Method for synthesizing agmatine sulfate
A technology of agmatine sulfate and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the industrial policy of energy saving and emission reduction, unpleasant smell of alkyl mercaptans, unfavorable Labor protection and other issues, to achieve great implementation value and social and economic benefits, to facilitate industrial production, and to achieve high sample purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
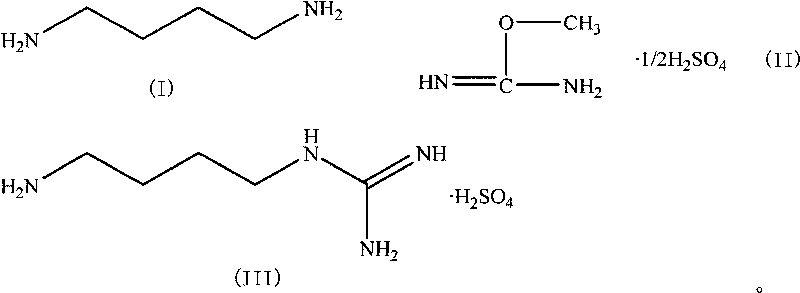
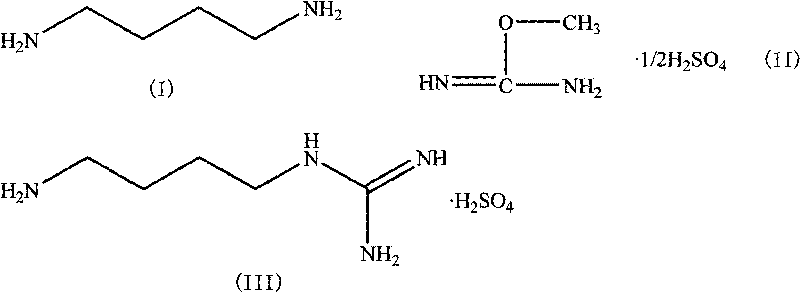
[0021] Weigh 3.22g (0.02mol) of 1,4-butylenediamine hydrochloride and 2.00g (0.05mol) of NaOH into a 250mL single-necked bottle, add water (10mL), stir magnetically, and distill under reduced pressure after the solid is dissolved , Collect the distillate at 120°C to obtain 1,4-butanediamine, which is used for the next step. Add 1,4-butanediamine, 3.075 g (0.025 mol) of O-methylisourea sulfate and 20 mL of water obtained in the previous step into a 250 mL three-necked flask, and then react with magnetic stirring in a water bath at 30 ° C. After 1 hour, the solution gradually becomes Turbidity, white insoluble matter is formed, continue to stir the reaction, TLC detects the reaction to the end point (about 3 hours), suction filtration, the filtrate is adjusted to pH1 H-NMR δ ppm (400 MHz, D2O) δ: 1.61 (m, 2H), 2.93 (t, 1H), 3.14 (t, 1H).
Embodiment 2
[0023] Weigh 3.22g (0.02mol) of 1,4-butylenediamine hydrochloride and 2.00g (0.05mol) of NaOH into a 250mL single-necked bottle, add water (10mL), stir magnetically, and distill under reduced pressure after the solid is dissolved , Collect the distillate at 120°C to obtain 1,4-butanediamine, which is used for the next reaction. Add 1,4-butanediamine, 3.075g (0.025mol) of O-methylisourea sulfate and 20mL of ethanol obtained in the previous step into a 250mL three-necked flask, and then react with magnetic stirring in a water bath at 30°C. After 2 hours, the solution gradually becomes Turbidity, white insoluble matter is generated, continue to stir the reaction, TLC detects the reaction to the end point (about 4 hours), suction filtration, the filtrate is adjusted to pH<7 with 25% sulfuric acid / ethanol solution, and concentrated under reduced pressure to a viscous transparent oil. Add water (4mL)-ethanol (6mL) for purification, crystallize at 0°C for 4 hours, filter with suction...
Embodiment 3
[0025] Weigh 3.22g (0.02mol) of 1,4-butylenediamine hydrochloride and 2.00g (0.05mol) of NaOH into a 250mL single-necked bottle, add water (10mL), stir magnetically, and distill under reduced pressure after the solid is dissolved , Collect the distillate at 120°C to obtain 1,4-butanediamine, which is used for the next reaction. Add 1,4-butanediamine, 3.075 g (0.025 mol) of O-methylisourea sulfate and 15 mL of water obtained in the previous step into a 250 mL three-necked flask, and then react with magnetic stirring in a water bath at 30 ° C. After 1 hour, the solution gradually becomes Turbidity, white insoluble matter is generated, continue to stir the reaction, TLC detects the reaction to the end point (about 4 hours), suction filtration, the filtrate is adjusted to pH<7 with 25% sulfuric acid / ethanol solution, and concentrated under reduced pressure to a viscous transparent oil. Add water (4mL)-ethanol (6mL) for purification, crystallize at 0°C for 4 hours, filter with suct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com