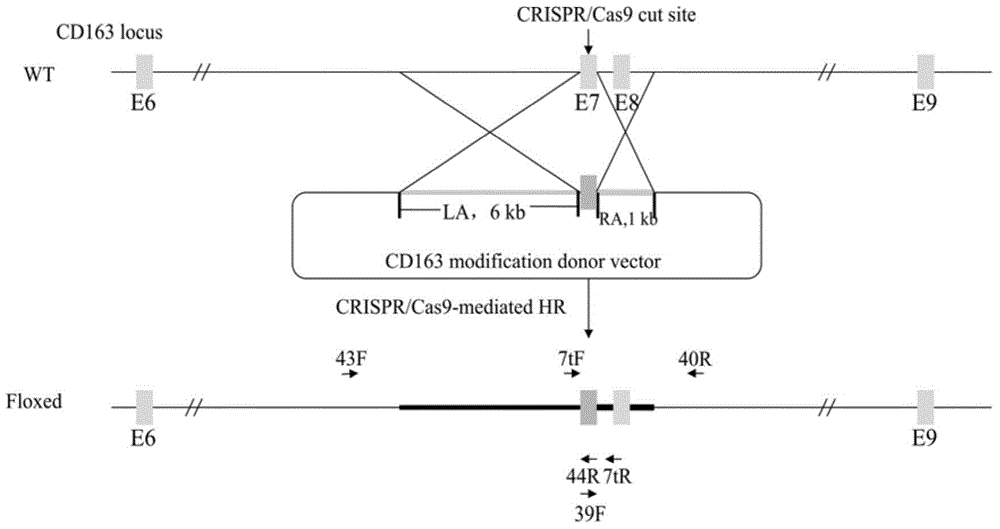
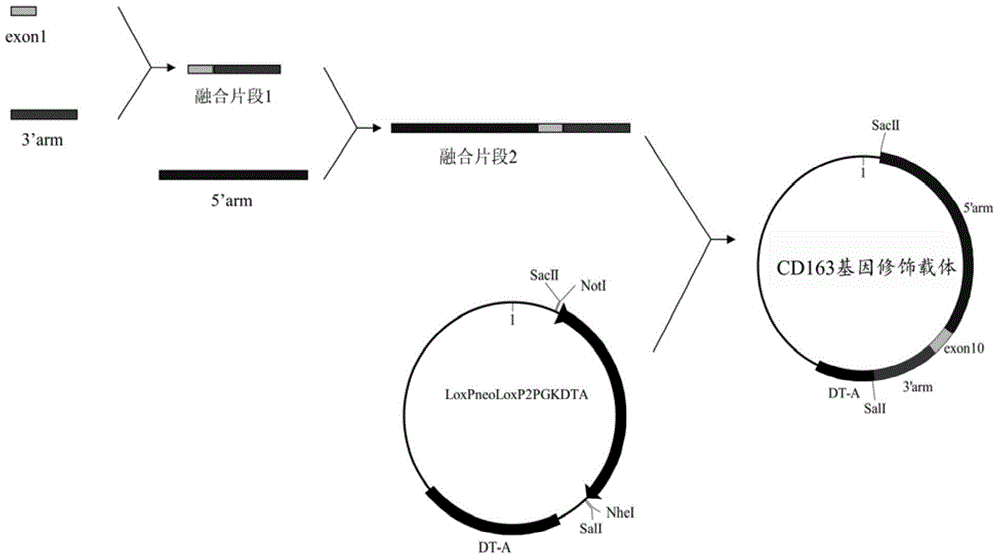
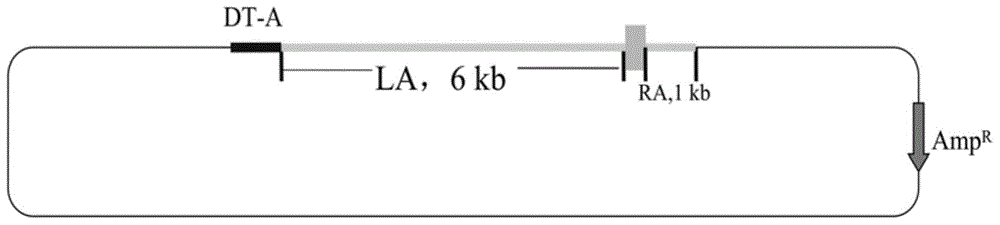
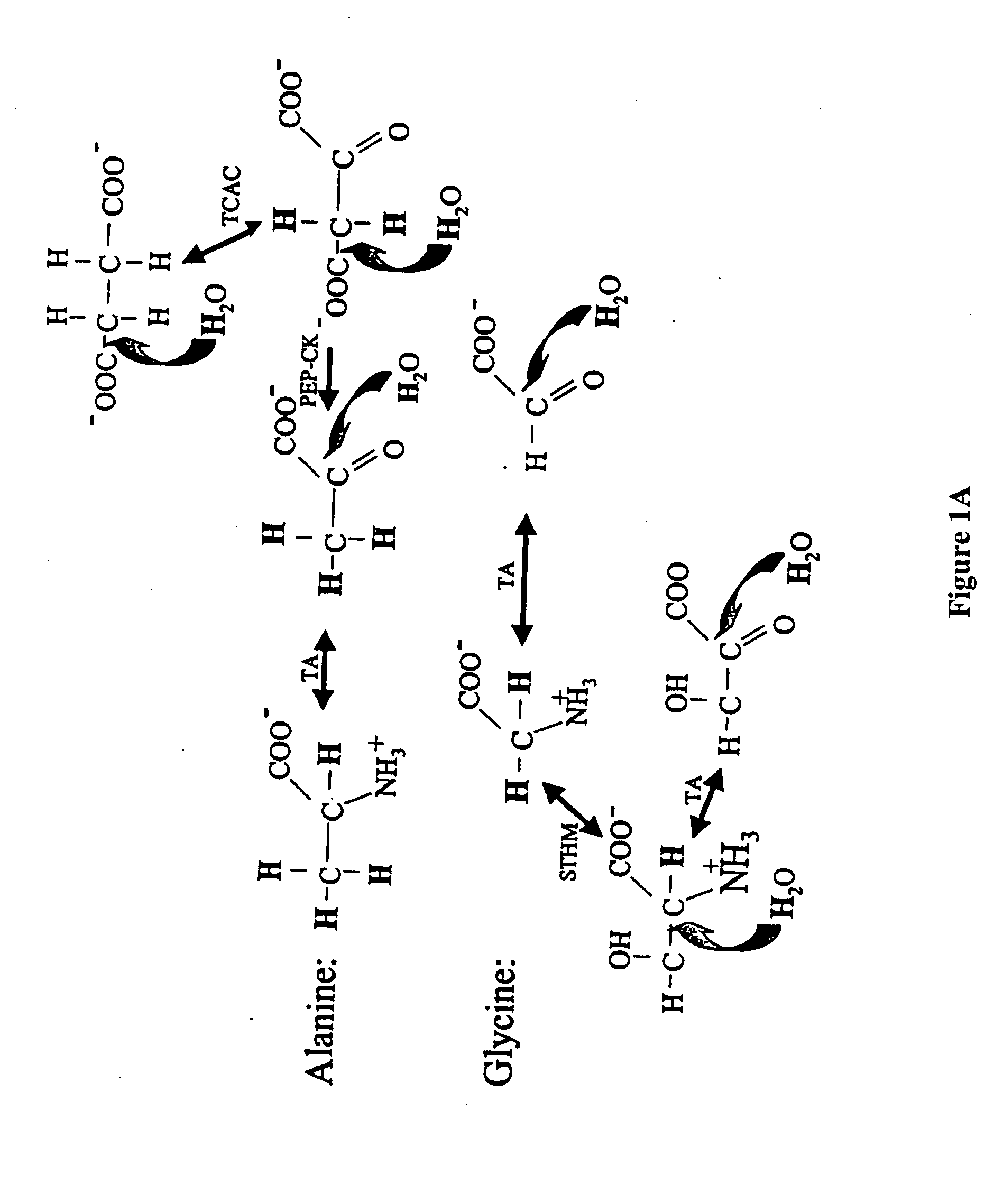
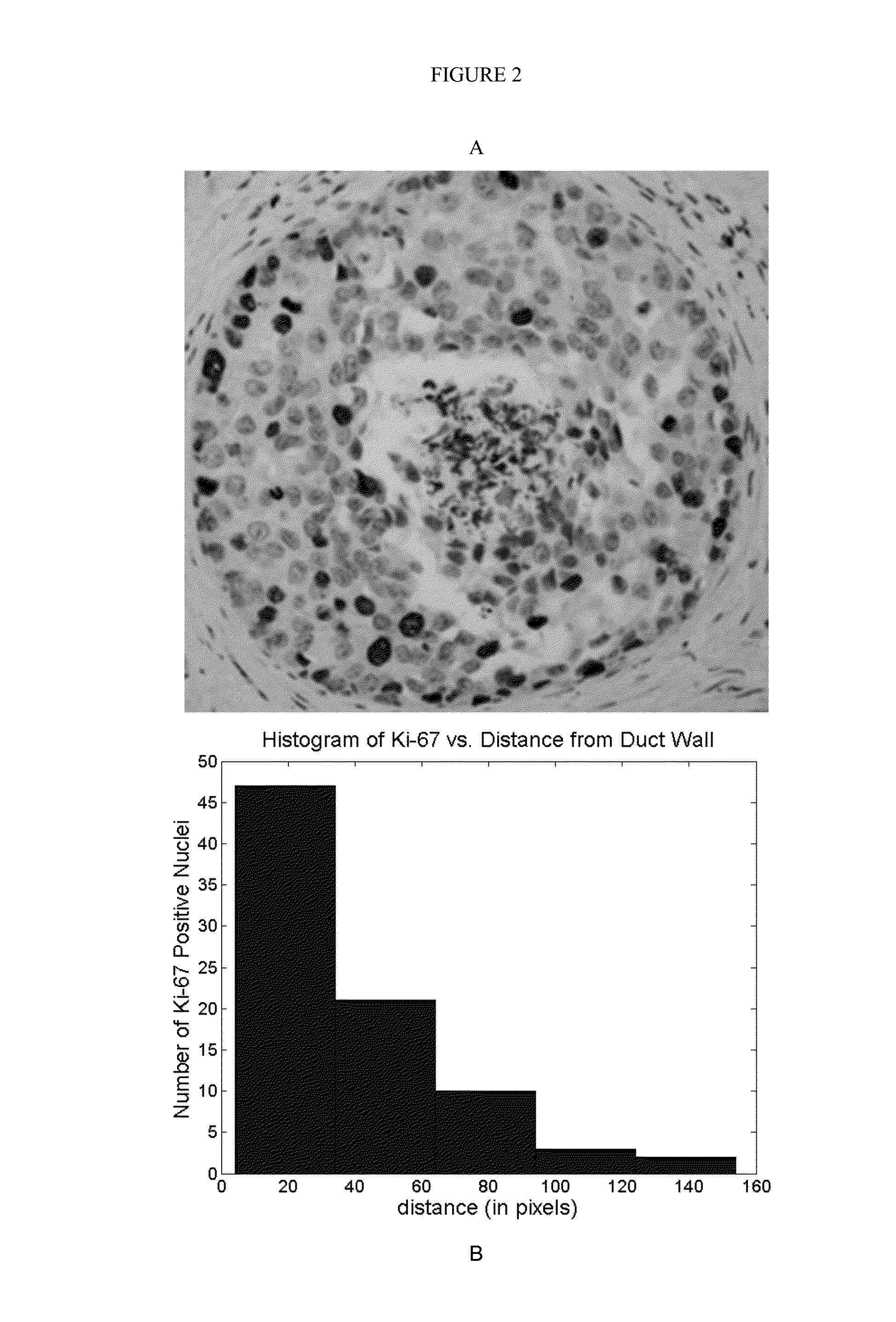
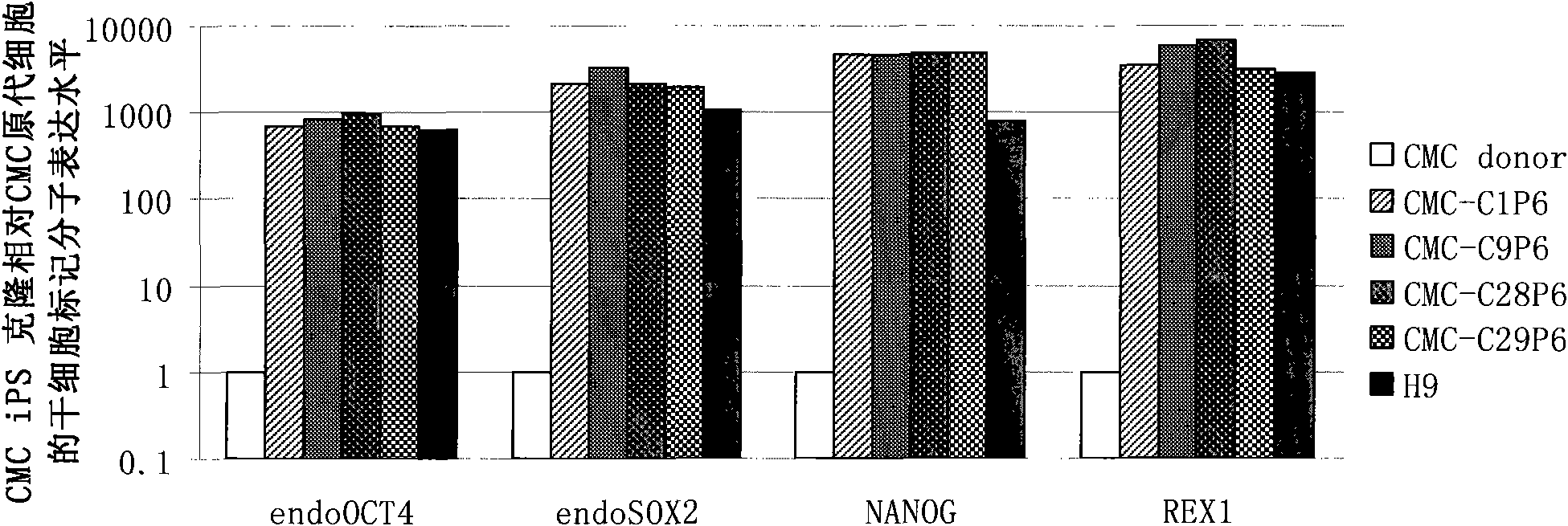
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
209 results about "Disease modelling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A disease model is a representation of the abnormal human or animal biology that occurs in a particular disease. The model may be a mouse with a condition that mimics a human disease, or it could be cells in a dish. Whatever the model, it must reproduce aspects of a disease, or even the whole disease pathology...
Method of cloning reproductive and respiratory syndrome resisting pig
Owner:CHINA AGRI UNIV
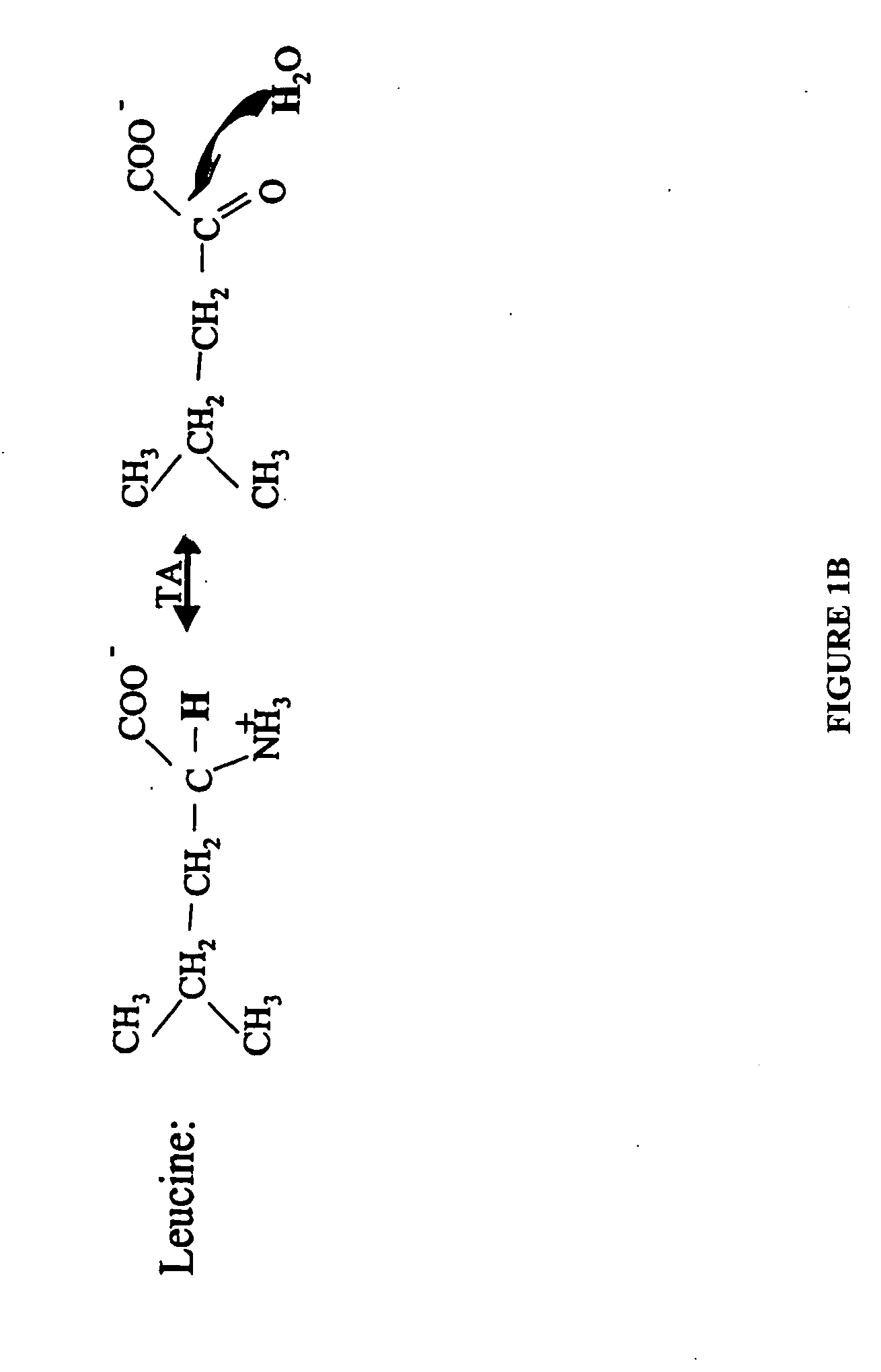
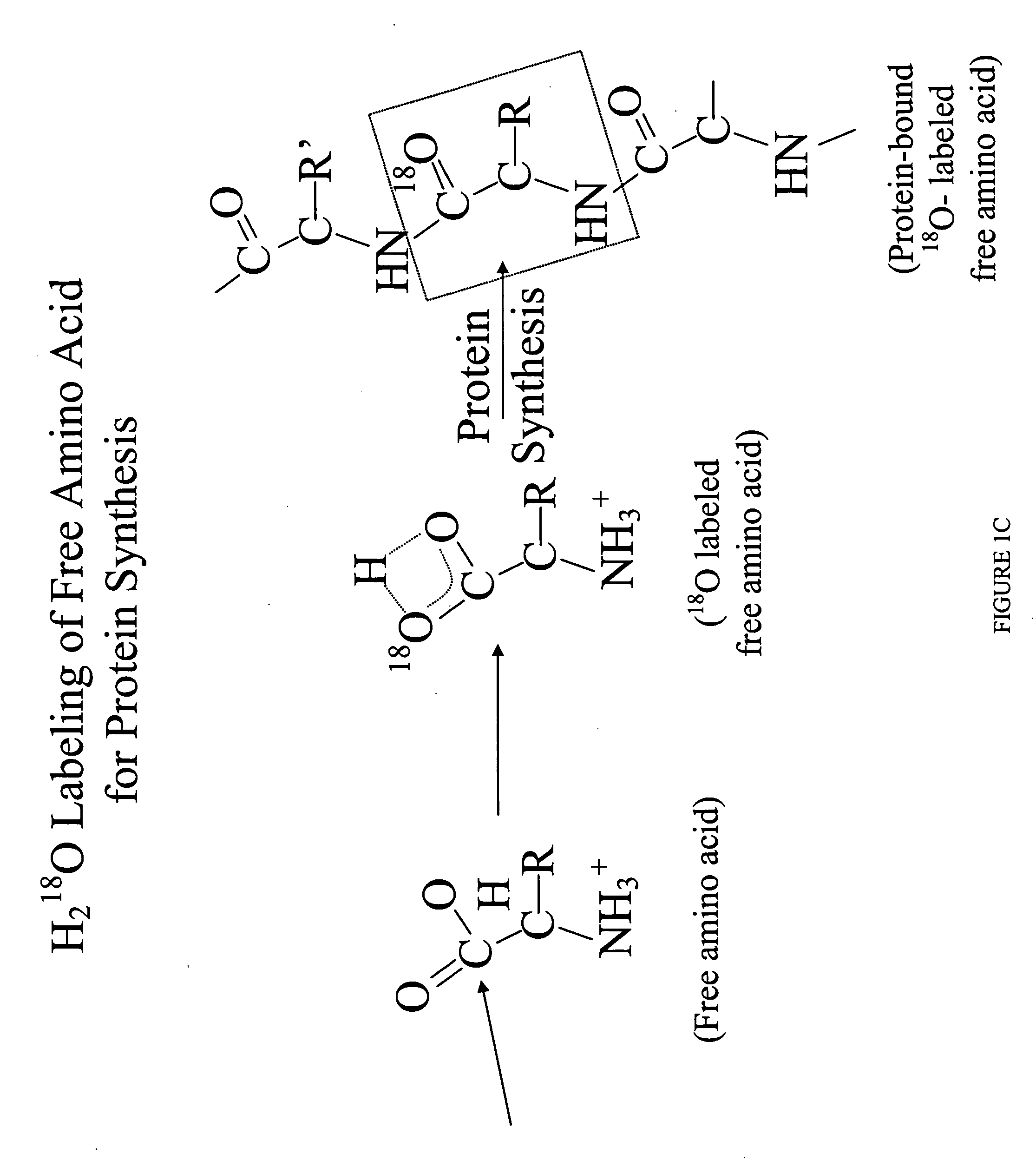
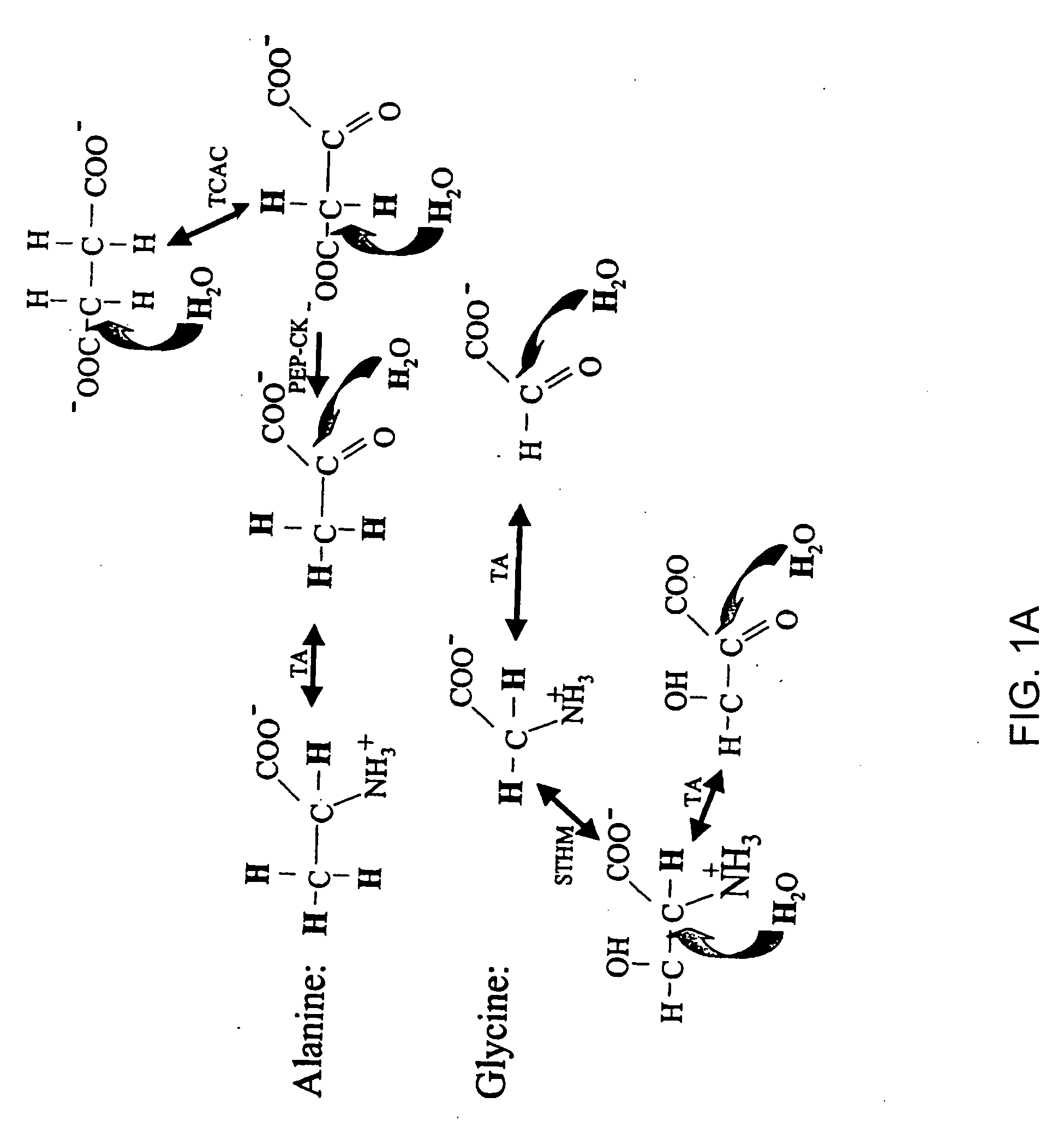
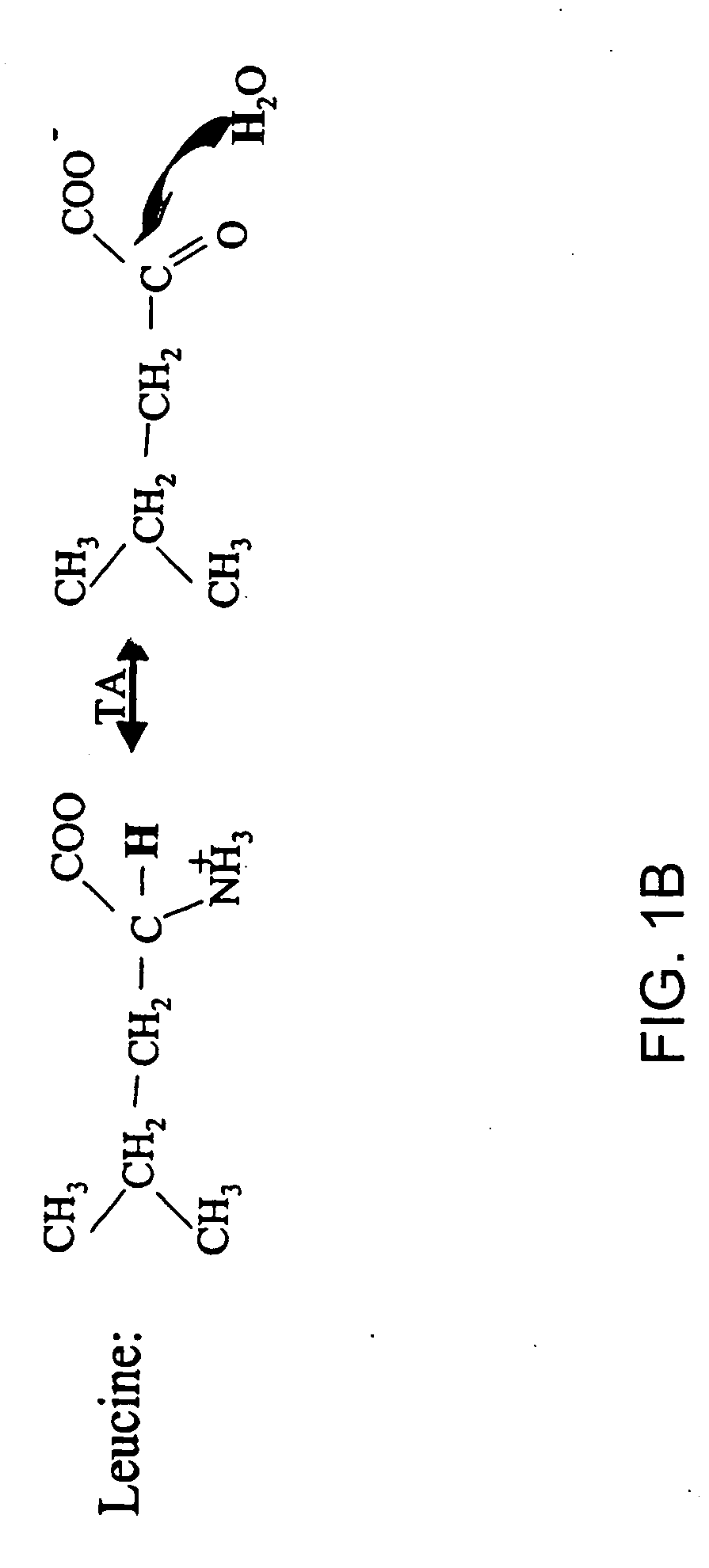
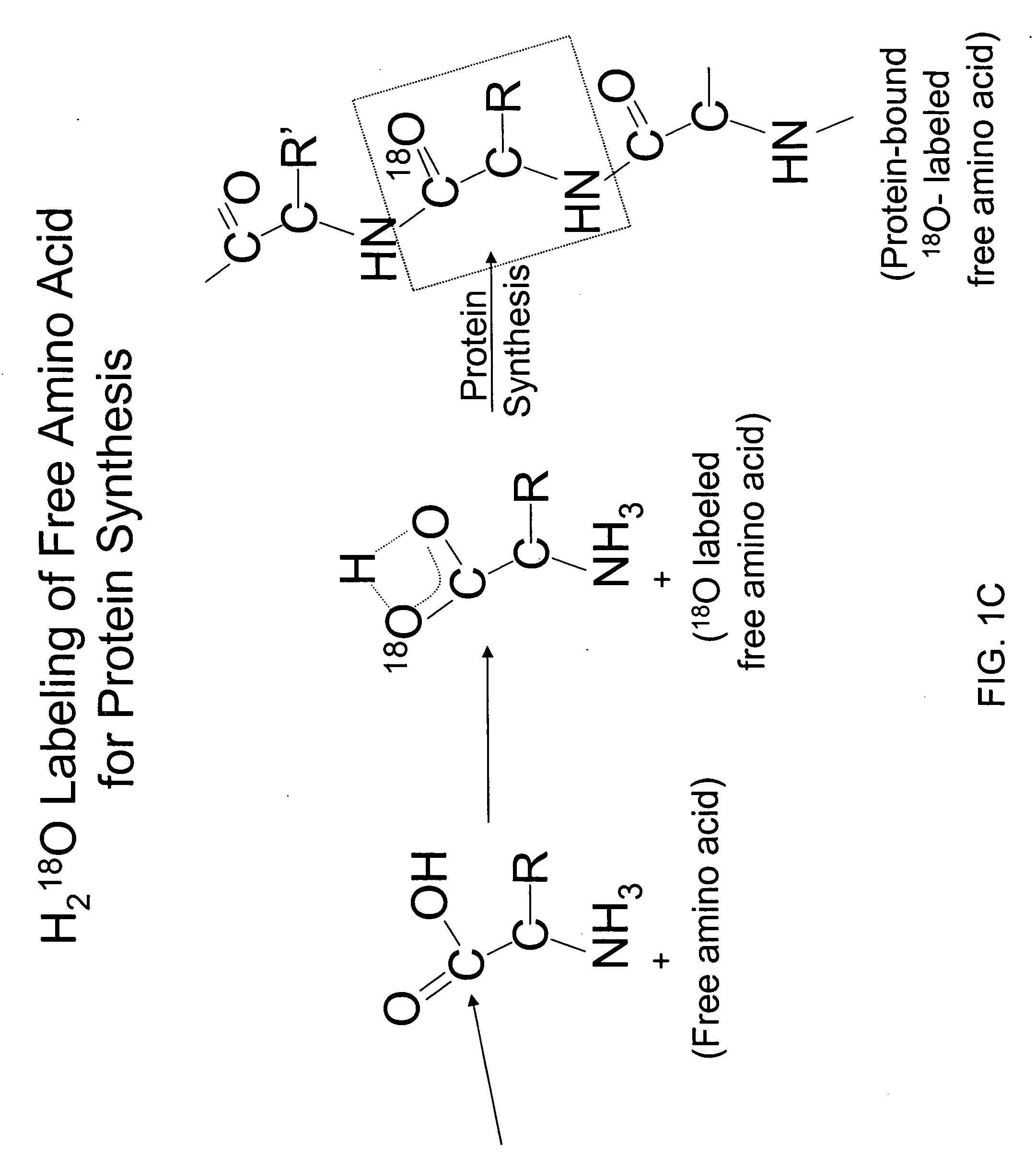
Methods for comparing relative flux rates of two or more biological molecules in vivo through a single protocol
ActiveUS20060204439A1Ease of of half-lifeEase of of levelsIn-vivo radioactive preparationsSugar derivativesDiseaseBio molecules
The invention relates to techniques for measuring and comparing relative molecular flux rates of different biological molecules by administering isotope-labeled water to one or more tissues or individuals and comparing the molecular flux rates of two or more biological molecules, including biological molecules in different chemical classes. The methods find use in several applications including diagnosing, prognosing, or monitoring a disease, disorder, or condition, the in vivo high-throughput screening of chemical entities and biological factors for therapeutic effects in various disease models, and the in vivo high-throughput screening of chemical entities and biological factors for toxic effects.
Owner:RGT UNIV OF CALIFORNIA
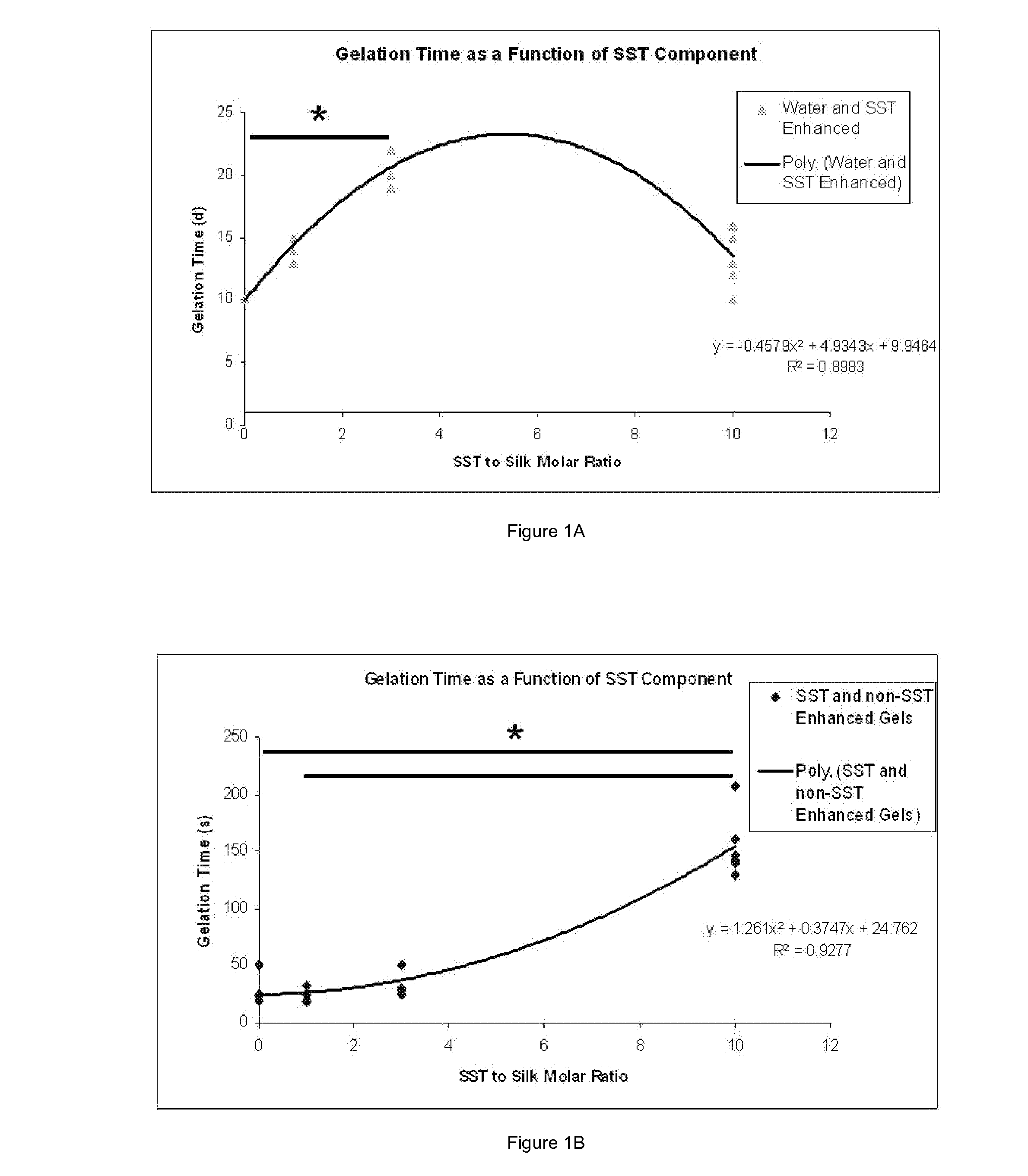
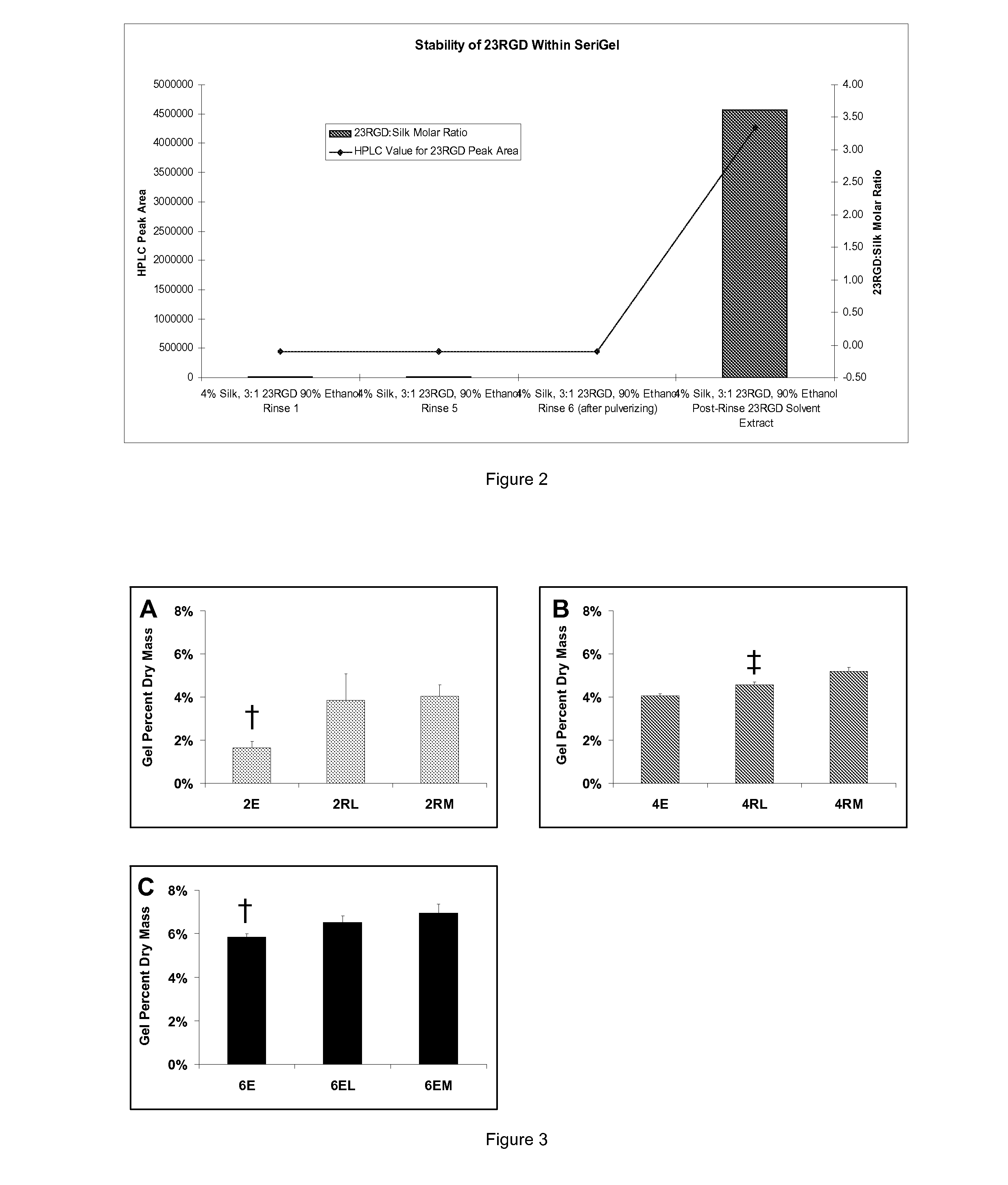
Silk Fibroin Hydrogels and Uses Thereof
InactiveUS20110014287A1Increase profitGood biocompatibilityCosmetic preparationsBiocideDiseaseMedical treatment
The present specification provides for methods for purifying fibroins, purified fibroins, methods of conjugating biological and synthetic molecules to fibroins, fibroins conjugated to such molecules, methods of making fibroin hydrogels, fibroin hydrogels and fibroin hydrogel formulations useful for a variety of medical uses, including, without limitation uses as bulking agents, tissue space fillers, templates for tissue reconstruction or regeneration, cell culture scaffolds for tissue engineering and for disease models, surface coating to improve medical device function, or drug delivery devices.
Owner:ALLERGAN INC
Methods for comparing relative flux rates of two or more biological molecules in vivo through a single protocol
ActiveUS20050019251A1Ease of of half-lifeEase of of levelsIn-vivo radioactive preparationsSugar derivativesHigh-Throughput Screening MethodsIsotopic labeling
The invention relates to techniques for measuring and comparing relative molecular flux rates of different biological molecules by administering isotope-labeled water to one or more tissues or individuals and comparing the molecular flux rates of two or more biological molecules, including biological molecules in different chemical classes. The methods find use in several applications including diagnosing, prognosing, or monitoring a disease, disorder, or condition, the in vivo high-throughput screening of chemical entities and biological factors for therapeutic effects in various disease models, and the in vivo high-throughput screening of chemical entities and biological factors for toxic effects.
Owner:RGT UNIV OF CALIFORNIA
Silk Fibroin Hydrogels and Uses Thereof
ActiveUS20110014263A1Increase profitGood biocompatibilityBiocideCosmetic preparationsDiseaseMedical treatment
The present specification provides for methods for purifying fibroins, purified fibroins, methods of conjugating biological and synthetic molecules to fibroins, fibroins conjugated to such molecules, methods of making fibroin hydrogels, fibroin hydrogels and fibroin hydrogel formulations useful for a variety of medical uses, including, without limitation uses as bulking agents, tissue space fillers, templates for tissue reconstruction or regeneration, cell culture scaffolds for tissue engineering and for disease models, surface coating to improve medical device function, or drug delivery devices.
Owner:ALLERGAN INC
Method for preparing high muscle content and hypertrophic cardiomyopathy model cloned pig
InactiveCN105463027AImprove convenienceShorten the timeNucleic acid vectorFermentationHypertrophic cardiomyopathyNuclear transfer
The invention provides application of a Trim63 gene in preparation of a high muscle content and hypertrophic cardiomyopathy model cloned pig. Somatic cells of the Trim63 gene of a modified pig are utilized as nuclear transfer donor cells, oocytes are utilized as nuclear transfer recipient cells, and a cloned embryo is obtained through the somatic cell nuclear transfer technology. The cloned embryo is transferred into a pig uterus to gestate to obtain the Trim63 gene modified high muscle content and hypertrophic cardiomyopathy model cloned pig, artificial intervention methods, such as operation and so on for the cloned pig are not needed any more, and the efficiency of establishment of a disease model is improved. According to the high muscle content and hypertrophic cardiomyopathy model cloned pig, paired Cas9n targeting vectors are utilized for the first time to perform gene editing for large animals. The method is low in cost, sharply shortens the time for obtaining a homozygote pig and lays a foundation for gene function research and the disease model establishment for the large animals by utilizing the CRISPR / Cas9 technology.
Owner:CHINA AGRI UNIV
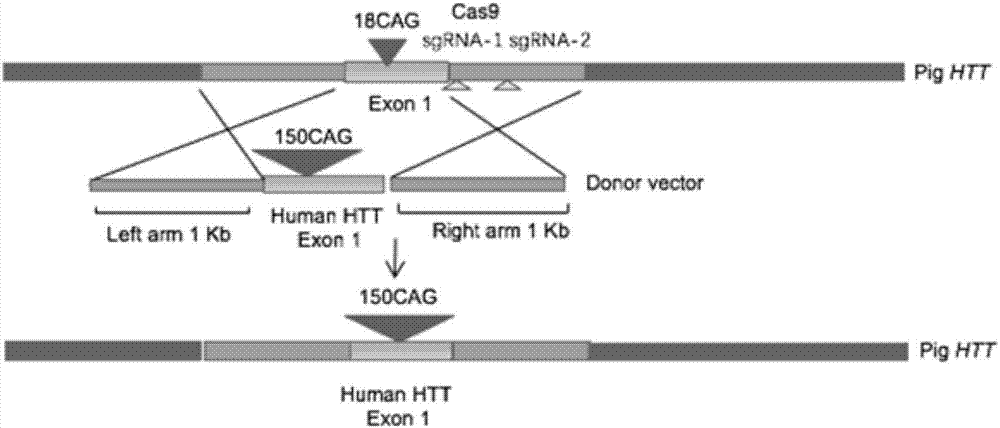
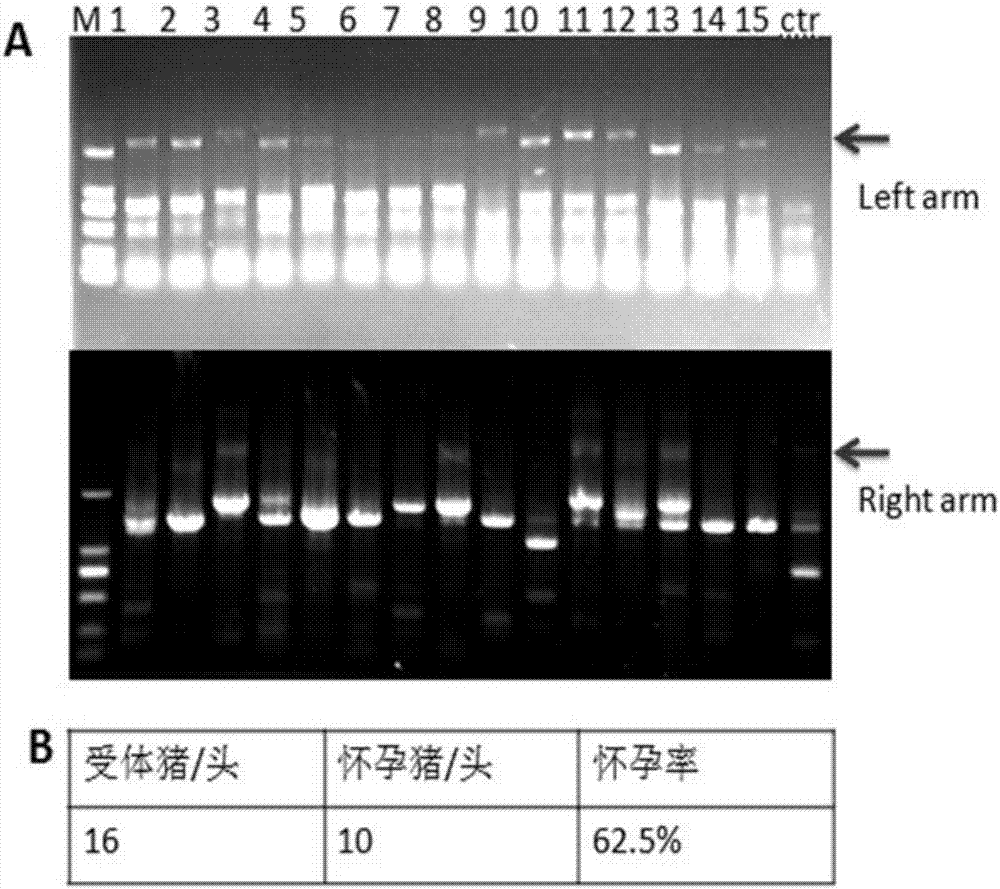
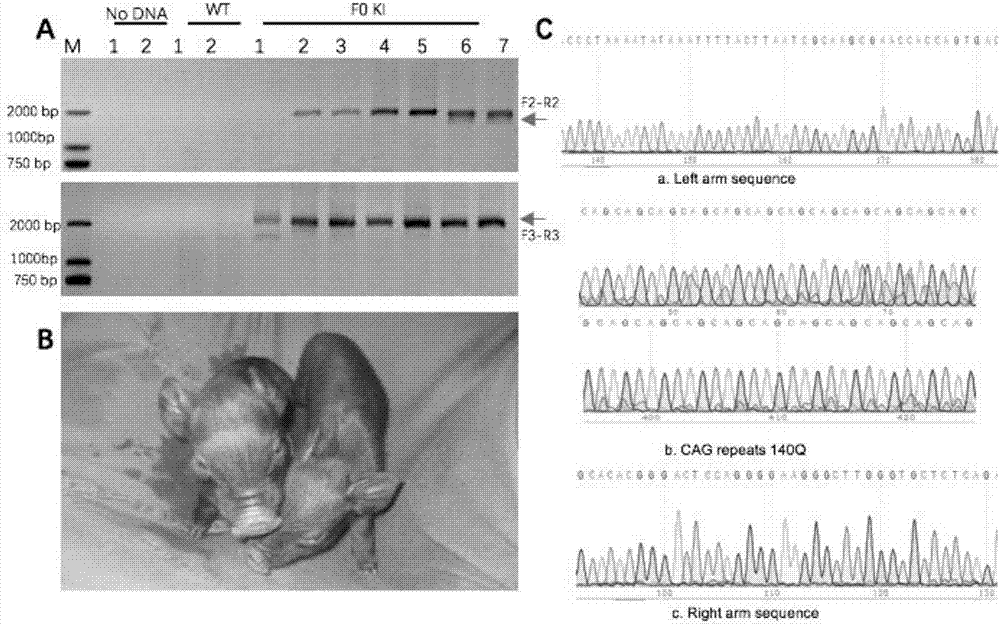
Recombinant vector for knock-in of human Huntington gene, construction method of recombinant vector and application of recombinant vector in construction of model pig
ActiveCN107988256AIncreased probability of knock-in positive clonesEfficient FeasibilityStable introduction of DNANucleic acid vectorHuman studiesExon
The invention discloses a recombinant vector for the knock-in of a human Huntington gene, a construction method of the recombinant vector and application of the recombinant vector in the constructionof a model pig. According to the recombinant vector, a human mutated Huntington exon gene is knocked in a fixed point manner for the first time, a virulence gene is knocked in by virtue of a CRISPR / Cas9 technique for the first time, and a donor vector is optimized by optimizing sfRNA, so that the probability that the gene is knocked into a positive cloned cell is increased; and by combining with apig cell nucleus transplantation technique, the probability that a directly obtained positive cell is knocked into the pig is increased, and a small human Huntington gene knock-in pig is obtained, thereby proving the efficient feasibility of the method for constructing gene modified pigs. The constructed Huntington gene knock-in model pig has behavioral characteristics such as respiratory disturbance and dyskinesia similar to human Huntington diseases, and stable heritable passage can be realized, so that a reliable model is provided for the research of the human Huntington diseases; and thenumber can be guaranteed so as to realize drug screening, gene treatment, stem cell treatment and the like, and the model can be a good human disease model.
Owner:JINAN UNIVERSITY +1
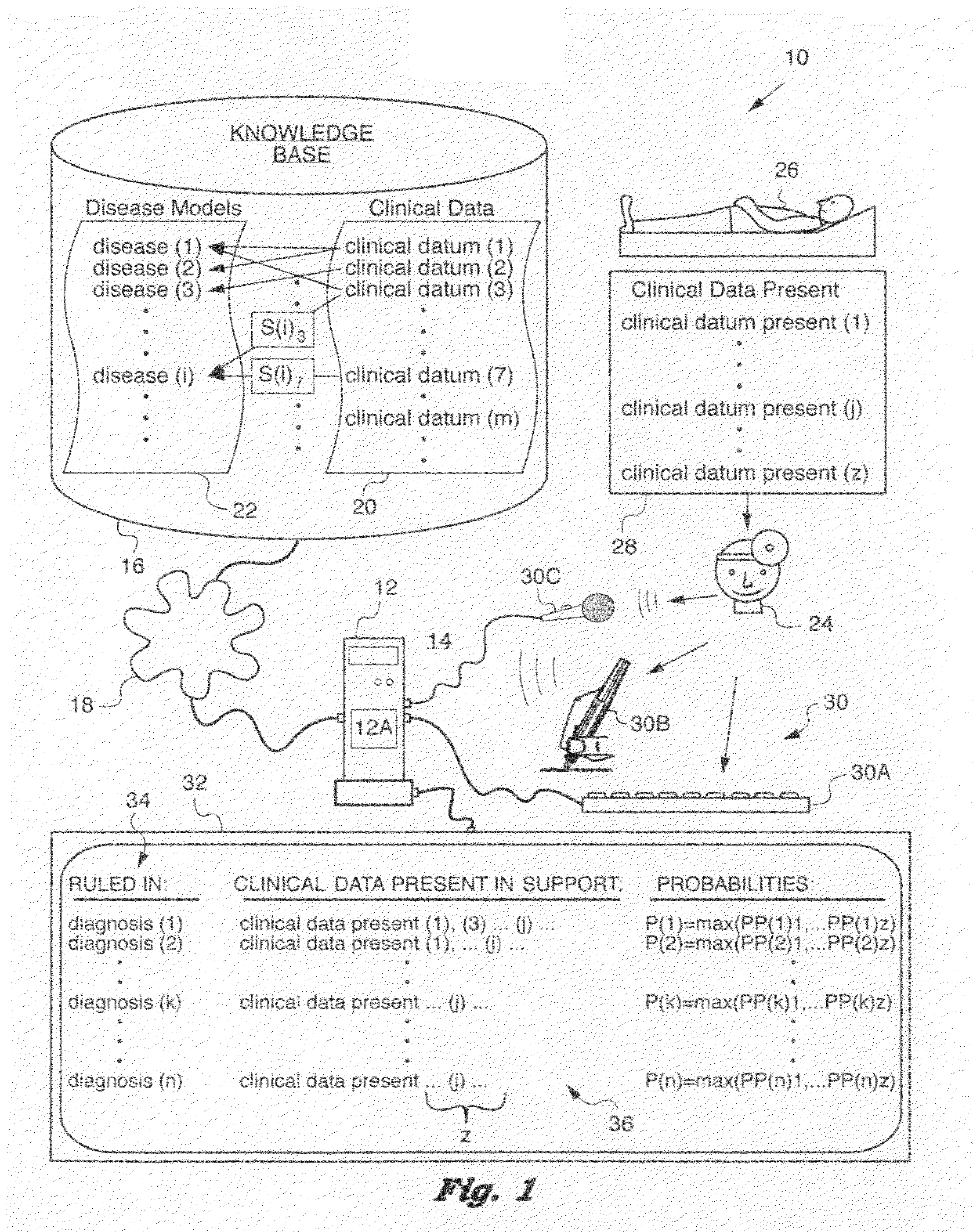
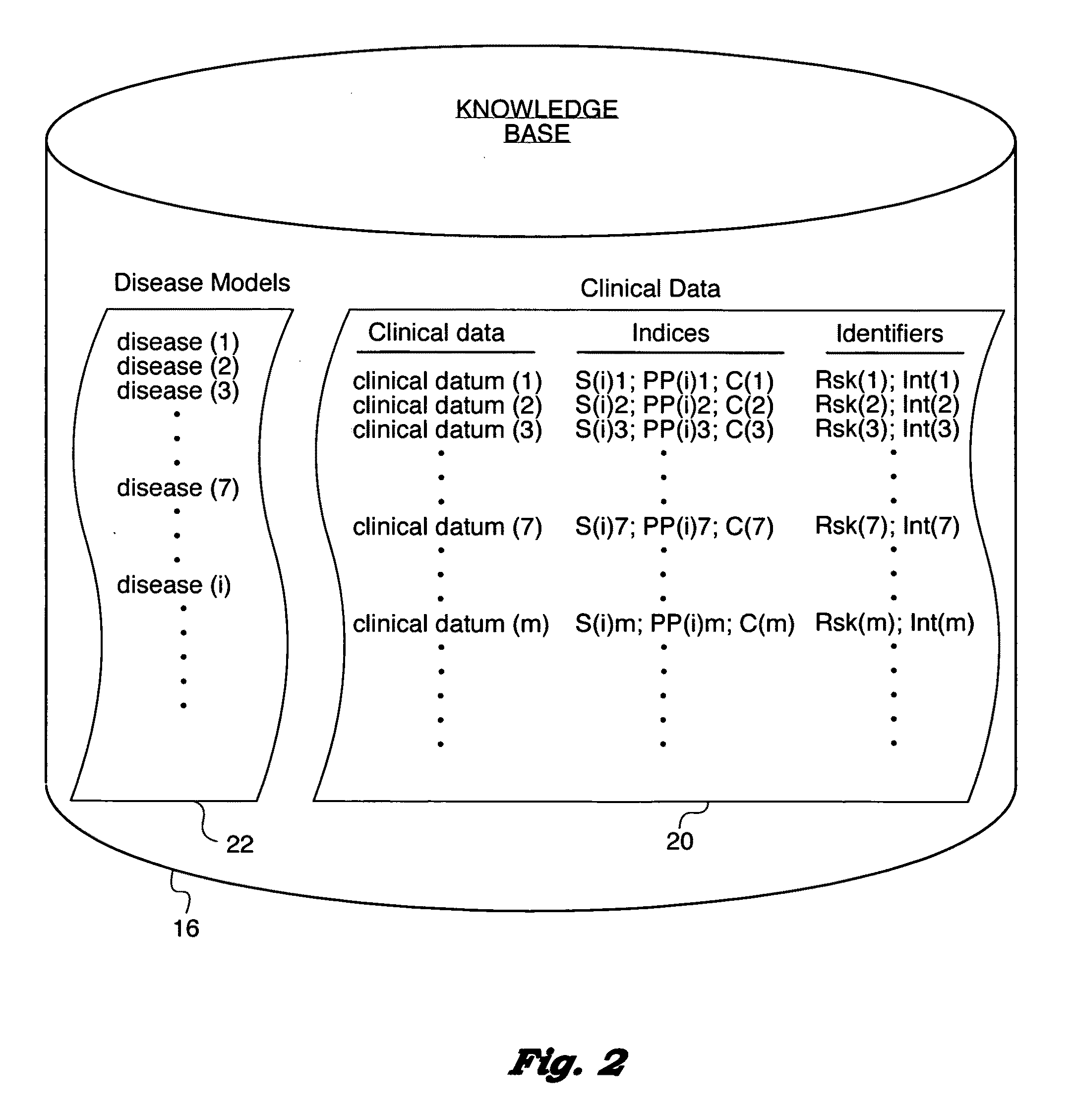
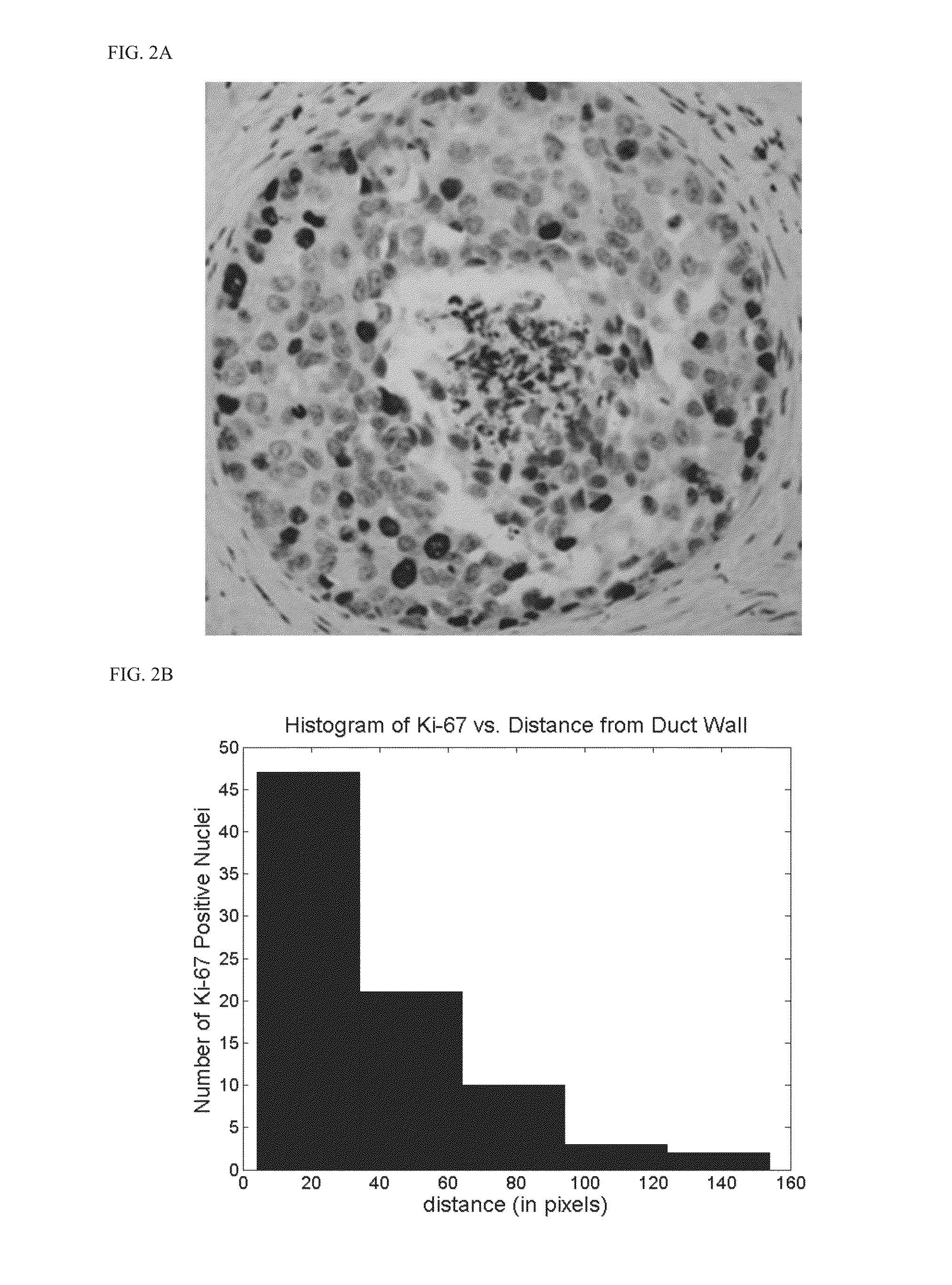
Computer-implemented medical analytics method and system employing a modified mini-max procedure
ActiveUS20090259494A1Simplify probabilistic determinationLimit natureFinanceForecastingDiseasePresent method
A method and system for medical analytics implemented on a computer and designed to aid a medical professional in diagnosing one or more diseases afflicting a patient. In contrast to prior art, the present method is based on using clinical data (m) that excludes subjective qualities of and also excludes prevalence of the one or more diseases (i). The method uses a knowledge base that contains disease (i) models exhibiting clinical data (m). Clinical data present (j) in the patient are input into the computer. Then, clinical data present (j) are matched with clinical data (m) in the knowledge base to enable the computer to compose a differential diagnosis list of ruled in diagnoses (k), where k=1 . . . n, for each of the disease (i) models that exhibits at least one clinical datum (m) that matches at least one clinical datum present (j) in the patient. In a key step, the computer computes a probability P(k) for each of the ruled in diagnoses (k) with the aid of a mini-max procedure that overcomes prior art limitations of the Bayes formulation and permits the analytics method to consider concurrent and competing diagnoses (k). Furthermore, the method composes pairs of clinical data present (j) and absent (r) in the patient to aid the medical professional in evaluating diagnoses and determining the most cost-effective clinical data to collect for conducting an effective and rapid diagnostic quest.
Owner:KNIDIAN INC
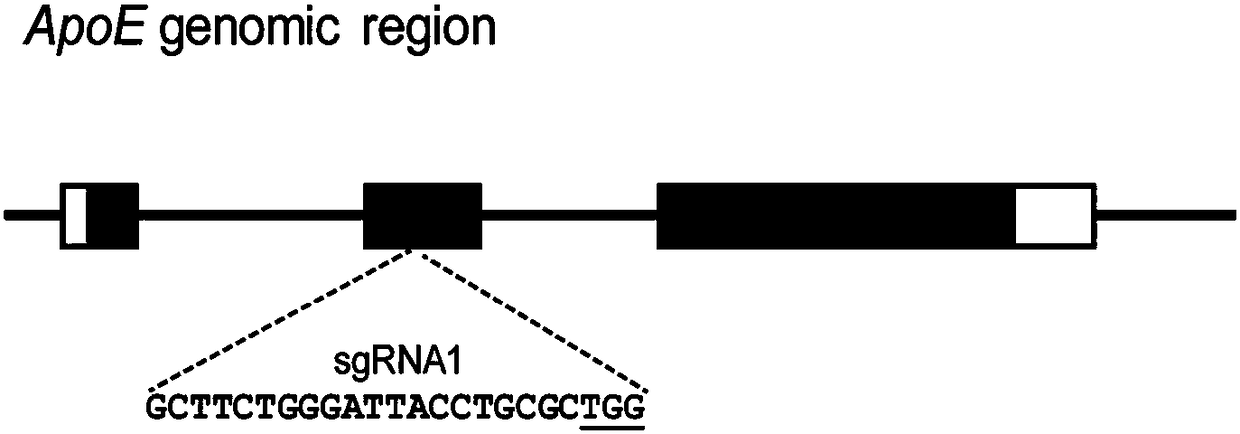
ApoE-CRISPR/Cas9 carrier and application thereof to ApoE gene knockout
InactiveCN108148837AAccelerated disease progressionSpeed up the modeling processApolipeptidesHydrolasesMammalCas9
The invention discloses a SgRNA (single chain guide ribonucleic acid) of the exon 2 of a specific targeted ApoE gene. The nucleotide sequence of the SgRNA is GCTTCTGGGATTACCTGCGC. The invention also discloses an ApoE-CRISPR / Cas9 carrier containing the SgRNA and application of the ApoE-CRISPR / Cas9 carrier to structuring ApoE gene knockout mammal models and to preparing ApoE gene knockout kits. TheApoE-CRISPR / Cas9 carrier structures the ApoE gene knockout mammal models; ApoE gene knockout can affect in vivo lipid metabolism of mammals and accelerate the pathogenic processes of lipid metabolismcorrelated diseases such as atherosclerosis to further accelerate the modeling process of corresponding mammal disease models and provide animal experiment basis for research on diseases such as atherosclerosis.
Owner:NANJING MEDICAL UNIV
Transgenic animal and methods
InactiveUS20020066117A1Easy to useCompounds screening/testingAnimal cellsNeurological disorderPathology diagnosis
A transgenic animal, preferably a mouse, that expresses human antichymotrypsin (ACT) in brain tissues is provided, together with animal tissue-derived cell lines and progeny animals of said transgenic animal. Progeny are obtained by mating the transgenic animal with select animal strains used as models of Alzheimer's disease, related neurological disorders, or amyloidogenic diseases. Methods utilizing the parent and progeny animals and cells derived therefrom are disclosed for testing compounds for use as anti-inflammatory drugs, inhibitors of amyloidogenesis, and / or inhibitors of tau protein pathology associated with Alzheimer's disease, in the treatment of a variety of neurological diseases.
Owner:SOUTH FLORIDA UNIVESITY OF
Transgenic mice containing glucagon receptor gene disruptions
InactiveUS6909031B2Enhance homologous recombinationLose weightAnimal cellsCell receptors/surface-antigens/surface-determinantsDiabetes mellitusRegulator gene
The present invention relates to transgenic animals, as well as compositions and methods relating to the characterization of gene function. Specifically, the present invention provides transgenic mice comprising mutations in a glucagon receptor gene. Such transgenic mice are useful as models for disease and for identifying agents that modulate gene expression and gene function, and as potential treatments for various disease states and disease conditions. The present invention also relates to diabetes and diabetic condition, as it demonstrates the role of the glucagon receptor in diabetes and diabetic conditions. The present invention further relates to weight gain and weight related conditions, such as obesity, and demonstrates the role of the glucagon receptor in weight gain and weight related conditions, such as obesity. In accordance with these aspects, the present invention provides methods and compositions useful in identifying, testing, and providing treatments for diabetes and diabetic conditions, weight gain and weight related conditions such as obesity.
Owner:DELTAGEN INC
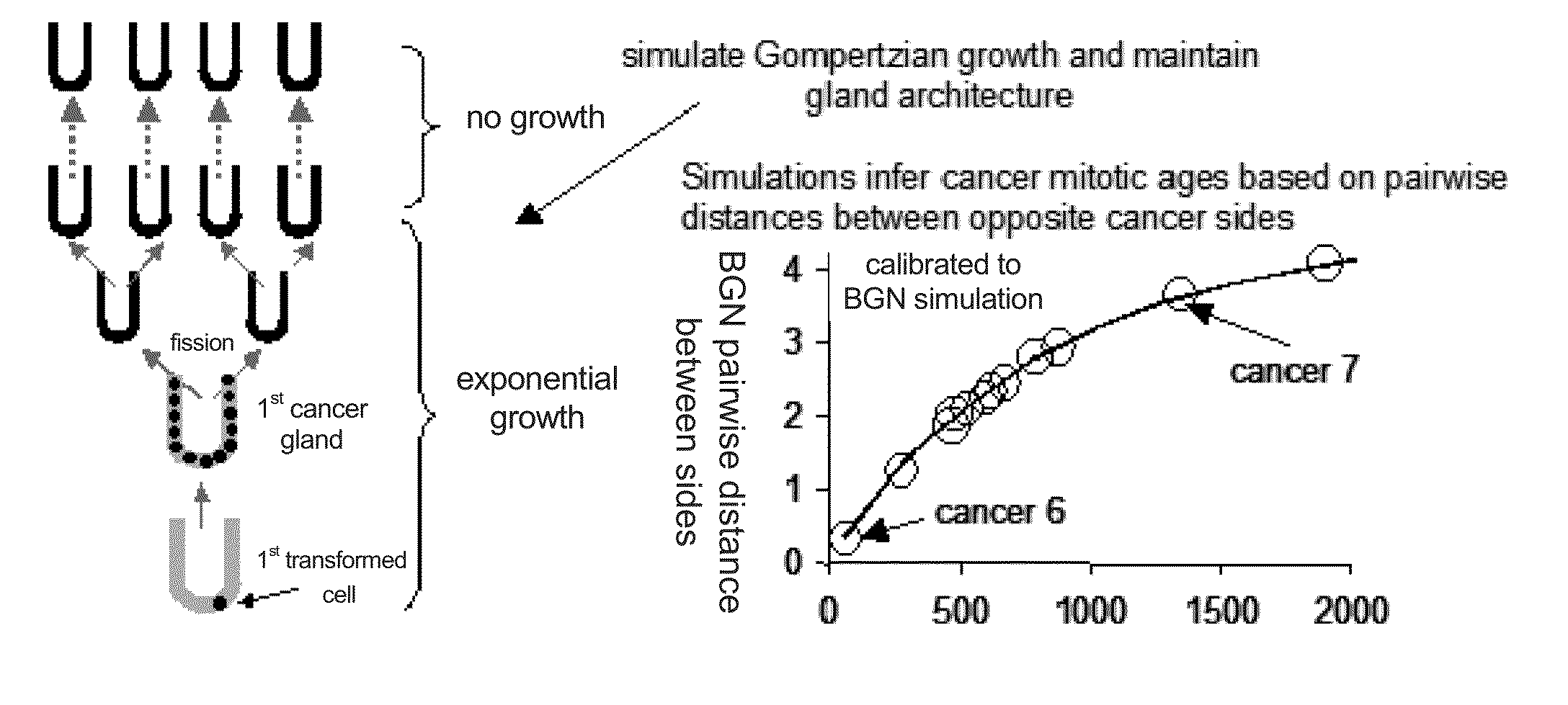
Multi-scale complex systems transdisciplinary analysis of response to therapy
Described herein are methods and systems to measure dynamics of disease progression, including cancer growth and response, at multiple scales by multiple techniques on the same biologic system. Methods and systems according to the invention permit personalized virtual disease models. Moreover, the invention allows for the integration of previously unconnected data points into an in silico disease model, providing for the prediction of disease progression with and without therapeutic intervention.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
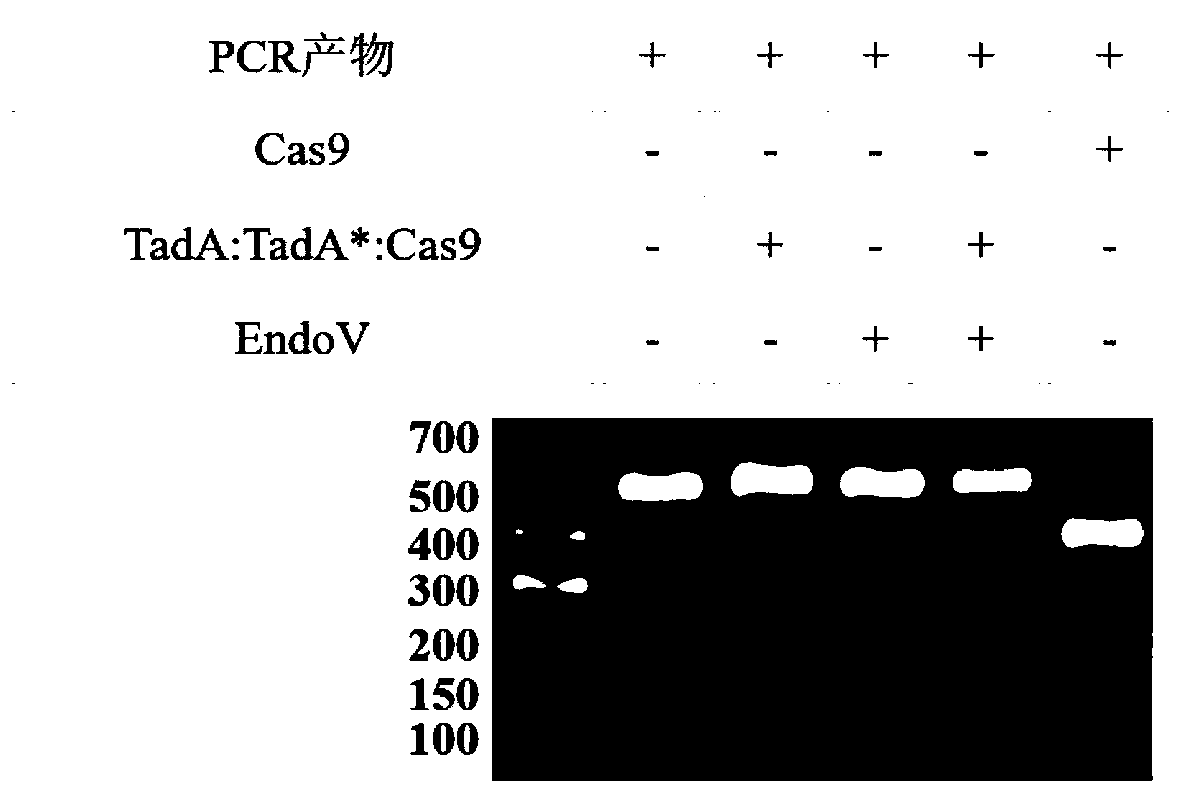
Method for detecting off-target effect of adenine base editor system based on whole genome sequencing, and its application in gene editing
PendingCN109295186AAccelerate cultivationMicrobiological testing/measurementAgainst vector-borne diseasesGenome editingWhole genome sequencing
The invention provides a method for detecting the off-target effect of an adenine base editor system based on whole genome sequencing, and its application in gene editing, The adenine base editor system comprises a TadA:TadA*:Cas9 fusion gene and gRNA. The system can catalyze the efficient replacement of adenine (A) at a target site with guanine (G), and has a broad application prospect in human disease gene editing therapy and disease model construction. The first method for detecting the off-target effect of the ABE system in a whole genome range, called EndoV-seq method for short, is developed in the invention. The EndoV-seq method provided by the invention has a broad application prospect in the field of gene editing, especially gene editing therapy.
Owner:SUN YAT SEN UNIV
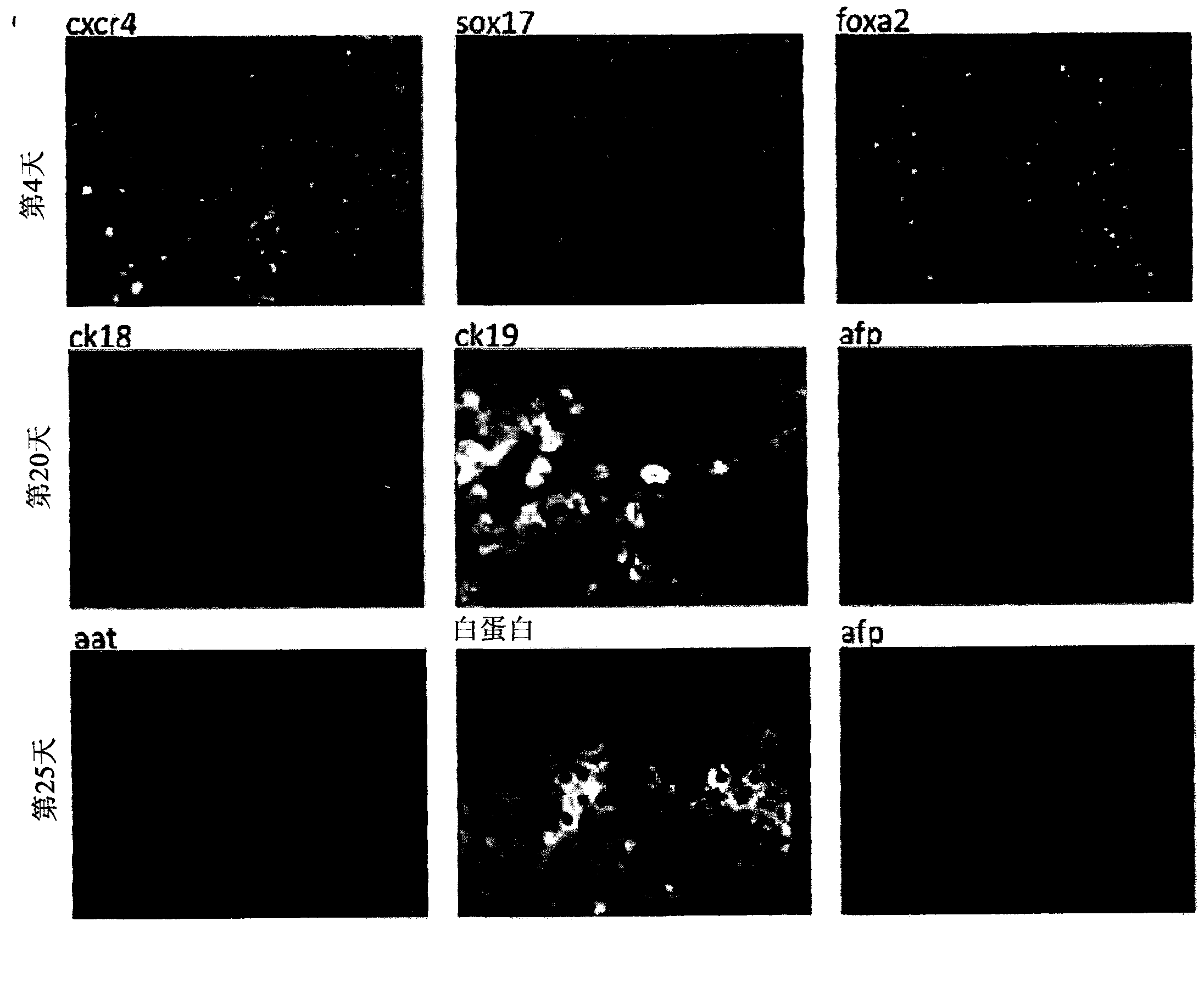
Vitro hepatic differentiation
Owner:CAMBRIDGE ENTERPRISE LTD
Methods and compositions for development of drug screening procedures and diagnostic tools
This invention defines novel research and clinical laboratory methodology and compositions related thereto appropriate for use in (a) determining the presence of a neurodegenerative disease selected from the group limited solely to Charcot-Marie-Tooth disease, familial Alzheimer's disease, familial Parkinson's disease, Huntington's disease, spinal muscular atrophy, Friedreich'a ataxia, giant axon neuropathy, juvenile ceroid-lipofuscinosis, familial motor neuron diseases, juvenile diabetic polyneuropathy and Down's syndrome, (b) monitoring the ongoing status of the physiological expression of said disease and (c) screening candidate therapeutic drug agents for possible effectiveness. The invention is based on the new and novel observation that the presence of a neurodegenerative disease can be characterized in part by the expression in cultured fibroblasts obtained from the patient of one or more proteins which are not the product of a defective disease-inducing gene, but which are stress proteins, one or more other proteins modified by conditions of oxidative stress or one or more other disease-related proteins. The invention depends on living cell material, namely fibroblasts, which are readily and, if necessary, repeatedly available from a patient. When adapted as a method and composition useful for the screening candidate therapeutic drug agents for possible effectiveness, this technology offers advantages in terms of (a) providing research opportunities which, in some cases, never existed before, (b) cost effectiveness when compared to alternative technologies, (c) ability to be used readily on a large scale, (d) ability to generate meaningful data in a comparatively short period of time, and (e) providing an early stage opportunity to obtain information based on direct interaction of a candidate drug and a living tissue disease model. Various aspects of diagnostic methods and compositions are also disclosed.
Owner:SHAPIRO HOWARD K
Anti-Alzheimer's Transgenic Drosophila Model and Its Application in Drug Screening
The invention relates to establishment and application of a model for screening medicines for treating Alzheimer disease, and aims to provide a transgenic Dosophila melanogaster model for studying molecular pathway of pathogenesis progress of Alzheimer disease and drug screening. The invention obtains stable heritable novel Drosophila melanogaster variety by successive crossing of available transgenic Drosophila melanogaster. The novel disease model can simulate the in vivo generation and metabolism processes of major pathogenic genes of Alzheimer disease, and reproduce the pathogenesis process of the disease. The Drosophila melanogaster model provided by the invention is proven by a variety of indicators closely related to the symptoms of disease, including survive curve, mobility and learning and memory indexes and in vivo experiments, to be distinctly different from other Alzheimer disease Dosophila melanogaster models in all respects.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
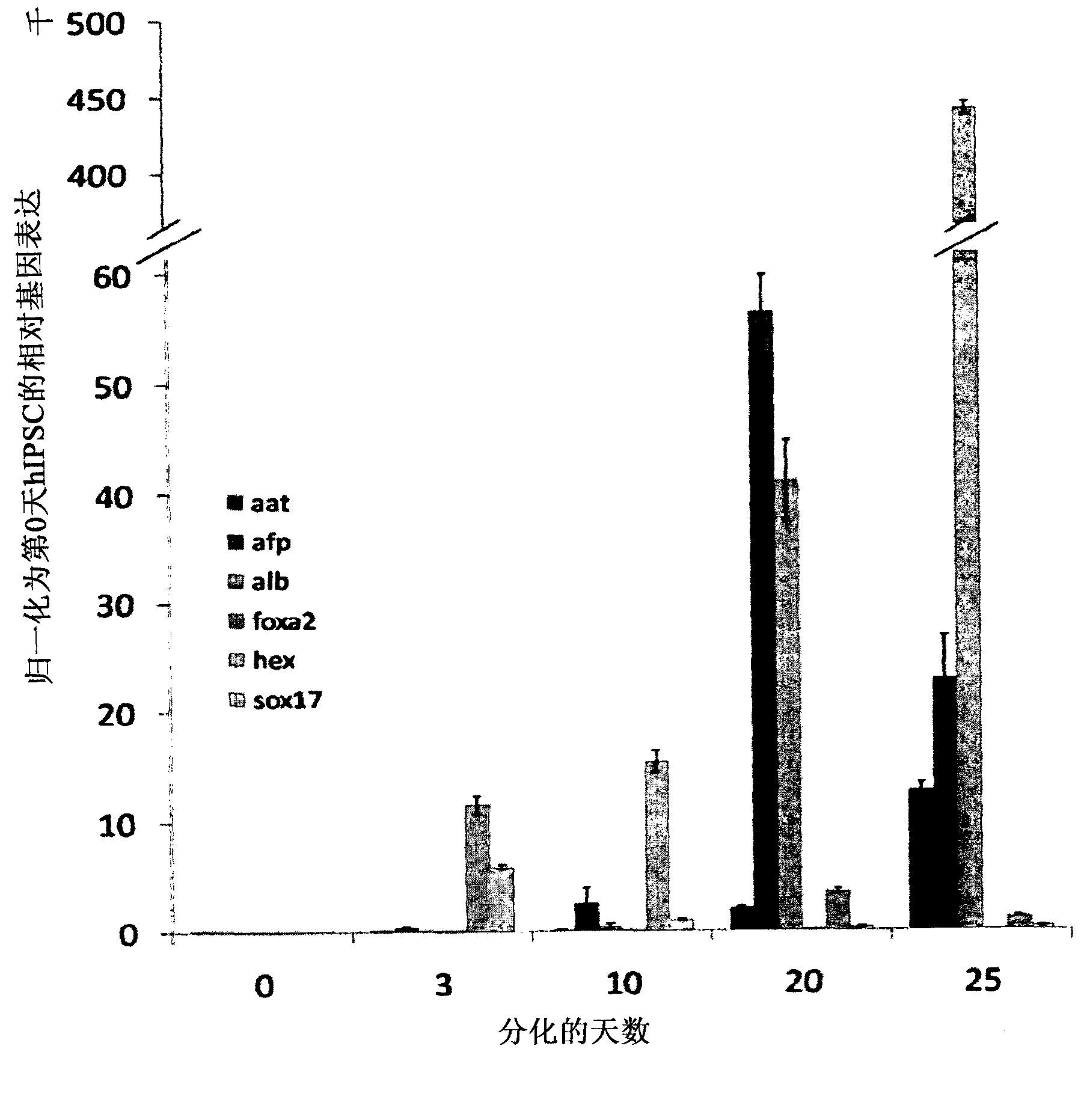
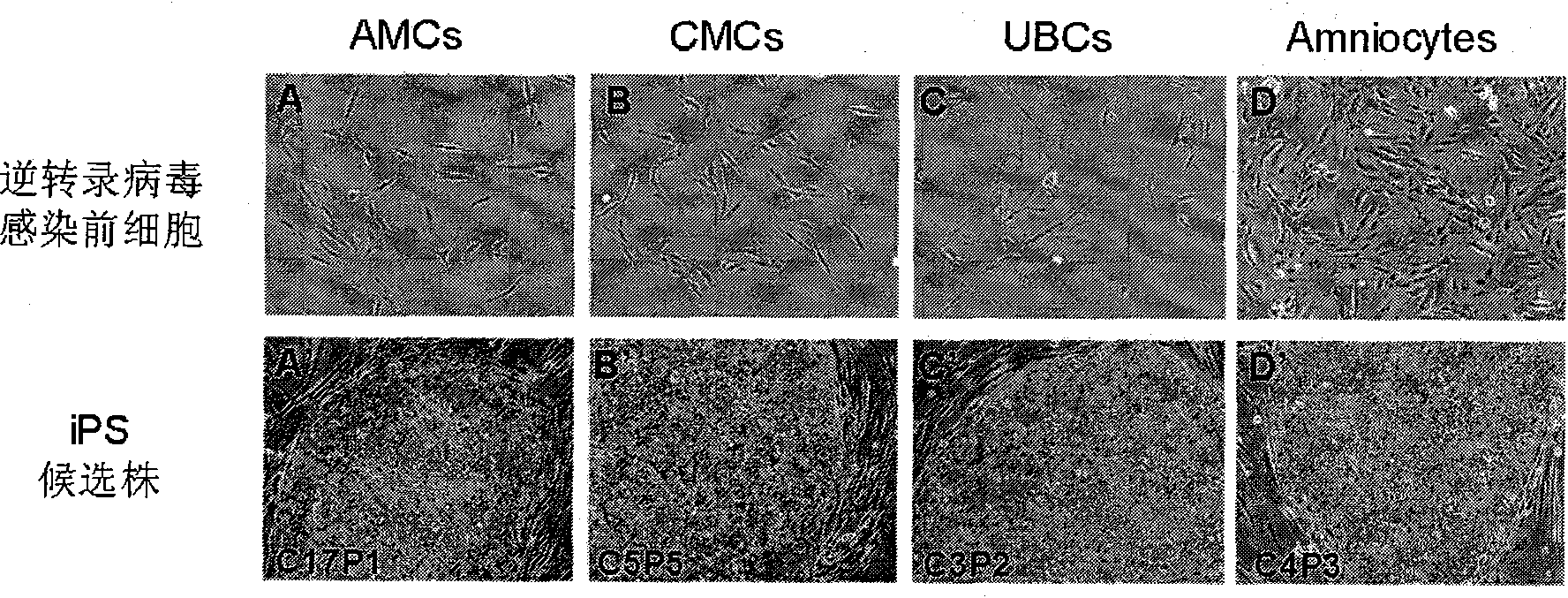
Cell type used for producing induced pluripotent stem (iPS) cells and preparation method and application thereof
InactiveCN101984050AGreat therapeutic application prospectsGood pluripotencyFermentationVector-based foreign material introductionControl orientedDisease
The invention discloses four cell types which have wide heteroplastic transplantation application prospect and can be effectively induced into induced pluripotent stem (iPS) cells. The origins of three cell types are placental tissue, namely amnion mesenchymal cell, chorion mesenchymal cell and umbilical cord mesenchymal cell; and the origin of the other one is amniotic fluid cell. Besides, the invention also discloses an induction reprogramming method for producing cells capable of producing induced pluripotent stem (iPS) cells by efficient induction, including the following steps: cDNA containing pluripotent stem cell factor is respectively introduced into the four primary culture cells; the four primary cells in which cDNA is introduced are respectively cultured on appropriate culture mediums, primary iPS is obtained and then quality of iPS is optimized on appropriate culture mediums, and cloning of pluripotent stem cell can be primarily evaluated. In the invention, three mesenchymal cells of placenta origin and amniotic fluid cell all can be induced into iPS, wherein the chorion mesenchymal cell is compared with fibroblast, efficiency of induction reprogramming is improved by over 100 times, efficiency is about 2.3%, and the efficiency is slightly higher than that of horny cell; and a means which is more effective and more pertinent is provided for building disease model, screening drug and controlling oriented differentiation.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
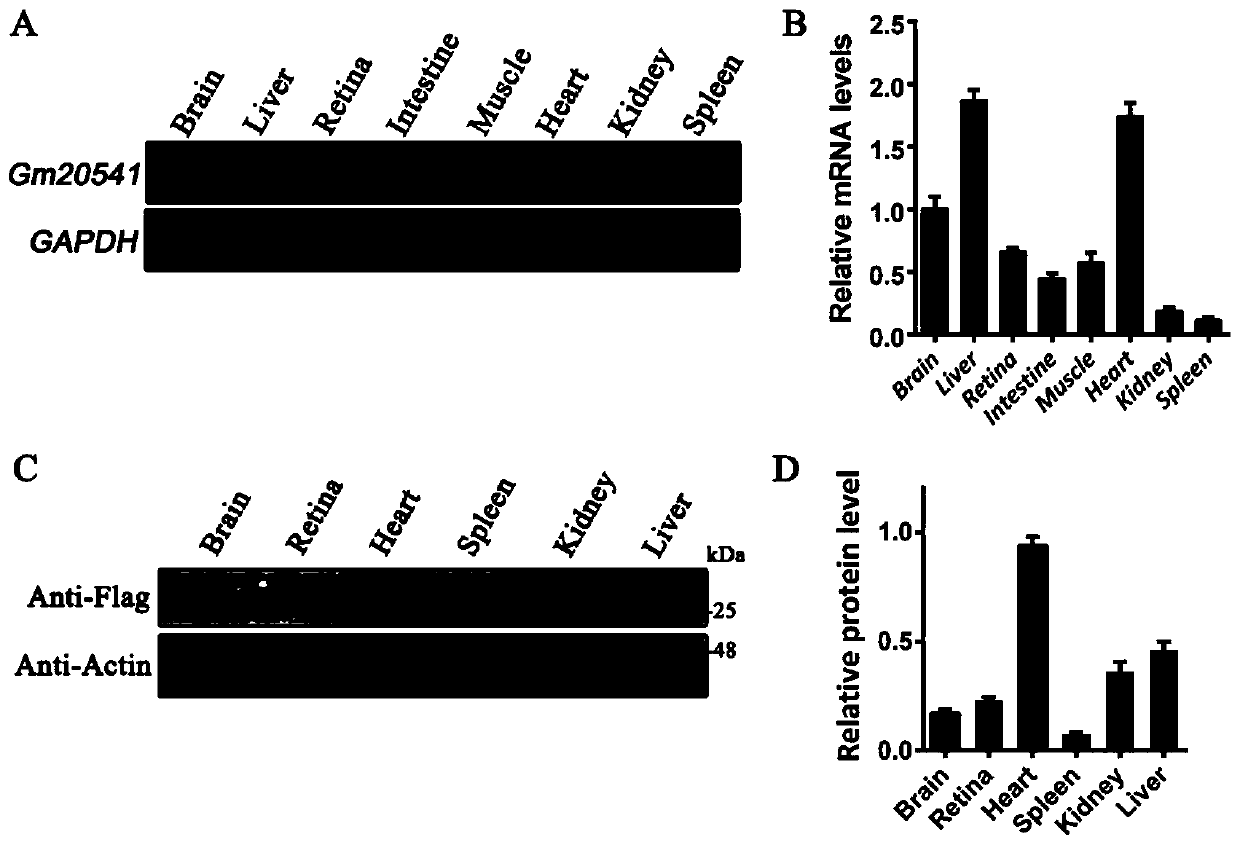
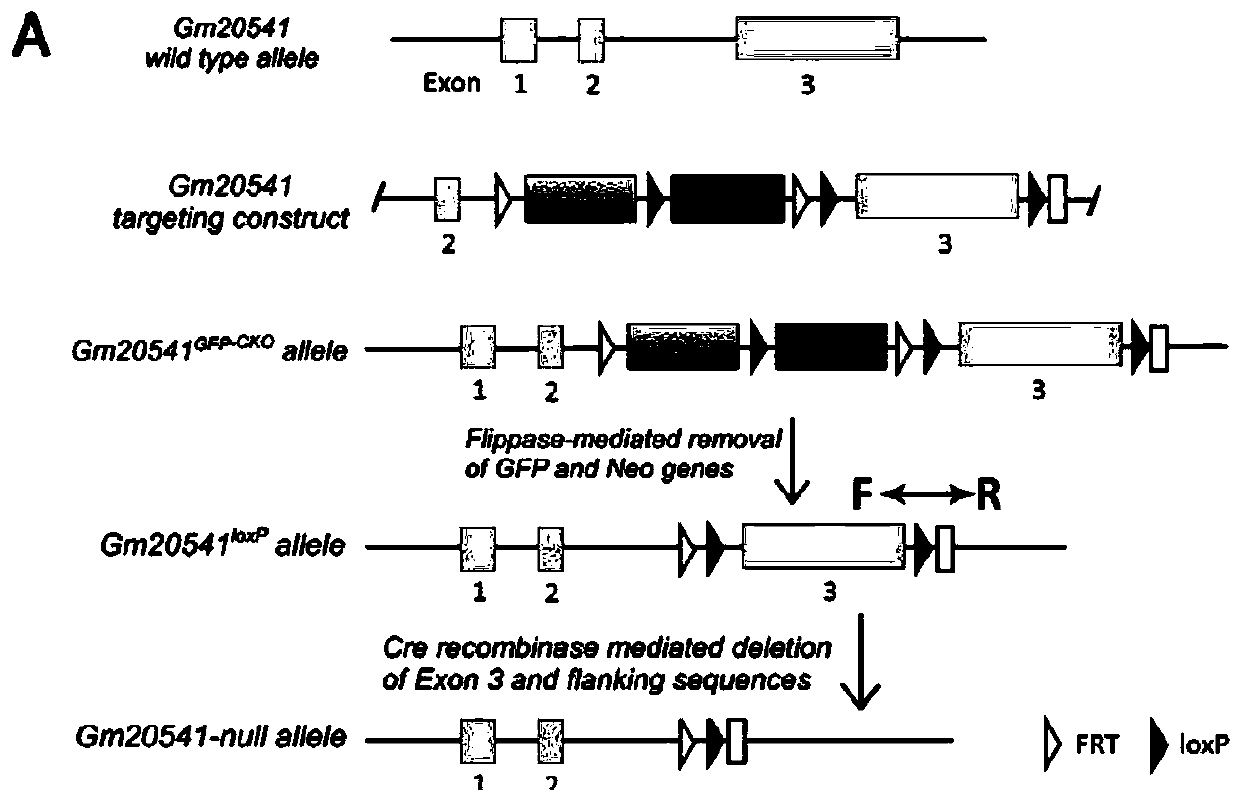
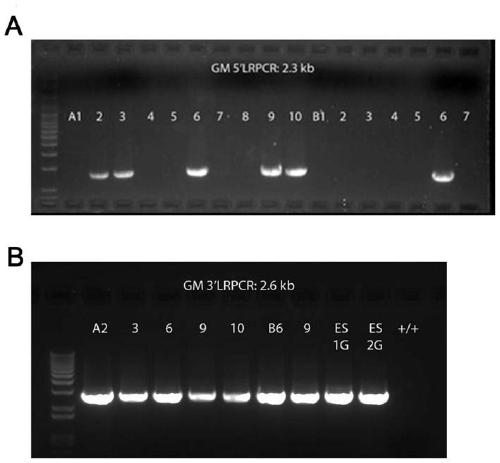
Method for constructing retinal pigment degeneration disease model by utilizing Gm20541 gene and application
The invention discloses a method for constructing a retinal pigment degeneration disease model by utilizing a Gm20541 gene and application, and relates to the technical field of medical engineering. According to the method disclosed by the invention, a Gm20541 gene sequence in a genome in retinal precursor cells is knocked out in a specific targeting manner; a target animal shows typical retinal pigment degeneration disease characteristics; a reliable animal model is provided for research of the Gm20541 gene related retinal pigment degeneration disease, the pathogenesis and mechanism of the retinal pigment degeneration disease can be helped to be illuminated, a new target is provided for treatment or prevention of the disease, and the application prospect is wide.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Methods of evaluating a test agent in a diseased cell model
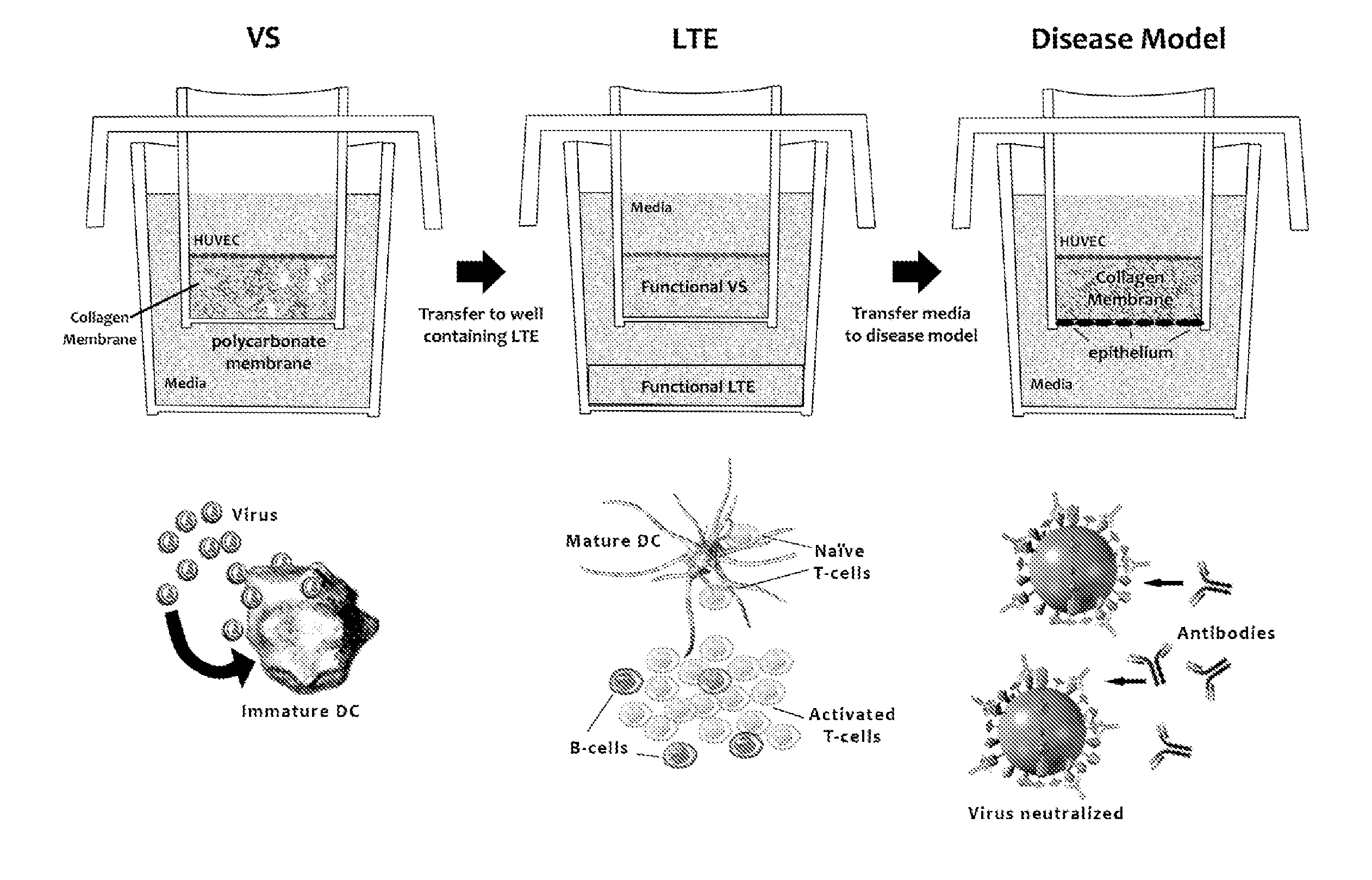
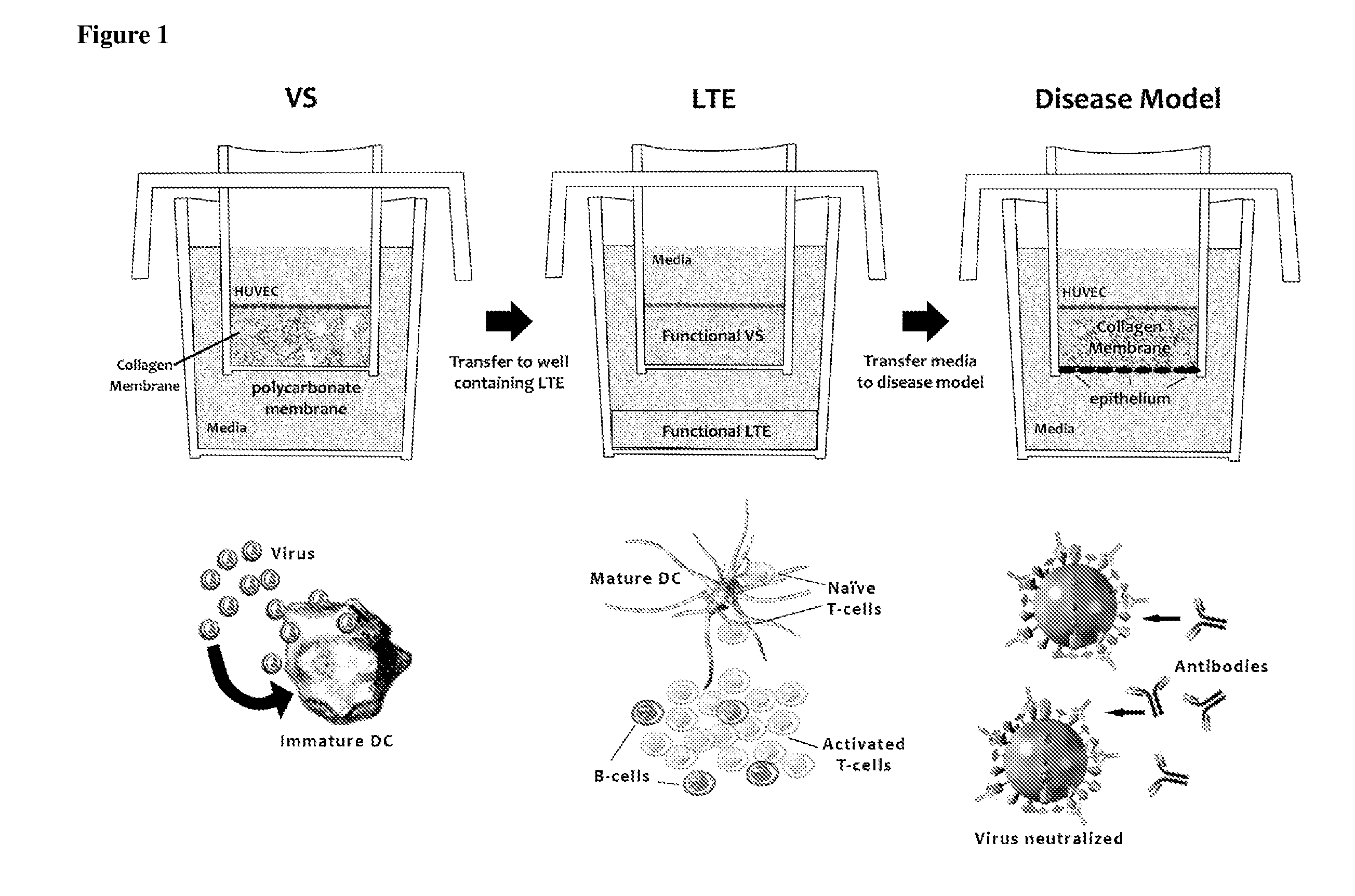
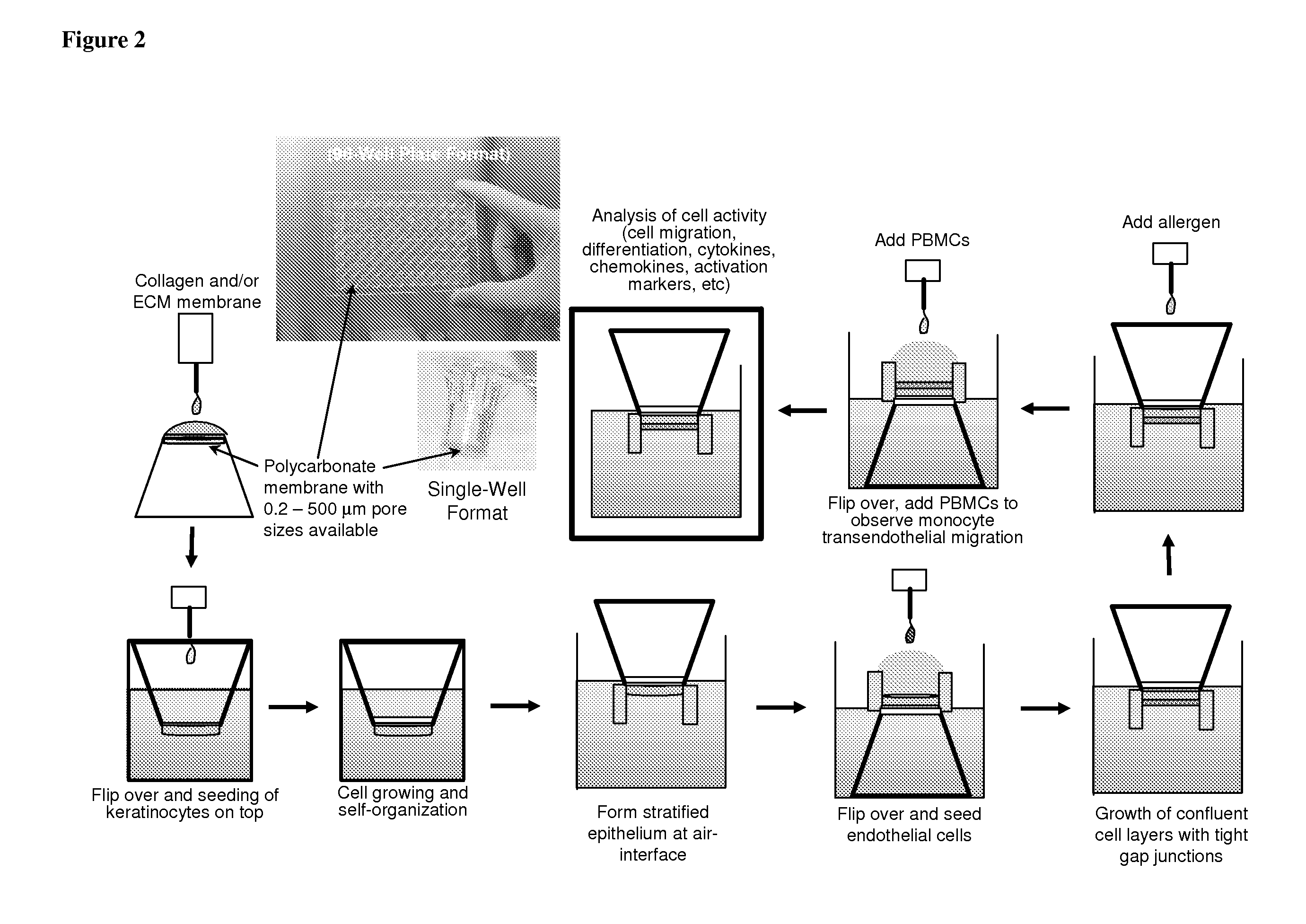
ActiveUS20100279277A1Effective therapyAvoid problemsEpidermal cells/skin cellsSkeletal/connective tissue cellsArtificial immune systemTest agent
The present invention relates to methods of constructing an integrated artificial immune system that comprises appropriate in vitro cellular and tissue constructs or their equivalents to mimic the tissues of the immune system in mammals. The artificial immune system can be used to test the efficacy of vaccine candidates and other materials in vitro and thus, is useful to accelerate vaccine development and testing drug and chemical interactions with the immune system, coupled with disease models to provide a more complete representation of an immune response.
Owner:SANOFI PASTEUR VAX DESIGN
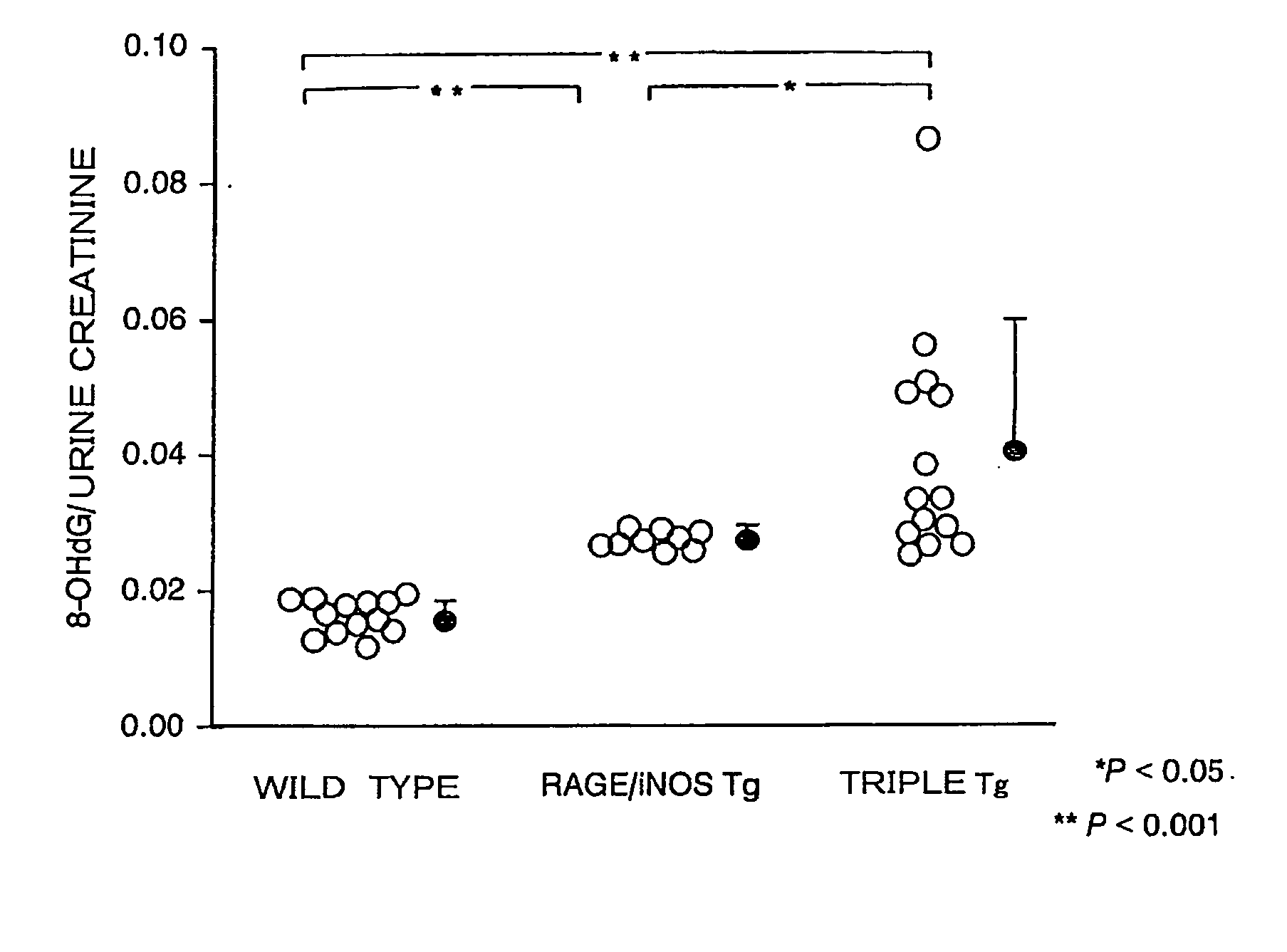
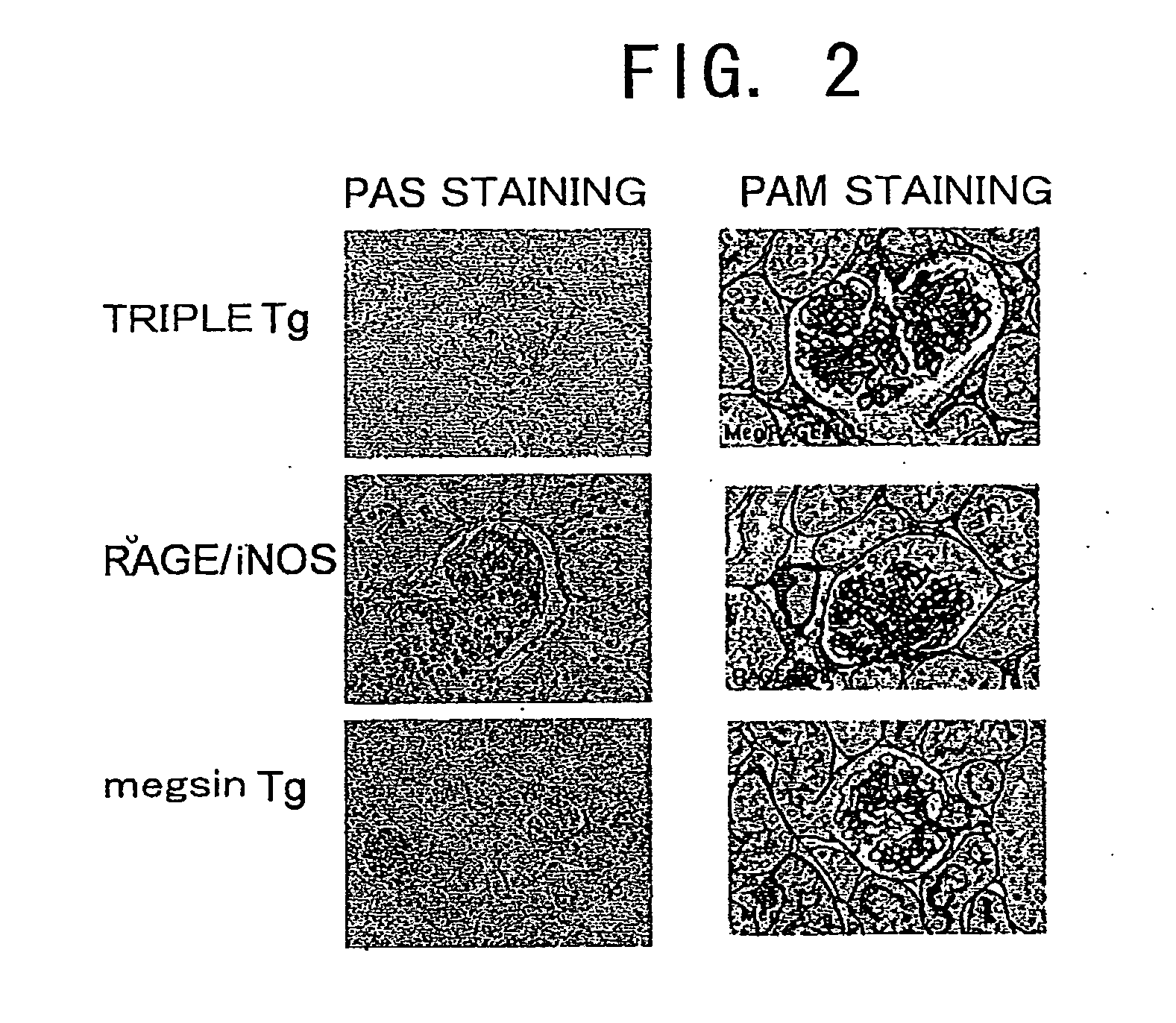
Megsin/Rage/Inos-Overexpressing Renal Disease Model Animals and Methods for Evaluating Compounds Using the Model Animals
InactiveUS20080263683A1Evaluating effectHighly accurate experimentsDisease diagnosisOxidoreductasesBiologyKidney
Triple Tg (megsin / RAGE / iNOS-Tg) was created by crossing megsin-Tg with RAGE / iNOS-Tg. The megsin / RAGE / iNOS-Tg develops marked pathologies of diabetic nephropathy unfound in conventional models at early stages, and various pathological conditions such as glomerular hypertrophy were observed uniformly in the megsin / RAGE / iNOS-Tg mice. In addition, it was also found that animals exhibiting these symptoms were useful as a disease model animal for diabetic nephropathy. Specifically, the disease model animals of the present invention overexpress the megsin gene, a gene encoding the receptor for advanced glycation end-products, and an inducible nitric oxide synthase gene. As a result, accompanying kidney function disorders of glomerular failure develop at early stages.
Owner:RENASCI +1
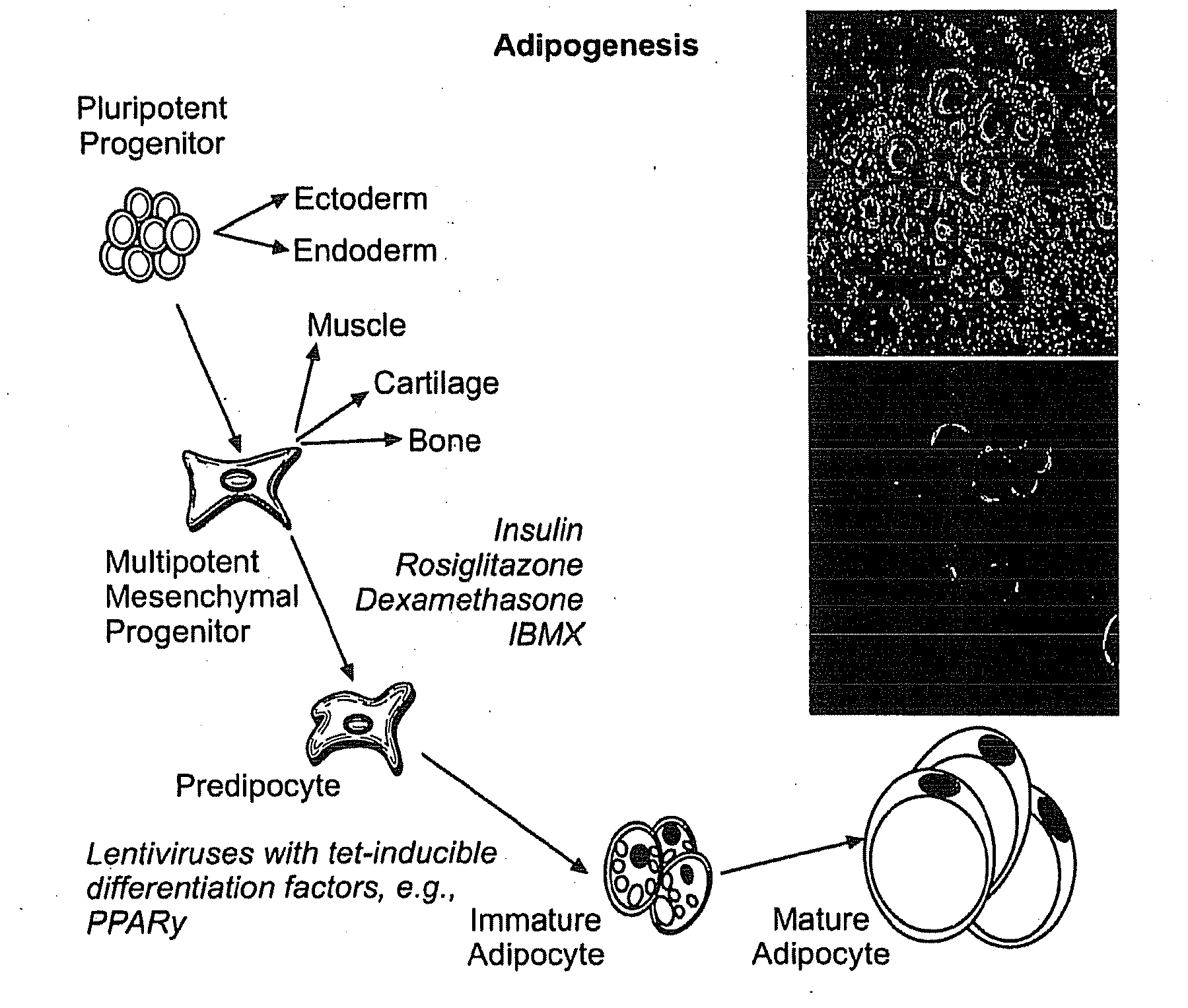
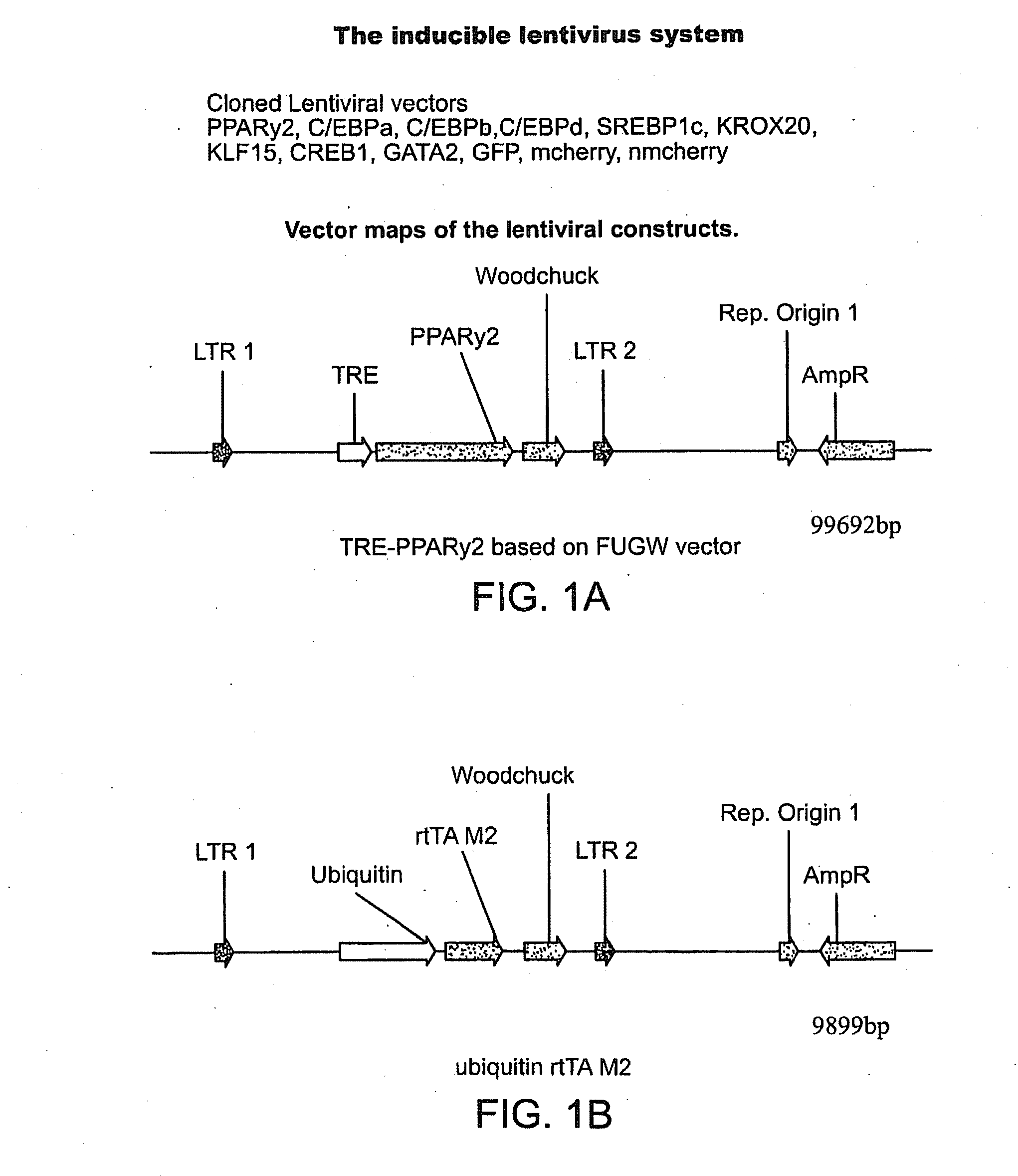
Compositions and methods of generating reprogrammed adipocyte cells and methods of use therefore
InactiveUS20120219530A1Reduce probabilityEasy to produceOptical radiation measurementFungiCell biologyDisease cause
The invention provides therapeutic compositions comprising reprogrammed adipocyte cells for use as disease models, therapeutic compositions comprising reprogrammed adipocyte cells for the treatment of conditions characterized by a reduction in cell number or tissue mass, and methods of generating such cells.
Owner:THE GENERAL HOSPITAL CORP
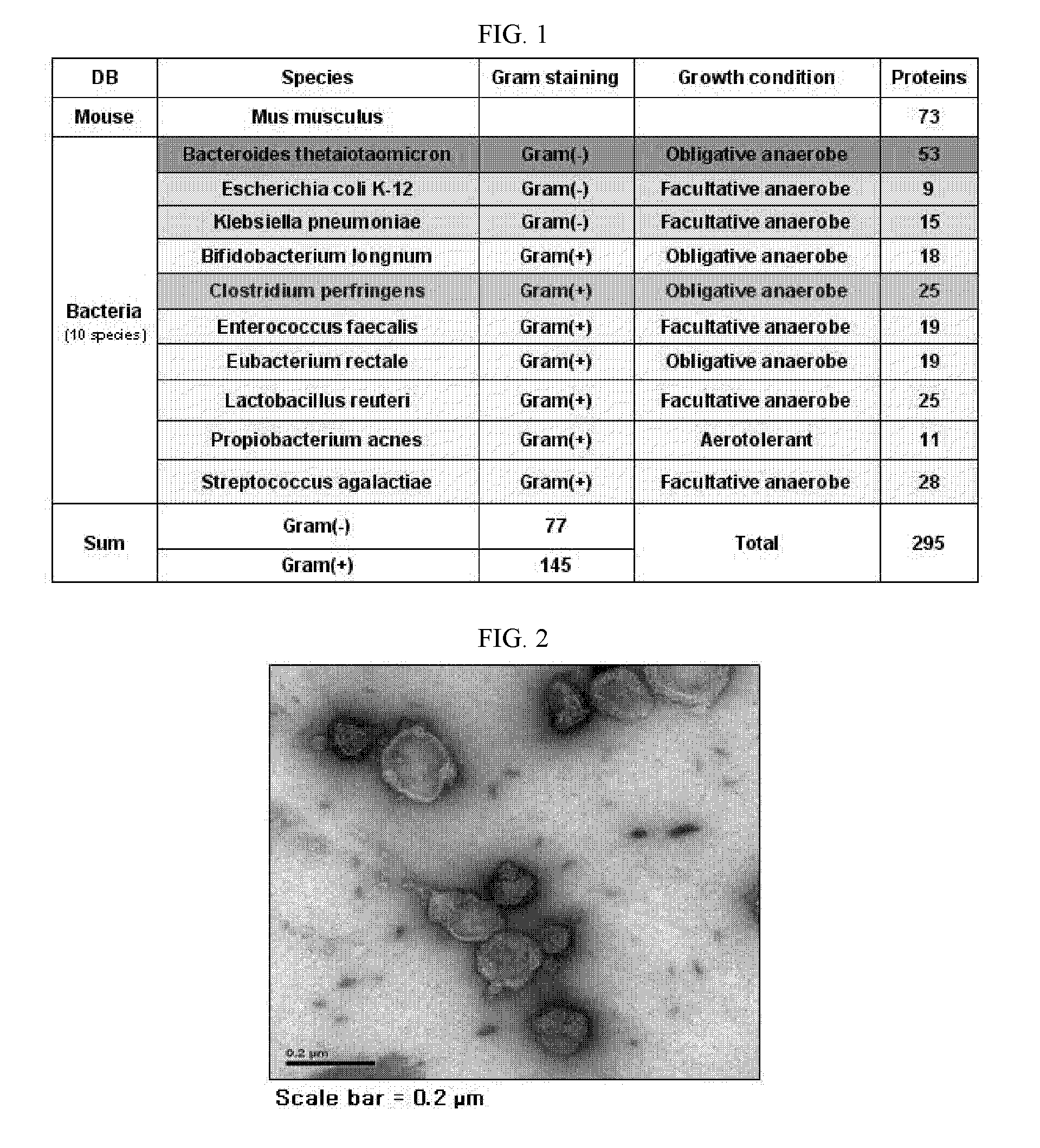
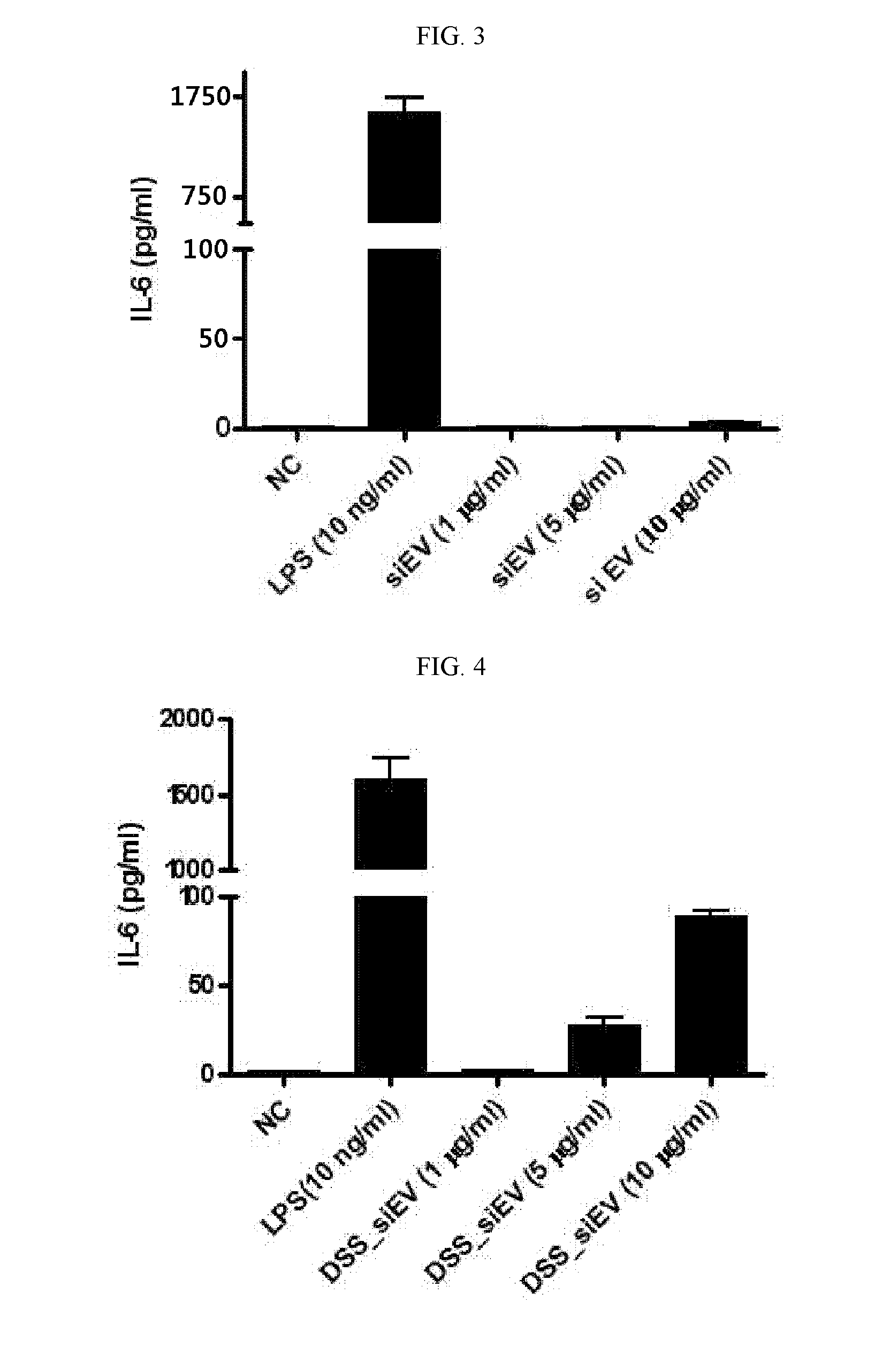
Gut flora-derived extracellular vesicles, and method for searching for a disease model, vaccine, and candidate drug and for diagnosis using the same
ActiveUS20120222142A1Efficient screeningEfficient miningAntibacterial agentsOrganic active ingredientsDrugDisease modelling
The present application relates to a composition including gut flora-derived extracellular vesicles, and to an animal disease model using same. In addition, the present application relates to a method for using the gut flora-derived extracellular vesicles to efficiently search for a candidate drug which may prevent or treat diseases that occur due to gut flora-derived extracellular vesicles, and to a vaccine which can efficiently prevent or treat infections caused by gut flora or diseases that occur due to gut flora-derived extracellular vesicles. Further, the development of diagnostic technology to discover, using the gut flora-derived extracellular vesicles of the present application, the etiology of diseases that occur due to gut flora-derived extracellular vesicles, can be achieved.
Owner:AEON MEDIX
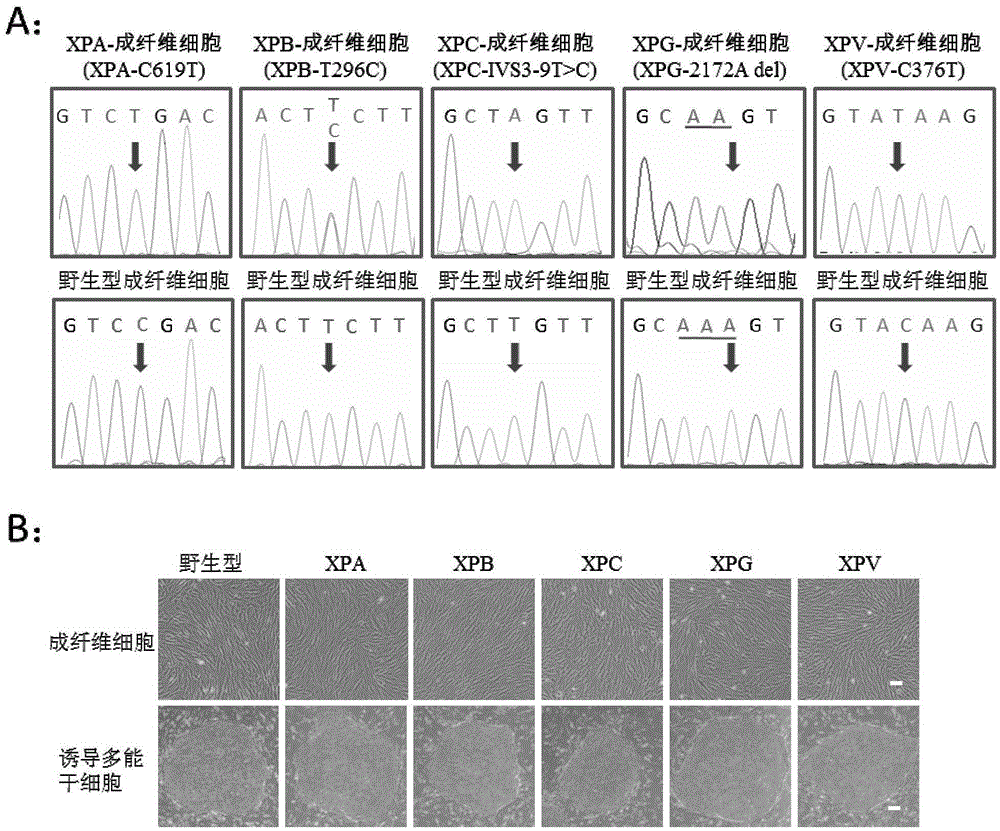
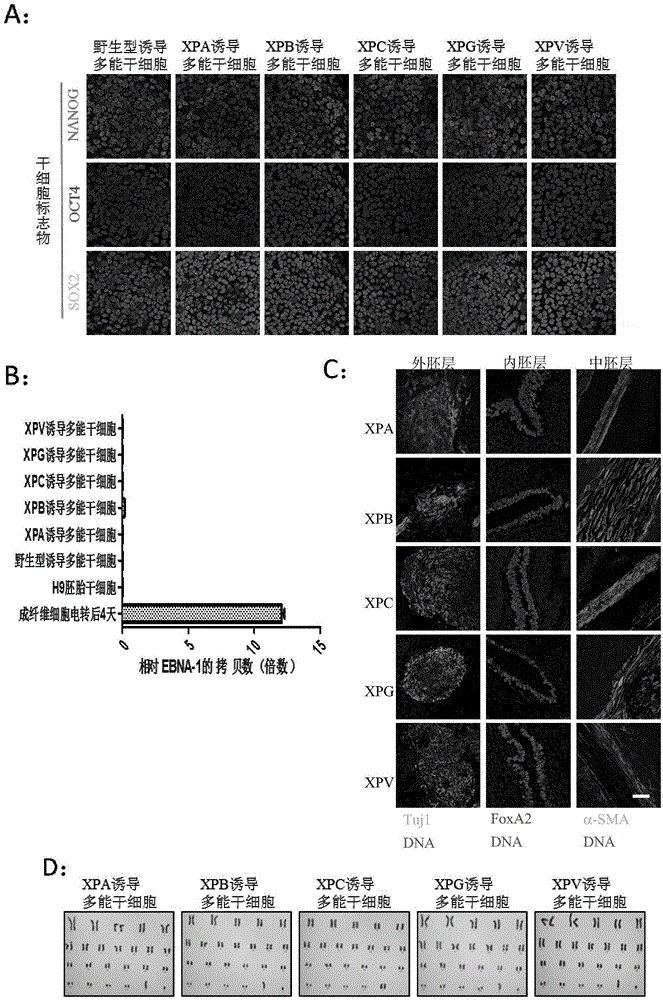
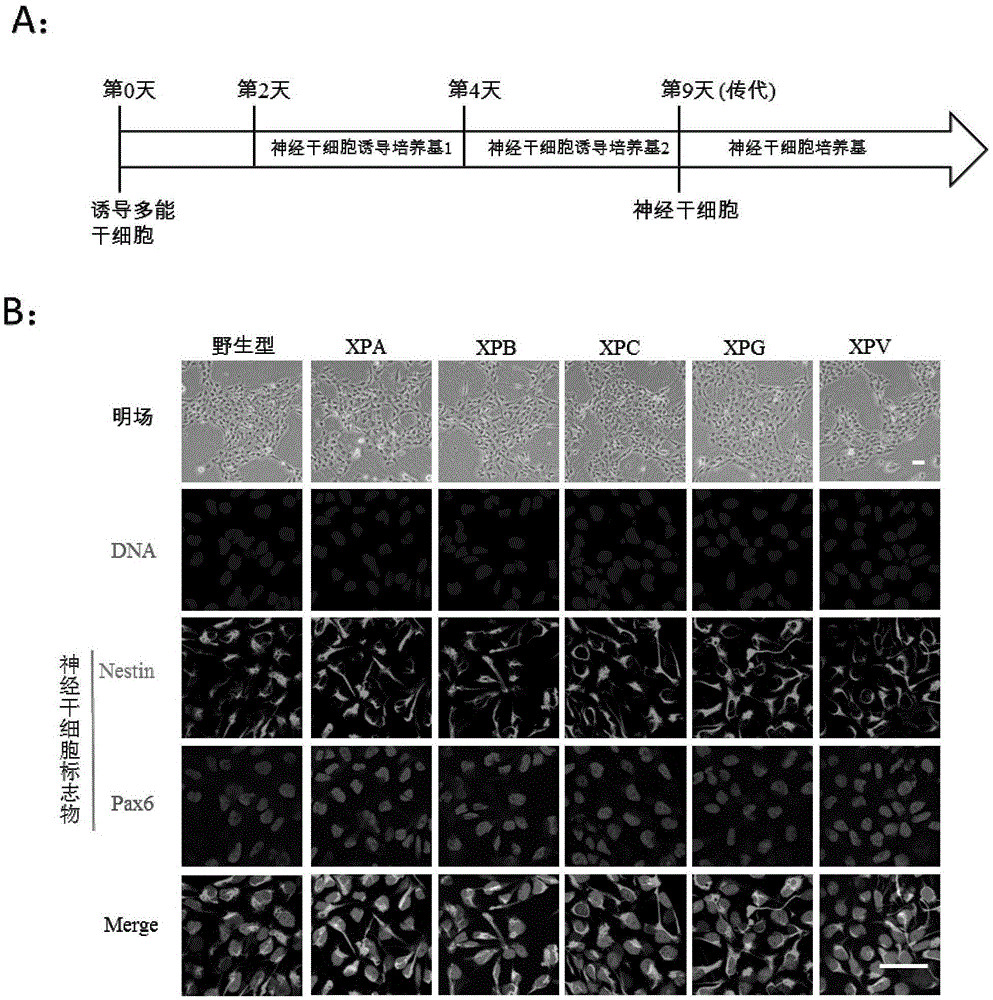
Preparation method of pluripotent stem cells of xeroderma pigmentosum patient
ActiveCN105567642ASafeAvoid troubleCompound screeningApoptosis detectionDirected differentiationApoptosis
The invention discloses a preparation method of pluripotent stem cells of a xeroderma pigmentosum patient. The method comprises the steps that by means of an iPSC technology, skin fibroblast cells of the xeroderma pigmentosum patient are reprogrammed into specific induced pluripotent stem cells through a non-integration episomal plasmid electrophoretic transfer method and directionally divided into nerve cells which show extreme sensitivities to UV in vitro, cell apoptosis is prone to occurrence, and some clues are provided for neurodegenerative diseases of the xeroderma pigmentosum patient. In addition, the mutational neural stem cells and neurones which carry genes of the patient can serve as an effective platform to conduct efficient and high-throughout personalized drug screening, and a foundation is laid for disease study, disease model development, research on pathogenesis of diseases and treatment on the diseases. A huge application prospect is achieved in personalized treatment and translational medicine.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Multi-scale complex systems transdisciplinary analysis of response to therapy
ActiveUS20160103971A1Medical simulationAnalogue computers for chemical processesOncologyComplex system
Described herein are methods and systems to measure dynamics of disease progression, including cancer growth and response, at multiple scales by multiple techniques on the same biologic system. Methods and systems according to the invention permit personalized virtual disease models. Moreover, the invention allows for the integration of previously unconnected data points into an in silico disease model, providing for the prediction of disease progression with and without therapeutic intervention.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
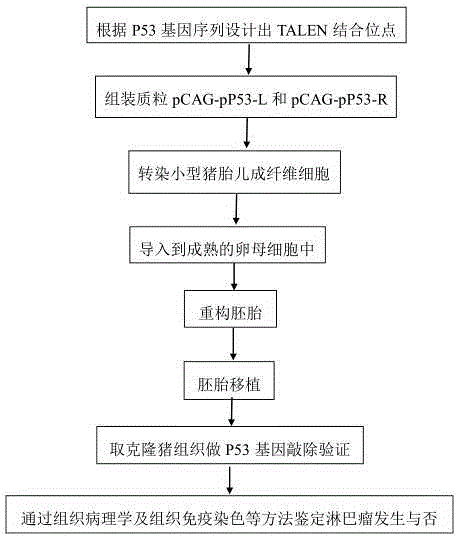
Method for obtaining lymphoma minipig disease model by knocking out P53 genes
The invention relates to a method for obtaining a lymphoma minipig disease model by knocking out P53 genes, and belongs to the field of medical animal disease models. The method mainly comprises the steps that TALEN target spots of the P53 genes are designed, P53 gene knockout TALEN plasmid pCAG-pP53-L and pCAG-pP53-R of the P53 genes are assembled, TALEN plasmid is transfected and screened to obtain a P53 gene knockout positive cell line, reconstructed embryos are constructed based on the somatic cell nuclear transplantation technology and transplanted into the body of a surrogate sow to continue to develop, and a piglet with the P53 genes knocked out is obtained; the function of the P53 genes is verified by detecting the expression level of RNA and protein of the P53 genes in tissue of the cloned piglet and downstream related genes, and lymphoma in the tissue of the piglet is identified through histopathology and an immunohistochemical method. The built lymphoma disease model has great significance in research and development of occurring, forming and medicine of human lymphoma, and the reliable model is provided for research and treatment of human lymphoma diseases.
Owner:魏红江 +1
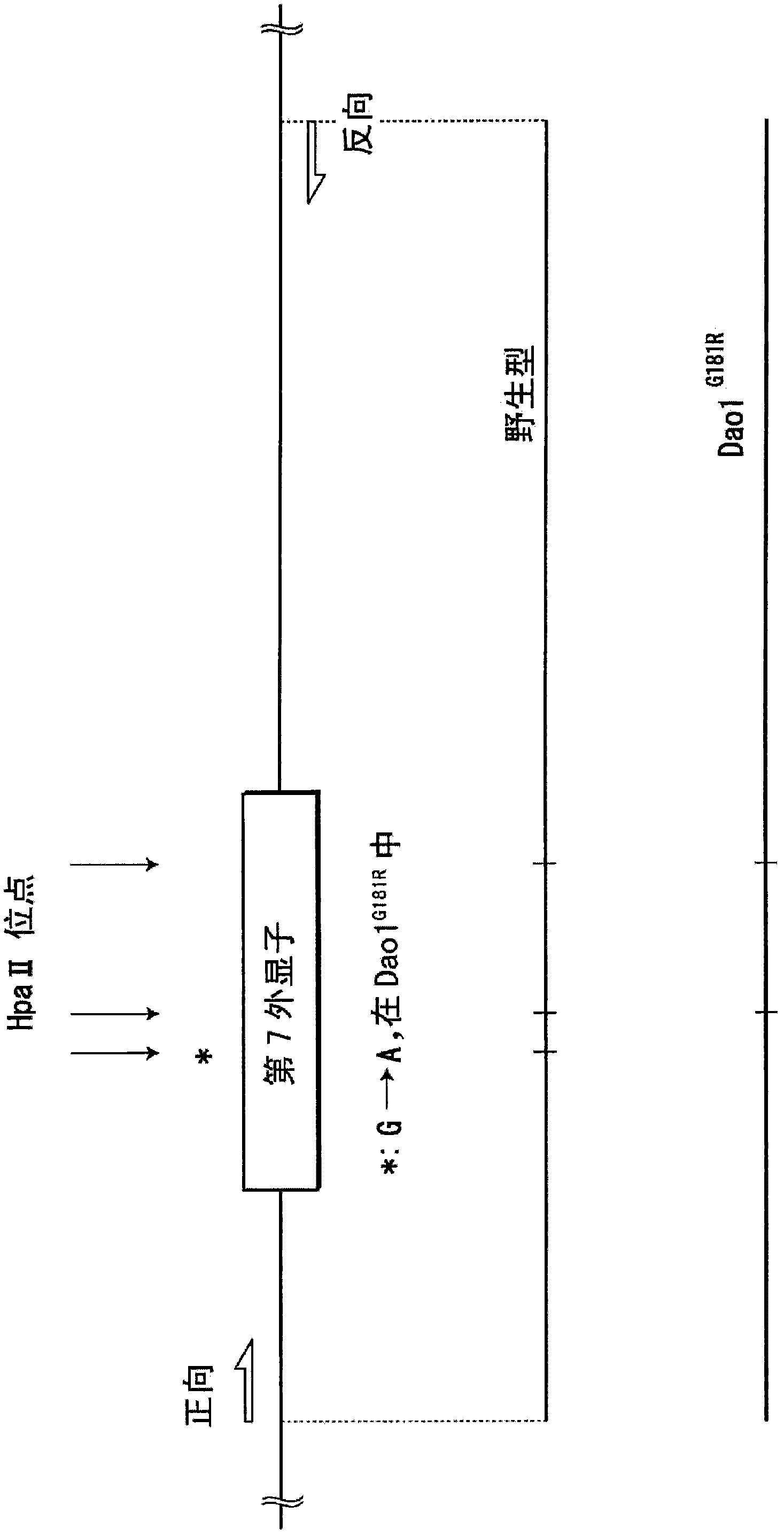
Evaluation/screening method for diseases associated with D-amino acid utilizing Dao1-/-mouse
Disclosed is an evaluation method which can select a Dao- / - homozygote rapidly among many animals produced by the mating experiment between a DAO oxygen-defect mouse and another disease model mouse to quantify a D-amino acid contained in many samples rapidly. Specifically disclosed is a method for evaluating the influence of test conditions on a mouse biological tissue or a cultured tissue cell derived from the biological tissue. The method comprises the steps of: providing a Dao1- / - mouse or the like; exposing a biological tissue from the Dao1- / - mouse or the like, or the like, to the test conditions; and analyzing the influence of the exposure of the biological tissue from the Dao1- / - mouse or the like, or the like, to the test conditions.
Owner:KYUSHU UNIV +1
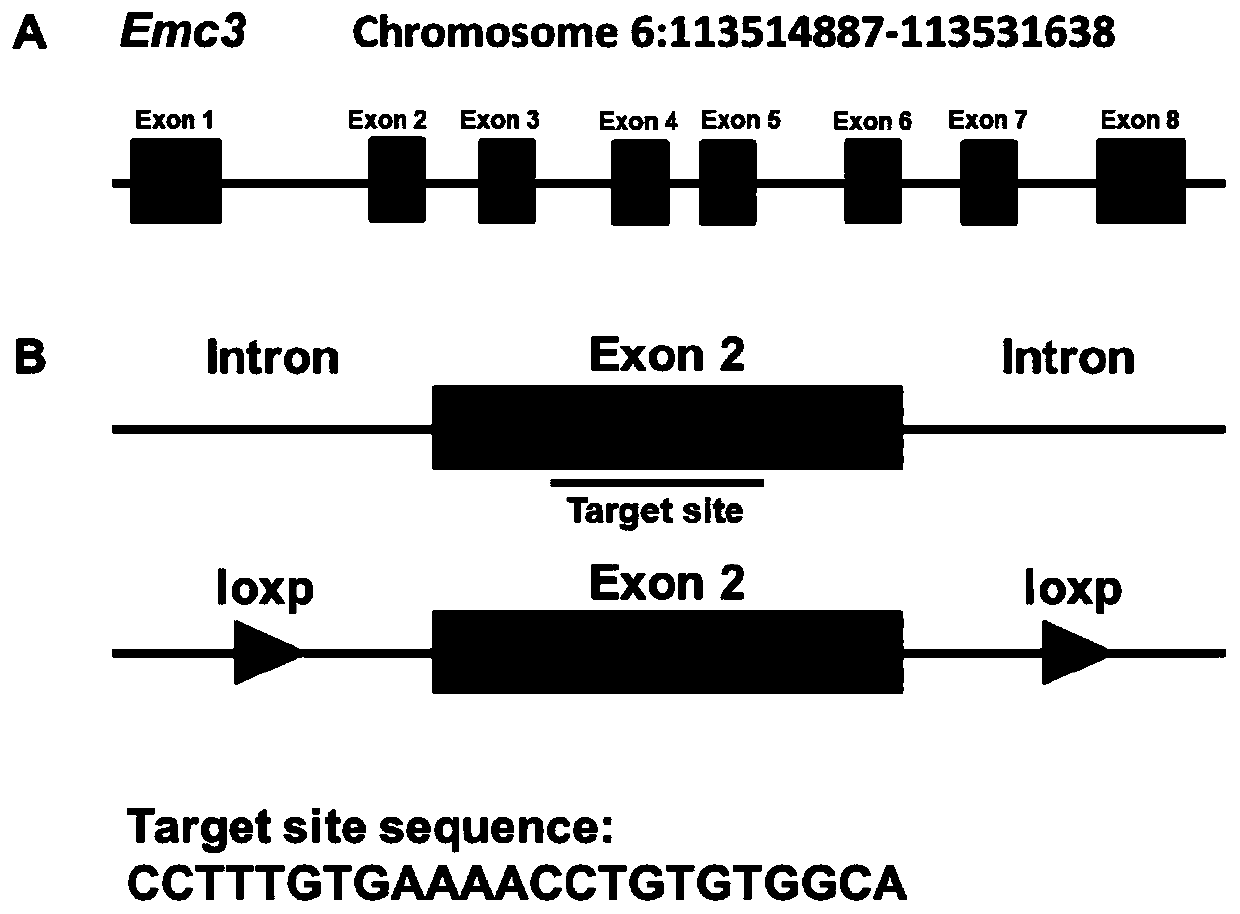
Method for constructing disease model on basis of gene operation strategy and application
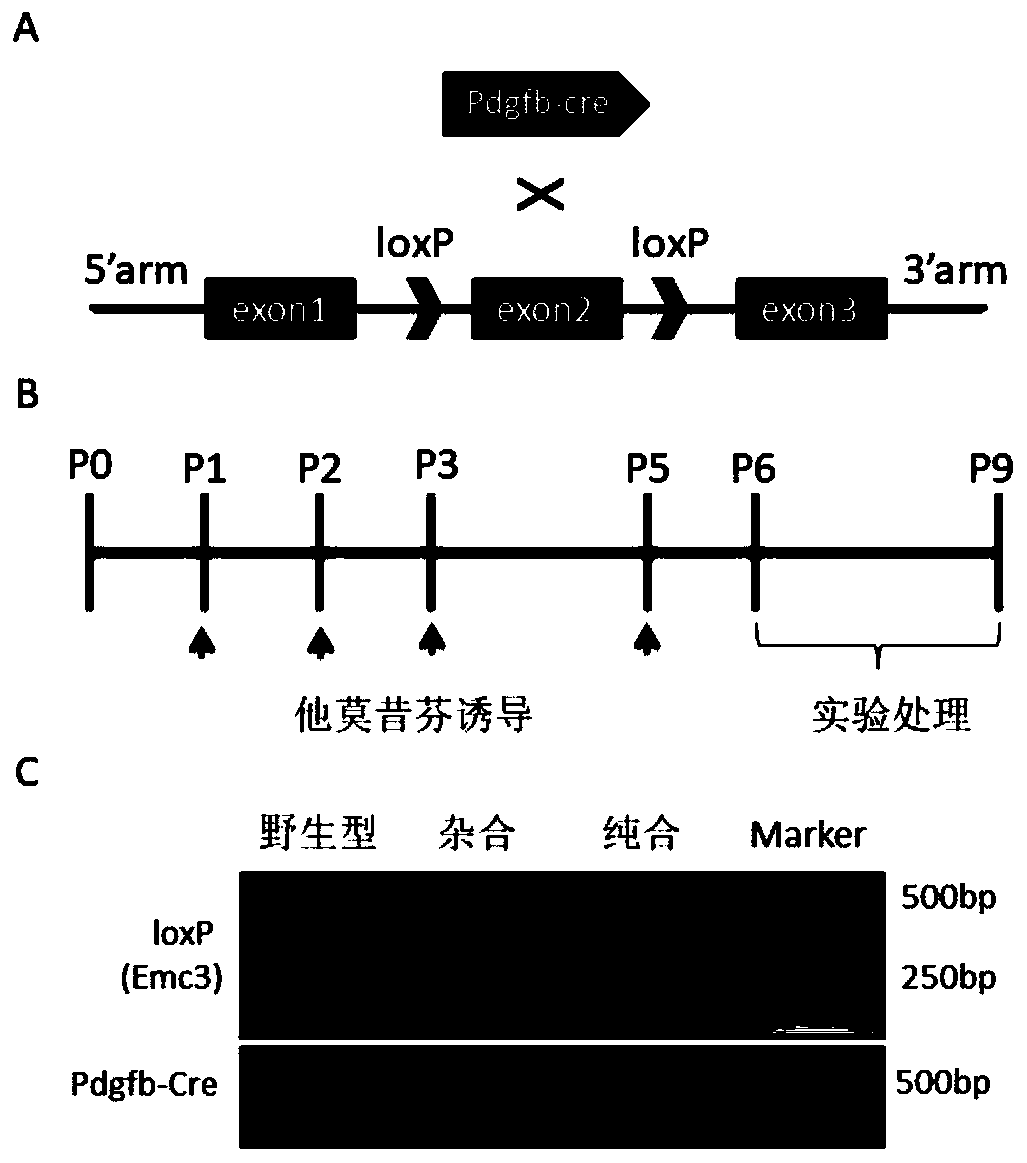
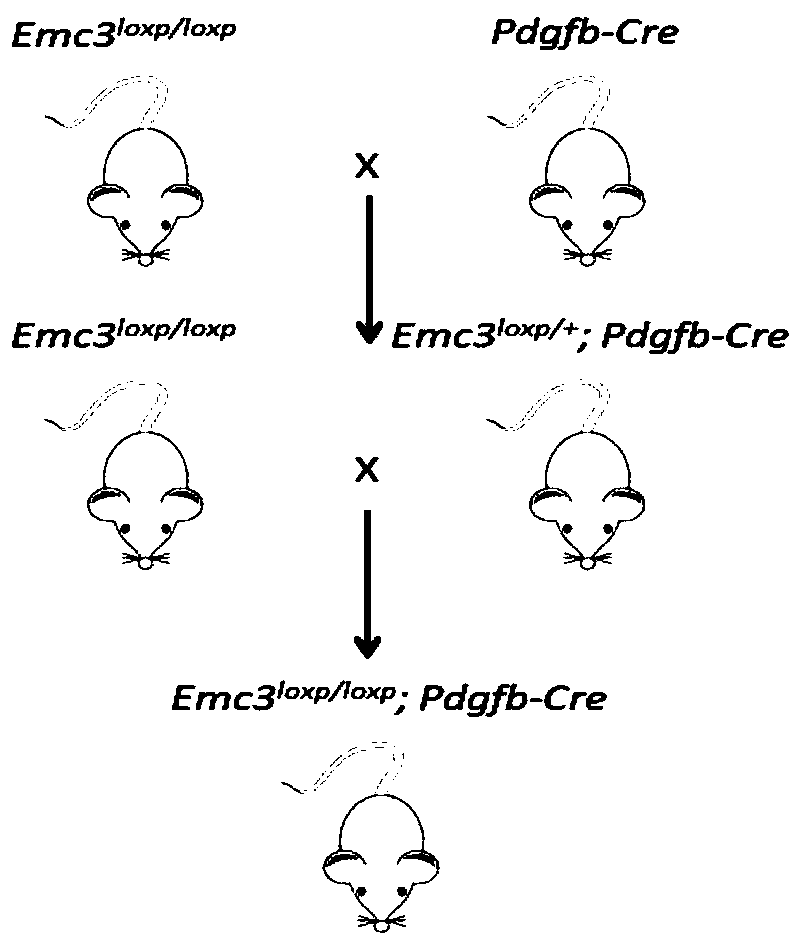
ActiveCN110100788AWide range of usesIncrease the speed of proliferationAnimal husbandryCerebellar ataxiaVascular endothelium
The invention discloses a method for constructing a disease model on the basis of a gene operation strategy and application, and relates to the technical field of biology. According to the method, anEmc3 gene is prevented from being expressed in vascular endothelial cells or cerebellum Purkinje cells of a target animal through a gene operation technology. By using the method, a disease animal model showing typical characteristics of retinal neovascularization diseases or cerebellar ataxia diseases can be obtained. The method provides a new thought or strategy for constructing a retinal neovascularization disease model or a cerebellar ataxia disease model. In addition, the disease model obtained by means of the method also provides a model basis for science researchers to study the retinalneovascularization diseases and screen medicines for treating the retinal neovascularization diseases or the cerebellar ataxia diseases.
Owner:SICHUAN ACADEMY OF MEDICAL SCI SICHUAN PROVINCIAL PEOPLES HOSPITAL
PirB gene-knock-in mouse animal model and construction method thereof
The invention discloses a PirB gene-knock-in mouse animal model, which comprises gRNA1 and gRNA2 for determining specific target sites to be knocked in by PirB gene, wherein the PirB gene is knocked into an intron 1 of ROSA26 gene of a C57BL / 6J mouse; the gene sequence of the gRNA1 is shown in SEQ ID NO.1; and the gene sequence of the gRNA2 is shown in SEQ ID NO.2. The invention further specifically discloses a construction method of the mouse animal model, the mouse animal model provides a good foundation for research on PirB gene function and in-vivo verification. Hybridization of PirB-knock-in animal model with different types of Cre mice can be used to study the functions of PirB in different organs or different cell types or different disease models.
Owner:XIAN MEDICAL UNIV
Application of prussian blue nanoparticles in preparation of drugs for preventing, delaying or treating nervous system degenerative diseases
ActiveCN111529547AEasy to makeEasy mass productionPowder deliveryHeavy metal active ingredientsNervous systemNerve cells
The invention relates to an application of prussian blue nanoparticles in preparation of drugs for preventing, delaying or treating nervous system degenerative diseases. Researches find that the prussian blue nanoparticles have remarkable effects in the aspects of preventing, delaying or treating nervous system degenerative diseases. Cell level test results show that the prussian blue nanoparticles can reduce the ROS level in nerve cells stimulated by hydrogen peroxide and increase the proportion of living cells in the nerve cells stimulated by hydrogen peroxide; and animal level test resultsshow that the prussian blue nanoparticles can significantly reduce the expression level of oxidative stress markers in hippocampus of nervous system degenerative disease model mice, significantly improve the learning and memory ability of nervous system degenerative disease model mice, and improve dyskinesia. The preparation process of the prussian blue nanoparticles is simple, the macro production is easy, the reaction condition is mild, and the surface modification is easy.
Owner:TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com