Protein polypeptide medicine duplex microsphere, preparation method of protein polypeptide medicine duplex microsphere, and insulin duplex microsphere
A technology of protein polypeptide and insulin, which is applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of lower encapsulation rate, obvious burst release effect, toxic and side effects, etc., achieve compact texture of microspheres, reduce burst release effect, The effect of uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
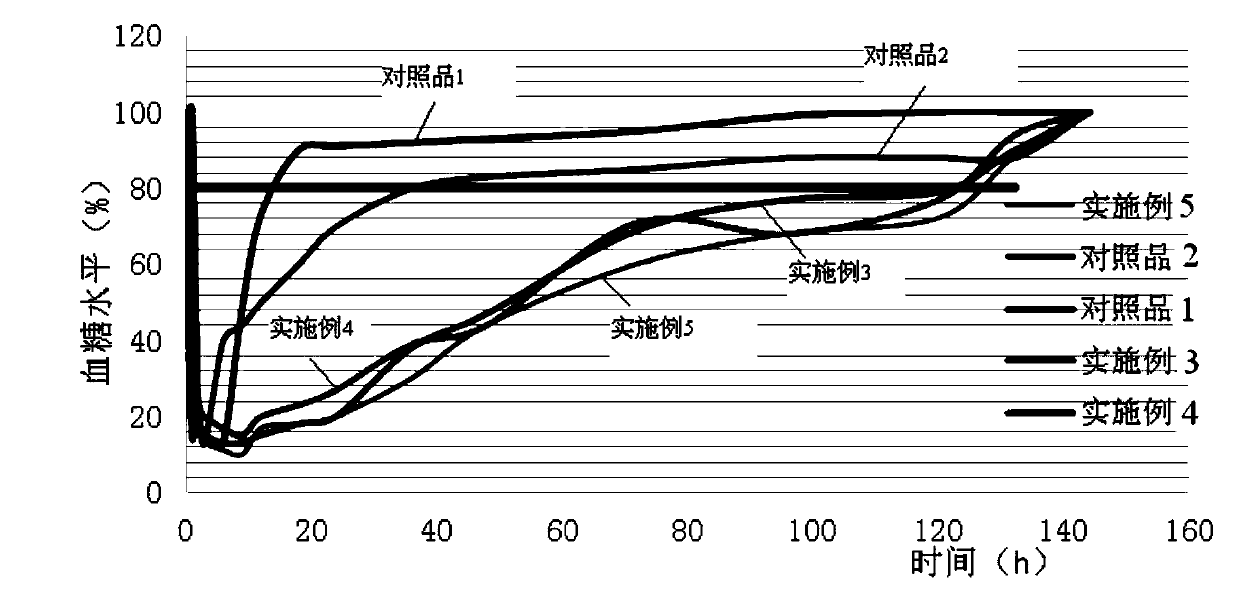
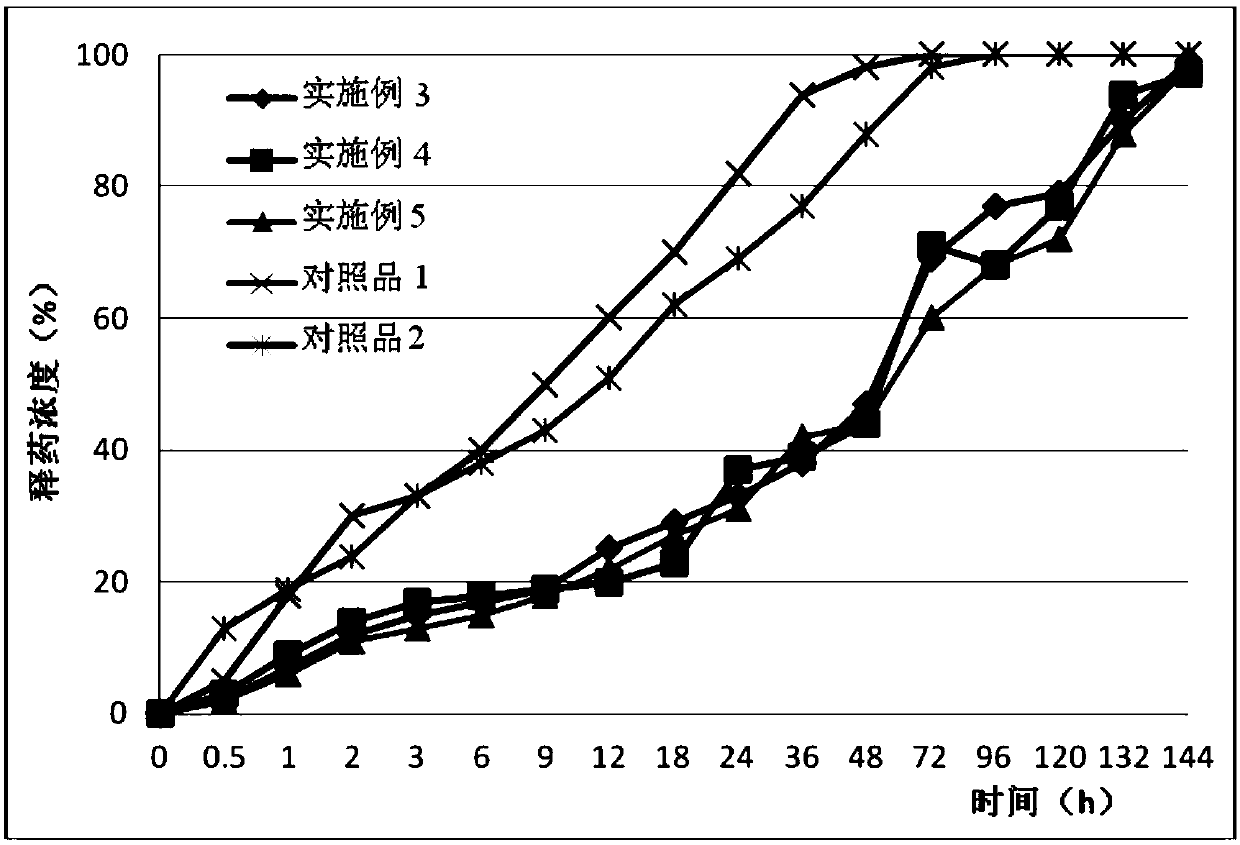
Examples
Embodiment 1
[0041] 1. Dissolve 0.01g of recombinant human insulin raw material and 20.00g of chitosan (CS) successively in 150mL of acetic acid solution with a pH of 5.0, and stir for 0.5h; Use water for injection as the exchange liquid to treat the above mixture for 4 cycles, discard the permeate each time, collect the reflux liquid, and then process the collected reflux liquid with a 30KD molecular weight membrane filtration system to collect nanospheres that meet the predetermined molecular weight Permeate; Finally, the collected permeate was centrifuged for 30min, the supernatant was discarded, the resulting precipitate was poured into a plate, and dried under reduced pressure at 4°C for 4h to obtain insulin-chitosan ( CS) nanoparticle solid powder.
[0042] 2. Dissolve 20.00g of polylactic acid with a molecular weight of 10000Da in acetonitrile, then add this solution to 150mL of acetate buffer solution with a pH of 5.0 and stir evenly, add 20mL to the buffer system (according to the...
Embodiment 2
[0048] 1. Dissolve 1.00g insulin aspart raw material drug and 20.00g chitosan (CS) successively in 150mL acetic acid solution with pH 5.0, and stir for 8h; The above mixture was treated for 6 cycles, and the permeate was discarded each time, and the reflux was collected, and then the collected reflux was treated with a membrane filtration system with a molecular weight of 30KD, and the nanosphere permeate that met the predetermined molecular weight was collected; finally, the The collected permeate was centrifuged for 30 minutes, the supernatant was discarded, the obtained precipitate was poured into a plate, and dried under reduced pressure at 4° C. for 10 hours to obtain insulin-chitosan (CS) nanoparticle solid powder.
[0049] 2. Dissolve 10.00 g of polylactic acid with a molecular weight of 10,000 Da in acetonitrile, then add this solution to 150 mL of acetate buffer solution with a pH of 5.0 and stir evenly, and add 3.4 mL (according to the density of 1.0×10 3 kg / m 3 Cal...
Embodiment 3
[0054] 1. Preparation of insulin degludec microspheres: Dissolve 1.00 g of insulin degludec raw material in 0.1 mol / L hydrochloric acid, stir until completely dissolved, and use a high-pressure homogenizer to repeatedly homogenize the obtained insulin solution to a nanometer After the particle size distribution of about 80% or more is 100nm as measured by a laser particle size analyzer, the insulin degludec microsphere powder is obtained by freeze-drying.
[0055]2. Dissolve 1.00 g of the insulin degludec microspheres and 20.00 g of chitosan (CS) successively in 150 mL of acetic acid solution with a pH of 5.0, and stir for 12 hours; first use a membrane filtration system with a molecular weight of 10KD, and use water for injection as an exchange The above mixture was treated with liquid for 8 cycles, the permeate was discarded each time, and the reflux liquid was collected, and then the collected reflux liquid was treated with a membrane filtration system with a molecular weigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com