Detection method for specific chromatogram of medicinal preparation and application thereof
A technology for characteristic maps and pharmaceutical preparations, which is applied in measuring devices, instruments, scientific instruments, etc., can solve the problem of less research on chemical components, achieve good reproducibility, reduce the possibility of artificial processing, and avoid one-sided effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] 1. Preparation of the test solution
[0076] The pharmaceutical preparation samples were prepared according to the ancient method recorded in the classic famous prescription "Synopsis of the Golden Chamber", that is, the fresh lilies were washed with clean water to remove the floating water on the surface, and 7 lilies were weighed. Wash it with clean water and set it aside; take fresh rehmannia glutinosa and squeeze 200mL of fresh rehmannia glutinosa juice into a juicer and boil it for later use. Take the above-mentioned lilies, add 400mL of water to a beaker, place them in a medicine pot, heat and decoct to 200mL, filter, add the above-mentioned fresh rehmannia juice to the filtrate, stir evenly, heat and decoct to 300mL, and the product is ready.
[0077] Take 3g of the drug preparation sample, accurately weigh it, put it in a 50ml measuring bottle, add water to dissolve, and dilute to the scale constant volume, shake well, and filter through a 0.45μm microporous mem...
Embodiment 2
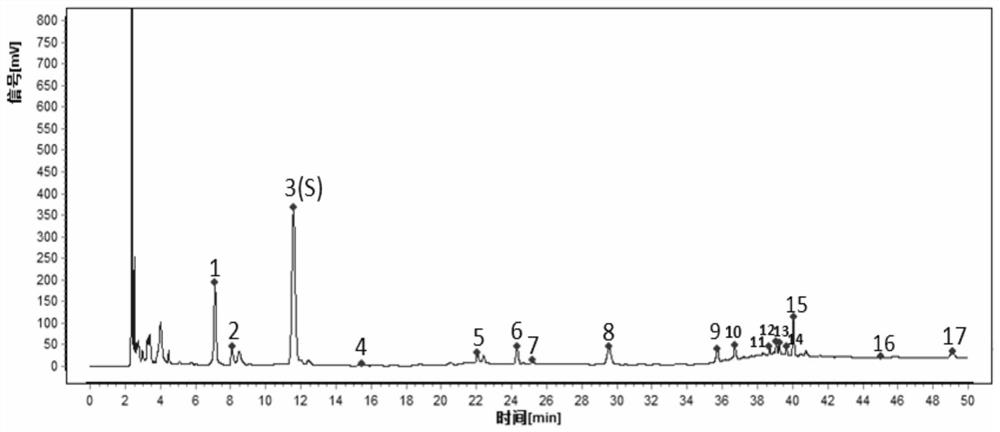
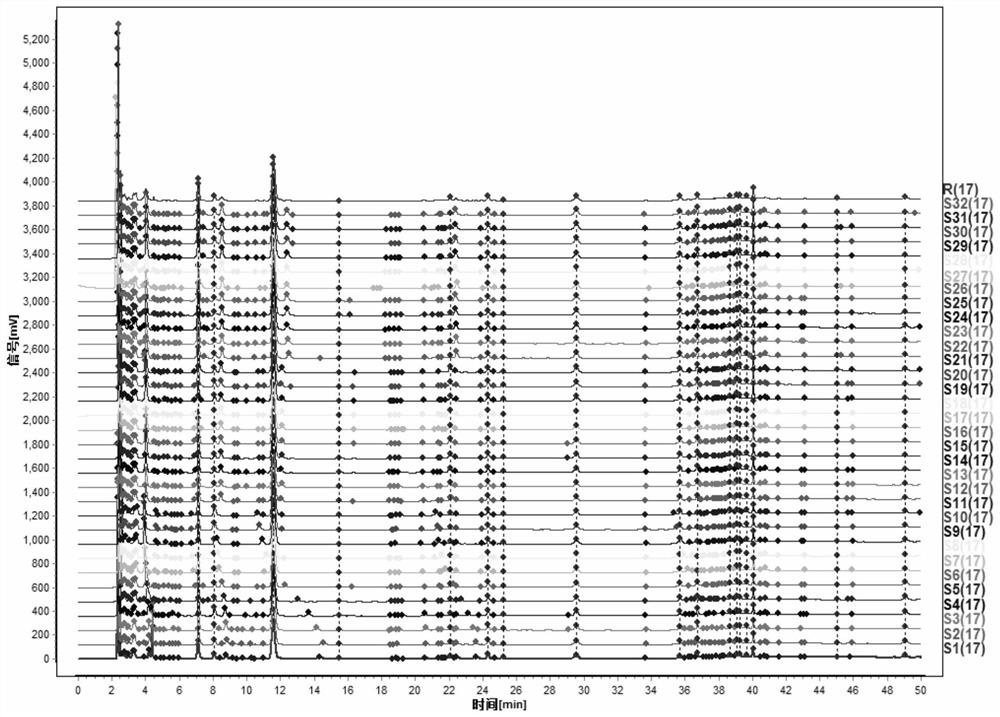
[0093] According to the method in Example 1, get 16 batches of pharmaceutical preparations and repeat the detection twice to obtain the characteristic spectra of a plurality of pharmaceutical preparation samples to generate a common contrast characteristic spectrum, and use the chromatographic peaks that exist in the spectra as the common characteristic peaks to determine the common characteristic peaks The relative retention time of the drug preparation is established to establish a standard characteristic map.
[0094] like figure 1 As shown, the standard feature spectrum of the pharmaceutical preparation includes 17 common fingerprint peaks, with No. 3 peak as the reference peak (S peak, retention time is 1.0000), and the relative retention time of other 16 common fingerprint peaks is No. 1 peak (0.6040 -0.6281), No. 2 peak (0.6840-0.7083), No. 4 peak (1.3314-1.3449), No. 5 peak (1.7815-1.9266), No. 6 peak (1.9625-2.1283), No. 7 peak (2.0377-2.2067), 8 No. peak (2.3913-2.5...
Embodiment 3
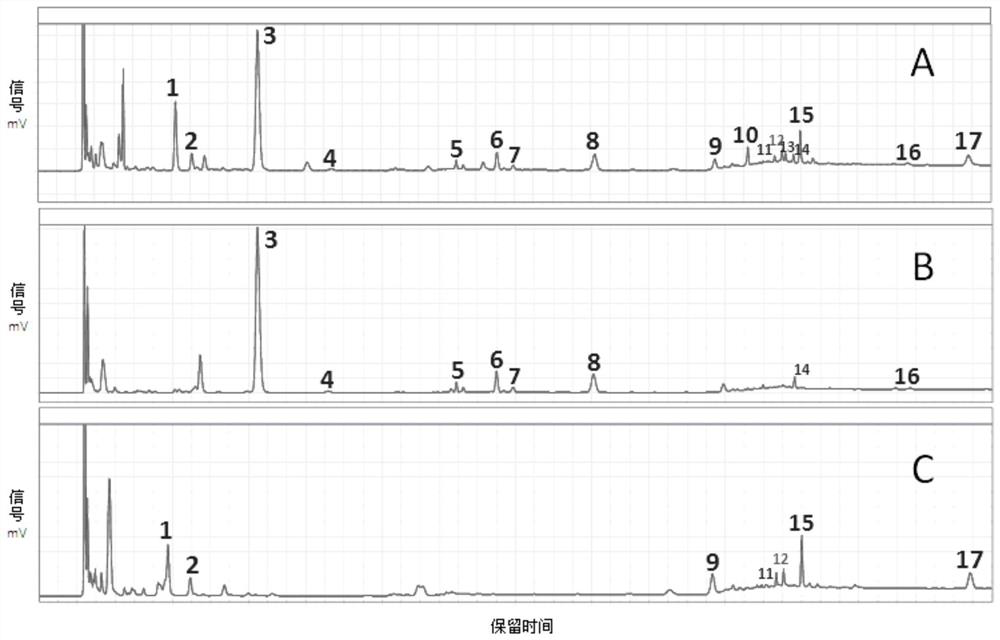
[0097] Remove any one of the medicinal materials from the pharmaceutical preparation samples containing rehmannia glutinosa and lily, and prepare according to step 1) of the detection method of the characteristic map of the pharmaceutical preparation in Example 1, and obtain 2 kinds of negative sample solutions without samples.
[0098] Adopt the high-performance liquid chromatography (HPLC) method of the same chromatographic condition in the step 3) of the detection method of the characteristic spectrum of the pharmaceutical preparation in Example 1 to measure 2 kinds of lacking sample negative sample solutions respectively, obtain 2 kinds of lacking sample negative samples respectively The characteristic spectrum of the solution.
[0099] Compare the characteristic spectra of the two kinds of lack-of-sample negative sample solutions with the standard characteristic spectra of pharmaceutical preparations established by the construction method of the standard characteristic map...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com