Glucan-agmatine polycation transgenic vector, and preparation method and application thereof
A technology of transgenic carrier and agmatine, which is applied in the direction of gene therapy, the use of carriers to introduce foreign genetic material, and pharmaceutical formulations, can solve problems affecting biological performance and glucan main chain damage, and achieve improved efficiency and performance Improved transfection efficiency and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
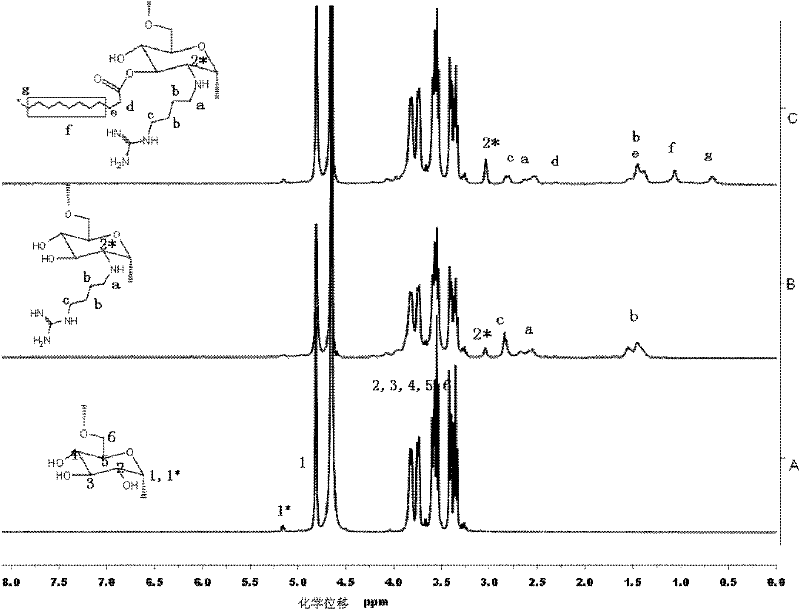
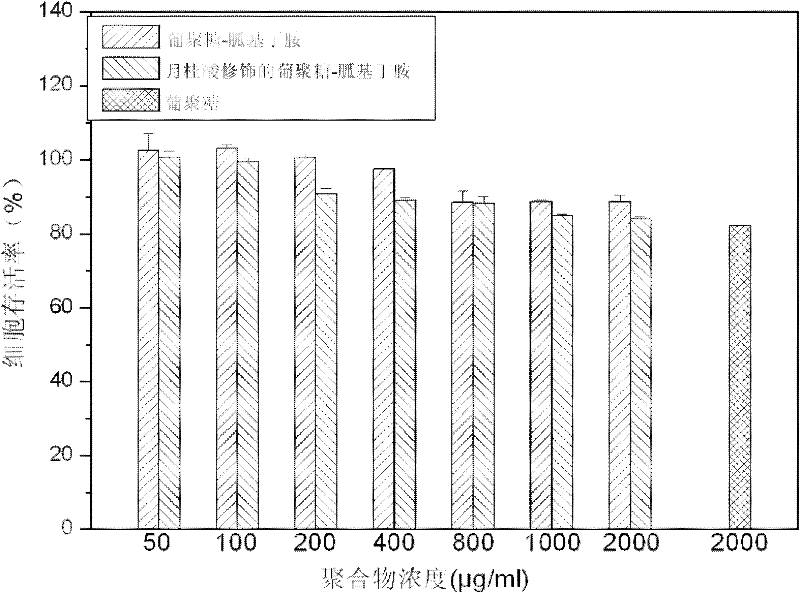
Image
Examples
Embodiment 1
[0041] (1) Dissolve dextran (2g, 12.4mmol) in 20mL of water, adjust the pH to 13 with NaOH, stir in an ice-water bath, then dissolve p-toluenesulfonyl chloride (2.4g, 12.4mmol) in 20mL of acetonitrile , and dropwise added to the aqueous solution of dextran within half an hour, a white precipitate will appear immediately, then react at room temperature for 3 hours, filter the precipitate with suction, lyophilize after dialysis for 5 days, and obtain p-toluenesulfonyl Dextran;
[0042] (2) Dissolve lauric acid (1.8mmol) and carbonyldiimidazole (CDI) (1.8mmol) in 3mL dimethyl sulfoxide (DMSO), respectively, and add the solution of CDI to the lauric acid solution, and react at 80°C for 3 Hours, p-tosylated dextran (6mmol) was dissolved in 5ml DMSO, and added to the solution of lauric acid and CDI, reacted at 80°C for 6 hours, then precipitated the product with 100mL ethanol, and washed several times, Suction filter the precipitate, dialyze for 5 days and freeze-dry to obtain laur...
Embodiment 2
[0046] (1) Dissolve dextran (2g, 12.4mmol) in 20mL of water, adjust the pH to 13 with NaOH, stir in an ice-water bath, then dissolve p-toluenesulfonyl chloride (4.8g, 24.8mmol) in 20mL of acetonitrile , and dropwise added to the aqueous solution of dextran within half an hour, a white precipitate will appear immediately, then react at room temperature for 3 hours, filter the precipitate with suction, lyophilize after dialysis for 5 days, and obtain p-toluenesulfonyl Dextran;
[0047] (2) Dissolve lauric acid (1.8mmol) and carbonyldiimidazole (CDI) (1.8mmol) in 3mL dimethyl sulfoxide (DMSO), respectively, and add the solution of CDI to the lauric acid solution, and react at 80°C for 3 Hours, p-tosylated dextran (6mmol) was dissolved in 5ml DMSO, and added to the solution of lauric acid and CDI, reacted at 80°C for 6 hours, then precipitated the product with 100mL ethanol, and washed several times, Suction filter the precipitate, dialyze for 5 days and freeze-dry to obtain laur...
Embodiment 3
[0051] (1) Dissolve dextran (2g, 12.4mmol) in 20mL of water, adjust the pH to 13 with NaOH, stir in an ice-water bath, then dissolve p-toluenesulfonyl chloride (7.2g, 37.2mmol) in 20mL of acetonitrile , and dropwise added to the aqueous solution of dextran within half an hour, a white precipitate will appear immediately, then react at room temperature for 3 hours, filter the precipitate with suction, lyophilize after dialysis for 5 days, and obtain p-toluenesulfonyl Dextran;
[0052] (2) Dissolve lauric acid (1.8mmol) and carbonyldiimidazole (CDI) (1.8mmol) in 3mL dimethyl sulfoxide (DMSO), respectively, and add the solution of CDI to the lauric acid solution, and react at 80°C for 3 Hours, p-tosylated dextran (6mmol) was dissolved in 5ml DMSO, and added to the solution of lauric acid and CDI, reacted at 80°C for 6 hours, then precipitated the product with 100mL ethanol, and washed several times, Suction filter the precipitate, dialyze for 5 days and freeze-dry to obtain laur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com