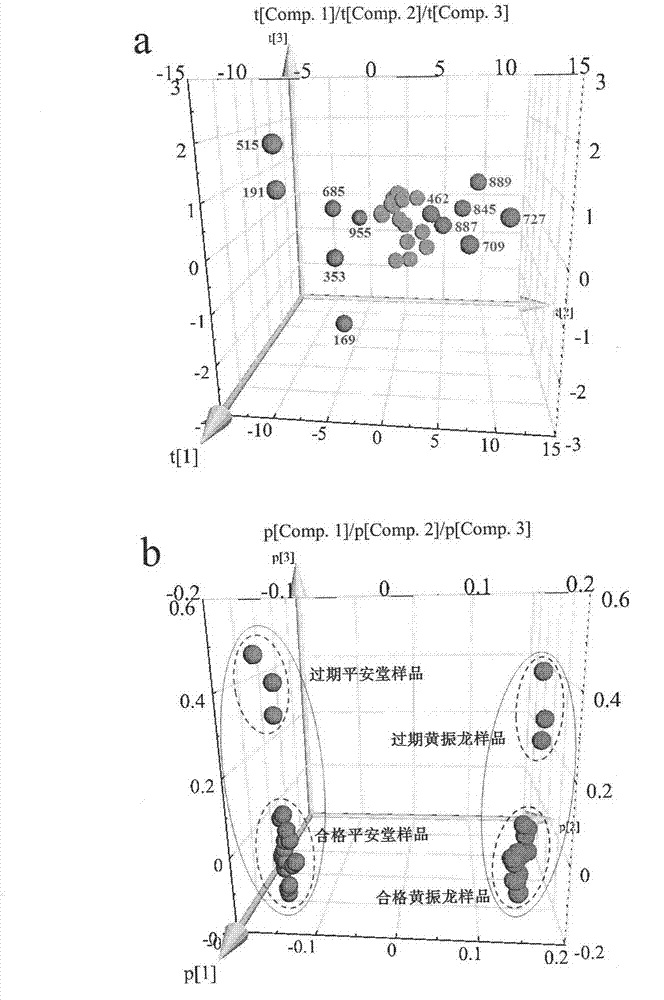
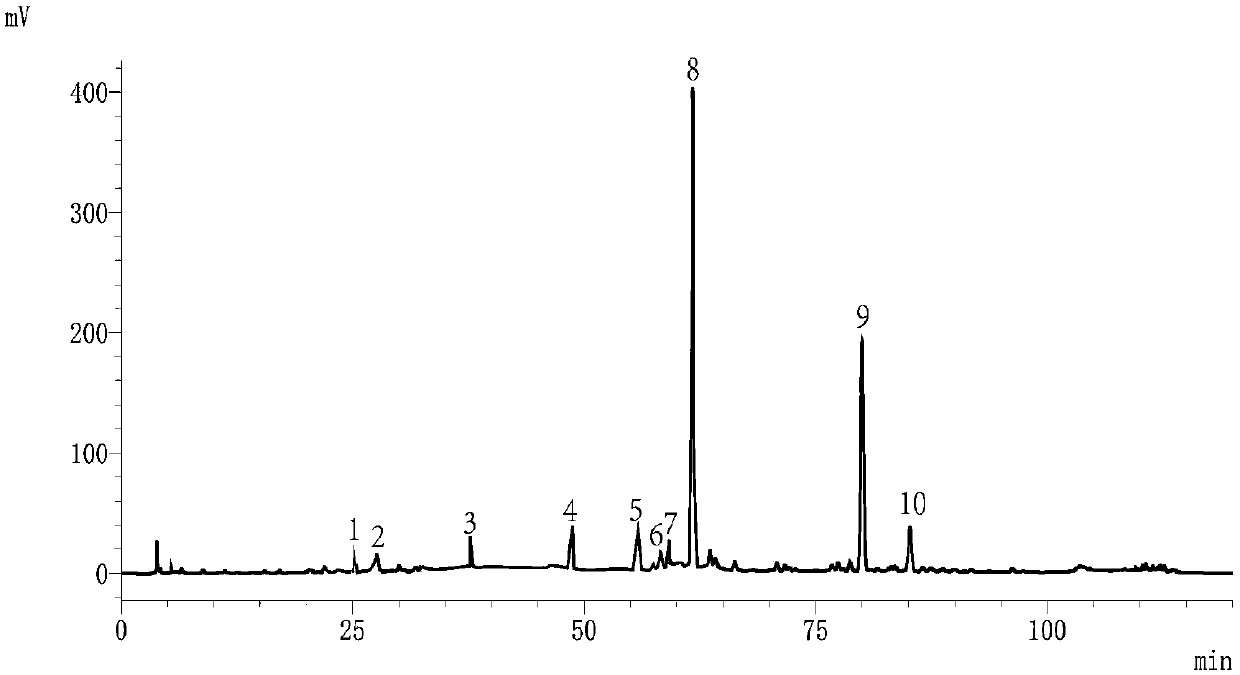
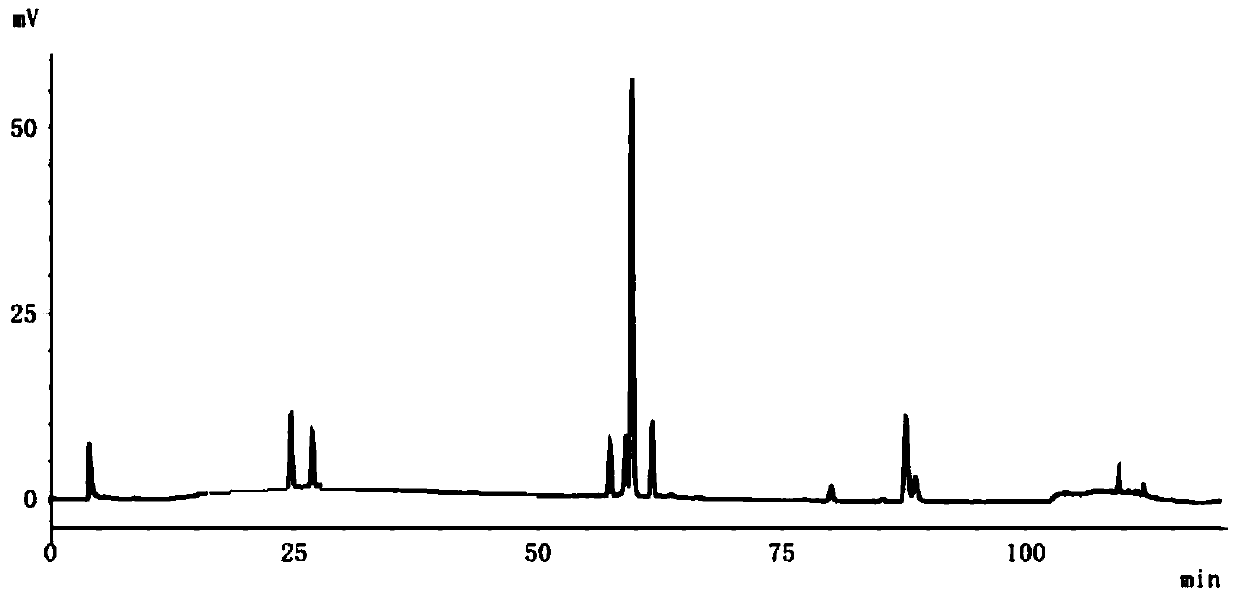
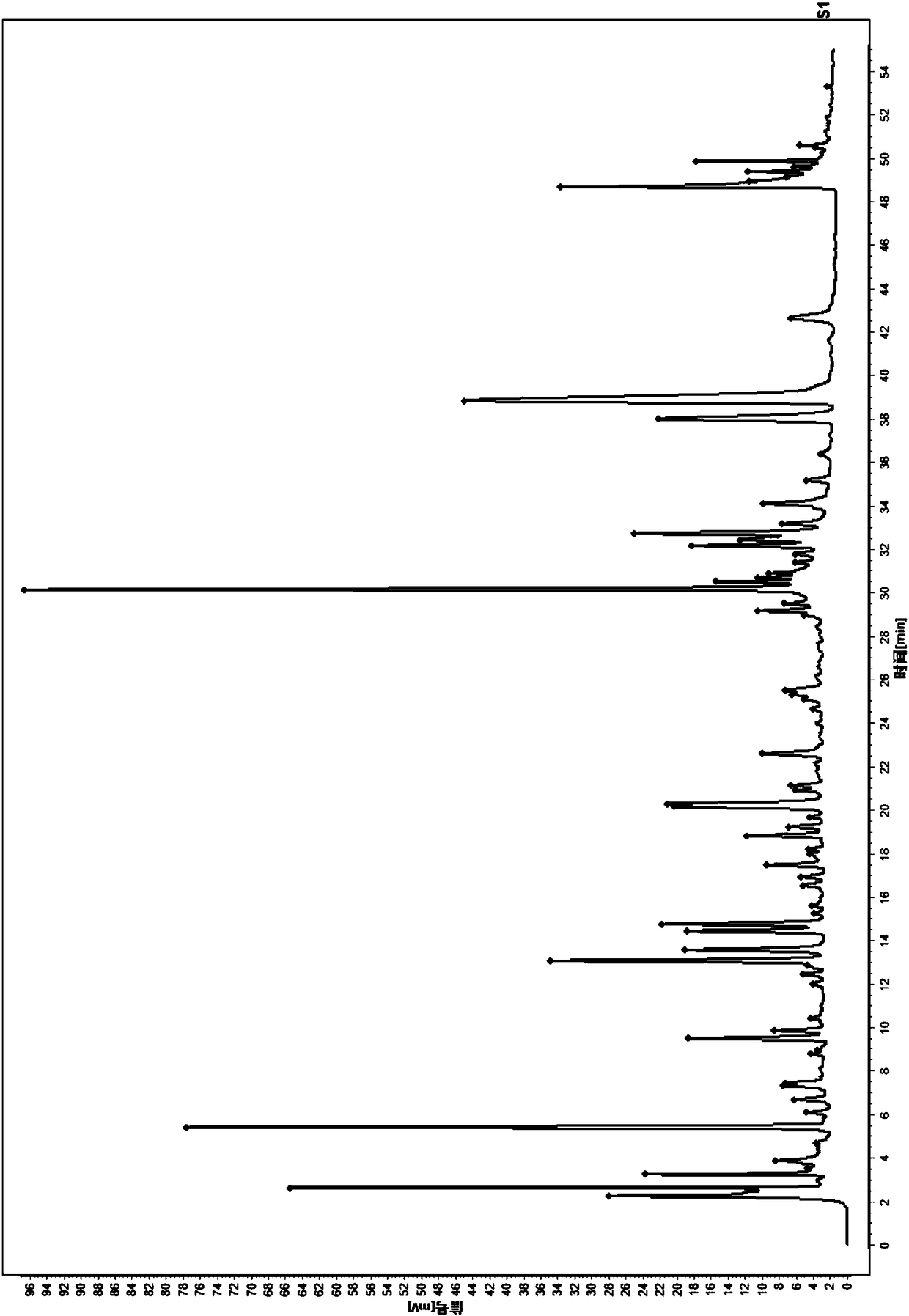
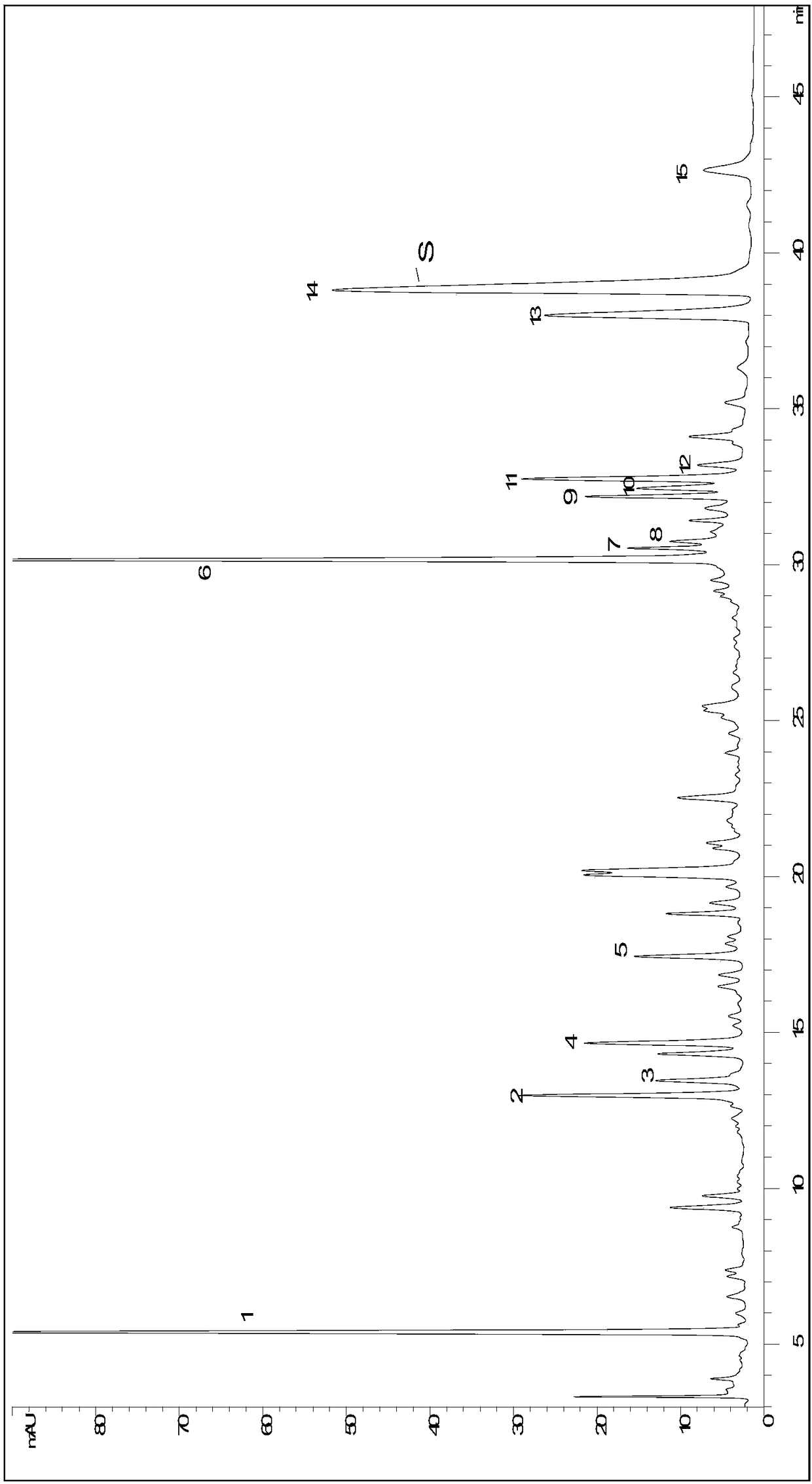
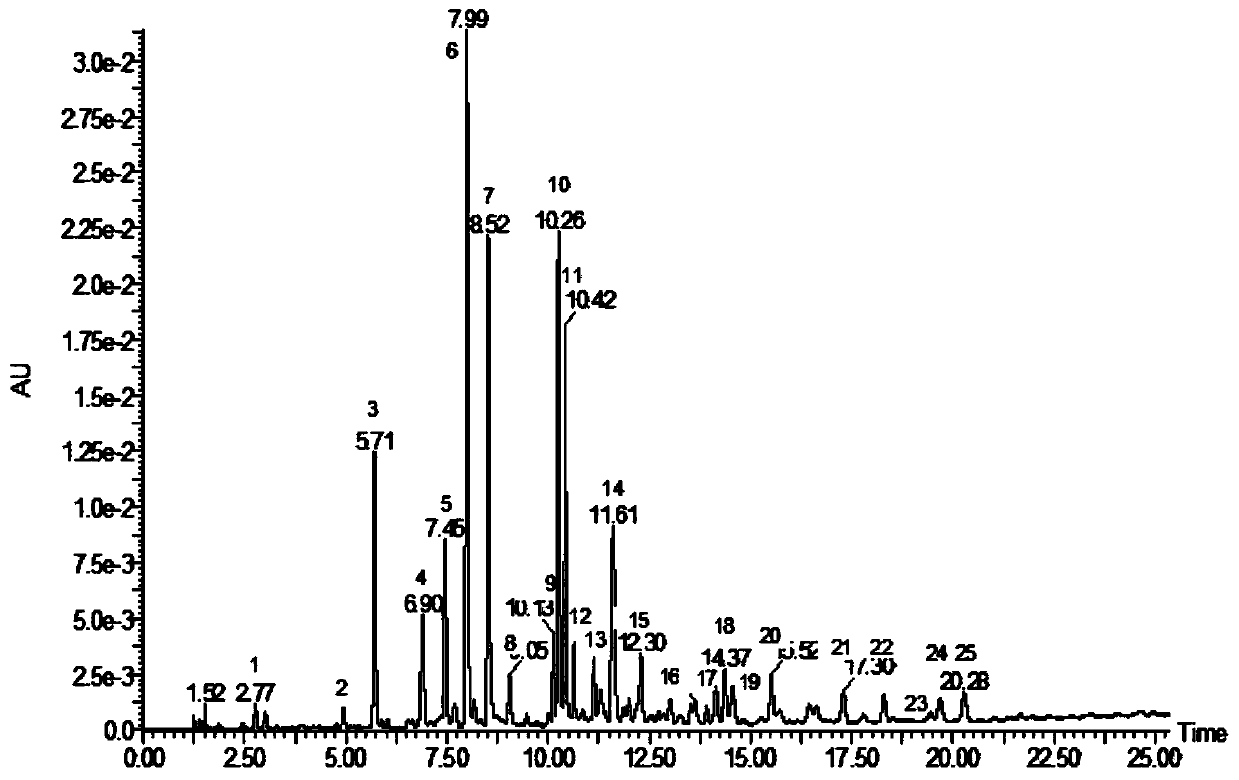
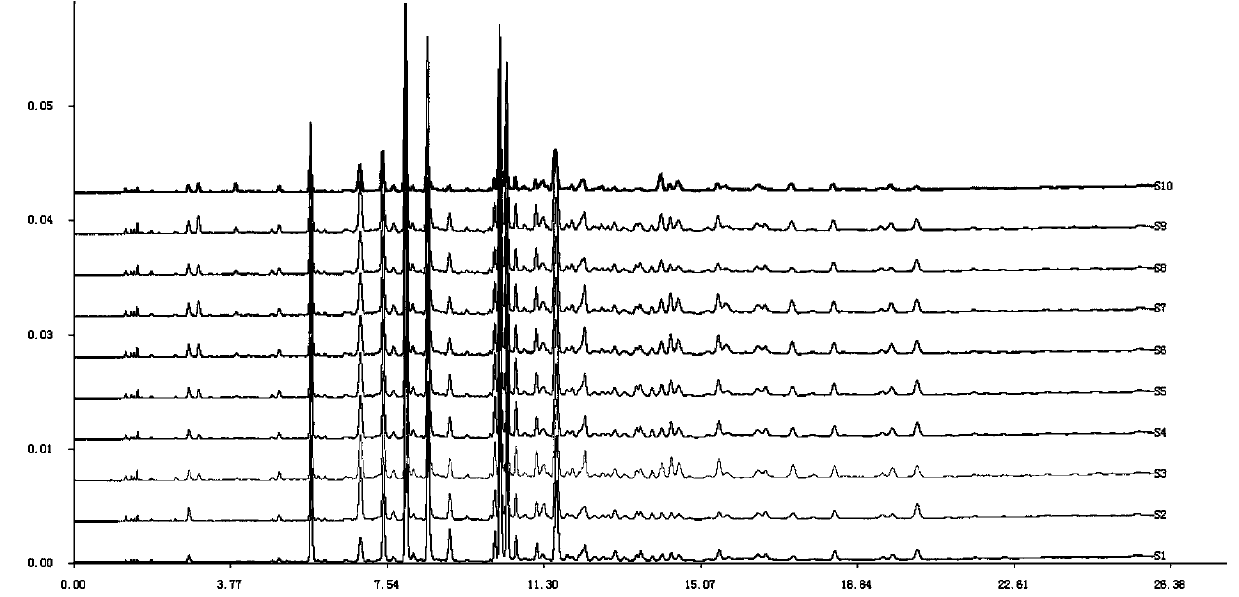
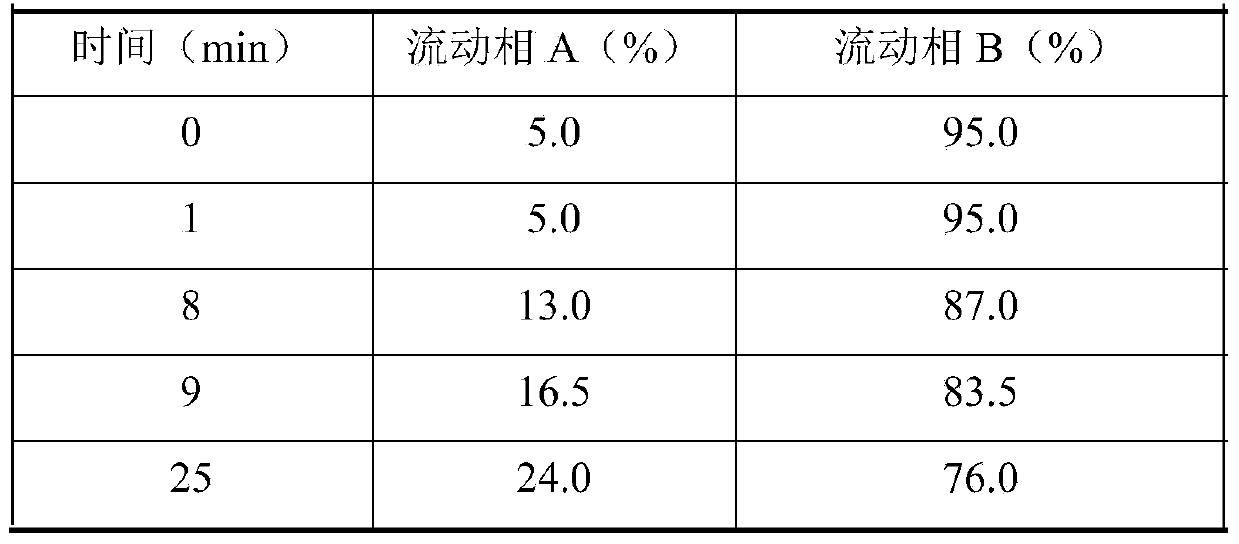
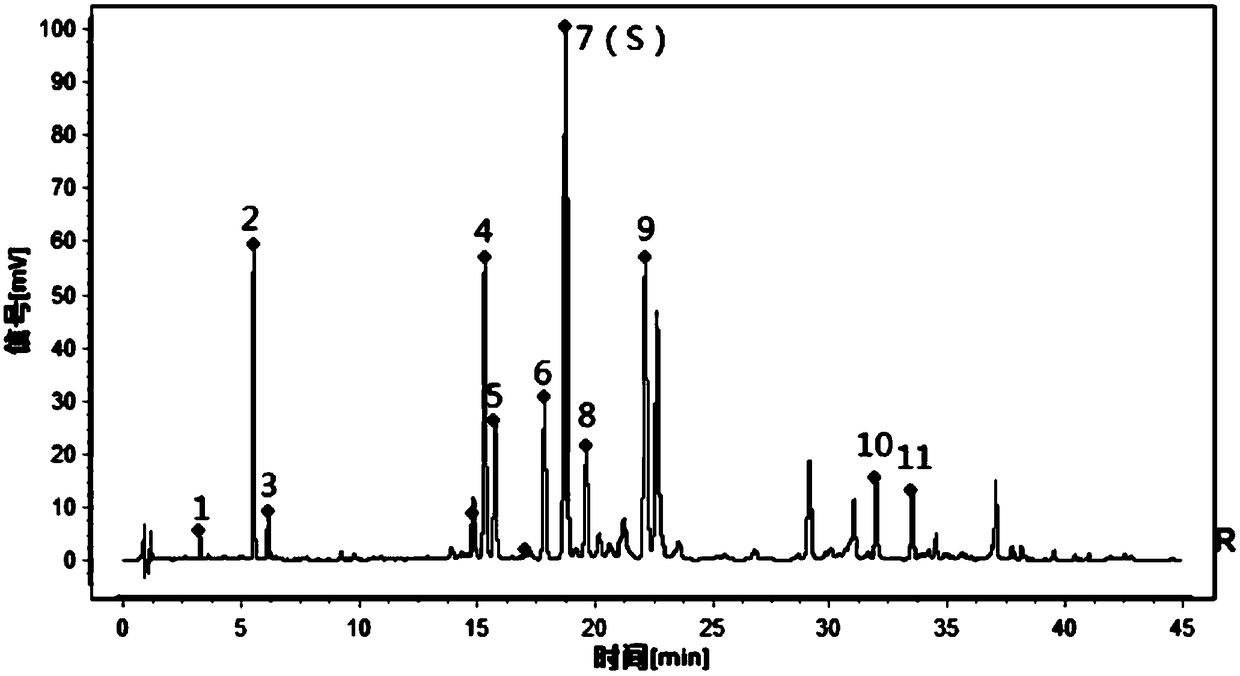
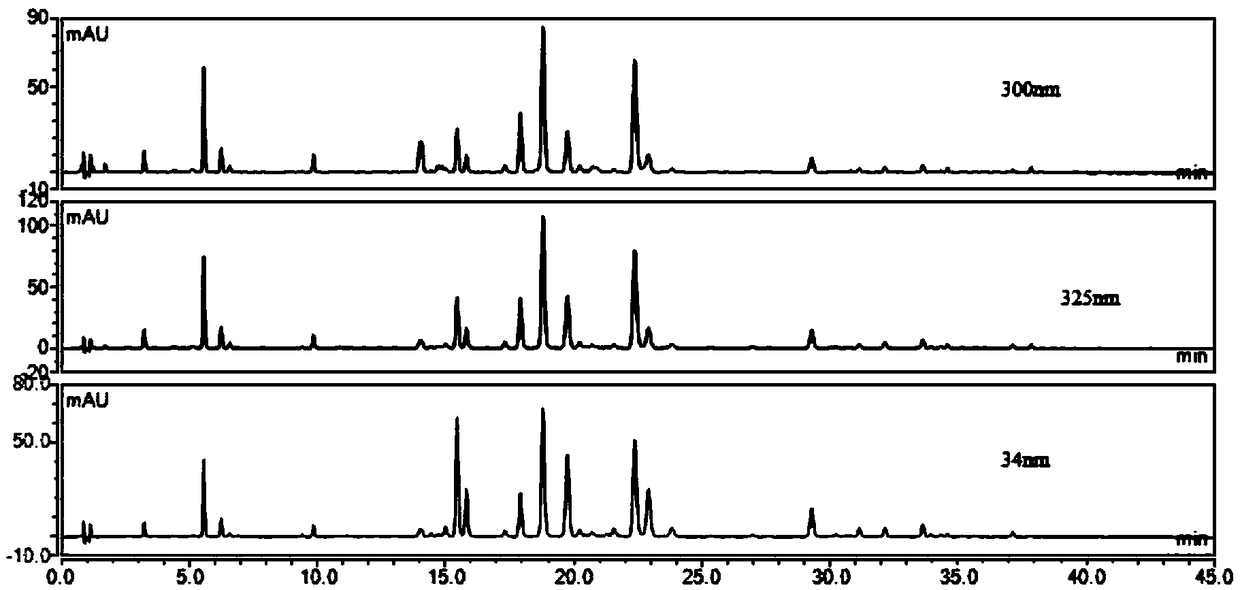
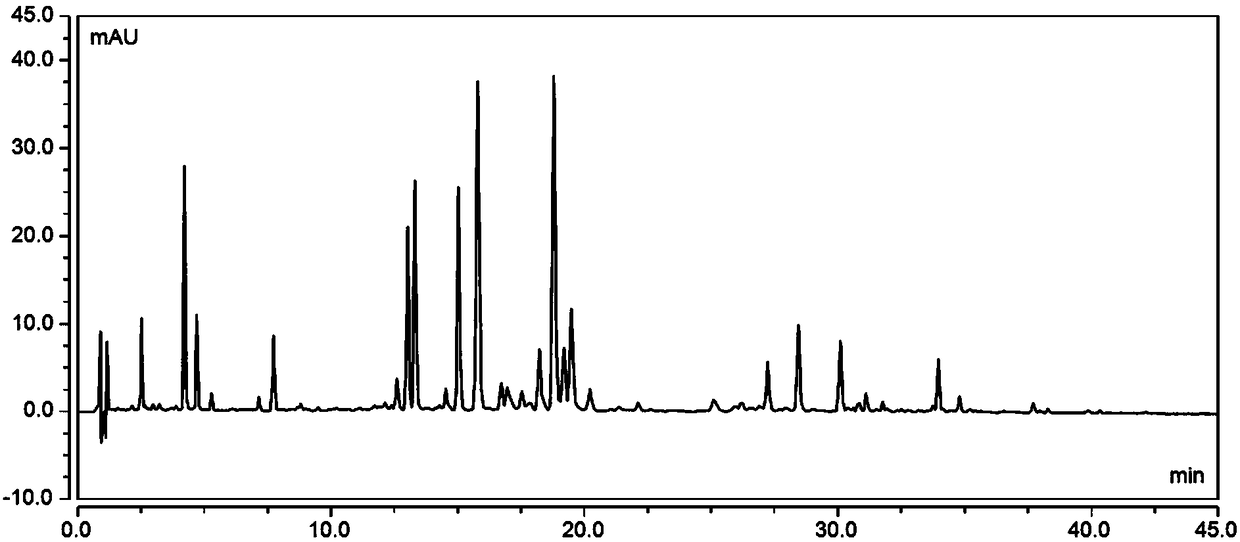
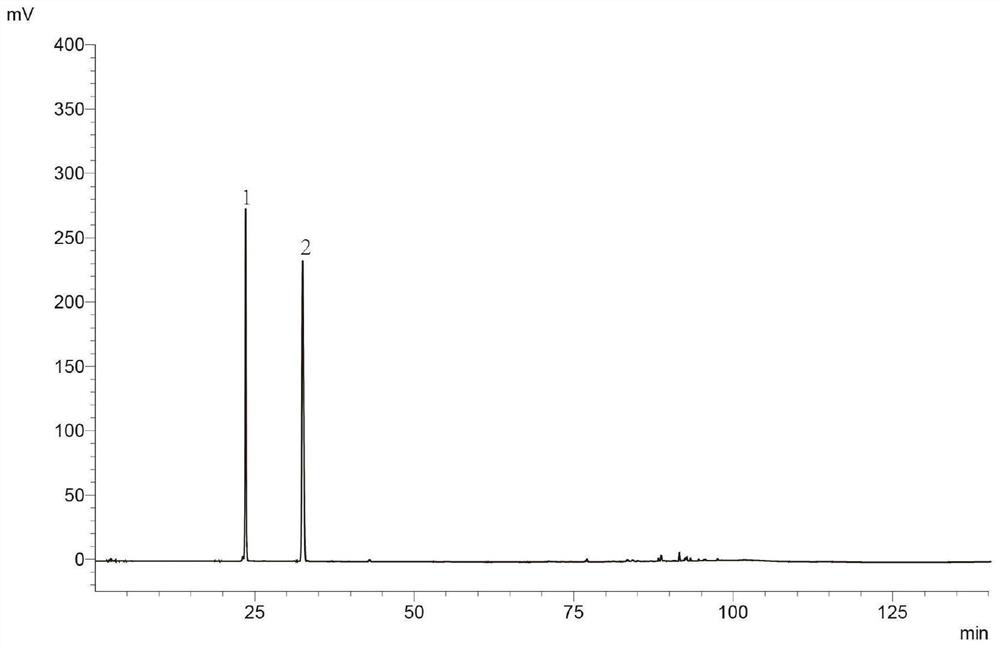
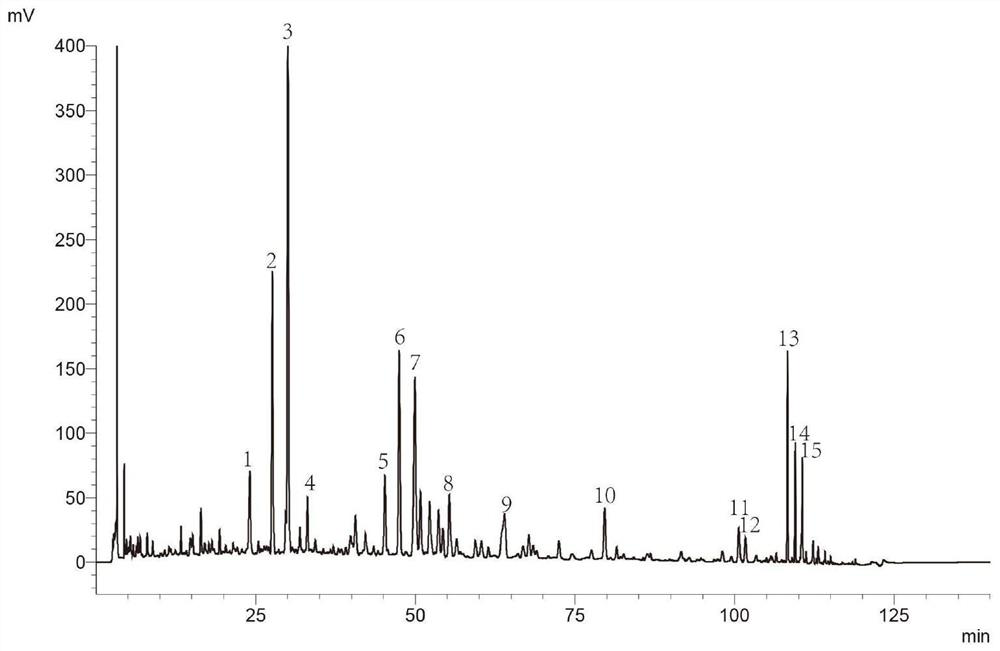
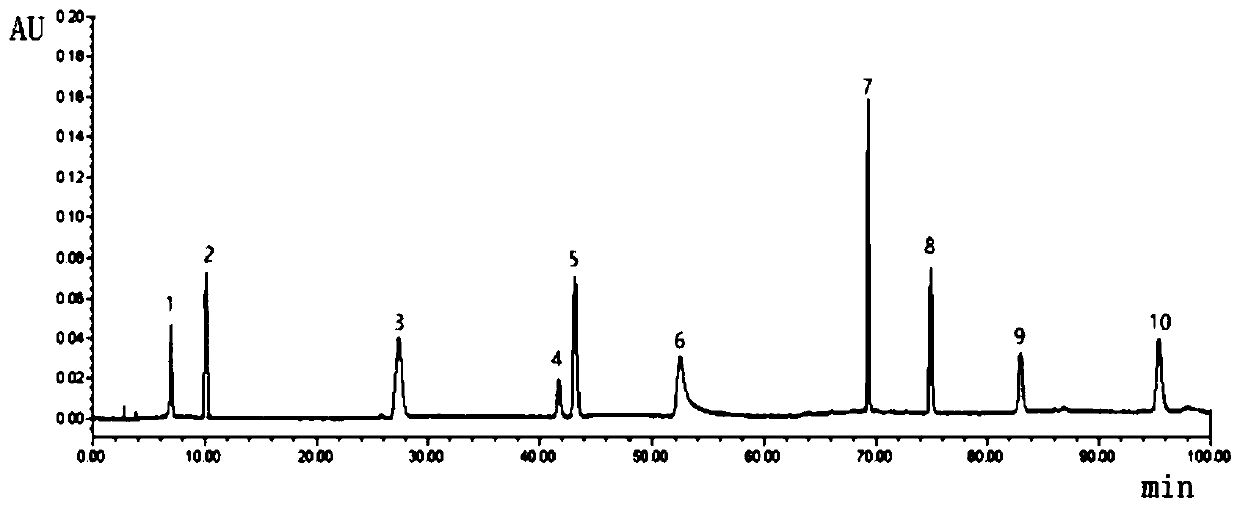
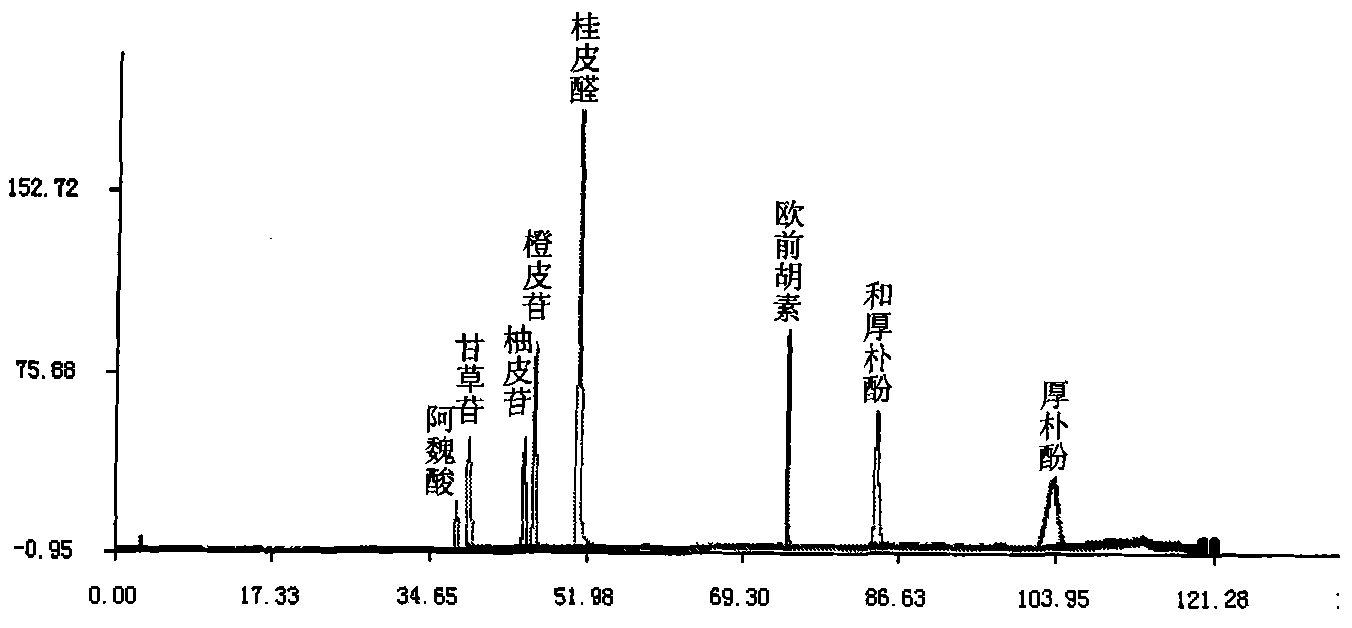
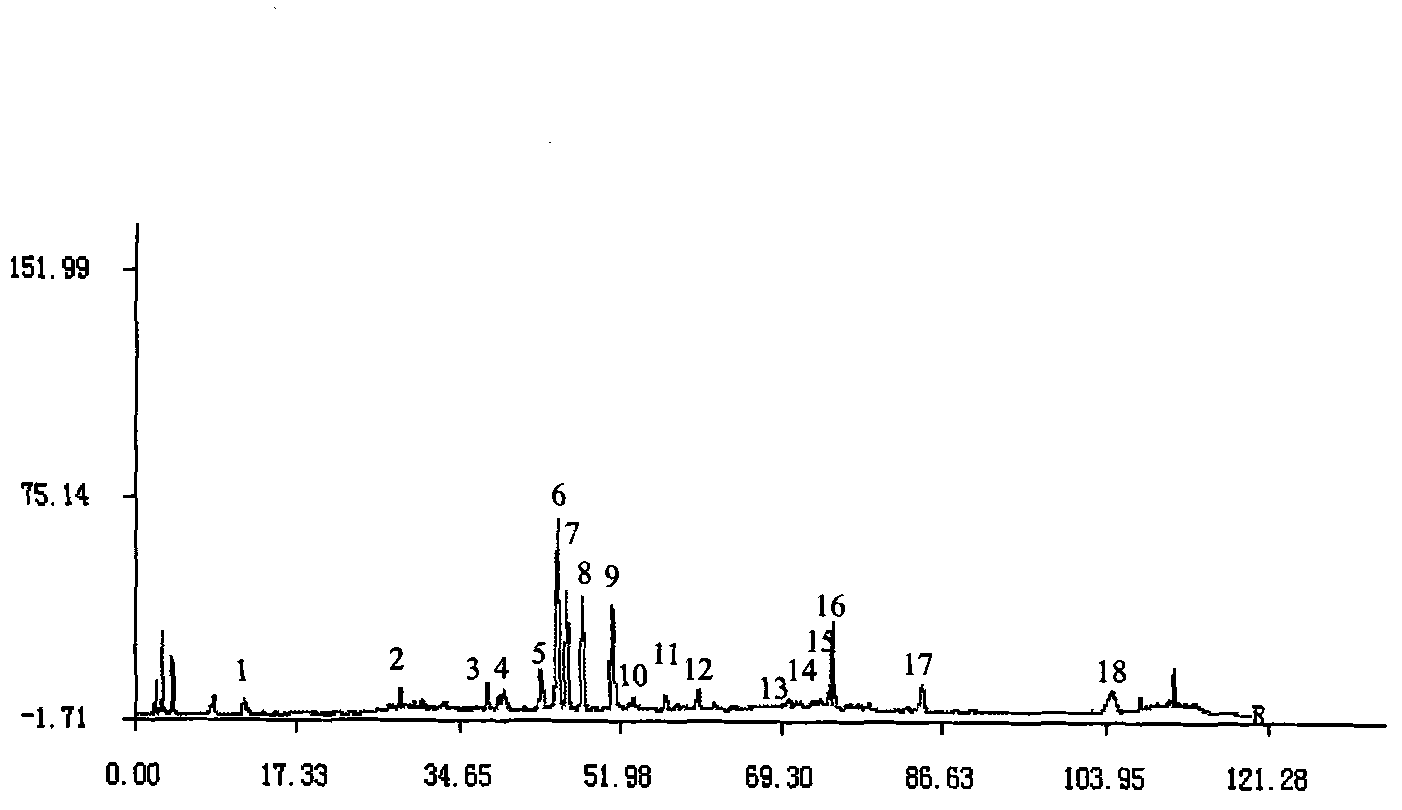
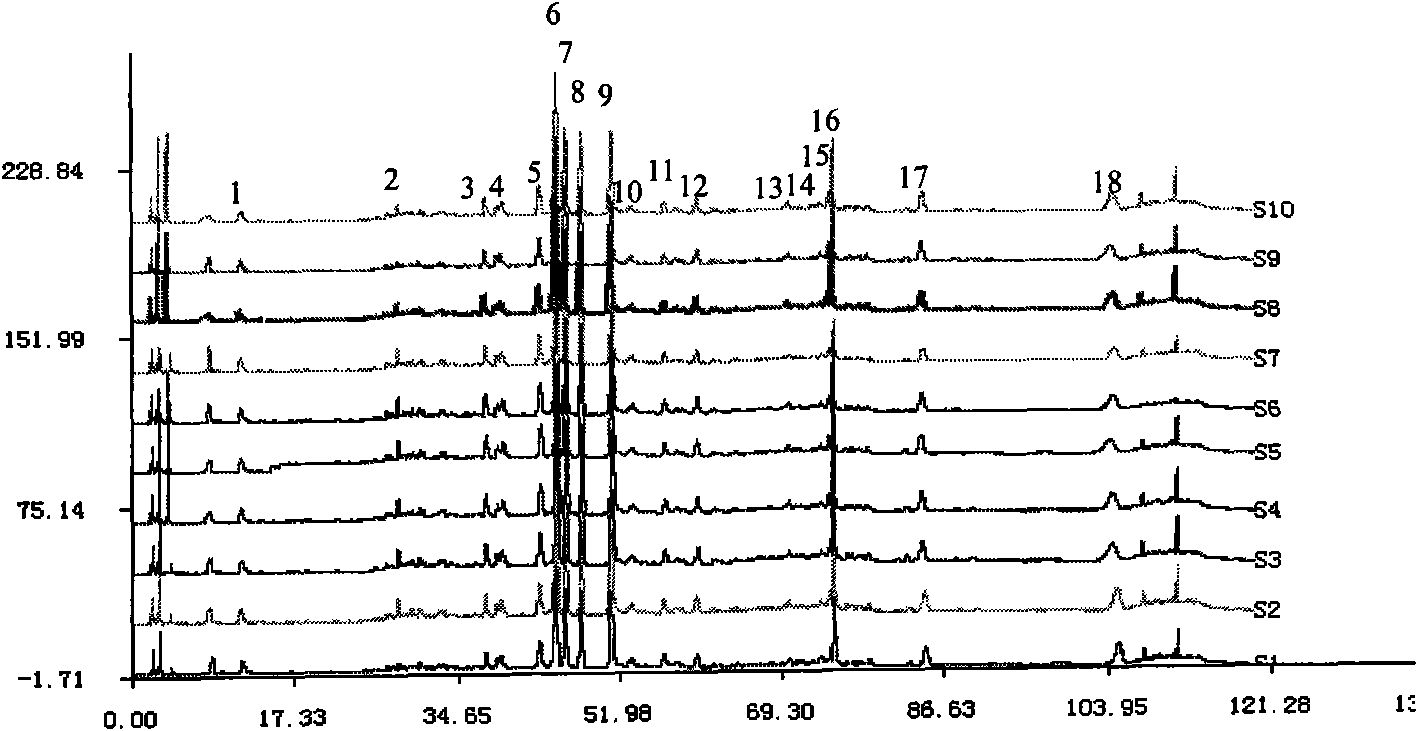
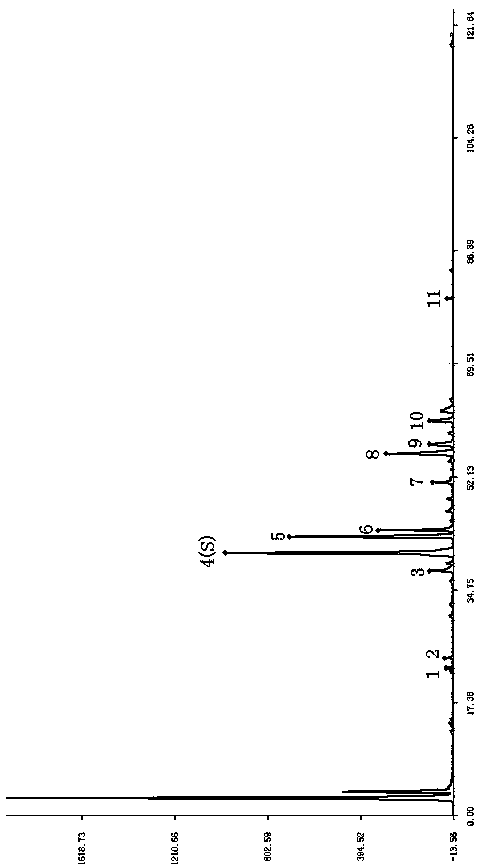
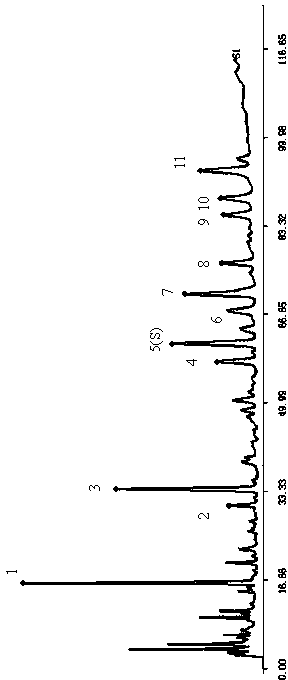
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
94results about How to "Reduce the possibility of human handling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
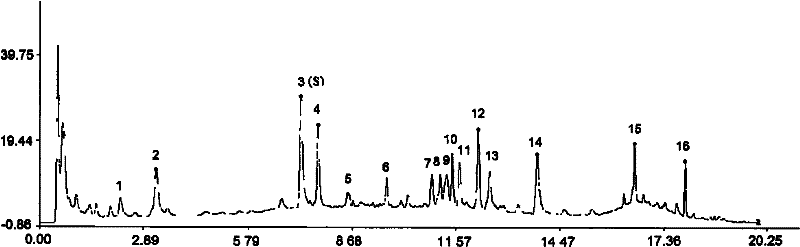
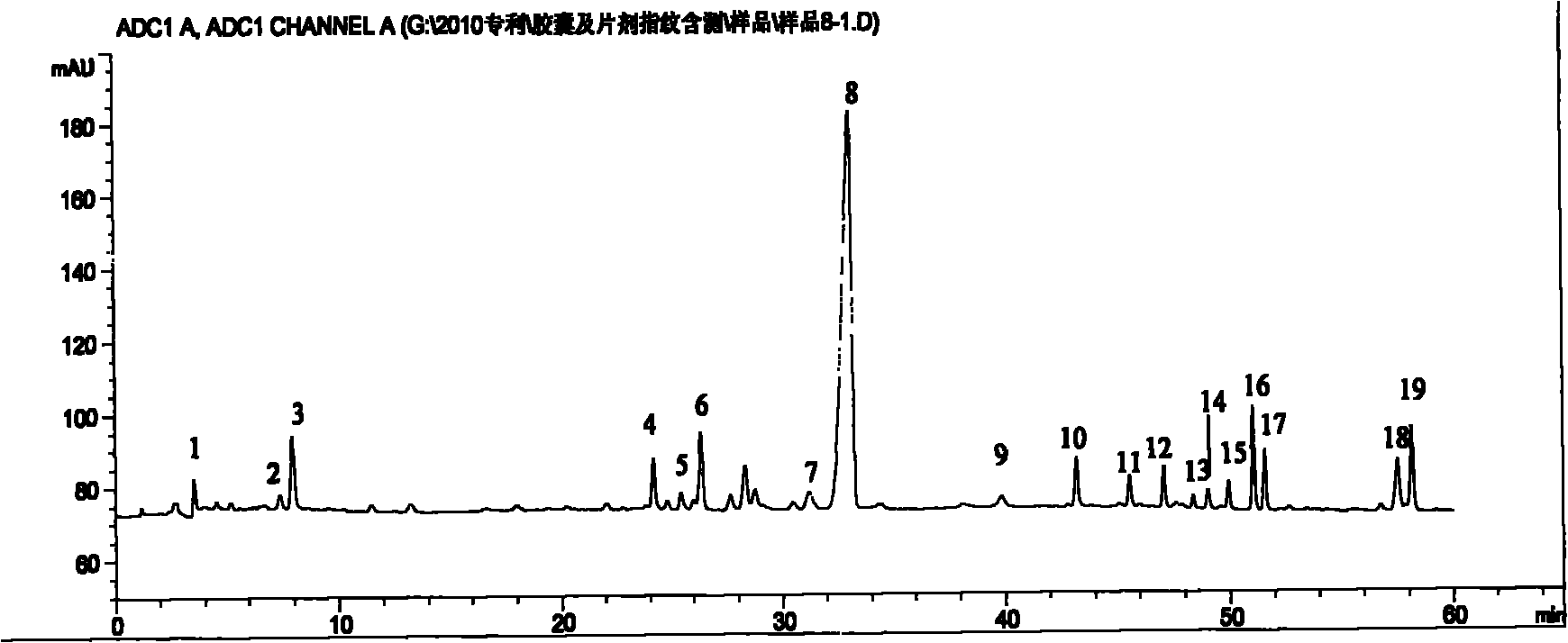
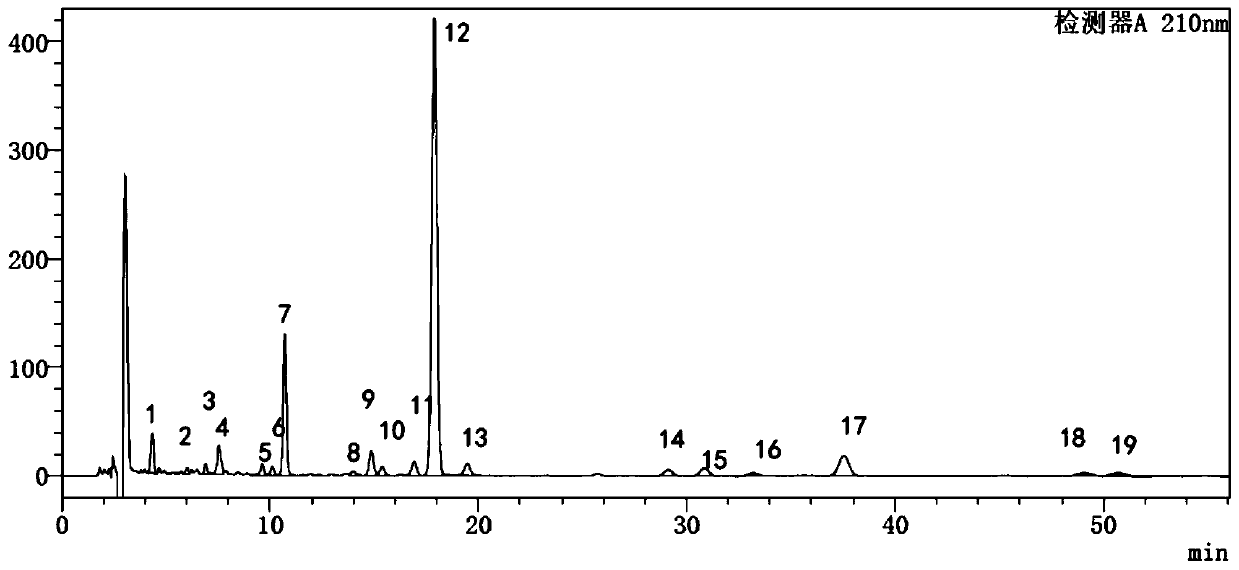
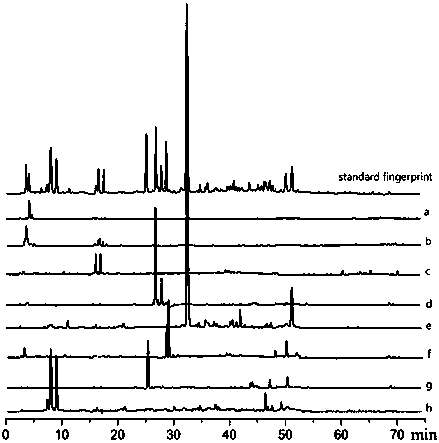
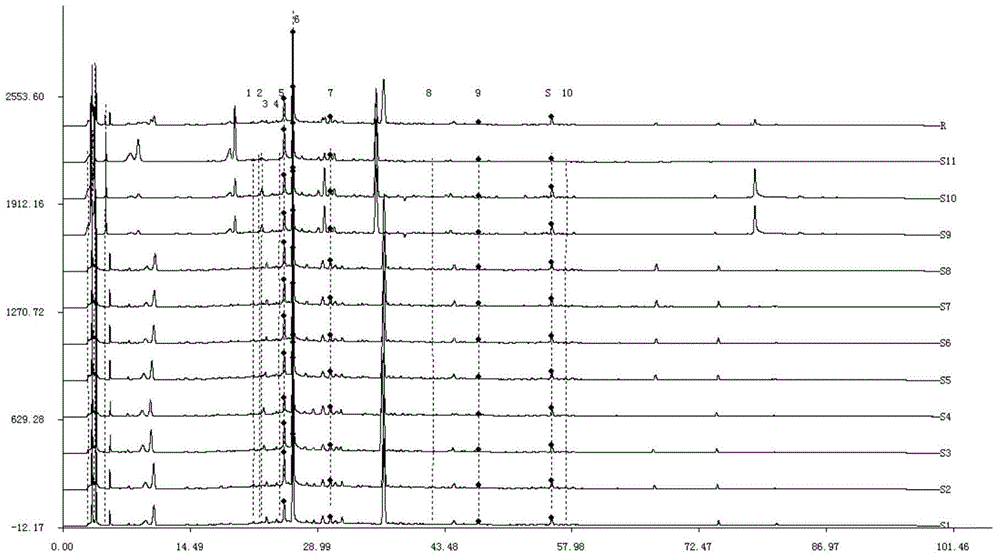
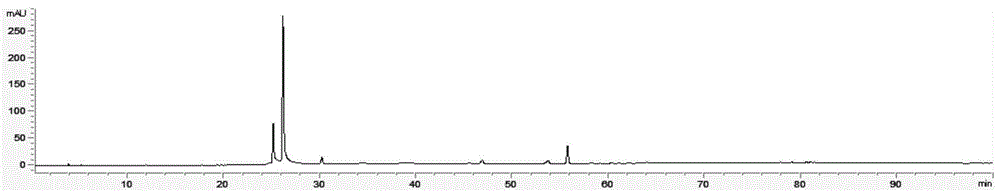
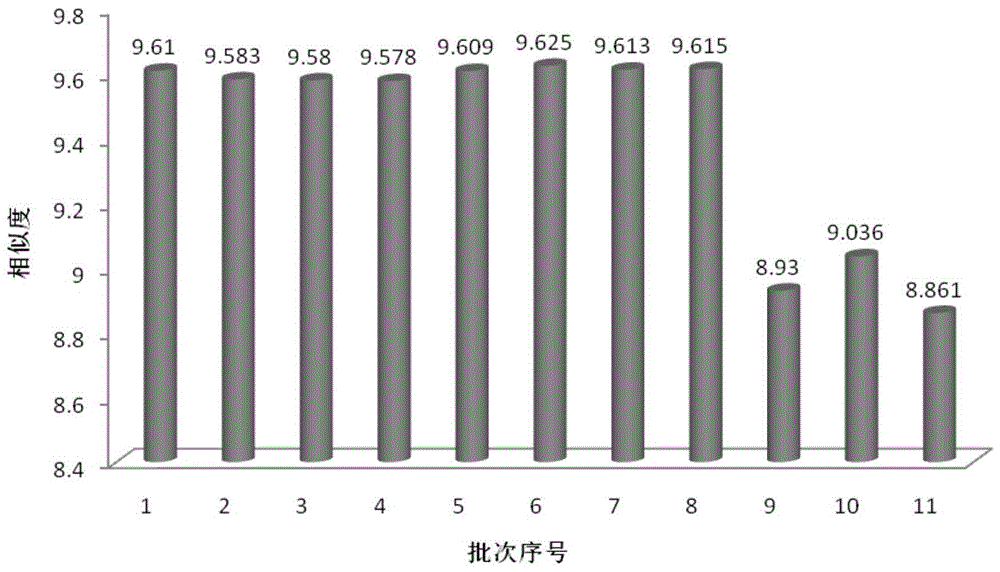
Method for detecting fingerprint spectrum of Guigui zhugan decoction
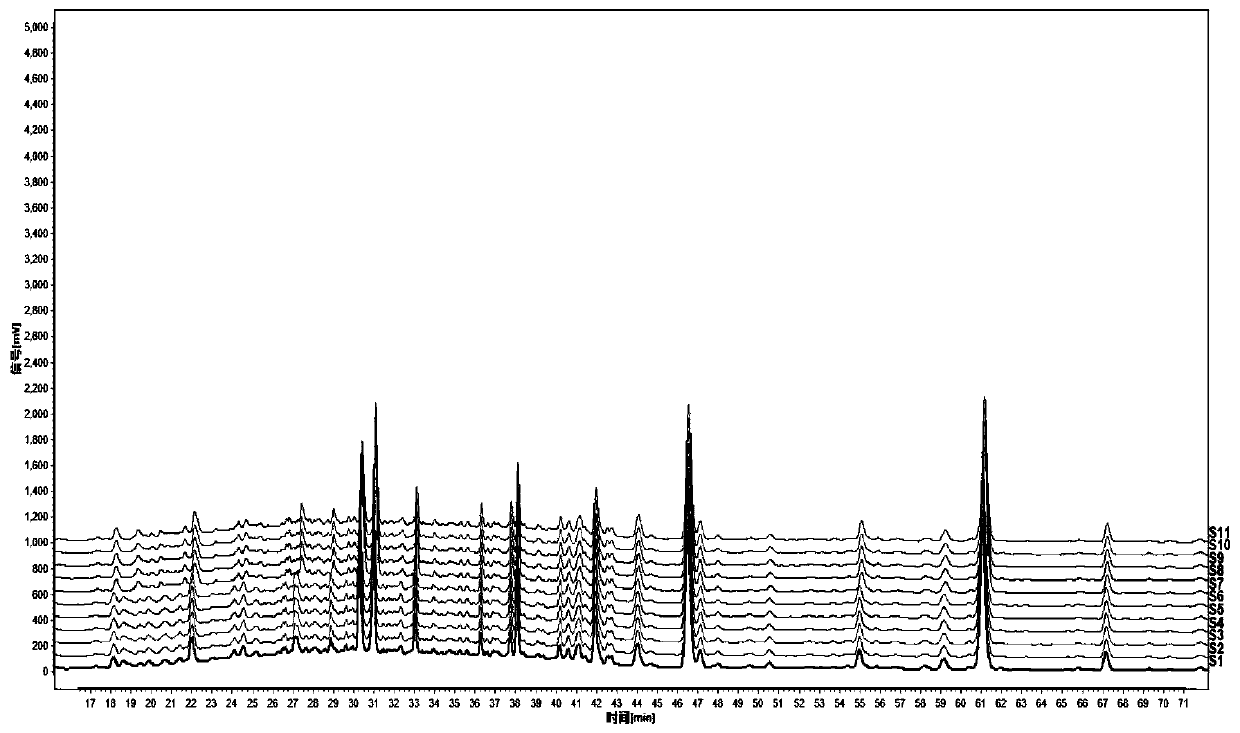
ActiveCN109709251AComprehensive detection effectComprehensive evaluationComponent separationTest sampleRetention time
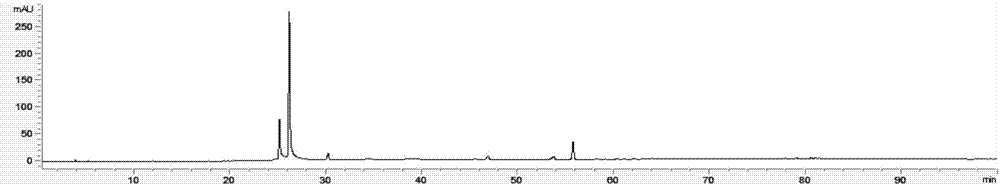
The invention discloses a method for detecting a fingerprint spectrum of Guigui zhugan decoction. The fingerprint spectrum is established by the following steps: S1, preparing a Guigui zhugan decoction test sample solution; S2, preparing a reference solution; S3, absorbing the test sample solution and the reference solution precisely and respectively and injecting the solutions into a liquid chromatograph, and recording a chromatogram map; and S4, outputting a Guigui zhugan decoction fingerprint spectrum obtained in the S3 into a traditional Chinese medicine chromatographic fingerprint similarity evaluation system, selecting chromatographic peaks exiting in all chromatogram maps of Guigui zhugan decoction with different batches as common peaks and generating a contrast fingerprint spectrumof the Guigui zhugan decoction based on an average value calculation method, and calculating relative retention time and relative peak areas of all common peaks. According to the invention, the detecting method having advantages of high stability and precision, and high reproducibility and the like is capable of analyzing the effective components in the Guigui zhugan decoction accurately and hasthe important significance in evaluating the quality of the Guigui zhugan decoction comprehensively and objectively.
Owner:NANJING HAICHANG CHINESE MEDICINE GRPCO LTD +2
Method for controlling quality of corydalis tuber and preparation thereof and drug effect thereof by using finger print
InactiveCN101288699AEffective representation qualitySuitable for useComponent separationCardiovascular disorderPalmatineCorydalis cava
The invention relates to a fingerprint map for controlling the quality and efficacy of rhizoma corydalis and preparation of the rhizoma corydalis and a preparation method of the fingerprint map. The invention discloses that the fingerprint map is one of anti-myocardial ischemic active site in the rhizoma corydalis, and the active site mainly comprises quaternary ammonium base components, wherein, columbamine, 13-methyldehydrocorydalmine, dehydrocorybulbine, palmatine, dehydrocorydaline are the main five components of quaternary ammonium base. The fingerprint map can identify the authenticity of samples and evaluate the quality and efficacy of the rhizoma corydalis or the preparation thereof, so that the quality of the rhizoma corydalis and the preparation thereof have real controllable standards, so as to ensure the product quality to be stable. The method has the advantages of being simple to operate and being accurate and reliable, which is applicable to controlling the quality and efficacy of the rhizoma corydalis and the preparation thereof.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
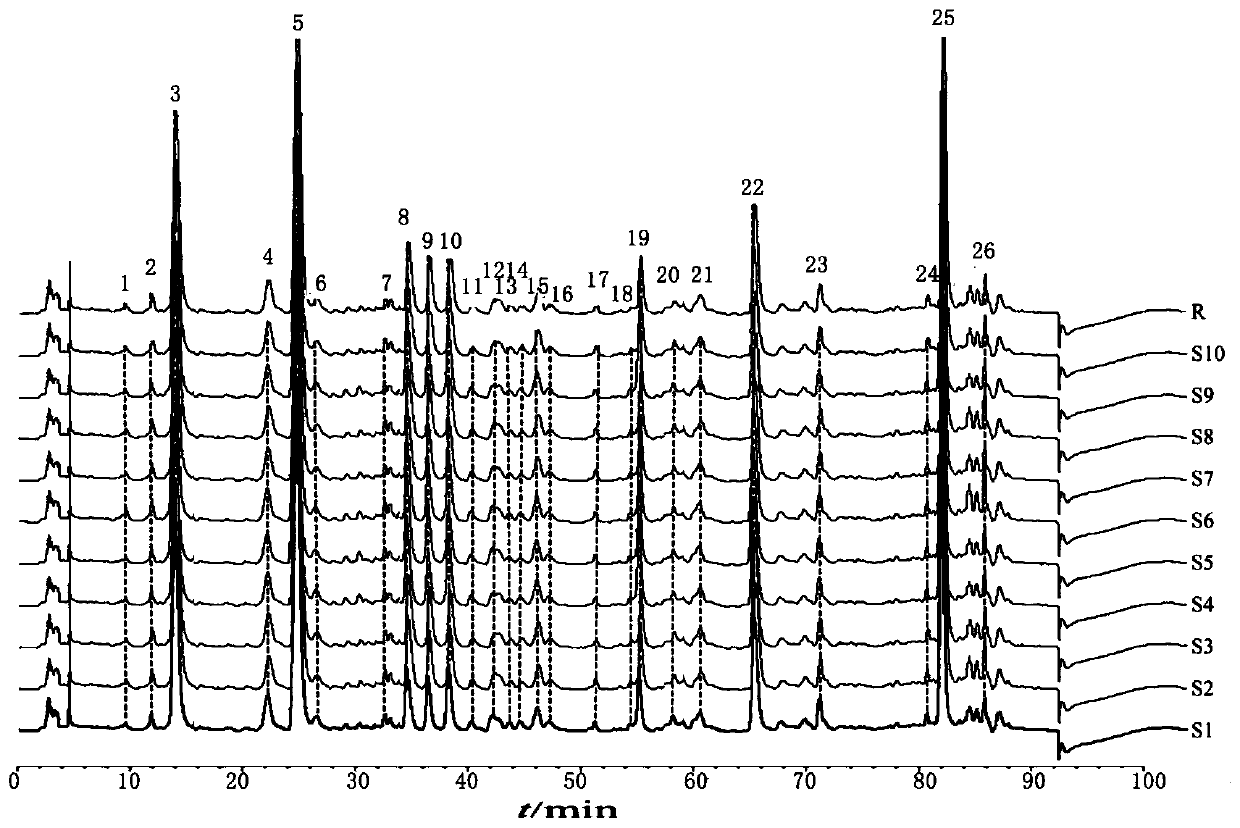
Canzhiling oral solution fingerprint map building method, fingerprint map and application thereof
ActiveCN104849364AImprove stabilityGood reproducibilityComponent separationSystems analysisChemical composition
The invention discloses a canzhiling oral solution fingerprint map building method. The map includes the following steps of preparation of a test solution, preparation of a reference solution, testing through a high performance liquid chromatograph and processing of data and a map. The invention further discloses a Canzhiling oral solution fingerprint map and a method for utilizing the fingerprint map to control quality of a Canzhiling oral solution. The Canzhiling oral solution fingerprint map building method is simple in operation, stable, reliable, high in accuracy and high in separation degree, the fingerprint map is high in stability and reproducibility and large in information quantity, and the fingerprint map is adopted as a quality control means for the Canzhiling oral solution, so that one-sidedness caused by judging of overall quality of a preparation by testing one or two chemical ingredients is avoided, and probability of artificial processing in order to enabling quality to be up to standards is lowered; samples of multiple batches are analyzed systematically, so that quality of the Canzhiling oral solution can be evaluated more comprehensively and scientifically, and product quality and efficacy are guaranteed.
Owner:SHANDONG UNIV
Method for overall monitoring kidney-tonifying body-strengthening tablet quality
InactiveCN101011480AStrong identification abilityComprehensive monitoringComponent separationDigestive systemProcess qualityMedicine
The invention relates to a method for detecting the quality of kidney tonifying tablet, which comprises that first, building the standard fingerprint diagram of barren wort, doddor, woodwardic, middle extract and final tablet; second, checking the fingerprints of tested barren wort, doddor, woodwardic, middle extract and final tablet; third, comparing the fingerprints with standard ones. The groups with met similarity can be used as material to be used in production, and the qualified middle extract is used in next process, and the final tablet is used as final product. The invention can realize full-process quality detection.
Owner:MASSON GROUP
Constructing method of electrospray ionization mass spectrometry fingerprint of Guangdong herbal tea granules and standard fingerprint
InactiveCN102914587AEffective massEffective source differentiationPreparing sample for investigationMaterial analysis by electric/magnetic meansAdditive ingredientPrincipal component analysis
The invention provides a constructing method of an electrospray ionization mass spectrometry fingerprint of Guangdong herbal tea granules and a standard fingerprint, and relates to a quality control method of a traditional Chinese medicine preparation, in particular to a method for establishing the electrospray ionization mass spectrometry fingerprint to achieve quality evaluation and source distinguishment of the Guangdong herbal tea granules. The method comprises the following steps: (1) preparation of a test solution; (2) mass spectrometry condition: electrospray ionization mass spectrometry is directly used for detection, mass spectrometry contour map is recorded in a full-scanning mode, and scanning quality range m / z is 100-1000; (3) detection: the fingerprint is directly obtained by the electrospray ionization mass spectrometry; (4) standard fingerprint: the fingerprint of a test product uses the fingerprint according to the Similarity Evaluation System for Chromatographic Fingerprint of TCM(2004 A edition); and the standard fingerprint is generated through calculation using a mean value method; and (5) main ingredient analysis: main ingredients of the fingerprint of the test product are analyzed by using statistics software, so as to evaluate the quality and distinguish sources. The constructing method of an electrospray ionization mass spectrometry fingerprint of the Guangdong herbal tea granules, provided by the invention, is beneficial for monitoring product quality and has the advantages as follows: the quality of the Guangdong herbal tea granules is rapidly, efficiently and reliably represented; simple and convenient method, stability, high sensitivity and good repetitiveness are achieved; requirements on high throughput analysis are satisfied; and the quality of a product can be rapidly and accurately identified.
Owner:CHINA NAT ANALYTICAL CENT GUANGZHOU
Method for establishing fingerprint of Deng's herbal tea granules and standard fingerprint thereof
InactiveCN102253135AMonitor qualityAvoid one-sidednessComponent separationPhosphoric acidColumn temperature
The invention discloses a method for establishing fingerprint of Deng's herbal tea granules and standard fingerprint thereof, and relates to a method for controlling quality of Chinese medicine preparation, in particular to a method for establishing HPLC (high performance liquid chromatography) standard fingerprint to detect quality of the Deng's herbal tea granules. The method comprises the following steps: (1) preparation of reference substance solution; (2) preparation of test substance solution; (3) chromatographic conditions: the chromatographic column takes octadecylsilane chemically bonded silica as filler; gradient elution is adopted, wherein the mobile phase of gradient eluent is 0.1-3%, and the gradient eluent consists of phosphoric acid and acetonitrile; the column temperature is 20-50 DEG C; the wavelength for ultraviolet detection is 240-262 nm; the flow velocity is 0.3-1.0 mL / min; and the time is 20-60 minutes; and (4) measurement: the fingerprint is obtained by HPLC. The method disclosed by the invention effectively represents the quality of Deng's herbal tea granules, is favorable for the monitoring of product quality, has the advantages of simplicity, stability, high precision and good reproducibility, and can quickly and accurately identify the true and false as well as the quality of the product.
Owner:CHINA NAT ANALYTICAL CENT GUANGZHOU
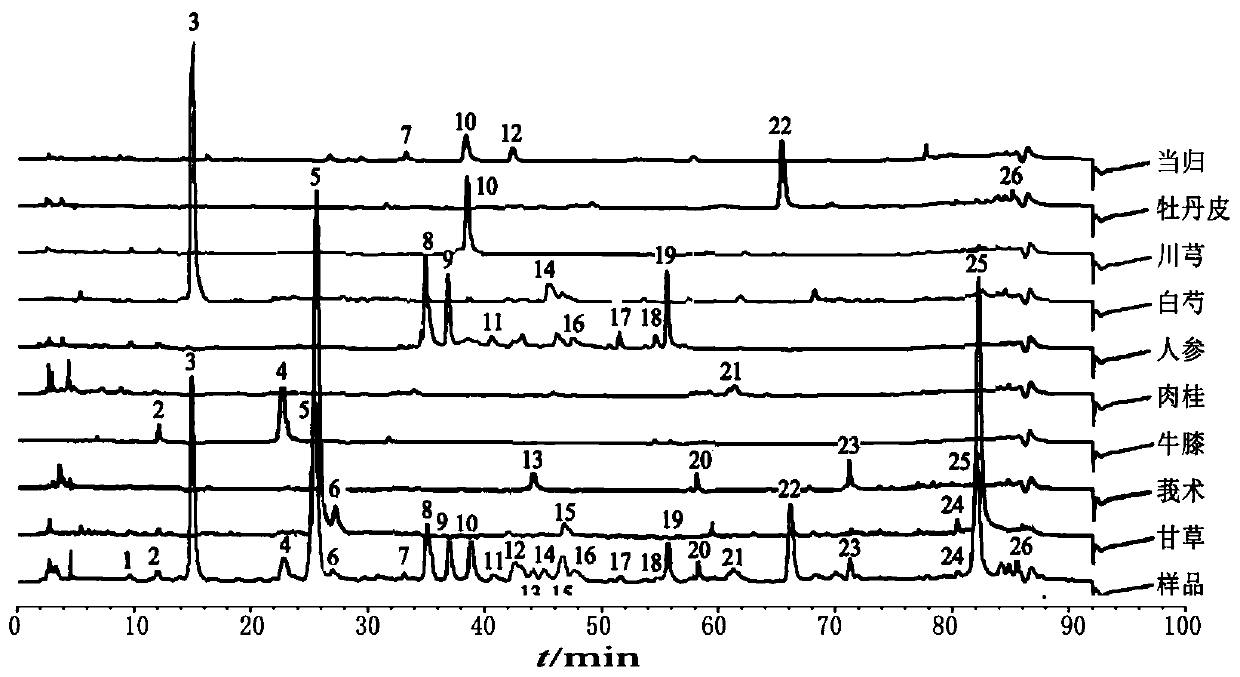
Fingerprint spectrum detection method for meridian warming decoction
ActiveCN110907553AImprove stabilityGood reproducibilityComponent separationLigusticum chuanxiongGinseng
The invention relates to a fingerprint spectrum detection method for meridian warming decoction. The method comprises the following steps: 1) preparing a test solution; weighing 1-2g of angelica sinensis, 1-2g of ligusticum wallichii, 1-2g of radix paeoniae alba, 1-2g of cinnamon, 1-2g of moutan bark, 1-2g of curcuma zedoary powder, weighing 3-4g of ginseng powder, 3-4g of liquorice powder and 3-4g of radix achyranthis bidentatae powder; adding 250-350mL of water, uniformly mixing, heating to boil with strong fire, slowly decocting with slow fire, filtering while the decoction is about 150-170mL, and adding water into the filtrate to dilute to 240-260mL; precisely sucking 8-12mL of the standard decoction of the meridian warming decoction, adding methanol to dilute to 40-60mL, sealing, weighing the mass, carrying out ultrasonic (the power is 180-220W and the frequency is 40-60kHz) treatment for 8-12min, standing overnight, weighing the mass again, supplementing the lost mass with methanol, uniformly shaking, filtering, and taking the subsequent filtrate, thereby obtaining the test solution; 2) detecting: taking and injecting 5-15 [mu]L of the test solution obtained in the previous step into a high performance liquid chromatograph, and obtaining a chromatogram; and 3) performing result judgment: comparing the chromatogram obtained in the previous step with a standard control fingerprint, and if the similarity is greater than 90%, the sample being qualified.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
Establishing method of fingerprint spectrum of honeysuckle-fructus forsythiae heat-clearing tablets and fingerprint spectrum
The invention discloses an establishing method of a fingerprint spectrum of honeysuckle-fructus forsythiae heat-clearing tablets and the fingerprint spectrum. The establishing method of the fingerprint spectrum of the honeysuckle-fructus forsythiae heat-clearing tablets includes the following steps: step 1, preparing a test sample solution of the honeysuckle-fructus forsythiae heat-clearing tablets; step 2, respectively precisely sucking the test sample solution and injecting into liquid chromatographs, and recording a chromatogram; step 3, exporting the honeysuckle-fructus forsythiae heat-clearing tablet fingerprint spectrum obtained in the step 2 from the instrument, and importing into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system; selecting chromatographic peaks existing in chromatograms of different batches of the honeysuckle-fructus forsythiae heat-clearing tablets as common peaks; generating a reference fingerprint spectrum of the honeysuckle-fructus forsythiae heat-clearing tablets by an average value calculation method; and calculating the relative retention time and the relative peak area of each common peak. The honeysuckle-fructus forsythiae heat-clearing tablet fingerprint spectrum established by the method provided by the invention can effectively characterize the quality of the honeysuckle-fructus forsythiae heat-clearing tablets, and is conducive to comprehensive supervisory control of the drug quality. The method has the advantages of being simple, convenient, stable, high in precision, good in reproducibility and the like.
Owner:JIANGSU KANION PHARMA CO LTD
Fingerprint detection method for compound wintercreeper preparation
InactiveCN101966223AQuality improvementEasy to detectNervous disorderComponent separationAstragalosideRepeatability
The invention provides a high performance liquid chromatography-evaporative light scattering detector (HPLC-ELSD) fingerprint detection method for a main saponin component of a compound wintercreeper preparation (including mixture, capsules and tablets). The fingerprint detection method of the invention comprises the steps of: preparing test solution and measuring the fingerprint under a certain chromatographic condition. Scientific tests prove that the fingerprint measured by the method has high repeatability, so that the method can be used as a method for checking and identifying the main saponin component of the compound wintercreeper preparation and can also be used for measuring astragaloside content and ginsenoside Rb1 content of the preparation.
Owner:广西中医学院
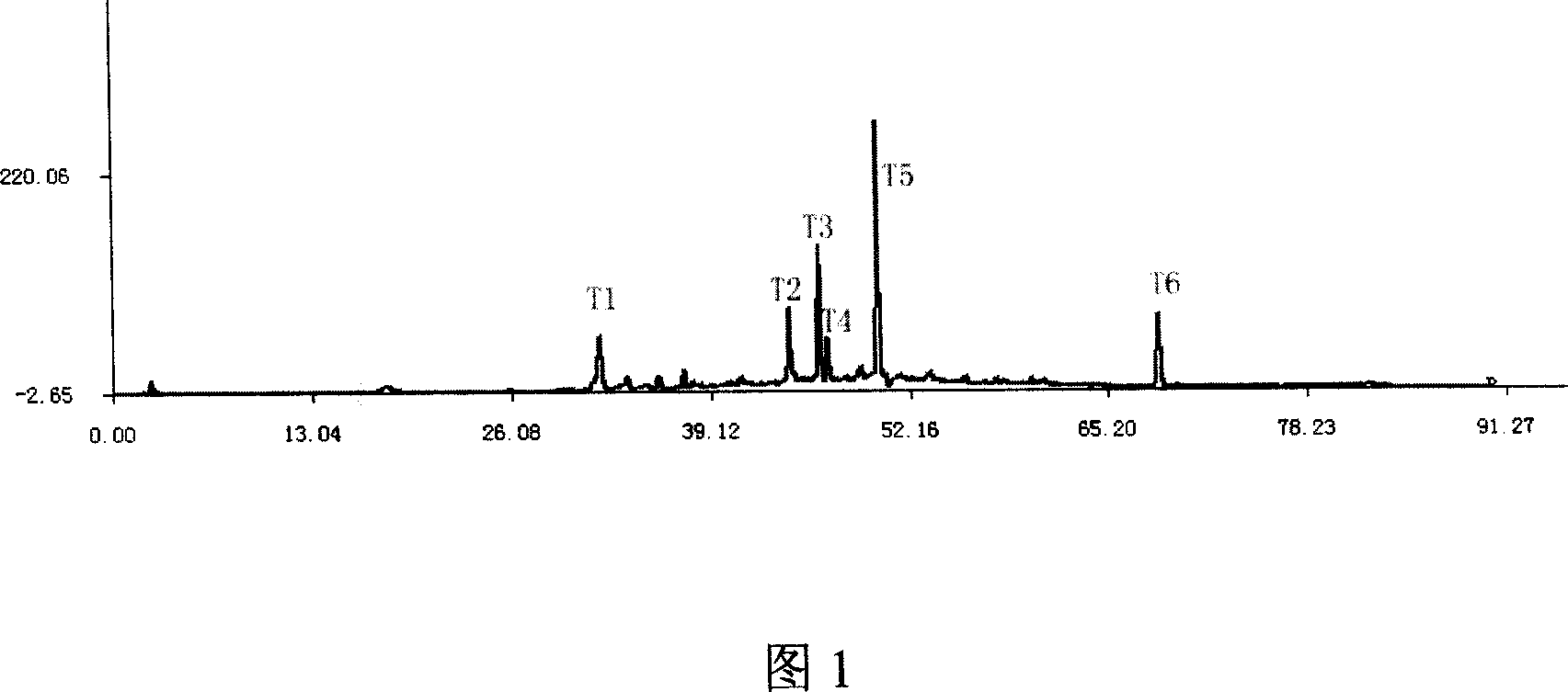
Method for constructing HPLC standard fingerprint pattern of semen cuscutae medicinal materials and quality identification
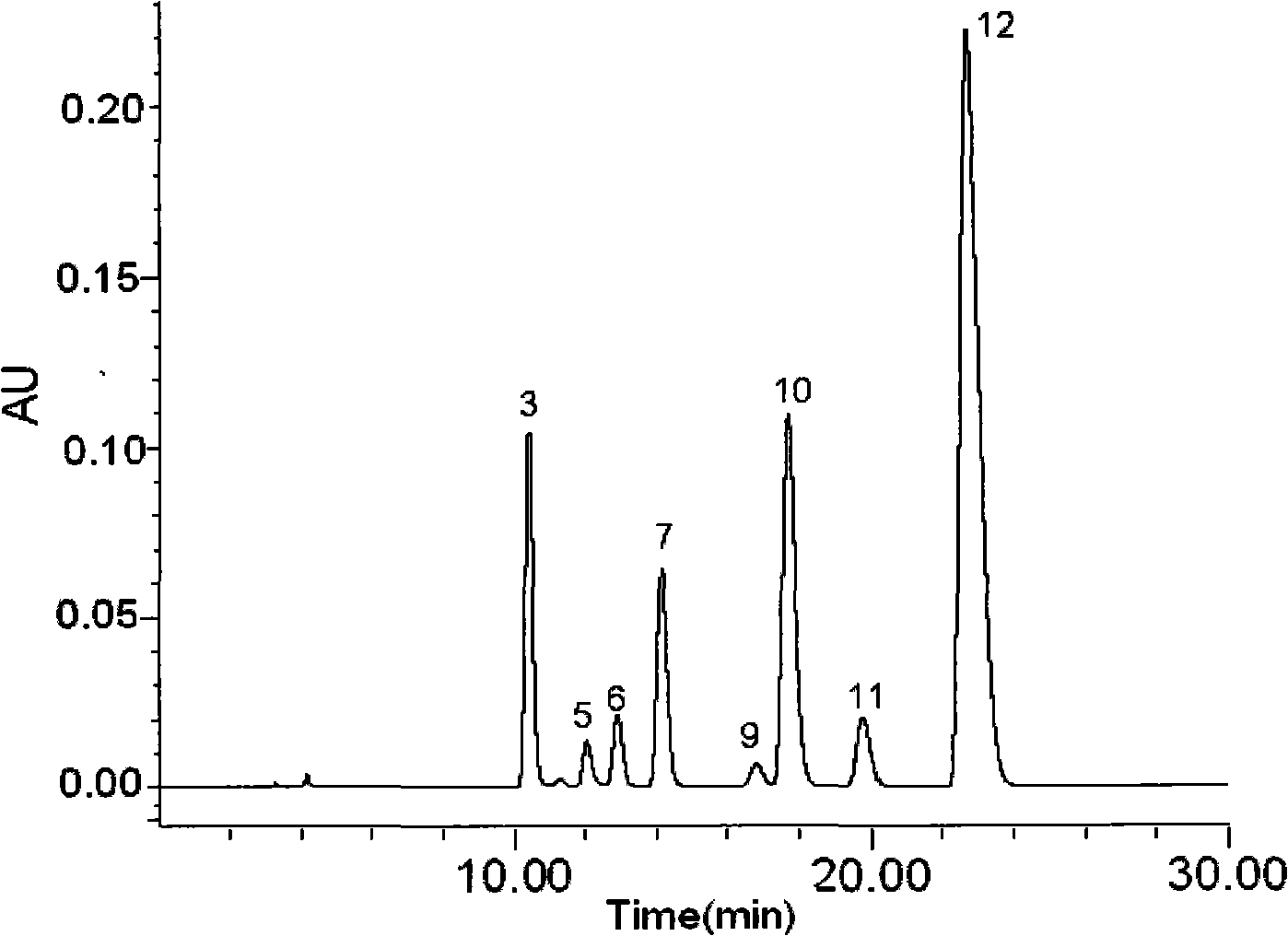
InactiveCN101013117AImprove discernmentHigh precisionComponent separationFingerprintGradient elution
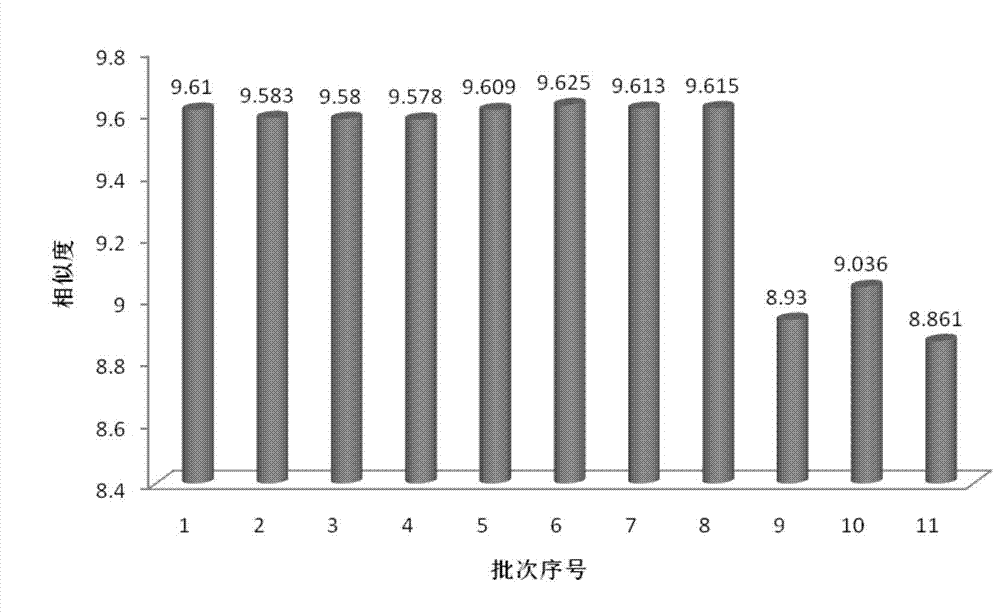
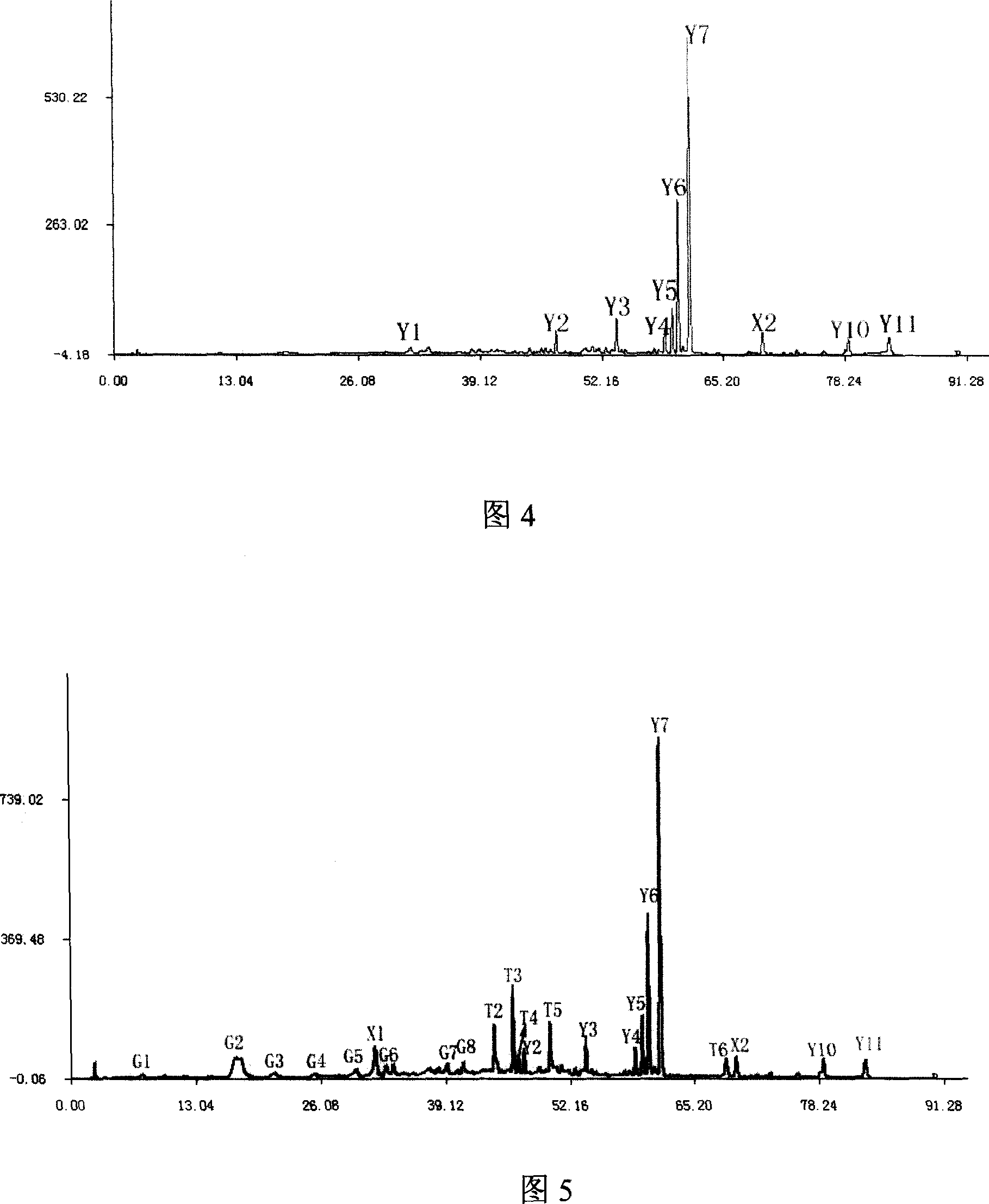
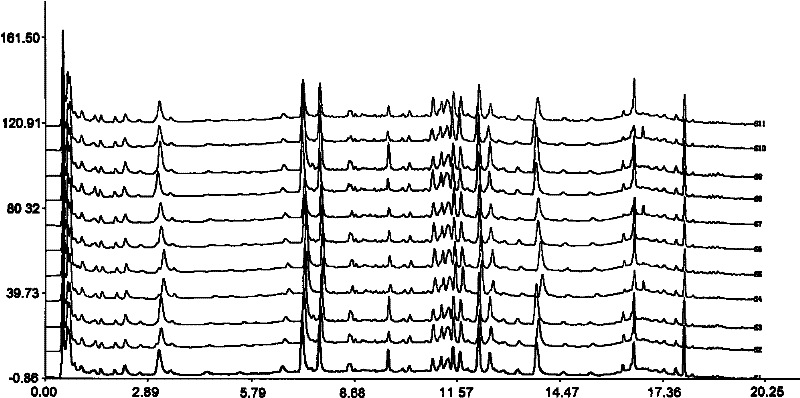
The invention relates to construction dodder medicines HPLC standard fingerprint pattern and identification medicine quality method, used for the dodder medicine quality identification, and it discloses the powder of the trial materials ultrasonically extracting 60mins in the 20ml80% methanol solution, and filtering to obtain the trial materials solution; HPLC conditions: chromatogram column Dumas C18 column (200X4.6 mm, 5mum), column temperature 30deg.C; mobile phase acetonitrile-1% acetic acid solution, gradient elution, flow rate 1ml / min; detection wavelength 350nm. Taking 10 groups Inner Mongolia dodder medicines to determine, to find common characterized peak, with software plausible to establish a standard fingerprint pattern, there are totally six common characterized peaks, T1 to T6 peaks, retention time being 31.8min, 44.3min, 46.2min, 46.7 min, 50.1min, 68.4min; and then, compare the fingerprint pattern determined by the trial medicine and the standard fingerprint pattern, the medicine with similarity more than 0.900 is applied in the product.
Owner:MASSON GROUP
Establishment of Xiasangju preparations fingerprint pattern and fingerprint pattern thereof
ActiveCN101034085AEffectively Characterize QualityMonitor qualityComponent separationPreparing sample for investigationHplc fingerprintAcetic acid
This invention relate to the found of summer mulberry chrysanthemum preparation finger print, refer methodical control of quality of Chinese traditional medicine preparation. The invention is a method of founding HPLC finger print to detect alcohol extracted thing in summer mulberry chrysanthemum preparation. The method include;(a) preparation of control article solution;(b) preparation of examining solution; (c) chromatographic condition; chromatographic column take octadecyl silane bonded silica gel as padding;adopt gradient elution, mobile phase is gradient elution liquid composed by 0.1 to 3 percent acetic acid and methanol; column temperature;25 to 50 deg; ultraviolet detecting wavelength is 285 to 295 nm;velocity of flow;0.5 to 1.5 ml per min; time;30 to 80 min;(d) measuring; high efficiency liquid chromatography to gain finger print. This invention can validly characterize quality of summer mulberry chrysanthemum preparation, in favor of monitoring product quality; possess merit of method handiness, stable, high degree of precision, good reappearance; the invention can quickly and exactly discriminate the product.
Owner:GUANGZHOU XINGQUN PHARMA
HPLC fingerprint spectrum determination method for cannabis sativa
InactiveCN110133148AAvoid one-sidednessReduce the possibilityComponent separationCannabidiolic acidFingerprint
The invention provides an HPLC fingerprint spectrum determination method for cannabis sativa. The method is characterized by comprising the steps of (1) preparing a test solution: taking the cannabissativa medicinal material, crushing, sieving and baking the cannabis sativa medicinal material, precisely weighing baked cannabis sativa medicinal material powder, adding an extracting agent, carryingout ultrasonic extraction, carrying out standing until the room temperature is reached, passing through a microporous filter membrane, and taking subsequent filtrate; (2) preparing a reference solution: taking cannabidiolic acid, cannabidivarin, cannabigerol, cannabidiol, delta 9-tetrahydrocannabinol, cannabichromene, tetrahydrocannabinol and geranylflavone A reference substances, and adding methanol to dissolve the reference substances so as to prepare the reference substance solution; and (3) performing determination: precisely sucking the reference solution and the test solution into an HPLC, performing determination, and recording a chromatogram.
Owner:HANYI BIO TECH CO LTD
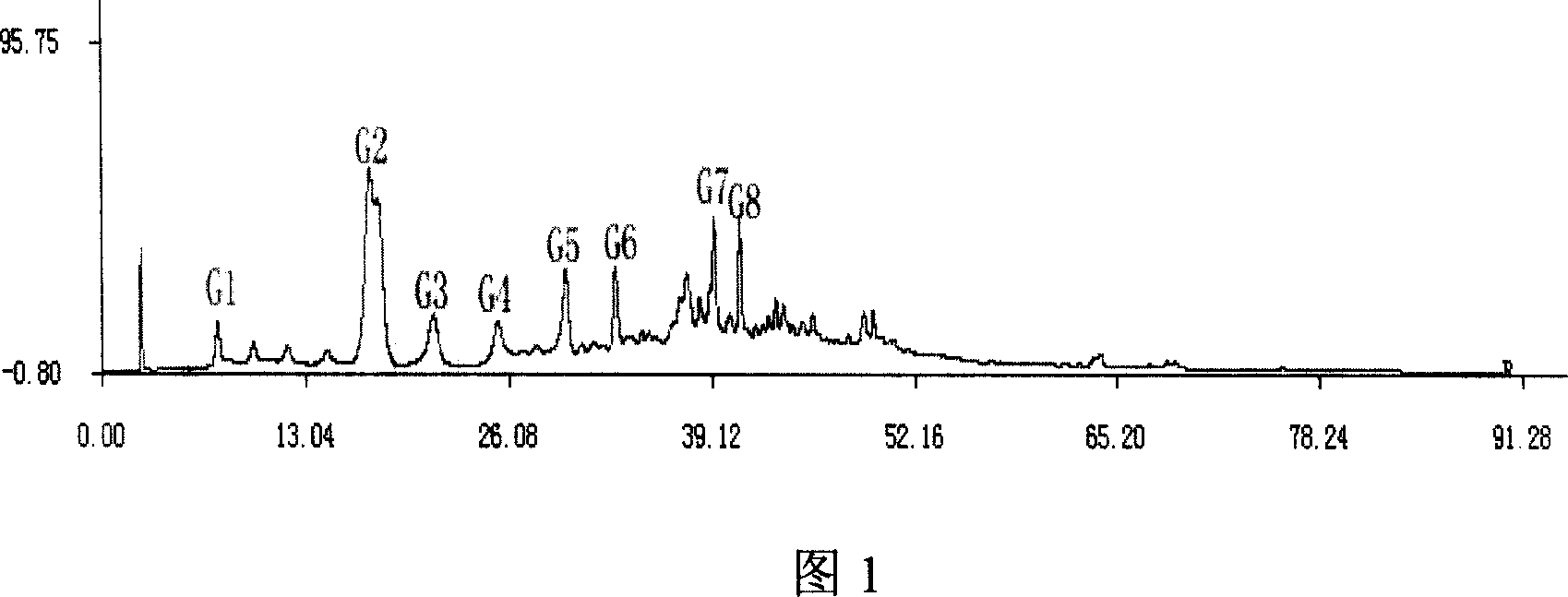
Method for constructing HPLC standard fingerprint pattern of dog-ridge medicinal materials and quality identification
InactiveCN101013118ARealize chemical composition detectionEffective representation qualityComponent separationRetention timeGradient elution
The invention relates to building barometz medicine HPLC standard fingerprint pattern and identification medicine quality method, to be used in the barometz medicine quality identification, and it discloses that after the trial materials powder ultrasonic extracting 60 minutes in 20ml80% methanol solution, to filter the trial materials solution. HPLC conditions: chromatogram column Dumas C18 column (200X4.6mm, 5mum), column temperature 30deg.C; mobile phase acetonitrile-1% acetic acid solution, gradient elution, flow rate 1ml / min; detection wavelength 350nm. Taking 10 groups Guangxi barometz medicines to determine, to find common characterized peak, with software plausible to establish a standard fingerprint pattern, there are totally eight common characterized peaks, G1 to G8 peaks, retention time being 7.4min, 17.3min, 21.3min, 25.5min, 29.8min, 33.0min, 39.3min, 41.0min. And then, compare the fingerprint pattern determined by the trial medicine and the standard fingerprint pattern, the medicine with similarity more than 0.950 is applied in the product.
Owner:MASSON GROUP
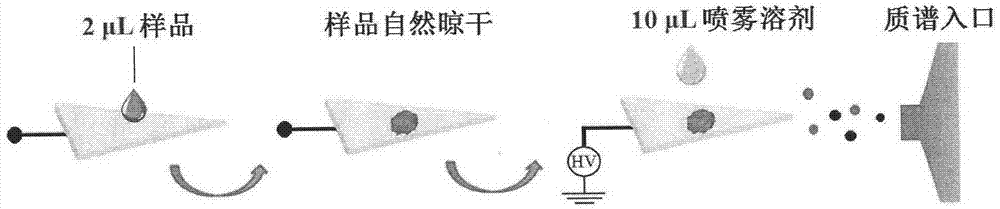
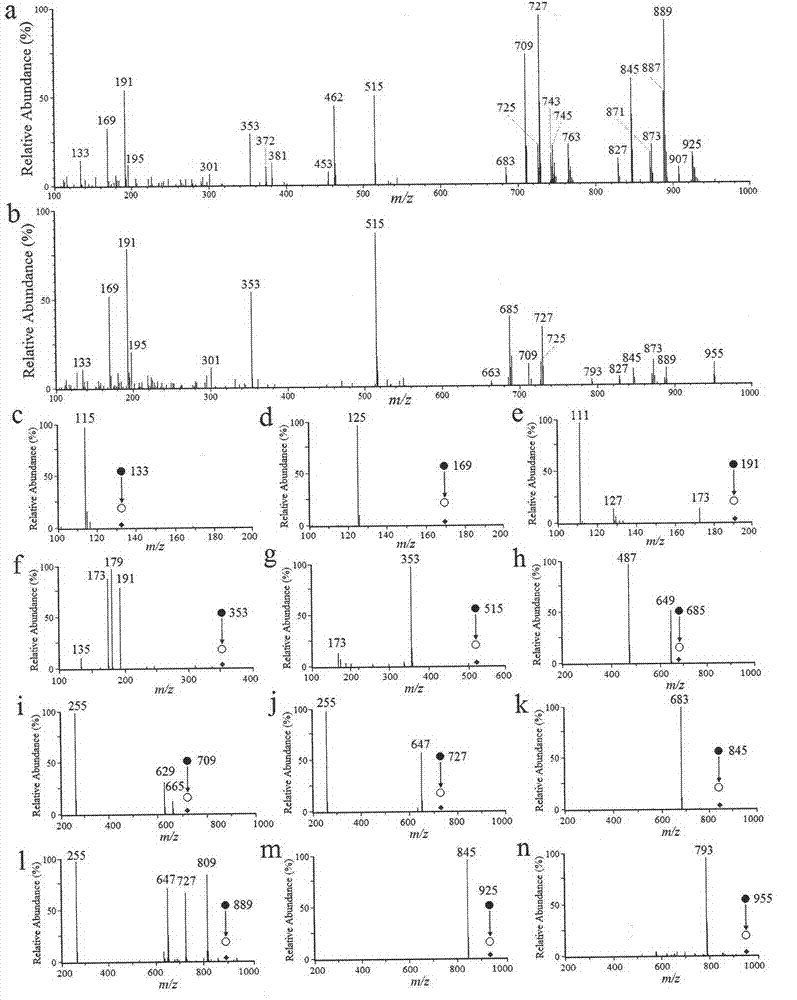
Paper-based electrospray ionization mass spectrometry fingerprint technology and construction method thereof
InactiveCN103499632AMonitor qualityAvoid one-sidednessMaterial analysis by electric/magnetic meansHerbal preparationsChromatographic separation
The invention provides a paper-based electrospray ionization mass spectrometry fingerprint technology and a construction method thereof. The paper-based electrospray ionization mass spectrometry fingerprint technolog comprises the steps of (1) manufacturing a paper-based carrier; (2) performing a paper-based eelctrospray test: aligning the tip end of the paper-based carrier to a mass spectrometry entrance, dropping a sample at the center position, loading a high-voltage electric field, dropping a spray solvent for dissolving active components in the sample, moving to the tip end of the paper-based carrier under the action of the high-voltage electric field to form a Taylor cone at the tip end, generating charged spray liquid drops, ionizing the active components and performing the mass spectrometry; (3) recording the mass spectrometry; (4) obtaining a fingerprint; (5) obtaining a standard fingerprint: calculating the standard fingerprint by an averaging method; (6) analyzing main components: performing quality evaluation, true and false identification and source distinguishing through main component analysis. The invention provides a brand-new high-flux mass spectrometry fingerprint analysis method which does not comprise sample pretreatment and chromatographic separation, thus quality monitoring for liquid Chinese herbal preparations or an extracting solution sample is facilitated.
Owner:CHINA NAT ANALYTICAL CENT GUANGZHOU
Glossy privet fruit fingerprint spectrum detection method
The invention discloses a glossy privet fruit fingerprint spectrum detection method. Building of glossy privet fruit fingerprint spectrums includes the following steps that 1, a test solution is prepared; 2, a mixed comparison product solution is prepared; 3, the test solution are accurately sucked to be injected into a liquid chromatograph, and chromatogram maps are recorded; and 4, the glossy privet fruit fingerprint spectrums obtained in the step 3 are subjected to instrument guiding-out, a traditional Chinese medicine chromatography fingerprint spectrum similarity evaluation system is guided in, chromatographic peaks existing in the chromatography maps of glossy privet fruits in different batches are selected as common peaks; comparison fingerprint spectrums of the glossy privet fruitsare generated by an average calculation method; and the relative retention time and the relative peak area of all common peaks are calculated. By means of glossy privet fruit fingerprint spectrums, the quality of the glossy privet fruits can be comprehensively and objectively represented. The fingerprint spectrum detection method has the advantages of the simple method, stability, high precision,good reproducibility and the like.
Owner:NANJING HAICHANG CHINESE MEDICINE GRPCO LTD +1
Establishment of pigeonpea leaf herb finger print, and finger print thereof
InactiveCN102980965AMonitor qualityAvoid one-sidednessComponent separationHplc fingerprintAcetic acid
The present invention provides establishment of a pigeonpea leaf herb finger print, and a finger print thereof, and relates to a quality control method for traditional Chinese medicine herbs, specifically to a method for establishing a HPLC finger print to detect a pigeonpea leaf herb extraction solution. The method comprises: (a) preparing a reference substance solution; (b) preparing a sample solution; (c) adopting the following chromatograph conditions, wherein a chromatographic column adopts octadecylsilane chemically bonded silica gel as a filler, gradient elution is performed, a mobile phase is a gradient elution solution comprising methanol and 1.0-3.0% glacial acetic acid, a column temperature is 20-40 DEG C, an ultraviolet detection wavelength is 220-300 nm, a flow rate is 0.5-1.2 mL.min<-1>, and a time is 100-140 min; and (d) determining, wherein a high performance liquid chromatography method is performed to determine the finger print. According to the present invention, quality of the pigeonpea leaf herb can be effectively characterized, herb quality monitoring can be easily achieved, the method has characteristics of simpleness, stability, high precision and good reproducibility, and authenticity of the herb can be quickly and accurately identified.
Owner:DONGGUAN MATHEMATICAL ENG ACAD OF CHINESE MEDICINE GUANGZHOU UNIV OF CHINESE MEDICINE
Finger-print spectrum detection method for Biling Weitong granules and finger-print spectrum thereof
The invention discloses a finger-print spectrum detection method for Biling Weitong granules. The method is established by an efficient liquid chromatography; chromatographic conditions are as follows: octadecylsilane bonded silica is taken as a filling agent, acetonitrile is taken as a mobile phase A; a 0.01mol / L potassium dihydrogen phosphate (phosphoric acid is used for adjusting the pH to 2 to4) water solution is taken as a mobile phase B; gradient elution is performed; the flow velocity is 0.5 to 1.5ml.min<-1>;the column temperature is 25 to 40 DEG C; the detection wave length is 210 to360nm; the number of theoretical plates is not less than 2,000 according to berberine hydrochloride peak. The detection method disclosed by the invention has the advantages of high analysis speed, high efficiency and high repeatability. The singleness and one-sidedness of the existing quality control are avoided, so that the quality of the Biling Weitong granules can be evaluated more comprehensively and accurately, and the method is of great significance to the clinical efficacy. The invention further provides a Biling Weitong granules fingerprint spectrum obtained through the finger-print spectrum detection method for the Biling Weitong granules particles.
Owner:YANGTZE RIVER PHARMA GRP JIA NGSU LONGFENGTANG TRADITIONAL CHINESE MEDICINE CO LTD +1
HPLC fingerprint determination method of cough relieving Bulbus fritillariae cirrhosae and loquat dripping pills
ActiveCN103487528AGuaranteed stabilityGuarantee the safety of useComponent separationHplc fingerprintHplc method
An HPLC (high performance liquid chromatography) fingerprint determination method of cough relieving Bulbus fritillariae cirrhosae and loquat dripping pills comprises the following steps: 1, cough relieving Bulbus fritillariae cirrhosae and loquat dripping pill tested substance solution preparation: preciously weighing the cough relieving Bulbus fritillariae cirrhosae and loquat dripping pills, and carrying out ultrasonic extraction of the cough relieving Bulbus fritillariae cirrhosae and loquat dripping pills by using 30-100% ethanol as an extraction solvent to prepare a tested substance solution; and 2, injecting the solution obtained in step 1 into a high performance liquid chromatograph, and determining to obtain a cough relieving Bulbus fritillariae cirrhosae and loquat dripping pill fingerprint. An HPLC method is adopted to establish the fingerprint according to the self characteristics of the cough relieving Bulbus fritillariae cirrhosae and loquat dripping pills, and optimal chromatogram conditions are found, so the determination result is precious, and has a good reappearance and a good stability.
Owner:津药达仁堂集团股份有限公司第六中药厂
Construction method and quality detection method of UPLC feature map of Hangzhou chrysanthemum medicinal material
ActiveCN109374786AEasy to harvestConvenient sourceComponent separationChemical compositionDicaffeoylquinic acid
The invention relates to a construction method and a quality detection method of a UPLC feature map of a Hangzhou chrysanthemum medicinal material. The construction method comprises the following steps: sample solution preparation; taking the Hangzhou chrysanthemum medicinal material, crushing, adding a solvent for extraction and filtering, wherein a filtrate is taken as a sample solution; reference solution preparation: taking chlorogenic acid, 3,5-O-dicaffeoylquinic acid, 4,5-O-dicaffeoylquinic acid, galuteolin and linarin and adding a solvent for dissolution, wherein an obtained solution isa reference solution; ultra performance liquid chromatography detection: absorbing the sample solution and the reference solution, injecting into a liquid chromatograph and detecting to obtain the UPLC feature map of the Hangzhou chrysanthemum medicinal material. The UPLC feature map is rich in characteristic peak information, can fully reflect the chemical composition of the Hangzhou chrysanthemum medicinal material and can also effectively characterize the qualities of a Hangzhou chrysanthemum decoction piece and standard decoction and identify the product authenticity.
Owner:GUANGDONG YIFANG PHARMA
Detection method for fingerprint of radix puerariae assorted Chinese herbal tea
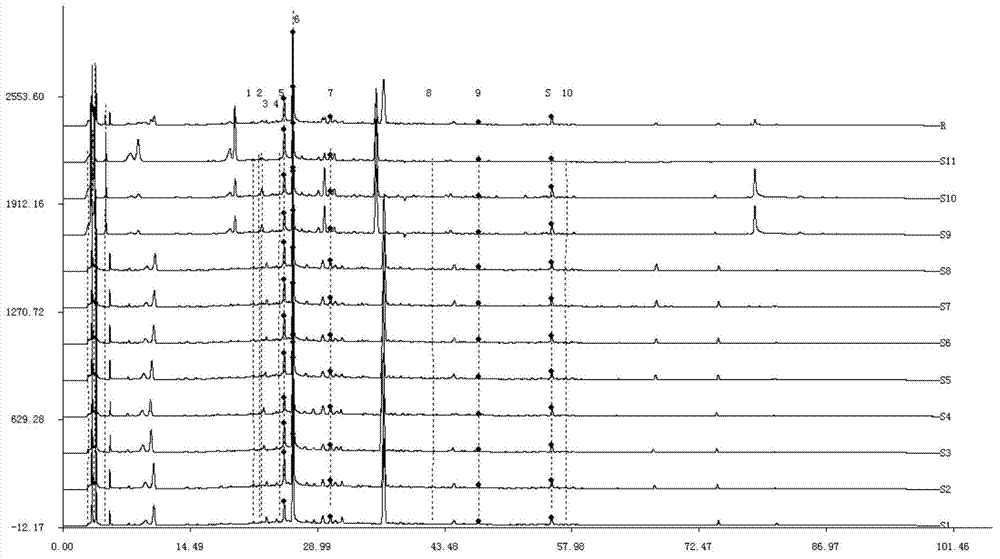
ActiveCN112748201AAvoid one-sidednessThe method is simpleComponent separationChinese herbologyFinger print
The invention discloses a detection method for the fingerprint of a radix puerariae assorted Chinese herbal tea. The detection method comprises the following steps: 1, preparing a radix puerariae assorted Chinese herbal tea test solution; 2, preparing a mixed reference substance solution; 3, respectively and precisely sucking the mixed reference substance solution and the test solution, injecting the mixed reference substance solution and the test solution into a liquid chromatograph, and recording chromatogram maps; and 4, exporting the radix puerariae assorted Chinese herbal tea fingerprint, importing the fingerprint into a traditional Chinese medicine chromatographic fingerprint similarity evaluation system, selecting chromatographic peaks existing in chromatograms of different batches of radix puerariae assorted Chinese herbal tea as common peaks, generating a contrast fingerprint spectrum of the radix puerariae assorted Chinese herbal tea by using an average value calculation method, calculating the relative retention time and the relative peak area of each common peak, comparing the fingerprint of the radix puerariae assorted Chinese herbal tea with the spectrum of the mixed standard substance, and identifying main component peaks. The radix puerariae assorted Chinese herbal tea fingerprint provided by the invention can comprehensively and objectively characterize the quality of the radix puerariae assorted Chinese herbal tea. And the detection method has the advantages of simplicity, stability, high precision, good reproducibility and the like.
Owner:NANJING HAICHANG CHINESE MEDICINE GRPCO LTD +1
Detection method for fingerprint spectrum of kidney-tonifying pregnancy-assisting granules
ActiveCN111175428AGuaranteed curative effectComprehensive detection effectComponent separationAgainst vector-borne diseasesPregnancyKidney
The invention discloses a detection method of a fingerprint spectrum of kidney-tonifying pregnancy-assisting granules. The detection method comprises the following steps: step 1, preparing a kidney-tonifying pregnancy-assisting granule test solution; step 2, preparing a mixed reference substance solution; step 3, precisely sucking the mixed reference substance solution and the test solution respectively, injecting the solutions into a liquid chromatograph, and recording chromatograms; step 4, exporting a fingerprint spectrum instrument of the kidney-tonifying pregnancy-assisting granules, importing the fingerprint spectrum instrument into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system, and selecting chromatographic peaks existing in chromatograms of different batches of kidney-tonifying pregnancy-assisting granules as common peaks; generating a reference fingerprint spectrum of the kidney-tonifying pregnancy-assisting granules by usingan average value calculation method; calculating the relative retention time and the relative peak area of each common peak; and comparing the fingerprint spectrum of the kidney-tonifying pregnancy-assisting granules with the spectrum of a mixed standard substance, and identifying the peak of main components. The fingerprint spectrum of the kidney-tonifying pregnancy-assisting granules provided bythe invention can comprehensively and objectively represent the quality of the kidney-tonifying pregnancy-assisting granules. And the detection method has the advantages of simplicity, convenience, stability, high precision, good reproducibility and the like.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Finger-print quality determination method on wuji powder preparation and intermediate thereof
ActiveCN101920001AGuaranteed stabilityGuarantee the safety of useComponent separationDigestive systemPhosphoric acidGradient elution
The invention discloses a finger-print quality determination method on wuji powder preparation and intermediate thereof. The finger-print quality determination method includes the following steps: quantitative wuji powder preparation or intermediate thereof is weighed, at least one of solvents of water, methyl alcohol or ethyl alcohol is used for extraction, constant volume is carried out, so as to obtain test solution; the test solution is sucked and injected into a liquid chromatograph, and high efficiency liquid chromatography and gradient elution are adopted for carrying out determination, so as to obtain the finger-print of wuji powder preparation or intermediate thereof; wherein the chromatographic conditions of the high efficiency liquid chromatography include that the filler of chromatographic column is octadecylsilane chemically bonded silica, mobile phase A is methyl alcohol, mobile phase B is phosphoric acid aqueous solution, the volume ratio of the mobile phase A and the mobile phase B is gradually increased along with time, and detection wavelength is 285-305nm. The invention has the characteristics that method is simple and stable, has high precision and good reproducibility and is easy to grasp.
Owner:JIUZHITANG
Marsdenia tenacissima medicinal material, method for establishing fingerprints of marsdenia tenacissima medicinal material preparation and application of method
The invention relates to a marsdenia tenacissima medicinal material, a method for establishing fingerprints of a marsdenia tenacissima medicinal material preparation, application of the method to quality control of the marsdenia tenacissima medicinal material and the preparation of marsdenia tenacissima medicinal material, and discloses fingerprints of phenolic acids and steroid saponins in the medicinal material and the preparation of marsdenia tenacissima, and a measurement method and an application of the fingerprints. The quality of the medicinal material and the preparation of marsdenia tenacissima is controlled by using the fingerprints of the phenolic acids and the steroid saponins, so that the identity, the producing area and the quality of the marsdenia tenacissima are identified and controlled, thus the quality of the medicinal material and the preparation of the marsdenia tenacissima has an actually controllable standard; stable quality of the medicinal material and the preparation of the marsdenia tenacissima is ensured. The method for controlling the quality of the marsdenia tenacissima through measuring the fingerprints of the phenolic acids and the steroid saponins in the medicinal material and the preparation of marsdenia tenacissima is simple, stable and reliable to operate.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Determination method for radix curcumae fingerprint and standard fingerprint thereof
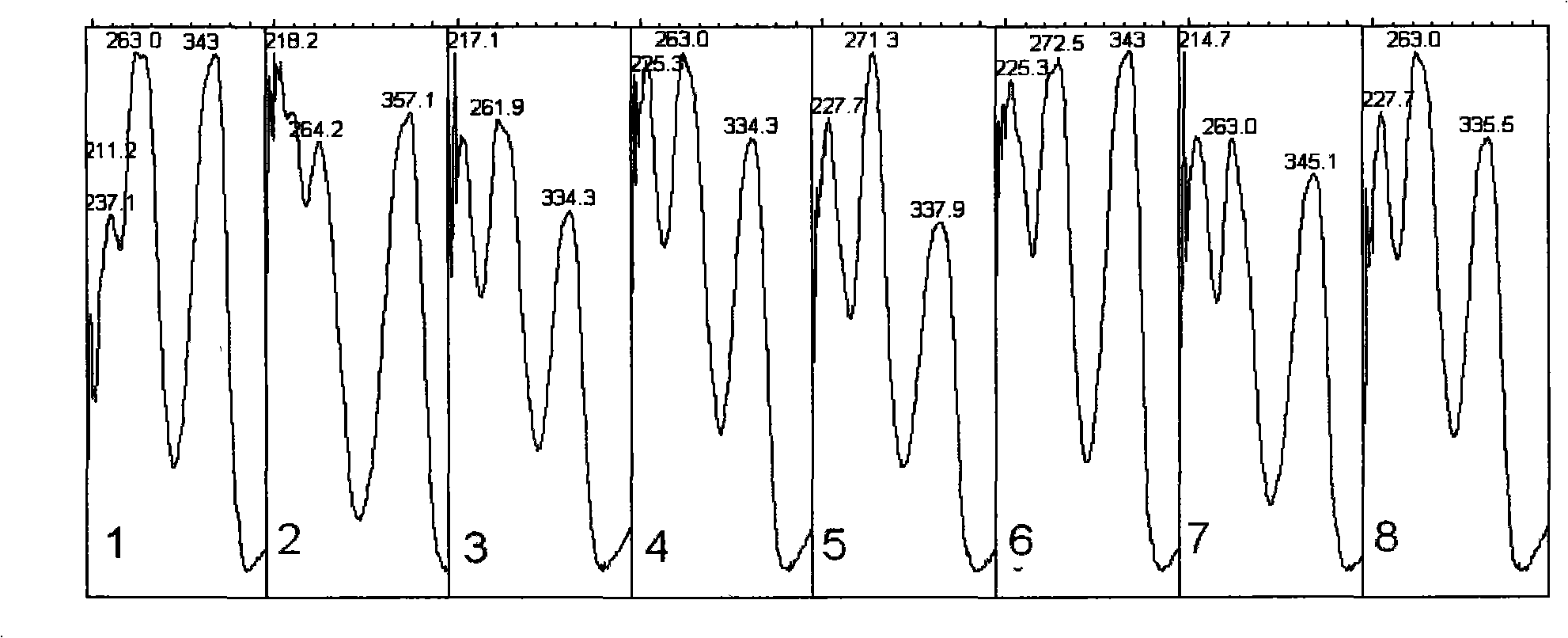
ActiveCN108693275AImprove the quality control technology of medicinal materialsEffective representation qualityComponent separationFingerprintChemistry
The invention relates to a determination method for radix curcumae fingerprint and a standard fingerprint thereof. The determination method comprises the following steps of preparing of a test samplesolution, preparing of a reference sample solution, determining by gas chromatography, and determining by liquid chromatography, so as to obtain a chromatogram map; contrasting the obtained standard radix curcumae gas fingerprint and the liquid fingerprint, wherein the qualified product is obtained when the obtained standard radix curcumae gas fingerprint is consistent with the liquid fingerprint.The determination method has the advantages that the determination method is simple, rapid, accurate, stable and reliable, and is used for controlling the quality of the radix curcumae; the quality of the radix curcumae can be more comprehensively, objectively and scientifically evaluated; the validity of the preparation is guaranteed.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
Establishment method and application of fingerprint spectrum of medicinal material, Marsdenia tenacissima and preparations of Marsdenia tenacissima
ActiveCN103884793ASimple and fast operationHigh precisionComponent separationMarsdenia tenacissimaSteroid Saponins
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Five magic skin healthy ointment fingerprint detection method
InactiveCN109490438AComprehensive detection effectComprehensive evaluationComponent separationMedicineRetention time
The invention discloses a five magic skin healthy ointment fingerprint detection method which includes the steps: 1 preparing five magic skin healthy ointment test solution; 2 preparing reference solution; 3 precisely sucking quantitative test solution and reference solution, injecting the solution into a liquid chromatograph and recording a chromatogram; 4 leading out a five magic skin healthy ointment fingerprint instrument obtained in the step 3, leading the chromatogram into a traditional Chinese medicine chromatographic fingerprint similarity evaluation system, selecting chromatographic peaks of chromatograms of five magic skin healthy ointments in different batches as common peaks, generating the reference fingerprint of the five magic skin healthy ointments by an average computing method, and calculating relative retention time and relative peak area of the common peaks. The researched five magic skin healthy ointment fingerprint detection method has the advantages that the method is simple, clear in fingerprint, high in repeatability and precision and the like. The quality of the five magic skin healthy ointments can be comprehensively and objectively represented.
Owner:NANJING HAICHANG CHINESE MEDICINE GRPCO LTD +1
Determination method for fingerprint chromatogram of HuangShiXiangShengWan preparation and standard fingerprint chromatogram thereof
ActiveCN104374838AEffective massEffective representation qualityComponent separationTest articleReference product
The invention relates to a determination method for a fingerprint chromatogram of a HuangShiXiangShengWan preparation and a standard fingerprint chromatogram thereof. The determination method comprises the steps of preparing a test article solution, preparing a reference product solution, injecting the test article solution and the reference product solution into a gas chromatograph to carry out determination, and on the basis of the standard fingerprint chromatogram, judging whether the quality of the obtained HuangShiXiangShengWan is qualified data by using the determination method. The determination method is simple, fast, accurate, stable and reliable, can be used for controlling the quality of a HuangShiXiangShengWan sample, can be used for evaluating the quality of the HuangShiXiangShengWan in a comprehensive, objective and scientific manner and can guarantee effectiveness of the preparation.
Owner:WUXI JIYU SHANHE PHARM CO LTD
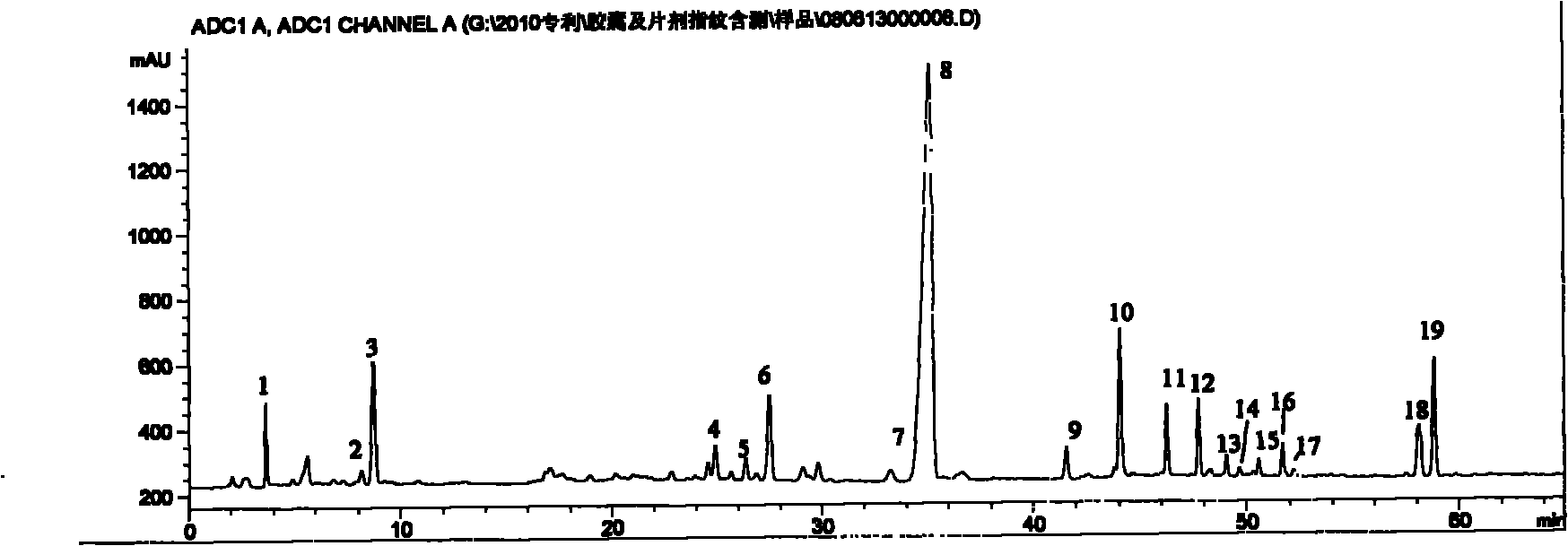
Detection method for specific chromatogram of medicinal preparation and application thereof
ActiveCN113447595AGuaranteed stabilityGuaranteed adaptabilityComponent separationAgainst vector-borne diseasesMedicinal herbsBiochemical engineering
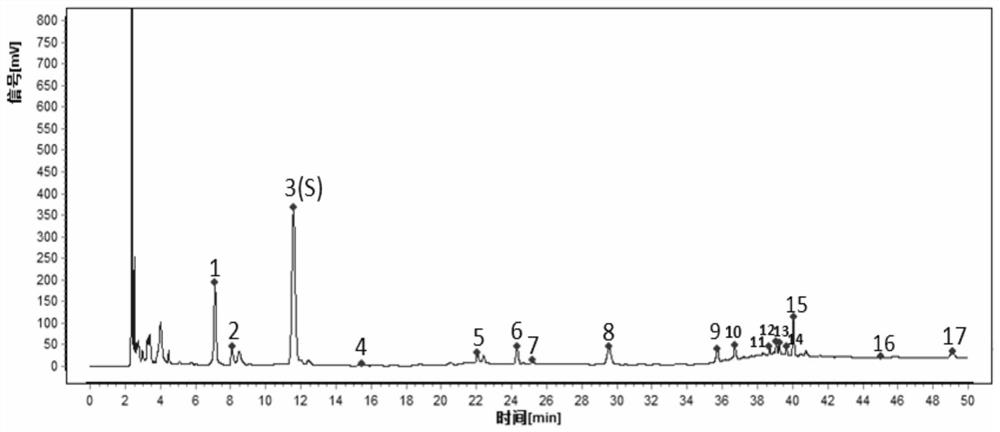
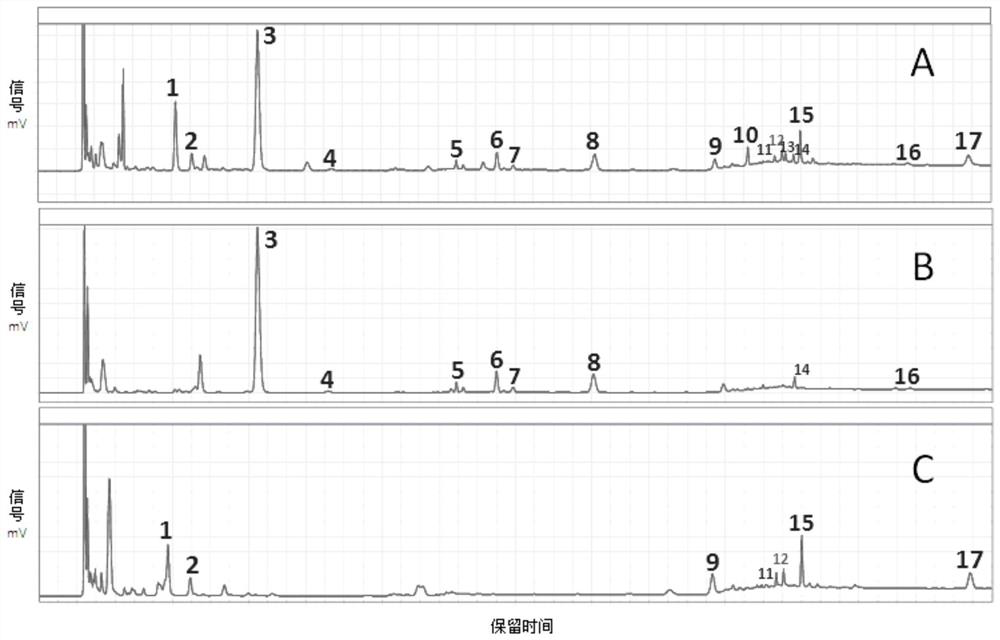
The invention provides a detection method for a specific chromatogram of medicinal preparation. The method comprises the following steps: 1) preparing a test solution; 2) preparing a reference substance solution; and 3) carrying out determination. The invention further provides application of the detection method of the specific chromatogram of the medicinal preparation in quality detection of the medicinal preparation, and a construction method of the standard specific chromatogram of the medicinal preparation, and a quality control method. The invention further provides a screening method of the specific chromatograms of multiple medicinal materials in the medicinal preparation. According to the method for detecting the specific chromatogram of the pharmaceutical preparation and the application of the method, the specific chromatogram of the pharmaceutical preparation containing 17 characteristic peaks can be obtained, 10 characteristic peaks in the specific chromatogram can be subjected to positioning attribution, the method is good in precision, stability and reproducibility, it is guaranteed that the amount of effective components in the pharmaceutical preparation is relatively stable, therefore, safety and effectiveness of clinical medication are guaranteed.
Owner:HEHUANG PHARMA SHANGHAI
Method for determining fingerprint chromatogram of radix codcnopsitis pilosulas, ramulus cinnamomi and poria cocos preparation
The invention relates to a method for detecting a pharmaceutical preparation, in particular to a method for constructing a fingerprint chromatogram of a traditional Chinese medicine preparation, namely, a radix codcnopsitis pilosulas, ramulus cinnamomi and poria cocos oral solution. High performance liquid chromatography is mainly adopted for detection, an acetonitrile-0.5% glacial acetic acid aqueous solution is taken as a mobile phase, A is acetonitrile, a 0.5% glacial acetic acid aqueous solution is taken as a mobile phase B, the detection wavelength is 203 nm, the column temperature is 30DEG C, the injection quantity is 10 mu l, and all the components are detected within 60 min.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
The establishment method of the fingerprint of Shenzhiling Oral Liquid, its fingerprint and its application
ActiveCN104849364BImprove stabilityGood reproducibilityComponent separationBiochemical engineeringInformation quantity
The invention discloses a canzhiling oral solution fingerprint map building method. The map includes the following steps of preparation of a test solution, preparation of a reference solution, testing through a high performance liquid chromatograph and processing of data and a map. The invention further discloses a Canzhiling oral solution fingerprint map and a method for utilizing the fingerprint map to control quality of a Canzhiling oral solution. The Canzhiling oral solution fingerprint map building method is simple in operation, stable, reliable, high in accuracy and high in separation degree, the fingerprint map is high in stability and reproducibility and large in information quantity, and the fingerprint map is adopted as a quality control means for the Canzhiling oral solution, so that one-sidedness caused by judging of overall quality of a preparation by testing one or two chemical ingredients is avoided, and probability of artificial processing in order to enabling quality to be up to standards is lowered; samples of multiple batches are analyzed systematically, so that quality of the Canzhiling oral solution can be evaluated more comprehensively and scientifically, and product quality and efficacy are guaranteed.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com