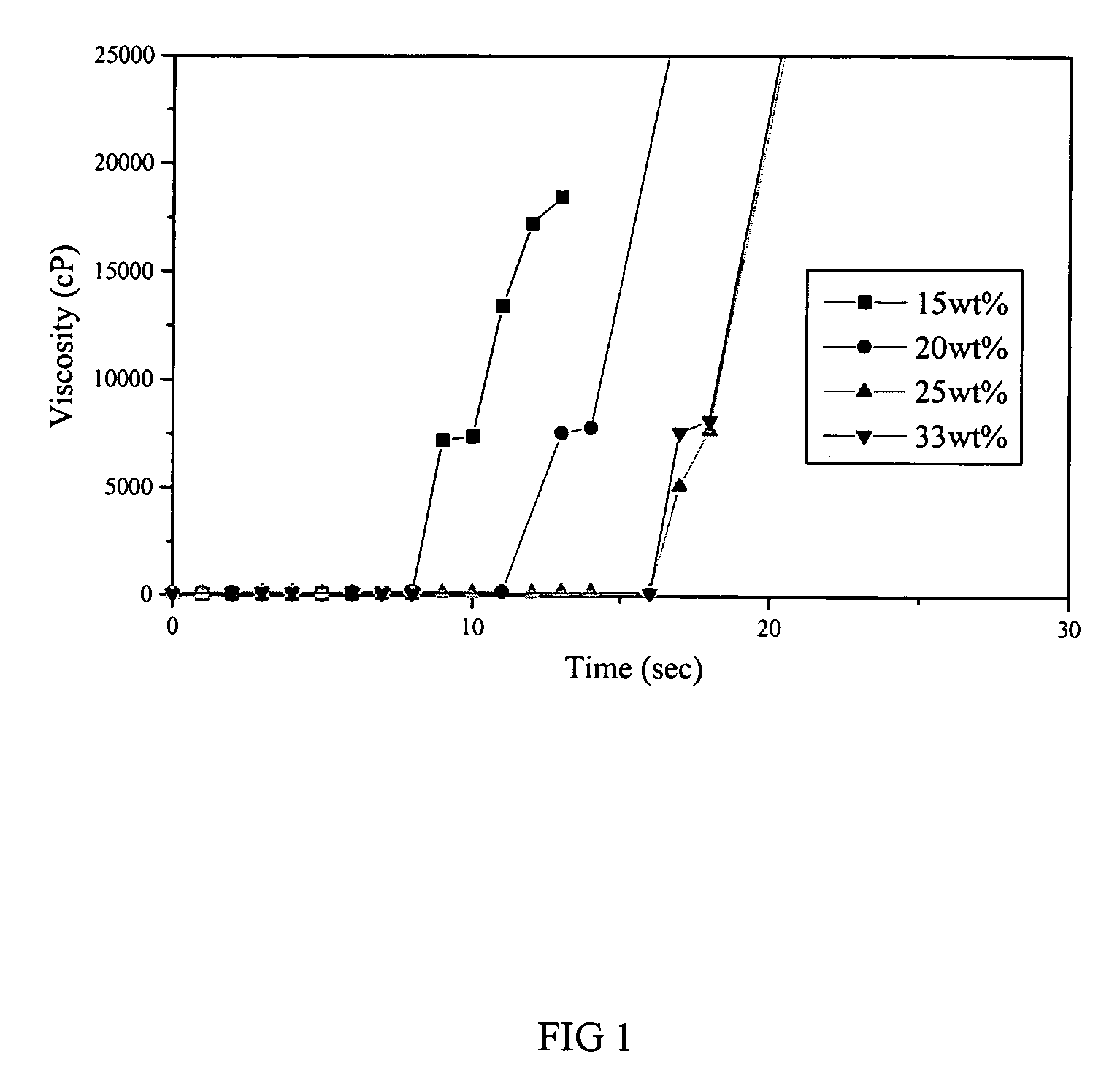
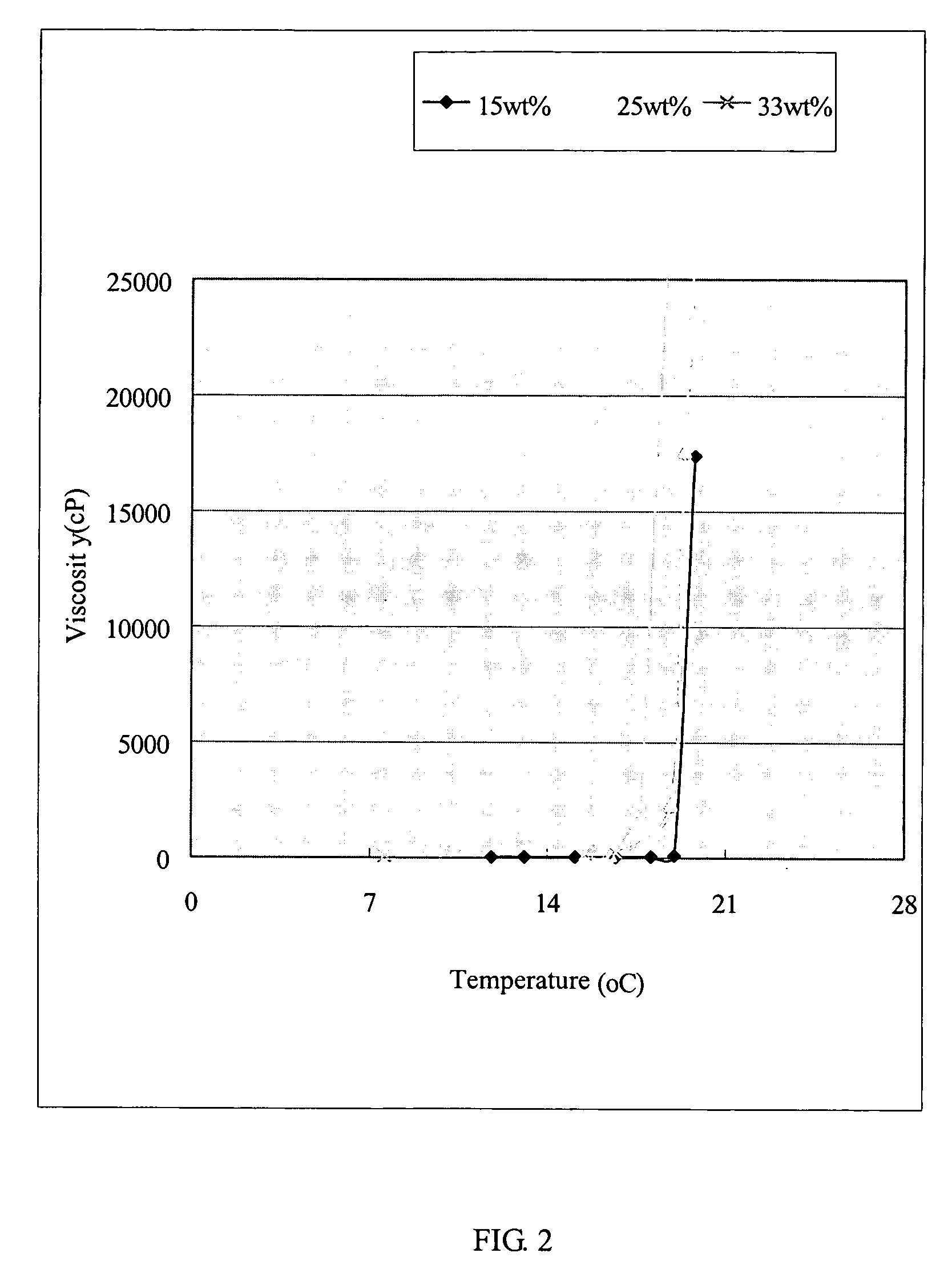
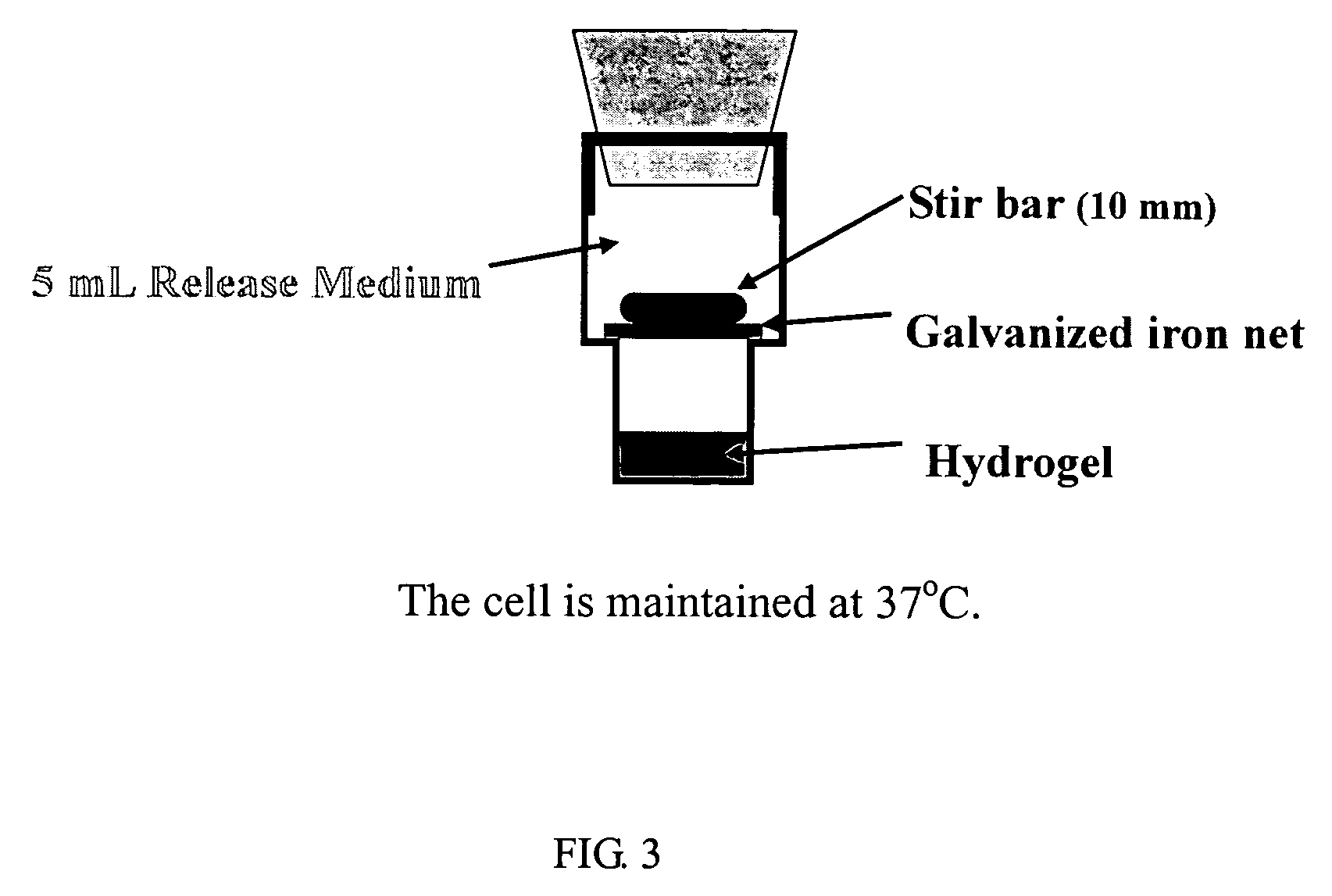
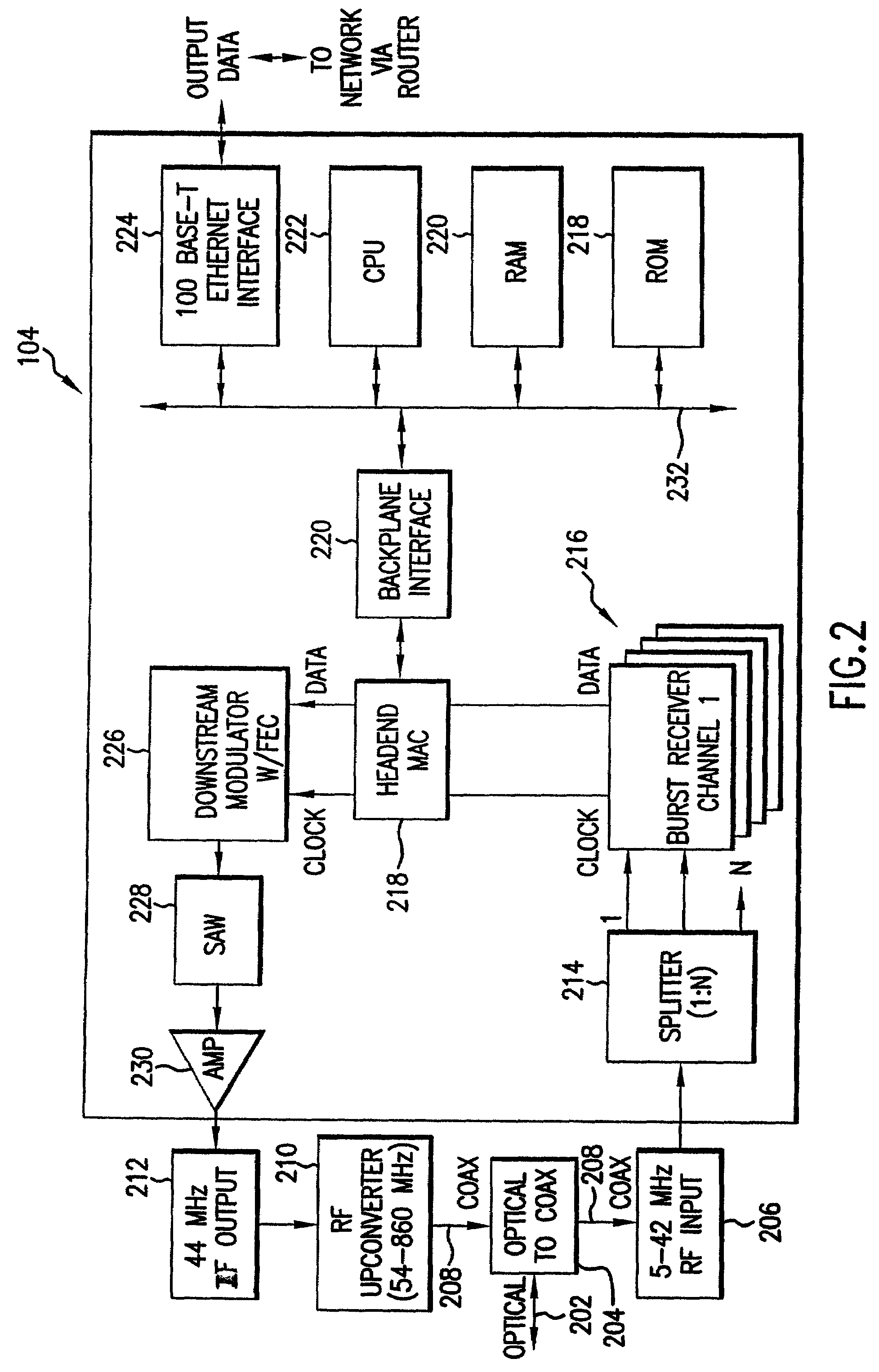
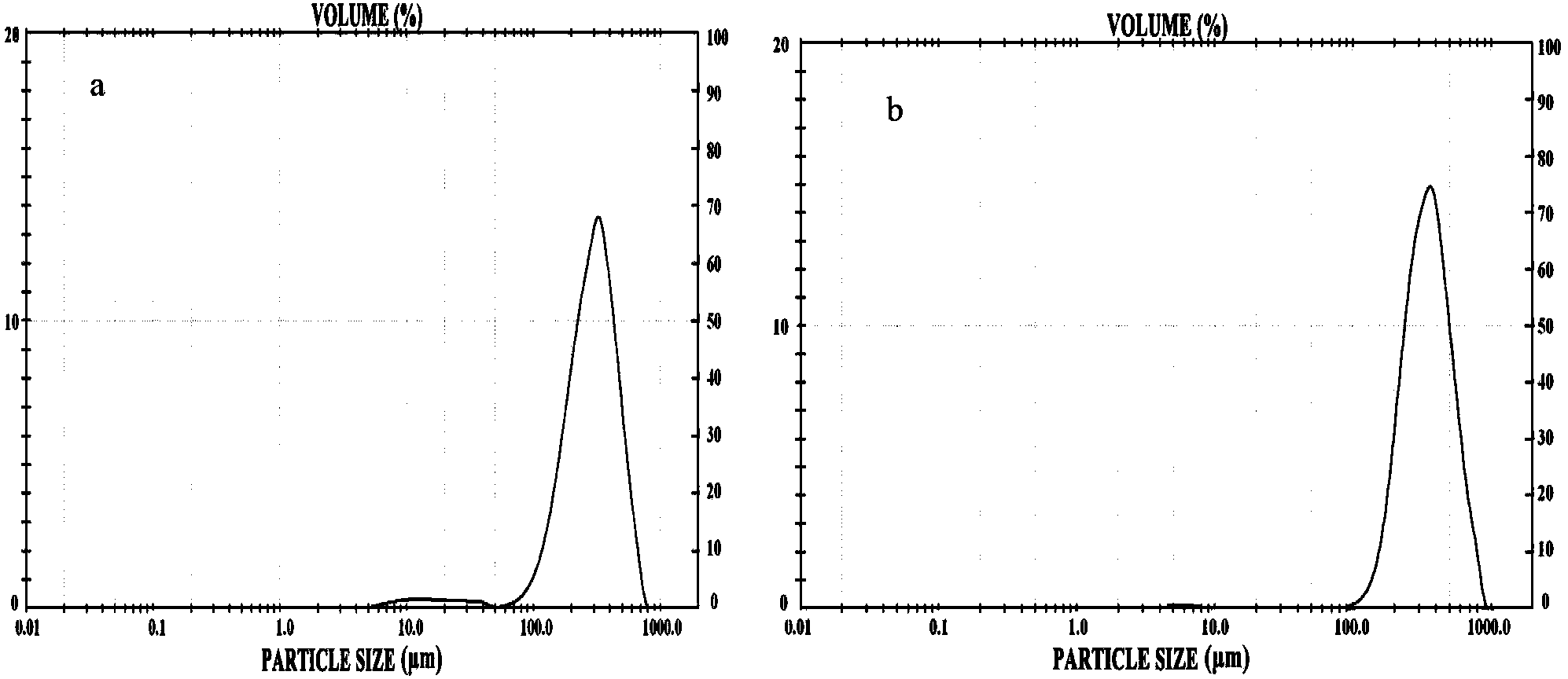
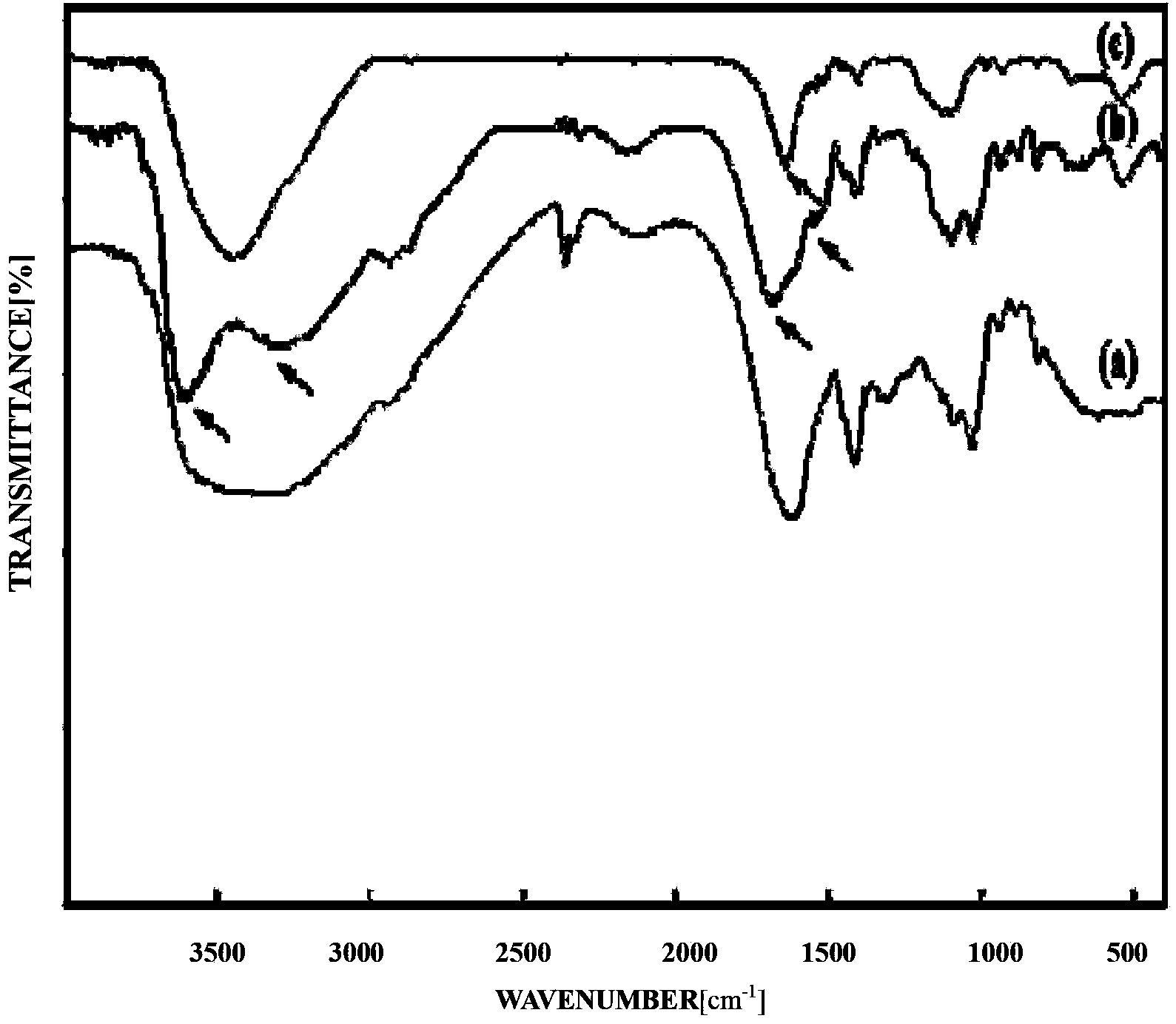
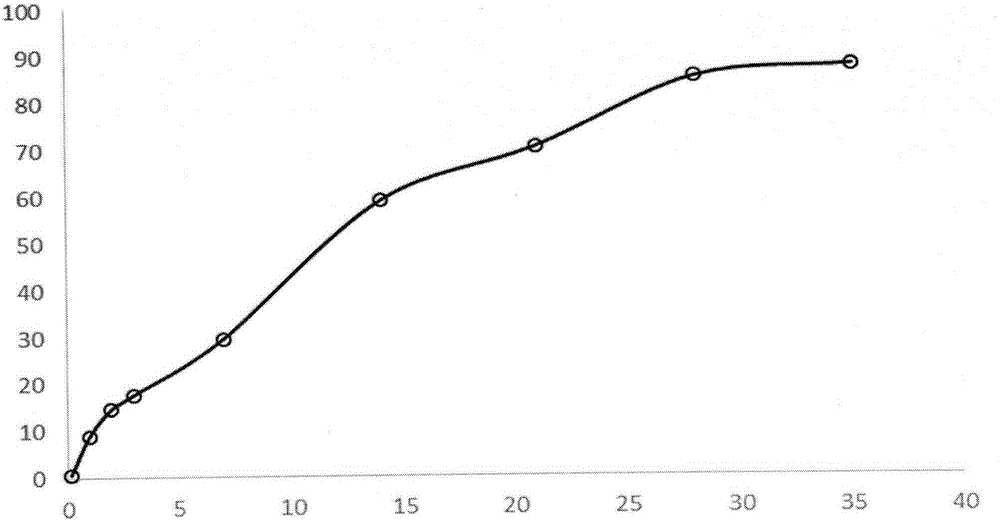
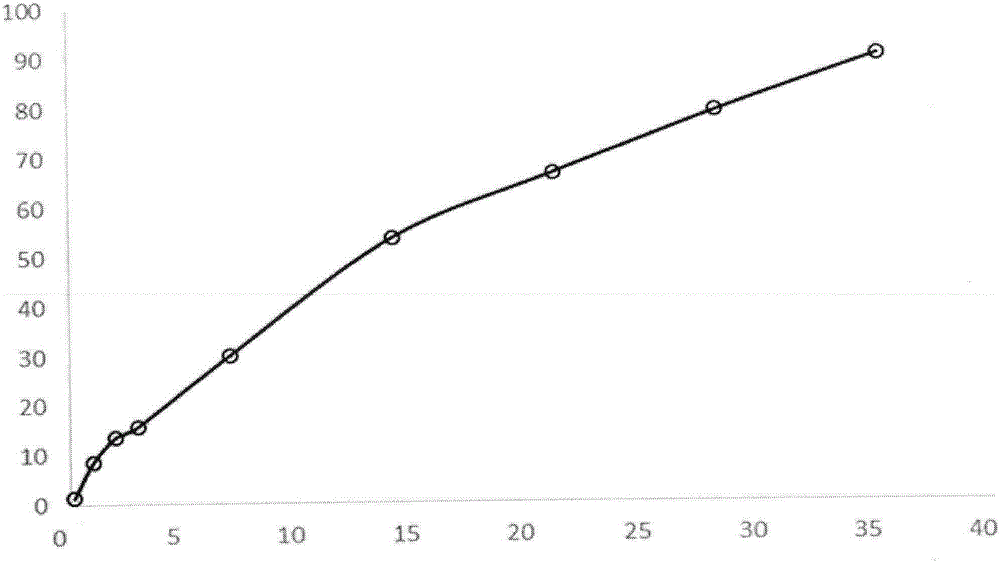
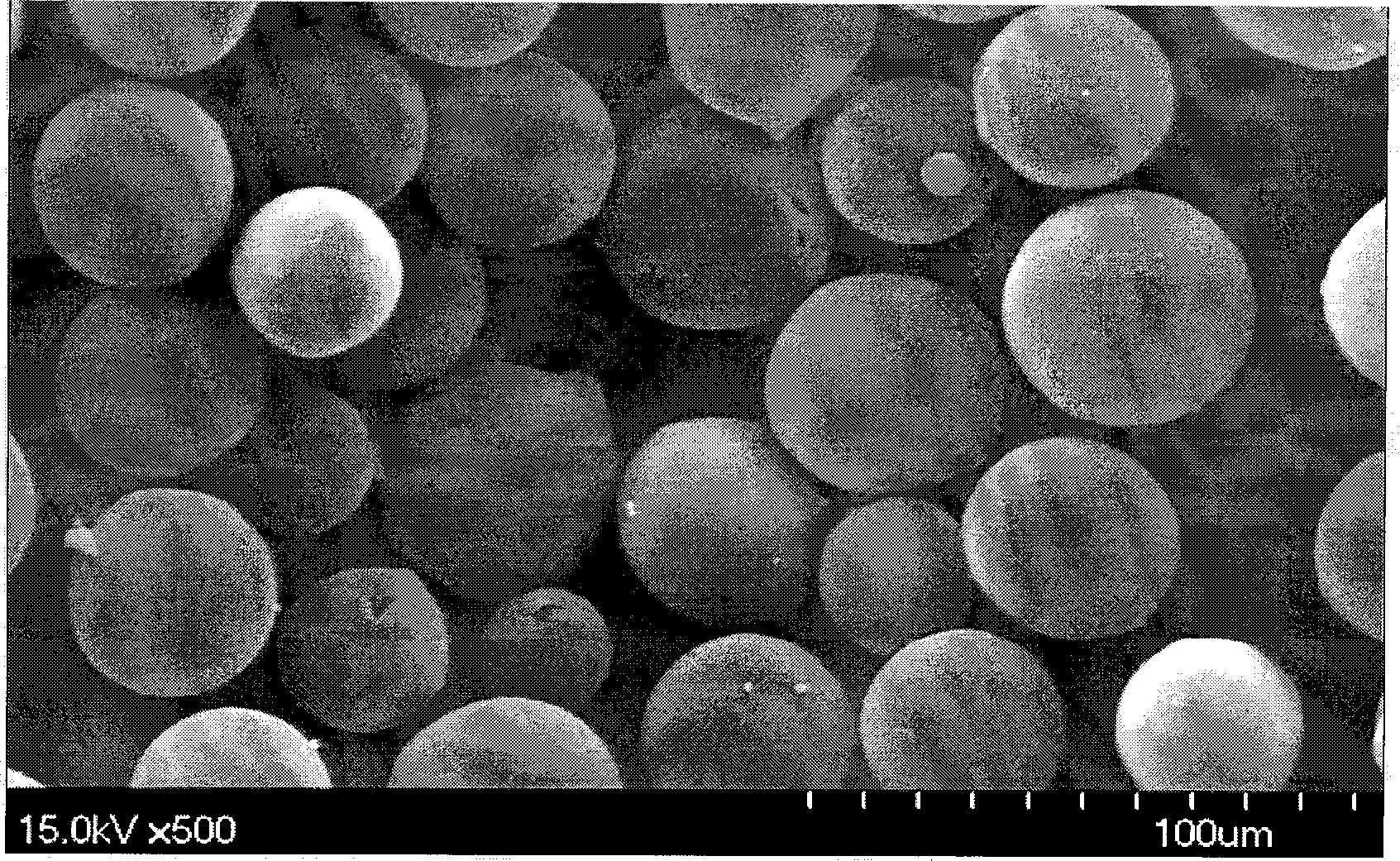Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69results about How to "Reduced burst effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical device with drug
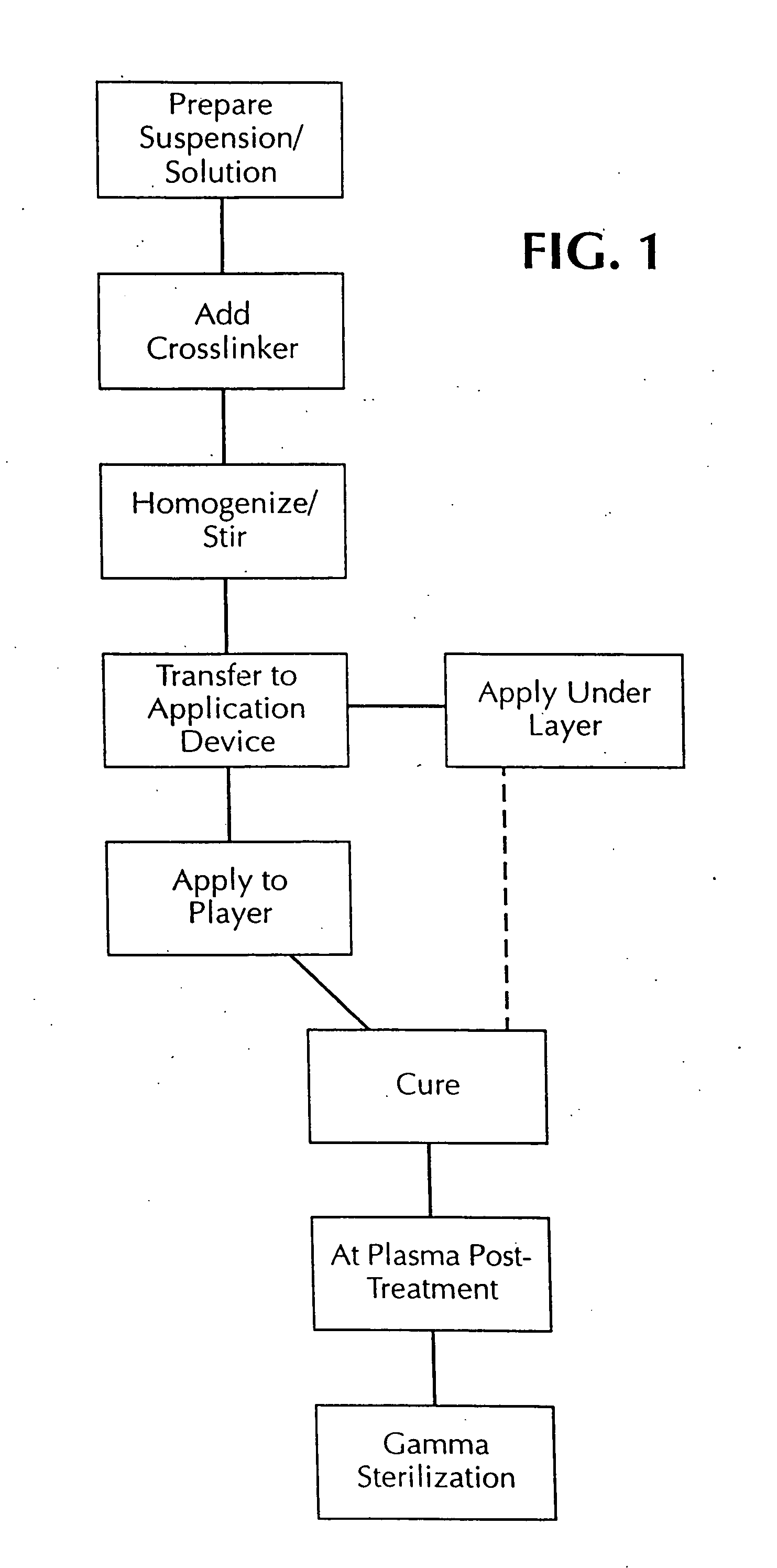
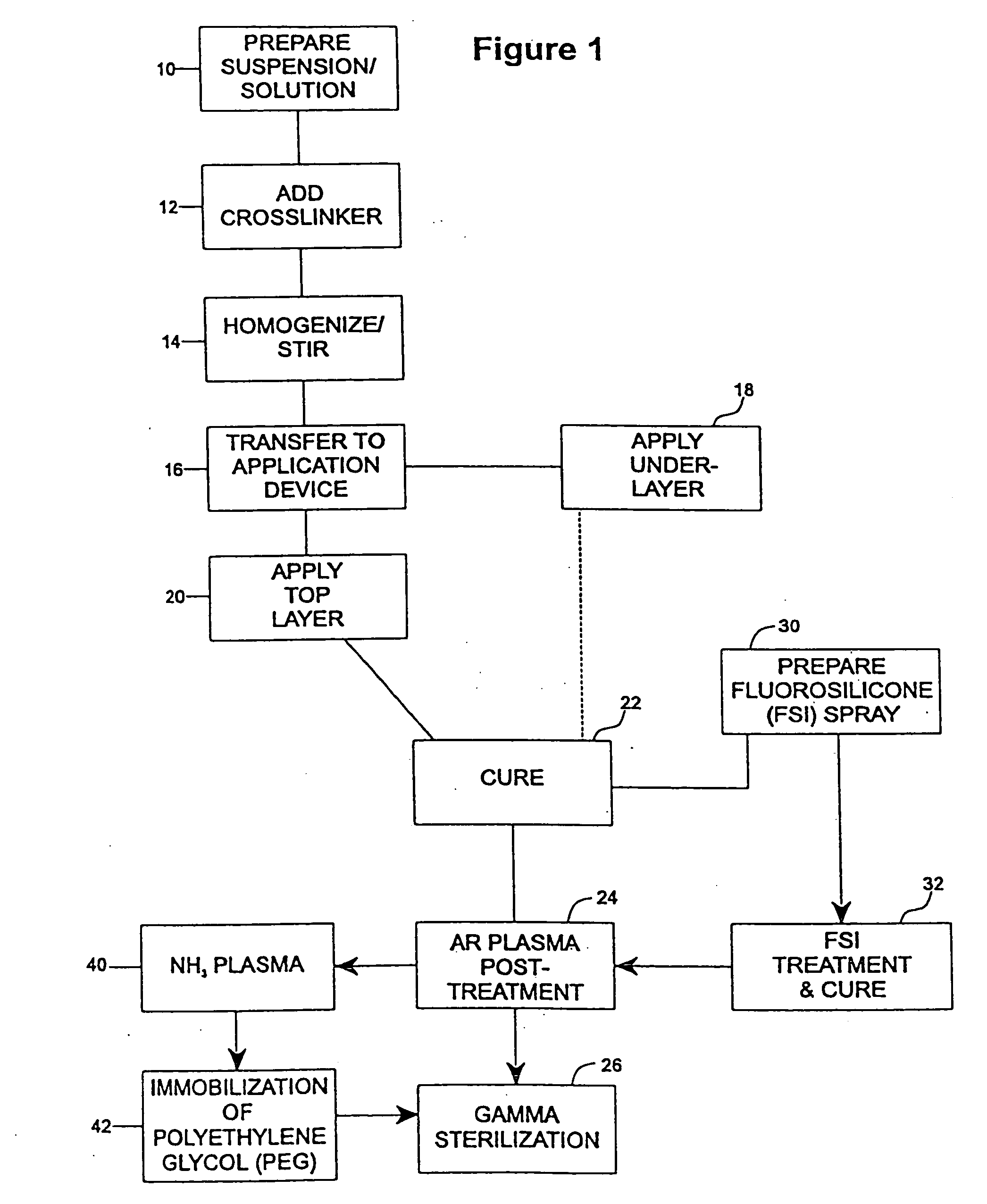
A method of coating implantable open lattice metallic stent prosthesis is disclosed which includes sequentially applying a plurality of relatively thin outer layers of a coating composition comprising a solvent mixture of uncured polymeric silicone material and crosslinker and finely divided biologically active species, possibly of controlled average particle size, to form a coating on each stent surface. The coatings are cured in situ and the coated, cured prosthesis are sterilized in a step that includes preferred pretreatment with argon gas plasma and exposure to gamma radiation electron beam, ethylene oxide, steam.
Owner:BOSTON SCI SCIMED INC
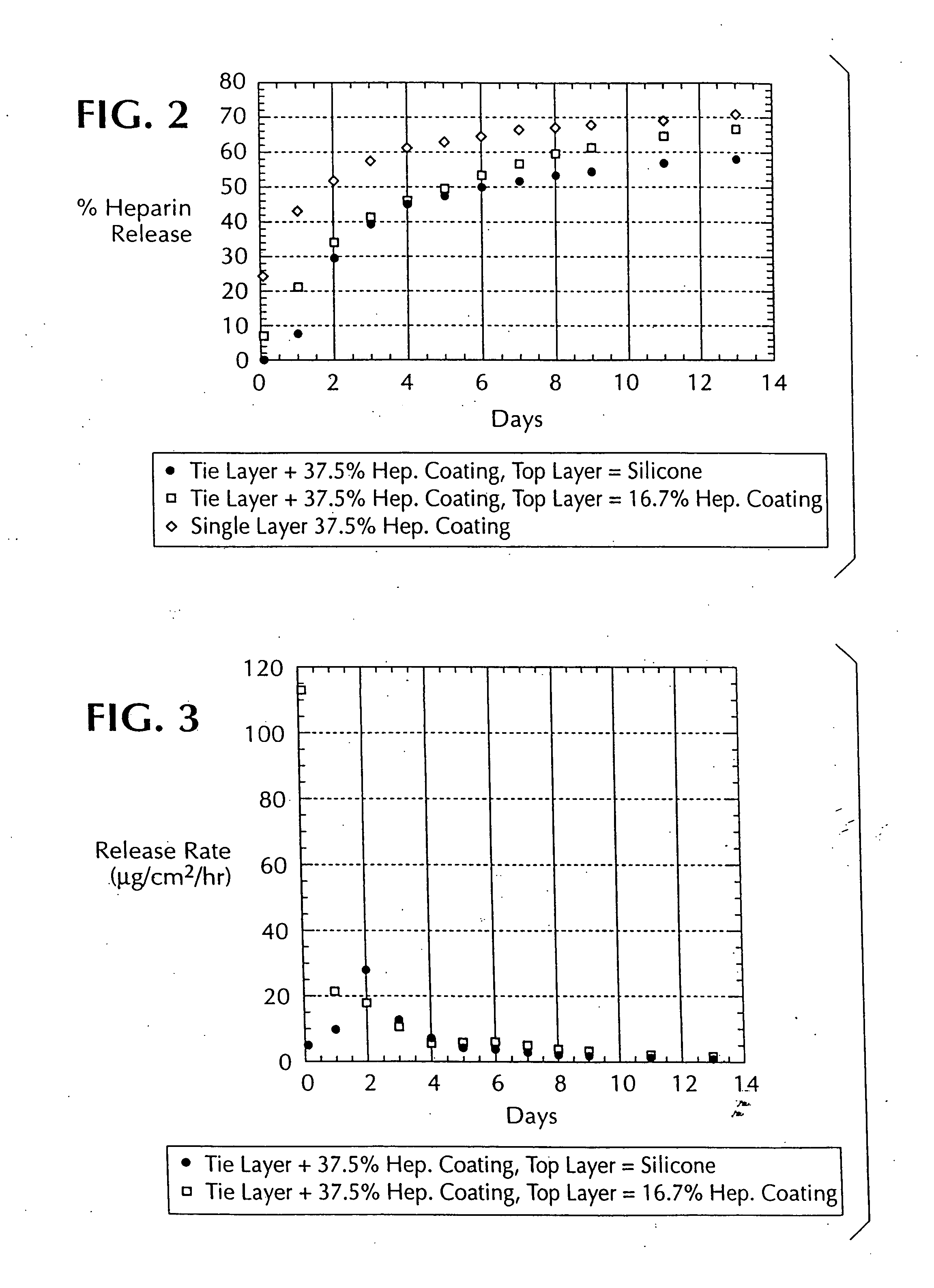
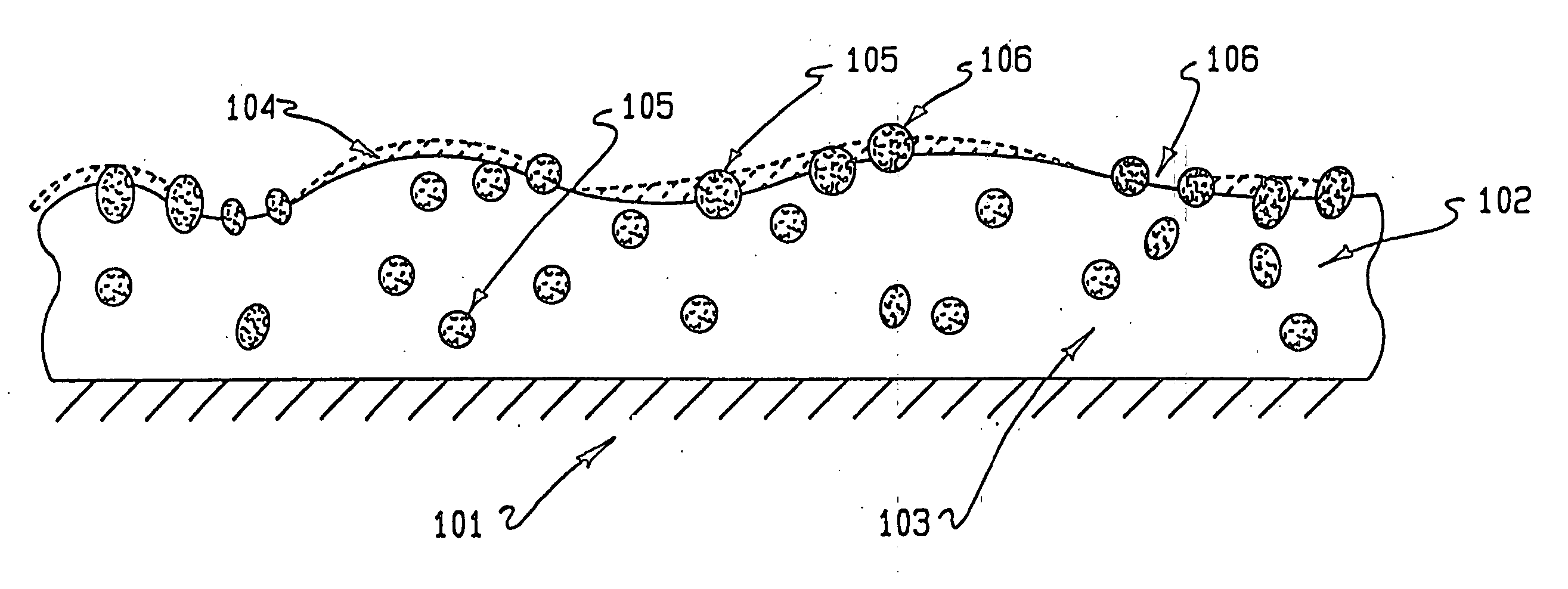
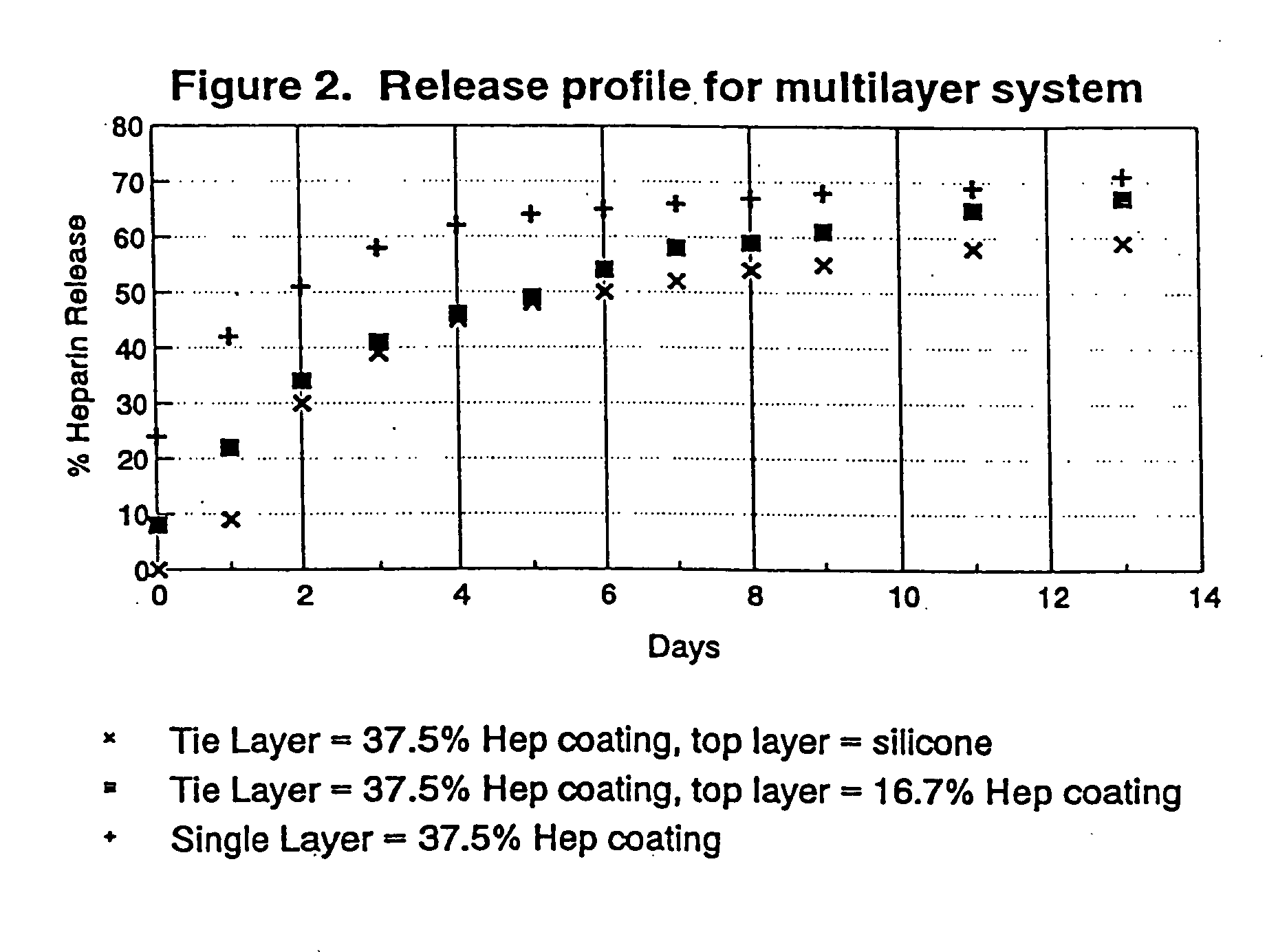
Drug coating with topcoat
InactiveUS20050187611A1Long release timeReduced burst effectStentsSurgeryThrombogenicityDrug coating
A coating and method for a coating an implantable device or prostheses are disclosed. The coating includes an undercoat of polymeric material containing an amount of biologically active material, particularly heparin, dispersed herein. The coating further includes a topcoat which covers less than the entire surface of the undercoat and wherein the topcoat comprises a polymeric material substantially free of pores and porosigens. The polymeric material of the topcoat can be a biostable, biocompatible material which provides long term non-thrombogenicity to the device portion during and after release of the biologically active material.
Owner:BOSTON SCI SCIMED INC
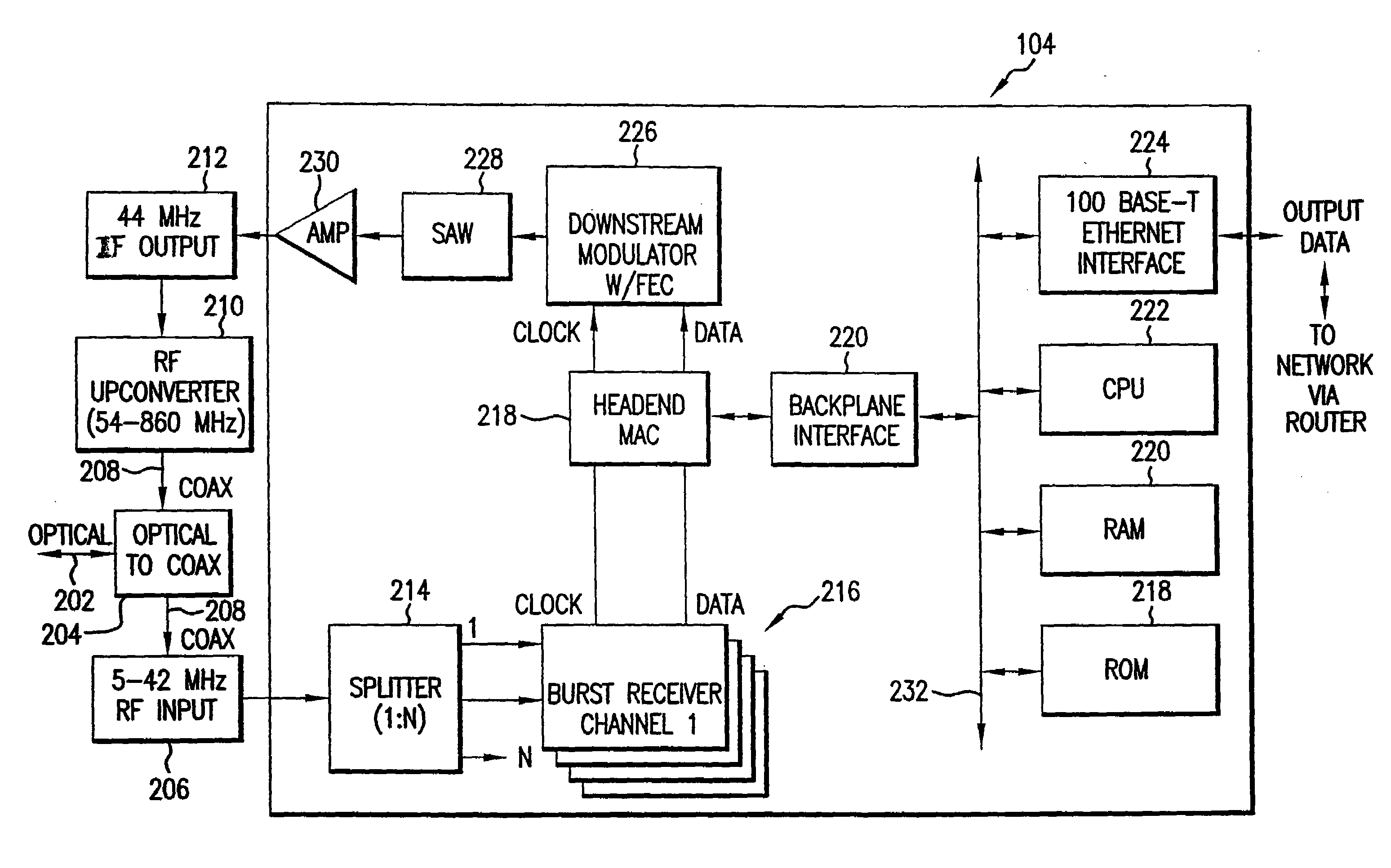
FEC block reconstruction system, method and computer program product for mitigating burst noise in a communications system
InactiveUS7089478B2Mitigate effectImprove robustnessError prevention/detection by using return channelCode conversionBlock codeReal-time computing
A system, method and computer program product is provided for mitigating the effects of burst noise on packets transmitted in a communications system. A transmitting device applies an outer code, which may include, for example, a block code, an exclusive OR (XOR) code, or a repetition code, to one or more packets prior to adaptation of the packets for transmission over the physical (PHY) layer of the communications system, wherein the PHY layer adaptation may include FEC encoding of individual packets. The outer coded packets are then separately transmitted over a channel of the communications system. A receiving device receives the outer coded packets, performs PHY level demodulation and optional FEC decoding of the packets, and then applies outer code decoding to the out6r coded packets in order to restore packets that were erased during transmission due to burst noise or other impairments on the channel.
Owner:AVAGO TECH INT SALES PTE LTD
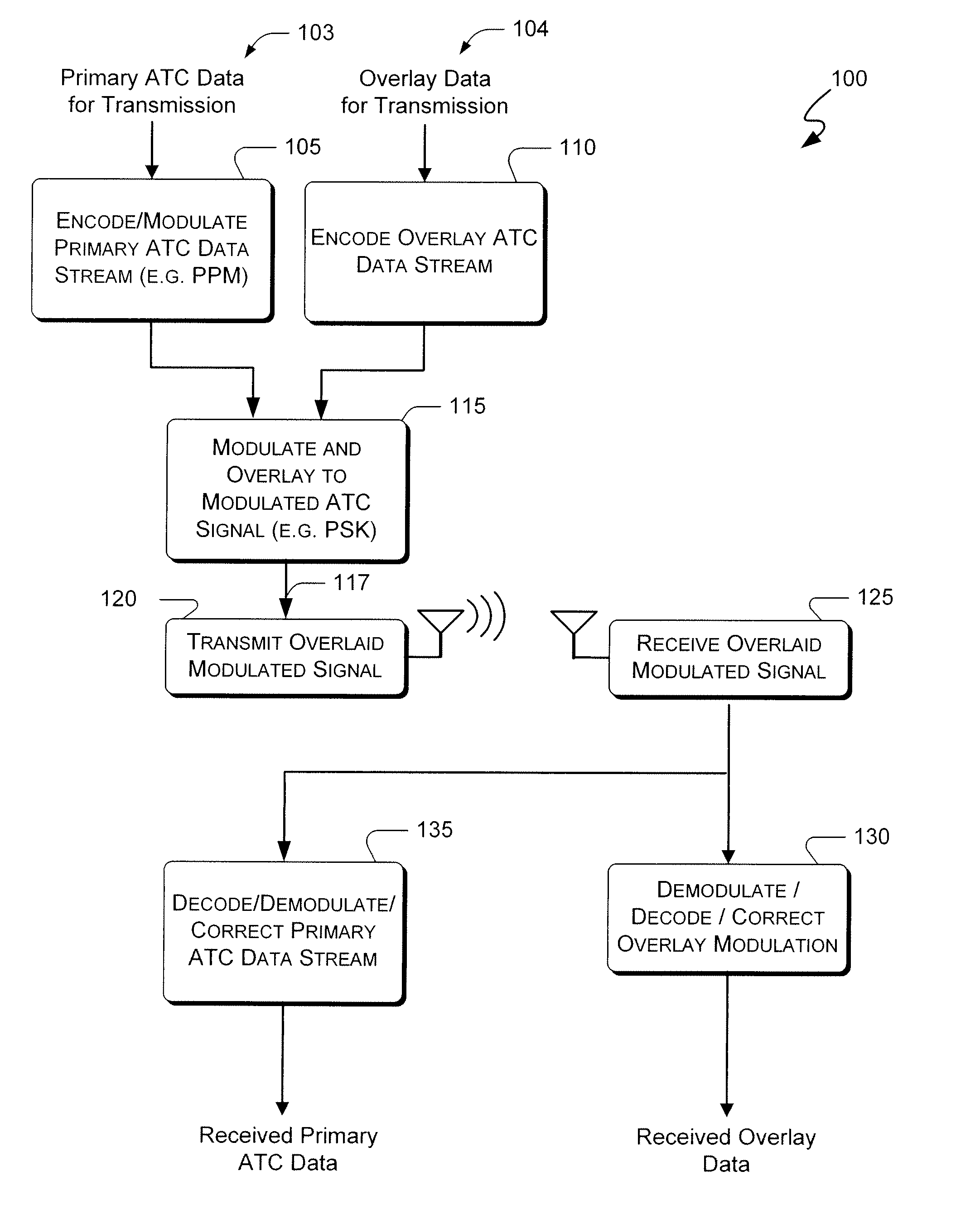
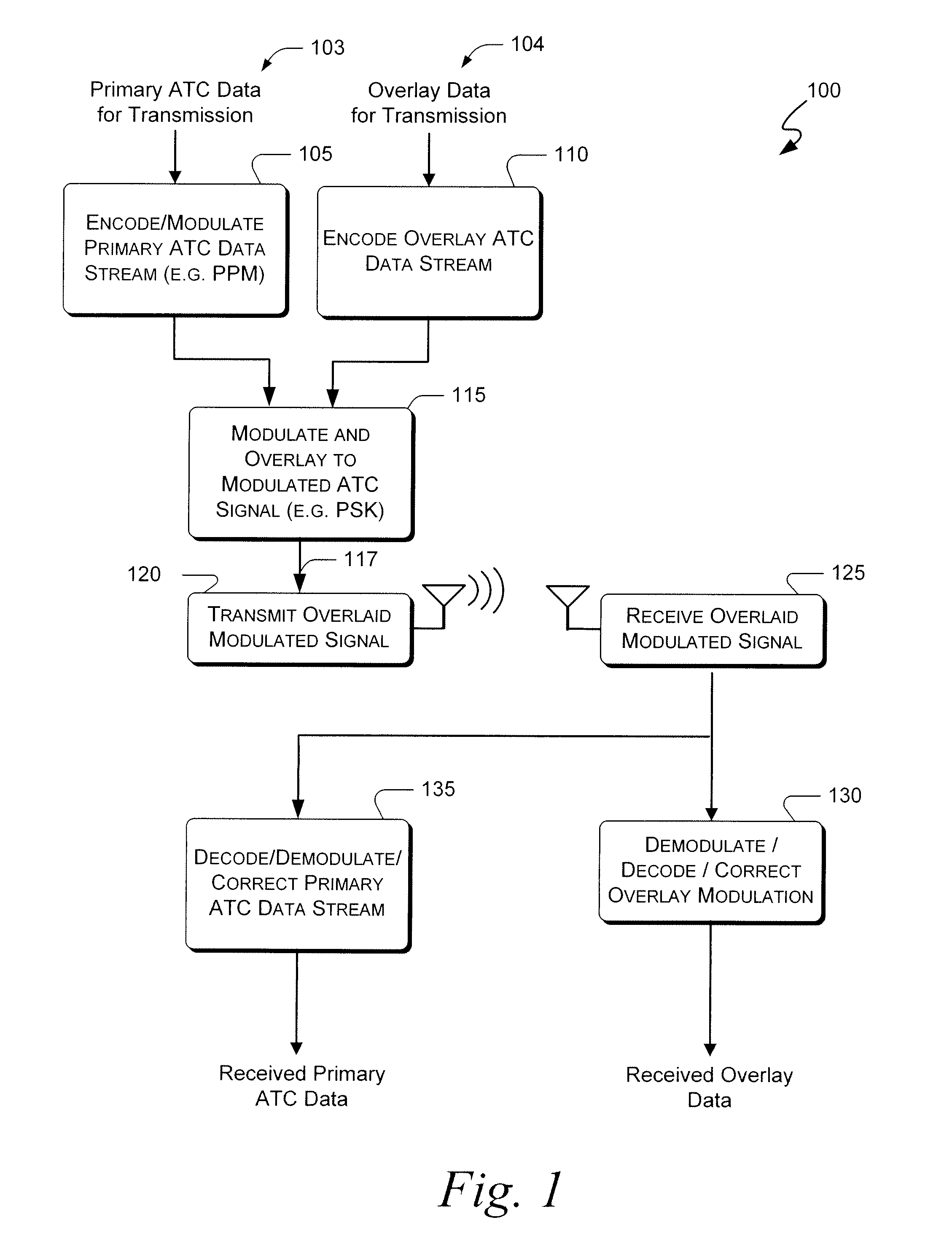
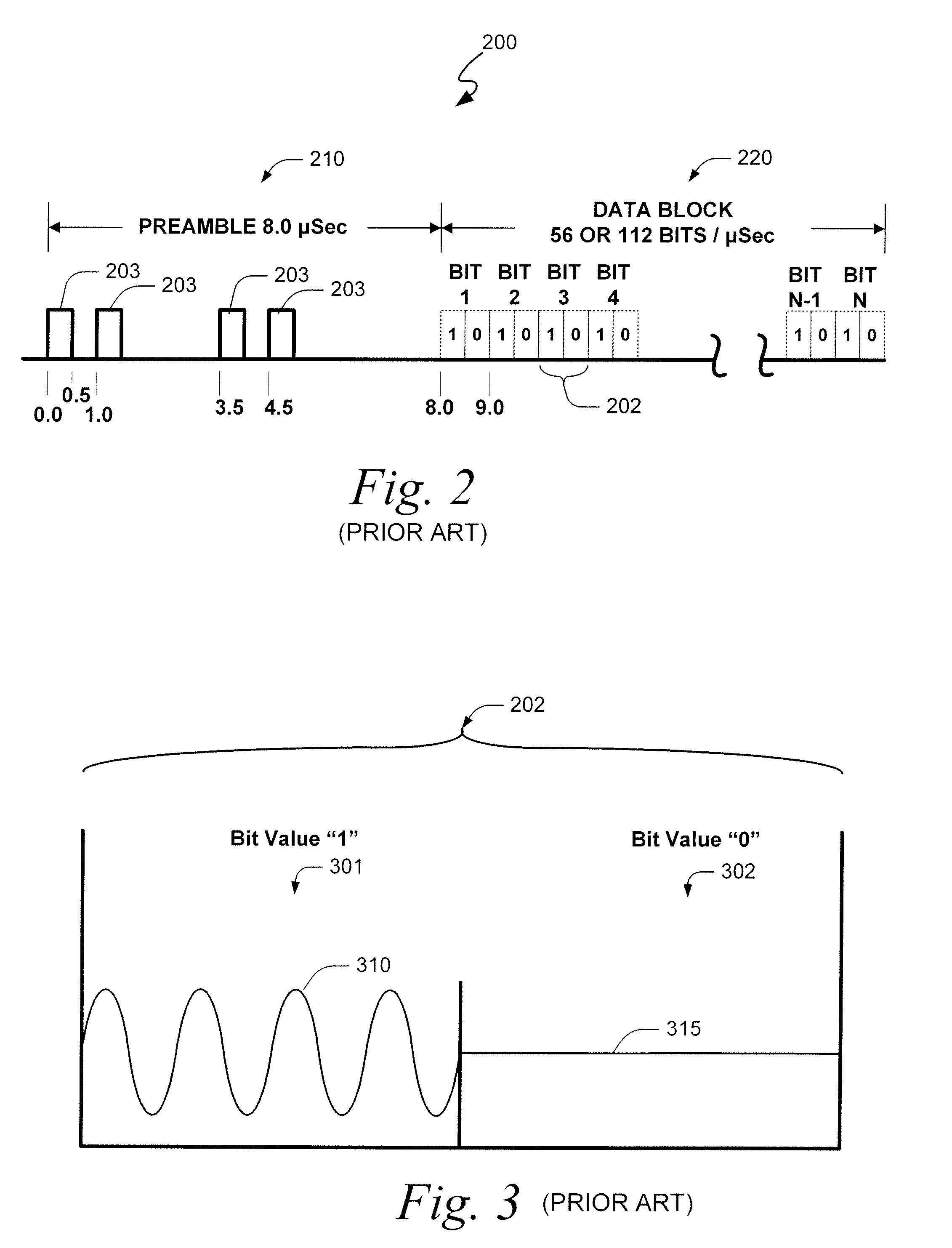
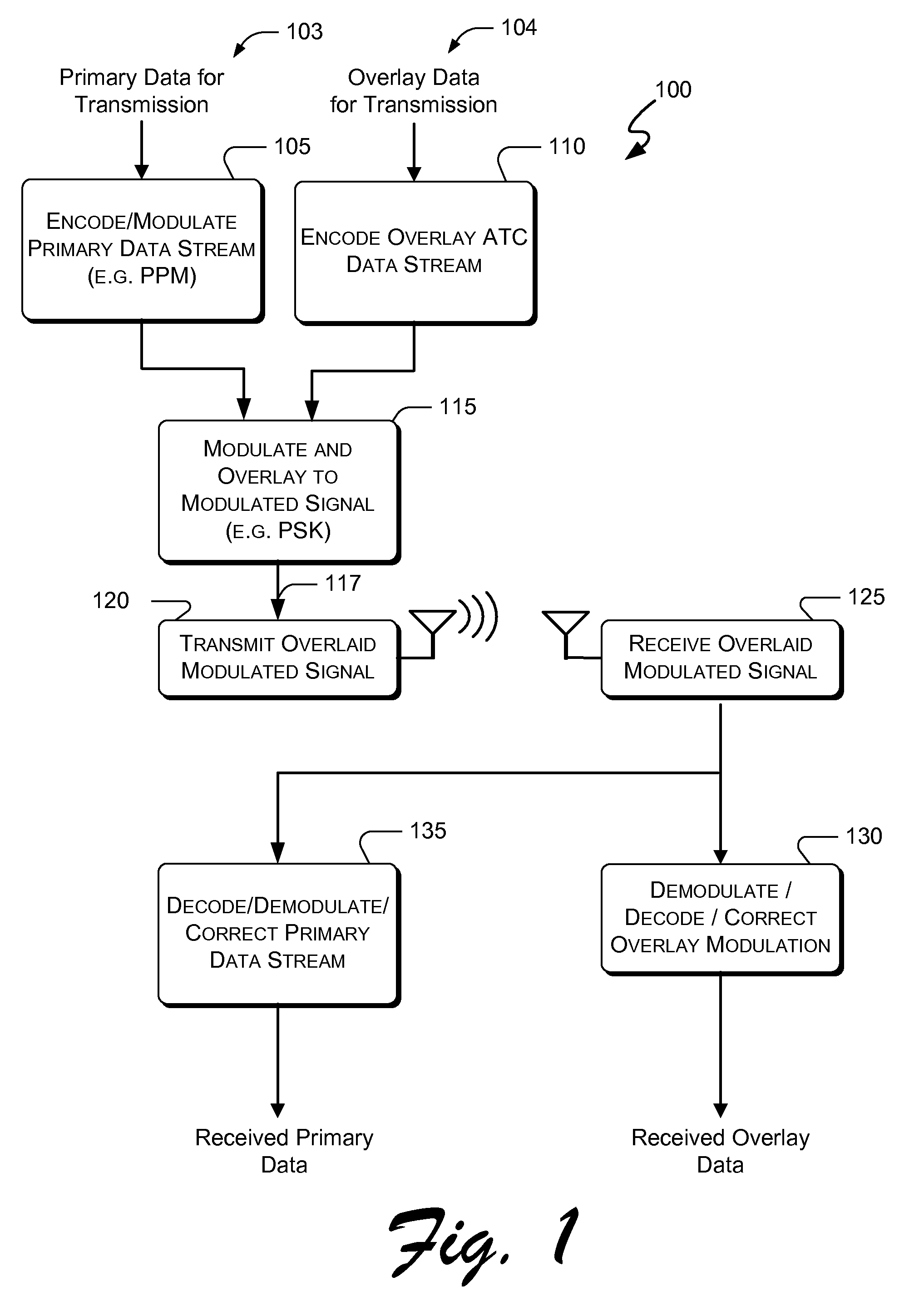
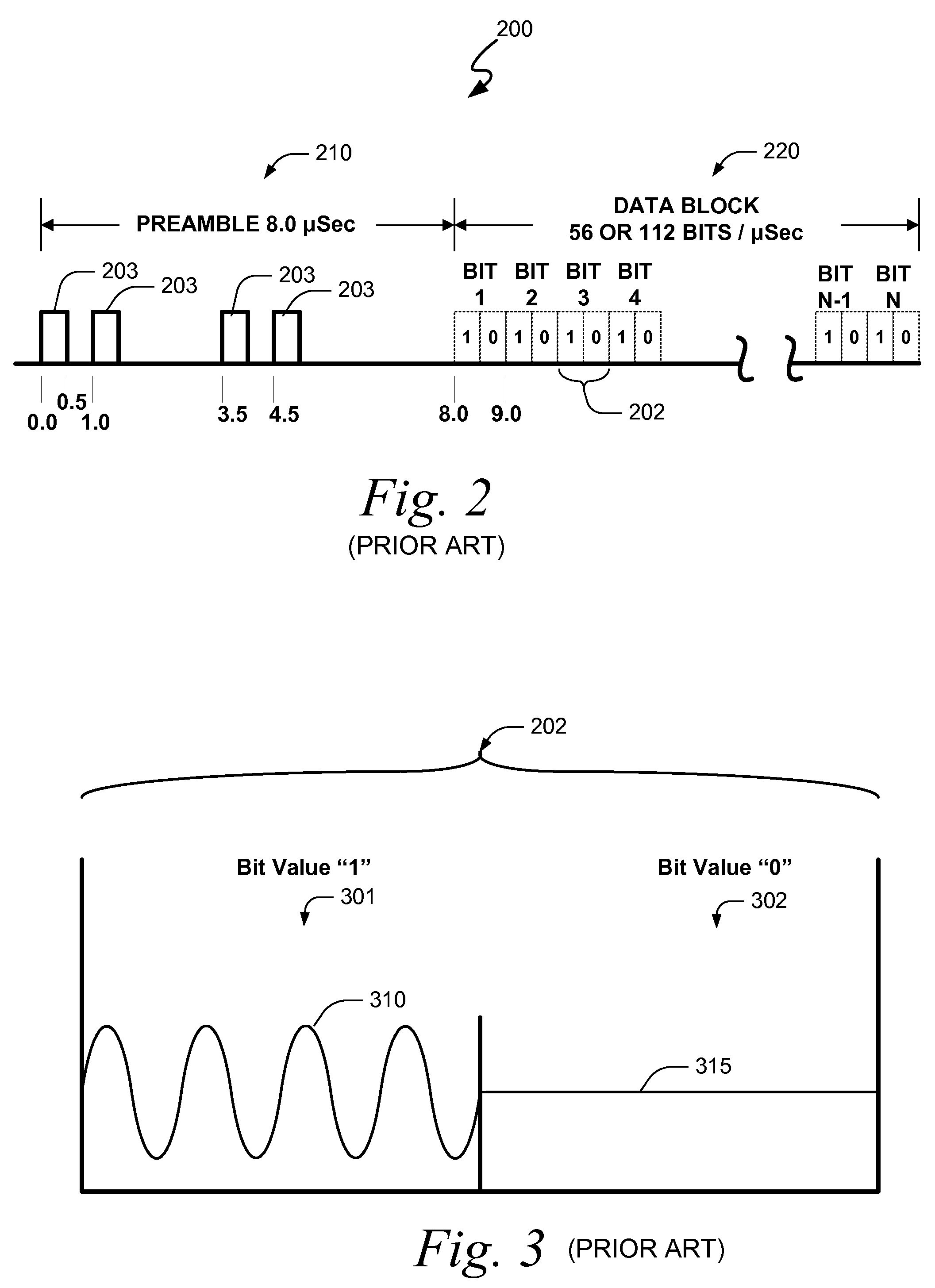
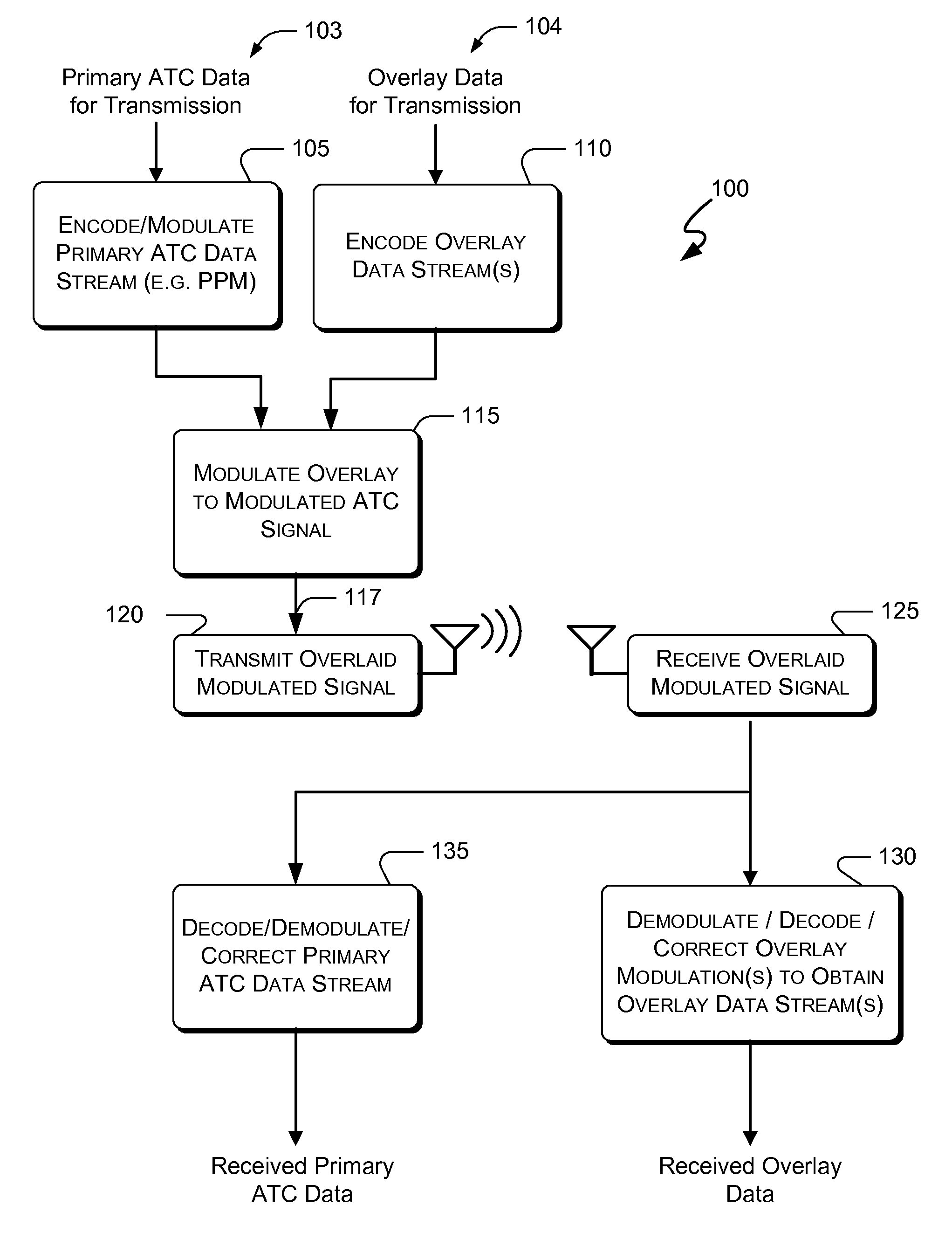
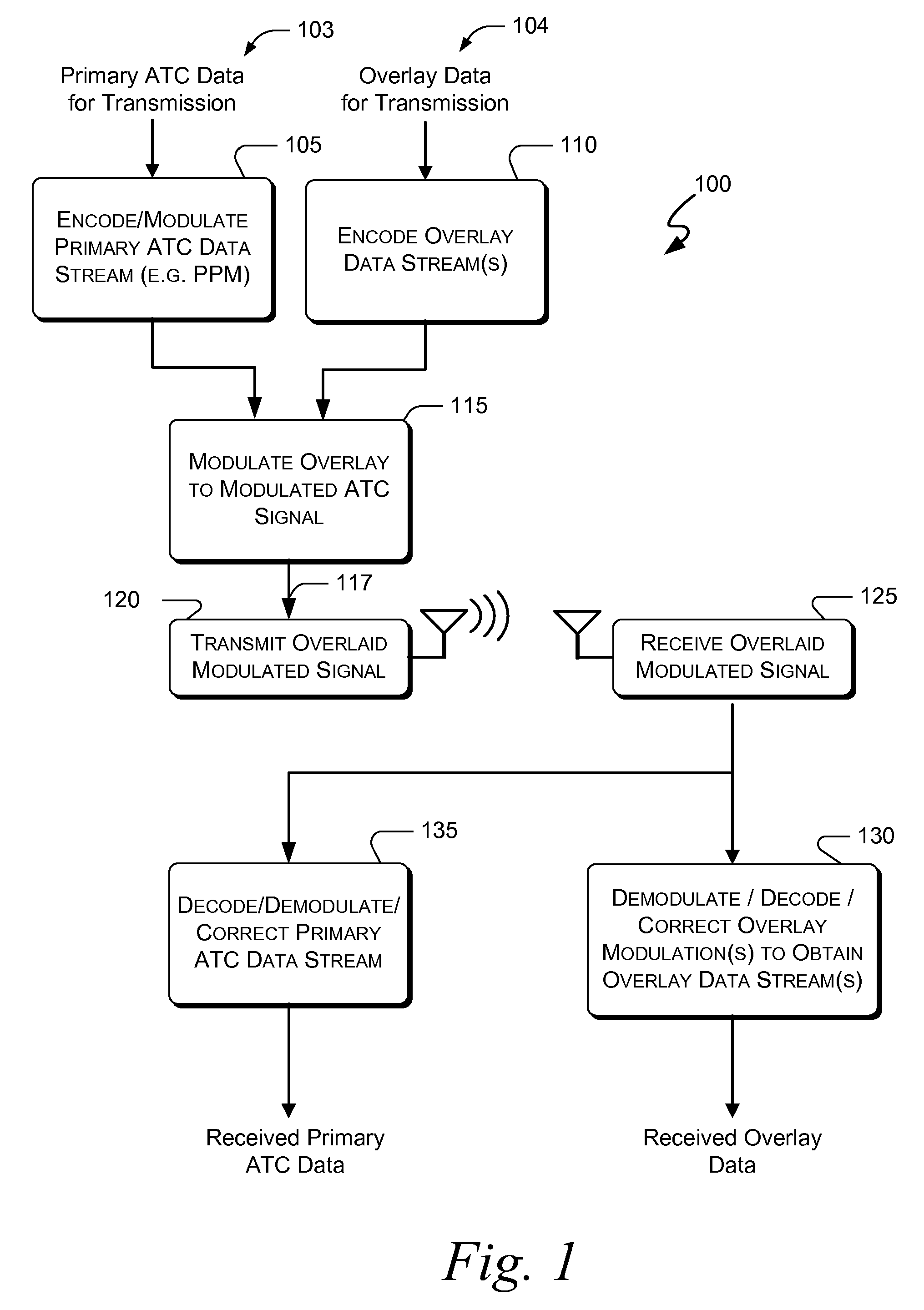
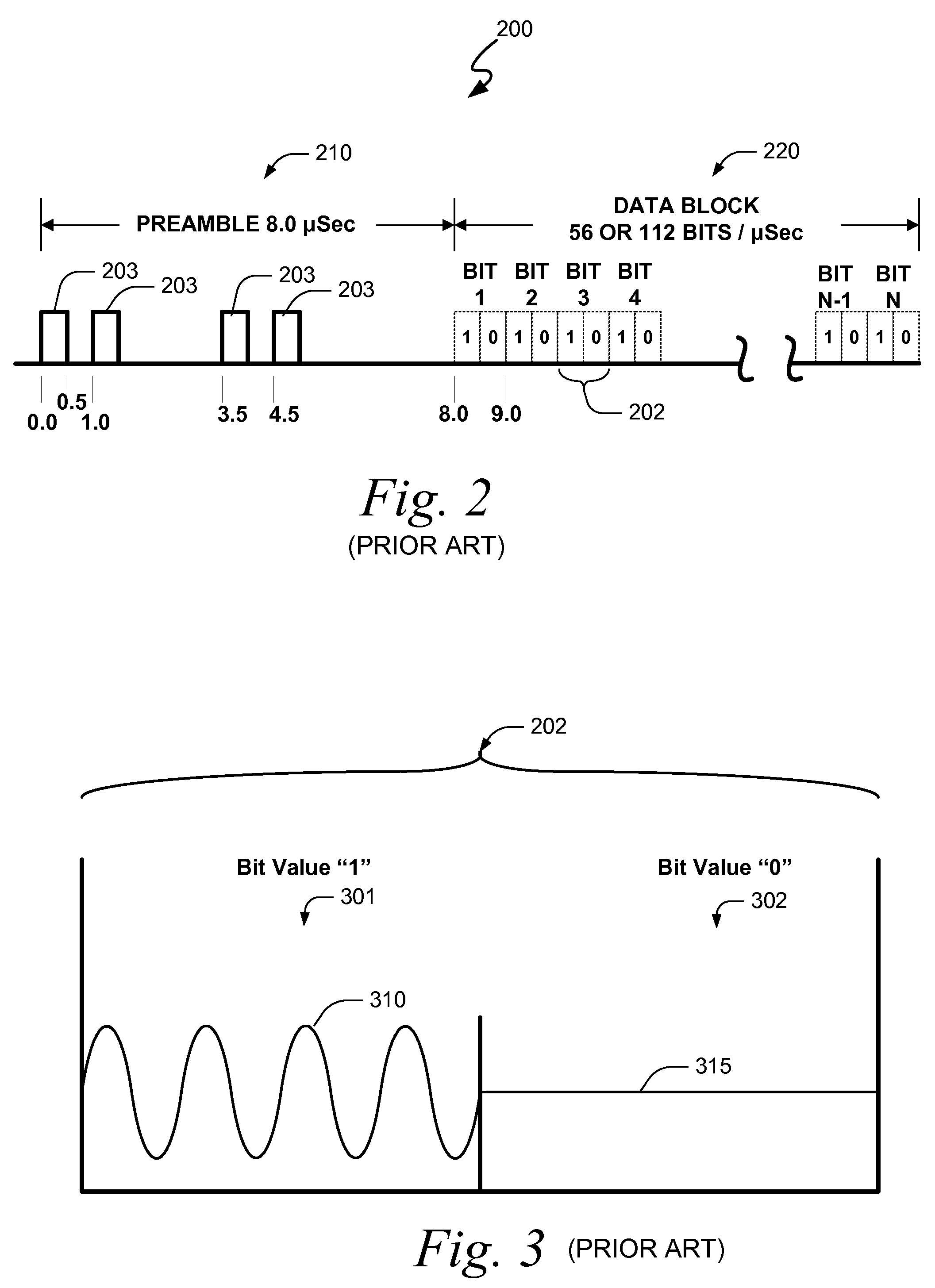
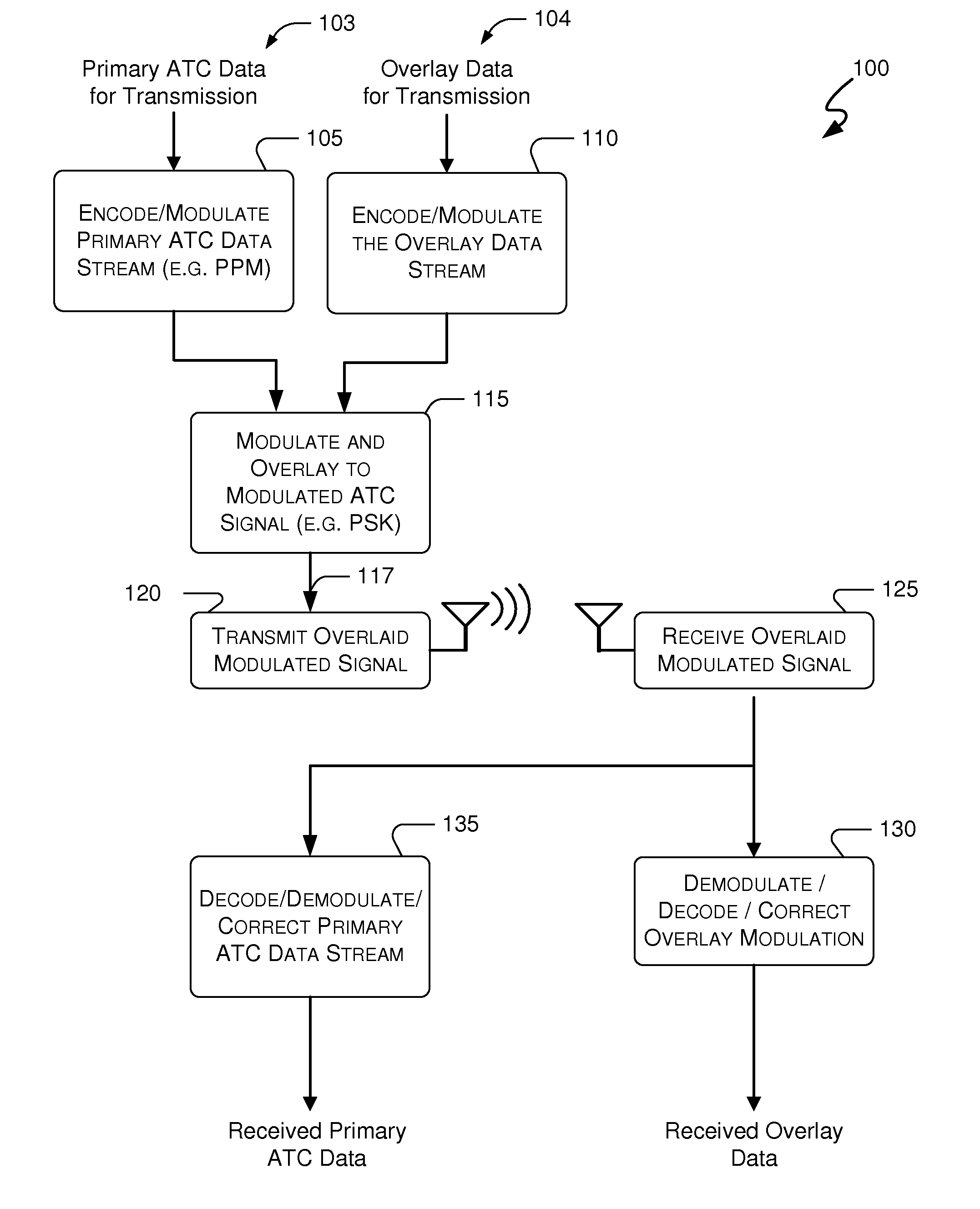
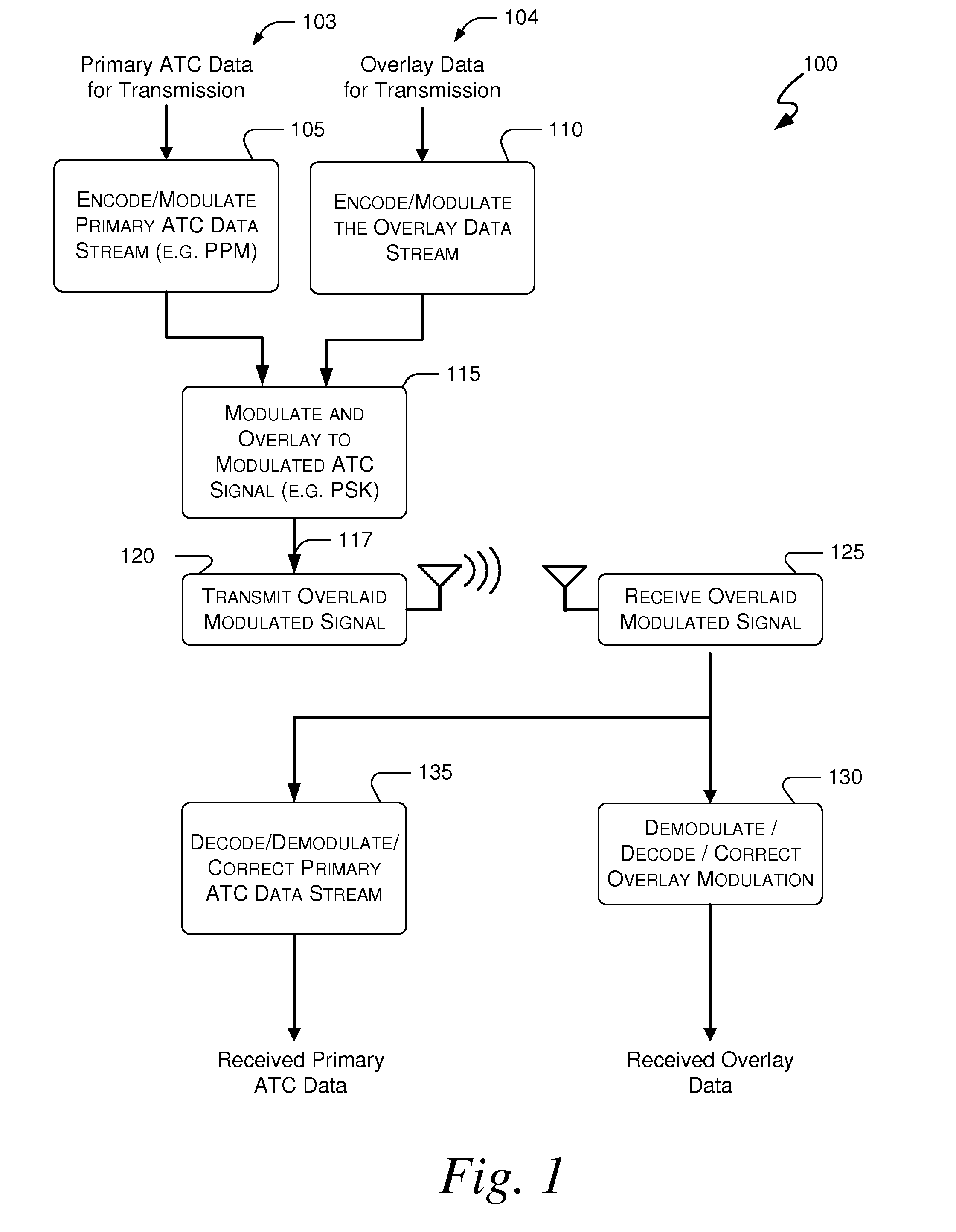
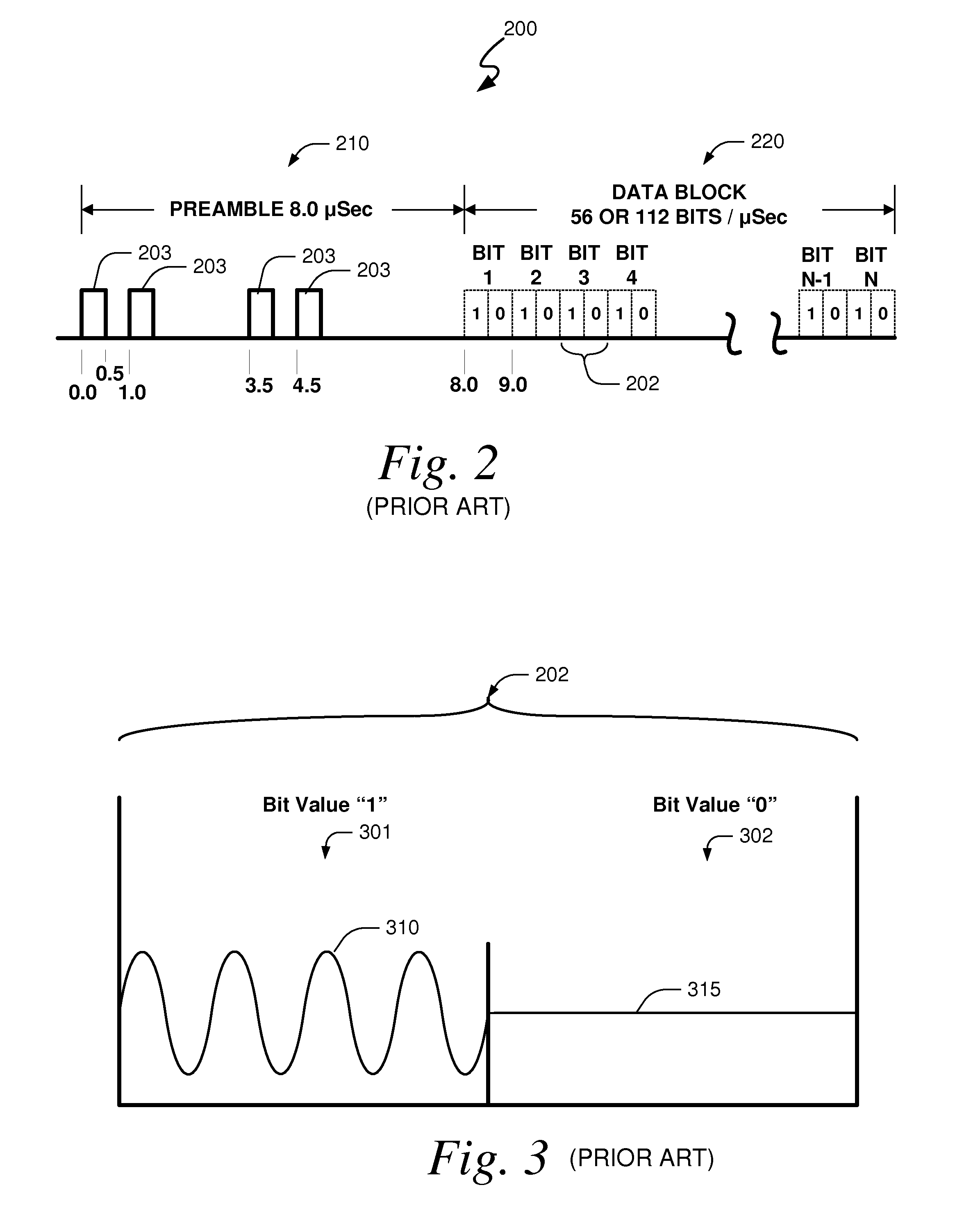
Systems and methods for providing an atc overlay data link
ActiveUS20090322587A1Reduce errorsOptimize noiseAmplitude-modulated carrier systemsFrequency-modulated carrier systemsComputer scienceData link
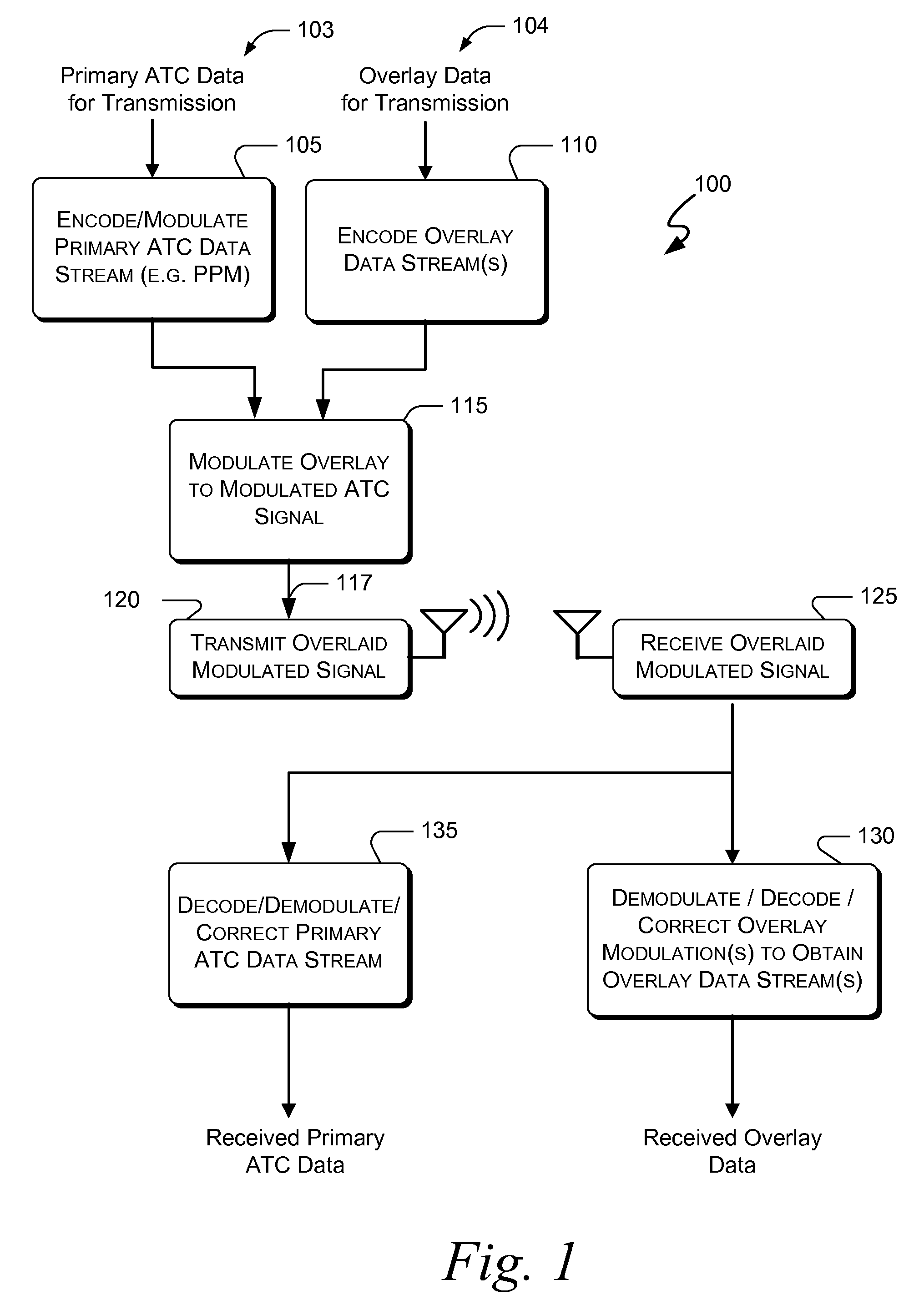
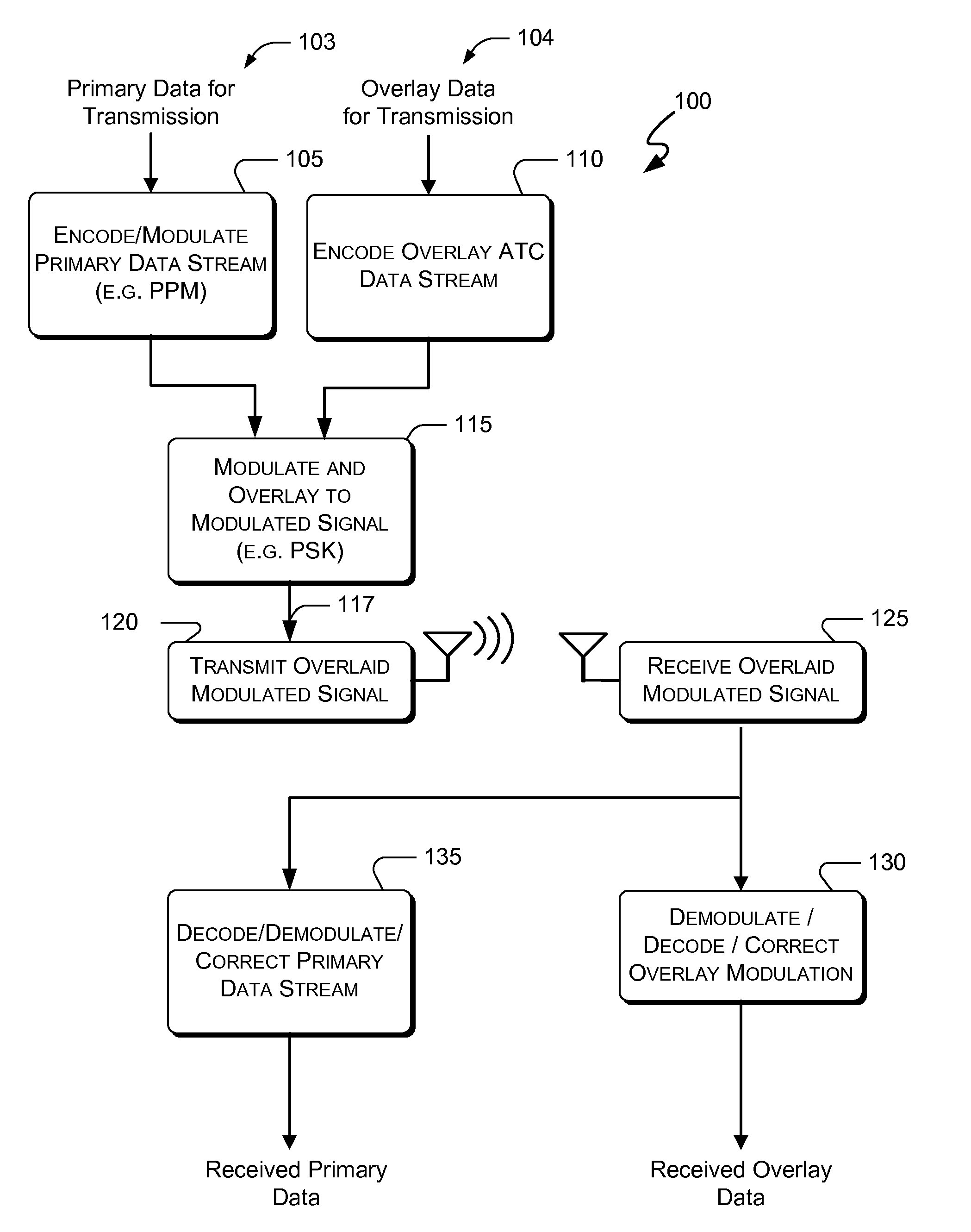
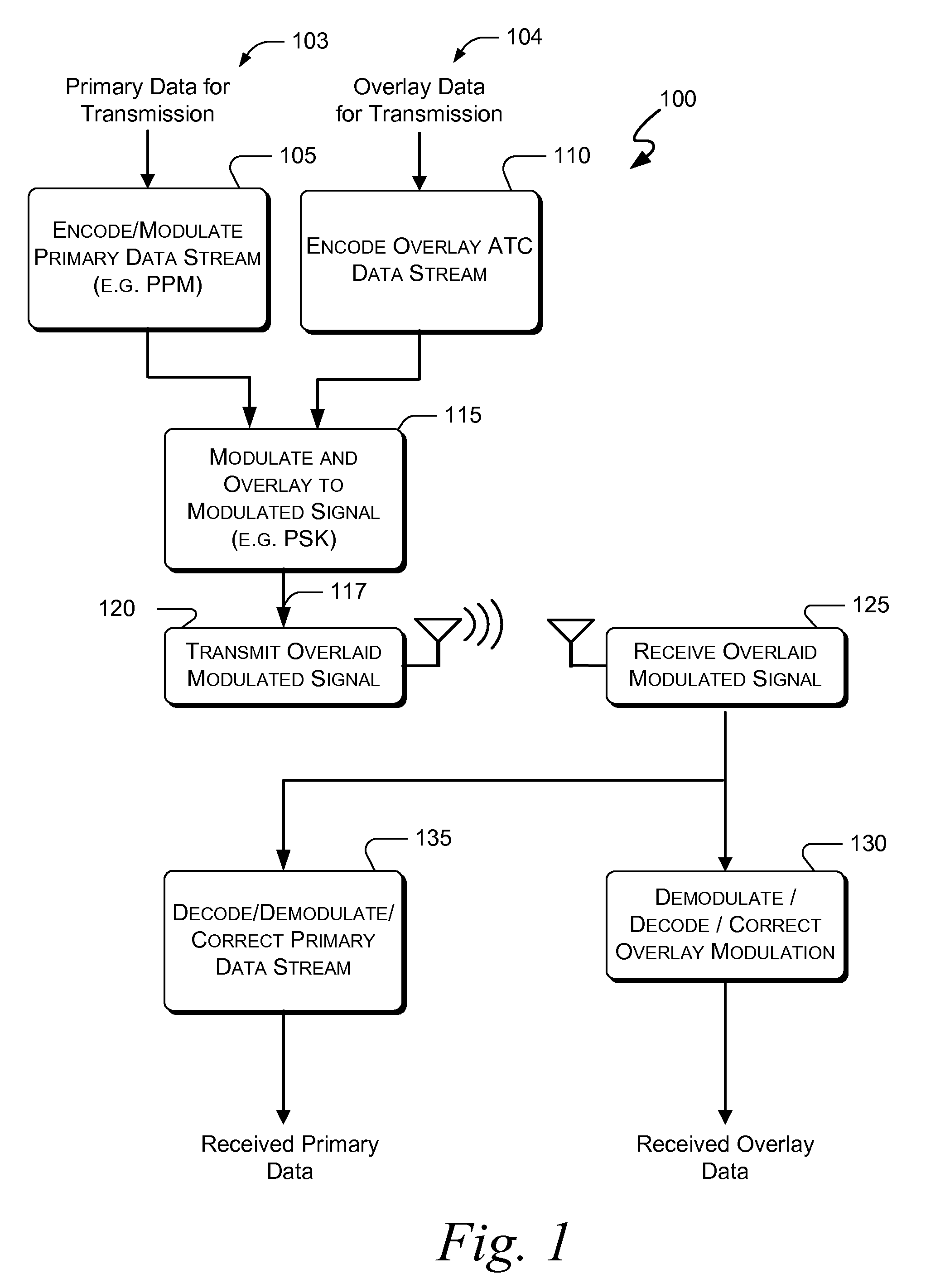
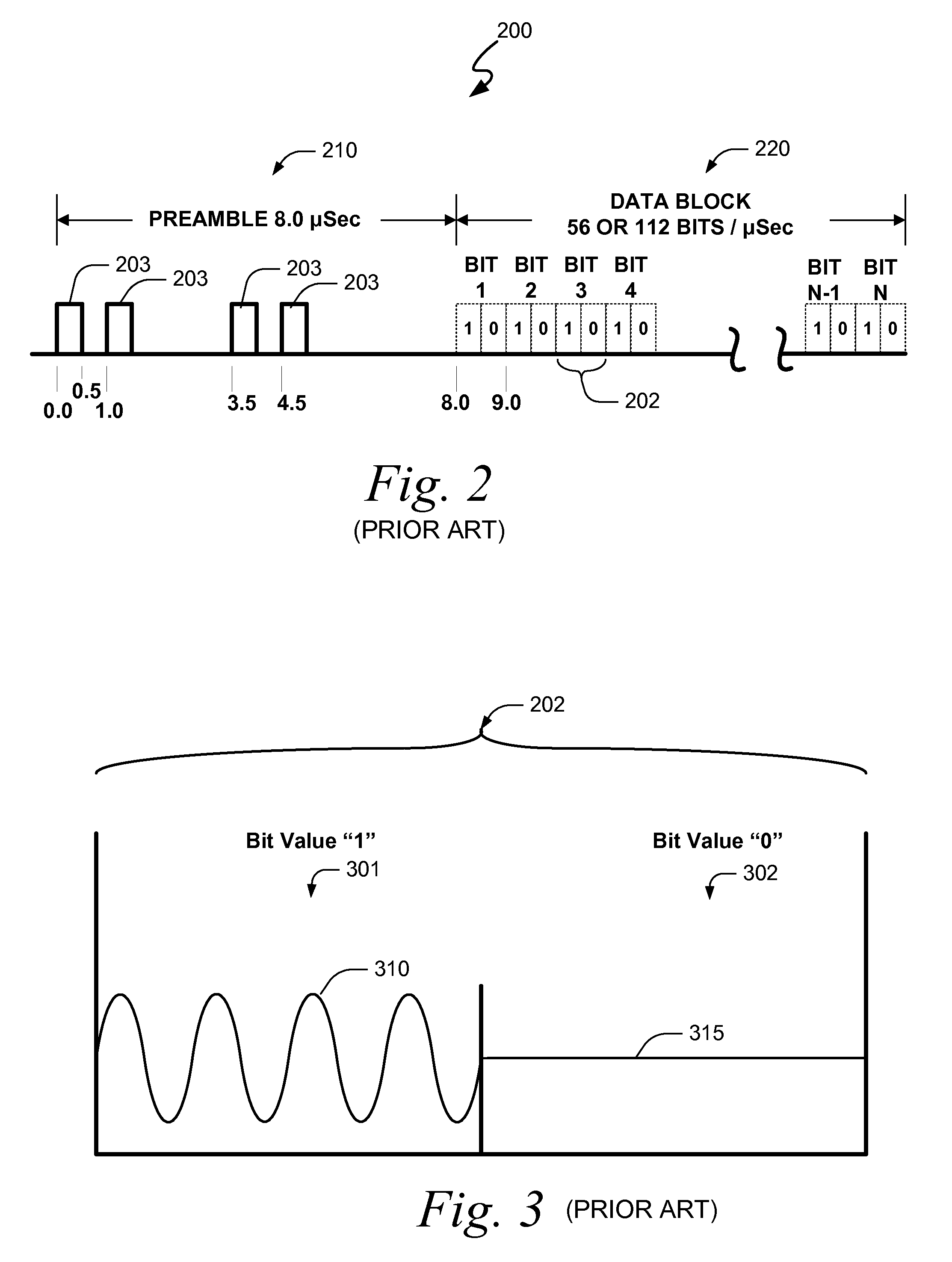
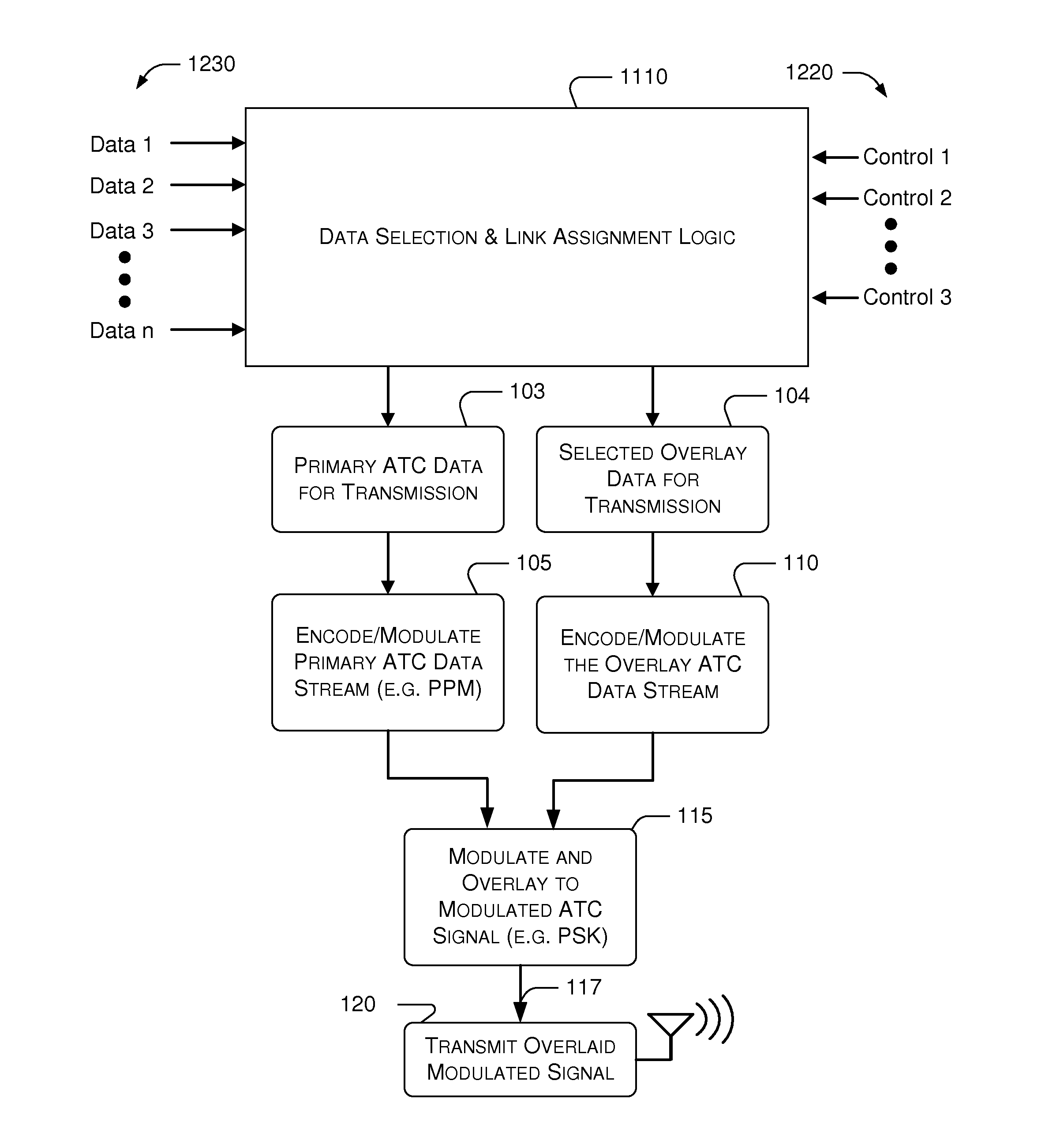
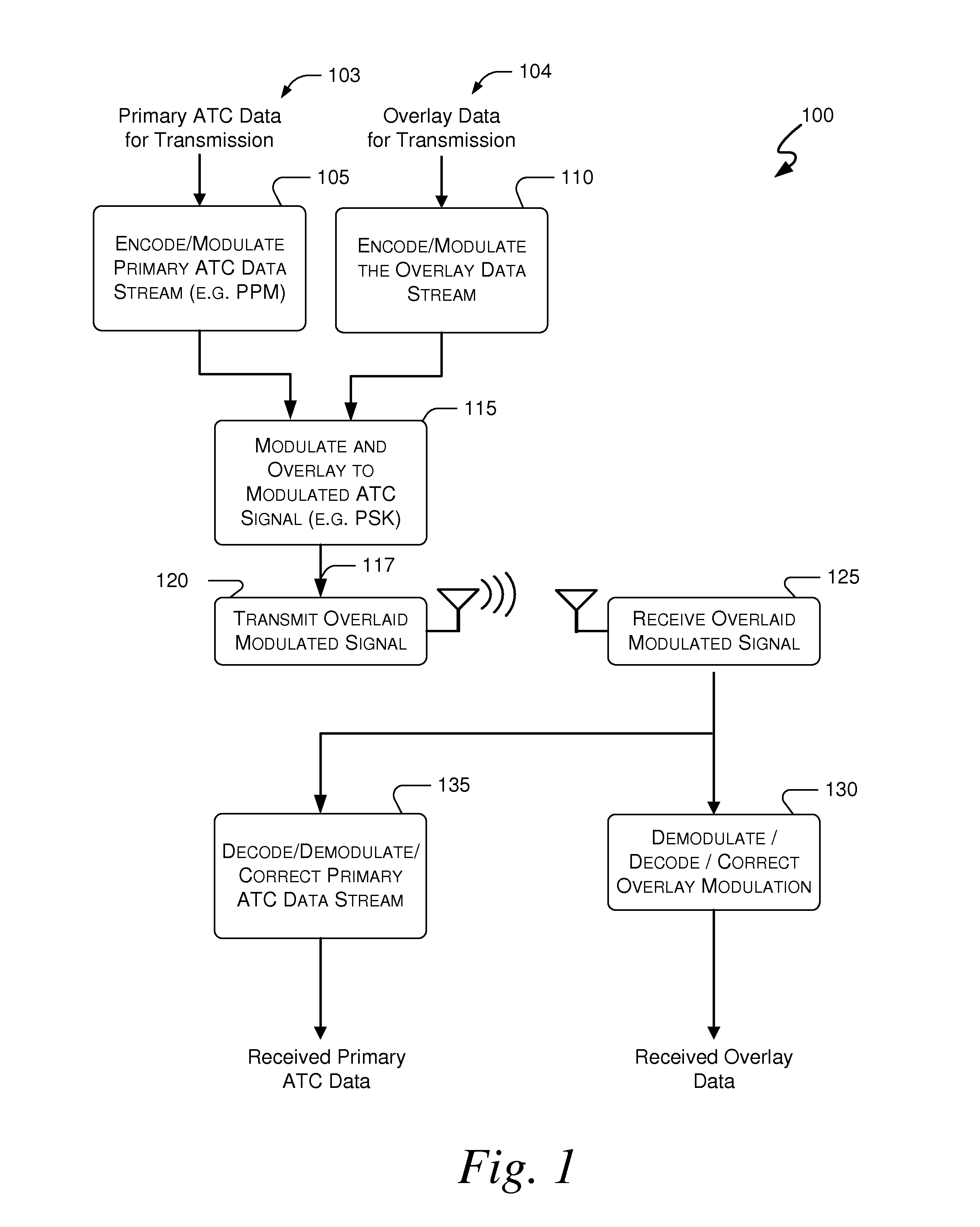
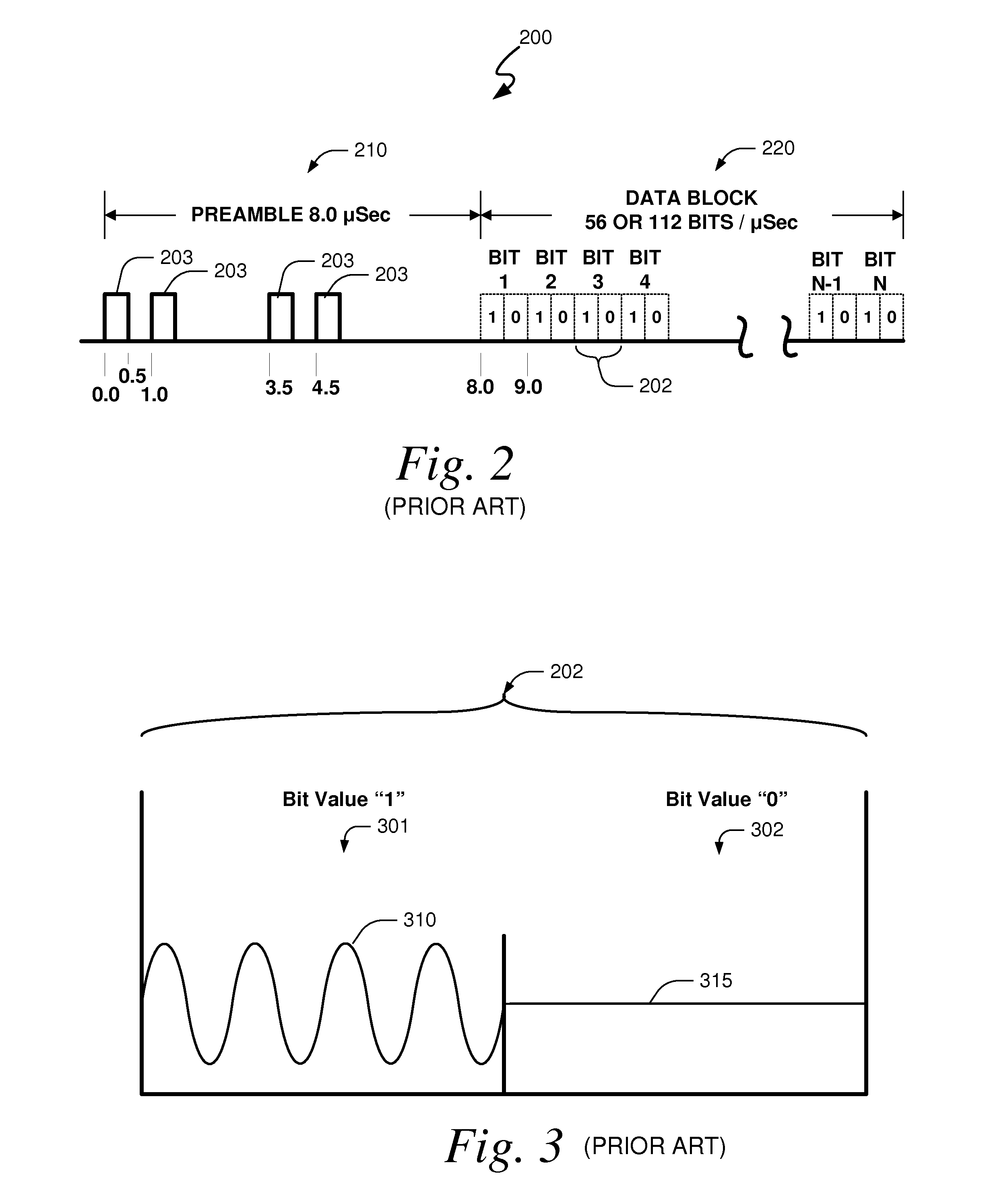
Embodiments of the present invention disclose systems and methods for providing an ATC Overlay data link. Through embodiments of the present invention, existing ATC (or other) modulated signals using existing standard frequencies may be utilized to transmit (e.g., from an aircraft transponder) additional information in a manner that does not render the transmitted signal unrecognizable by legacy ATC equipment. Legacy equipment will be able to demodulate and decode information that was encoded in the transmitted signal in accordance with preexisting standard modulation formats, and updated equipment can also extract the additional information that was overlaid on transmitted signals.
Owner:AVIATION COMM AMP SURVEILLANCE SYST LLC
Systems and methods for providing airborne aircraft weather reporting and supplemental occupant services
ActiveUS20100311354A1Reduce errorsOptimize noiseAmplitude-modulated carrier systemsFrequency-modulated carrier systemsTransceiverComputer science
An embodiment of the present invention delineates a method for relaying information between a first transceiver and a second provided transceiver. The method comprises generating a signal for transmission from the first transceiver to the second provided transceiver. The method also modulates the signal with a first data pattern, the first data pattern comprising aircraft state data. The method also modulates the signal with a second data pattern, the second data pattern comprising information other than aircraft state data. The method also transmits the signal including both the first data pattern and the second data pattern from the first transceiver to the second provided transceiver. Other related system and method embodiments are set forth.
Owner:AVIATION COMMUNIATION & SURVEILLANCE SYST
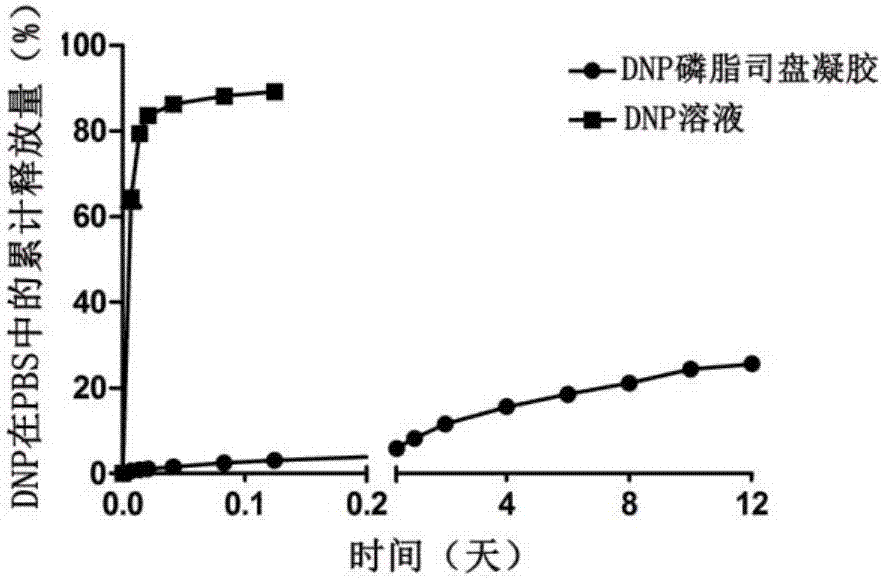
Small molecule drug in-situ phase-change gel sustained release system and preparation method thereof
ActiveCN107049932AGood slow releaseImprove liquidityHydroxy compound active ingredientsAerosol deliveryHypodermoclysisSubcutaneous injection
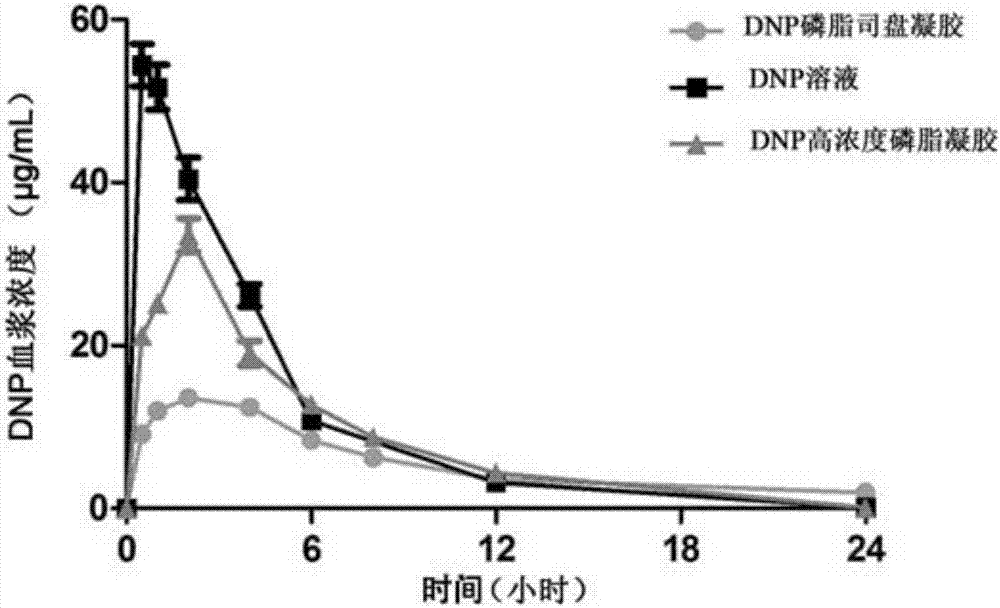
The invention provides a small molecule drug in-situ phase-change gel sustained release agent with phospholipid and span as matrices and a preparation method thereof. By the adoption of the simple method, the phospholipid and span sustained release agent is prepared from phospholipid, span, active pharmaceutical ingredients and ethanol solutions of different concentrations, and has the advantages that the biocompatibility is good, the adverse reaction is low, the burst-release and inhibition capability is high, and the releasing time is prolonged; the sustained release agent is suitable for various drug administration modes such as subcutaneous injection and external drug administration, the amount of carried drugs can be adjusted conveniently according to the clinical medication dosage of the drugs, and the sustained release agent and the preparation method thereof have broad application prospects.
Owner:SICHUAN UNIV +1
Aripiprazole sustained-release microspheres and preparation method thereof
ActiveCN105310997AImprove complianceGood treatment effectOrganic active ingredientsNervous disorderAcetic acidMicrosphere
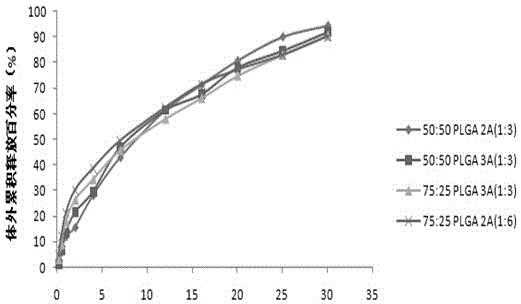
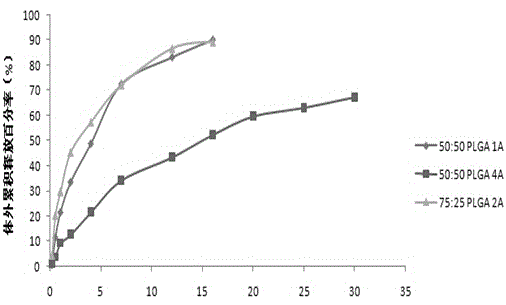
The invention relates to aripiprazole sustained-release microspheres and a preparation method thereof. The sustained-release microspheres include aripiprazole and a bio-degradable pharmaceutical high-molecular material PLGA, wherein the ratio of lactic acid to hydroxyacetic acid in the PLGA is 75:50-25:50. The PLGA is 25000-35000 Dolton in molecular weight. The addition weight ratio of the aripiprazole to the PLGA is 1:1-20. The aripiprazole accounts for 3.01-21.09% of total weight of the microsphere. The aripiprazole sustained-release microspheres have high drug embedding rate, is high in drug loading capacity, is smooth and round in surface and can release more than 90% of the drug in 30 days.
Owner:CHONGQING PHARMA RES INST
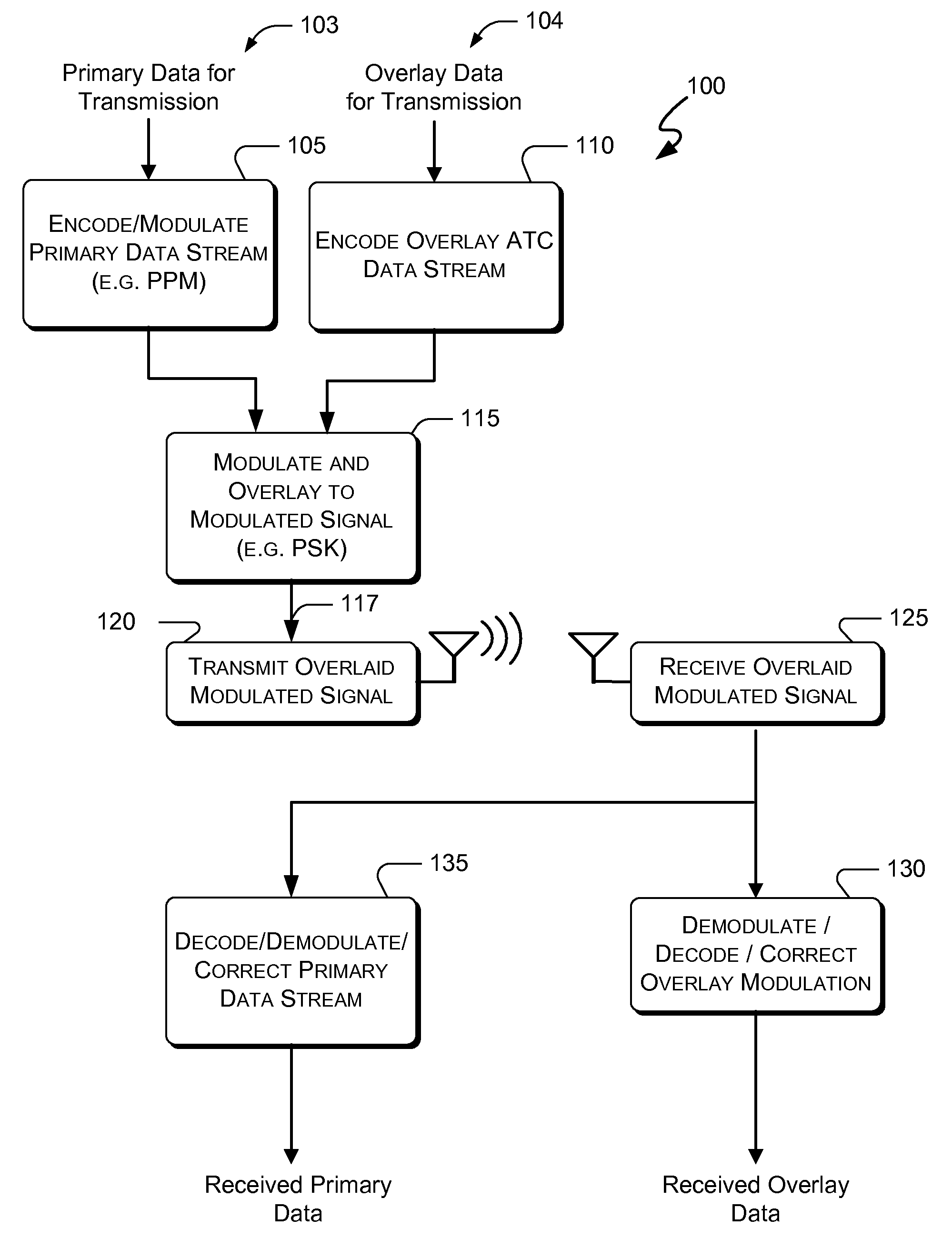
Systems and methods for enhanced atc overlay modulation
ActiveUS20100315282A1Reduce errorsOptimize noiseAmplitude-modulated carrier systemsFrequency-modulated carrier systemsComputer scienceData link
Embodiments of the present invention disclose systems and methods for providing an enhanced data link using overlaid modulation. Through embodiments of the present invention, existing ATC (or other) modulated signals using existing standard frequencies may be utilized to transmit (e.g., from an aircraft transponder) additional information in a manner that does not render the transmitted signal unrecognizable by legacy ATC equipment. Legacy equipment will be able to demodulate and decode information that was encoded in the transmitted signal in accordance with preexisting standard modulation formats, and updated equipment can also extract the additional information that was overlaid on transmitted signals.
Owner:AVIATION COMM AMP SURVEILLANCE SYST LLC
Systems and methods for enhanced ATC overlay modulation
ActiveUS8031105B2Reduce errorsOptimize noiseAmplitude-modulated carrier systemsFrequency-modulated carrier systemsComputer scienceData link
Embodiments of the present invention disclose systems and methods for providing an enhanced data link using overlaid modulation. Through embodiments of the present invention, existing ATC (or other) modulated signals using existing standard frequencies may be utilized to transmit (e.g., from an aircraft transponder) additional information in a manner that does not render the transmitted signal unrecognizable by legacy ATC equipment. Legacy equipment will be able to demodulate and decode information that was encoded in the transmitted signal in accordance with preexisting standard modulation formats, and updated equipment can also extract the additional information that was overlaid on transmitted signals.
Owner:AVIATION COMM AMP SURVEILLANCE SYST LLC
Thermosensitive biodegradable copolymer
ActiveUS7179867B2Reduce harm to tissuesSolve lowSurgical adhesivesPharmaceutical delivery mechanismBiodegradable copolymersChemistry
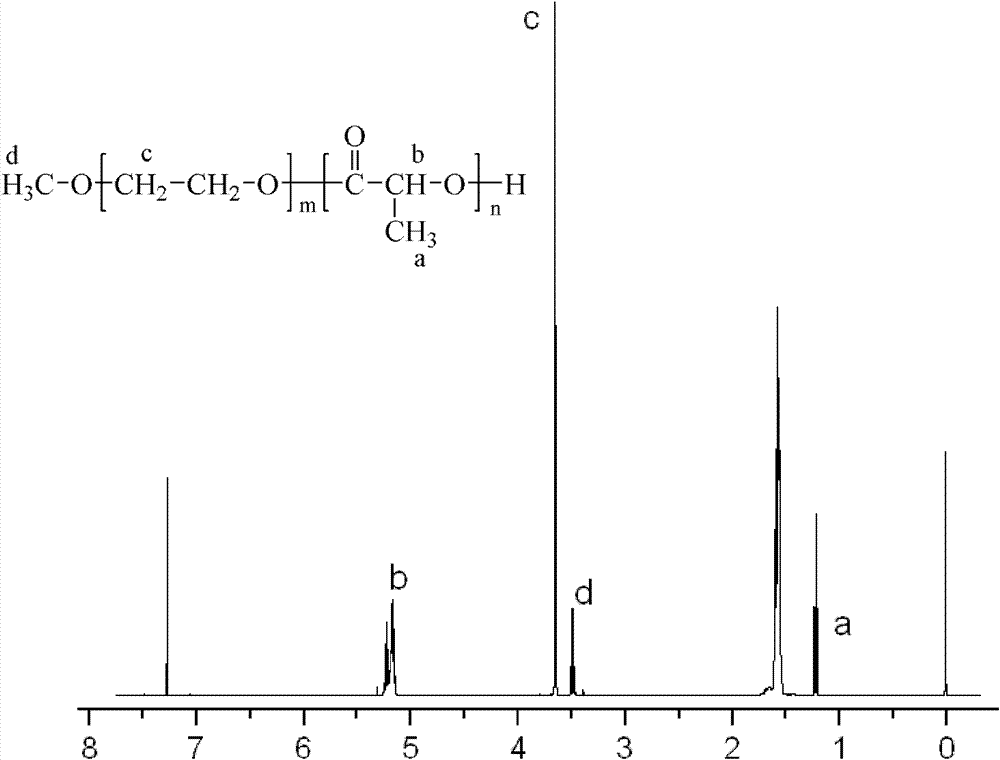
A thermo-sensitve copolymer of following formula;is disclosed; wherein R1 is hydrogen, or —C(═O)—R2; R2 is C7-30 alkyl substituted or unsubstituted with functional groups; R3 is hydrogen, or C1-6 alkyl; and x, y or z individually is an integer greater than 0. The thermo-sensitive copolymers disclosed here are easy to be implanted into a human body through injection. The biodegradability is greatly improved and the cytotoxicity of the copolymers is low.
Owner:IND TECH RES INST
Asiatic acid injectable sustained-release microballoons and preparation method thereof
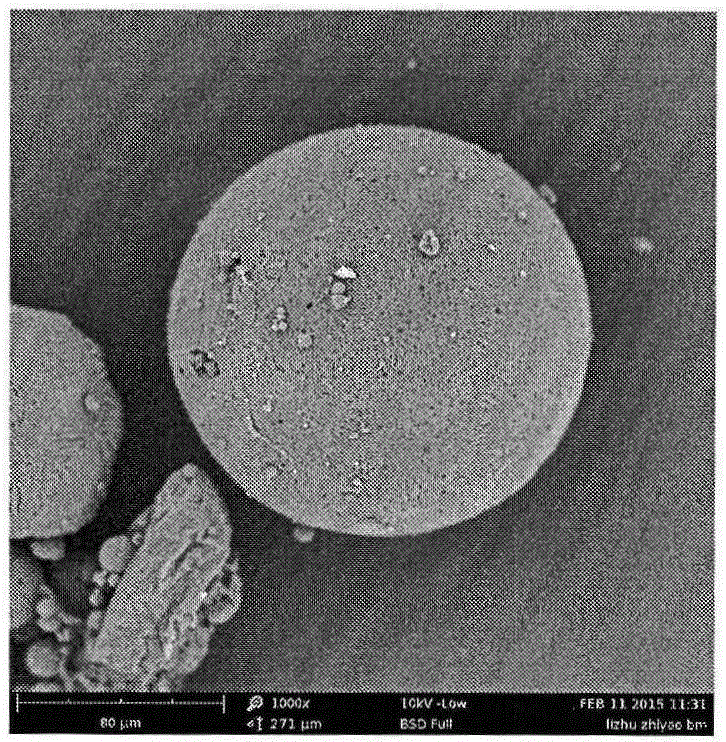
InactiveCN101474157ABiodegradableStable release rateOrganic active ingredientsPharmaceutical non-active ingredientsCross-linkBurst effect
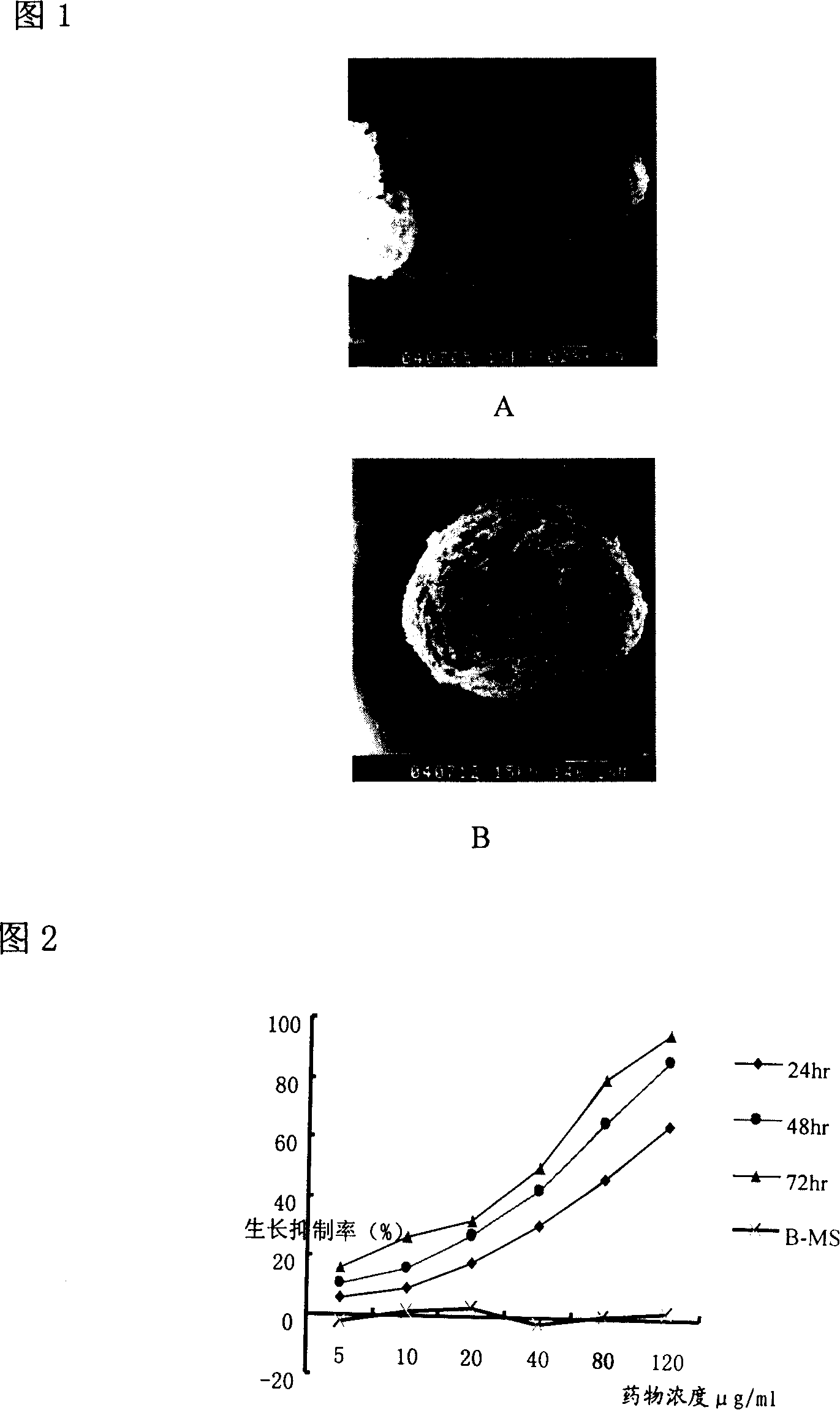
The invention relates to the technical field of pharmaceutical preparation, in particular to an asiatic acid sustained-release microsphere used for injection and a preparation method thereof. A biodegradable chitosan is taken as a carrier, an asiatic acid is taken as principal agent, and the asiatic acid sustained-release microsphere used for injection is obtained by the technology of cross-linked emulsion. Due to the obtained medicament carried microspheres, the surfaces are relatively smooth; the particle diameters range from 20mum to 110mum; and the medicament loading rate of the microspheres ranges from 4.8% to 15.6%. The asiatic acid microsphere has the characteristics that the burst effect is modest (the burst size on the first day is smaller than 15%); the release rate is stable; the sustained release time is long (4-10 weeks); and the asiatic acid microsphere is biodegradable. The implantation and the removal by operation before and after the medicament is used are avoided.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
System and Method For Mitigating Burst Noise In A Communications System
ActiveUS20090327845A1Avoid packet lossImprove robustnessError prevention/detection by using return channelTransmission systemsCommunications systemBlock code
Owner:AVAGO TECH INT SALES PTE LTD
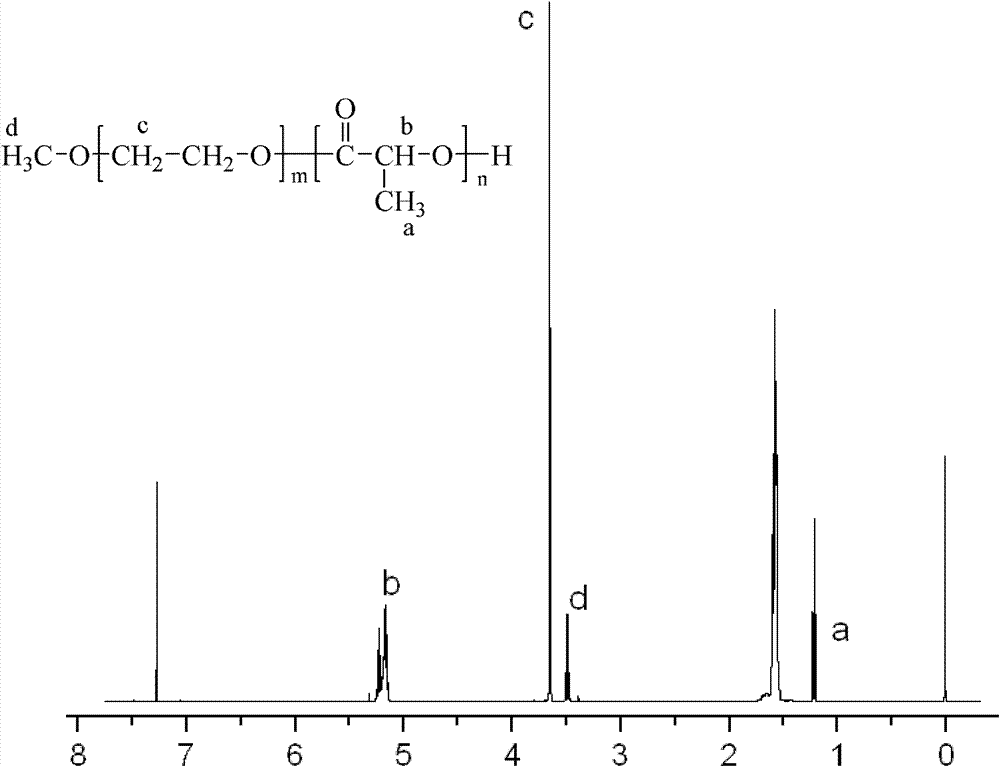
Microsphere preparation encapsulating hydrophilic medicine and preparation method thereof
ActiveCN102266294BAvoid sudden releaseImprove hydrophilicityPharmaceutical non-active ingredientsGranular deliveryParaffin waxMicrosphere
The invention discloses a microsphere preparation encapsulating a hydrophilic medicine, and the microsphere preparation comprises the following components in percentage by weight: a hydrophilic medicine 0.1-40%, methoxy poly(ethylene glycol)-poly(lactic acid) block copolymers 0.1-99%, and poly(lactic acid) or poly(lactic acid-glycollic acid) copolymers 0.1-99%. The microsphere preparation has round and intact surface, uniform and controllable particle diameter, high encapsulation rate, and controllable drug release behaviors. The invention also discloses a preparation method of the microsphere preparation encapsulating the hydrophilic medicine, and the preparation method has simple process and good controllability and comprises the following steps: adding a water solution of the hydrophilic medicine into an organic solution of the methoxy poly(ethylene glycol)-poly(lactic acid) block copolymers, and vortexing to form water-in-oil type primary emulsion; adding the primary emulsion intoliquid paraffin containing emulsifier I, and vortexing to form water-in-oil type multiple emulsion; and adding the multiple emulsion into liquid paraffin containing emulsifier II, continuing stirringfor 4-24 h, centrifuging, collecting pellets, washing, and drying to obtain the microsphere preparation encapsulating the hydrophilic medicine.
Owner:ZHEJIANG UNIV
System, method and computer program product for mitigating burst noise in a communications system
ActiveUS7631242B2Reduce the impactIncrease robustness of communicationError prevention/detection by using return channelCode conversionCommunications systemBlock code
A system, method and computer program product is provided for mitigating the effects of burst noise on packets transmitted in a communications system. A transmitting device applies an outer code, which may include, for example, a block code, an exclusive OR (XOR) code, or a repetition code, to one or more packets prior to adaptation of the packets for transmission over the physical (PHY) layer of the communications system, wherein the PHY layer adaptation may include FEC encoding of individual packets. The outer coded packets are then separately transmitted over a channel of the communications system. A receiving device receives the outer coded packets, performs PHY level demodulation and optional FEC decoding of the packets, and then applies outer code decoding to the outer coded packets in order to restore packets that were erased during transmission due to burst noise or other impairments on the channel.
Owner:AVAGO TECH INT SALES PTE LTD
Iloperidone sustained release microsphere and preparation method thereof
InactiveCN103599074AHigh encapsulation efficiencyHigh drug loadingOrganic active ingredientsNervous disorderMicrosphereGlycolic acid
The invention relates to an iloperidone sustained release microsphere and a preparation method thereof. The sustained release microsphere mainly comprises iloperidone and a biodegradable pharmaceutical polymer material PLGA (poly lactic-co-glycolic acid), wherein the molar ratio of lactic acid of PLGA to glycolic acid is (75-50):(25-50), the dosing weight ratio of the iloperidone to the PLGA is 1:(1-10), and the iloperidone is 3.5-15.5% of the total weight of the microsphere. According to the prepared iloperidone sustained release microsphere, the medicine encapsulation efficiency is high, the drug loading capacity is high, and the surface of the microsphere is smooth and round. The sustained release microsphere is used for curing psychosis, can prolong the action time of the medicine, reduces the dosing times, and greatly improves the obedience of patients in taking medicine.
Owner:CHONGQING PHARMA RES INST
Biological adhesive microsphere with nuclear shell structure and preparation method of microsphere
InactiveCN104069228AHigh drug loadingReduce lossesDigestive systemPharmaceutical non-active ingredientsOral medicineSmart hydrogels
The invention relates to a biological adhesive microsphere with a nuclear shell double-layer structure and a preparation method of the microsphere. By taking Zujin pill total alkaloids as a model medicine, taking natural high-molecular polymers such as chitosan, sodium alga acid and gelatin as carrier materials, an intelligent hydrogel medicine release system with biological adhesiveness is constructed. Due to the biological adhesive microsphere with the nuclear shell structure, detention time of the medicine in stomach is prolonged, medicine absorption is increased, and the biological availability of oral medicine for treating gastrohelcosis is improved. The biological adhesive microsphere is a reasonable and effective novel dosage form of gastrohelcosis-resisting medicine developed by using ancient prescription into a modern research method. The invention further illuminates in-vitro medicine release performance of the microsphere and in-vitro biological adhesion effect of the microsphere.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Preparation method of triptorelin acetate sustained-release microsphere
ActiveCN105169366ASmall burst effectSlow release curvePowder deliveryPeptide/protein ingredientsFreeze-dryingTriptorelin Acetate
The invention relates to a preparation method of triptorelin acetate sustained-release microsphere. The preparation method comprises the following steps: (1) adding water into triptorelin acetate to prepare a solution A, and adding PLGA into an organic solvent to prepare a solution B; (2) mixing the solution A and solution B, subjecting the mixed solution to an ultrasonic treatment to form primary emulsion, adding the primary emulsion into a PVA water solution, which has been saturated by an organic mixed solvent, and homogenizing and emulsifying the solution to obtain multiple emulsion; (3) stirring the multiple emulsion at a room temperature for 1 hour, then heating the multiple emulsion to a temperature of 40 to 45 DEG C, maintaining the temperature for 1 hours, cooling to 10 DEG C, sieving, collecting the particles, and freeze-drying the particles. The provided technology can solve the problem that the adverse reactions are enhanced by the burst release of triptorelin acetate, moreover, the blood concentration of the prepared microsphere is very smooth and stable, and thus the sustained-release microsphere is suitable for long-term drug administration.
Owner:LIVZON PHARM GRP INC
Systems and Methods for Providing ADS-B Mode Control Through Data Overlay
ActiveUS20150338503A1Quantity maximizationReduce radio frequency interferenceAmplitude-modulated carrier systemsFrequency-modulated carrier systemsInput selectionMode control
Embodiments of the present invention disclose systems and methods for providing enhanced features using an ATC Overlay data link. Further, there are provided systems and methods for ADS-B Mode Control that enable a transponder to selectively transmit data on a desired link, such as the ADS-B or ATC Overlay link, based on the control inputs such as current active ADS-B applications, thus reducing RF interference while maximizing the amount of pertinent data being transmitted. In various embodiments, ADS-B Mode Control also offers a mechanism to include pilot-entered data onto the ADS-B or ATC Overlay link, thereby producing flexibility for future ADS-B In applications.
Owner:AVIATION COMM AMP SURVEILLANCE SYST LLC
Ligustrazine microcosmic salt liposome medicine and preparing method
InactiveCN101176720ASmall doseGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsAdditive ingredientPhosphate
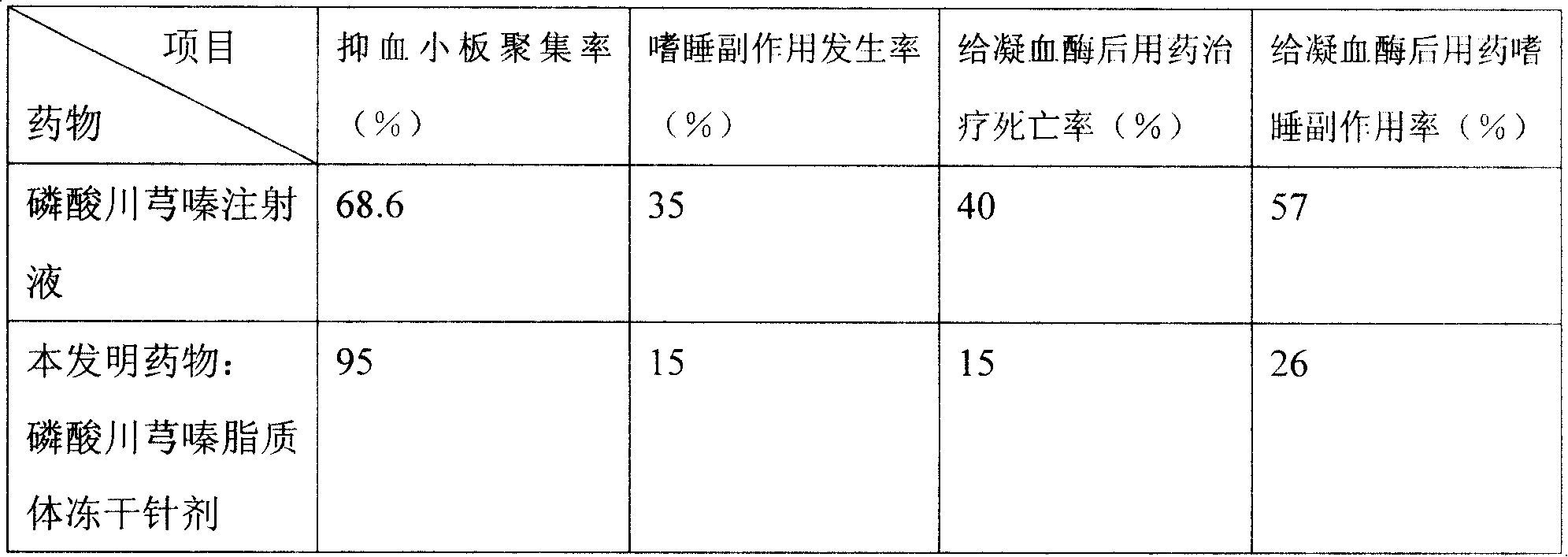
The invention relates to a tetramethylpyrazine phosphate liposome drug and a preparation method; wherein, the drug is characterized in that: the effective efficacy components and weight accounts of the liposome drug are as following: a mixture 200 to330 with a weight ratio 4 to 1 of egg yolk lecithin and sheep cerebral lecithin, a mixture 170 to 280 with a weight ratio 3 to 1of stigmasterol and campestrol, reduction glutathione 2.5 to 10, tetramethylpyrazine phosphate 15 to 25, a mixture 720 to 1200 with a weight ratio 1 : 0.01 to 0.05 : 0.02 to 0.07 of tert-butyl alcohol, ethanol, acetone, a mixture 140 to 160 with a weight ratio 4:05 to 1 of polyethylene glycol-2000 and tween 801, and hydroxyl propyl methyl cellulose 100 to 120. The invention also provides the preparation method for the liposome drugs. The invention has the advantages of adopting the provided drugs, enabling to decrease the drug dosage by two third and once a day, and improving the efficacy above fifteen percent.
Owner:HUNAN KANGDU PHARMA
Systems and methods for providing an advanced ATC data link
ActiveUS8344936B2Reduce errorsOptimize noiseError preventionAmplitude-modulated carrier systemsAviationEngineering
Embodiments of the present invention disclose systems and methods for providing an avionics overlay data link. Through embodiments of the present invention, existing ATC (or other) modulated signals using existing frequencies (or other frequencies) may be utilized to transmit (e.g., from an aircraft transponder) additional information in a manner that does not render the transmitted signal unrecognizable by legacy ATC equipment. In various embodiments, legacy equipment may demodulate and decode information that was encoded in the transmitted signal in accordance with preexisting standard modulation formats, and updated equipment can also extract the additional information that was overlaid on transmitted signals.
Owner:AVIATION COMM AMP SURVEILLANCE SYST LLC
Long-acting cefotaxime sodium injection and preparation method thereof
ActiveCN104644547AImprove efficacyStable blood concentrationAntibacterial agentsOrganic active ingredientsRoom temperatureChitosan succinate
The invention relates to a long-acting cefotaxime sodium injection and a preparation method of the injection. The injection comprises a biodegradable polymer material, wherein the biodegradable polymer material comprises an injectable hydrogel. The preparation method comprises the following steps: mixing the medicines and N-chitosan succinate solution at a certain temperature and stirring rate, and mixing the mixture with carboxymethyl chitosan solution; adding oxidized chondroitin sulfate solution and glutaraldehyde solution in a certain time while stirring, injecting the solution into a circular mold with the bottom diameter of 10mm after stirring, forming drug-loading hydrogel, and performing vacuum drying to constant weight at room temperature.
Owner:BEIJING RED SUN PHARMA
Preparation method of acetic acid goserelin microspheres
ActiveCN104840429AConcentration is smooth and stableOvercoming the problem of increased adverse reactionsPeptide/protein ingredientsPharmaceutical non-active ingredientsDrugs solutionMicrosphere
The invention provides a preparation method of acetic acid goserelin microspheres. The preparation method includes: step 1), mixing acetic acid goserelin with water to obtain a drug solution A, and mixing PLGA (polylactic-co-glycolic acid) with an organic solution to obtain a solution B; step 2), mixing the solution A with the solution B to form colostrum through ultrasound, and adding the colostrum to a PVA (polyvinyl acetate) water solution saturated by an organic mixed solution to obtain a compound emulsion through homogeneous emulsification; step 3), stirring the compound emulsion at the room temperature for one hour, increasing the temperature to 40-45 DEG C, keeping the compound emulsion for one hour, reducing the temperature to 10 DEG C, sieving the compound emulsion to collect particles and subjecting the particles to freeze-frying. The preparation method has the advantages that the problem of adverse drug reaction increase caused by high sudden release effect of the acetic acid goserelin microspheres can be solved, and the microspheres prepared by the method have quite smooth and stable blood concentration and are beneficial to long-term medication.
Owner:LIVZON PHARM GRP INC
Preparation method of drug-carrying liposome
ActiveCN102188379AReduce leakageHigh encapsulation efficiencyPharmaceutical non-active ingredientsLiposomal deliveryFreeze-dryingWater soluble
The invention relates to a preparation method of drug-carrying liposome. In the method, a two-step freeze-drying method is used for preparing the liposome for coating and carrying water soluble drugs, wherein a primary freeze drying process is performed in a water phase system, and a secondary freeze drying process is performed in an organic phase system. The water soluble drugs are evenly coated and carried in the liposome by the two-step freeze drying method, thus improving the envelopment rate of the drugs and reducing the initial burst release effect of the liposome for coating and carrying the water soluble drugs. The liposome prepared by the preparation method is suitable for coating various kinds of water soluble drugs and has a wide range of applicable dosage forms.
Owner:ZHEJIANG HISUN PHARMA CO LTD
A kind of preparation method of triptorelin acetate sustained-release microspheres
ActiveCN105169366BSmall burst effectSlow release curvePowder deliveryPeptide/protein ingredientsDrugs solutionMicrosphere
The invention relates to a preparation method of triptorelin acetate sustained-release microspheres, comprising the following steps: step 1) adding water to triptorelin acetate to form a drug solution A; adding PLGA to an organic solvent to form a solution B; step 2) mixing Solution A and solution B are mixed and ultrasonicated to form colostrum, and the colostrum is added to a PVA aqueous solution saturated with an organic mixed solvent, and homogeneously emulsified to obtain double emulsion; Step 3) Stir the double emulsion at room temperature for 1 hour and then heat up to 40°C-45°C The temperature was kept at ℃ for 1 hour, and then the temperature was lowered to 10 ℃, and the particles were collected by sieving and freeze-dried. On the one hand, the technology involved in the present invention can overcome the problem of increased adverse drug reactions caused by the sudden release of triptorelin acetate microspheres; on the other hand, the blood drug concentration of the prepared microspheres is very smooth and stable, and is suitable for long-term administration treat.
Owner:LIVZON PHARM GRP INC
Protein polypeptide medicine duplex microsphere, preparation method of protein polypeptide medicine duplex microsphere, and insulin duplex microsphere
ActiveCN109568601ATight textureControlled burstPowder deliveryPeptide/protein ingredientsGraft reactionMicrosphere
The invention provides a protein polypeptide medicine duplex microsphere and a preparation method thereof. The method comprises the following steps of S1, performing medicine loading on protein polypeptide medicine and chitosan in weak acidic solvents; obtaining a nanometer particle solution; sequentially performing film filtering and reduced pressure drying to obtain the medicine coated chitosannanometer particles; S2, under the effect of coupling agents, performing grafting reaction on the medicine coated chitosan nanometer particles and polylactic acid to obtain the protein polypeptide medicine duplex microsphere. The invention also provides an insulin duplex microsphere, which is prepared by the method. The insulin duplex microsphere has the advantages that the use is safer; the patient tolerance is better; the effect is longer; the medicine effect is more stable; the sudden release is obviously reduced.
Owner:JILIN HUISHENG BIOPHARMACEUTICAL CO LTD
Potassium dehydroandrographolide succinate/potassium dehydroandrographolide succinate liposome composition and production method thereof
InactiveCN1907266AEliminate adverse reactionsPrevent spoilageOrganic active ingredientsAntiviralsPalmitoylcholinePotassium
The invention discloses a potassium dehydroandrographolide succinate liposome combined drug and preparing method, which comprises the following parts: 1100-3500g phosphatidyl ethanolamine, dikitool bursine, egg lecithin at 1:1:2-6, 1000-3200g campesterol and cholesterol at 1:1-4; 30-50g vitamin E, 50-80g carbowax 2000 and carbowax 6000 at random proportion, 100-160g glycine and clockwise sugar 40 at 1: 1-3, 300-1000g potassium dehydroandrographolide succinate liposome.
Owner:刘祥华 +1
Systems and methods for providing ADS-B mode control through data overlay
ActiveUS9465097B2Quantity maximizationReduce radio frequency interferenceAmplitude-modulated carrier systemsFrequency-modulated carrier systemsMode controlComputer science
Embodiments of the present invention disclose systems and methods for providing enhanced features using an ATC Overlay data link. Further, there are provided systems and methods for ADS-B Mode Control that enable a transponder to selectively transmit data on a desired link, such as the ADS-B or ATC Overlay link, based on the control inputs such as current active ADS-B applications, thus reducing RF interference while maximizing the amount of pertinent data being transmitted. In various embodiments, ADS-B Mode Control also offers a mechanism to include pilot-entered data onto the ADS-B or ATC Overlay link, thereby producing flexibility for future ADS-B In applications.
Owner:AVIATION COMM AMP SURVEILLANCE SYST LLC
Tumour interposition suppository norcantharidin-alginic acid/poly-acid anhydride control-release microsphere
InactiveCN101108262ARelease stabilitySimple preparation processSurgeryEmbolization AgentTumor vessel
The invention belongs to the pharmaceutical preparation filed and relates to a tumor interventional embolization agent, which takes norcantharidin as the active ingredient and biodegreadable material aliginate and polyanhydride as the accessories to establish emulsion-chemical crossline preparation method and prepare norcantharidin-alginic acid / poly acid anhydride micro-spheres. The norcantharidin micro spheres are applied for tumor interventional treatment through the hepatic artery. It can keep embolizing the tumor as long as one month and cause the tumor necrosis. At the same time, the microspheres can targetingly disperse in the tumor tissues to release the medicine slowly, prolong the active time of the medicine to the tumor tissues, increase partial medicine density and decrease the tumor toxicity. The invention has the advantages of targeting dispersed microspheres in the tumor tissues, long time for embolization, slow drug release, high anti-cancer effect and security.
Owner:SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE PUTUO DISTRICT CENT HOSPITAL
Method for preparing microsphere preparation coated with hydrophilic medicaments
ActiveCN102302455BAvoid sudden releaseImprove hydrophilicityPharmaceutical non-active ingredientsGranular deliveryMicrosphereCentrifugation
The invention discloses a method for preparing microsphere preparation coated with hydrophilic medicaments. The method has simple process and good controllability, and comprises: adding hydrophilic medicament water solution into an organic solution containing a polylactic acid-polyethylene glycol monomethyl ether segmented copolymer, and carrying out vortex to form water-in-oil initial emulsion; adding the initial emulsion into liquid paraffin containing an emulsifying agent I, and carrying out the vortex to form water-in-oil compound emulsion; and adding the compound emulsion into petroleum ether containing an emulsifying agent II, continuing stirring for 10 minutes-60 minutes, collecting microspheres through centrifugation, washing and drying to obtain the microsphere preparation coated with the hydrophilic medicaments.
Owner:ZHEJIANG UNIV
Method for preparing medicine-carrying hydroxyapatite/poly glycolide-co-lactide (PLGA)/chitosan demixing microspheres
ActiveCN102579361AHigh encapsulation efficiencyLong release timePharmaceutical non-active ingredientsGranular deliveryAcetic acidPolyvinyl alcohol
The invention discloses a method for preparing medicine-carrying hydroxyapatite / poly glycolide-co-lactide (PLGA) / chitosan demixing microspheres. The method comprises the following steps of: dissolving isoniazide in deionized water, adding hydroxyapatite powder, stirring in a dark place, and freeze-drying to obtain powder; mixing PLGA and the powder uniformly to obtain a hydroxyapatite / PLGA commixed solution containing the isoniazide; dissolving chitosan in an acetic acid aqueous solution to obtain a chitosan solution; mixing the chitosan solution and a polyvinyl alcohol aqueous solution to obtain a chitosan / polyvinyl alcohol solution; and pouring the hydroxyapatite / PLGA commixed solution containing the isoniazide into the chitosan / polyvinyl alcohol solution, stirring under vacuum, washingby using water, and freeze-drying to obtain the medicine-carrying hydroxyapatite / PLGA / chitosan demixing microspheres. The prepared medicine-carrying composite microspheres are regular in spherical shapes, uniform in particle size distribution, high in envelop rate of medicines, long in in-vitro medicine release time and small in burst release; and a preparation process is simple, raw materials are readily available, and industrialization is easy to realize.
Owner:广州智园生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com