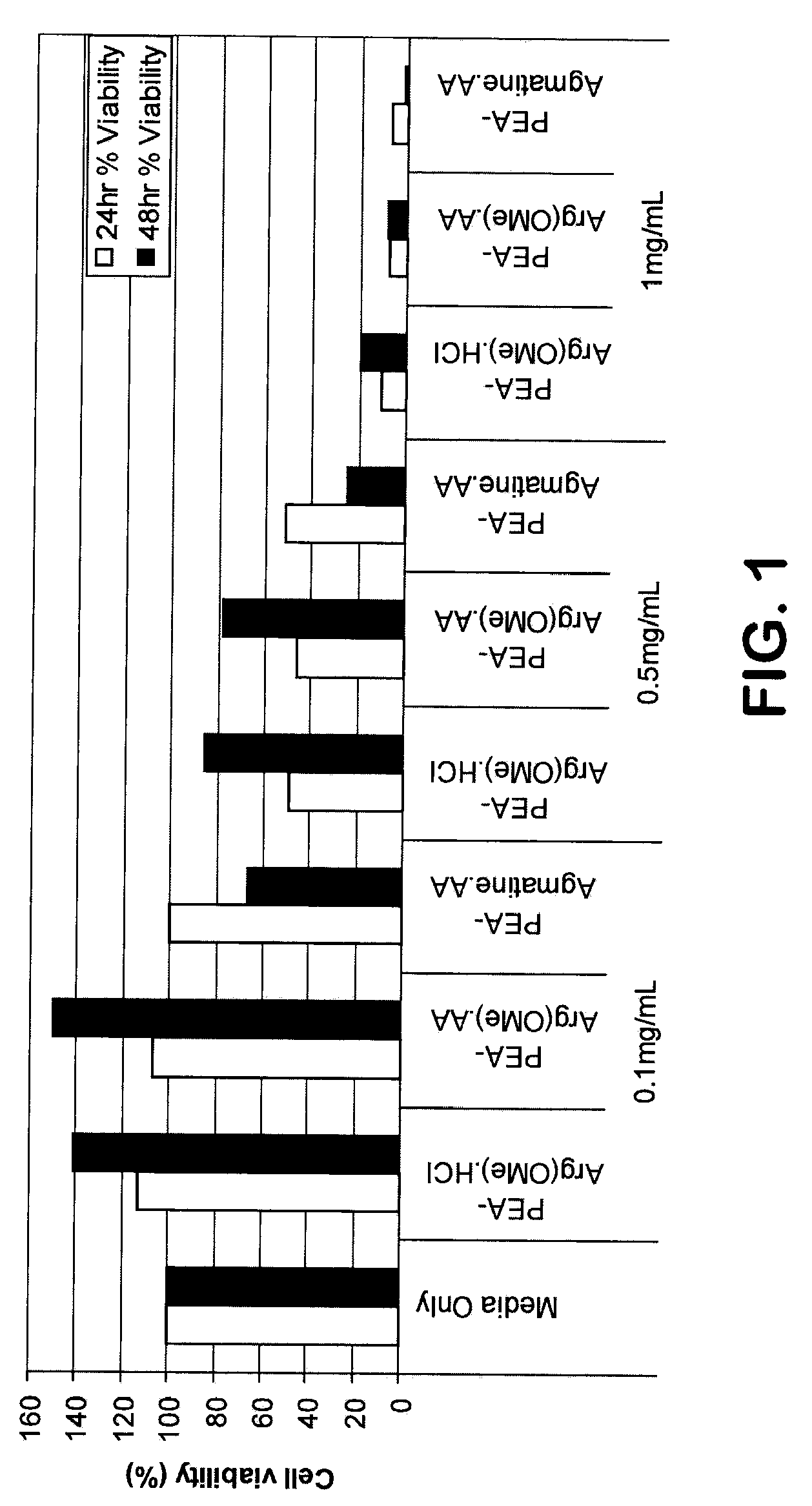
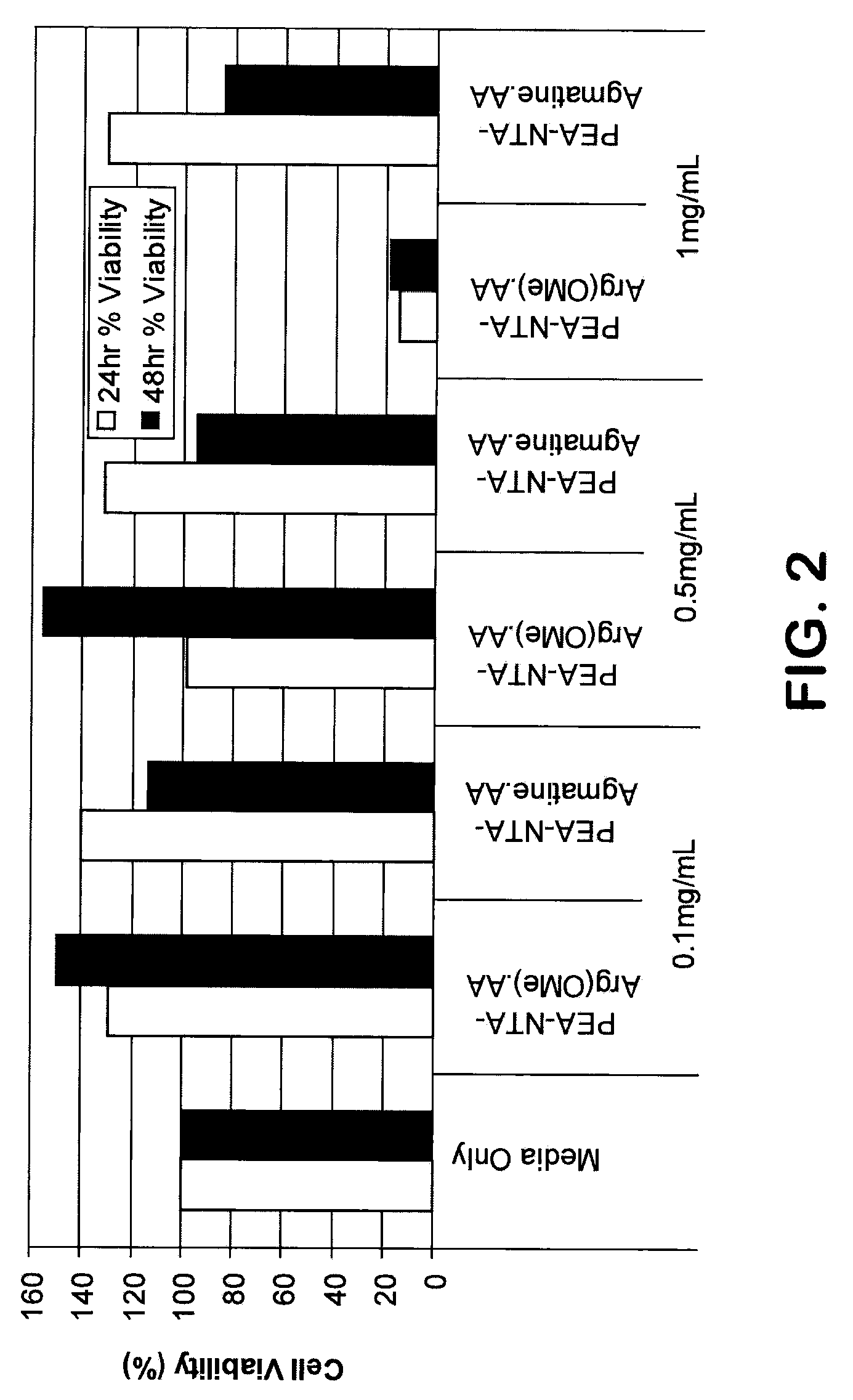
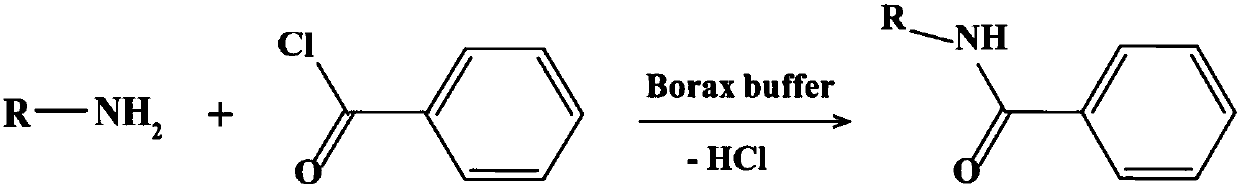
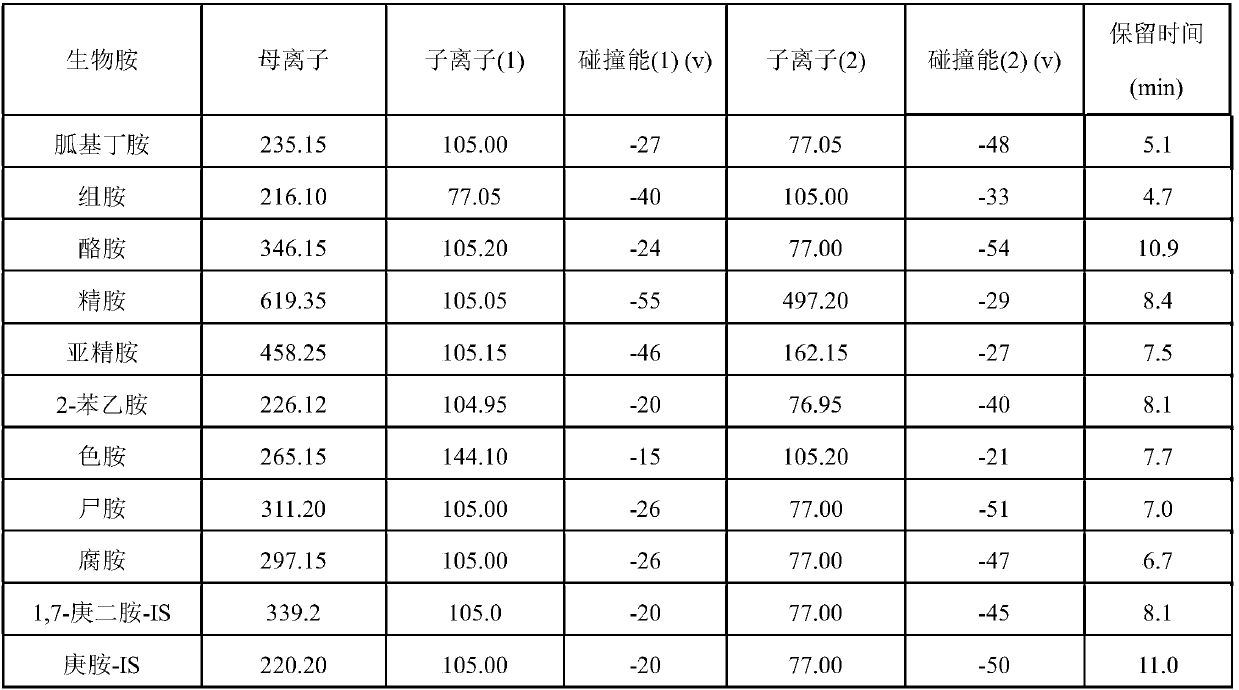
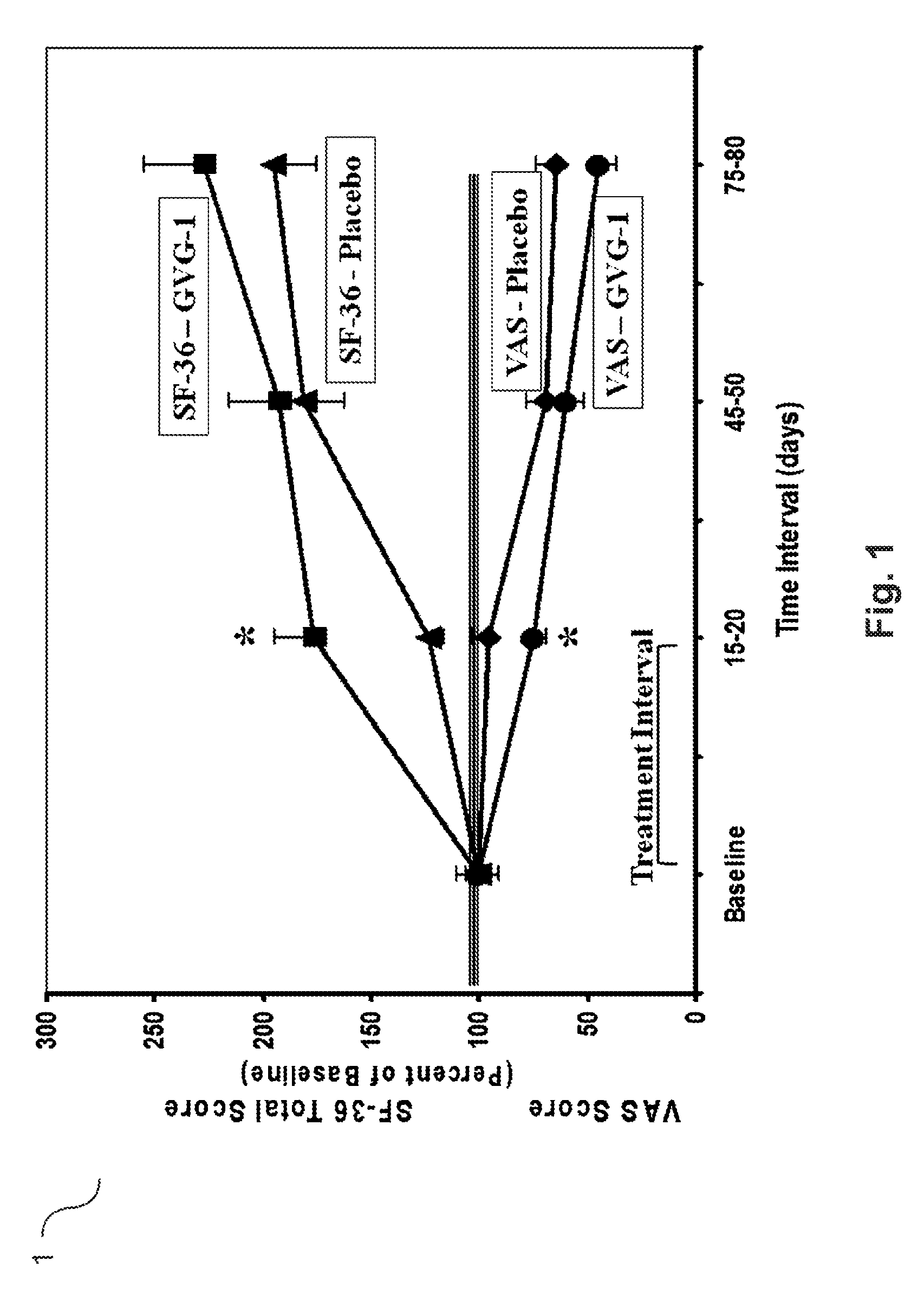
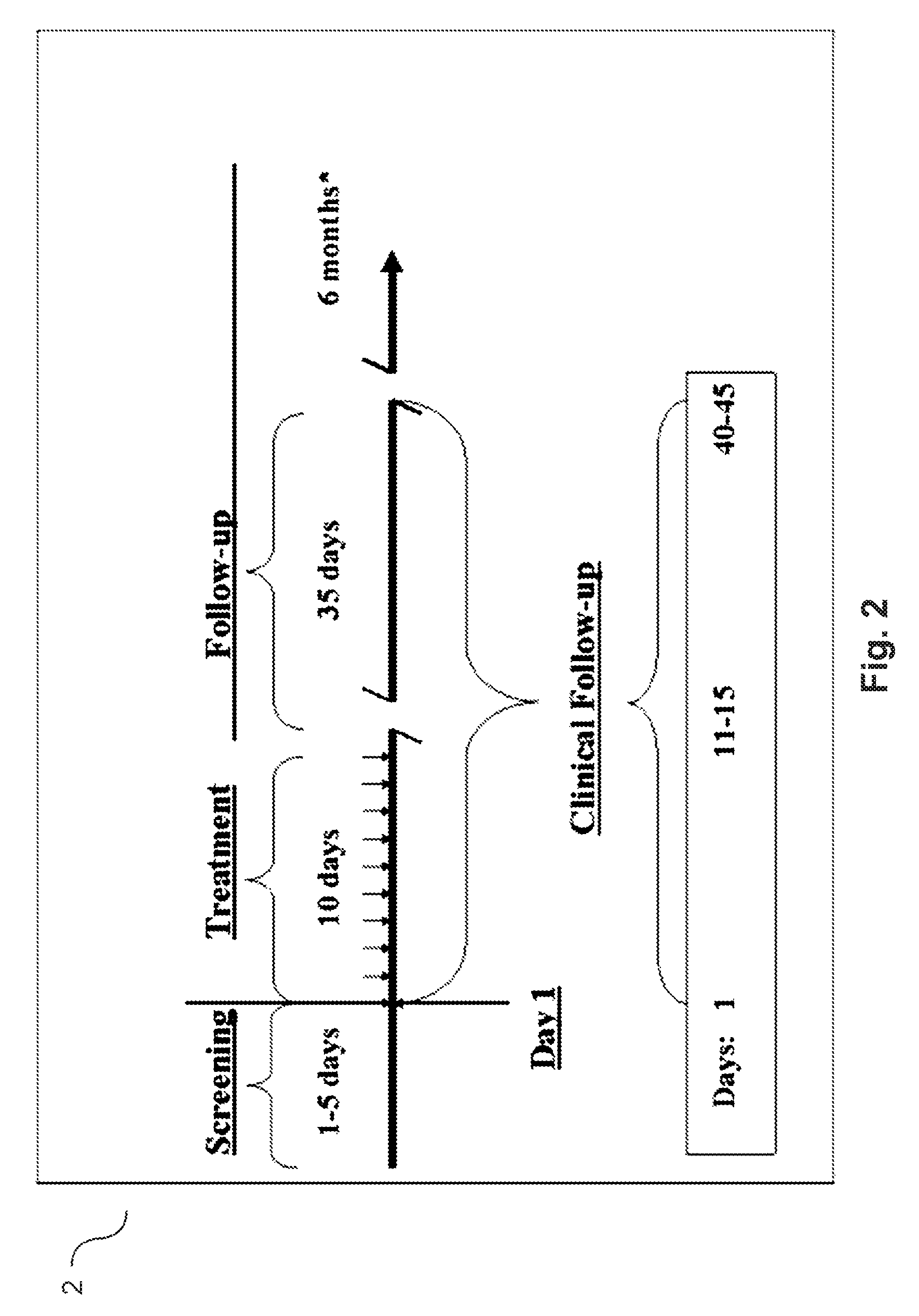
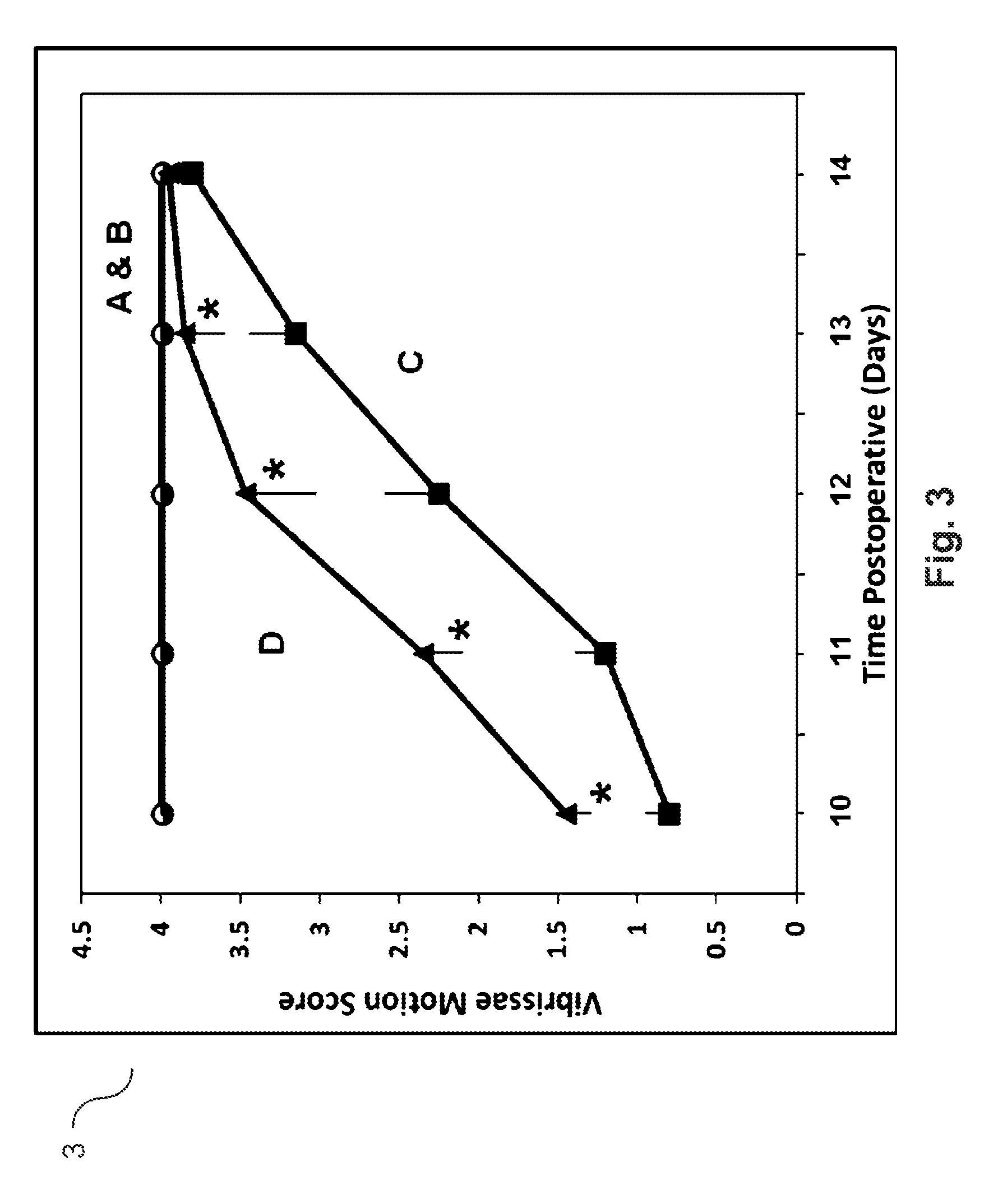
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
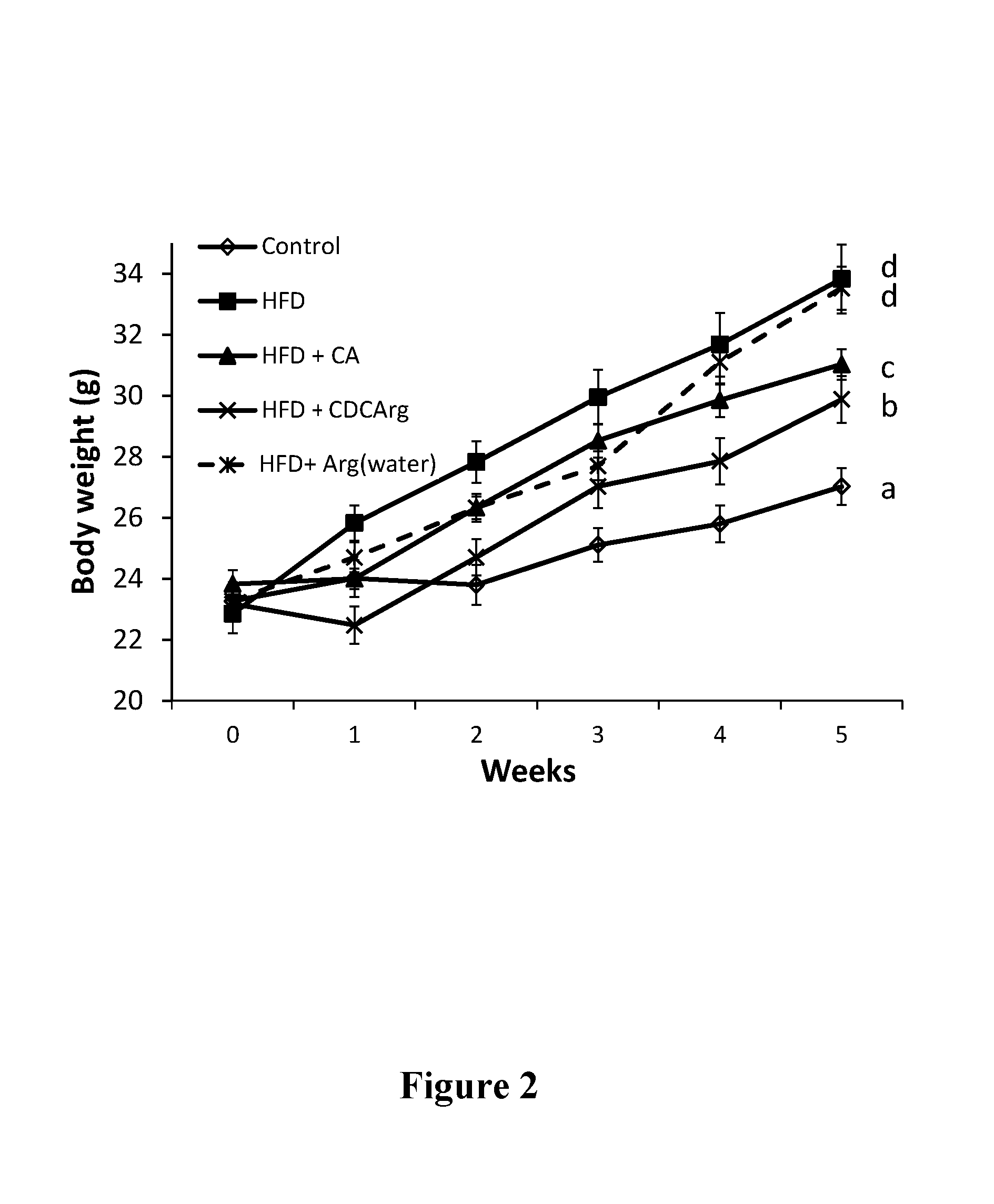
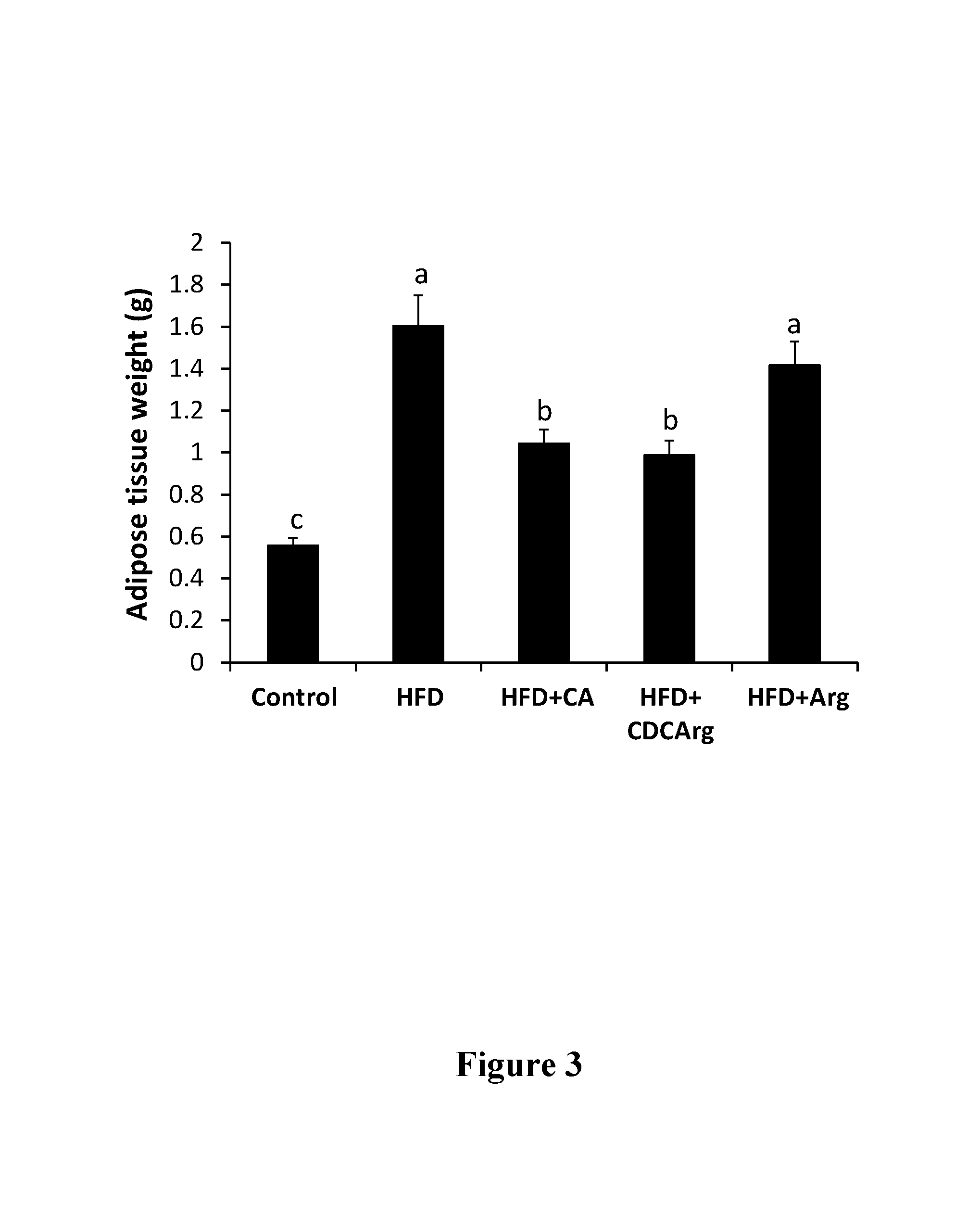
76 results about "Agmatine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
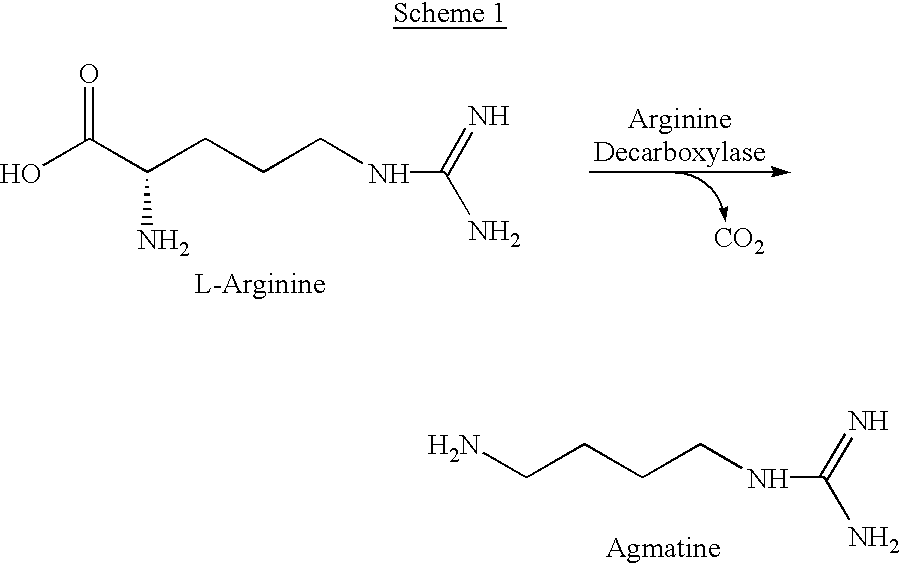
Agmatine, also known as (4-aminobutyl)guanidine, is an aminoguanidine that was discovered in 1910 by Albrecht Kossel. Agmatine is a chemical substance which is naturally created from the chemical arginine. Agmatine has been shown to exert modulatory action at multiple molecular targets, notably: neurotransmitter systems, ion channels, nitric oxide (NO) synthesis and polyamine metabolism and this provides bases for further research into potential applications.
Agmatine, and polyaminoguanidine-bound heterocyclic compounds for neurotrauma and neurodegenerative diseases
InactiveUS6114392ABiocideIsocyanic acid derivatives preparationPhenothiazine derivativeDegenerative Disorder
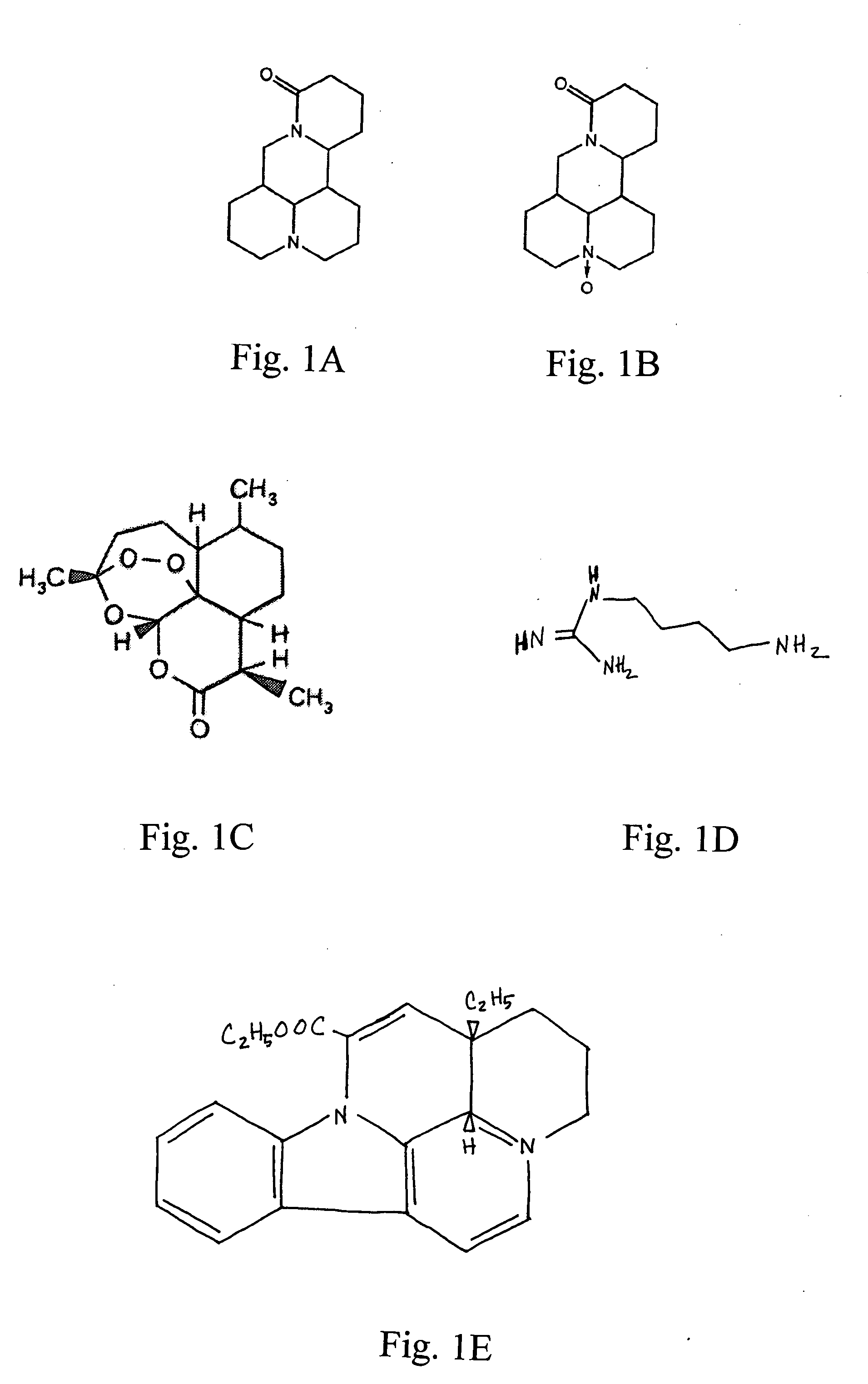
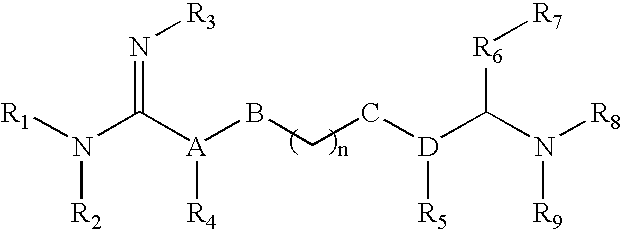
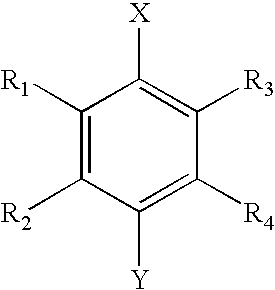
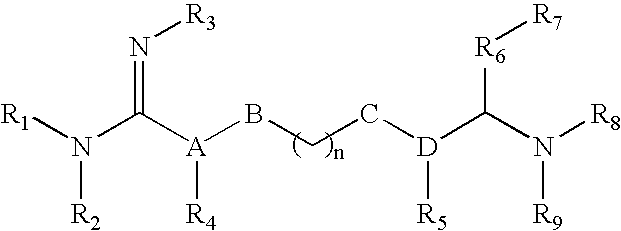
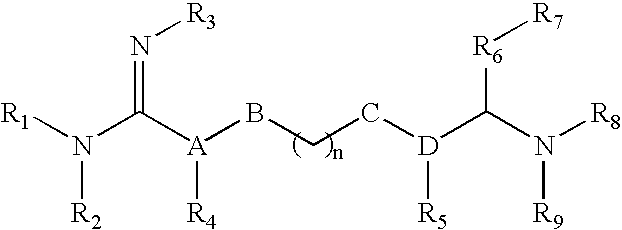
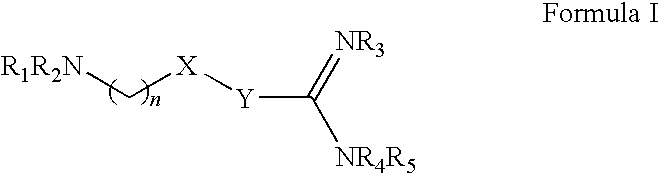
The invention relates to the use of agmatine, in the treatment of acute neurotrauma (such as stroke) and degenerative disorders of the central and peripheral nervous system (such as dementia). The invention further provides novel compounds of general formula I (which are quinuclidine derivatives), formula II (which are norbornane derivatives), formula III (which are adamantane derivatives), and formula IV (which are phenothiazine derivatives): wherein R1, R2 and R3 are each independently hydrogen, hydroxy, substituted or unsubstituted C1-4 alkyl, substituted or unsubstituted C1-4 alkoxy, halogeno, amino, phenyl, or R4NR5; R4 and R5 are each independently hydrogen, or (CH2)n-[NH(CH2)x]y-NHR6, or (CH2)n-[NH(CH2)x]y-NH-NHR6, or (CH2)n-[NH(CH2)x]y-(NR7=)CNHR6, or (CH2)n-[NH(CH2)x]y-NH(NR7=)CNHR6 wherein n is from 0-5, y is from 0-5 and each x is independently from 1-5; R6, and R7 are each independently hydrogen, hydroxy, substituted or unsubstituted C1-4 alkyl, substituted or unsubstituted C1-4 alkoxy, or halogeno; and pharmaceutically acceptable salts and optically active isomers thereof.
Owner:GILAD GAD M +1
Compositions and methods for treating cellular proliferation disorders
Compositions and methods for treating patients suffering from a proliferation disorder characterized by an increased voltage gated ion-channel uptake are described. Included are compositions comprised of a compound selected from the group consisting of matrine, oxymatrine, artemisinin, agmatine, and vinpocetine.
Owner:NUTRICOLOGY INC
Regulation of mammalian hair growth
The present invention relates to a topical skin care composition containing a safe and effective amount of a skin care active comprising agmatine, and its salt; a safe and effective amount of a first additional skin care active selected from the group consisting of BHT or BHA, hexamidine, cetyl pyridinium chloride, green tea catechins, phytosterols, ursolic acid, compounds derived from plant extracts, their salts and derivatives; and a dermatologically acceptable carrier for the agmatine composition. The present invention also relates to methods of using such agmatine compositions to regulate hair growth and the condition of mammalian skin. Said methods generally comprise the step of topically applying the composition to the skin of a mammal needing such treatment, a safe and effective amount of such compositions.
Owner:THE PROCTER & GAMBLE COMPANY
Synthesis and characterization of biodegradable cationic poly(propylene fumarate-co-ethylene glycol) copolymer hydrogels modified with agmatine for enhanced cell adhesion
InactiveUS7629388B2Improve cell adhesionSignificant positive effectBiocidePeptide preparation methodsCross-linkCell adhesion
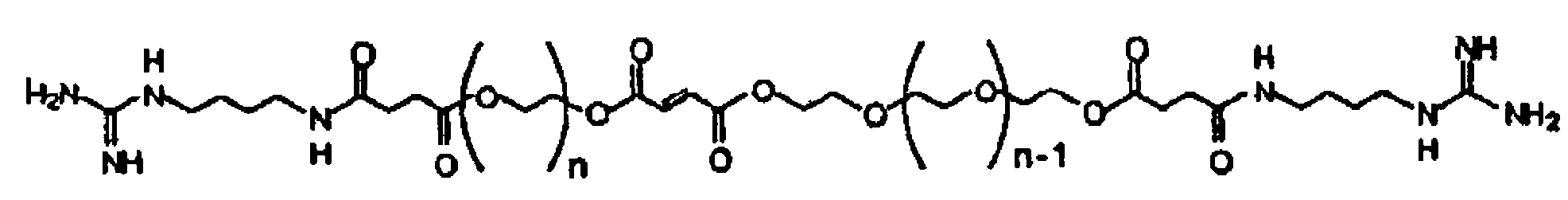
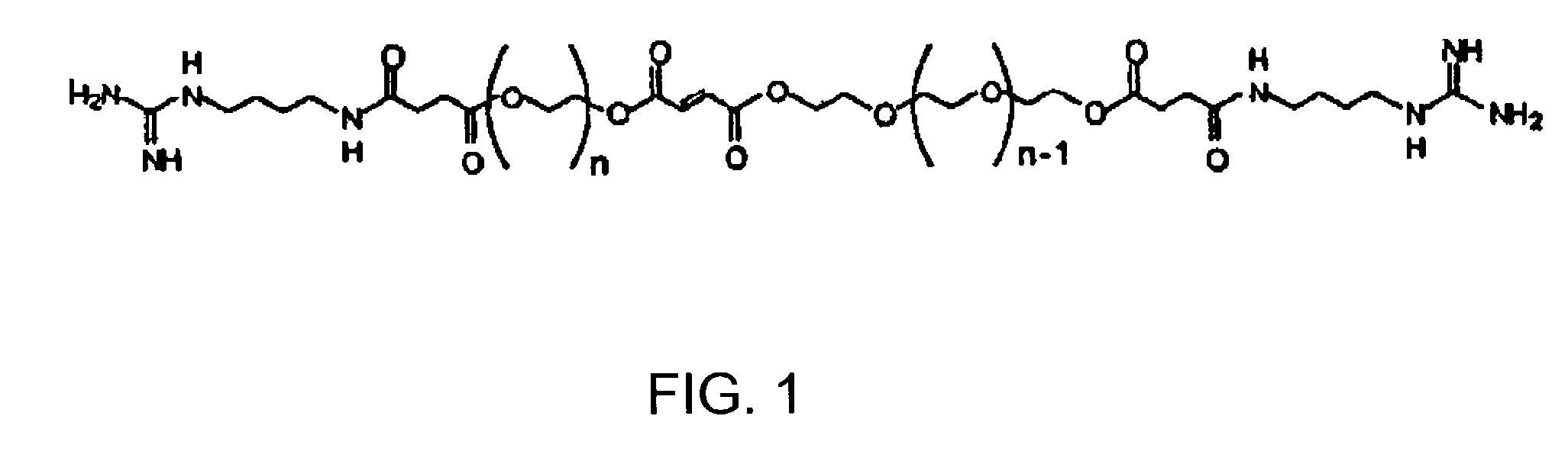
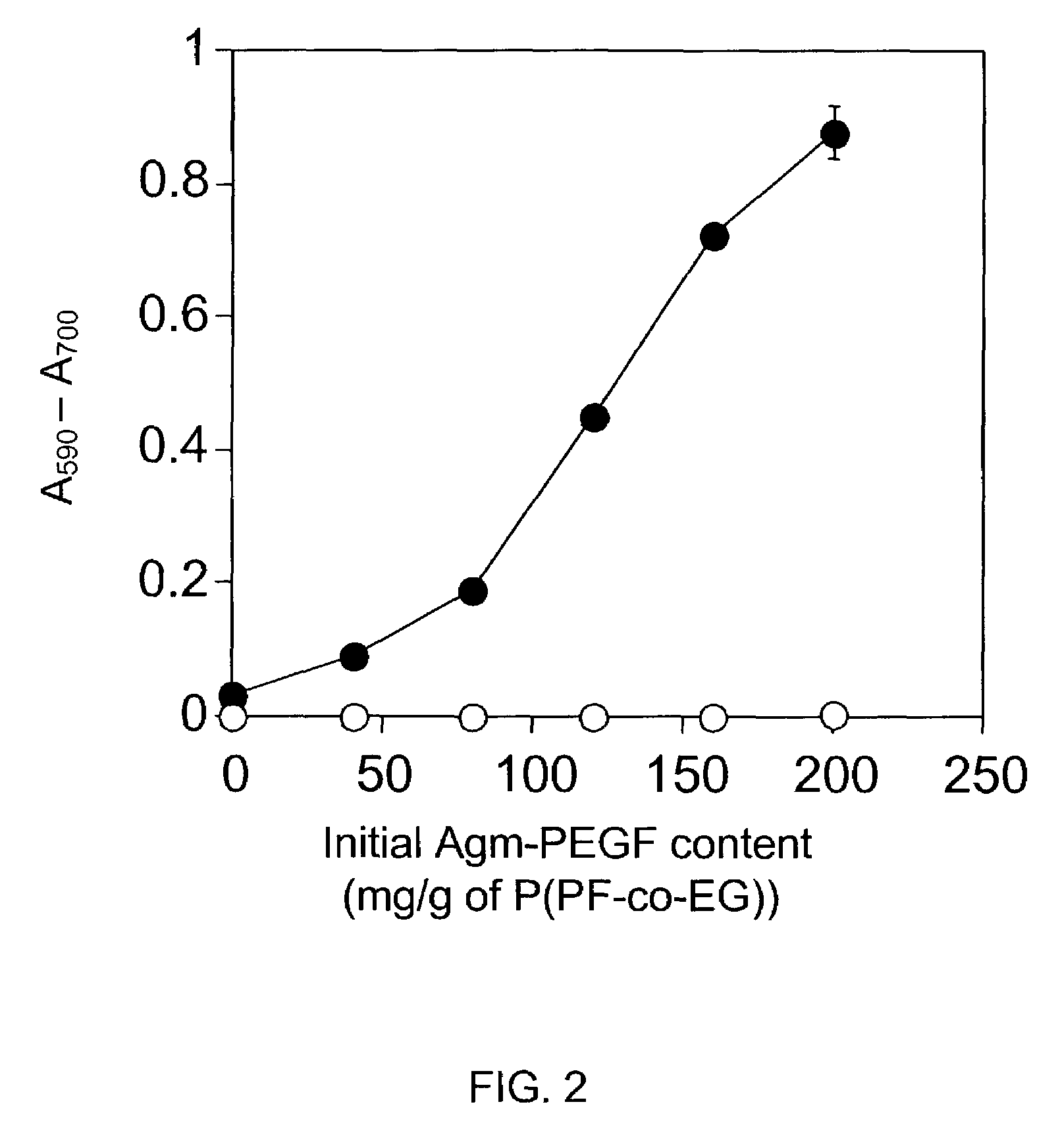
A cross-linkable monomer comprises a fumaric acid functional group having a first end and a second end, a first spacer group affixed to said first end and comprising at least repeating unit, a first terminal group affixed to said first spacer group, a second spacer group affixed to said second end and comprising at least one ethylene glycol repeating unit, and a second terminal group affixed to said second spacer group. A hydrogel formed by cross-linking the present monomer and a method for making the monomer. A method for forming a hydrogel, comprises the steps of a) synthesizing a copolymer of poly(propylene fumarate) (PPF) and poly(ethylene glycol (PEG) so as to produce P(PF-co-EG), b) synthesizing a PEG-tethered fumarate (PEGF), c) coupling agmatine sulfate to the PEGF to produce PEGF modified with agmatine (Agm-PEGF), and d) cross-linking the P(PF-co-EG) from step a) with Agm-PEGF from step c).
Owner:RICE UNIV
Cationic alpha-amino acid-containing biodegradable polymer gene transfer compositions
The invention provides gene transfer compositions using as the gene carrier a biodegradable polymer that contains one or more cationic alpha amino acids, such as arginine or agmatine. The compositions form a tight soluble complex with a poly nucleic acid suitable for transfecting target cells to effect translation of the cargo poly nucleic acid by the target cell. Thus, such compounds are useful both in vitro and in vivo.
Owner:MEDIVAS LLC
Rapid analysis method of biogenic amine in fish
ActiveCN107782834AReduce the impactHigh sensitivityComponent separationTryptamineSulfosalicylic acid
The invention discloses a rapid analysis method of biogenic amine in fish. The common biogenic amine (nine types of biogenic amine comprising agmatine, histamine, tyramine, spermine, spermidine, 2-phenylethylamine, tryptamine, cadaverine and putrescine) is analyzed in a targeted manner by taking 5-sulfosalicylic acid as an extraction solvent, adopting bead agitating and grinding as an extraction method, taking benzoyl chloride as a derivatization reagent and adopting an LC (liquid chromatogram)-triple tandem quadrupole mass spectrometry. The biogenic amine is detected in a multi-reactions monitoring manner by taking ion pairs with optimum response, which are obtained by optimization, as quantitative ion pairs and taking ion pairs with following response as auxiliary qualitative ion pairs.According to the method, the sample extraction time and the biogenic amine derivation time can be greatly shortened, so that the analysis time of the whole analysis process is greatly shortened. The analysis method has the advantages of being simple in sample pretreatment, rapid in derivation, high in detection sensitivity, high in repeatability and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Use of agmatine for protection of retinal ganglion cells
ActiveUS20100130783A1Curing and preventing eye diseaseBiocideOrganic active ingredientsApoptosisFactor ii
A use method of agmatine or a pharmaceutically allowable salt thereof, and a pharmaceutical composition comprising the same are disclosed. The method and pharmaceutical composition of the present invention can effectively cure or prevent eye diseases preferably including glaucoma, retinopathy, and optic neuropathy associated with apoptosis in retinal ganglion cells (RGCs), particularly hypoxia-induced or tumor necrosis factor-α (TNF-α)-induced apoptosis.
Owner:SEONG GONG JE +3
Method for simultaneously determining 20 organic amines, biogenic amines and cations in fruit juice beverage
ActiveCN110261530AImprove detection efficiencyEfficient separationComponent separationFruit juiceIon chromatography
The invention discloses a method for simultaneously determining 20 organic amines, biogenic amines and cations in a fruit juice beverage, belonging to the field of analytical chemistry. In the method, a conductivity inhibition-ion chromatography method is adopted, and under the condition of multi-stage gradient elution, the experimental determination conditions are optimized by researching a plurality of experimental influence factors, so that the method for simultaneously determining 20 organic amines, biogenic amines and cations (methylamine, dimethylamine, trimethylamine, diethanolamine, butylamine, histamine, cadaverine, putrescine, agmatine, heptanediamine, spermidine, spermine, Li+, Na+, NH4+, K+, Mg2+, Ca2+, Sr2+ and Ba2+) in the fruit juice beverage is established. The method is rapid, simple and convenient, high in high in sensitivity and good in stability, and achieves the aims of rapid analysis, green analysis and multi-component simultaneous analysis.
Owner:日照海关综合技术服务中心
LIPOSOMAL REDUCED GLUTATHIONE AND l-ARGININE, INCLUDING WITH OTHER INGREDIENT(S), CAPABLE OF MULTIPATH ADMINISTRATION FOR REVERSAL AND PREVENTION OF OBESITY AND FOR MITOCHONDRIAL BIOGENESIS
InactiveUS20120219616A1Reduce productionReduce weightBiocideOrganic active ingredientsArginineAdditive ingredient
The invention enables management of mammalian disease related to decreased energy production in the mitochondria by a combination of liposomal reduced glutathione and 1-arginine. For individuals whose inability to lose weight is related to inefficiency of the biochemical pathways facilitating mitochondrial function and energy production, the invention proposes to assist in weight loss by improving the inefficient production of energy by the respiratory transport chain of mitochondria. The invention is useful for the management of the metabolic syndrome, a group of metabolic factors associated with an increased risk of vascular disease problems. The invention is also useful for the resolution of fatigue that accompanies both weight gain and illnesses. The ability of the invention to increase the production of the biochemical agmatine in the central nervous system as well as generally in the body is part of the benefit of the combination of liposomal reduced glutathione and 1-arginine.
Owner:YOUR ENERGY SYST
Acid-resistant threonine production bacterium and establishment method and application thereof
InactiveCN105385702AImprove acid resistanceReduce the burden onFermentationVector-based foreign material introductionThreonineOxidative enzyme
The invention provides an establishment method of an acid-resistant threonine production bacterium. The acid-resistant threonine production bacterium obtained through the method can be used for preparing threonine, the deamination reaction is conducted on glutamic acid through a glutamic acid oxidase gene, glutamic acid is converted into alpha-ketoglutarate, and generated free NH3 and H2O2 can neutralize redundant intracellular protons so that the acid-resistant capacity of thalli can be improved. The acid-resistant system can be effectively applied to fermentation production of biologics, the system can not generate gamma-aminobutyric acid or agmatine which has a thallus restraining effect, a gene for expressing gamma-aminobutyric acid or agmatine transport protein is not needed, and the burdens of recombined strains are reduced; alpha-oxoglutarate generated in the reaction can directly enter TCA circulation, energy is provided for thallus growth, the use amount of liquid ammonia is effectively decreased, and the influences of pH fluctuation on fermentation are effectively avoided.
Owner:TIANJIN UNIV OF SCI & TECH
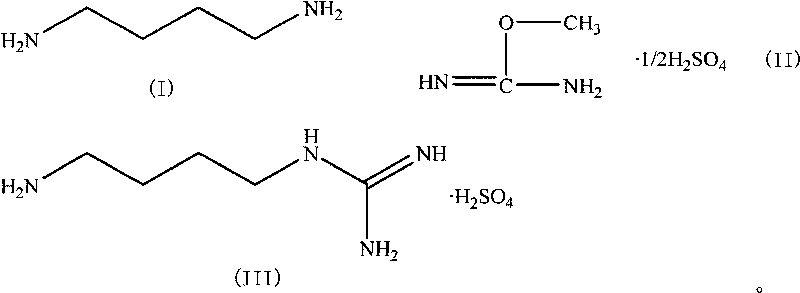
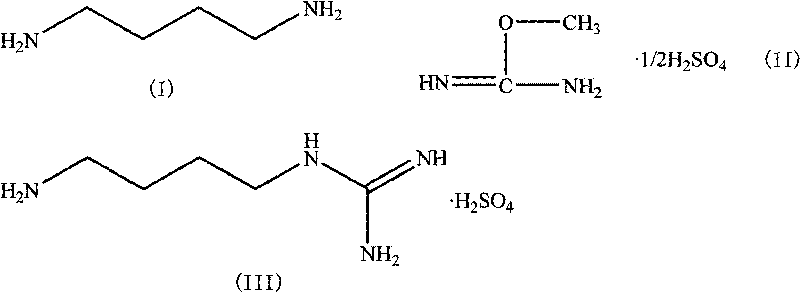
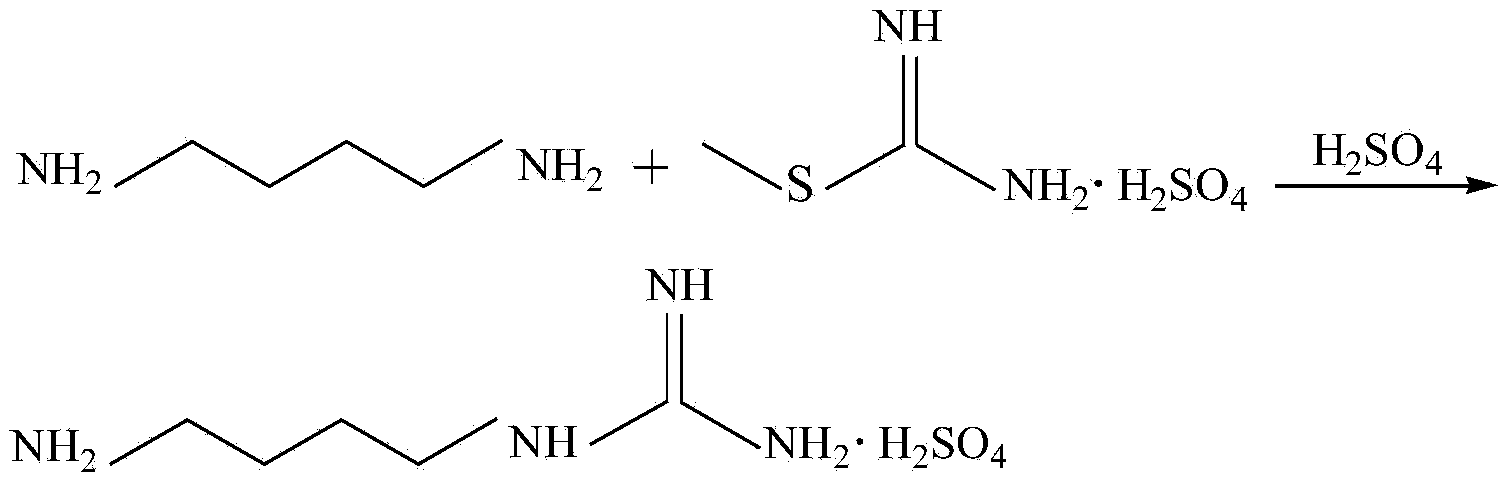
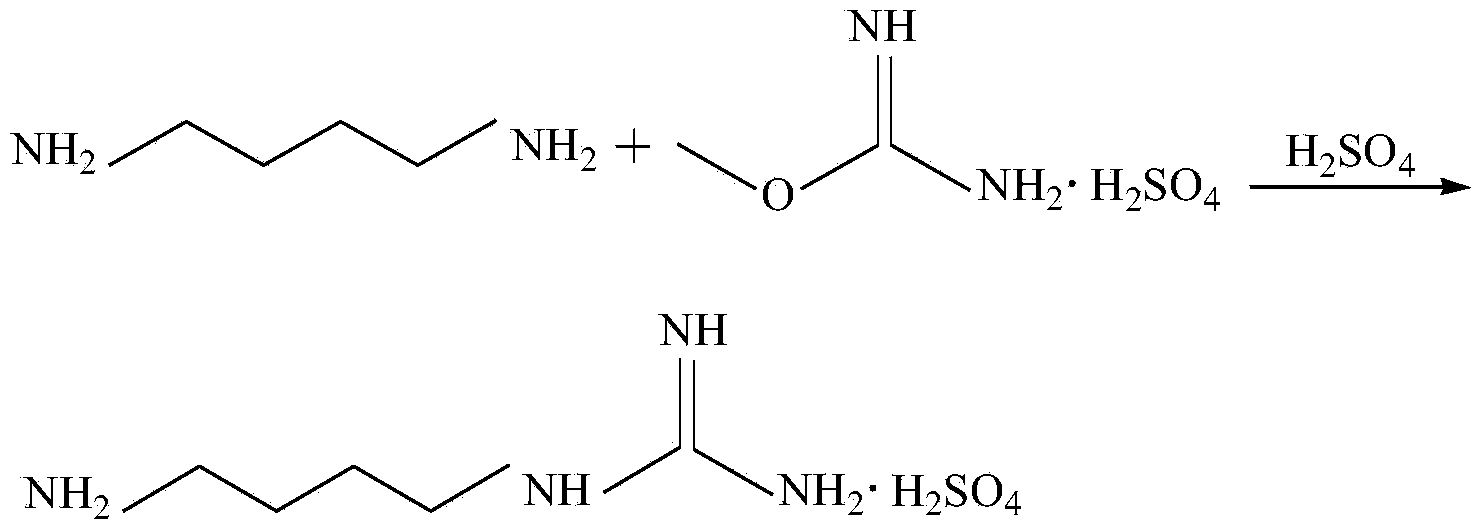
Method for synthesizing agmatine sulfate
ActiveCN101717352AHigh purityAvoid it happening againOrganic chemistryOrganic compound preparationO-methylisourea sulfateAlcohol
The invention discloses a method for synthesizing agmatine sulfate. The method comprises the following steps of: in a reaction solvent, performing a condensation reaction of 1,4-butanediamine and O-methylisourea sulfate at the temperature of between 20 DEG C below zero and 100 DEG C, utilizing the TLC to detect if the reaction is finished, filtering the reaction solution under vacuum, adding alcoholic solution of sulfuric acid into the obtained filtrate dropwise, adjusting a pH value of the filtrate to below 7, condensing the filtrate to dryness, and re-crystallizing residues to obtain the agmatine sulfate, wherein the reaction solvent is water or a C1 to C3alcohol, the adding mol ratio of the 1,4-butanediamine and the O-methylisourea sulfate is 1:1-1:2.0, and the volume dosage of the reaction solvent is 4 to 20ml / g based on the mass of the O-methylisourea sulfate. The method has the advantages of easy and safe operation, high reaction yield, low production cost, basically no emissionof three wastes, and bigger application values and social economic benefits.
Owner:ZHEJIANG UNIV OF TECH
Regulation of mammalian hair growth
The present invention relates to a topical skin care composition containing a safe and effective amount of a skin care active comprising agmatine, and its salt; a safe and effective amount of a first additional skin care active selected from the group consisting of BHT or BHA, hexamidine, cetyl pyridinium chloride, green tea catechins, phytosterols, ursolic acid, compounds derived from plant extracts, their salts and derivatives; and a dermatologically acceptable carrier for the agmatine composition. The present invention also relates to methods of using such agmatine compositions to regulate hair growth and the condition of mammalian skin. Said methods generally comprise the step of topically applying the composition to the skin of a mammal needing such treatment, a safe and effective amount of such compositions.
Owner:THE PROCTER & GAMBLE COMPANY
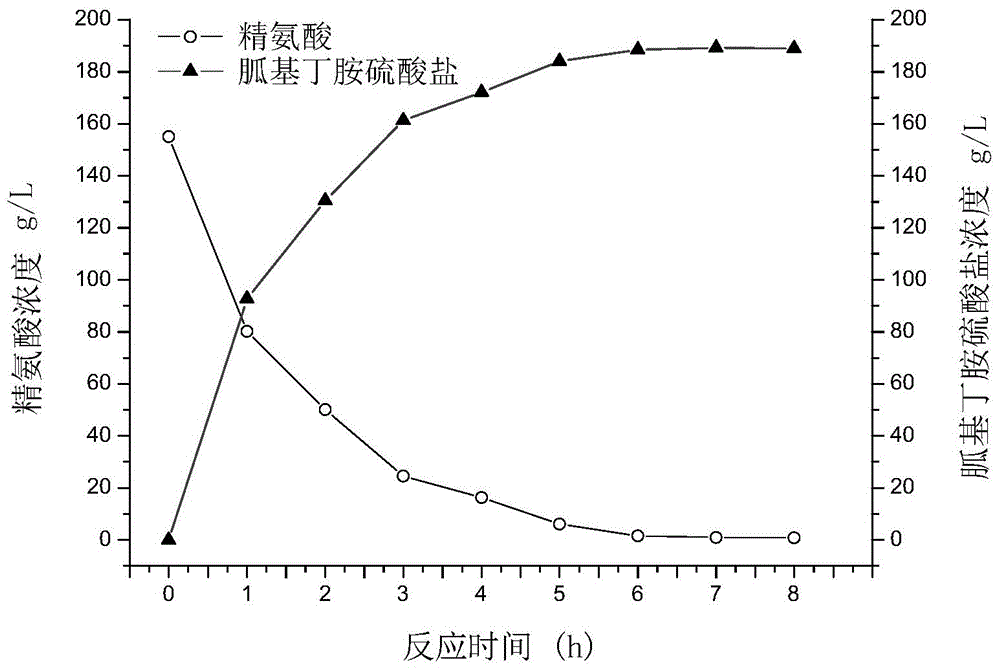
Biological preparation method of agmatine sulfate
InactiveCN104911223ASolve the costSettlement yieldMicroorganism based processesFermentationChemical synthesisArginine
The invention belongs to the technical field of biological catalysis, and relates to a biological preparation method of agmatine sulfate, which comprises the following steps: by using an L-arginine sulfate solution as a substrate raw material, carrying out decarboxylation reaction in the presence of a biocatalyst and a coenzyme to obtain agmatine sulfate, and discharging gas CO2, wherein the biocatalyst adopts L-arginine decarboxylase, and the coenzyme adopts phosphopyridoxal. The L-arginine sulfate solution used as the substrate is stirred and mixed with the L-arginine decarboxylase and the coenzyme (phosphopyridoxal) to perform decarboxylation reaction under certain conditions, thereby obtaining the agmatine sulfate. The conversion rate is greater than or equal to 98%, and the continuous production yield can reach 90%. The technical route has the advantages of simple preparation technique, high production efficiency, low cost and the like. The fermentation and conversion techniques of the recombinant L-arginine decarboxylase can achieve the industrial preparation level, thereby basically solving the problems of high chemical synthesis cost, low yield, and great environmental hidden danger and the like.
Owner:CHANGXING PHARMA
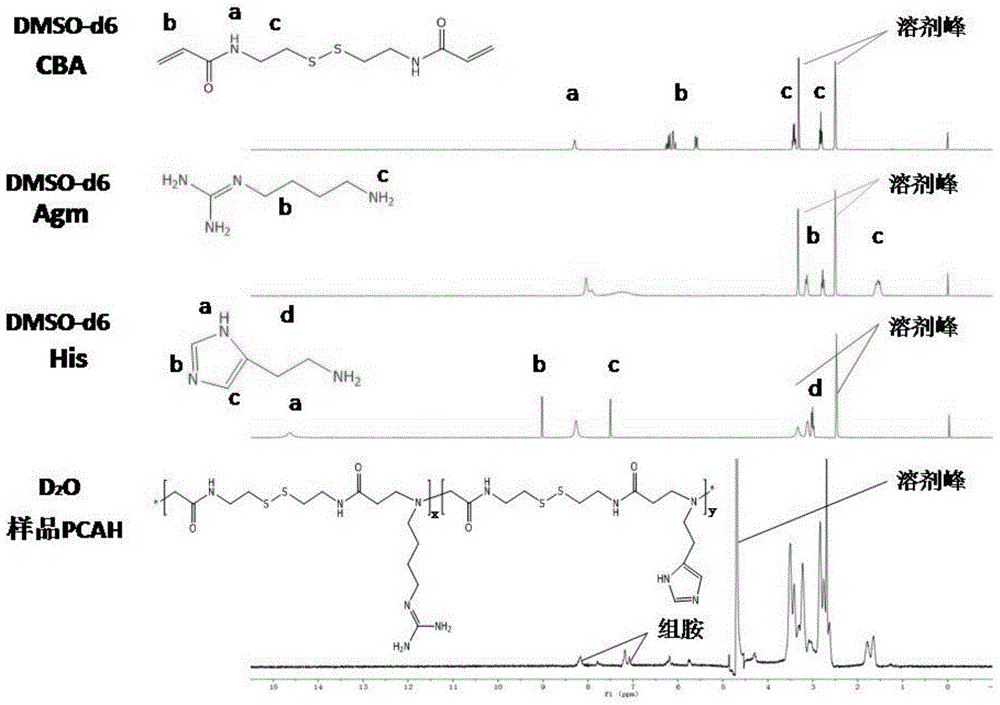
Histone simulated gene vector and preparation method and application thereof
InactiveCN105063090AEfficient compressionGood biocompatibilityNervous disorderMetabolism disorderHigh cellDisease
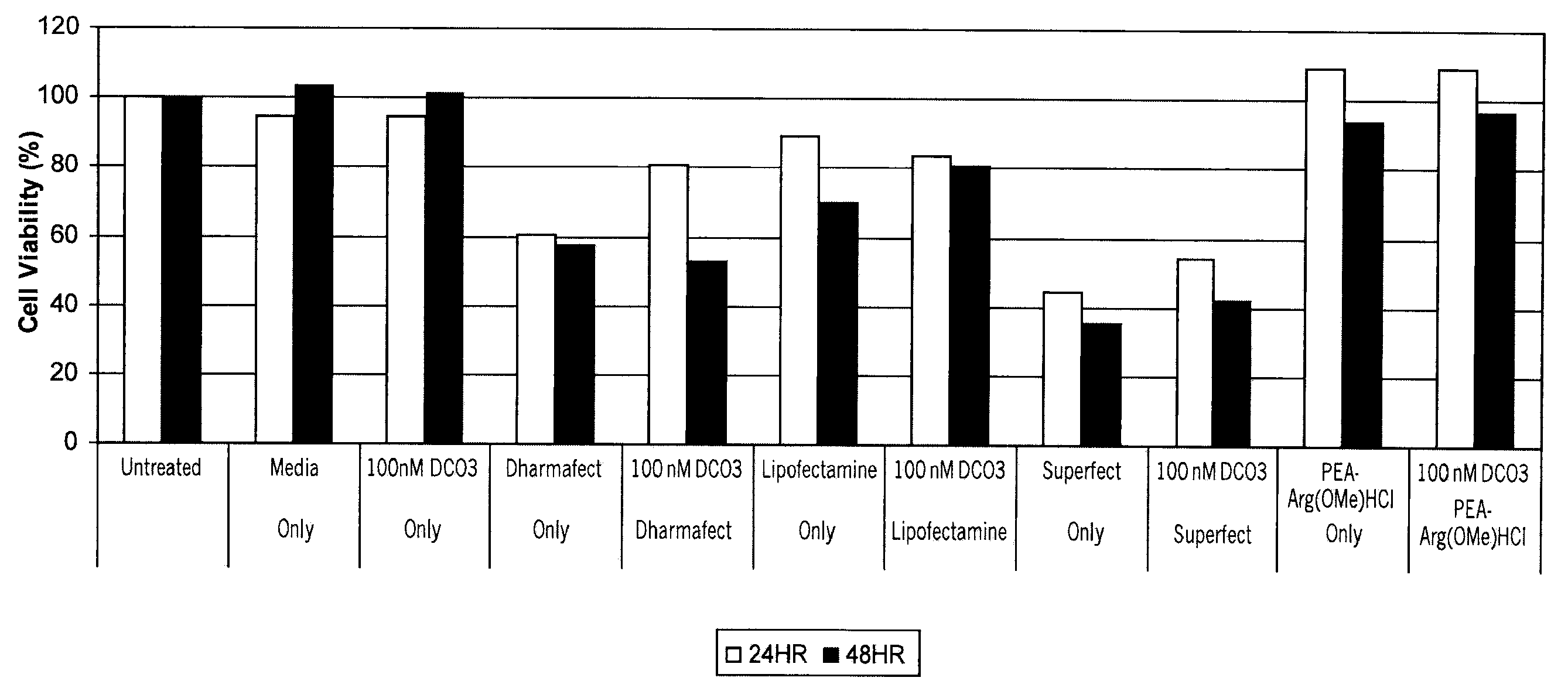
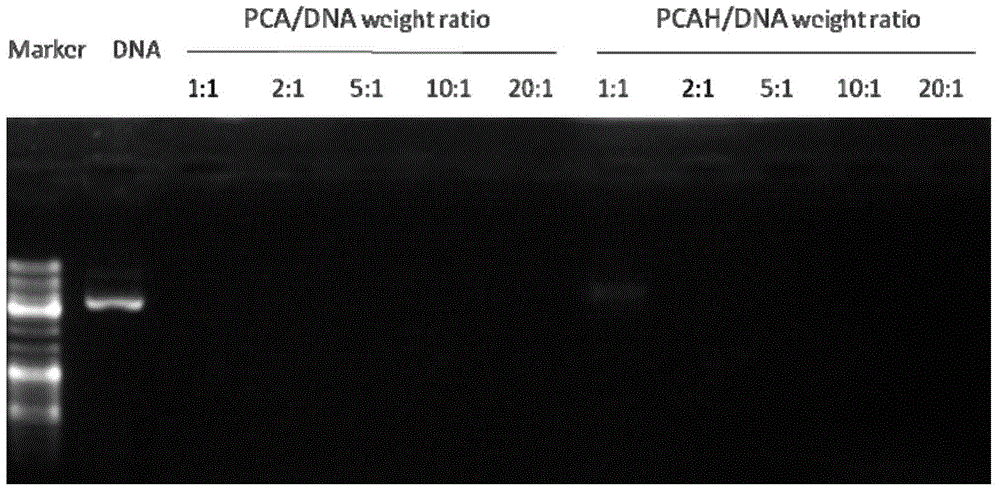
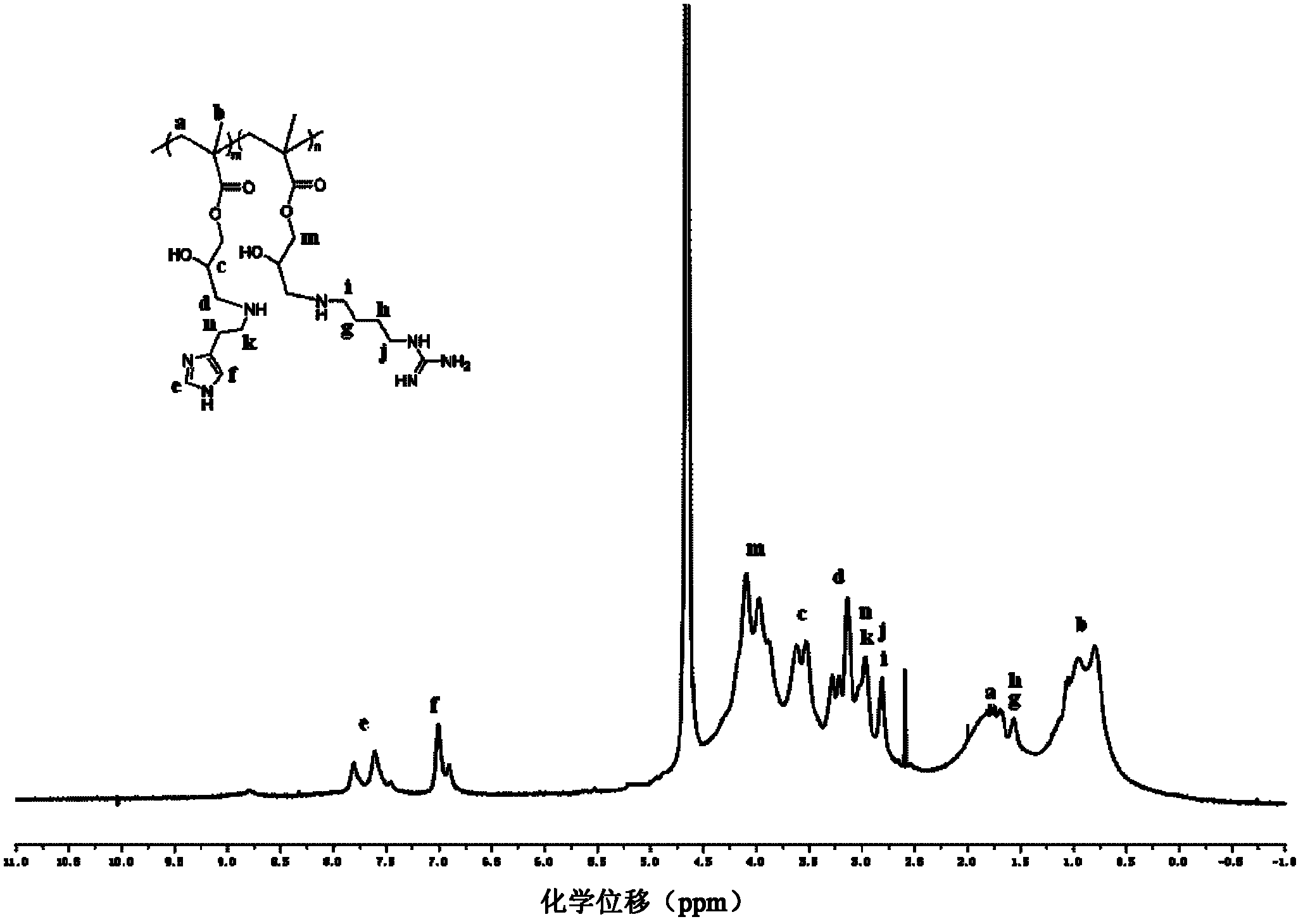
The present invention discloses a histone simulated gene vector and a preparation method and application thereof, and in particular relates to a histone imitated gene vector PCAH capable of gene delivery. The invention also provides a gene vector system which is obtained through electrostatic adsorption self-assembly between nucleic acids and a polymer synthesized from arginine and histidine basic amino acid decarboxylated products, agmatine, histamine and bis(acryloyl)cystamine. The invention also discloses the preparation method of the histone simulated gene vector and application of the histone simulated gene vector to gene therapy. The histone simulated gene vector has good cell penetration function, high cell transfection efficiency and low cytotoxicity, and provides an effective mean for gene therapy of diseases.
Owner:CHINA PHARM UNIV
Biotransformation method of agmatine sulfate
ActiveCN105713938ASimple processMild reaction conditionsOrganic chemistryOrganic compound preparationChemical reactionArginine
The invention discloses a biotransformation method of agmatine sulfate and belongs to the field of pharmaceutical intermediates. The method comprises steps as follows: L-arginine taken as a substrate and arginine decarboxylase taken as a biotransformation catalyst are subjected to a decarboxylic reaction to produce a coarse agmatine product, the coarse agmatine product is acidized with dilute sulfuric acid, and a coarse agmatine sulfate product is obtained. The method adopts the simple technological process, has no special requirement for equipment and is applicable to industrial production; compared with a simple chemical method, the method has the advantages that the reaction condition is mild, the operation is simple and convenient, the cost is lower, the pollution is low, and an enzyme catalytic reaction which is difficult to conduct in the chemical reaction can be finished.
Owner:JIANGHAN UNIVERSITY
Histamine-modified agmatine-based linear-polymer transgenic carrier and preparation method and applications thereof
InactiveCN102633934ATransmembraneGood biocompatibilityOther foreign material introduction processesGlycidyl methacrylateEnd-group
The invention discloses a histamine-modified agmatine-based linear-polymer transgenic carrier and a preparation method and applications thereof. The transgenic carrier is prepared through reacting open loops of epoxy groups of glycidyl methacrylate with amino end groups of agmatine and histamine, chaining the agmatine and the histamine to the glycidyl methacrylate through chemical bonds, initiating a modified glycidyl methacrylate monomer to carry out free-radical atactic polymerization, and finally chaining the electropositive agmatine and the histamine with a proton buffering function to macromolecule chains. The agmatine carrier has good biocompatibility and promotes the transmembrane capacity of composites. The modification of the histamine increases the endosome escape capability of composites on the premise of not affecting the cell toxicity of the carrier, thereby effectively improving the efficiency and properties of the agmatine as a transgenic carrier; and the carrier can be used as an efficient non-viral gene carrier for gene therapy, realizes the improvement on the gene transfection efficiency of the agmatine carrier, and extends the role of the in-vivo circulation time.
Owner:樊世田
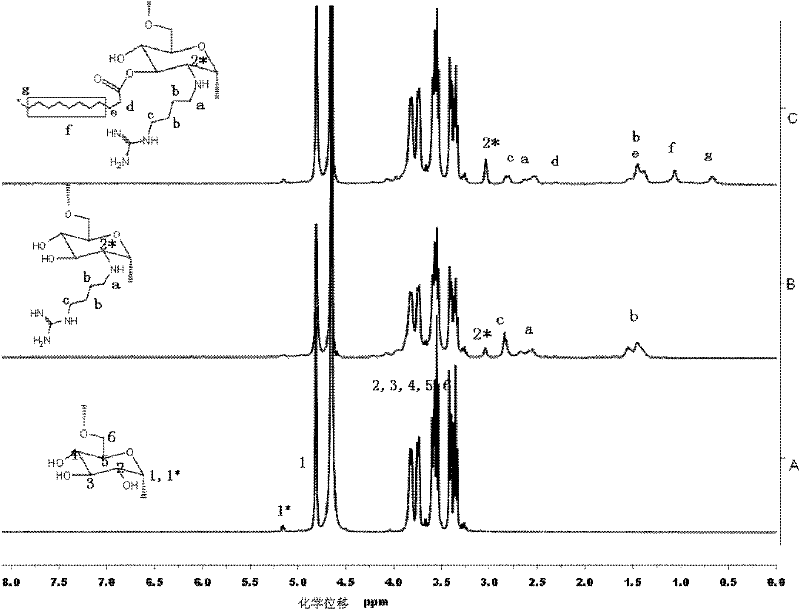
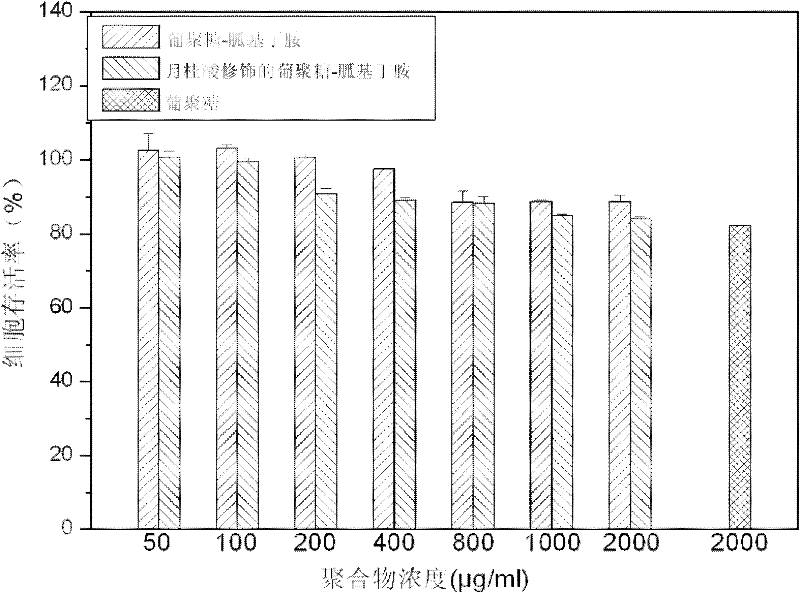
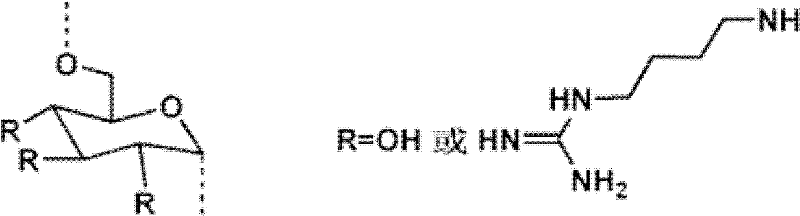
Glucan-agmatine polycation transgenic vector, and preparation method and application thereof
InactiveCN102337296ABiodegradableGood biocompatibilityGenetic material ingredientsPharmaceutical non-active ingredientsBiocompatibility TestingFatty acid
The invention discloses a cationic high-polymer transgenic vector having good biocompatibility, degradability and high stability, and a preparation method thereof. A glucan molecule is used as a main chain, and agmatine and fatty acid bonds are respectively connected to the main chain of the glucan molecule through hydroxyl groups on the main chain. Guanylation glucan and primary amine of agmatine are subjected to nucleophilic reaction through tosylation glucan, and such copolymerization mode can not destroy the main chain of the glucan and can change the primary amine into secondary amine. Meanwhile, in order to improve the transfection efficiency, the nontoxic fatty acid is introduced to enhance the hydrophobic property of the glucan-agmatine polycation.
Owner:TIANJIN UNIV
Regulation of Mammalian Hair Growth
The present invention relates to a topical skin care composition containing a safe and effective amount of a skin care active comprising agmatine, and its salt; a safe and effective amount of a first additional skin care active selected from the group consisting of BHT or BHA, hexamidine, cetyl pyridinium chloride, green tea catechins, phytosterols, ursolic acid, compounds derived from plant extracts, their salts and derivatives; and a dermatologically acceptable carrier for the agmatine composition.The present invention also relates to methods of using such agmatine compositions to regulate hair growth and the condition of mammalian skin. Said methods generally comprise the step of topically applying the composition to the skin of a mammal needing such treatment, a safe and effective amount of such compositions.
Owner:PROCTER & GAMBLE CO
Method for synthesis of acrylamide derivatives
ActiveUS20070106090A1Improve responseHigh purityOrganic chemistryOrganic compound preparationAcid derivativeAqueous solution
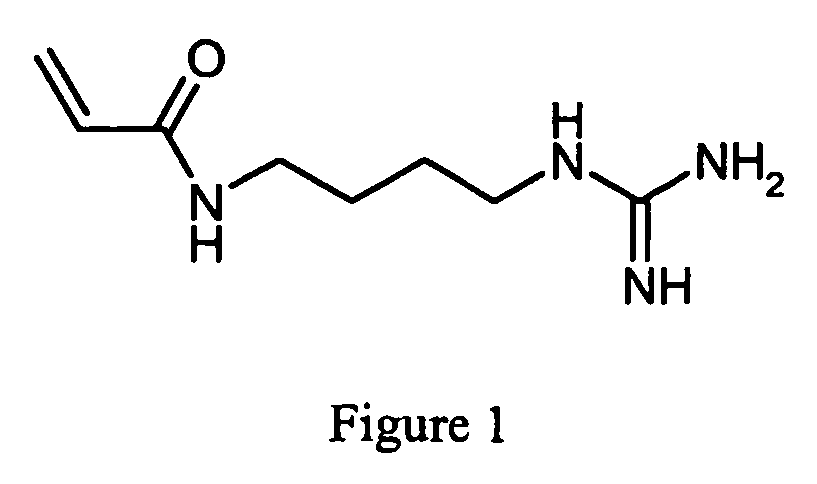
The present invention relates to a method for synthesis of an acrylamide derivative, starting with dissolving a salt of a nucleophilic amine in water to form an aqueous solution and desalting said solution with a base, comprising the following steps: a) addition of dissolved activated acrylic acid derivative to said solution; b) acidification of aqueous phase; and c) extraction of said aqueous phase. In a preferred embodiment the acryl amide derivative is an immobiline, preferably acrylamido agmatine. The preferred use of the immobilines is for production of 2D gels.
Owner:CYTIVA SWEDEN AB
Bile acid-basic amino acid conjugates and uses thereof
InactiveUS20160120880A1Ameliorate liver steatosisIncrease body weightOrganic active ingredientsMetabolism disorderChenodeoxycholic acidHistidine
Compositions and methods for treating diseases or disorders associated with the metabolic syndrome using conjugates of bile acids with basic amino acids or a decarboxylated amino acid such as agmatine are provided. Further provided are bile acid-basic amino acid conjugates including chenodeoxycholic acid with an amino acid selected from arginine, lysine, histidine, ornithine or a decarboxylated amino acid such as agmatine.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Agmatine containing dietary supplements, nutraceuticals, and foods
ActiveUS20100172890A1High cytoprotective amountGood effectBiocideNervous disorderDietary supplementHigh doses
The invention is dietary supplements, nutraceutical compositions, medical foods, and animal feeds that have cytoprotective (cell and tissue protection) and health promoting effects. The compositions contain a high dose range of agmatine and nutraceutical acceptable salts thereof as dietary fortification for providing effective long-term cytoprotection and affording for soft stool. The compositions may contain agmatine alone or in combination with other dietary ingredients having health promoting effects. The compositions can be prepared with dietary accepted excipients and compatible forms of carriers, including but not limited to, powders, tablets, capsules, controlled release carriers, lozenges and chewable preparations, liquid suspensions, suspensions in an edible supporting matrix or foodstuff and oral rehydration solutions, to enable consumption of said compositions.
Owner:GILAD GAD +1
Agmatine and agmatine analogs in the treatment of epilepsy, seizure, and electroconvulsive disorders
Pharmaceutical preparations containing of agmatine, congeners, analogs or derivatives thereof for use in preventing or treating epilepsy, seizures and other electroconvulsive disorders are provided. Embodiments include administering an effective amount of agmatine, an agmatine analog or a pharmaceutically acceptable salt thereof to a human subject in need of treatment or prevention of epilepsy, seizure or other electroconvulsive disorder to treat, reduce, or prevent the disorder in the subject.
Owner:UNIV OF KENTUCKY RES FOUND
Recombinant corynebacterium crenatum capable of producing agmatine, and application thereof
PendingCN111718883AIncrease productionLow costBacteriaMicroorganism based processesArginineMicrobiology
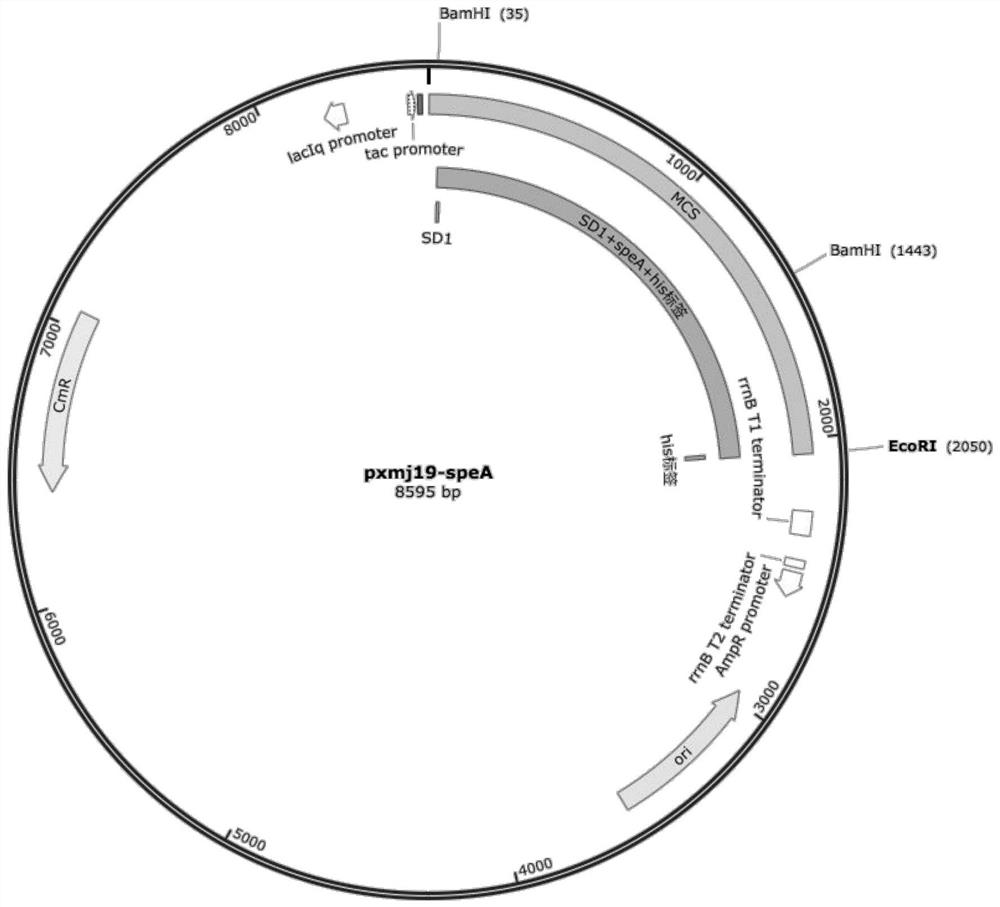
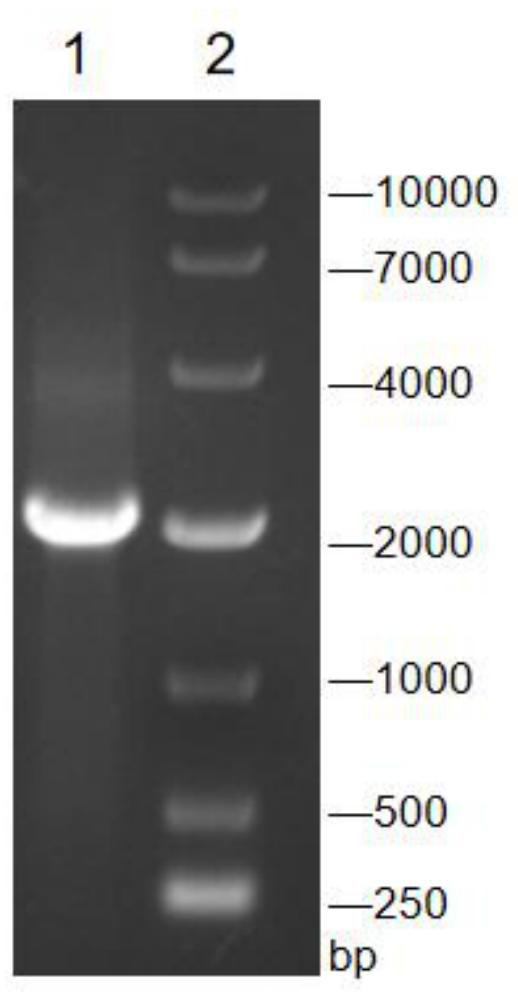
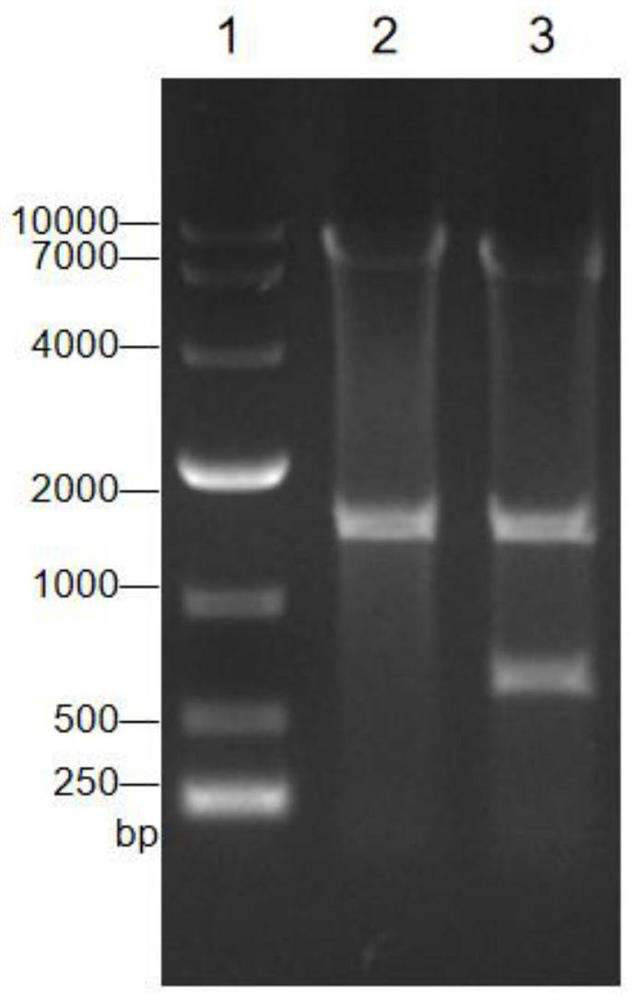
The invention discloses a recombinant corynebacterium crenatum capable of producing agmatine, and application thereof, and belongs to the technical field of biology. The invention provides a recombinant corynebacterium crenatum C.crenatum SYPA5-5 / pXMJ19-speA; the recombinant corynebacterium crenatum is inoculated into a fermentation culture medium containing glucose to perform shake-flask fermentation for 72 h; therefore, the yield of agmatine in fermentation liquid is up to 9.7 g / L; simultaneously, the content of a by-product, namely L-arginine, is only 0.23 g / L; the recombinant corynebacterium crenatum is inoculated into the fermentation culture medium containing glucose to perform fermentor fermentation for 72 h; therefore, the yield of agmatine in the fermentation liquid is up to 37.44g / L; simultaneously, the content of the by-product, namely L-arginine, is only 0.76 g / L; and furthermore, L-arginine does not need to be added in the whole fermentation process.
Owner:JIANGNAN UNIV
Composition of Athletic and Fitness Supplements, Method for Production and Method for Pulmonary Administration of Same
InactiveUS20200306187A1Improve athletic performanceIncrease physical strengthPowder deliverySpray deliveryTyrosineGlycerol
Disclosed is a performance-enhancing supplement for pulmonary administration wherein the supplement consists of a base e-liquid and a selected combination of supplement ingredients. The base liquid is selected from vegetable glycerin or propylene glycol, and the combination of ingredients is selected from at least three of pineapple lime extract, riboflavin, L-tyrosine, L-theanine, taurine, caffeine, agmatine sulfate, citrulline malate, and beetroot. The resulting performance-enhancing supplement is provided in a standard vape cartridge for use in an electronic cigarette device.
Owner:V RUSH VAPES LLC
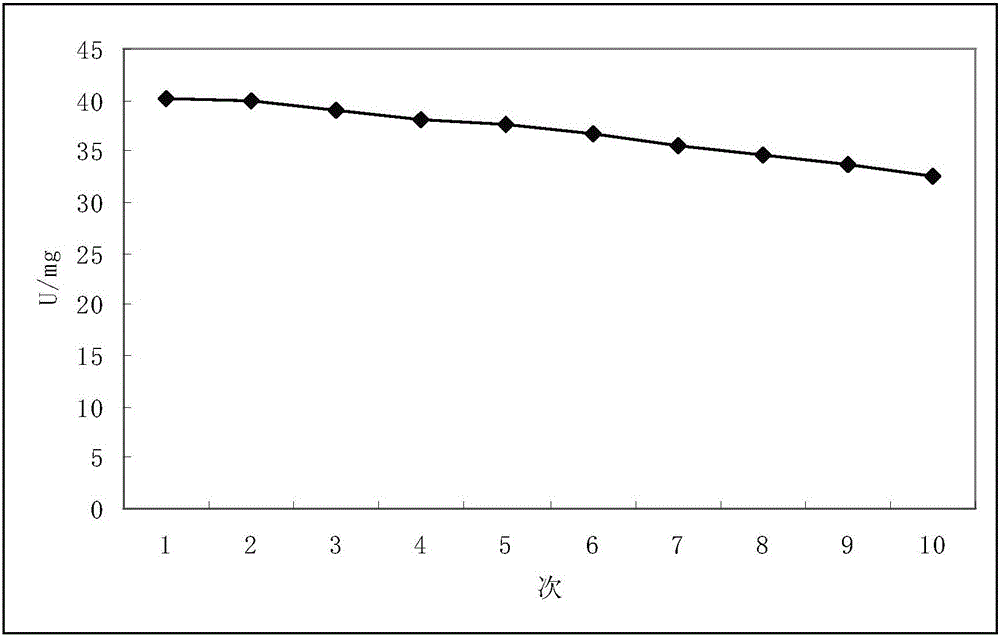
Arginine decarboxylase mutant and application thereof in production of agmatine
ActiveCN111748548AIncreased half-inhibition constantIncrease contentBacteriaMicroorganism based processesArginineArginine decarboxylase
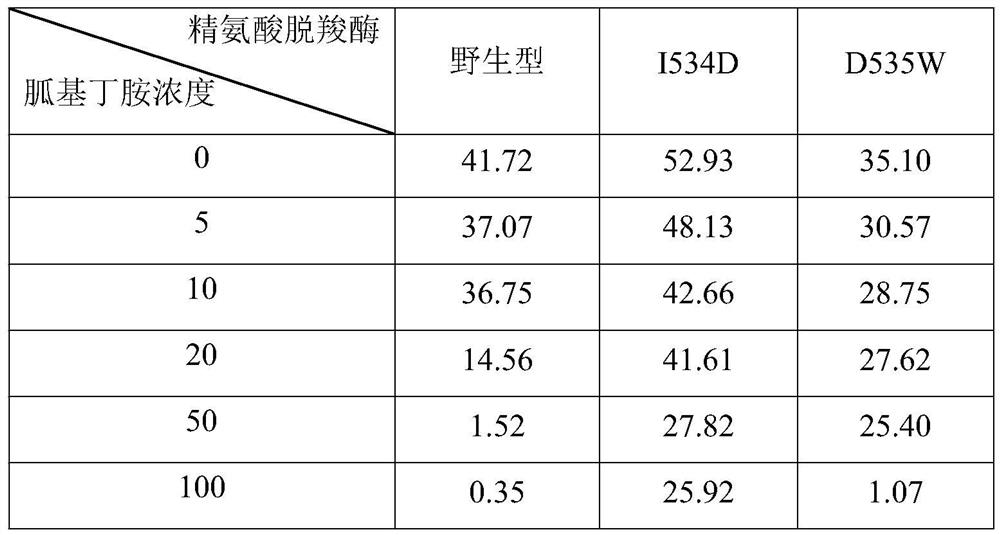
The invention discloses an arginine decarboxylase mutant and an application of the arginine decarboxylase mutant in production of agmatine, which belong to the technical field of biology. The invention provides arginine decarboxylase mutants I534D and D535W which are less influenced by product inhibition. The arginine decarboxylase mutant I534D is obtained by mutating isoleucine at the 534th siteof wild-type arginine decarboxylase of which the amino acid sequence is shown as SEQ ID NO.1 into aspartic acid. The half inhibition constant of the arginine decarboxylase can reach 0.66 mol / L and isincreased by 5.5 times compared with that of wild arginine decarboxylase; the arginine decarboxylase mutant D535W is obtained by mutating aspartic acid at the 535th site of wild type arginine decarboxylase with an amino acid sequence shown as SEQ ID NO.1 into tryptophan, the half inhibition constant of the arginine decarboxylase can reach 0.50 mol / L, and is improved by 4.2 times compared with thatof wild arginine decarboxylase.
Owner:JIANGNAN UNIV
Agmatine containing dietary supplements, nutraceuticals, and foods
ActiveUS8916612B2High cytoprotective amountGood effectBiocideNervous disorderDietary supplementNutrition
The invention is dietary supplements, nutraceutical compositions, medical foods, and animal feeds that have cytoprotective (cell and tissue protection) and health promoting effects. The compositions contain a high dose range of agmatine and nutraceutical acceptable salts thereof as dietary fortification for providing effective long-term cytoprotection and affording for soft stool. The compositions may contain agmatine alone or in combination with other dietary ingredients having health promoting effects. The compositions can be prepared with dietary accepted excipients and compatible forms of carriers, including but not limited to, powders, tablets, capsules, controlled release carriers, lozenges and chewable preparations, liquid suspensions, suspensions in an edible supporting matrix or foodstuff and oral rehydration solutions, to enable consumption of said compositions.
Owner:GILAD GAD +1
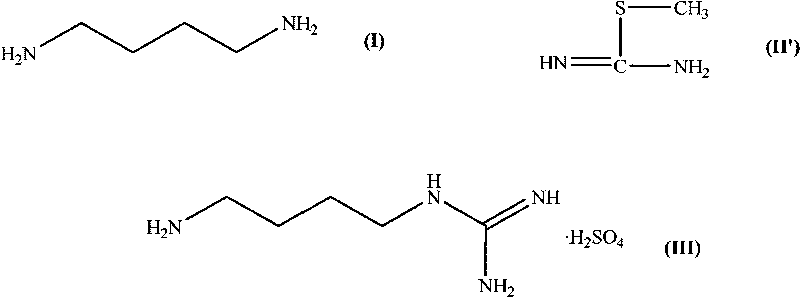
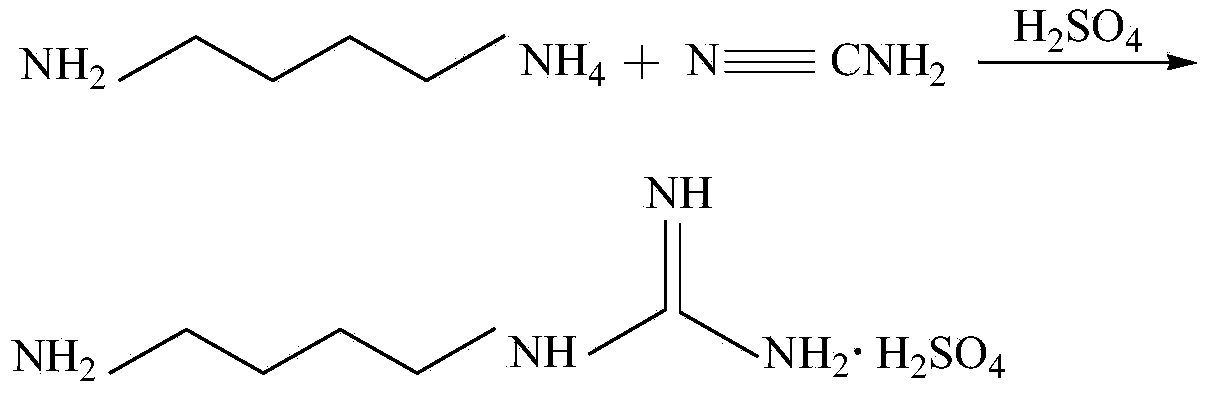
Synthesis method of agmatine sulfate
InactiveCN103755598AImprove protectionMild reaction conditionsOrganic chemistryOrganic compound preparationSynthesis methodsSulfate
The invention provides a synthesis method of agmatine sulfate. The method comprises the steps of carrying out addition reaction by taking 1,4-butanediamine as a raw material and a cyanamide water solution as a guanidination reagent to obtain agmatine, and forming salt with sulfuric acid to obtain agmatine sulfate. The synthesis method disclosed by the invention, by taking cyanamide water solution as the guanidination reagent, is moderate in reaction condition, easy to control, light in pollution, favorable for environmental protection, low in cost, and suitable for industrial production and application on a large scale.
Owner:SHANGHAI XUXIN CHEM
Agmatine Containing Dietary Supplements, Nutraceuticals and Foods
ActiveUS20150086523A1High cytoprotective amountGood effectMilk preparationBiocideDietary supplementHigh doses
The invention is dietary supplements, nutraceutical compositions, medical foods, and animal feeds that have cytoprotective (cell and tissue protection) and health promoting effects. The compositions contain a high dose range of agmatine and nutraceutical acceptable salts thereof as dietary fortification for providing effective long-term cytoprotection and affording for soft stool. The compositions may contain agmatine alone or in combination with other dietary ingredients having health promoting effects. The compositions can be prepared with dietary accepted excipients and compatible forms of carriers, including but not limited to, powders, tablets, capsules, controlled release carriers, lozenges and chewable preparations, liquid suspensions, suspensions in an edible supporting matrix or foodstuff and oral rehydration solutions, to enable consumption of said compositions.
Owner:GILAD GAD +1
A kind of biotransformation method of agmatine sulfate
ActiveCN105713938BSimple processMild reaction conditionsOrganic chemistryOrganic compound preparationChemical reactionArginine
The invention discloses a biotransformation method of agmatine sulfate, which belongs to the field of pharmaceutical intermediates. The method comprises: using L-arginine as a substrate, using arginine decarboxylase as a biotransformation catalyst, performing a decarboxylation reaction to generate a crude agmatine product, and acidifying the crude agmatine product with dilute sulfuric acid , to obtain the crude product of agmatine sulfate. The process of the method is simple, has no special requirements for equipment, and is suitable for industrial production. Compared with the pure chemical method, the method has mild reaction conditions, easy operation, low cost, and less pollution, and can complete chemical reactions that are difficult to carry out. enzyme-catalyzed reaction.
Owner:JIANGHAN UNIVERSITY
A kind of preparation method of agmatine hydrochloride
ActiveCN106631909BWon't wasteHigh active ingredientOrganic chemistryOrganic compound preparationSocial benefitsActivated carbon
The invention discloses an agmatine hydrochloride preparation method. The method includes steps: concentrating crystallization mother liquor generated in production of agmatine sulfate to remove a solvent and obtain concentrated liquid; allowing the concentrated liquid to overflow regenerated 717 anion resin to adsorb sulfate ions, collecting lower column liquid until sulfate ions flow out through an outlet, and stopping overflowing; concentrating the collected lower column liquid to obtain crystallization liquid; adopting hydrochloric acid for adjusting pH of the crystallization liquid to 3-6; adding activated carbon, stirring to remove impurities, and filtering to remove carbon; crystallizing to obtain an agmatine hydrochloride product. The agmatine hydrochloride preparation method has advantages that the mother liquid obtained after production of agmatine can be completely used, waste of agmatine is avoided while the agmatine hydrochloride product is obtained, the content of effective components of the hydrochloride product is higher than that of effective components of a sulfate product equal to the hydrochloride product in mass, and accordingly carbon emission is reduced, social benefits are increased, and the production requirement on environmental friendliness is met.
Owner:JING JING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com