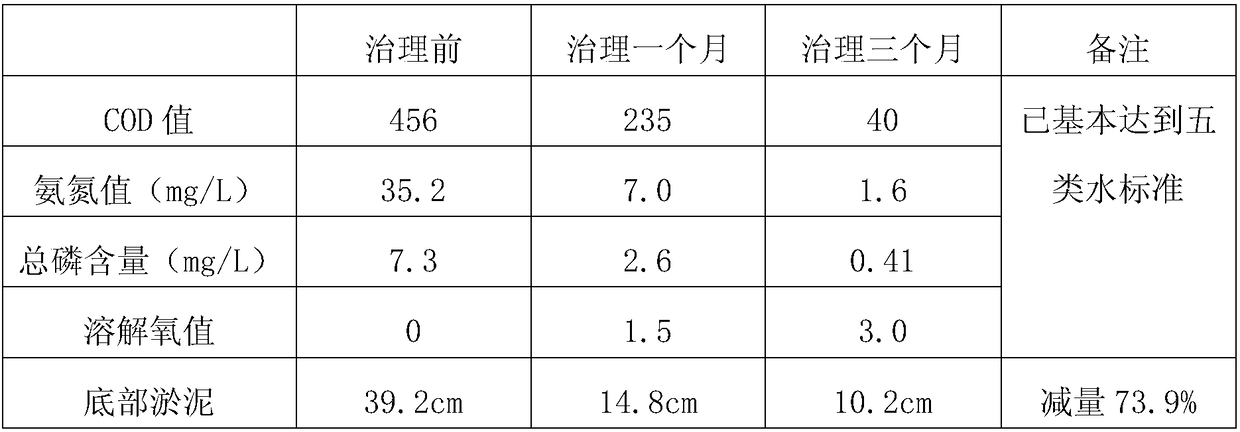
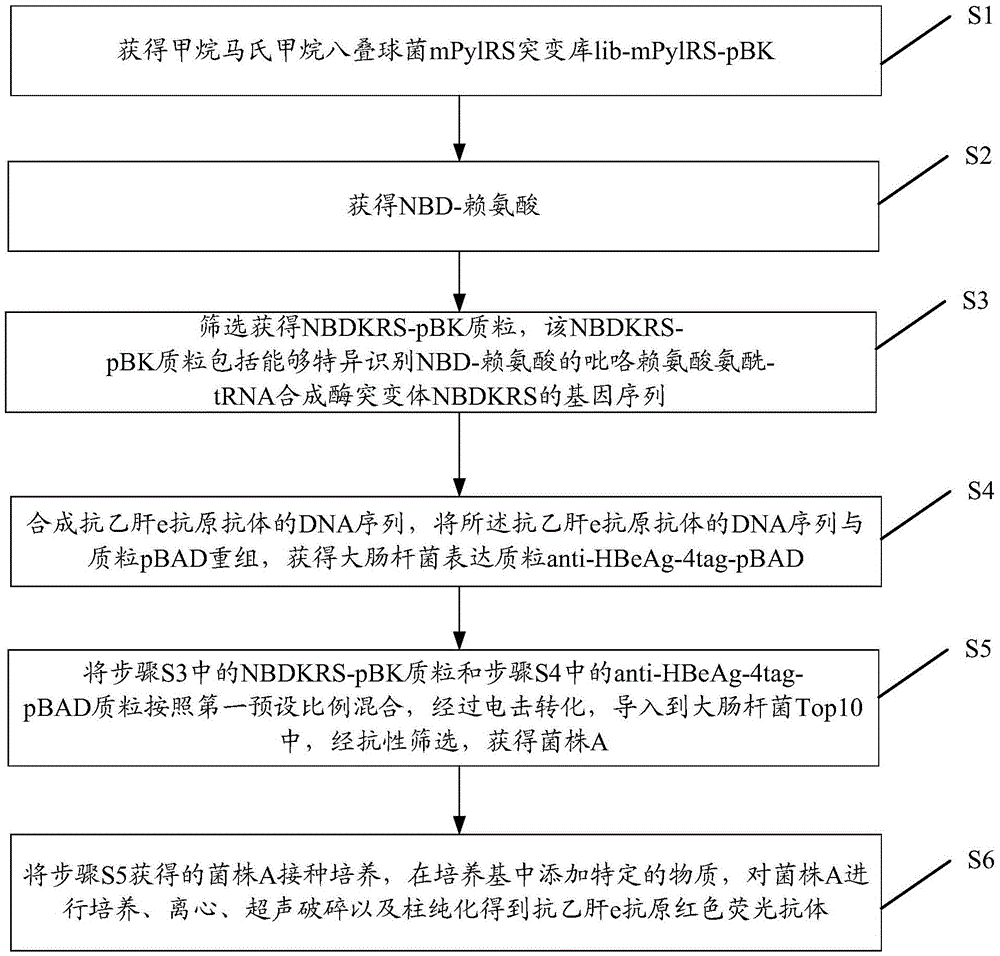
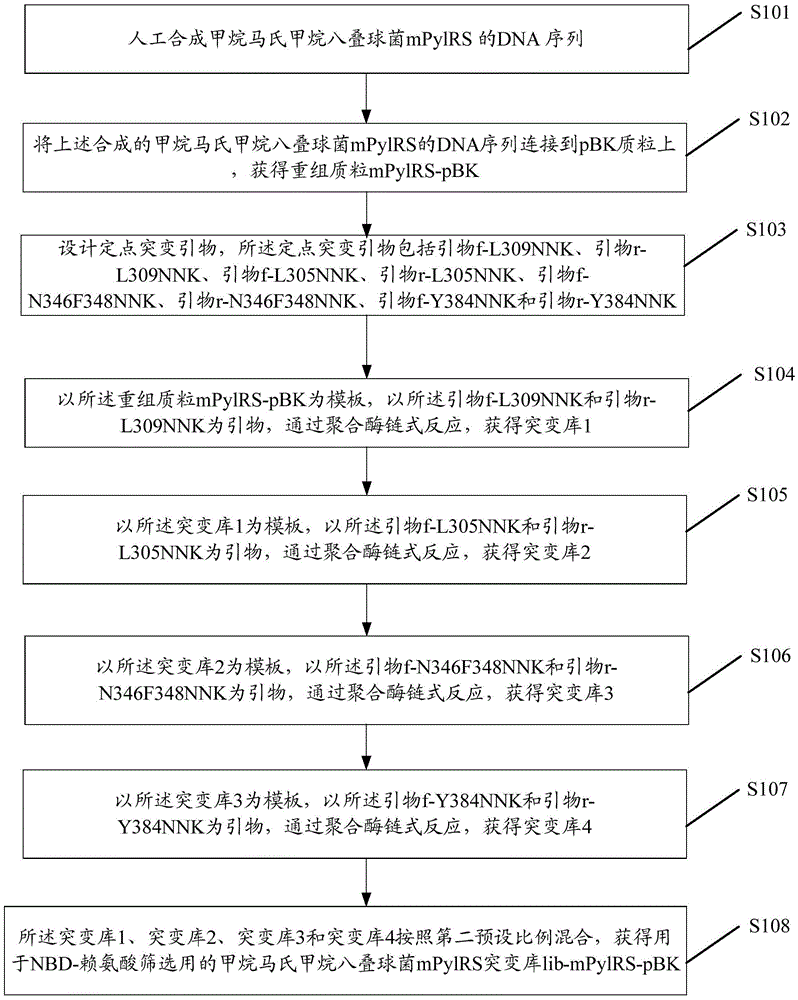
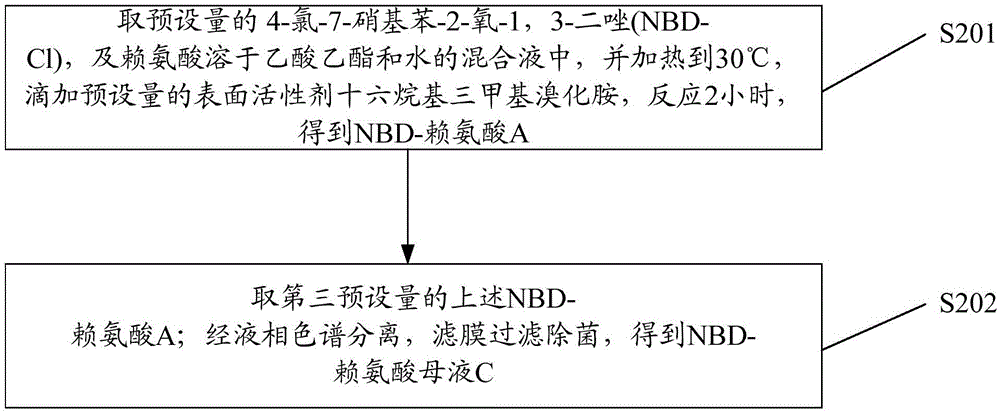
Preparation method of red fluorescent antibody resisting HBeAg (hepatitis B virus e antigen)
InactiveCN105647918AAchieve preparationHigh sensitivityImmunoglobulins against virusesPeptide preparation methodsAntigenFluorescence
The invention discloses a preparation method of a red fluorescent antibody resisting HBeAg (hepatitis B virus e antigen). Genes of an mPylRS (pyrrolysyl-tRNA synthetase) of a synthesized methanosarcina mazei species and a plasmid pBK are recombined, and mPylRS-pBK is obtained; a mutant library lib-mPyl1RS-pBK is established with mPylRS-pBK as a template; lib-mPylRS-pBK is subjected to electroporation and then screened; NBDKRS-pBK plasmids are screened and recombined; DNA (deoxyribonucleic acid) resisting the HBeAg antibody is recombined with pBAD, and anti-HBeAg-4tag-pBAD is obtained; the plasmidsanti-HBeAg-4tag-pBAD and NBDKRS-pBK are transformed in a competent state, and a biological expression system for preparing the red fluorescent antibody resisting HBeAg is obtained. With the adoption of the preparation method, the red fluorescent antibody resisting HBeAg can be prepared, and the detection sensitivity is improved.
Owner:SHENZHEN SHENGBIZHI TECH DEV CO LTD
Vaccine pharmaceutical composition for transdermal administration
ActiveUS20170216431A1Improve complianceImprove the quality of lifeBacterial antigen ingredientsViral antigen ingredientsAntigenAcidocella
The present invention provides a vaccine pharmaceutical composition for transdermal administration which is safe, usable as a prophylactic or therapeutic agent for cancer or infectious diseases, and capable of safely and effectively inducing a systemic immune response. It can be administered to a human or animal skin, the vaccine pharmaceutical composition including: a lipopolysaccharide or its salt derived from at least one gram-negative bacterium such as Serratia, Lelercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, as an immunostimulant; and at least one antigen, the vaccine pharmaceutical composition having a mass ratio between the immunostimulant and the antigen, the total mass of the immunostimulant / the total mass of the antigen, of 0.002 to 500.
Owner:NITTO DENKO CORP
Mucosal vaccine composition
ActiveUS10092642B2Safe and effectiveAntibacterial agentsSsRNA viruses negative-senseMucosal Immune ResponsesSex organ
The present invention aims at providing a mucosal vaccine composition that can be administered to an intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, or rectal mucous membrane, that is useful as a prophylactic or therapeutic agent for infectious diseases or cancers, and is capable of safely and effectively inducing the systemic immune response and mucosal immune response. The present invention provides a mucosal vaccine composition to be administered to at least one mucous membrane selected from the group consisting of a human or animal intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, and rectal mucous membrane, containing: at least one antigen; and as an adjuvant, a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
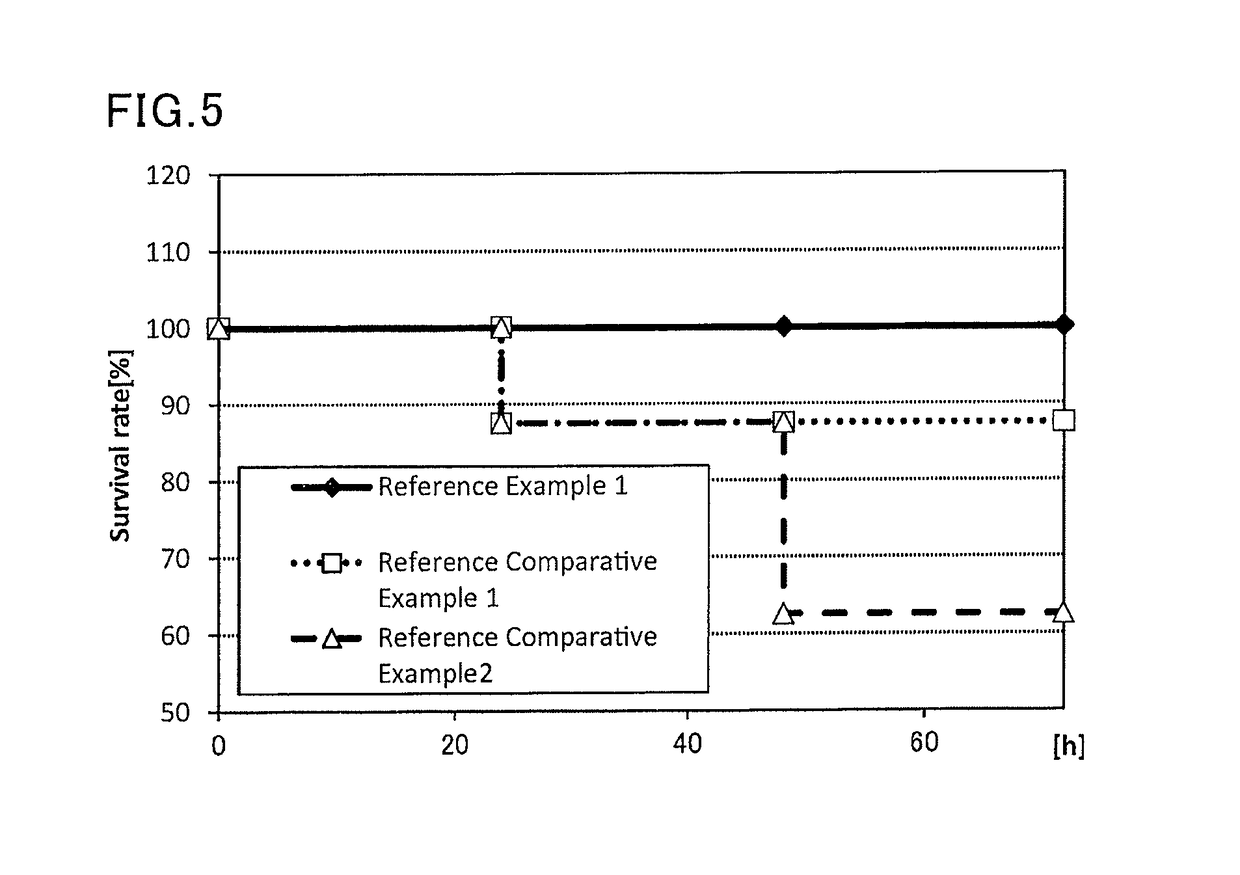
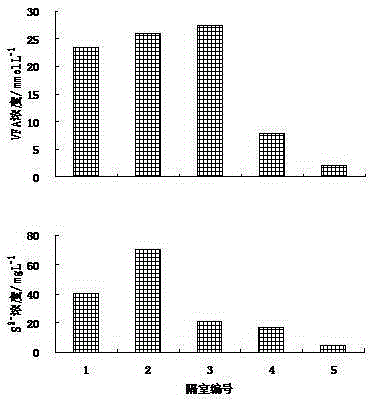
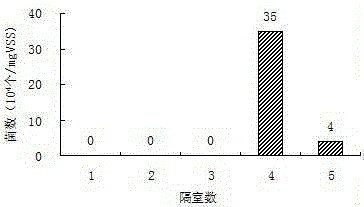
Method for Enrichment of Methanosarcina and Methanothrix in Treatment of Sulphate Organic Wastewater
ActiveCN104150591BWaste based fuelTreatment with anaerobic digestion processesGenus MethanosarcinaSulfate
The invention discloses a method for enriching methanosarcina and methanothrix during sulfate organic wastewater treatment. According to the method, anaerobic reactors of at least four compartments connected in series, the starting mode of a sulfate organic wastewater anaerobic treatment system and a running control mode of a sulfate organic wastewater anaerobic treatment system are adopted. The method can be used for solving the difficulty that the growth and activity of methanosarcina and methanothrix during the process of sulfate organic wastewater anaerobic treatment are inhibited, realizing the separation of a sulfate reducing phase from a methane producing phase and the separation of methanosarcina from methanothrix and achieving the purpose of directionally enriching the methanosarcina and methanothrix.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Anaerobic active sludge process
InactiveCN100398463CHigh removal rateImprove utilization rateTreatment with aerobic and anaerobic processesSequencing batch reactorHigh concentration
Owner:TAIYUAN UNIV OF TECH
A bacterial agent for rapid start-up and efficient operation of anaerobic digestion of food waste
ActiveCN104762237BReduce startup timeAnaerobic digestion is efficientBacteriaMicroorganism based processesGenus ThermusMicrobial agent
The invention provides a microbial agent for quick start and efficient operation of anaerobic digestion of kitchen waste. The microbial agent comprises the following strains: bright yellow clostridium DSM19732, saccharophilous sewage bacillus DSM22681, proteolysis feces thermus DSM5265, thermophilic anaerolineae DSM14523, fast-growing thermobrachium DSM8682, riparian high temperature bacillus DSM21630, syntrophomonas DSM4212, methanothermobacter thermautotrophicus DSM1053, thermophilic methanosarcina DSM1825, Luminy Marseille methanococcus DSM25720 and methanobacterium formicium DSM3637. By using the microbial agent as inoculum, the quick start and efficient operation of anaerobic digestion of kitchen waste can be realized.
Owner:BIOGAS SCI RES INST MIN OF AGRI
Mucosal vaccine composition
InactiveCN105530958AImmune inductionSsRNA viruses negative-senseAntibacterial agentsMucosal Immune ResponsesGenus Serratia
The purpose of the present invention is to provide a mucosal vaccine composition that is effective and safe as a prophylactic or therapeutic drug for infections and cancer, which effectively induces a systemic immune response and mucosal immune response, and which can be administered to the oral mucosa, ocular mucosa, ear mucosa, genital mucosa, pharyngeal mucosa, respiratory mucosa, bronchial mucosa, pulmonary mucosa, gastric mucosa, intestinal mucosa, or rectal mucosa. The present invention is a mucosal vaccine composition administered to at least one mucous membrane of a person or animal, said mucous membrane being selected from the group consisting of oral mucosa, ocular mucosa, ear mucosa, genital mucosa, pharyngeal mucosa, respiratory mucosa, bronchial mucosa, pulmonary mucosa, gastric mucosa, intestinal mucosa, and rectal mucosa. The mucosal vaccine composition is characterized by including at least one type of antigen, and, as an immunostimulant, a lipopolysaccharide or a salt thereof derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter.
Owner:NITTO DENKO CORP
Culture medium for methanosarcina
InactiveCN106867925AIncrease proliferation rateImprove training effectBacteriaMicroorganism based processesGenus MethanosarcinaActinidia
Owner:天津植草园生物科技有限公司
Vaccine pharmaceutical composition for transdermal administration
InactiveUS10420837B2Improve the quality of lifeImprove complianceBacterial antigen ingredientsViral antigen ingredientsAntigenInfectious illness
The present invention provides a vaccine pharmaceutical composition for transdermal administration which is safe, usable as a prophylactic or therapeutic agent for cancer or infectious diseases, and capable of safely and effectively inducing a systemic immune response. It can be administered to a human or animal skin, the vaccine pharmaceutical composition including: a lipopolysaccharide or its salt, derived from at least one gram-negative bacterium such as Serratia, Lelercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, as an immunostimulant; and at least one antigen, the vaccine pharmaceutical composition having a mass ratio between the immunostimulant and the antigen, the total mass of the immunostimulant / the total mass of the antigen, of 0.002 to 500.
Owner:NITTO DENKO CORP
A kind of biogas fermentation initiator and preparation method thereof
InactiveCN104152494BReduce startup timeIncrease gas productionMicroorganism based processesWaste based fuelStart timeSludge
The invention discloses a biogas fermentation initiator and a preparation method thereof, and belongs to the technical field of biogas preparation through a biological fermentation method. The present problems of slow start, low gas yield efficiency, and no gas yield in the fermentation process are solved. The initiator is composed of the following raw materials: sludge in fishpond, wheat straw, animal manure, ammonium bicarbonate, and bacterium strains; wherein the bacterium strains are bacteroides succinoge-nes, butyrivibrio fibrisolvens, bacillus subtilis, saccharomycetes, methanosarcina, and methanosaeta. The biogas fermentation initiator is applied to biogas fermentation, can greatly shorten the start time (biogas can be produced after one day), and is capable of increasing the biogas yield and biogas generation speed.
Owner:INST OF AGRI RESOURCES & ENVIRONMENT SHANDONG ACADEMY OF AGRI SCI
Probiotic honey grapefruit tea and preparation method thereof
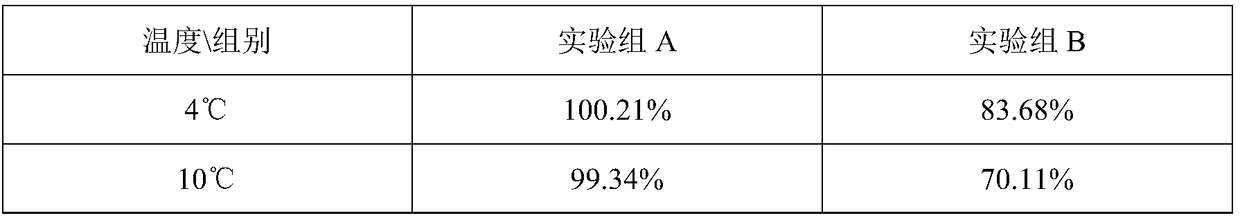
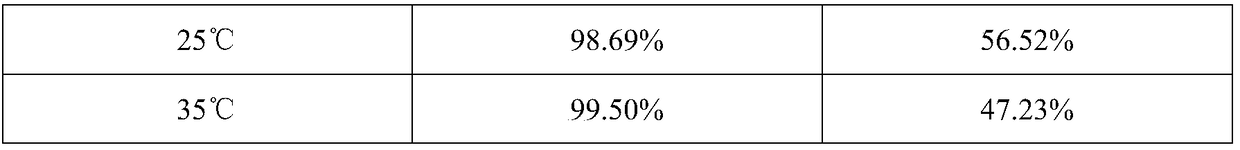
InactiveCN108887437AGuaranteed accumulationDoes not affect flavorTea substituesMethanosarcina barkeriStreptococcus fecalis
The invention provides a probiotic honey grapefruit tea and a preparation method thereof. In the technical scheme, lactobacillus casei and streptococcus faecium are taken as probiotic components and are blended with a conventional beverage, so that an intestinal tract probiotic effect is achieved. On such basis, the invention researches an approach for inhibiting growth of two probiotics, in orderto prevent the lactobacillus casei and streptococcus faecium from propagating in the beverage and influencing the flavor of the beverage. An experiment proves that an antagonism effect on propagationefficiency can be achieved when two probiotics are co-cultured with pyrococcus furiosus and methanosarcina barkeri; on such basis, an intergrowth environment and a nutritional condition of four microorganisms are researched; a treating method according to the invention can guarantee the biomass accumulation in early stage and can achieve the balance of flora scale of four microorganisms after mixed cultivation; the blending of the beverage and the mixed bacteria acquired according to the method is capable of keeping bacteria propagation basically stagnated under storage and transportation environments, so that the flavor of the beverage is prevented from being influenced.
Owner:江西韩金实业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com