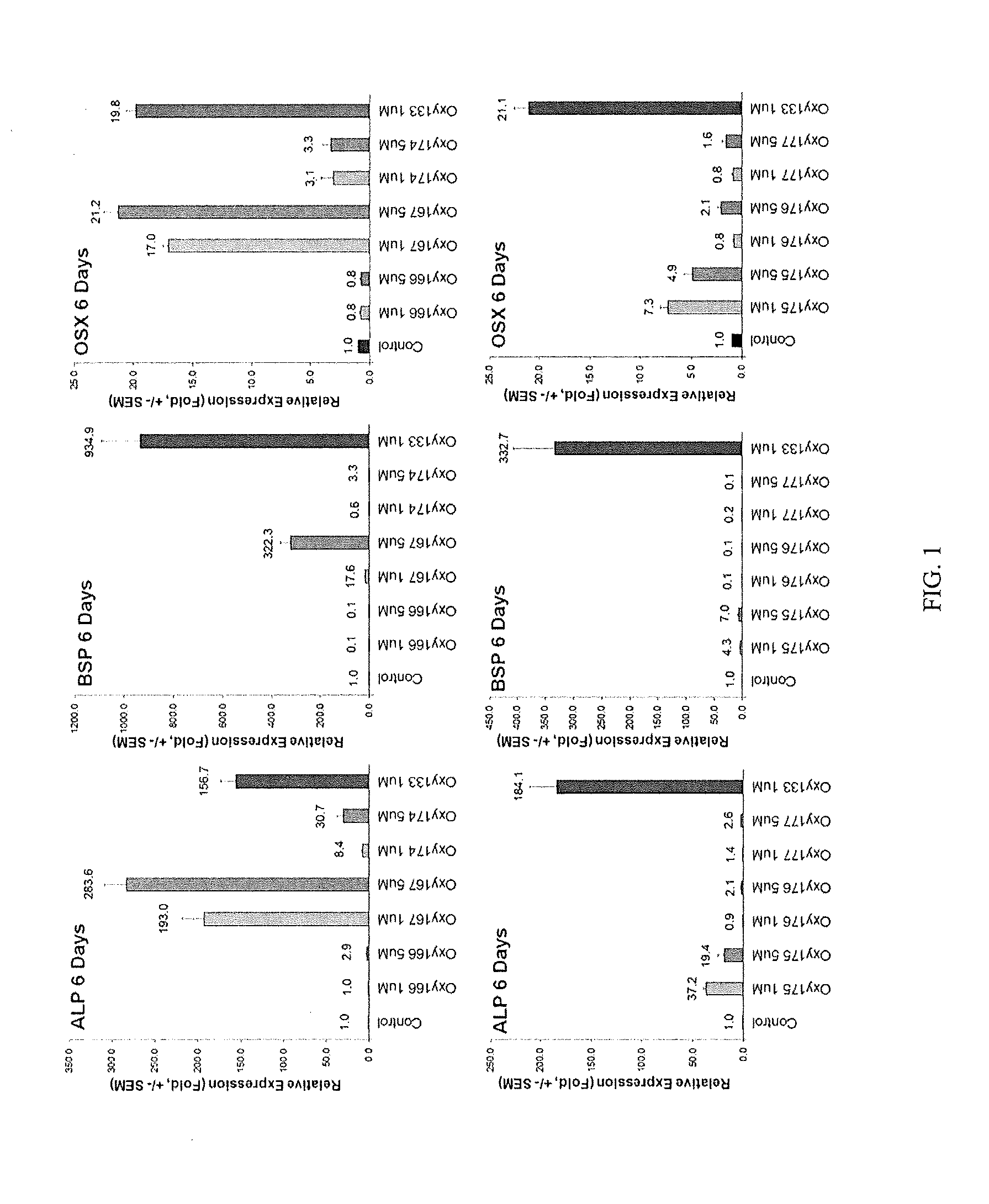
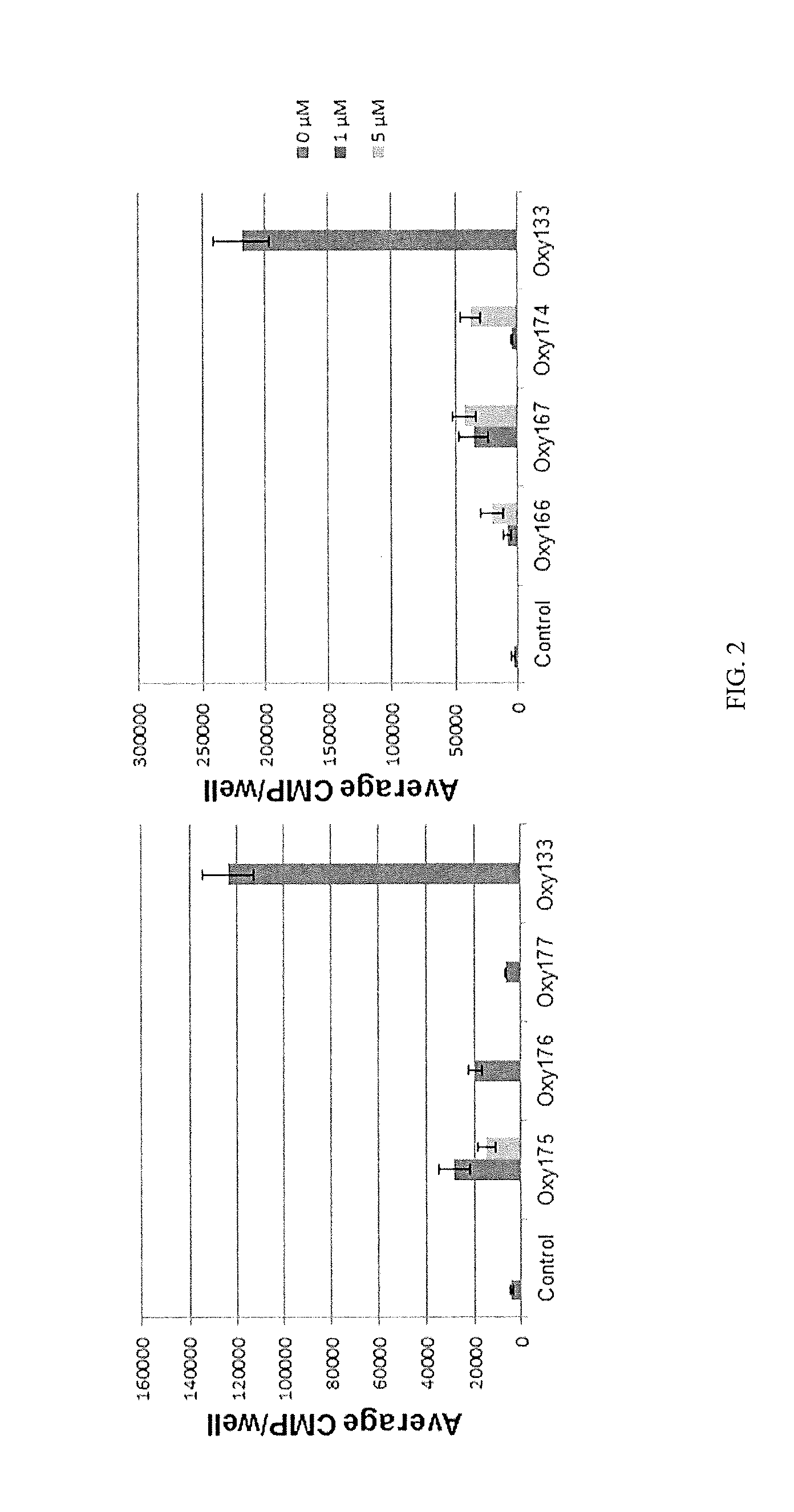
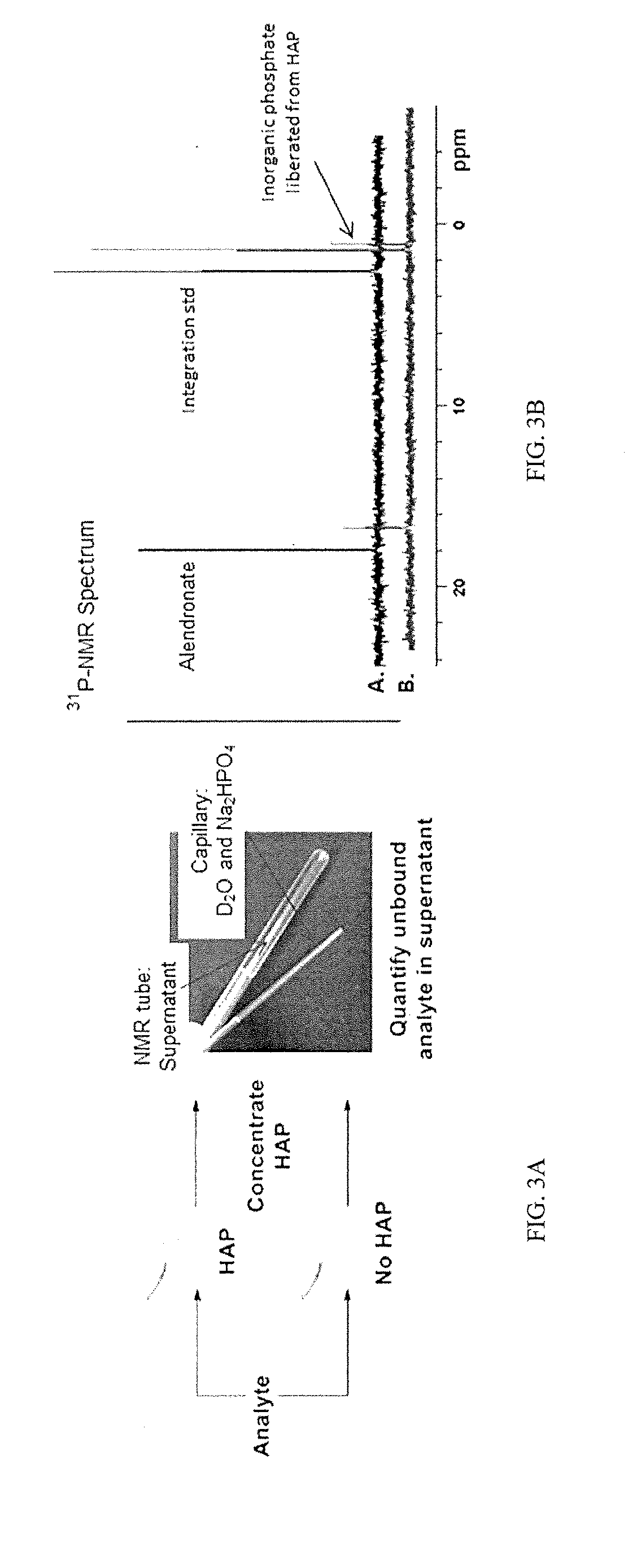
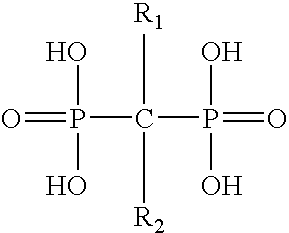
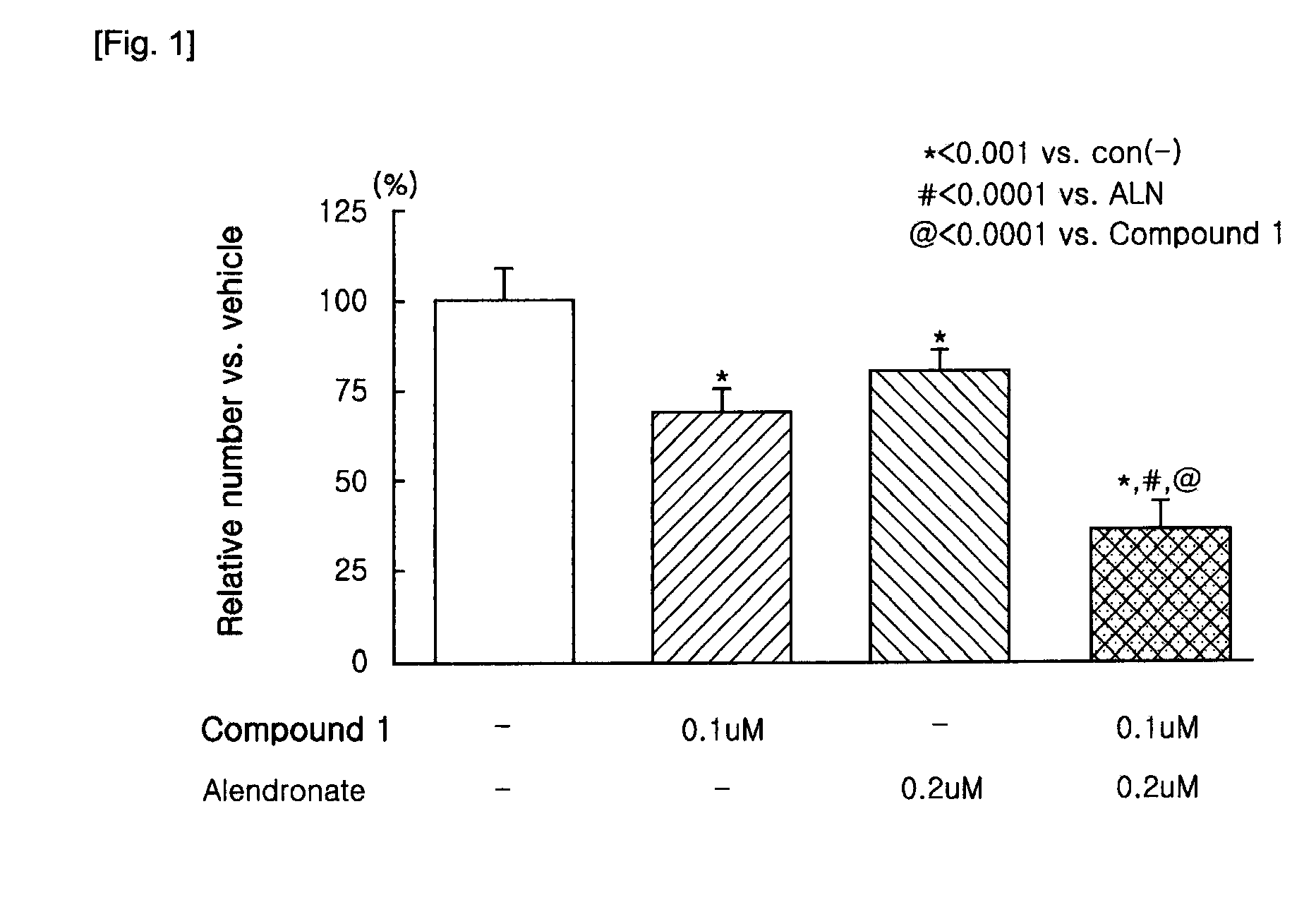
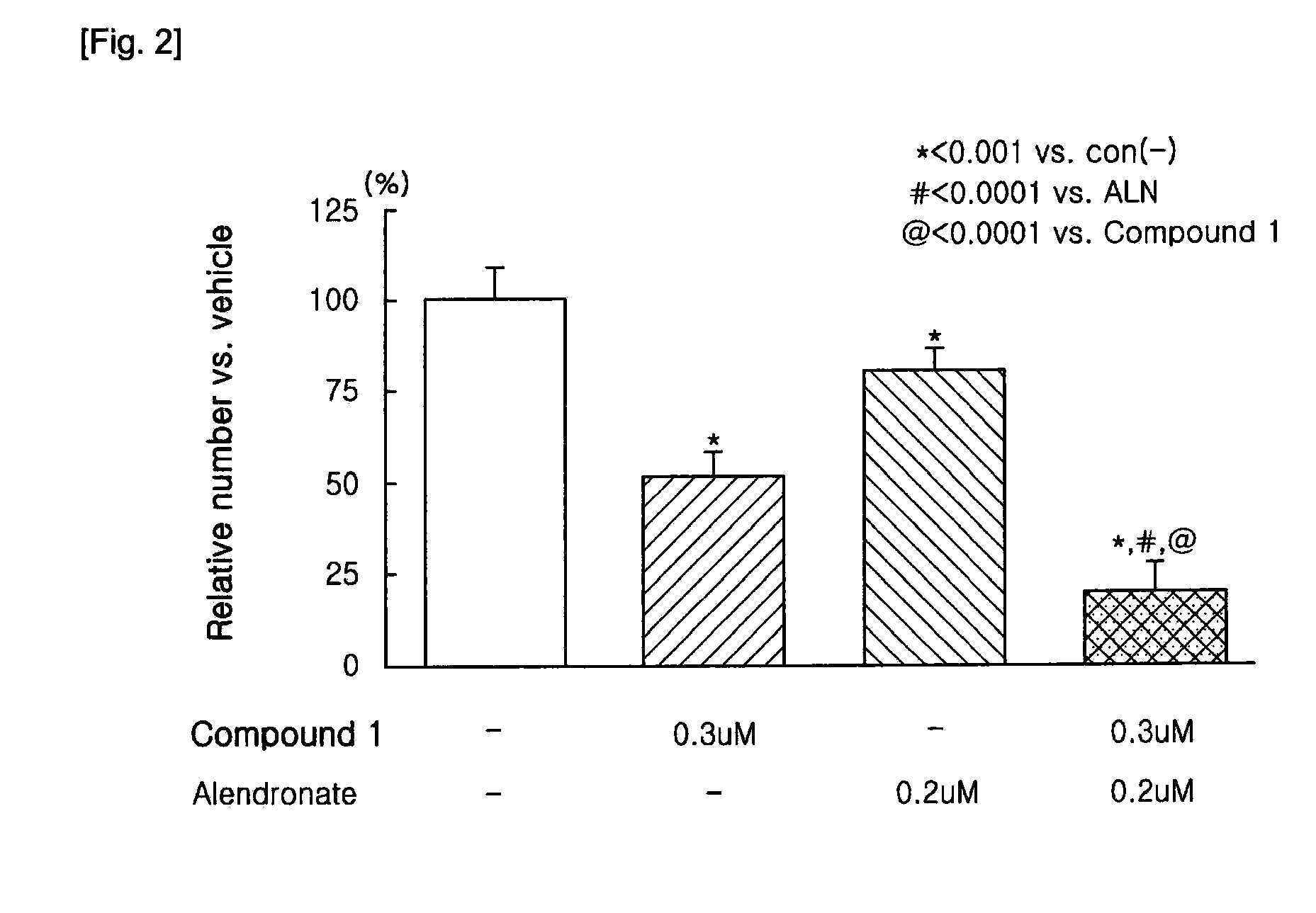
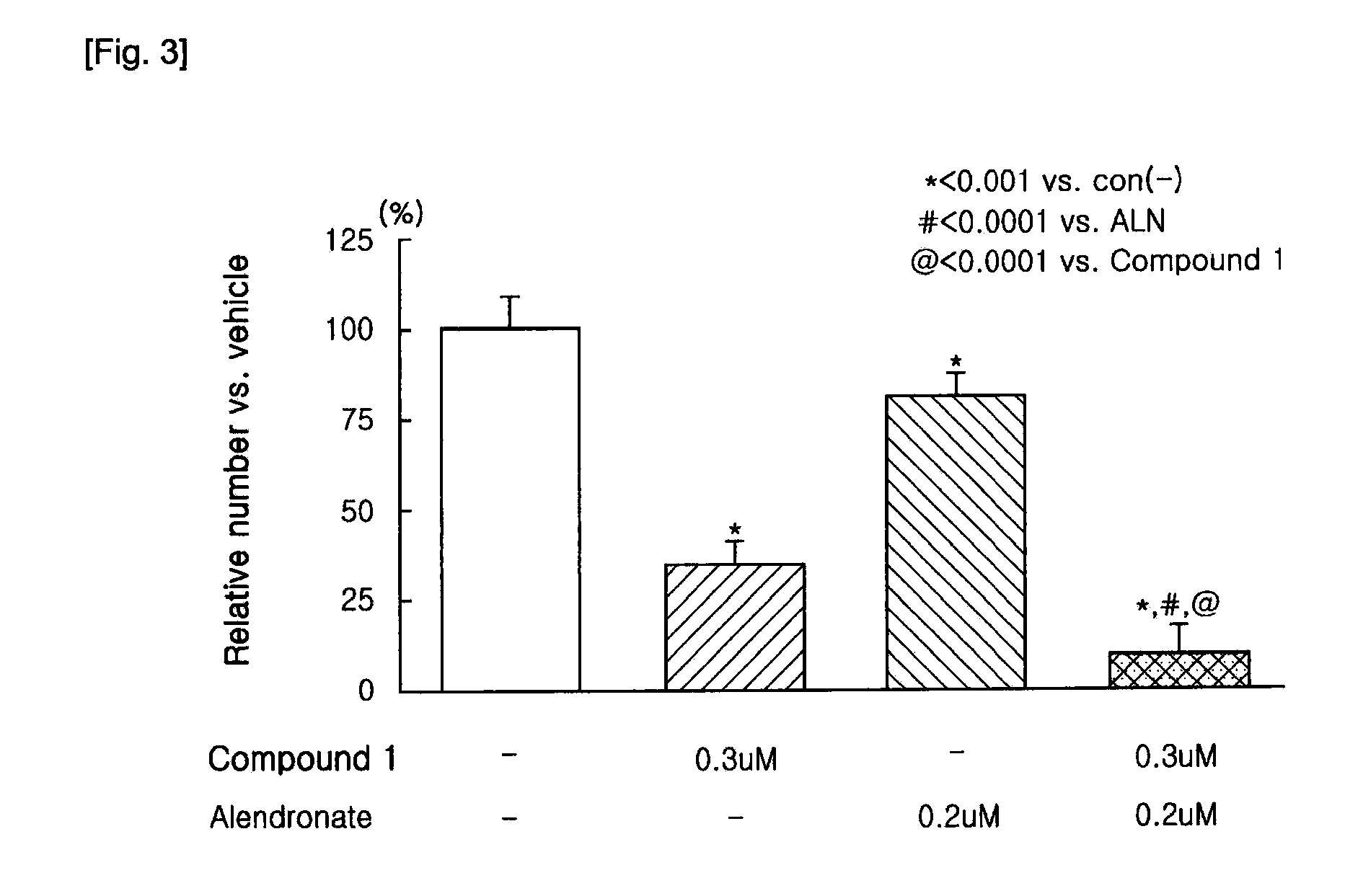
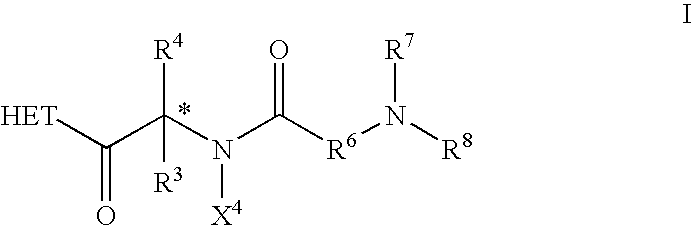
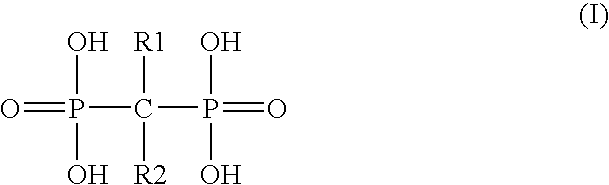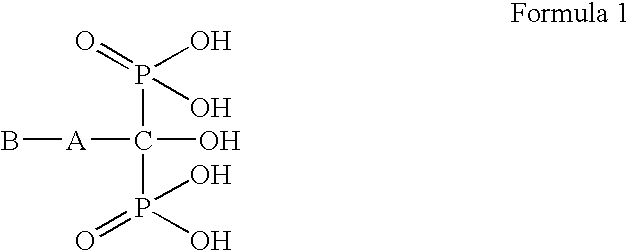Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Alendronic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
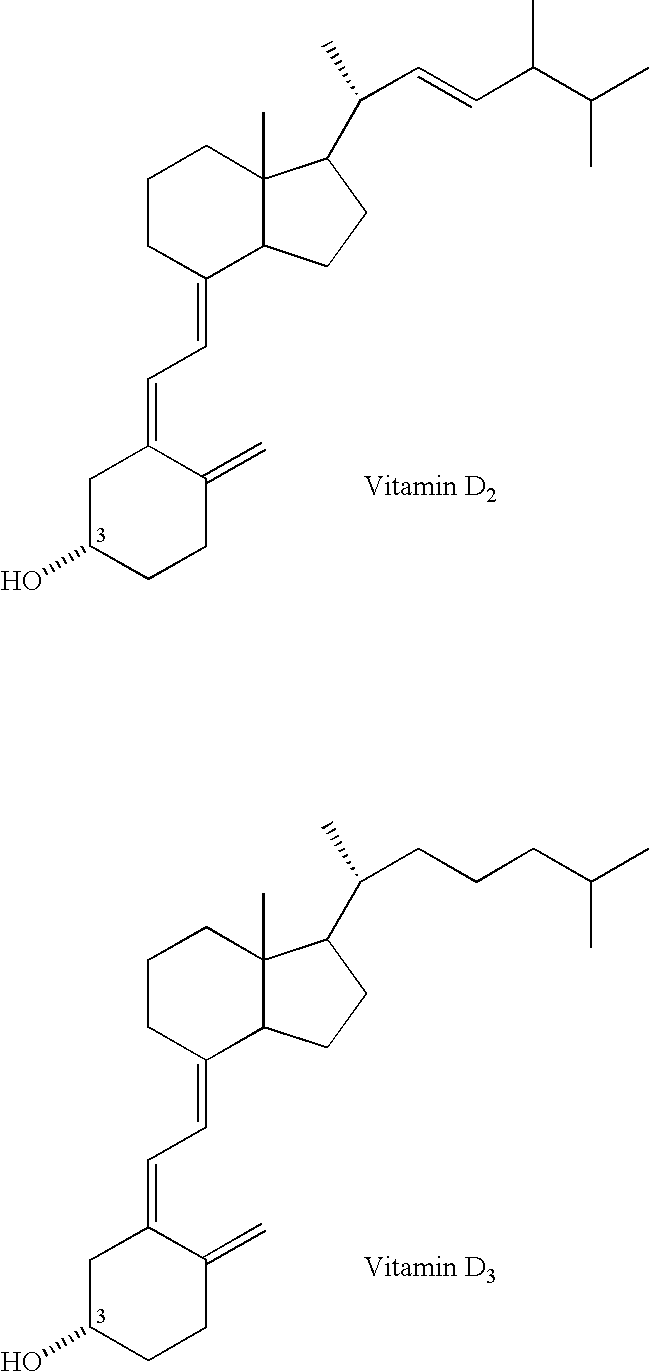
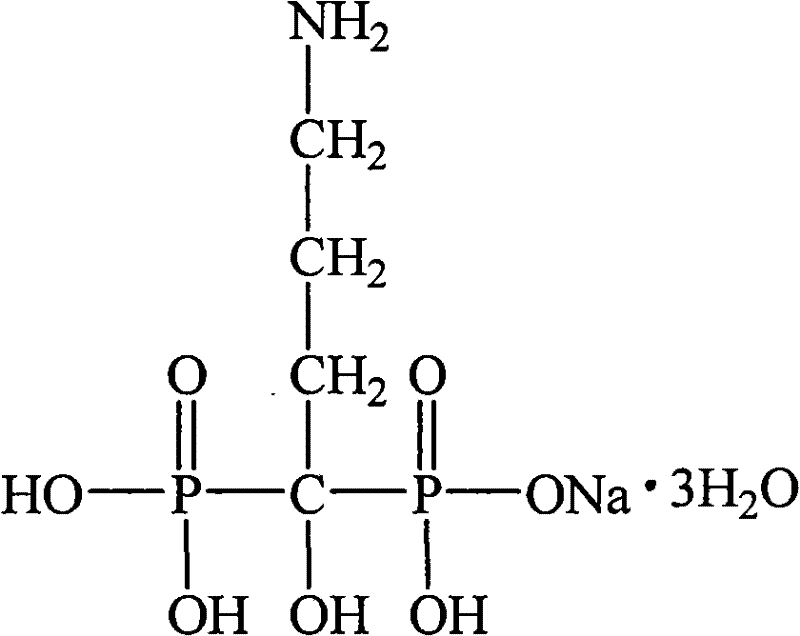
Alendronic acid, sold under the brand name Fosamax among others, is a bisphosphonate medication used to treat osteoporosis and Paget's disease of bone. It is taken by mouth. Use is often recommended together with vitamin D, calcium supplementation, and lifestyle changes.
Method of increasing bioavailability of alendronate or other bis-phosphonate by predose administration of vitamin D derivative
InactiveUS20050026871A1Improve bioavailabilityBiocidePhosphorous compound active ingredientsBioavailabilityVitamin D+Metabolites
The present invention relates to a method of increasing the bioavailability of a bis-phosphonate such as alendronate by administering an effective predose of a vitamin D derivative at least 6 hours before administering a therapeutic dose of the bis-phosphonate.
Owner:TEVA PHARM USA INC
Softgel formulations of bisphosphonates bone resorption inhibitors
InactiveUS20050142185A1Improve bioavailabilityInhibiting bone resorptionBiocidePhosphorous compound active ingredientsSoftgelGlycerol
The present invention provides a pharmaceutical formulation suitable for filling softgel capsules comprising: (a) from about 1% to about 90% by weight of a bisphosphonic acid or a pharmaceutically acceptable salt; and (b) from about 40% to about 80% by weight of a liquid carrier comprising 50% to 80% by weight polyethylene glycol; 5% to 15% by weight of glycerin; and 5% to 20% by weight water. The invention also describes a method for preparing alendronate or its pharmaceutical acceptable salts in encapsulated therapeutic dosage form, comprising the steps of reducing the size of alendronate particles to an average size no larger than about 80 microns, then mixing the micronized particles of alendronate with a solvent essentially consisting predominantly of polyethylene glycol of a molecular weight no greater than about 1000, and heating the mixture at a temperature of from about 40° C. to about 50° C. until the alendronate is dissolved in the solvent, and then encapsulating therapeutic doses of the dissolved alendronate in gelatin capsules soluble in water but insoluble in said solvent.
Owner:BELENO ALFREDO BERTHEL +1
Formulations of bisphosphonate drugs with improved bioavailability
This invention relates to formulations of bisphosphonates such as alendronate. The formulations taught herein enhance bioavailability of bisphosphonates and reduce esophageal and gastric ulcerations associated with them. Also taught herein are methods of preparing the formulations and their clinical use in the treatment of osteoporosis and other bone diseases.
Owner:BANNER PHARMACAPS
Pharmaceutical compositions and preparations for treatment of metabolic bone disease
The present invention relates to a pharmaceutical composition for the treatment of metabolic bone disease and the method of preparation thereof, and more particularly, to an improved pharmaceutical composition for the therapeutic treatment of metabolic disease and the method of preparation thereof, wherein said composition is prepared as a composite pharmaceutical agent which comprises calcitriol; which reduces the rate of spine fractures and increases bone density; alendronate, a bone resorption inhibitor, as two main active ingredients in an optimal mixing ratio to exert the greatest synergistic therapeutic effect; and adequate amount of other additives such as a resorption fortifier of alendronate. Thereof, the pharmaceutical composition according to the present invention can inhibit hypercalcemia caused when administered by calcitriol alone, compensate the inhibitory activity of bone remodeling caused by alendronate due to the presence of calcitriol, and improve drug compliance associated with the usual difficulty in administration as well as a side effect in esophagus, thus effectively preventing the occurence of osteoporosis.
Owner:YUYU IND
Enteric coated formulation for bisphosphonic acids and salts thereof
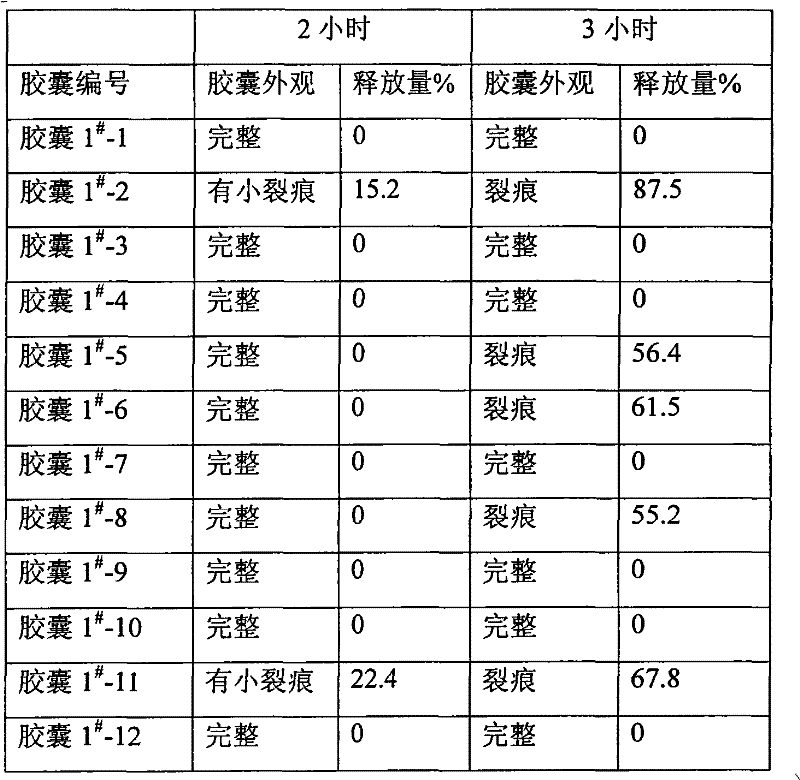
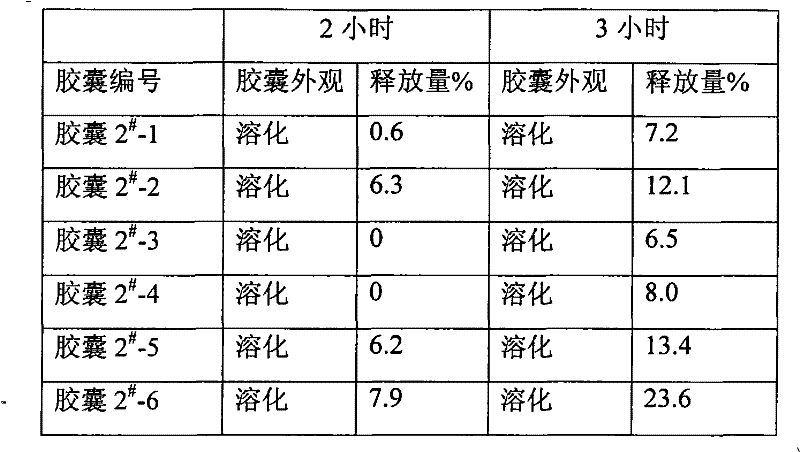
Pharmaceutical compositions and methods of using the composition are provided. The pharmaceutical composition comprises an inert core surrounded by an active coating containing one or more bisphosphonic acids or salts thereof, a seal coating surrounding the active coating and an enteric coating surrounding the seal coating. Alendronic acid and alendronate sodium trihydrate are the preferred active ingredients. The composition may be provided in the form of pellets in a capsule or Peltabs. The invention further provides methods for the treatment of disorders caused by the abnormal dissolution or deposition of calcium salts using the inventive compositions.
Owner:CIPLA LTD
Process for preparation of bisphosphonic acid compounds
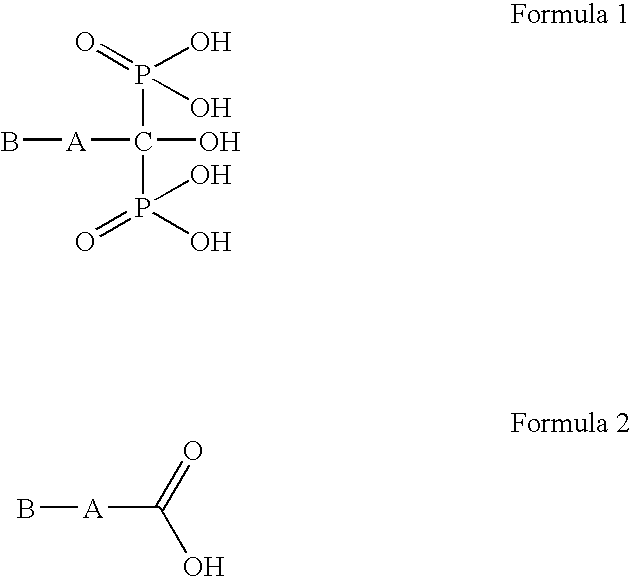
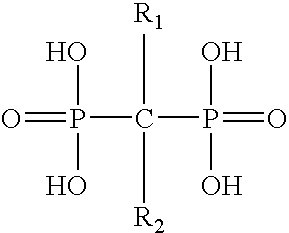
The present invention provides a novel process for preparation of bisphosphonic acids or salts thereof, e.g. alendronic acid, by reacting a carboxylic acid, phosphorous acid and a halophosphorous compound in a water miscible neutral solvent.
Owner:SUN PHARMA INDS
Method of extending the dose range of vitamin D compounds
InactiveUS7259143B2Inhibiting hypercalcemiaMinimal dangerBiocideOrganic active ingredientsDiseaseCalcification
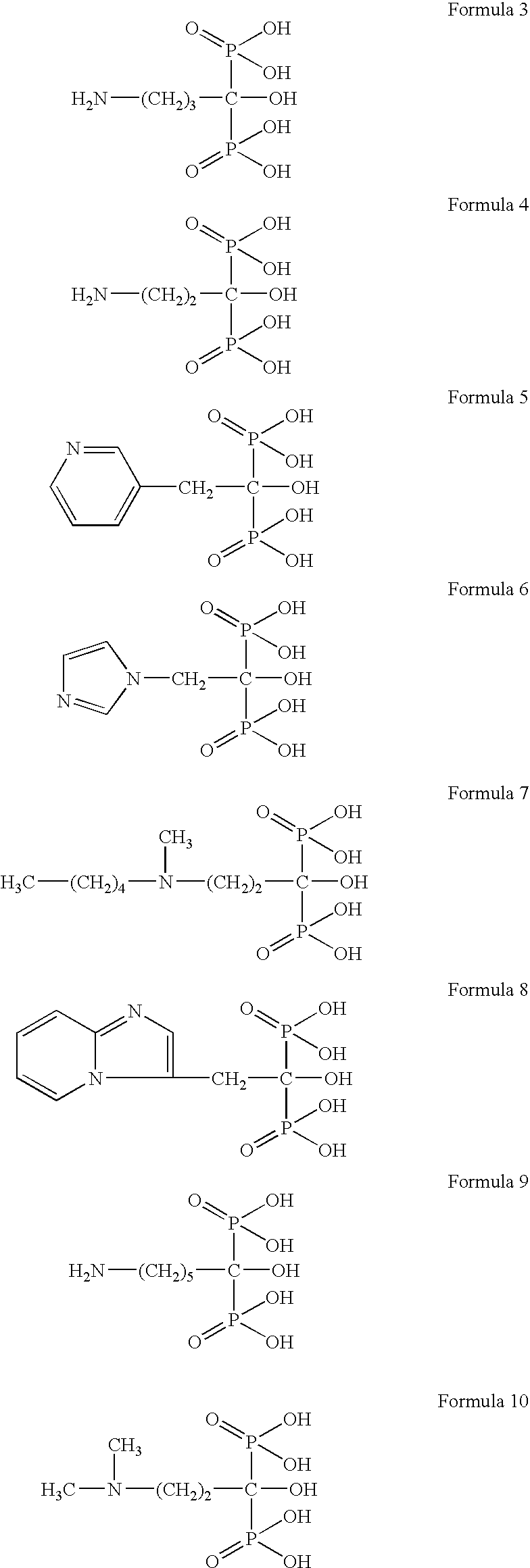
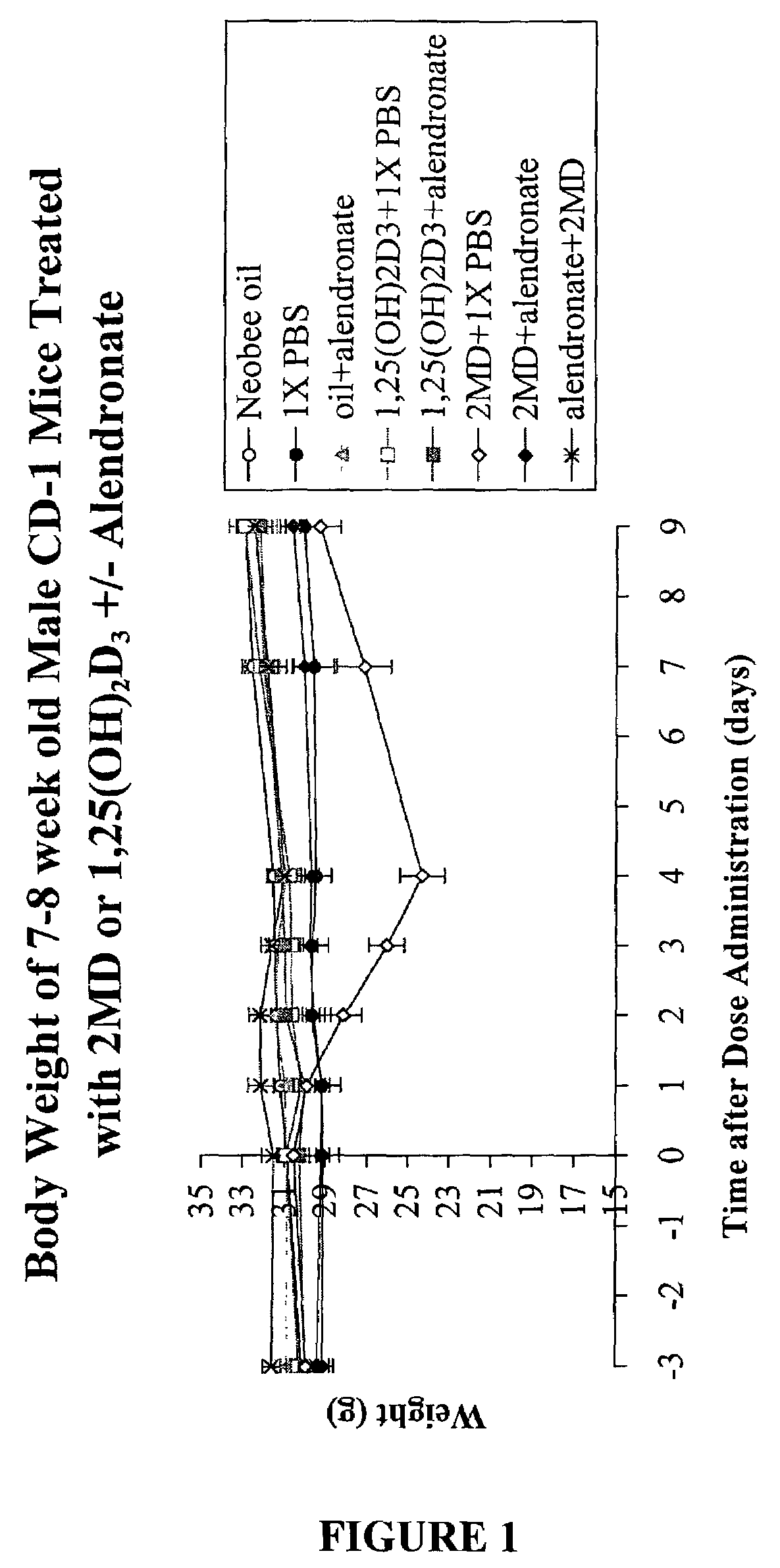
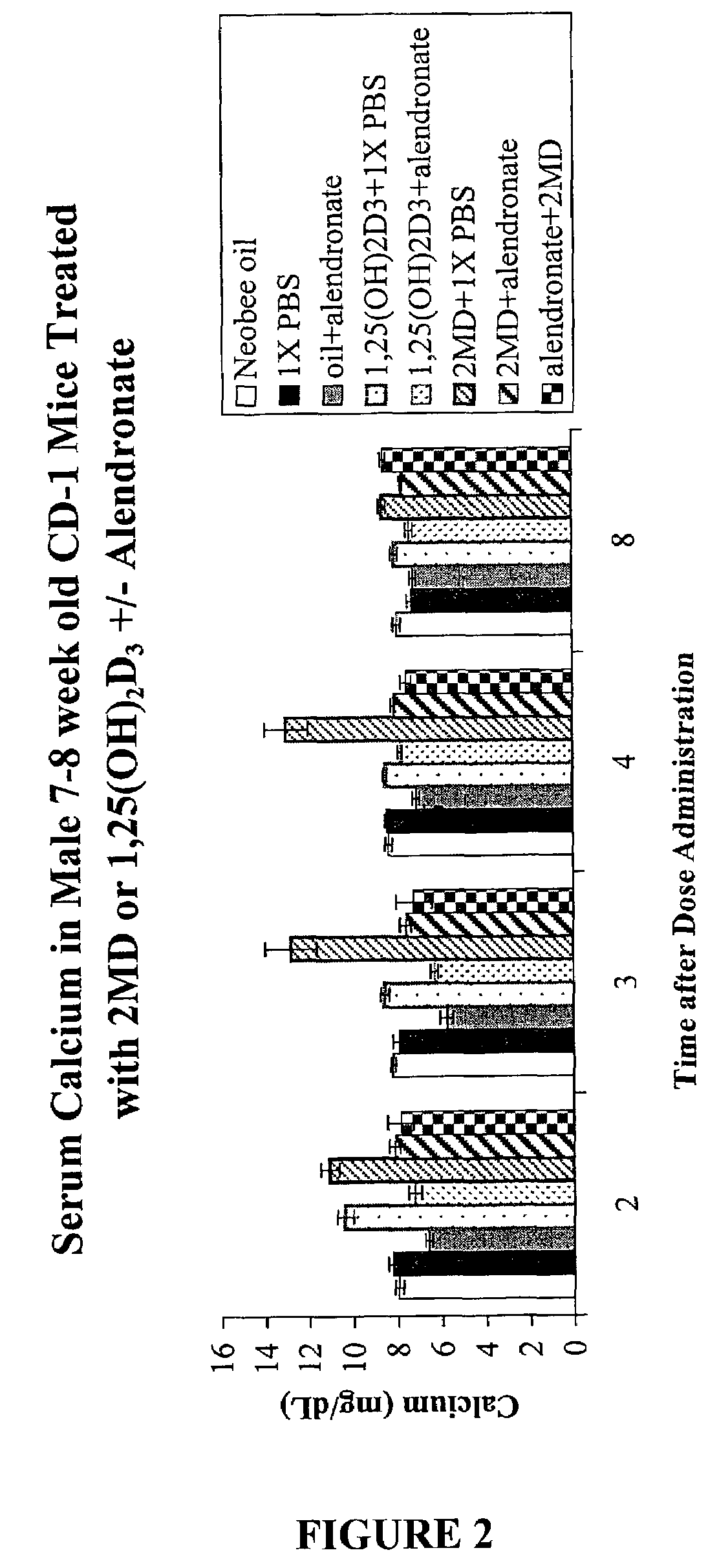
Inhibitors of bone calcium resorption are administered to allow high doses of vitamin D compounds or mimetics to be given with the intent of treating non-calcium related diseases such as cancer, psoriasis, and autoimmune disease without the dangers of calcification of kidney, heart, and aorta. Inhibitors of bone calcium resorption include the bis-phosphonates, OPG (osteoprotegerin) or the soluble RANKL (receptor activator of NF-κB ligand) receptor known as sRANK (soluble RANK which is the protein expressed by the NF-κB gene), and function to block the availability of calcium from bone thereby preventing hypercalcemia and the resulting calcification of soft tissues. Thus, high doses of 1α,25-dihydroxyvitamin D3 (1,25-(OH)2D3), its analogs, prodrugs, or mimetics can be utilized with minimal risk to a patient. Specifically, alendronate is shown to block the bone calcium mobilization activity of both 1,25-(OH)2D3 and its very potent analog, 2-methylene -19-nor-(20S)-1α,25-dihydroxyvitamin D3.
Owner:WISCONSIN ALUMNI RES FOUND
Process for preparation of bisphosphonic acid compounds
InactiveUS20060293524A1Good yieldEasy to usePhosphorus organic compoundsPhosphorous acidCarboxylic acid
The present invention provides a novel process for preparation of bisphosphonic acids or salts thereof, e.g. alendronic acid, by reacting a carboxylic acid, phosphorous acid and a halophosphorous compound in a water miscible neutral solvent.
Owner:SUN PHARMA INDS
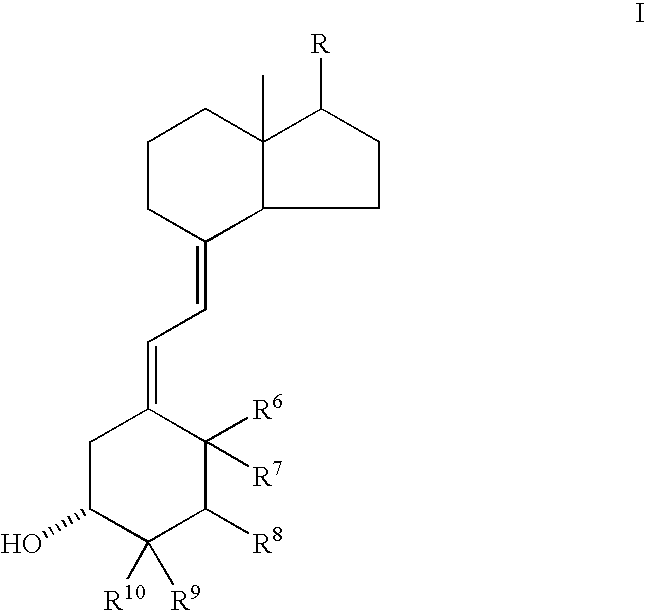
Bone-selective osteogenic oxysterol bisphosphonate analogs
Oxysterol-bisphosphonate and oxysterol-alendronic acid compounds, compositions including them, and methods using them for the treatment of bone disorders.
Owner:RGT UNIV OF CALIFORNIA
Method for inhibiting bone resorption with an alendronate and vitamin d formulation
Composition and method for preventing or treating abnormal bone resorption in mammals, the composition characterized as containing, a supplementary effective amount of a non-activated metabolite of vitamin D2 and / or D3 and a pharmaceutically effective amount of bisphosphonate to provide vitamin D nutrition during treatment to facilitate normal bone formation and mineralization, while minimizing the occurrence of or potential for the complications associated with vitamin D insufficiency, such as hypocalcemia and osteomalacia. The method of preventing or treating may be further characterized by concomitantly administering the components simultaneously or alternately at dosing intervals selected from once-weekly, twice-weekly, bi-weekly, monthly, and bimonthly.
Owner:DAIFOTIS ANASTASIA G +2
Medicine composition for treating osteoporosis or preventing bone fracture caused by osteoporosis and its application
InactiveCN101125201AInhibition of mineralizationStrong anti-bone resorption effectOrganic active ingredientsPowder deliveryDysostosisBone formation
The present invention provides a pharmaceutical composition containing alendronate or the pharmaceutical acceptable salt thereof and parathyroid hormone or the derivatives thereof, the pharmaceutical composition can also be added with vitamin D and calcium salt. The pharmaceutical composition of the present invention can be used for the treatment of osteoporosis, on the one hand, the present invention can inhibit the bone resorption fundamentally, on the other hand, the present invention can increase the bone mass and improve the bone formation, the two-pronged approach can ensure the patients to get the scientific and comprehensive treatment, so as to achieve the purposes of treatment of osteoporosis, the prevention of occurrence of bone fracture and the treatment of the fractures caused by osteoporosis.
Owner:CSPC OUYI PHARM CO LTD
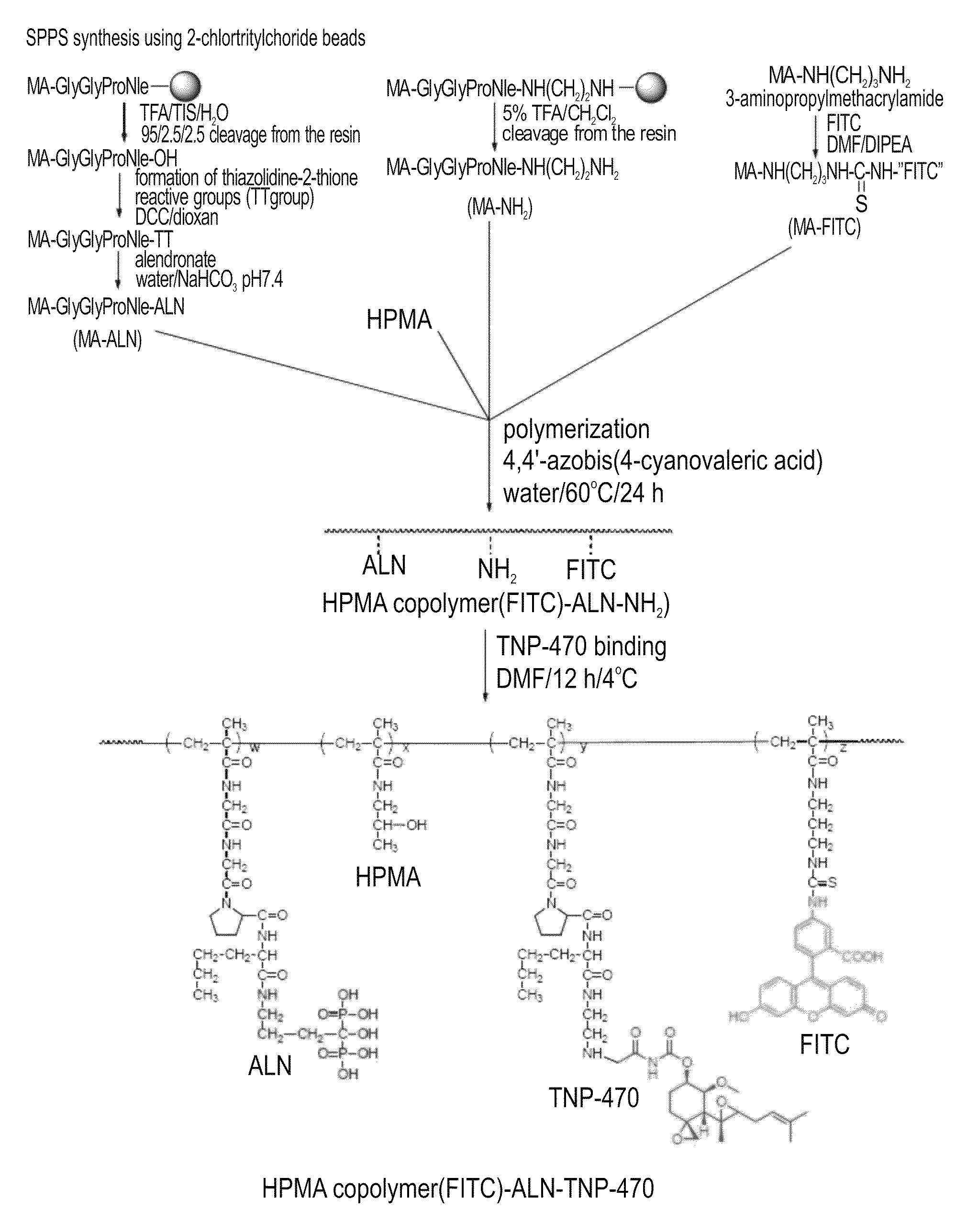
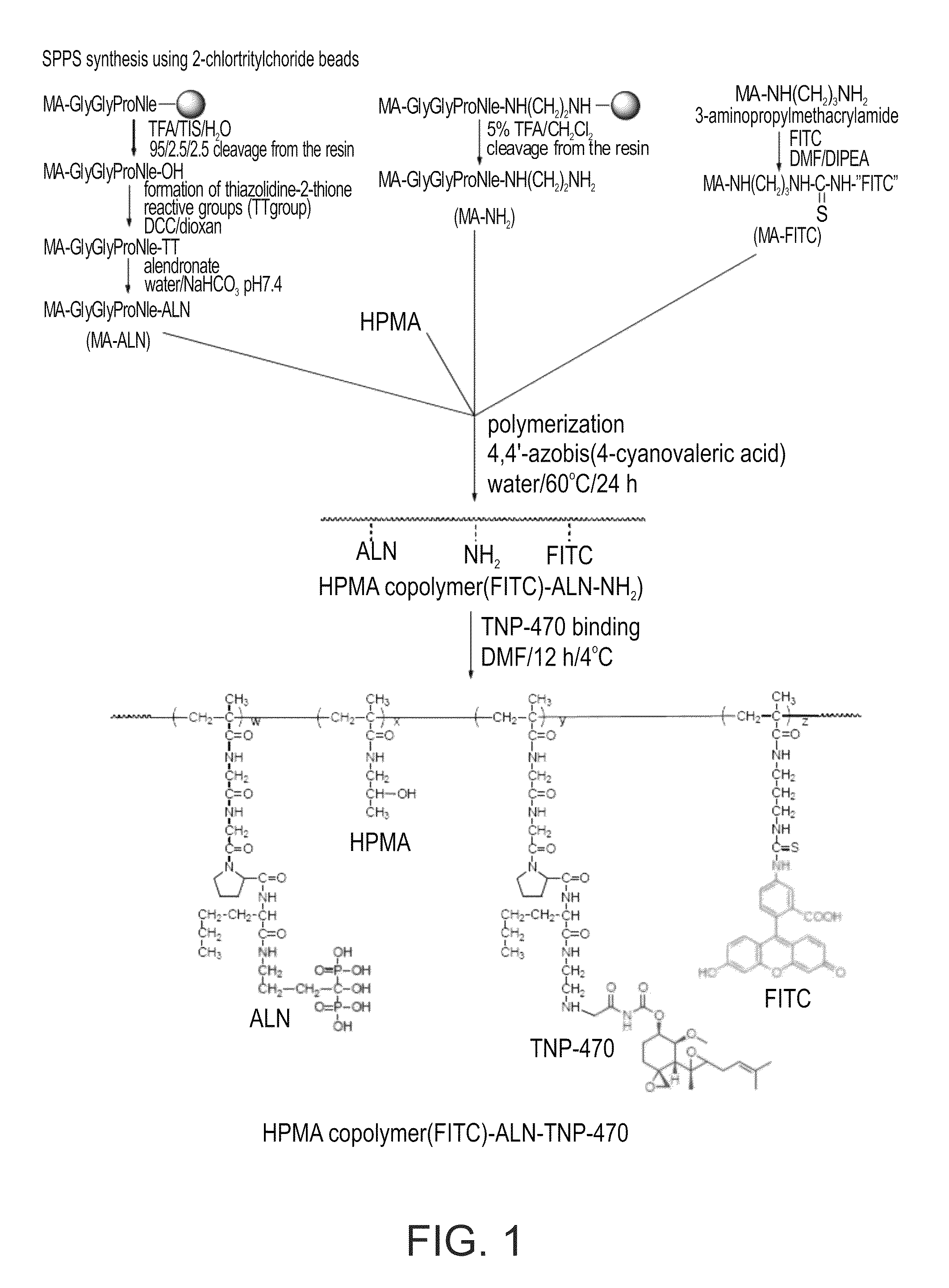
Conjugate of a polymer, an anti-angiogenesis agent and a targeting moiety, and uses thereof in the treatment of bone related angiogenesis conditions
InactiveUS8703114B2Increase loadReduce polydispersitySkeletal disorderTripeptide ingredientsAngiogenesis growth factorBone targeting
Owner:RAMOT AT TEL AVIV UNIV LTD +1
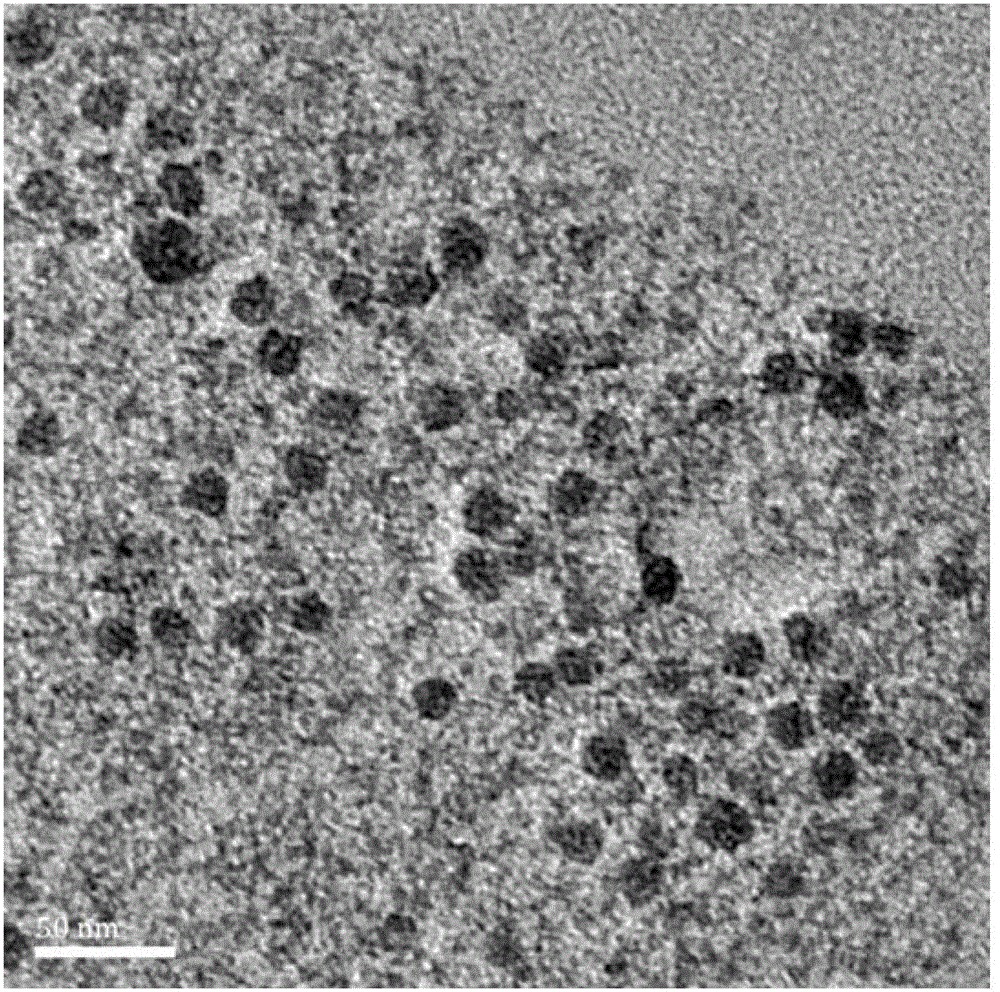
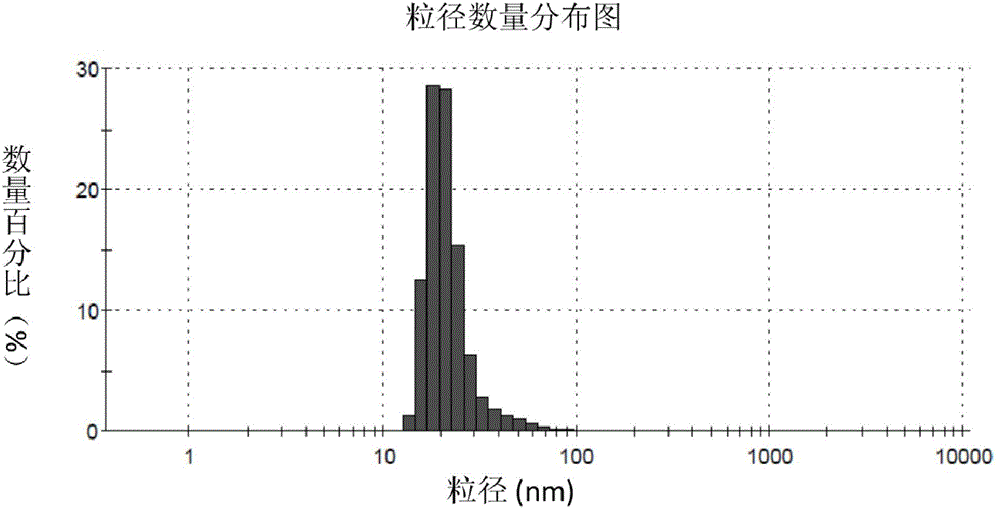
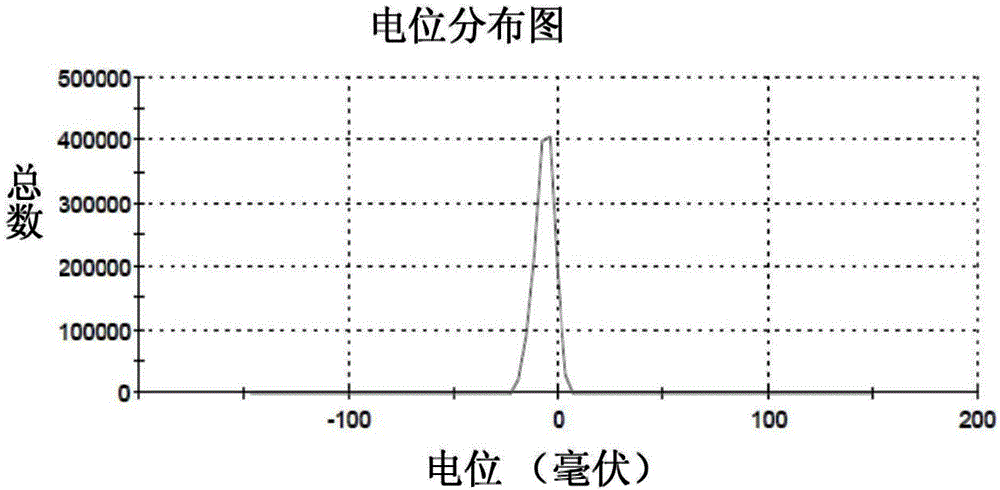
Nano particles modified by alendronic acid functionalized polyethylene glycol and preparation method of nano particles
ActiveCN104147614AExtension of timeLow costGenetic material ingredientsPharmaceutical non-active ingredientsCalcium biphosphateMacromolecular drug
The invention provides nano-particles modified by alendronic acid functionalized polyethylene glycol. The nano particles are calcium phosphate particles or calcium carbonate particles wrapped with target delivery matters; the target delivery matters are selected from DNA, siRNA and protein acromolecular drugs or micro-molecular chemical drugs; alendronic acid functionalized polyethylene glycol covers the surfaces of the nano particles; and the particle size of the nano particles modified by alendronic acid functionalized polyethylene glycol is 20-50 nm. The nano particles modified by alendronic acid functionalized polyethylene glycol are small in particle size; polyalendronic acid-polyethylene glycol can stably cover the surfaces of phosphate-genetic particles, and therefore, the nano particles can be effectively stabilized for a long time; the body circulation time is prolonged; the nano particles disclosed by the invention are low in cost and toxicity, safe and effective. The invention further provides a preparation method of the nano particles modified by the alendronic acid functionalized polyethylene glycol.
Owner:SHENZHEN INST OF ADVANCED TECH
Enteric soluble preparation of Alun phosphorate and its preparing method
ActiveCN1582949AGood effectEliminate irritationOrganic active ingredientsSkeletal disorderMedicineEnteric coating
An enteric allenphosphonate in the form of tablet or capsule is prepared from allenphosphonate, medicinal auxiliary and enteric coating.
Owner:CSPC OUYI PHARM CO LTD
Therapy using a combination of raloxifene and alendronate
Provided are methods of treating bone disease including, but not limited to, osteoporosis, metastatic bone disease, or Paget's disease, by administering a combination of raloxifene and alendronate in a manner that mitigates the formation of ulcerative adverse events.
Owner:TEVA PHARM USA INC
Method of treating or preventing osteoporosis comprising administering to a patient in need thereof an effective amount of pharmaceutical composition comprising benzamidine derivatives or their salts, and alendronic acid or its salt
InactiveUS20100029595A1Evaluate effectBiocidePhosphorous compound active ingredientsBenzamidineOsteopoikilosis
The present invention relates to a pharmaceutical composition for preventing and treating osteoporosis, comprising N-hydroxy-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)phenoxy]pentoxy}benzamidine, 4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)phenoxy]pentoxy}benzamidine, or salts thereof, and alendronic acid or a salt thereof.As a prophylactic or therapeutic composition for osteoporosis, the combination treatment of N-hydroxy-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)phenoxy]pentoxy}benzamidine, 4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)phenoxy]pentoxy}-benzamidine, or salts thereof and alendronic acid or a salt thereof exhibits excellent inhibitory effect on osteoclast differentiation, as compared to each individual treatment, thereby being useful for the prevention or treatment of osteoporosis.
Owner:DONG WHA PHARM CO LTD
Formulations of lycopene in combination with bisphosphonates bone resorption inhibitors
InactiveUS20140356423A1Improve bioavailabilityInhibiting bone resorptionBiocideHydrocarbon active ingredientsLycoperseneSoftgel
The present invention provides a pharmaceutical formulation suitable for filling softgel capsules comprising: (a) from about 1% to about 90% by weight of a bisphosphonic acid or a pharmaceutically acceptable salt; (b) from 1% to about 99% by weight of lycopene and (c) from about 40% to about 80% by weight of a liquid carrier comprising 50% to 80% by weight polyethylene glycol; 5% to 15% by weight of glycerin; and 5% to 20% by weight water. The invention also describes a method for preparing alendronate or its pharmaceutical acceptable salts in encapsulated therapeutic dosage form in combination with lycopene.
Owner:ANGRES ISAAC A
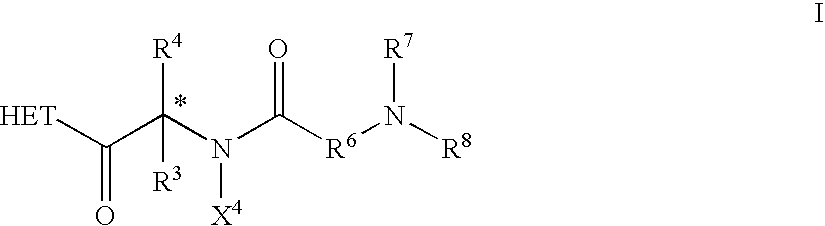
Dipeptide derivatives as growth hormone secretagogues
This invention is directed to compounds of the formula ##STR1## and the pharmaceutically-acceptable salts thereof, where the substituents are as defined in the Specification, which are growth hormone secretogogues and which increase the level of endogenous growth hormone. The compounds of this invention are useful for the treatment and prevention of osteoporosis and / or frailty, congestive heart failure, frailty associated with aging, obesity; accelerating bone fracture repair, attenuating protein catabolic response after a major operation, reducing cachexia and protein loss due to chronic illness, accelerating wound healing, or accelerating the recovery of burn patients or patients having undergone major surgery; improving muscle strength, mobility, maintenance of skin thickness, metabolic homeostasis or renal homeostasis. The compounds of the present invention are also useful in treating osteoporosis and / or frailty when used in combination with: a bisphosphonate compound such as alendronate; estrogen, premarin, and optionally progesterone; an estrogen agonist or antagonist; or calcitonin, and pharmaceutical compositions useful therefor. Further, the present invention is directed to pharmaceutical compositions useful for increasing the endogenous production or release of growth hormone in a human or other animal which comprises an effective amount of a compound of the present invention and a growth hormone secretagogue selected from GHRP-6, Hexarelin, GHRP-1, growth hormone releasing factor (GRF), IGF-1, IGF-2 or B-HT920. The invention is also directed to intermediates useful in the preparation of compounds of Formula I.
Owner:PFIZER INC
Bisphosphonate-prostatic acid phosphatase inhibitor conjugates to treat prostate cancer bone metastasis
InactiveUS20110065672A1Growth inhibitionReduce the overall heightBiocidePhosphorous compound active ingredientsMedicineProstatic acid phosphatase
The present invention concerns conjugate compounds comprising a bisphosphonate covalently bonded to a prostatic acid phosphatase inhibitor and compositions comprising such conjugates. Methods for treating and inhibiting prostate cancer bone metastases, and determining whether a conjugate is useful for such treatment are also provided. In some instances, the bisphosphonate is alendronate, and it is covalently bonded to either tartaric acid or glyceric acid.
Owner:MT SINAI SCHOOL OF MEDICINE
Pharmaceutical composition for treating post-chemotherapeutic osteoporosis and use thereof
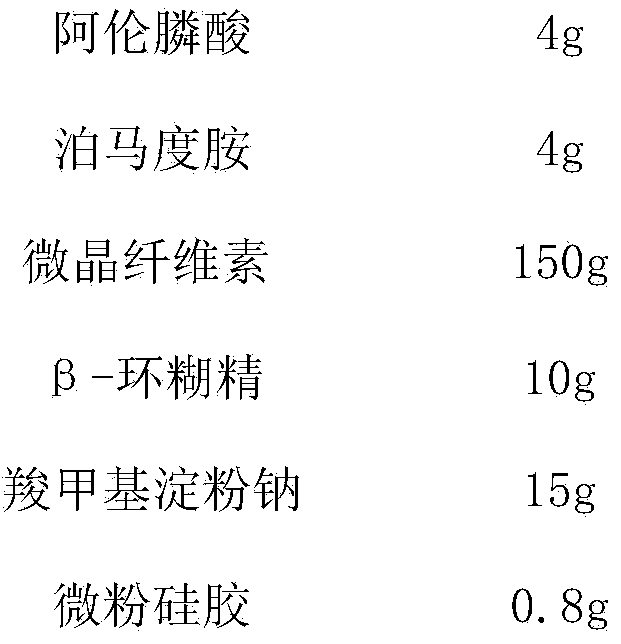
InactiveCN104161762AGood treatment effectExact therapeutic effectOrganic active ingredientsSkeletal disorderWeight coefficientTherapeutic effect
The invention relates to a pharmaceutical composition for treating post-malignant tumor-chemotherapeutic osteoporosis and use thereof and belongs to the field of medicines. In order to overcome the technical defect that the medicine for treating post-malignant tumor-chemotherapeutic osteoporosis in the prior art is poor to control symptoms, the invention provides a pharmaceutical composition containing alendronic acid and pomalidomide. The pharmaceutical composition used for treating post-malignant tumor-chemotherapeutic osteoporosis is good to control symptoms and has a remarkable effect in increasing bone mineral density and bone weight coefficient as well as increasing content of serum inorganic phosphorus and blood calcium, so that the pharmaceutical composition is suitable for clinically treating tumor chemotherapy induced osteoporosis.
Owner:王鹏
Dipeptide derivatives
InactiveUS6924280B2Reduced responseReducing cachexia and protein lossBiocideNervous disorderProgesteronesConjugated estrogen
This invention is directed to compounds of the formula and the pharmaceutically-acceptable salts thereof, where the substituents are as defined in the Specification, which are growth hormone secretogogues and which increase the level of endogenous growth hormone. The compounds of this invention are useful for the treatment and prevention of osteoporosis and / or frailty, congestive heart failure, frailty associated with aging, obesity; accelerating bone fracture repair, attenuating protein catabolic response after a major operation, reducing cachexia and protein loss due to chronic illness, accelerating wound healing, or accelerating the recovery of burn patients or patients having undergone major surgery; improving muscle strength, mobility, maintenance of skin thickness, metabolic homeostasis or renal homeostasis. The compounds of the present invention are also useful in treating osteoporosis and / or frailty when used in combination with: a bisphosphonate compound such as alendronate; estrogen, conjugated estrogens, and optionally progesterone; an estrogen agonist or antagonist; or calcitonin, and pharmaceutical compositions useful therefor. Further, the present invention is directed to pharmaceutical compositions useful for increasing the endogenous production or release of growth hormone in a human or other animal which comprises an effective amount of a compound of the present invention and a growth hormone secretagogue selected from GHRP-6, Hexarelin, GHRP-1, growth hormone releasing factor (GRF), IGF-1, IGF-2 or B-HT920. The invention is also directed to intermediates useful in the preparation of compounds of Formula I.
Owner:PFIZER INC
Tablet obtained by direct compression comprising 4-amino-1-hydroxybutylidene-1,1-bisphosphonic acid as active ingredient
InactiveUS20030161878A1Decrease of tablet stabilityImprove stabilityBiocidePhosphorous compound active ingredientsDiluentLactose
A tablet, obtainable by direct compression, comprising the active ingredient 4-amino-1-hydroxybutylidene-1,1-bis-phosphonic (alendronic) acid or its pharmaceutically acceptable salts in an amount of 5 to 140 mg, based on the pure acid, a dry binder, a disintegrating agent, a lubricant, the tablet comprising, as the diluent, a combination of at least two diluents except lactose.
Owner:ZENTIVA AS
Delivery of a bioactive material
InactiveUS8753683B2Reduced bioavailabilityRapid onset of releaseBiocidePowder deliveryActive agentSurface-active agents
A solid pharmaceutical composition comprising a water-soluble bioactive material and an encapsulating material which is present in the composition in the form of continuous solid phase, and in which solid particles of the bioactive material are dispersed and encapsulated in the continuous solid phase of the encapsulating material, wherein each of the bioactive material and the encapsulating material is normally a solid at room temperature and the melting point of the encapsulating material is lower than the melting point of the bioactive material, the bioactive material being preferably a bisphosphonate, most preferably alendronate, and the encapsulating material includes an enhancer, preferably a mono- or di-glyceride, or an encapsulating surfactant, preferably a polyoxyethylene / polyoxypropylene block copolymer having surface active properties, and a process for preparing the composition in which solid particles of the bioactive material are mixed with and dispersed in the encapsulating material which is in molten (liquid) form; and cooling the molten form of the encapsulating material to form a solid pharmaceutical composition having the solid particles of the bioactive material dispersed and encapsulated in a continuous solid phase of the encapsulating material.
Owner:NOVO NORDISK AS
Synthetic method of clinic effect of alendronate
ActiveCN101284848AOvercoming the disadvantages of solidification and inability to stirAvoid spontaneous combustion, dangerous problemsGroup 5/15 element organic compoundsSkeletal disorderPhosphorous acidOperability
The invention provides a method for synthesizing alendronic acid. According to the invention, 4-aminobutyric acid, phosphorous acid, phosphorus trichloride or phosphorus pentachloride are reacted in aprotic polar solvent, and then the mixture obtained through the reaction is hydrolyzed and crystallized to obtain the alendronic acid. The method has high safety and reliability, easily separated product, strong operability, high yield and low cost, and is particularly suitable for large-scale production.
Owner:CSPC OUYI PHARM CO LTD
Preparation method for alendronic acid
ActiveCN101125863AReduce manufacturing costControl concentrationGroup 5/15 element organic compoundsFiltrationYield rate
The invention provides a novel preparation method of alendronic acid, pertaining to medicine and chemical technical field. The method solves the run-away safety hidden trouble caused by self-putting heat flush materials in the existing preparation method of alendronic acid. The novel preparation method of the alendronic acid comprises the steps: (1) 2-pyrrolidone is added in acidic aqueous solution, thus obtaining Gamma -aminobutyrate after reaction; (2) the Gamma -aminobutyrate system that is obtained from reaction is added into organic solvent of 2-pyrrolidone, and phosphorus tricholoride is added; (3) water is added in the reaction system to lower the temperature, remove organic horizon, and aqueous layer reflows, and active carbon is added to decolor, the solution is added in alcohol solution to be crystallized after filtration and concentration, thus obtaining alendronic acid through filtration and drying. The novel preparation method of the alendronic acid has the advantages of low cost, high yield rate, and good industrial application value.
Owner:ZHEJIANG LEPU PHARMA CO LTD
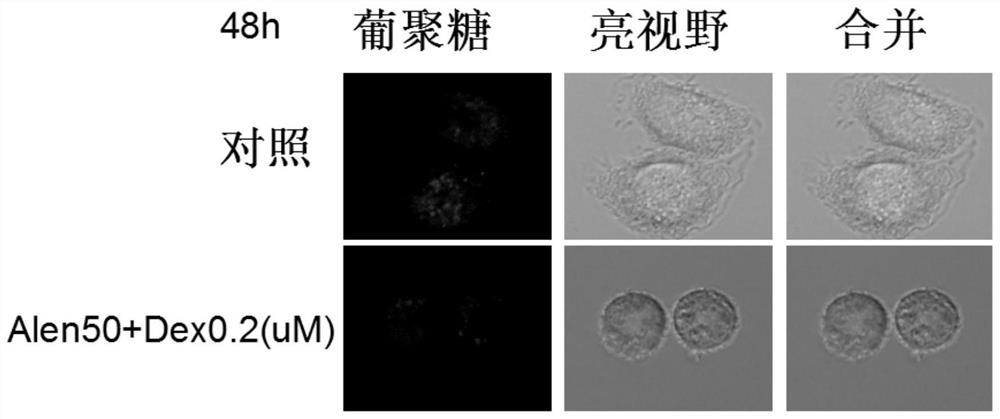
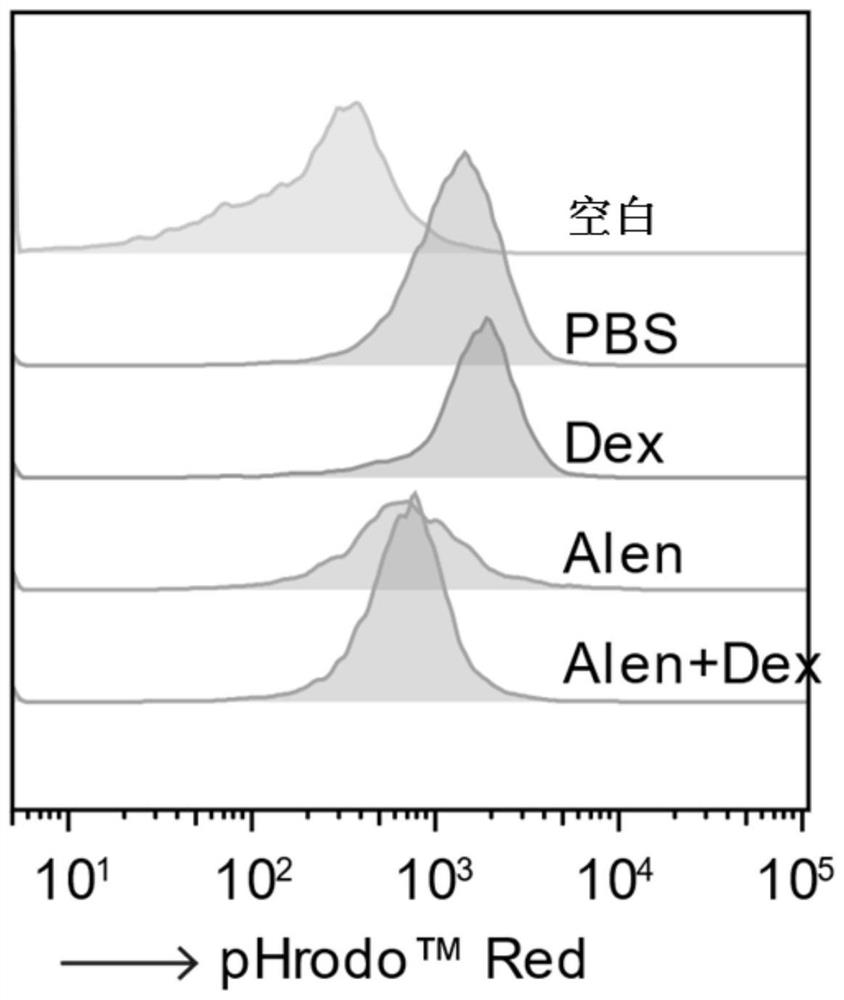
Application of nitrogenous bisphosphonate combined glucocorticoid in prevention or treatment of viral pneumonia
ActiveCN114028571AOrganic active ingredientsPharmaceutical delivery mechanismPropanoic acidFluticasone propionate
The invention provides application of nitrogenous bisphosphonate combined glucocorticoid in prevention or treatment of viral pneumonia. In particular, the nitrogenous bisphosphonate combined glucocorticoid is used to treat or ameliorate coronavirus infections, wherein the nitrogen-containing bisphosphonate is selected from pamironate, alendronate, risedronate, ibandronate, zoledronate, minodronate and pnaphonate, and the glucocorticoid is selected from the group consisting of dexamethasone, rimesolone, prednisolone, fluorometholone, hydrocortisone, mometasone, fluticasone, beclomethasone, flunivoxone, antiphlogistic, fluticasone propionate, beclomethasone dipropionate, budesonide and mometasone furoate.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Alendronate oral liquid formulations
InactiveUS20090170815A1Easy to manageImprove complianceBiocidePharmaceutical delivery mechanismMedicineOral solutions
The invention features an oral pharmaceutical solution comprising a therapeutically effective amount of alendronate or a salt thereof and a pharmaceutically acceptable liquid carrier. The solution is substantially free from degradation products, with the proviso that the solution has no buffer and no complexing agent. The oral solution avoids the difficulties in swallowing tablets of the prior art. Moreover, the oral solution is surprisingly stable without the use of buffering systems and complexing agents of the prior art.
Owner:ROXANE LAB
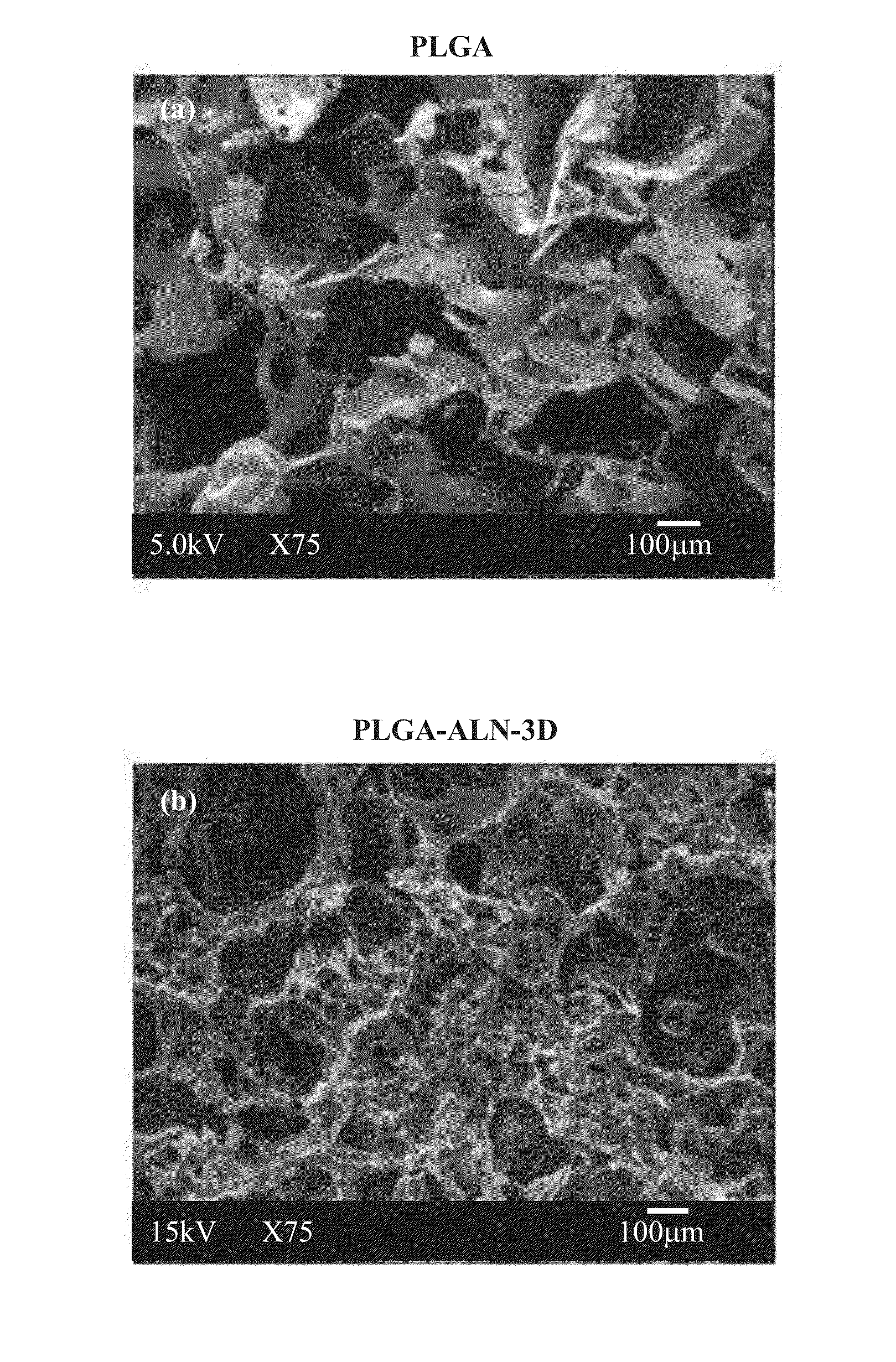
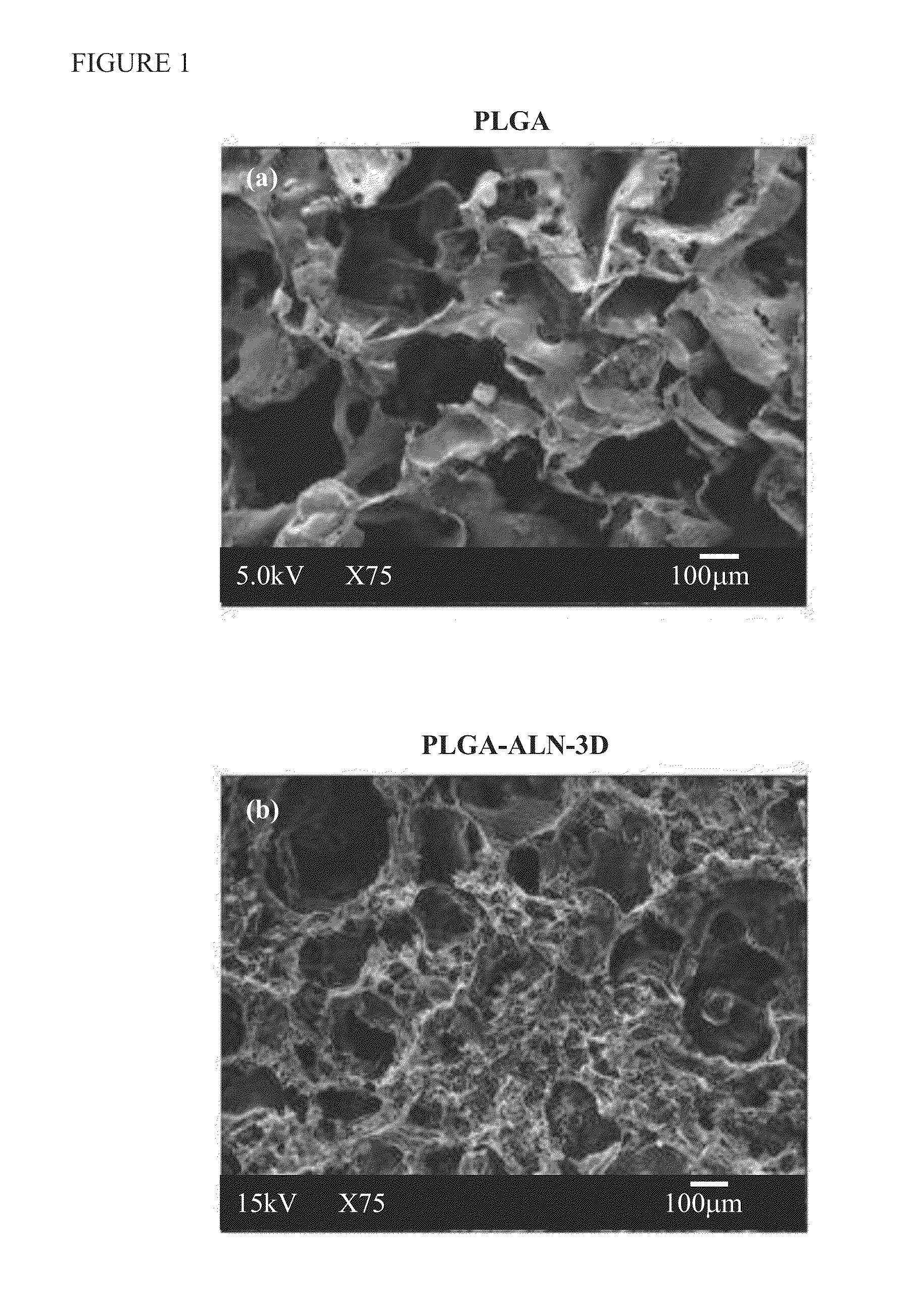
Method for bone formation by administering poly(lactic-co-glycolic acid) cross-linked alendronate
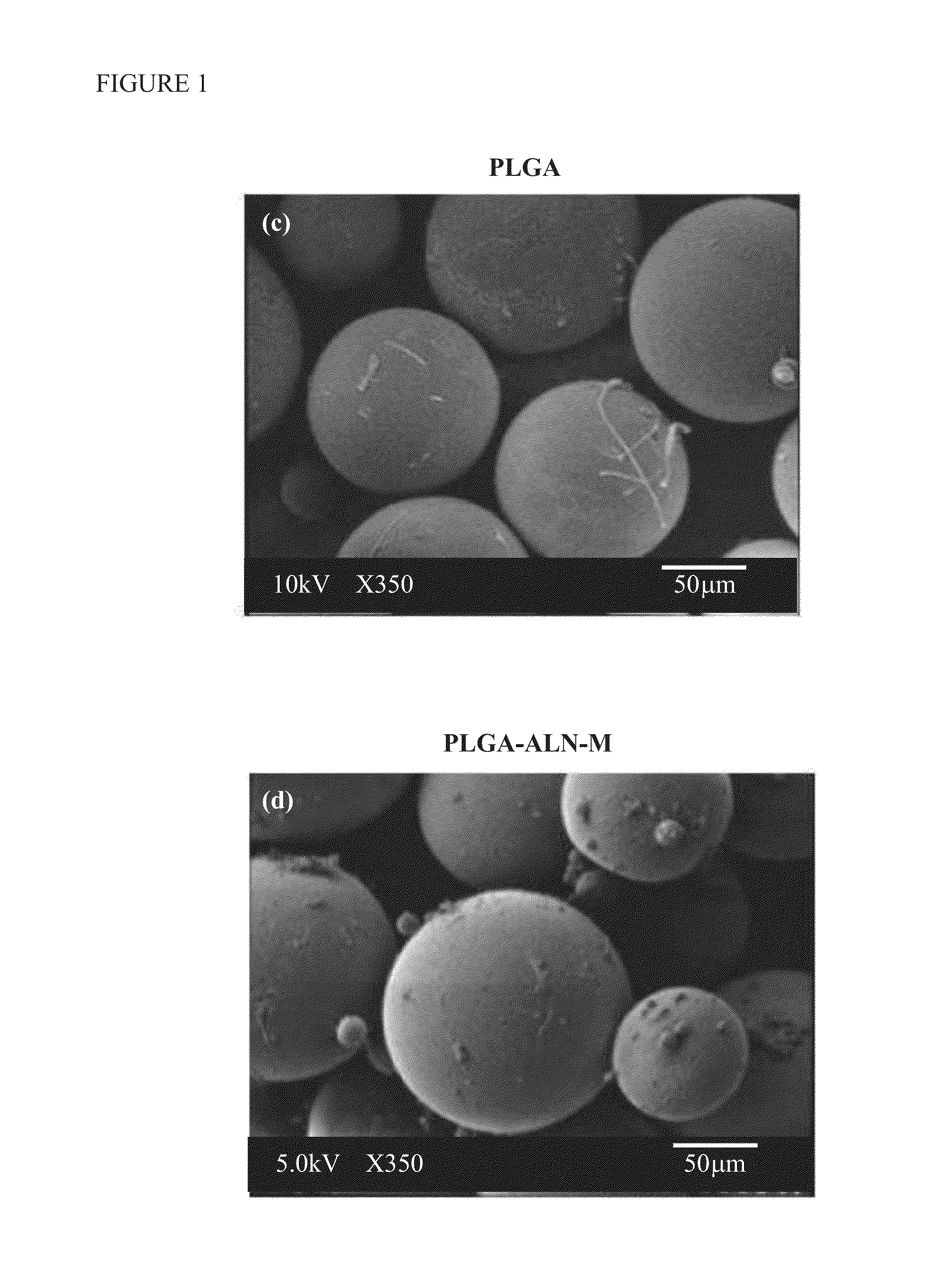
A method for bone regeneration which comprises administering a short term release composition into a bone area of a subject in need thereof, wherein the composition comprises a poly(lactic-co-glycolic acid) cross-linked alendronate (PLGA-ALN), wherein the composition releases the alendronate into the bone area, wherein the bone tissue of the bone area is exposed in situ to a therapeutically effective amount of the alendronate over 9 days.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Targeted liposomes comprising n-containing bisphosphonates and uses thereof
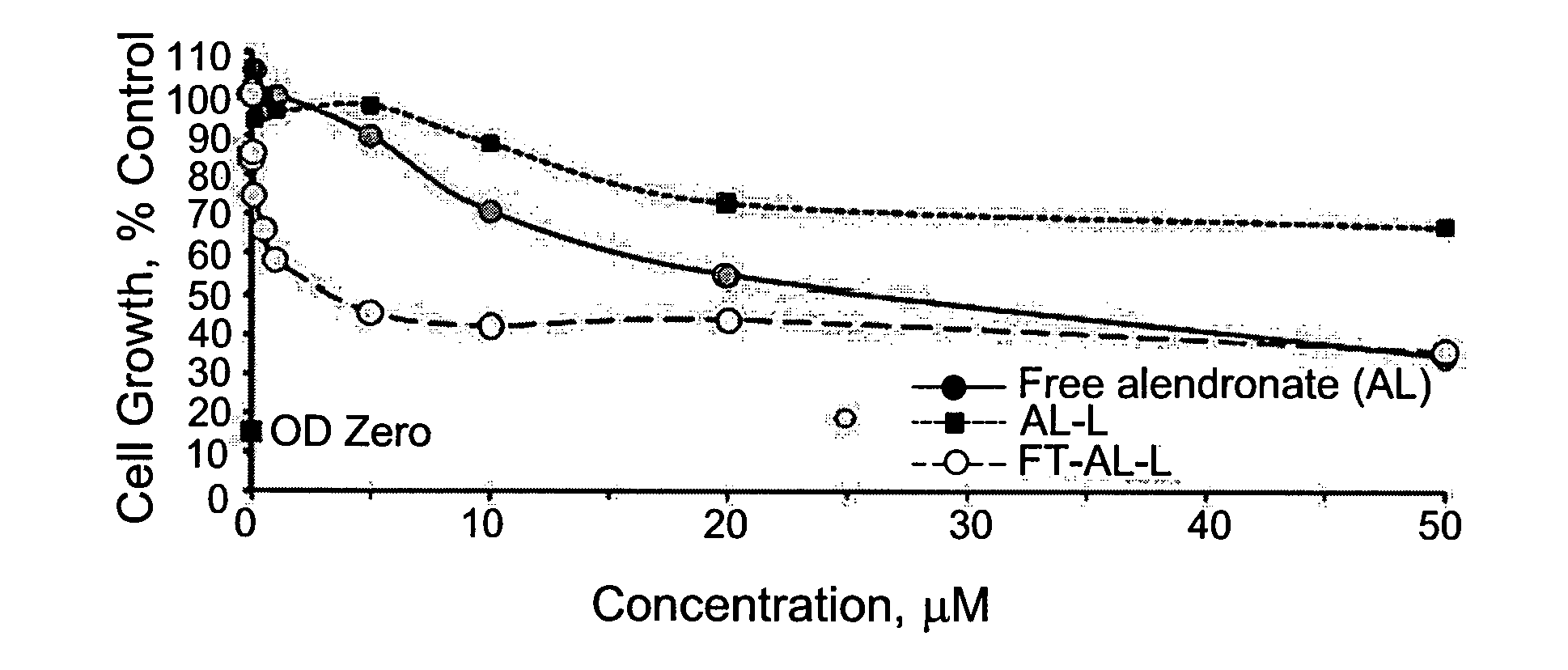
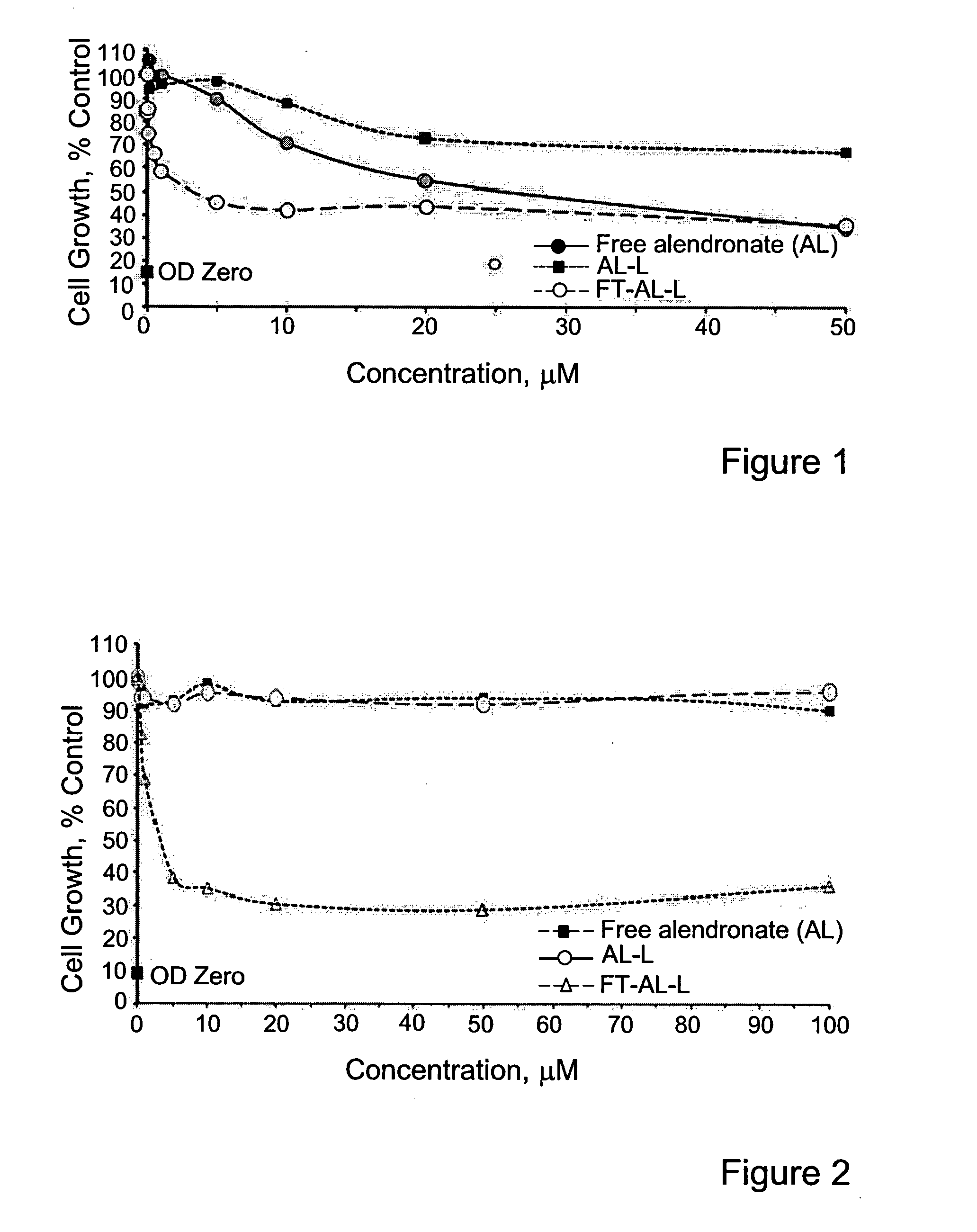
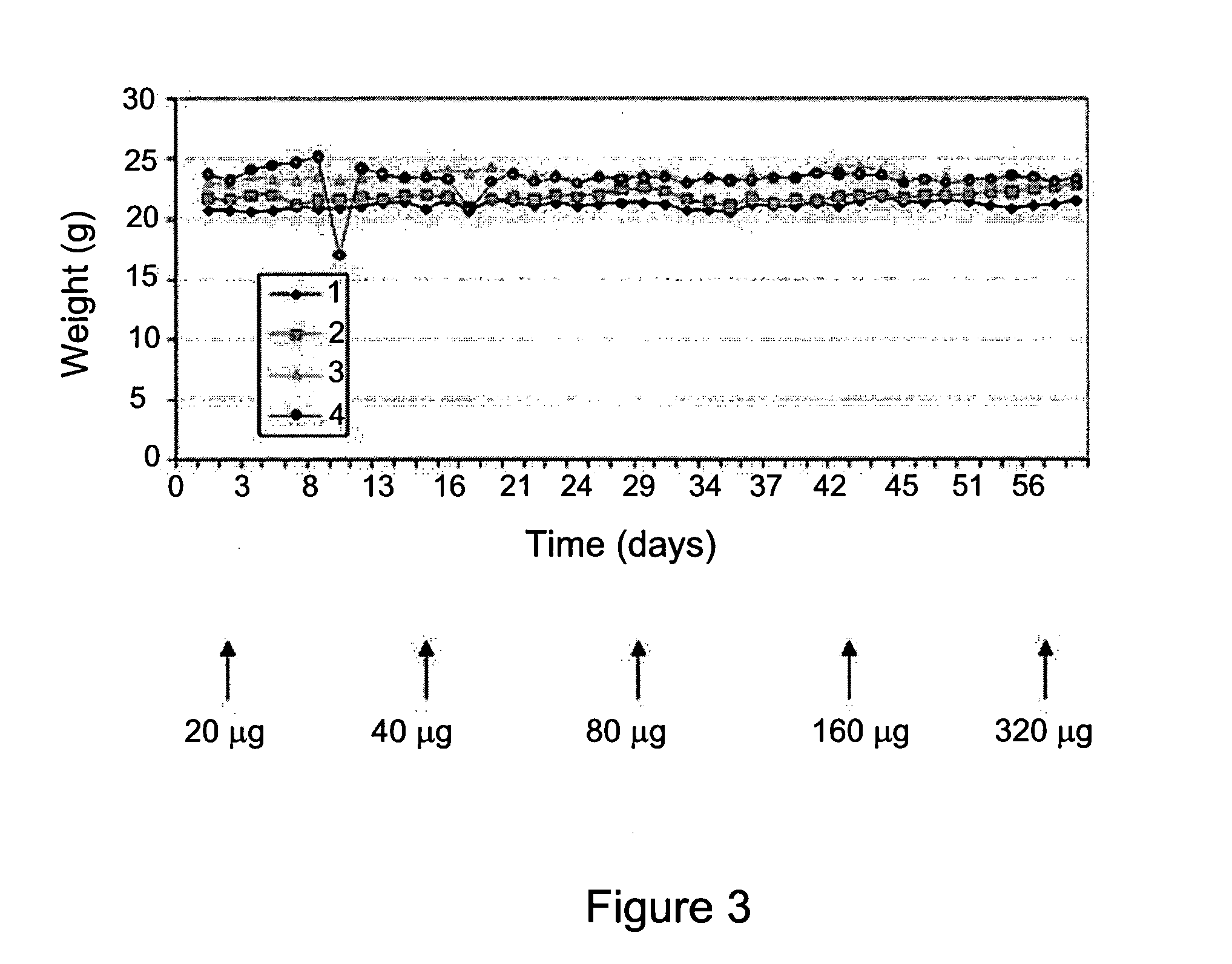
The present is based on the finding that folate targeted liposomal alendronate (FT-AL-L) was significantly more potent against two tested cancer cell lines than the free alendronate (AL) or the non-targeted liposomal alendronate (AL-L), as observed by the increased cytotoxicity of the folate targeted liposomal alendronate. Thus, the present disclosure provides targeted liposomes comprising a membrane and an intraliposomal core, the membrane comprising at least one liposome forming lipid and a targeting moiety, such as folate, exposed at the membrane's outer surface; and the intraliposomal core comprising encapsulated therein least one N-containing bisphosphonate. Also provided by the present disclosure are methods of use of the targeted liposomes such as for the treatment of a disease or disorder.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Alendronate sodium intestine-sol capsule and preparation method thereof
ActiveCN101601662BEnsure safetyGuaranteed releaseOrganic active ingredientsSkeletal disorderIntestinal structurePollution
The invention discloses an alendronate sodium intestine-sol capsule and a preparation method thereof; the intestine-sol capsule comprises the following compositions by weight parts: 10-20 parts of alendronate sodium, 70-100 parts of diluents, 0.5-5 parts of protective agents and 0.5-2 parts of lubricants. The alendronate sodium intestine-sol capsule is particularly added in the protective agents,so as to effectively prevent basic remedy from being released in oral cavity, esophagus and esophagus before reaching the absorption site of the intestinal canal, thereby improving the medicine quality, reducing the adverse reaction and improving the compliance of a patient; the preparation process is simple, the pollution to the environment is reduced by energy consumption and social cost is saved.
Owner:JIANGSU SHENLONG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com