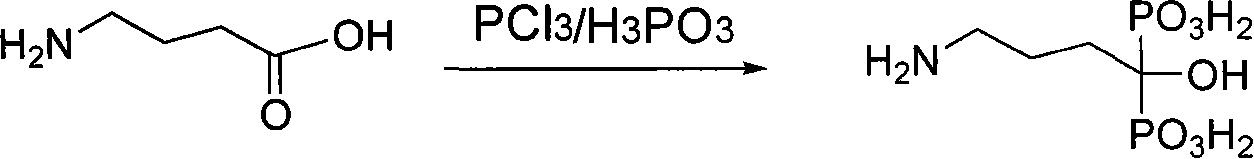Preparation method for alendronic acid
A technology of acid new and acid aqueous solution, applied in the field of medicine and chemistry, can solve the problems of hidden danger, high cost of γ-aminobutyric acid, easy to be oxidized, etc., and achieve the effect of reducing production cost, good industrial application value and improving safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 51g of 2-pyrrolidone and 100g of 70% sulfuric acid aqueous solution were hydrolyzed at a controlled temperature of 80°C for 20 hours. After the reaction was completed, 300ml of chlorobenzene was added, the temperature was stirred at 60°C, and 182g of phosphorus trichloride was added dropwise, and the addition was completed within 2 hours. After the addition, the temperature was controlled at 75°C to continue the reaction for 6 hours. After the reaction was over, 350ml of distilled water was added to the reaction system, the temperature was lowered to 20°C, and the organic layer was separated. After concentration under reduced pressure, it was added to 600ml of 40°C methanol for crystallization, cooled, filtered, and dried to obtain 93.1g of alendronic acid, with a yield of 62.3% and a content (HPLC) of 99.6%.
Embodiment 2
[0029] 51g of 2-pyrrolidone and 90g of 80% phosphoric acid aqueous solution were hydrolyzed at a controlled temperature of 100°C for 16 hours. After the reaction was completed, 200ml of fluorobenzene-difluorobenzene mixed solvent was added, stirring and temperature controlled at 63°C, and phosphorus trichloride was added dropwise. 175g, control the addition in 2.5 hours, after the addition, control the temperature at 80°C and continue the reaction for 5 hours. After the reaction is over, add 200ml of distilled water to the reaction system, lower the temperature to 25°C, separate the organic layer, and heat the water layer to reflux for 3 hours. Add 8 g of activated carbon for decolorization, filter, concentrate under reduced pressure, add to 310 ml of methanol at 45° C. to crystallize, cool, filter, and dry to obtain 73.6 g of alendronic acid, with a yield of 49.3% and a content (HPLC) of 99.5%.
Embodiment 3
[0031] 51g of 2-pyrrolidone and 150g of 95% methanesulfonic acid aqueous solution were hydrolyzed at a controlled temperature of 120°C for 30 hours. After the reaction was completed, 100ml of petroleum ether (bp: 60-90°C) was added after the reaction was completed, and the temperature was stirred at 56°C. Add 168g of phosphorus trichloride, and control the addition for 4.5 hours. After the addition, control the temperature at 60°C to continue the reaction for 8 hours. After the reaction is over, add 500ml of distilled water to the reaction system, cool it down to 10°C, separate the organic layer, and refill the water layer. Heat up and reflux for 1.5 hours, add 10 g of activated carbon for decolorization, filter, concentrate under reduced pressure, add to 400 ml of ethanol at 55°C to crystallize, cool, filter, and dry to obtain 79.5 g of alendronic acid, with a yield of 53.2% and a content (HPLC) of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com