Marsdenia tenacissima medicinal material, method for establishing fingerprints of marsdenia tenacissima medicinal material preparation and application of method
A technique of fingerprinting and establishing methods, which is applied in the field of quality control of traditional Chinese medicinal materials and their preparations, can solve problems such as inability to fully reflect the overall characteristics of medicinal materials and injections, drug efficacy, and poor control of the quality of injections, etc., to achieve Easy operation, high precision, good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
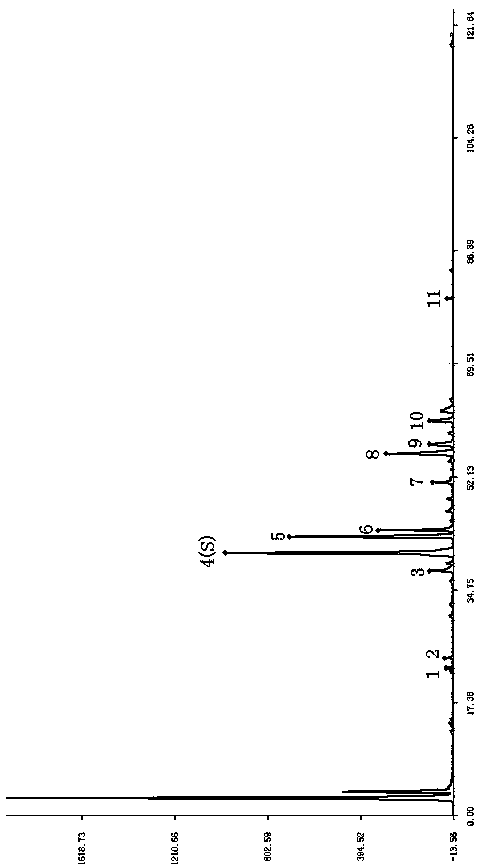
[0062] Methodological verification of fingerprints of steroidal saponins in Tengguan Teng medicinal material:
[0063] Chromatographic conditions: Column: Agilent ZORBAX SB-C 18 (250×4.6mm, 5μm) mobile phase A is water, mobile phase B is acetonitrile, gradient elution: 0~23min: 75%A→65%A, 25%B→35%B: 23~48min: 65% A→50%A, 35%B→50%B: 48~60min: 50%A→50%A, 50%B→50%B: 60~90min: 50%A→20%A, 50%B→ 80%B: 90~100min: 20%A→10%A: 80%B→90%B: 100~101min: 10%A→75%A, 90%B→25%B: 101~120min: 75% A→75%A, 25%B→25%B: column temperature: 35°C, flow rate: 0.8ml / min, detector: Alltech2000ES evaporative light scattering detector; atomization gas flow rate: 2.9L / min; drift tube Temperature: 106.5°C. The injection volume is 20 μL.
[0064] precision test
[0065] Take the same test solution, continuously inject 6 times, calculate the relative retention time and relative peak area of the characteristic peak, the RSD is less than 0.04% and 1.4% respectively, and the similarity is greater than 0.95, ...
Embodiment 2
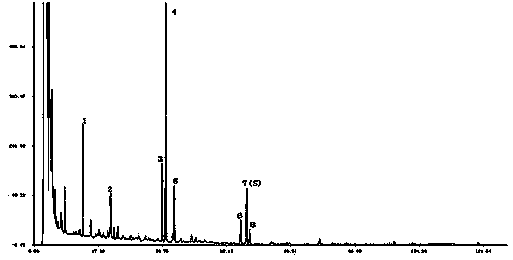
[0077] Methodological verification of fingerprints of phenolic acids in Tongguanteng injection:
[0078] Chromatographic conditions: Column: Agilent ZORBAX SB-C 18 (250×4.6mm, 5μm); mobile phase A is 0.05% phosphoric acid aqueous solution, mobile phase B is 0.05 phosphoric acid acetonitrile solution, gradient elution: 0~20min: 98A→96%A, 2%B→4%B: 20 ~70min: 96%A→93%A, 4%B→7%B: 70~110min: 93%A→90%A, 7%B→10%B: 110~115min: 90%A→85%A , 10%B→15%B: 115~116min: 85%A→98%A, 15%B→2%B: equilibration time 10 minutes; flow rate 1.0ml / min: detection wavelength 300nm; column temperature 25°C.
[0079] (batch number 200706301), 6 consecutive injections (S1~A6), 20 μl each time, after the integration of the obtained chromatogram, the similarity with the control fingerprint was calculated by the fingerprint software, all above 0.95, see Table 4 and Figure 7 .
[0080] Table 4 Similarity of phenolic acids fingerprints in Tongguanteng Injection
[0081]
S1
S2
S3
S4 ...
Embodiment 3
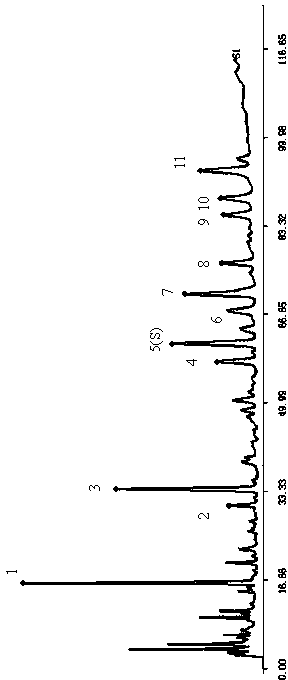
[0089] Methodological verification of fingerprints of steroidal saponins in Tongguanteng injection:
[0090] Chromatographic conditions: Column: Agilent ZORBAX SB-C 18 (250×4.6mm, 5μm): mobile phase A is water, mobile phase B is acetonitrile, gradient elution: 0~110.0min: 90%A→44%A, 10%B→56%B: 110.0~110.1min : 44%A→90%A, 56%B→10%B: 110.1~120.0min: 98%A→90%A, 10%B→10%B: flow rate 0.8ml / min; column temperature 35℃, detection Detector: evaporative light scattering detector; atomization gas flow rate: 2.9 L / min; drift tube temperature: 106.5°C, injection volume 20 μl.
[0091] precision test
[0092] Take Tongguanteng Injection (batch number: 2000810241), and inject 6 times continuously, calculate the relative retention time and relative peak area according to the characteristic peaks, the RSDs are less than 1% and 3% respectively, and the obtained chromatograms are integrated and then calculated and compared by fingerprint software The similarity of the fingerprints is above 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com