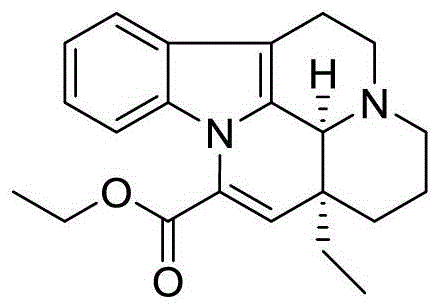
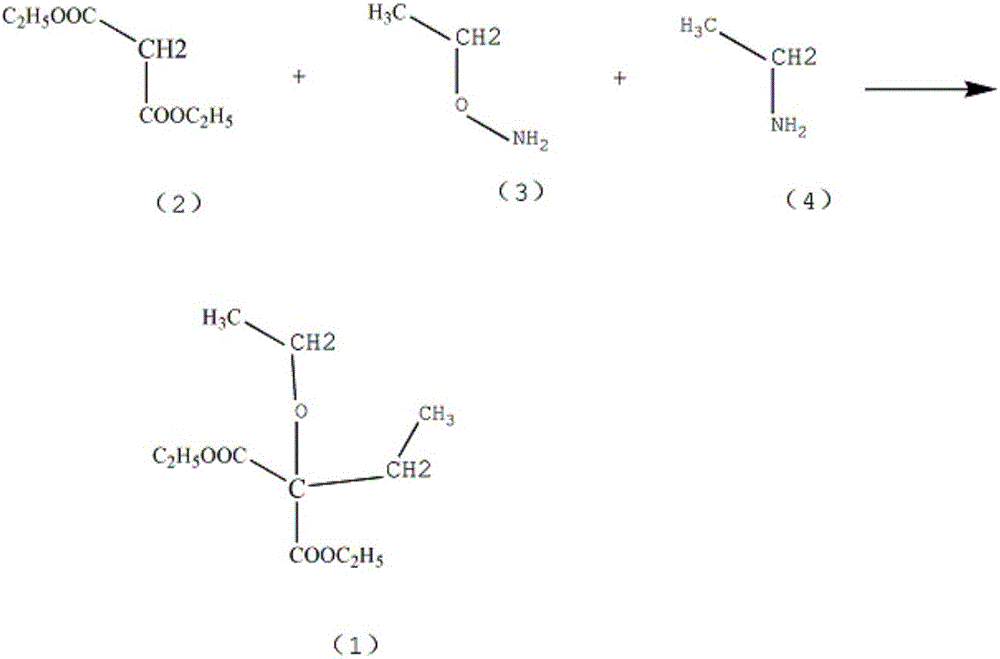
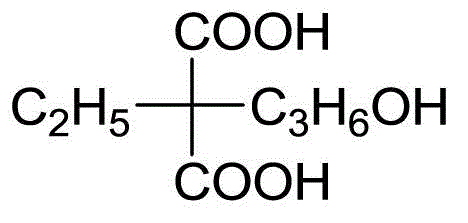Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Ethylmalonic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
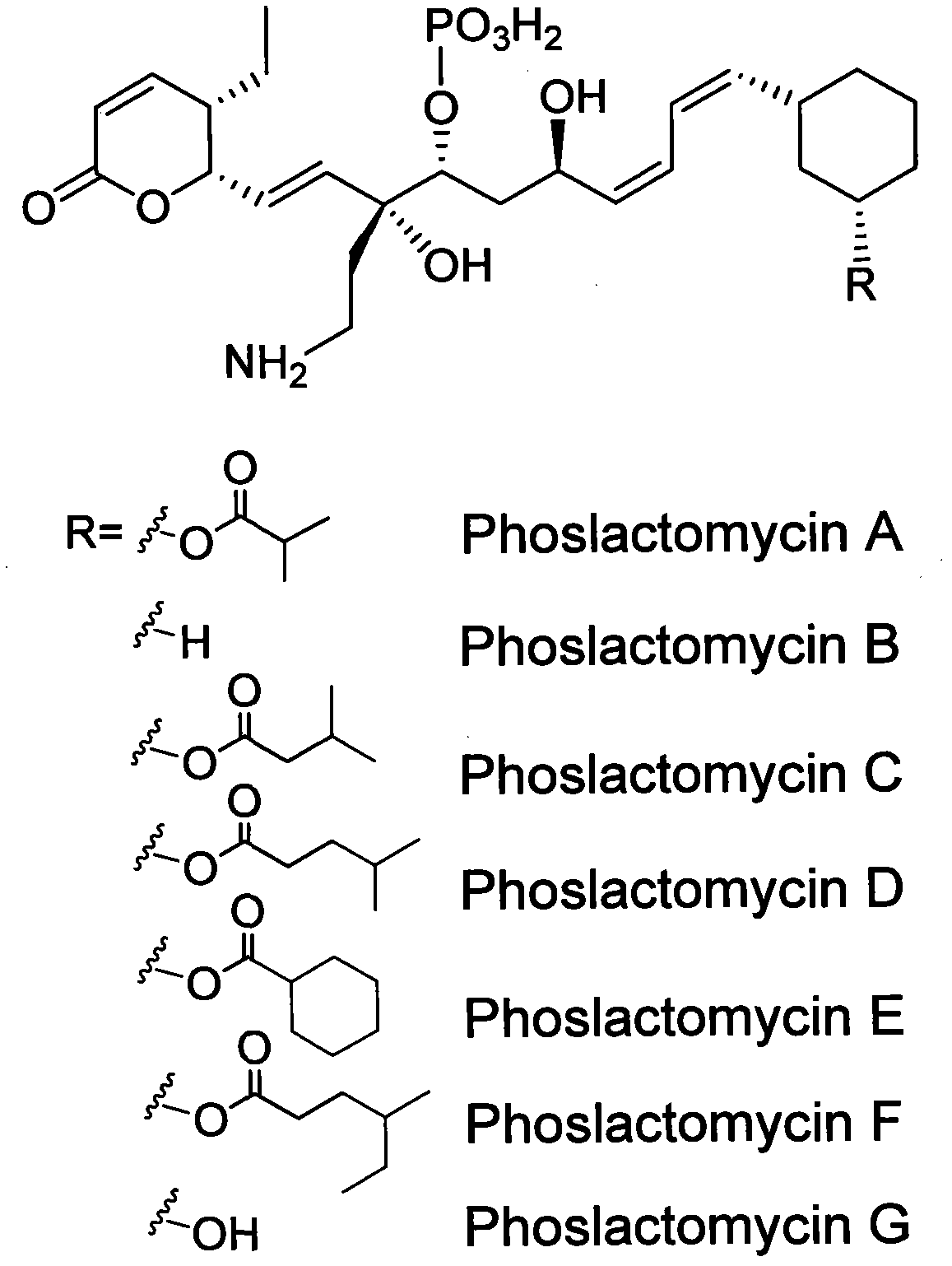
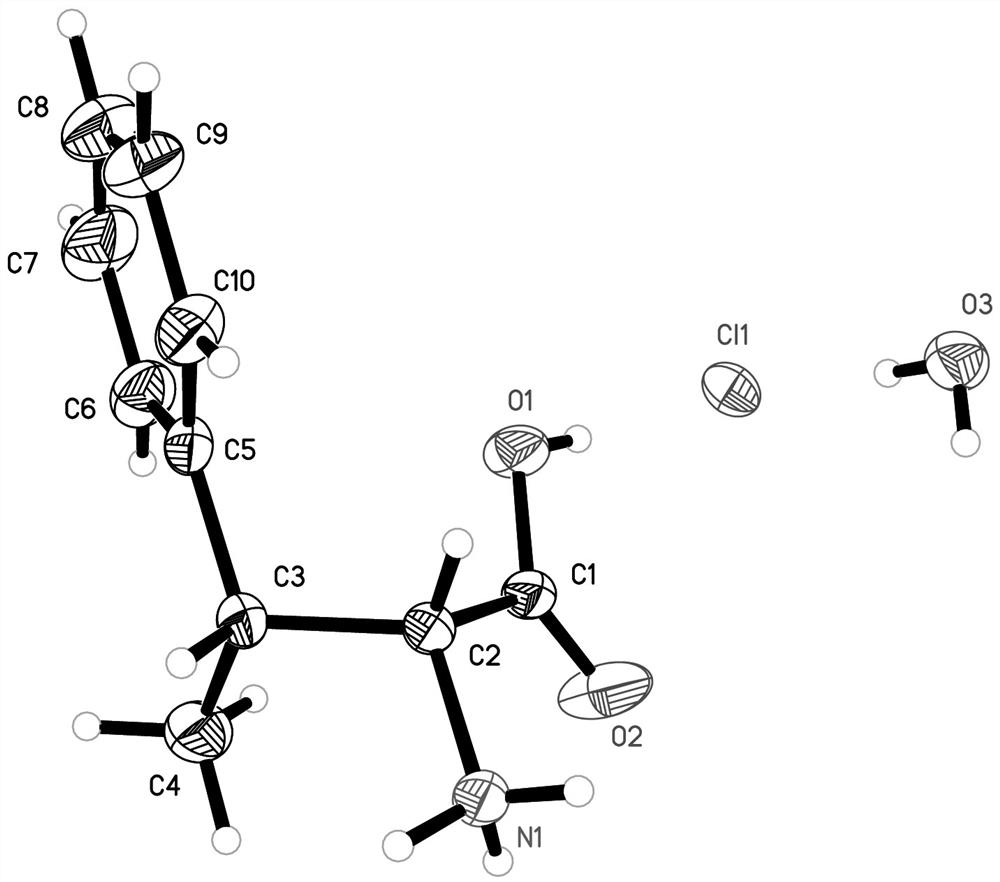
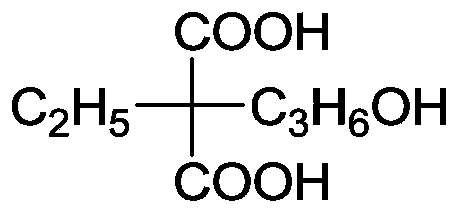
Ethylmalonic acid, also known as alpha-carboxybutyric acid or ethylmalonate, is a member of the class of compounds known as branched fatty acids.
Stabilized Polypeptide Formulations
InactiveUS20090060861A1Improve physical stabilityPeptide/protein ingredientsMetabolism disorderEthylmalonic acidShikimic acid
The present invention relates to a pharmaceutical formulation comprising a polypeptide and a buffer selected from the group consisting of diethylmalonic acid, trimellitic acid, shikimic acid, glycinamid, 2-amino-2-methyl-1,3-propanediol (AMPD) and tetraethylammonium (T.E.A.) or salts thereof. Further more the invention relates to a method for improving stability of a polypeptide in a purification process comprising the step of applying a buffer selected from the group consisting of diethylmalonic acid, trimellitic acid, shikimic acid, glycinamid, AMPD and T.E.A. or salts thereof to said purification process.
Owner:NOVO NORDISK AS
Biosynthetic gene cluster of phoslactomycins
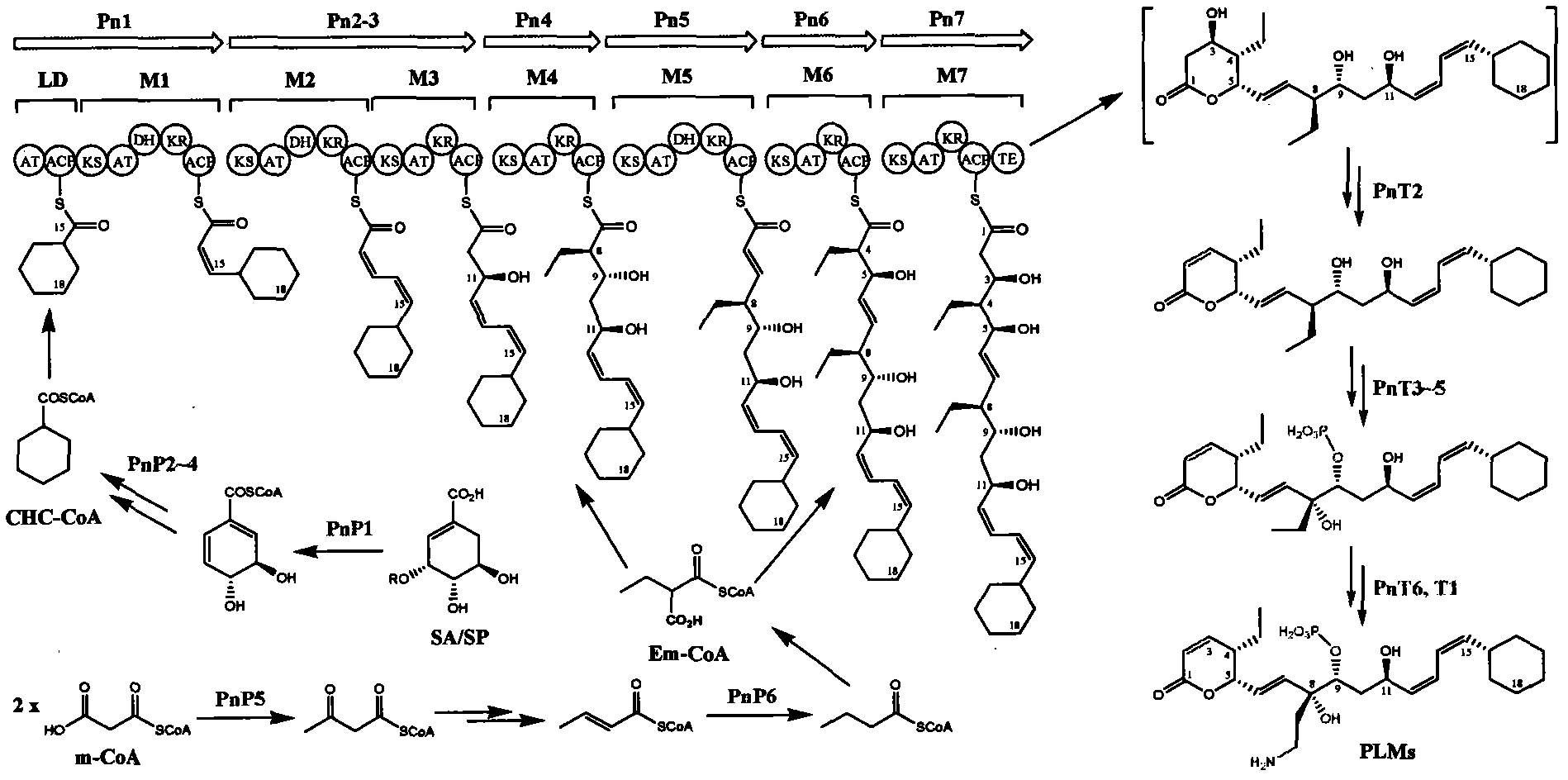
InactiveCN102061299AUnderstanding Biosynthetic MechanismsFermentationDNA/RNA fragmentationEthylmalonic acidBiosynthetic genes
The invention discloses a biosynthetic gene cluster of phoslactomycins produced by Streptomyces platensis SAM-0654. The whole gene cluster contains twenty twelve genes as follows: seven I-type linear polyketone synthetase (PKS) genes, two ethylmalonyl CoA synthetase genes, four cyclohexylformyl-CoA synthetase genes, six post-modified enzyme genes, two regulation genes and a resistance gene. Through carrying out genetic operation on the biosynthetic gene cluster, the synthesis of the phoslactomycins can be blocked. The gene cluster provided by the invention can be used for output variation of the phoslactomycins, combination generation of polyketone compounds and the like, and also can be used for searching and finding compounds or genes for medicine, industry or agriculture.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Preparation of 5,5-diethylmalonylurea
ActiveCN101323598AProcess stabilityMild reaction conditionsOrganic chemistryAlcoholEthylmalonic acid
The invention provides a new technology for preparing 5,5-diethyl-barbituric acid compound, which comprises steps as follows: malonic ester reacts with bromoethane under the condition of 'boiling in one pot' while alcobolic solution of sodium alcoholate exists to obtain 5,5- diethyl-malonic ester; the bromoethane reacts with urea while the alcobolic solution of sodium alcoholate exists; the reaction is completed in a concentration process; after the concentration is finished, water or (mother solution) is added for dissolution and acidification is carried out to obtain crude 5,5-diethyl-barbituric acid; water or ethanol water is recrystallized to prepare 5- ethyl-5-isopentyl balbituric acid compound. The technology is stable and carbethoxy and diethyl ester are completed under the condition of 'boiling in one pot'; the synthesis reaction with the urea is completed during distillation process; the technology has no particular requirement on the concentration of sodium alcoholate and has the advantages of easily controllable reaction conditions, short reaction time, simple operation, less three wastes, good product quality, high yield, low production cost and being more applicable to industrialized production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Method for preparing high-quality mercaptoacetic acid from tail solution from O-alkyl-N-alkyl thinocarbamate production
ActiveCN105254547AHigh recovery rateHigh purityOrganic chemistryOrganic compound preparationGlutaric acidSuspended organic matter
The invention discloses a method for preparing high-quality mercaptoacetic acid from a tail solution from O-alkyl-N-alkyl thinocarbamate production. The method comprises the following steps: (1) performing acidizing treatment on the tail solution by using an inorganic acid, and controlling the pH value to be 0.5-1.5; (2) leaving to stand for 6-48 hours, separating suspended organic matters, extracting by using an organic solvent, and extracting by using an extraction agent, thereby obtaining an extraction liquid, wherein the volume ratio of the total amount of the organic solvent to an acidifying liquid is (2.5:0.01)-(2.5:0.1), the organic solvent consists of 40-60wt% of glutaric acid diethyl ester and 40-060wt% of ethyl malonic acid diethyl ester, the volume ratio of the total amount of the extracting agent to a new acidifying liquid is (2:0.1)-(2:2), and the extracting agent is prepared by mixing n-amyl ether, isoamyl ether and sec-butyl methyl ether in a mass ratio of (1-3):(1-3):(1-3); (3) removing the solvent and water from the extraction liquid, thereby obtaining crude acid; (4) performing flash evaporation on the crude acid. The method is high in recycling rate, high in product purity, good in quality and simple in process.
Owner:QINGDAO LNT CHEM
Method for purifying high-purity phenyl ethyl malonate
ActiveCN101633619ASimple processHigh purityOrganic compound preparationCarboxylic acid esters preparationPurification methodsEthylmalonic acid
The invention relates to a method for purifying phenyl ethyl malonate, comprising the following steps: dissolving coarse phenyl ethyl malonate into a nonpolar solvent; decoloring by active carbon; filtering; mixing and crystallizing at the temperature of -10 DEG C to -5 DEG C; filtering to obtain a first filter cake; decompressing and recovering the solvent by the first filter cake to obtain colorless oil liquid as a fine product; dissolving the fine product into the nonpolar solvent; stirring and crystallizing at the temperature of -10 DEG C to -5 DEG C; filtering to obtain a second filter cake; decompressing the second cake to recover the solvent; and obtaining colorless transparent liquid as a phenyl ethyl malonate pure product. The purity of the phenyl ethyl malonate is greater than or equal to 99.5 percent, 2-phenyl-2-ethyl malonate impurities are less than 0.1 percent, and the total yield is greater than 90.0 percent.
Owner:南通森萱药业有限公司
Method and kit for detecting metabolites in dried blood spots
ActiveCN109142577AImprove diagnostic efficiencyReduce false positive rateComponent separationHomocystinemiaMetabolite
The invention relates to a method and a kit for detecting metabolites in dried blood spots. The metabolites include malonic acid, methyl malonic acid, ethyl malonic acid, me-citrate and total homocysteine, extraction agents containing internal standards are added into the dried blood spots to perform extraction, vibration incubation and derivatization on the dried blood spots, and the malonic acid, the methyl malonic acid, the ethyl malonic acid, the me-citrate and the total homocysteine in treated dried blood spot samples are detected by a liquid chromatography-tandem mass spectrometry method. The concentration of the malonic acid, the methyl malonic acid, the ethyl malonic acid, the me-citrate and the total homocysteine in the filter paper dried blood spot samples can be measured, the method and the kit are used for auxiliary diagnosis of methylmalonic acidemia, methylmalonic acidemia combined homocystinemia, homocystinemia and propionic acidemia, diagnosis efficiency is high, and diagnosis time can be shortened.
Owner:GENERAL HOSPITAL OF PLA
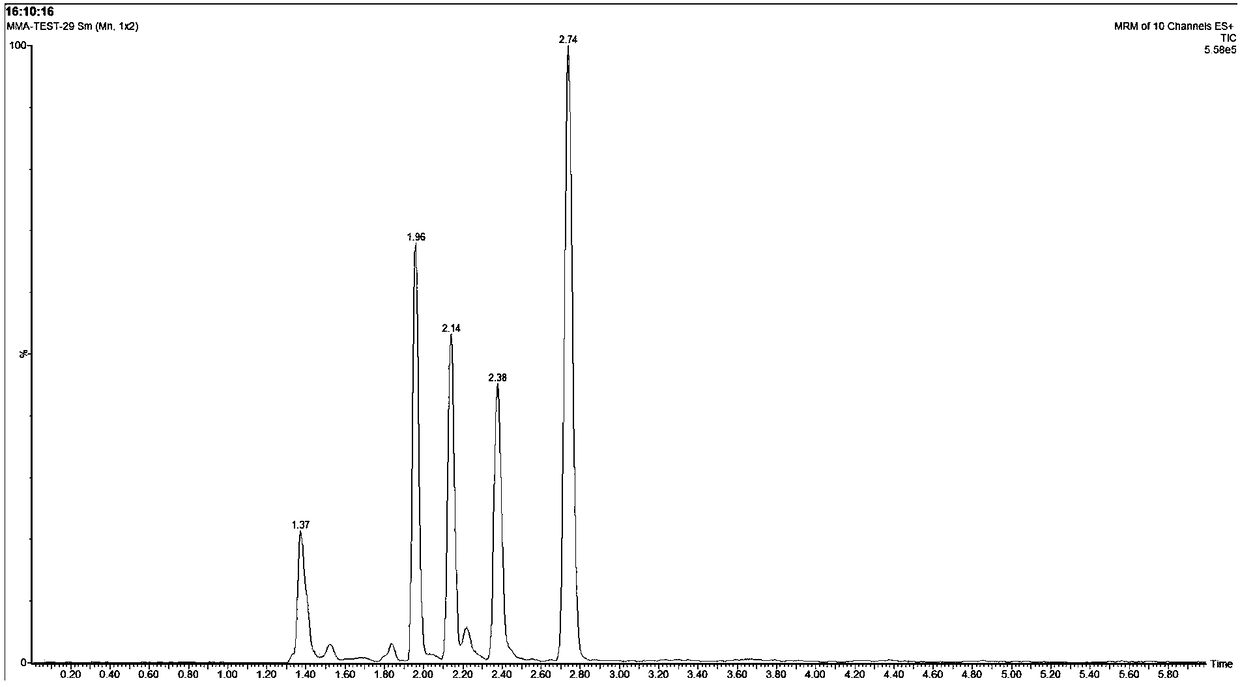
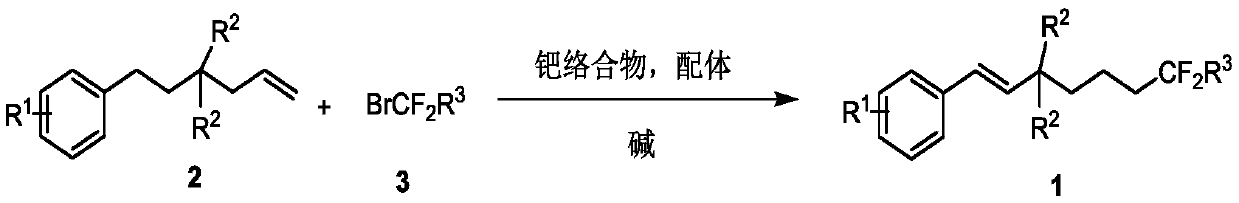
Method for synthesizing remote fluorinated aryl olefin
ActiveCN111004114AOrganic compound preparationCarboxylic acid esters preparationIsomerizationEthylmalonic acid
The invention discloses a method for synthesizing remote fluorinated aryl olefin, and belongs to the field of organic chemistry. The preparation method comprises the following steps: carrying out a reaction by taking 2-allyl-2-phenethyl diethyl malonate and a derivative thereof as raw materials in the presence of a phosphorus ligand, an inorganic alkali and an organic solvent to obtain the fluorinated olefin compound triethyl (E)-1,1-difluoro-7-phenylhept-6-ene-1,5,5-tricarboxylic acid. According to the method, the method can be completed in one step under the catalysis of palladium, difluoroalkylation is achieved while olefin isomerization is conducted, and a direct and effective way is provided for synthesis of the compound.
Owner:XINYANG NORMAL UNIVERSITY
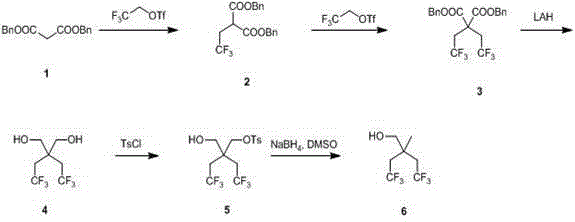
Synthetic method of 2,2-bis (trifluoroethyl) propanol
The invention relates to a synthetic method of 2,2-bis (trifluoroethyl) propanol and mainly solves the technical problem that the existing synthetic method is lack of industrialization prospect. The method includes subjecting dibenzyl ester malonate and trifluoroethyl triflate to a step-by-step reaction in the presence of an alkalization agent to obtain 2,2-bis (trifluoroethyl) dibenzyl ester malonate 2; reducing the composition 2 through lithium aluminum hydride to obtain 2,2-bis (trifluoroethyl)-1,3-propylene glycol; and using tosyl to protect one hydroxyl and then using sodium borohydride for reduction to obtain the 2,2-bis (trifluoroethyl) propanol. The 2,2-bis (trifluoroethyl) propanol can be prepared quickly and conveniently through the method.
Owner:WUXI BIOLOGICS CO LTD
Method for purifying high-purity phenyl ethyl malonate
ActiveCN101633619BSimple processHigh purityOrganic compound preparationCarboxylic acid esters preparationPurification methodsEthylmalonic acid
The invention relates to a method for purifying phenyl ethyl malonate, comprising the following steps: dissolving coarse phenyl ethyl malonate into a nonpolar solvent; decoloring by active carbon; filtering; mixing and crystallizing at the temperature of -10 DEG C to -5 DEG C; filtering to obtain a first filter cake; decompressing and recovering the solvent by the first filter cake to obtain colorless oil liquid as a fine product; dissolving the fine product into the nonpolar solvent; stirring and crystallizing at the temperature of -10 DEG C to -5 DEG C; filtering to obtain a second filter cake; decompressing the second cake to recover the solvent; and obtaining colorless transparent liquid as a phenyl ethyl malonate pure product. The purity of the phenyl ethyl malonate is greater than or equal to 99.5 percent, 2-phenyl-2-ethyl malonate impurities are less than 0.1 percent, and the total yield is greater than 90.0 percent.
Owner:南通森萱药业有限公司
Polymer supported chrome catalyst for olefins polymerization
InactiveUS20100022727A1Easy to shapeHigh bulk densityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationChromium CompoundsCross-link
A highly active supported chromium catalyst composition for ethylene and other olefins polymerization and also for ethylene copolymerization with efficient incorporation of comonomer, produces polymers with superior spherical morphology, improved bulk density and almost 0% fines. The catalyst composition component includes at least one chromium compound, mainly chromium acetylacetonate, or chromium hexaflouroacetonylacetonate, or chromium diethylmalonate. One magnesium compound, or aluminum compound, metal alkoxy compound and defined polymer particles mainly chloromethylated cross linked styrene-DVB copolymer or polyvinylchloride. The catalyst composition, when used in conjunction with an organoaluminum compound or a mixture of organoaluminum compounds, can be used for olefin polymerization to produce medium or high density polyethylene and copolymers of ethylene with alpha-olefins having about 3 to 18 carbon atoms.
Owner:AL ARIFI ABDULLAH SAAD N
Fingolimod hydrochloride process
ActiveUS9643914B2Cost effectiveOrganic compound preparationOrganic chemistry methodsOrganic solventEthylmalonic acid
A process for preparation of diethyl 2-aetamido-2-(4-octyl phenyl)ethyl malonate (III), a key intermediate of fingolimod hydrochloride comprising reaction of 2-(4-octylphenyl)ethyl iodide (IV) with diethyl acetamido malonate in presence of a base and an iodinating agent and in an organic solvent. The compound of formula (III) thus obtained provided fingolimod hydrochloride (Ia) having associated impurities below the regulatory limits.
Owner:EMCURE PHARAMACEUTICALS LTD
Method for preparing vinpocetine intermediate gamma-hydroxypropyl-ethylmalonic acid
ActiveCN103626651BEliminate reaction stepsShort reaction timeOrganic compound preparationCarboxylic acid esters preparationChemical industryFiltration
The invention discloses a method for preparing vinpocetine intermediate gamma-hydroxypropyl-ethylmalonic acid, and belongs to the chemical industry synthesis field. The preparation method comprises the following steps: 1, adding sodium hydride into an organic solvent, adding 2-ethyl diethylmalonate and 1-bromo-3-chloropropane, extracting by using ethyl acetate, and carrying out reduced pressure concentration of a solvent to obtain gamma-chloropropyl-ethyl diethylmalonate; and 2, adding gamma-chloropropyl-ethyl diethylmalonate to an ethanol-water solvent of sodium hydroxide, refluxing, adding a proper amount of water, adjusting the pH value to 1 by using concentrated hydrochloric acid, crystallizing, and carrying out pumping filtration to obtain a white solid gamma-hydroxypropyl-ethylmalonic acid, wherein a molar ratio of 2-ethyl diethylmalonate to 1-bromo-3-chloropropane to sodium hydride in step 1 is 1:1-1.2:1-1.3; and a reaction temperature in the step 1 is 10-40DEG C, and a reaction time of 1-bromo-3-chloropropane, 2-ethyl diethylmalonate and 1-bromo-3-chloropropane is 8-15h. The intermediate is gamma-hydroxypropyl-ethylmalonic acid, and the preparation method has the advantages of cheap and easily available raw materials, low cost, short reaction steps, simple operation, good product quality, and benefit for protecting the green resource.
Owner:NORTHEAST PHARMA GRP
Synthetic method for intermediate diethyl diethylmalonate of barbiturate
InactiveCN106397199AReduce intermediate linksLow reaction temperatureOrganic compound preparationCarboxylic acid esters preparationDistillationEthylmalonic acid
he invention provides a synthetic method for an intermediate, i.e., diethyl diethylmalonate, of barbiturate. The synthetic method comprises the following steps: adding 1.13 mol of ethanolamine and 0.5 mol of cuprous chloride into a reaction vessel and controlling a stirring speed to be 130 to 160 rpm; after complete dissolving of ethanolamine, adding 0.65 mol of diethyl malonate drop by drop within 2 to 3 h; heating a solution obtained in the previous step to 70 to 75 DEG C and maintaining the heating state for 50 to 70 min; carrying out cooling, wherein a solid is precipitated; then adding 1.1 to 1.3 mol of ethylamine drop by drop within 2 to 3 h and maintaining a reflux state for 4 to 5 h; adding 300 ml of a potassium chloride solution and decreasing the temperature of the solution to 5 to 8 DEG C; carrying out extraction with cyclohexane three to five times and combining extract; successively carrying out washing with a salt solution, dehydrating with a dehydrating agent and evaporation of cyclohexane; then carrying out pressure-reduced distillation and collecting a fraction obtained at a temperature of 185 to 195 DEG C; and subjecting the fraction to re-crystallization in acetonitrile so as to obtain a crystal, i.e., diethyl diethylmalonate; wherein the mass fraction of the potassium chloride solution is 15 to 20%, the mass fraction of cyclohexane is 80 to 85%, and the salt solution is any one selected from a group consisting of sodium nitrate and potassium sulfate.
Owner:XIAMEN AN PU DUN INFORMATION TECH CO LTD
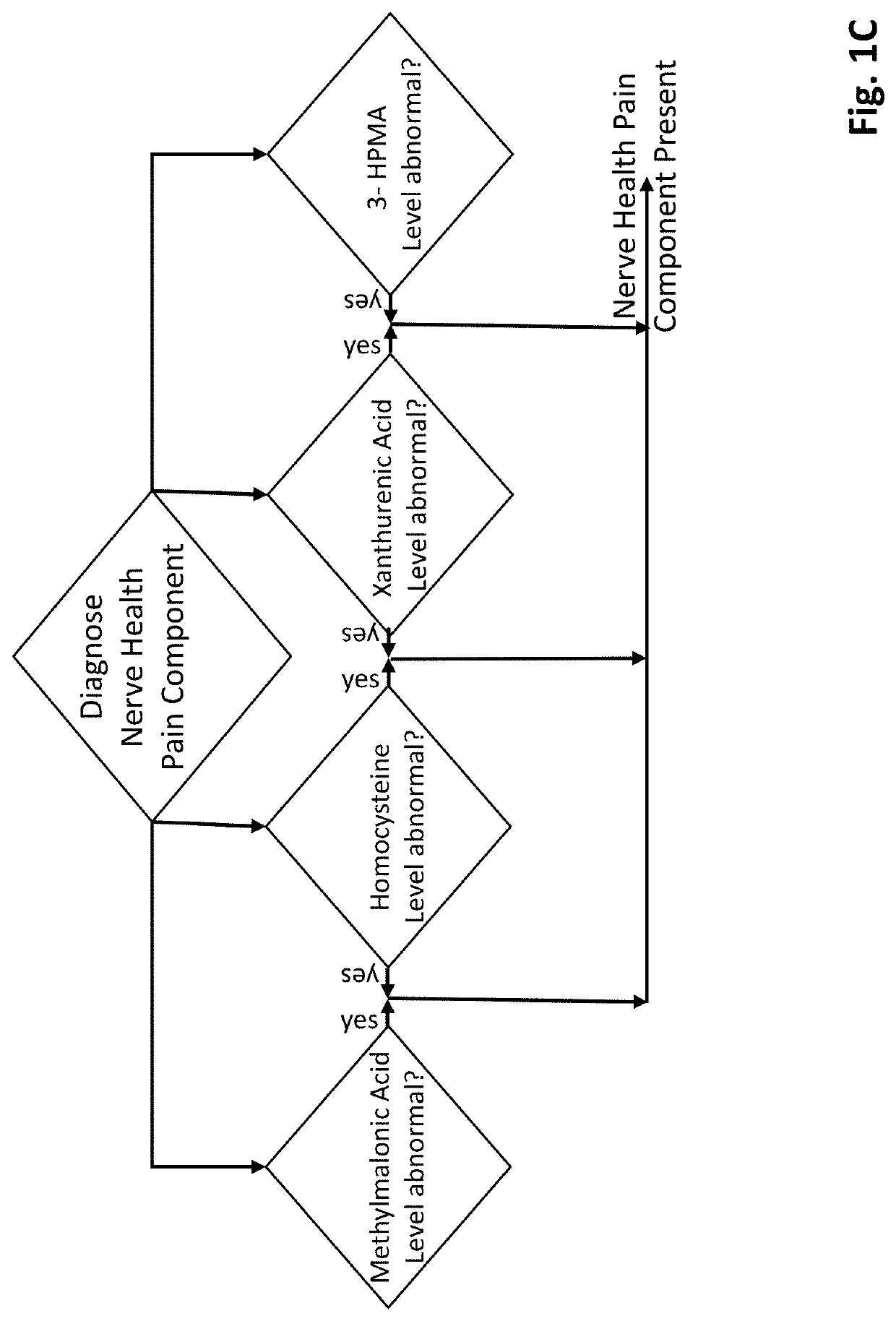
Methods of diagnosing and treating particular causal components of chronic pain in a patient
PendingUS20200363433A1Reduce chronic painEasy to manageOmicsDisease diagnosisOpioidergicEthylmalonic acid
The present disclosure teaches systems and methods of diagnosing and treating the distinct biologic components that contribute to chronic pain symptoms experienced by patients. A biologic sample is obtained from a patient. Levels of two or more biomarkers (e.g., methylmalonic acid, homocysteine, xanthurenic acid, 3-hydroxypropyl mercapturic acid (3-HPMA), pyroglutamate, hydroxymethylglutarate (HMG), quinolinic acid, kynurenine acid, 5-hydroxyindoleacetate (5-HIAA), vanilmandelate (VMA), and ethylmalonic acid) in the biologic sample are experimentally determined. Based on the existence of abnormal results in one or more biomarkers the patient is diagnosed as having the nerve health, oxidative stress, chronic inflammation pain, and / or neurotransmitter pain components to their chronic pain. Based on the resulting diagnoses administration of certain support compounds is directed. The patient may retest after a sufficient period of time to observe any longitudinal differences in the test results and adjust treatment accordingly. Further, the biomarker data gathered from pain-neutral and chronic pain patients (particularly those using opioid therapies) will be used to characterize biochemistries going forward.
Owner:ETHOS RES & DEV LLC
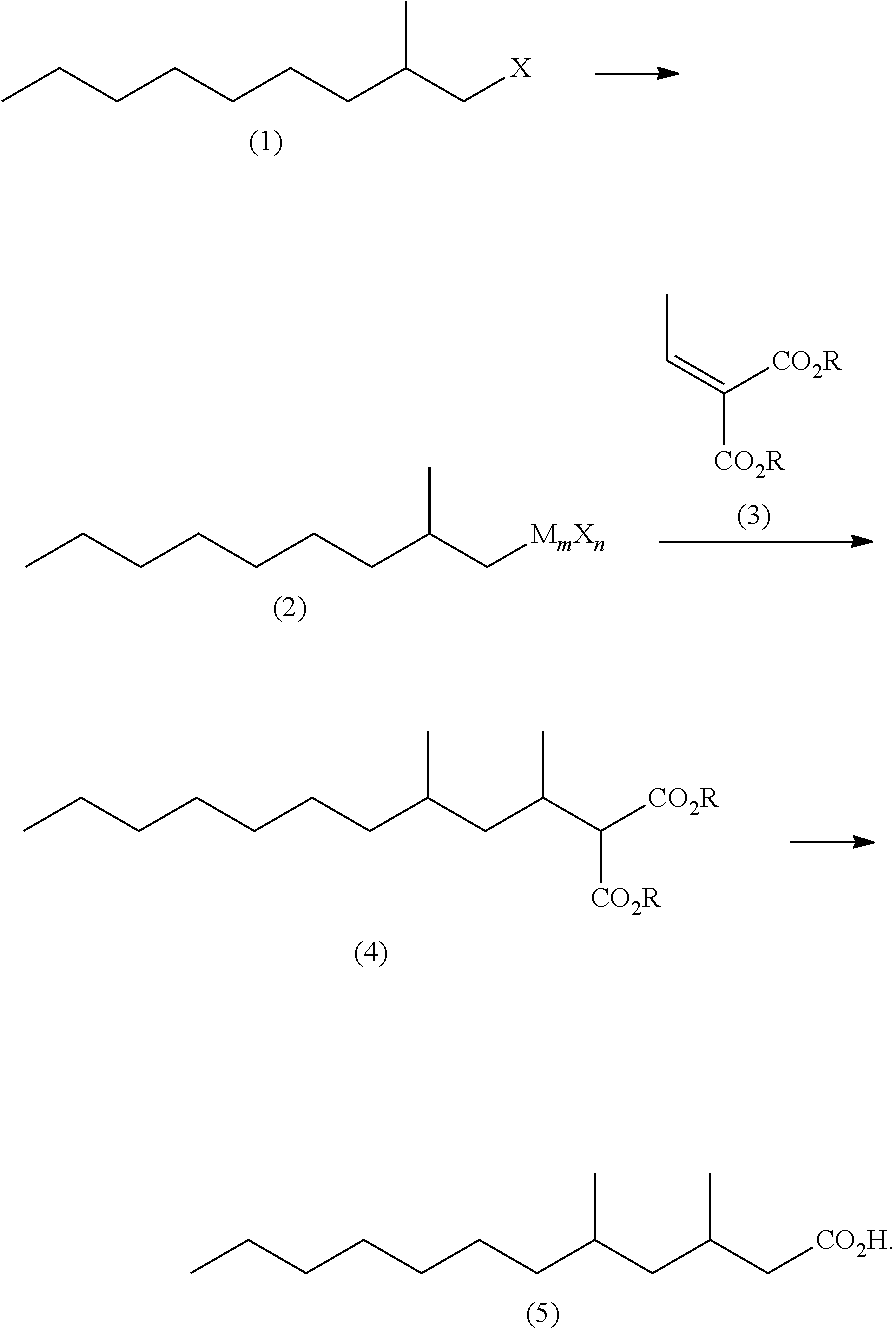
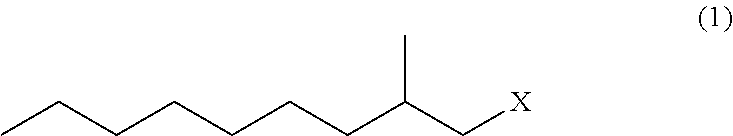
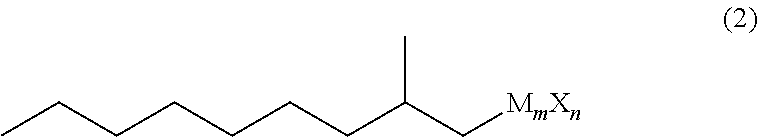
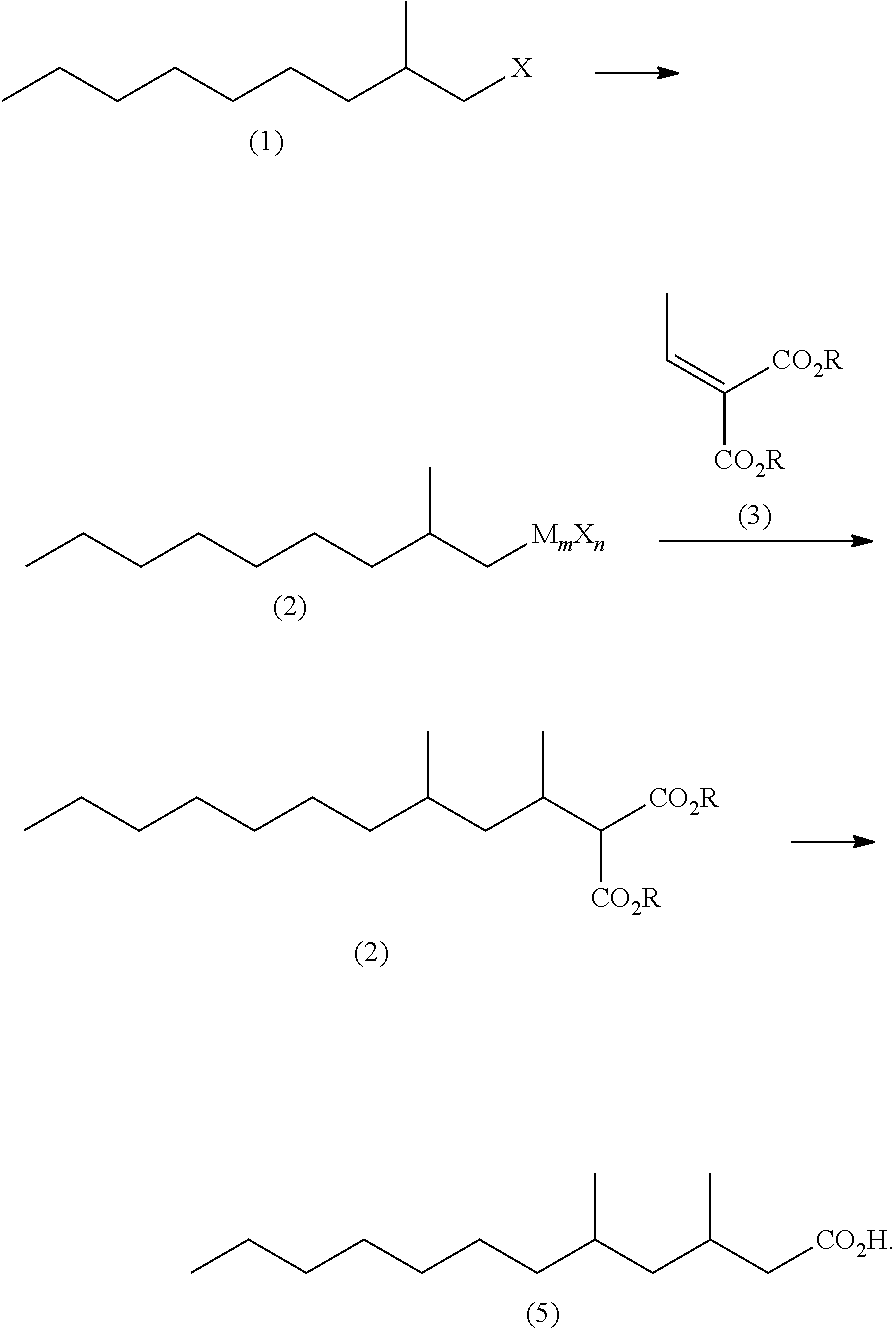
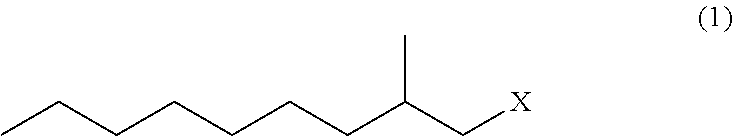
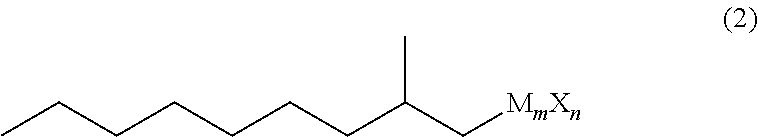
Method for producing 3,5-dimethyldodecanoic acid
ActiveUS9278900B2Simply and efficiently producingShort processOrganic compound preparationCarboxylic acid esters preparationMalonic acidEthylmalonic acid
Provided is a process for producing 3,5-dimethyldodecanoic acid, which is an active ingredient of the pheromone of California prionus. More specifically provided is a method for producing 3,5-dimethyldodecanoic acid (5) comprising the steps of converting 2-methylnonyl halide (1) to a 2-methylnonyl metal reagent (2), and reacting the 2-methylnonyl metal reagent (2) with 2-ethylidene malonic acid ester (3) to form 2-(1,3-dimethyldecyl)malonic acid ester (4) as a result of 1,4-addition of the 2-methylnonyl metal reagent (2) to the 2-ethylidene malonic acid ester (3), as shown in the following scheme:
Owner:SHIN ETSU CHEM IND CO LTD
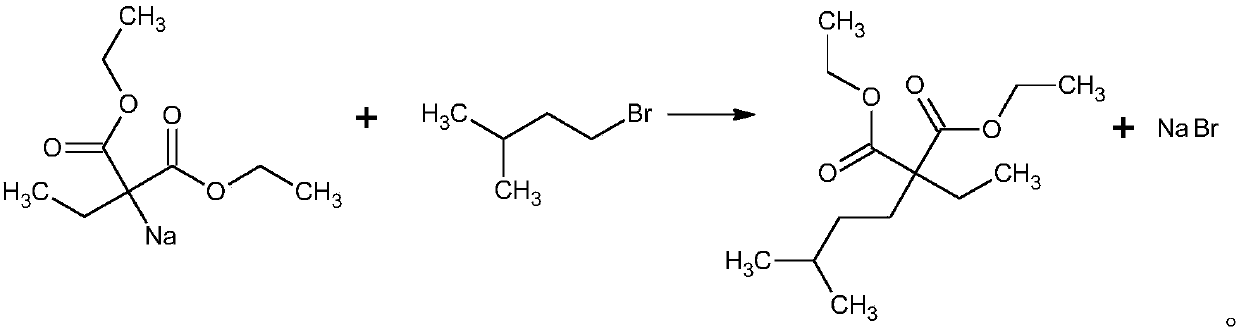
The preparation method of ethyl isopentyl malonate diethyl ester
ActiveCN105646214BHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationEthylmalonic acidSodium salt
The invention discloses a preparation method of diethyl ethylisopentylmalonate. The preparation method comprises following steps: 1), ethanol and sodium react to generate sodium ethoxide; 2), diethyl ethylmalonate is added to sodium ethoxide obtained in the step 1), and diethyl ethylmalonate sodium salt is generated; 3), 1-bromo-3-methylbutane is added to the diethyl ethylmalonate sodium salt obtained in the step 2), and diethyl ethylisopentylmalonate is generated after reaction. Diethyl ethylisopentylmalonate is prepared from ethanol, sodium, diethyl ethylmalonate and 1-bromo-3-methylbutane as the raw materials, and prepared diethyl ethylisopentylmalonate has high yield and high purity and meets the drug quality standard.
Owner:WEIFANG JINGRUN CHEM CO LTD
Method for producing 3,5-dimethyldodecanoic acid
ActiveUS20150344396A1Simply and efficiently producingShort processOrganic compound preparationCarboxylic acid esters preparationMalonic acidEthylmalonic acid
Provided is a process for producing 3,5-dimethyldodecanoic acid, which is an active ingredient of the pheromone of California prionus. More specifically provided is a method for producing 3,5-dimethyldodecanoic acid (5) comprising the steps of converting 2-methylnonyl halide (1) to a 2-methylnonyl metal reagent (2), and reacting the 2-methylnonyl metal reagent (2) with 2-ethylidene malonic acid ester (3) to form 2-(1,3-dimethyldecyl)malonic acid ester (4) as a result of 1,4-addition of the 2-methylnonyl metal reagent (2) to the 2-ethylidene malonic acid ester (3), as shown in the following scheme:
Owner:SHIN ETSU CHEM IND CO LTD
Method for preparing high-quality thioglycolic acid from the tail liquid of producing o-alkyl-n-alkylthiocarbamate
ActiveCN105254547BHigh recovery rateHigh purityOrganic chemistryOrganic compound preparationCarbamateGlutaric acid
The invention discloses a method for preparing high-quality mercaptoacetic acid from a tail solution from O-alkyl-N-alkyl thinocarbamate production. The method comprises the following steps: (1) performing acidizing treatment on the tail solution by using an inorganic acid, and controlling the pH value to be 0.5-1.5; (2) leaving to stand for 6-48 hours, separating suspended organic matters, extracting by using an organic solvent, and extracting by using an extraction agent, thereby obtaining an extraction liquid, wherein the volume ratio of the total amount of the organic solvent to an acidifying liquid is (2.5:0.01)-(2.5:0.1), the organic solvent consists of 40-60wt% of glutaric acid diethyl ester and 40-060wt% of ethyl malonic acid diethyl ester, the volume ratio of the total amount of the extracting agent to a new acidifying liquid is (2:0.1)-(2:2), and the extracting agent is prepared by mixing n-amyl ether, isoamyl ether and sec-butyl methyl ether in a mass ratio of (1-3):(1-3):(1-3); (3) removing the solvent and water from the extraction liquid, thereby obtaining crude acid; (4) performing flash evaporation on the crude acid. The method is high in recycling rate, high in product purity, good in quality and simple in process.
Owner:QINGDAO LNT CHEM
A kind of method for synthesizing remote fluorinated aryl olefins
ActiveCN111004114BOrganic compound preparationCarboxylic acid esters preparationIsomerizationEthylmalonic acid
The invention discloses a method for synthesizing remote fluorinated aryl olefins, belonging to the field of organic chemistry. Taking 2-allyl-2-diethyl phenethylmalonate and its derivatives as raw materials, and reacting in the presence of phosphorus ligands, inorganic bases and organic solvents, the fluoroolefin compound three of the present invention is obtained. Ethyl (E)-1,1-difluoro-7-phenylhept-6-ene-1,5,5-tricarboxylic acid. The method of the invention can be completed in one step by palladium catalysis, and at the same time of isomerizing the olefin, difluoroalkylation is realized, which provides a direct and effective way for the synthesis of such compounds.
Owner:XINYANG NORMAL UNIVERSITY
Platinum (II) complexes having antitumor activities, and preparation methods thereof
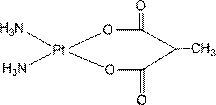
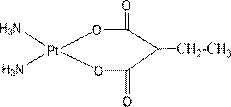
InactiveCN103130836AHas in vitro tumor growth inhibitory activityGroup 8/9/10/18 element organic compoundsAntineoplastic agentsMethylmalonic acidPlatinum
The invention relates to platinum (II) complexes having antitumor activities, and preparation methods thereof. The platinum (II) complexes treat an ammonia molecule as an accompanying group, and treat methylmalonic acid and ethylmalonic acid as leaving groups respectively. The series of the above compounds have the antitumor activities. The structure of the compounds is shown in the specification.
Owner:KUNMING GUIYAN PHARMA
Preparation method of (2R, 3R)-3-methyl-3-phenylalanine
ActiveCN113004161ALow costEasy to operateOrganic compound preparationOrganic chemistry methodsEthylmalonic acidPhenylalanine
The invention relates to a preparation method of (2R, 3R)-3-methyl-3-phenylalanine. The technical problems that an existing preparation method is long in route, expensive in used reagent, incapable of achieving large-scale production and the like are mainly solved. According to the technical scheme, the preparation method of the (2R, 3R)-3-methyl-3-phenylalanine comprises the following steps: condensing 2-acetamidomalonic acid diethyl ester and 1-bromoethyl benzene under the action of alkali to obtain 2-acetamido-2-(1-phenylethyl) malonic acid diethyl ester; heating and hydrolyzing in concentrated hydrochloric acid, concentrating and crystallizing to obtain erythro 3-methyl-3-phenylalanine; then performing acylation to obtain the erythro 2-acetamido-3-methyl 3-phenylalanine; splitting under the action of acetamido transferase, and obtaining (2R, 3R)-2-acetamido-3-methyl 3-phenylalanine; and finally, heating and hydrolyzing by using hydrochloric acid to obtain the product (2R, 3R)-3-methyl-3-phenylalanine (hydrochloride).
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Fingolimod hydrochloride process
ActiveUS20160289166A1Cost-effectiveOrganic compound preparationOrganic chemistry methodsOrganic solventEthylmalonic acid
A process for preparation of diethyl 2-aetamido-2-(4-octyl phenyl)ethyl malonate (III), a key intermediate of fingolimod hydrochloride comprising reaction of 2-(4-octylphenyl)ethyl iodide (IV) with diethyl acetamido malonate in presence of a base and an iodinating agent and in an organic solvent. The compound of formula (III) thus obtained provided fingolimod hydrochloride (Ia) having associated impurities below the regulatory limits.
Owner:EMCURE PHARAMACEUTICALS LTD
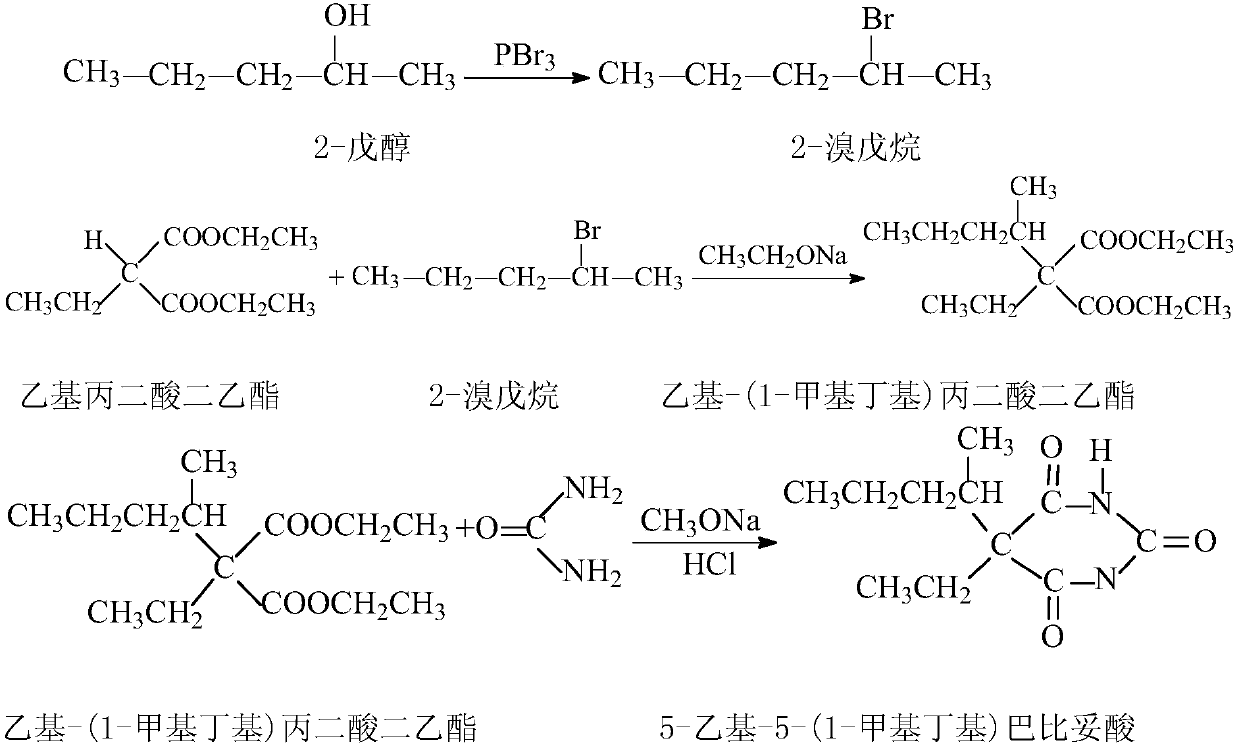
High-purity 5-ethyl-5-(1-methylbutyl)barbituric acid preparation method
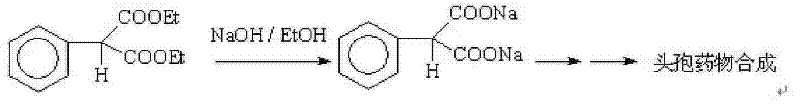
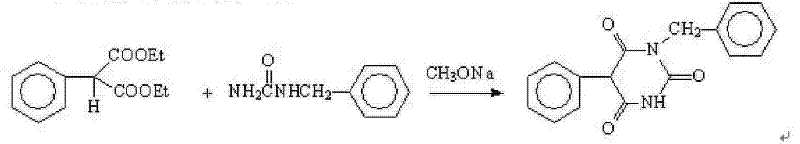
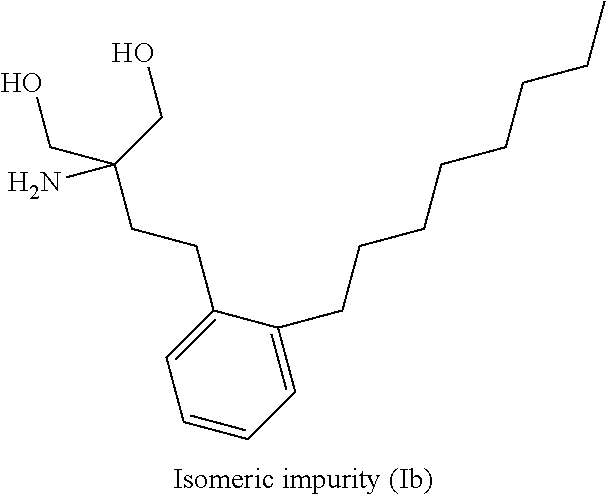
The present invention relates to a preparation method of a drug pentobarbital (1) for sedation, hypnosis, pre-anesthesia administration and anti-convulsion, and provides a new preparation process for preparing high-purity pentobarbital (I) from diethyl ethylmalonate, wherein the process comprises three steps: A, bromination; B, alkylation; and C, cyclization, acidification, and purification. According to the present invention, the method has advantages of simple operation, short production cycle, low energy consumption, mother liquor circulation, less three-waste, stable process, good product quality, high product purity, isomer impurity content of less than 0.1%, high yield and low production cost, and is suitable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Method for preparing vinpocetine intermediate gamma-hydroxypropyl-ethylmalonic acid
ActiveCN103626651AEliminate reaction stepsShort reaction timeOrganic compound preparationCarboxylic acid esters preparationChemical industryFiltration
The invention discloses a method for preparing vinpocetine intermediate gamma-hydroxypropyl-ethylmalonic acid, and belongs to the chemical industry synthesis field. The preparation method comprises the following steps: 1, adding sodium hydride into an organic solvent, adding 2-ethyl diethylmalonate and 1-bromo-3-chloropropane, extracting by using ethyl acetate, and carrying out reduced pressure concentration of a solvent to obtain gamma-chloropropyl-ethyl diethylmalonate; and 2, adding gamma-chloropropyl-ethyl diethylmalonate to an ethanol-water solvent of sodium hydroxide, refluxing, adding a proper amount of water, adjusting the pH value to 1 by using concentrated hydrochloric acid, crystallizing, and carrying out pumping filtration to obtain a white solid gamma-hydroxypropyl-ethylmalonic acid, wherein a molar ratio of 2-ethyl diethylmalonate to 1-bromo-3-chloropropane to sodium hydride in step 1 is 1:1-1.2:1-1.3; and a reaction temperature in the step 1 is 10-40DEG C, and a reaction time of 1-bromo-3-chloropropane, 2-ethyl diethylmalonate and 1-bromo-3-chloropropane is 8-15h. The intermediate is gamma-hydroxypropyl-ethylmalonic acid, and the preparation method has the advantages of cheap and easily available raw materials, low cost, short reaction steps, simple operation, good product quality, and benefit for protecting the green resource.
Owner:NORTHEAST PHARMA GRP
The preparation method of ethyl 2-oxo-3-piperidinecarboxylate
ActiveCN108484484BRaw materials are easy to getEasy to operateOrganic chemistryPtru catalystEthylmalonic acid
The invention provides a preparation method of 2-oxo-3-ethyl piperidinecarboxylate. The preparation method comprises the following steps: S1, mixing diethyl malonate and a base catalyst uniformly, andthen dropwise adding acrylonitrile at 10 to 50 DEG C for reaction to obtain diethyl 2-(2-cyanoethyl)malonate; S2, reacting 2-(2-cyanoethyl)malonate, an organic solvent and a Raney cobalt catalyst at75-130 DEG C in a hydrogen atmosphere, and carrying out recrystallization to obtain 2-oxo-3-ethyl piperidinecarboxylate. Compared with the prior art, the preparation method has the beneficial effectsthat the raw materials are easily available, the operation is simple, and the total yield reaches 77% or higher compared with a classic N.F.Albertsm method (which is improved by 20%), so that the preparation method is very suitable for industrial production.
Owner:上海泾维化工科技有限公司
Polymer supported chrome catalyst for olefins polymerization
InactiveUS8247342B2Easy to shapeGreat freeOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationCross-linkChromium Compounds
A highly active supported chromium catalyst composition for ethylene and other olefins polymerization and also for ethylene copolymerization with efficient incorporation of comonomer, produces polymers with superior spherical morphology, improved bulk density and almost 0% fines. The catalyst composition component includes at least one chromium compound, mainly chromium acetylacetonate, or chromium hexaflouroacetonylacetonate, or chromium diethylmalonate. One magnesium compound, or aluminum compound, metal alkoxy compound and defined polymer particles mainly chloromethylated cross linked styrene-DVB copolymer or polyvinylchloride. The catalyst composition, when used in conjunction with an organoaluminum compound or a mixture of organoaluminum compounds, can be used for olefin polymerization to produce medium or high density polyethylene and copolymers of ethylene with alpha-olefins having about 3 to 18 carbon atoms.
Owner:AL ARIFI ABDULLAH SAAD N
Preparation method of ketorolac tromethamine intermediate
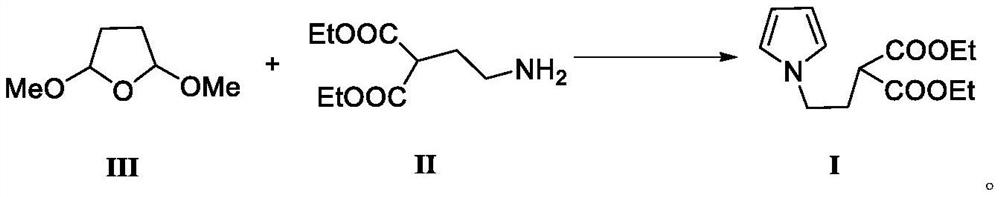
The invention belongs to the technical field of medicine synthesis, and relates to a preparation method of a ketorolac tromethamine intermediate. According to the preparation method, 2, 5-dimethoxy tetrahydrofuran reacts with a compound 2-(2-aminoethyl) diethyl malonate to generate the important intermediate 2-(2-(1H-pyrrole-1-yl) ethyl) diethyl malonate of ketorolac tromethamine. According to the novel method for synthesizing the ketorolac intermediate, use of dangerous chemical reagents is avoided, the synthesized intermediate does not generate new impurities, a traditional catalyst is replaced with a green catalyst, the reaction is milder, economical and environmentally friendly, the yield is high, and the method is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Synthesis method of barbitone drug intermediate diethyl diethylmalonate
InactiveCN105439862AReduce intermediate linksLow reaction temperatureOrganic compound preparationCarboxylic acid esters preparationEthylmalonic acidSynthesis methods
The invention relates to a synthesis method of a barbitone drug intermediate diethyl diethylmalonate, which comprises the following steps: adding 1.13 mol of ethanolamine and 0.5 mol of cuprous chloride into a reaction vessel, controlling the stirring rate at 130-160 rpm until the ethanolamine is completely dissolved, dropwisely adding 0.65 mol of diethyl malonate within 2-3 hours, heating the solution to 70-75 DEG C, keeping the heating state for 50-70 minutes, cooling to precipitate a solid, dropwisely adding 1.1-1.3 mol of ethylamine within 2-3 hours, keeping the reflux state for 4-5 hours, adding 300ml of potassium chloride solution, cooling the solution to 5-8 DEG C, extracting with cyclohexane 3-5 times, merging the extracting solutions, washing with a salt solution, dehydrating with a dehydrating agent, distilling to remove the cyclohexane, distilling under reduced pressure, collecting the 185-195-DEG C fraction, and recrystallizing in acetonitrile to obtain the crystal diethyl diethylmalonate, wherein the mass percent of the potassium chloride solution is 15-20%, the mass percent of the cyclohexane is 80-85%, and the salt solution is any one of sodium nitrate and potassium sulfate.
Owner:CHENGDU KA DI FU TECH
Synthetic method of 2,2-bis (trifluoroethyl) propanol
The invention relates to a synthetic method of 2,2-bis (trifluoroethyl) propanol and mainly solves the technical problem that the existing synthetic method is lack of industrialization prospect. The method includes subjecting dibenzyl ester malonate and trifluoroethyl triflate to a step-by-step reaction in the presence of an alkalization agent to obtain 2,2-bis (trifluoroethyl) dibenzyl ester malonate 2; reducing the composition 2 through lithium aluminum hydride to obtain 2,2-bis (trifluoroethyl)-1,3-propylene glycol; and using tosyl to protect one hydroxyl and then using sodium borohydride for reduction to obtain the 2,2-bis (trifluoroethyl) propanol. The 2,2-bis (trifluoroethyl) propanol can be prepared quickly and conveniently through the method.
Owner:WUXI BIOLOGICS CO LTD
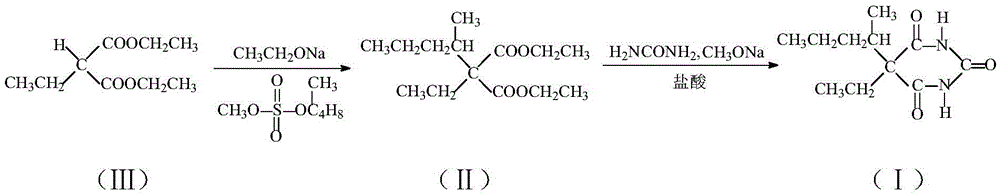
Method for preparing 5-ethyl-5-(1-methylbutyl)malonylurea
InactiveCN105541731AProcess stabilityMild reaction conditionsOrganic chemistrySodium methoxideContinuous use
The invention belongs to the technical field of drug synthesis and particularly relates to a method for preparing 5-ethyl-5-(1-methylbutyl)malonylurea. A mixture which is obtained after ethyl-propanedioic acid diethyl ester (III) reacts with an alcohol solution of sodium ethoxide reacts with methanesulfonic acid (2-pentyl) ester under the condition that toluene exists, and an ethyl-(1-methylbutyl) diethyl malonate compound (II) is obtained through rectification; the compound (II) reacts with urea under the condition that a methanol solution of sodium methoxide exists, and a fine 5-ethyl-5-(1-methylbutyl)malonylurea (I) product is obtained through hydrochloric acid acidizing and re-crystallization. The technology is stable, reaction conditions are moderate, control is easy, the obtained product is high in purity and high in yield, the conversion rate nearly reaches 100%, post-processing is easy and convenient, energy consumption is low, three wastes are few, mother liquor can be continuously used, production cost is low, and the method is suitable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com