Preparation method of red fluorescent antibody resisting HBeAg (hepatitis B virus e antigen)
A technology of red fluorescence and antigen antibody, which is applied in the field of preparation of red fluorescent antibody, can solve problems such as low sensitivity, misjudgment of hepatitis B e antigen detection, and non-detection of hepatitis B e antigen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
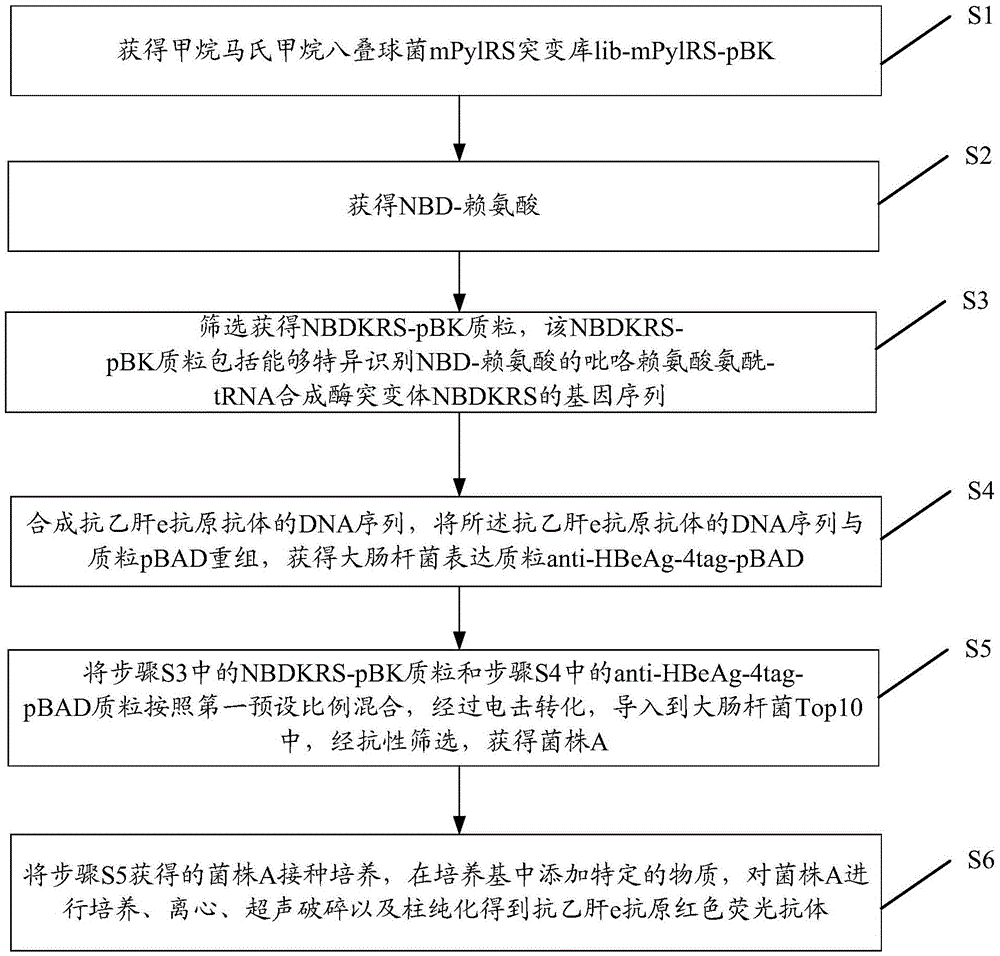
[0047] like figure 1 as shown, figure 1 It is a schematic flowchart of the preparation method of the red fluorescent antibody against hepatitis B e antigen of the present invention. The preparation method of the red fluorescent antibody of anti-hepatitis B e antigen provided by the invention comprises the steps:
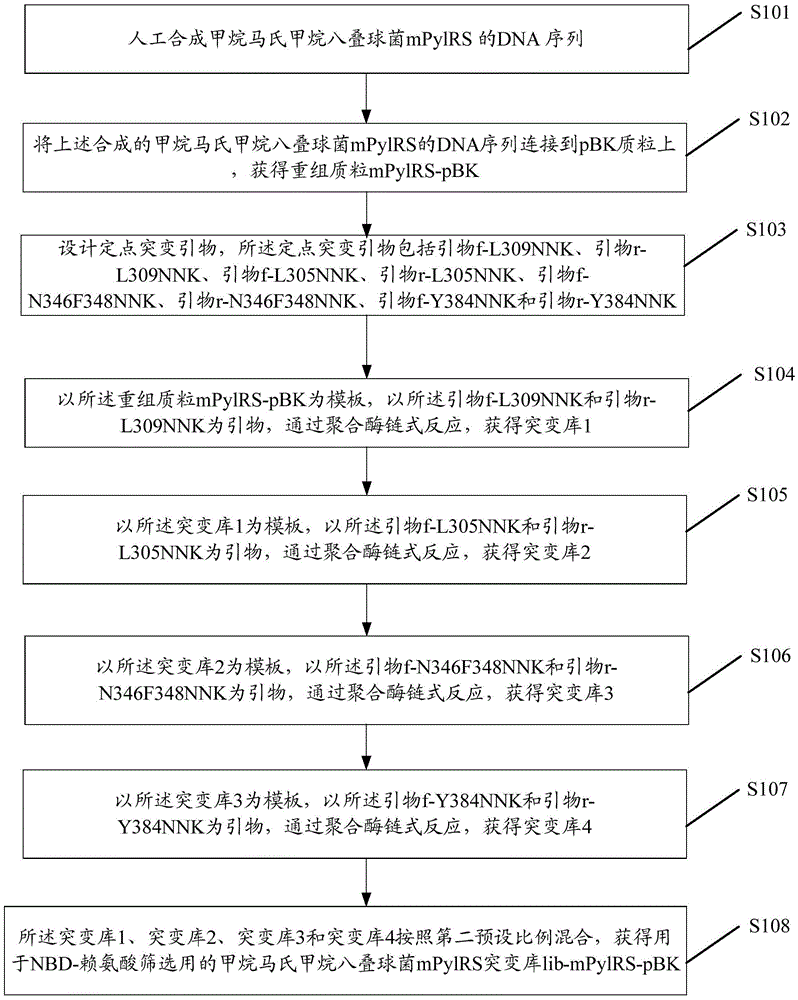
[0048]S1: Obtain the mPylRS mutant library lib-mPylRS-pBK of Methanosarcina mazei;
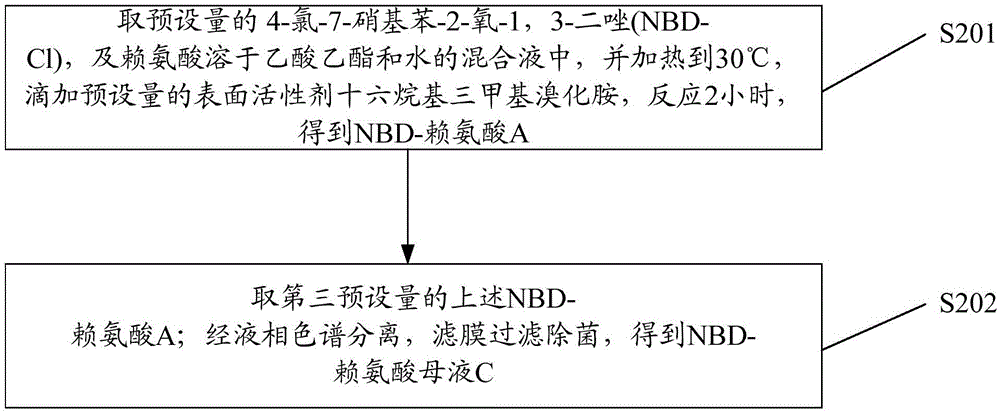
[0049] S2: obtaining NBD-lysine;
[0050] S3: screening to obtain the NBDKRS-pBK plasmid, the NBDKRS-pBK plasmid includes the gene sequence of the pyrrolysine aminoacyl-tRNA synthetase mutant NBDKRS capable of specifically recognizing NBD-lysine;
[0051] S4: Synthesizing the DNA sequence of the anti-hepatitis B e antigen antibody, recombining the DNA sequence of the anti hepatitis B e antigen antibody with the plasmid pBAD to obtain the Escherichia coli expression plasmid anti-HBeAg-4tag-pBAD;
[0052] S5: Mix the NBDKRS-pBK plasmid in step S3 and the anti-HBeAg-4tag-pBAD plasmi...
example 1
[0091] The sensitivity of the fluorescent antibody prepared by the present invention is tested by using low-concentration hepatitis B e antigen as a sample, and the specific steps are as follows.
[0092] S120: Take hepatitis B e antigen, dilute hepatitis B e antigen with phosphate buffered solution (PBS), and prepare the concentration as 0ng / mL, 0.2ng / mL, 0.5ng / mL, 1ng / mL, 1.5ng / mL, 2ng / mL mL, 2.5ng / mL, 10ng / mL, 15ng / mL, 20ng / mL10 gradients of hepatitis B e antigen solution for use.
[0093] S130: Add 10 μL of the anti-hepatitis B e antigen fluorescent antibody and 90 μL of phosphate buffer solution to 11 wells of the microtiter plate, and add 10 μL of non-fluorescence-labeled hepatitis B e antigen fluorescent antibody and phosphate buffer solution to the 11th well. Solution 90μL, respectively named as 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and incubated at room temperature for 12h.
[0094] S140: Discard the liquid in the microplate in S130, re-add 100 μL of phosphate buffer to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com