Synthesis method of barbitone drug intermediate diethyl diethylmalonate
A technology of diethyl malonate and synthesis method, which is applied in the field of synthesis of diethyl malonate, an intermediate of barbiturates, and can solve the problem of prolonging the opening time of chlorine channels and increasing the influx of Cl and other problems, to achieve the effect of reducing reaction temperature and reaction time, increasing reaction yield and reducing intermediate links
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
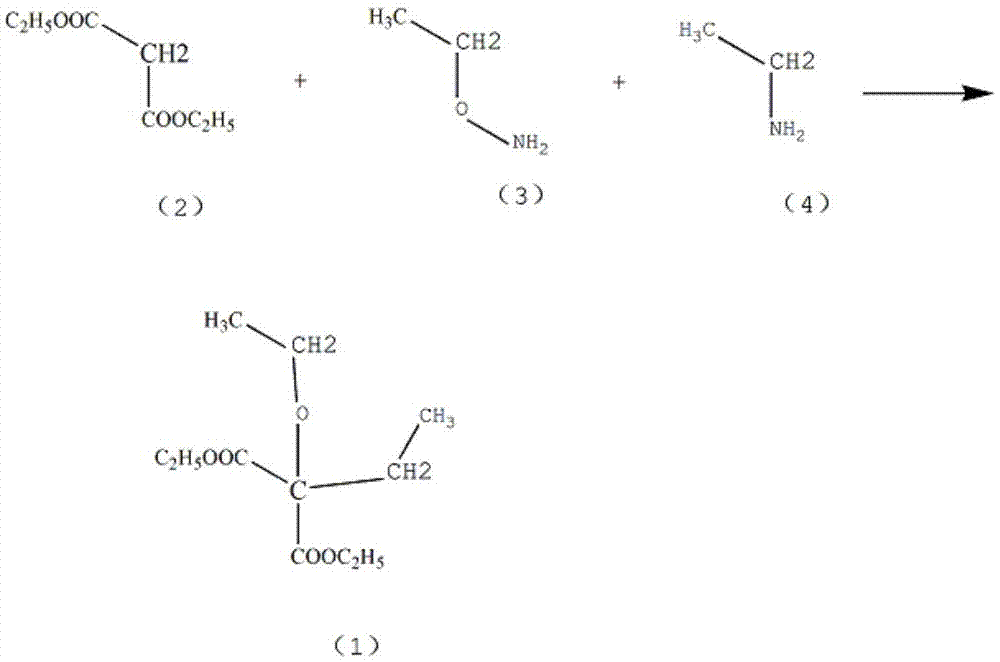
[0012] Add 1.13mol of ethanolamine (3) and 0.5mol of cuprous chloride into the reaction vessel, and control the stirring speed at 130rpm. After the ethanolamine is completely dissolved, add 0.65mol of diethyl malonate (2) dropwise, and the dropping time is controlled at 2h , raise the temperature of the solution to 70°C, maintain the heating state for 50 minutes, and solid precipitates after cooling, add 1.1 mol of ethylamine (4) dropwise, control the dropping time at 2 hours, keep the reflux state for 4 hours, and add 15% chloride by mass fraction Potassium solution 300ml, lower the temperature of the solution to 5°C, extract 3 times with cyclohexane, combine the extracts, wash with sodium nitrate solution, dehydrate with calcium sulfate, distill out cyclohexane, then distill under reduced pressure at 1.6kPa, collect at 185--195°C The diethyl diethyl malonate was recrystallized in 90% acetonitrile by mass fraction to obtain 120.74 g of crystalline diethyl malonate, with a yiel...
example 2
[0014] Add 1.13mol of ethanolamine (3) and 0.5mol of cuprous chloride into the reaction vessel, and control the stirring speed at 140rpm. After the ethanolamine is completely dissolved, add 0.65mol of diethyl malonate (2) dropwise, and the dropping time is controlled at 2h , raise the temperature of the solution to 72°C, maintain the heating state for 60 minutes, and solid precipitates after cooling, add 1.2 mol of ethylamine (4) dropwise, control the dropping time at 2 hours, keep the reflux state for 4 hours, add 17% chlorinated Potassium solution 300ml, lower the temperature of the solution to 7°C, extract 4 times with cyclohexane, combine the extracts, wash with potassium sulfate solution, dehydrate activated alumina, distill cyclohexane, and then distill under reduced pressure at 1.65kPa to collect 185--195 The fraction at ℃ was recrystallized in acetonitrile with a mass fraction of 925% to obtain 124.96 g of crystalline diethyl malonate, with a yield of 89%.
example 3
[0016] Add 1.13mol of ethanolamine (3) and 0.5mol of cuprous chloride to the reaction vessel, and control the stirring speed at 160rpm. After the ethanolamine is completely dissolved, add 0.65mol of diethyl malonate (2) dropwise, and the dropping time is controlled at 3h , Raise the temperature of the solution to 75°C, maintain the heating state for 70 minutes, and solid precipitates after cooling, add 1.3 mol of ethylamine (4) dropwise, control the dropping time at 3 hours, keep the reflux state for 5 hours, add 20% chlorinated Potassium solution 300ml, lower the solution temperature to 8°C, extract 5 times with cyclohexane with a mass fraction of 85%, combine the extracts, wash with sodium nitrate solution, dehydrate calcium sulfate, distill cyclohexane, then distill under reduced pressure at 1.7kPa, collect The fraction at 185--195°C was recrystallized in acetonitrile with a mass fraction of 95% to obtain 130.57 g of diethyl diethylmalonate in a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com