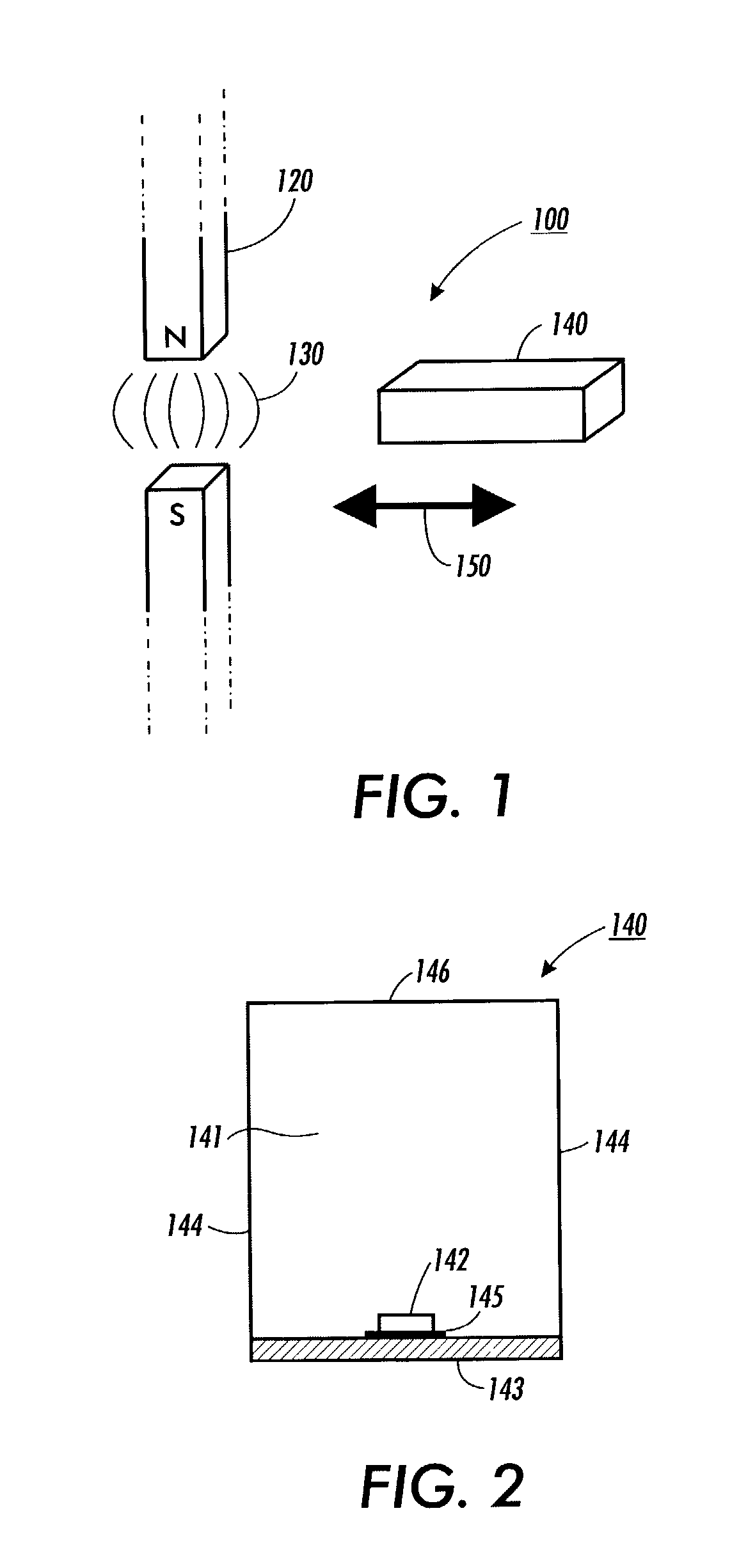
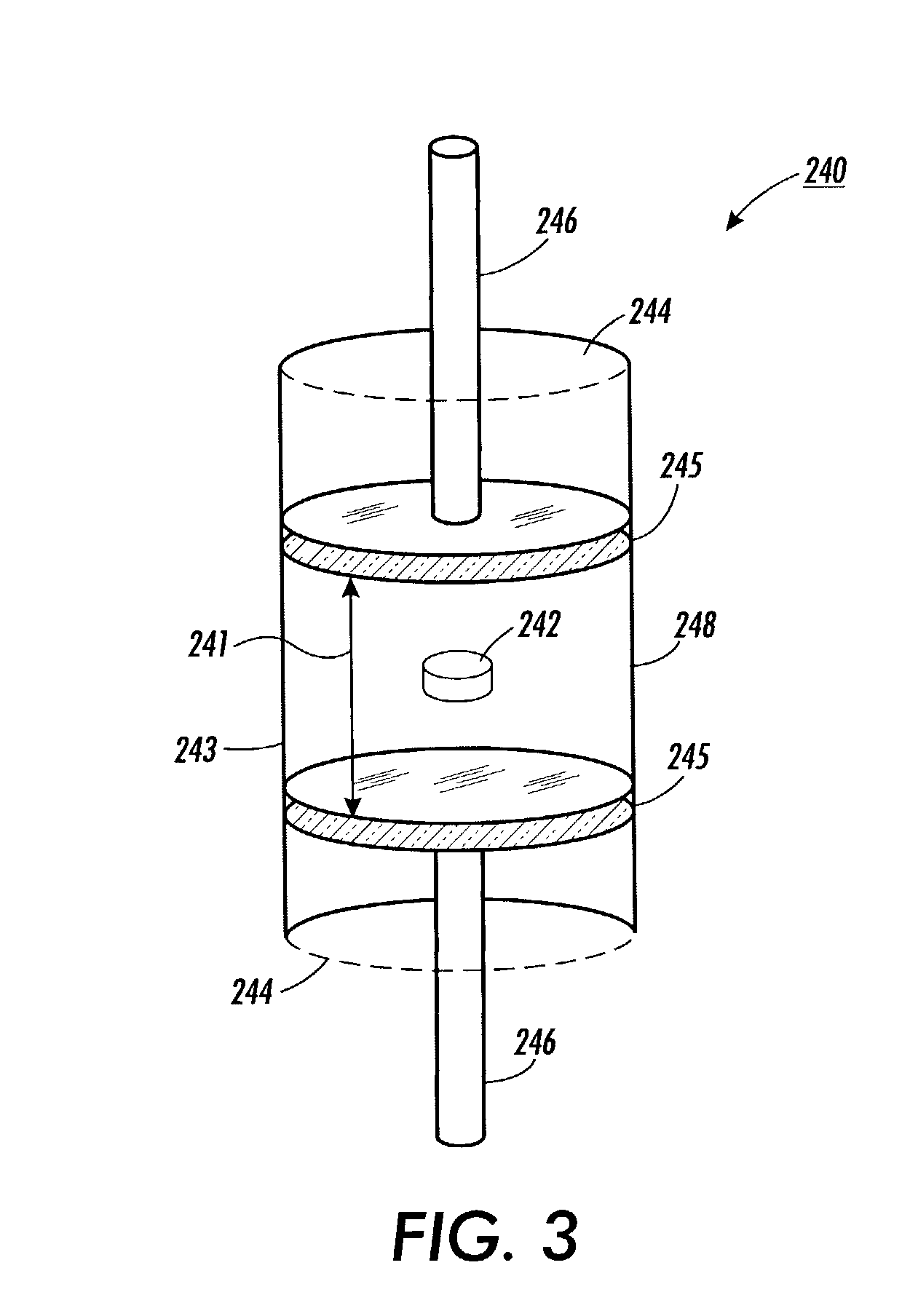
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61 results about "Molecular magnets" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
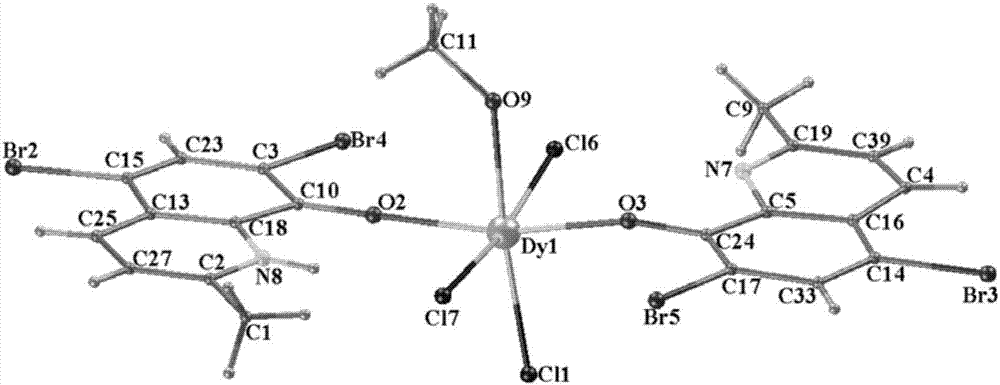
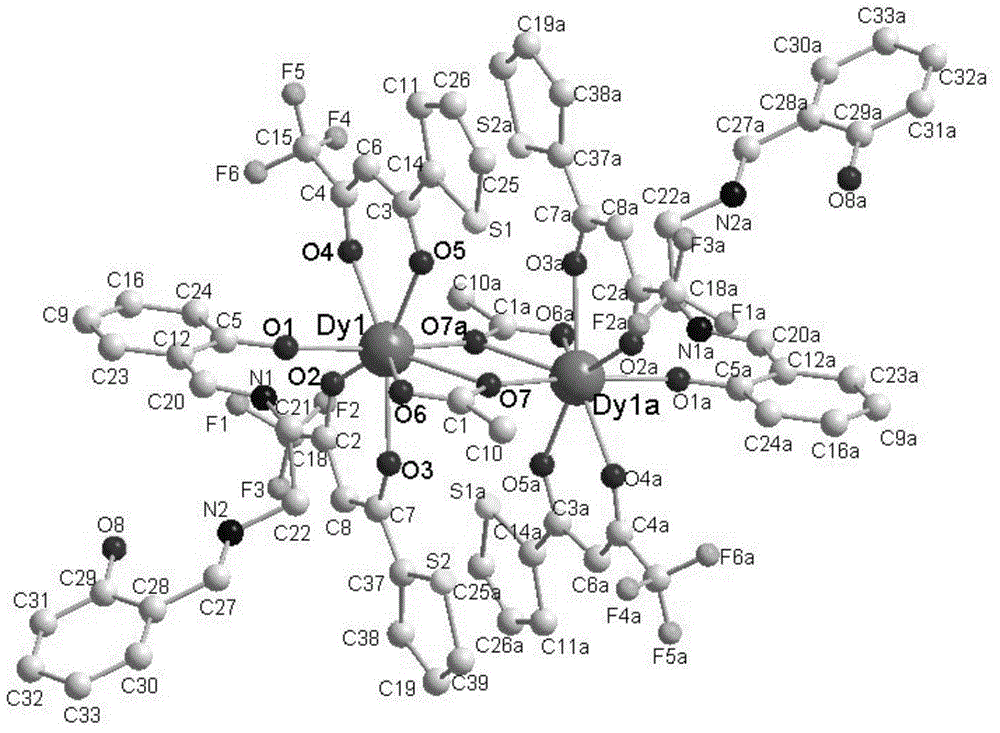
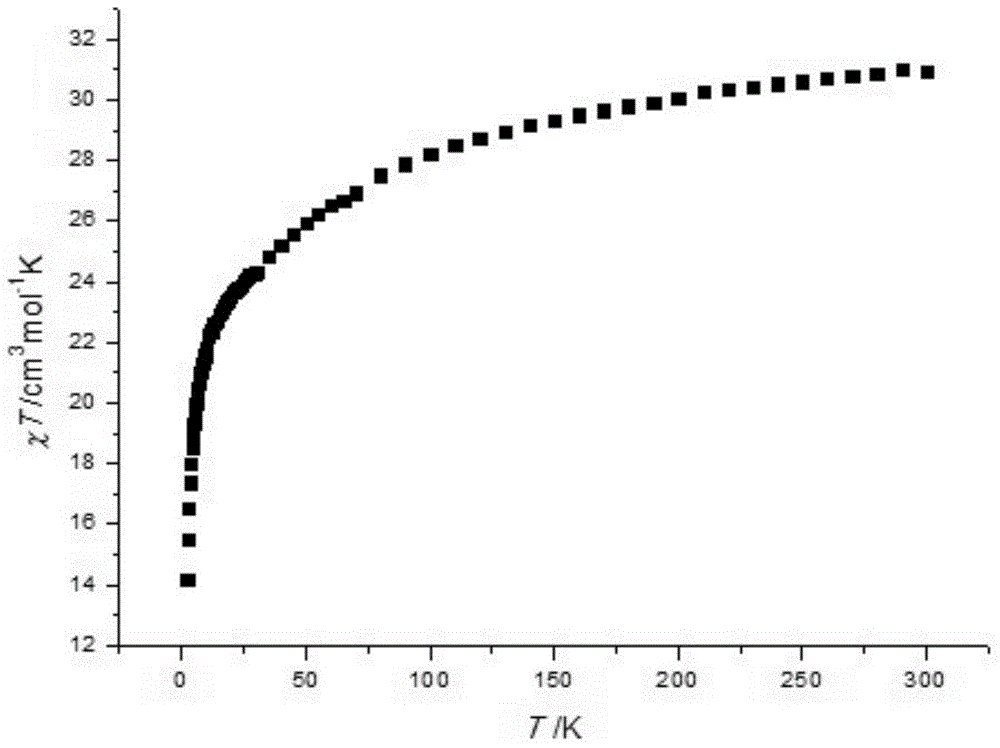
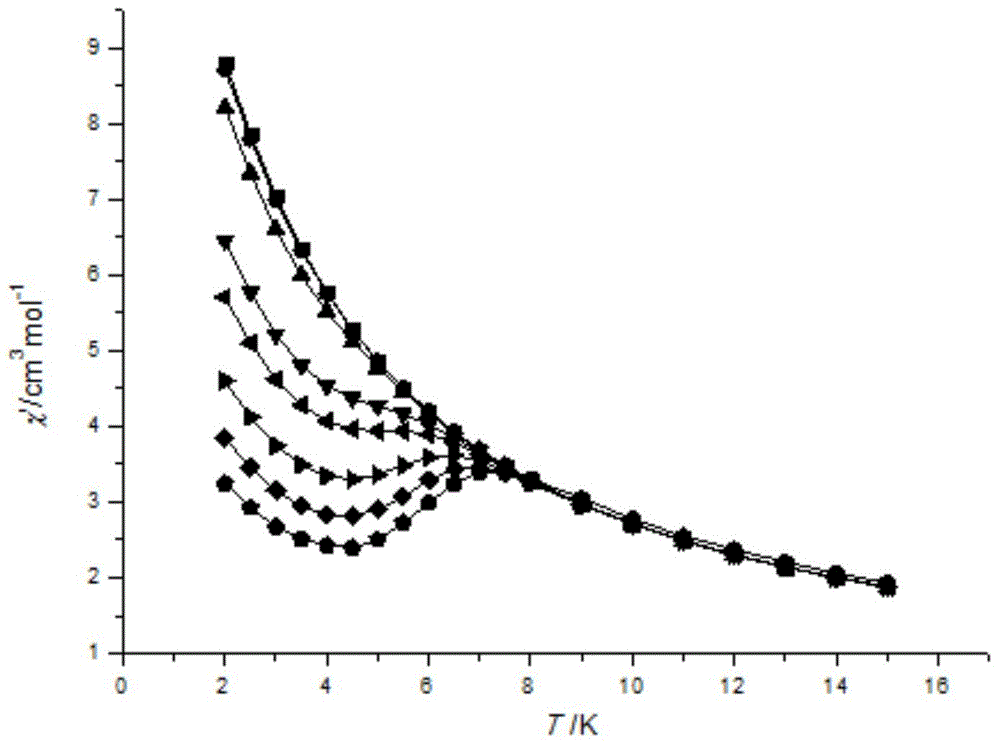
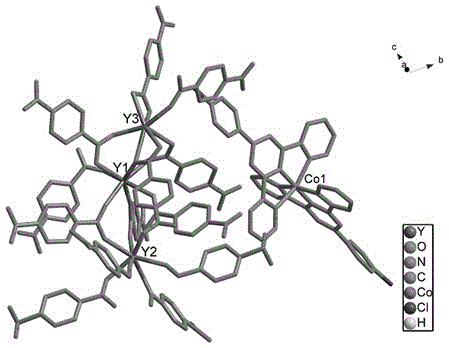
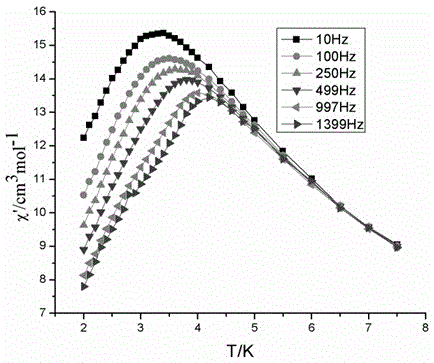
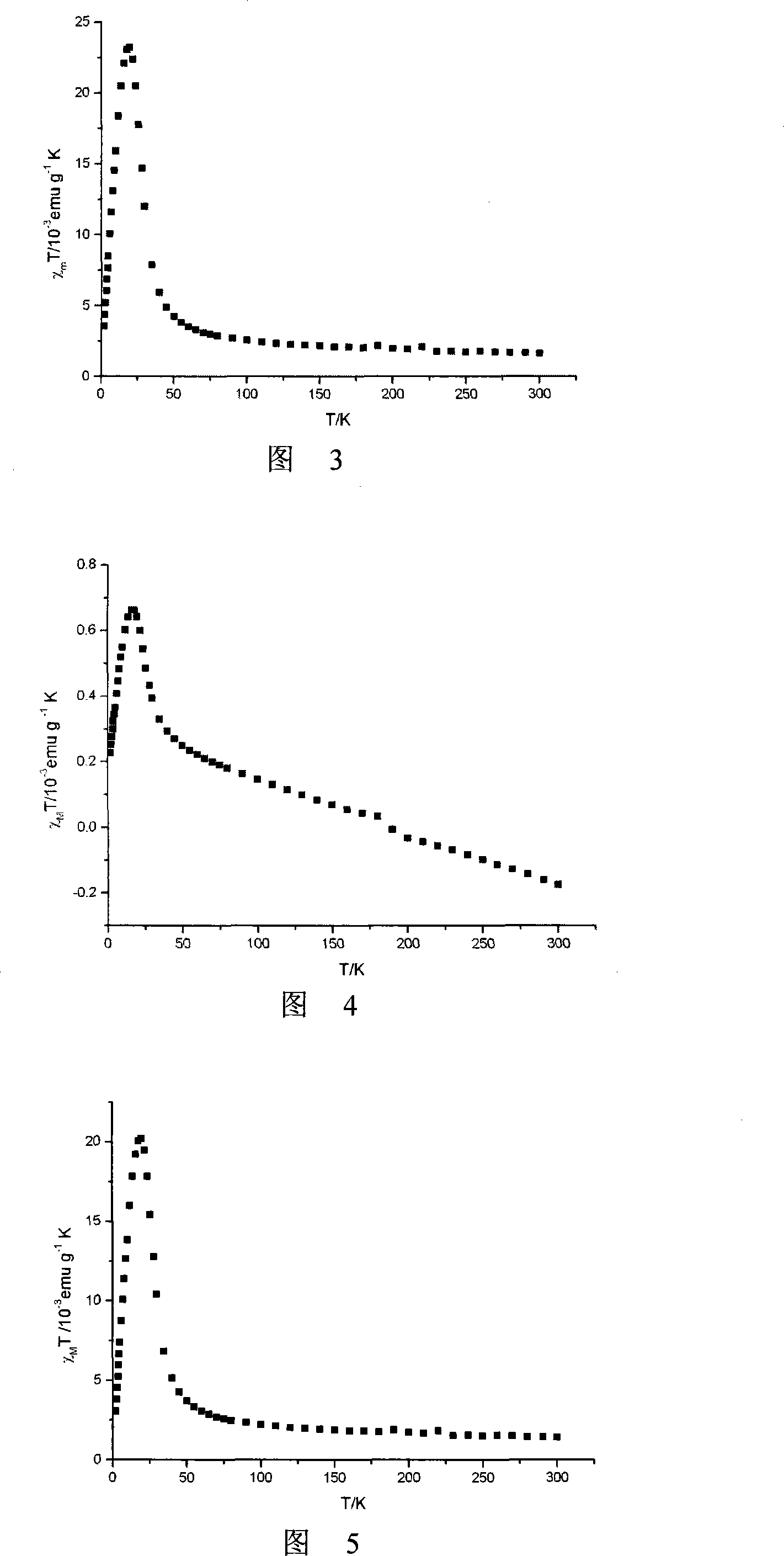
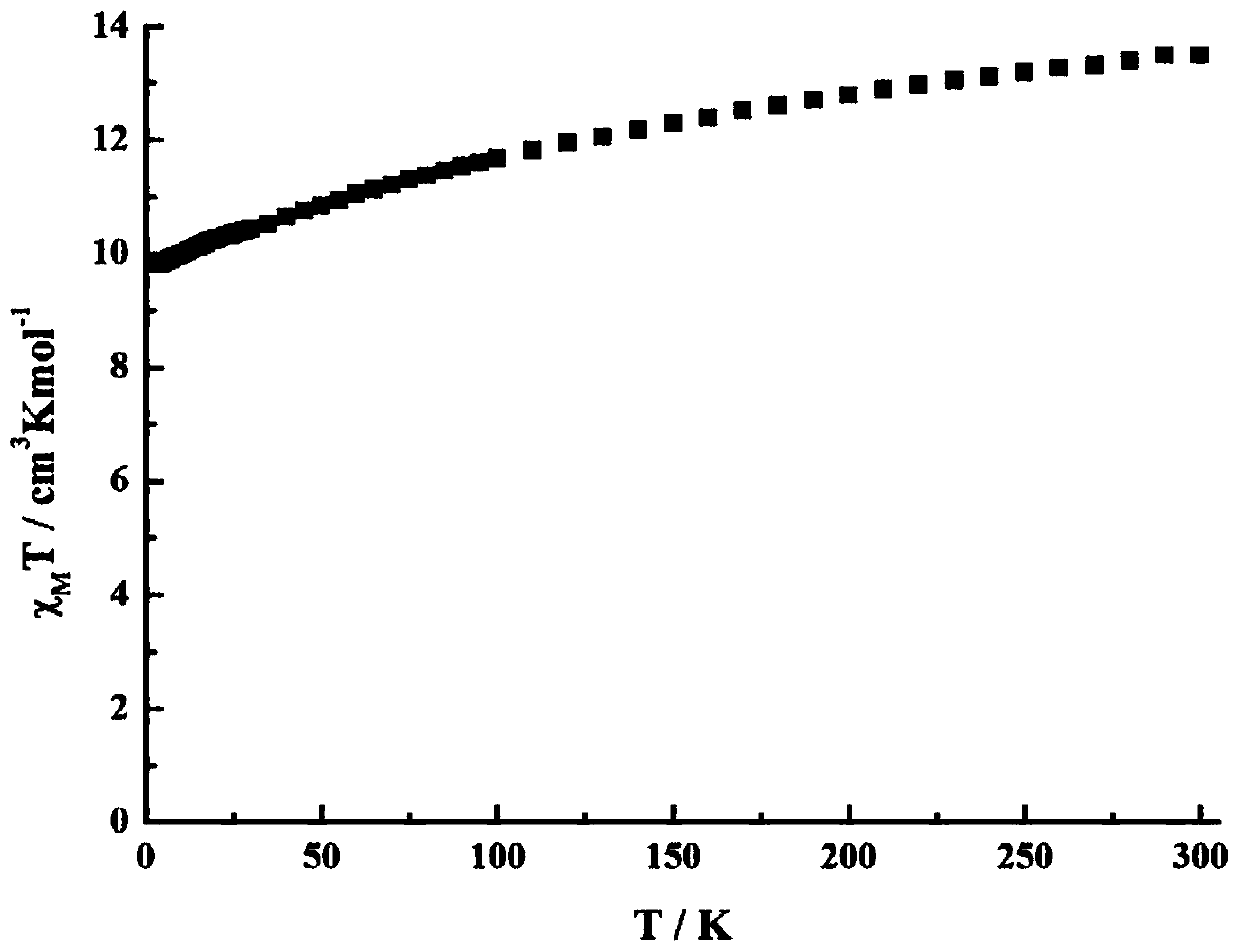
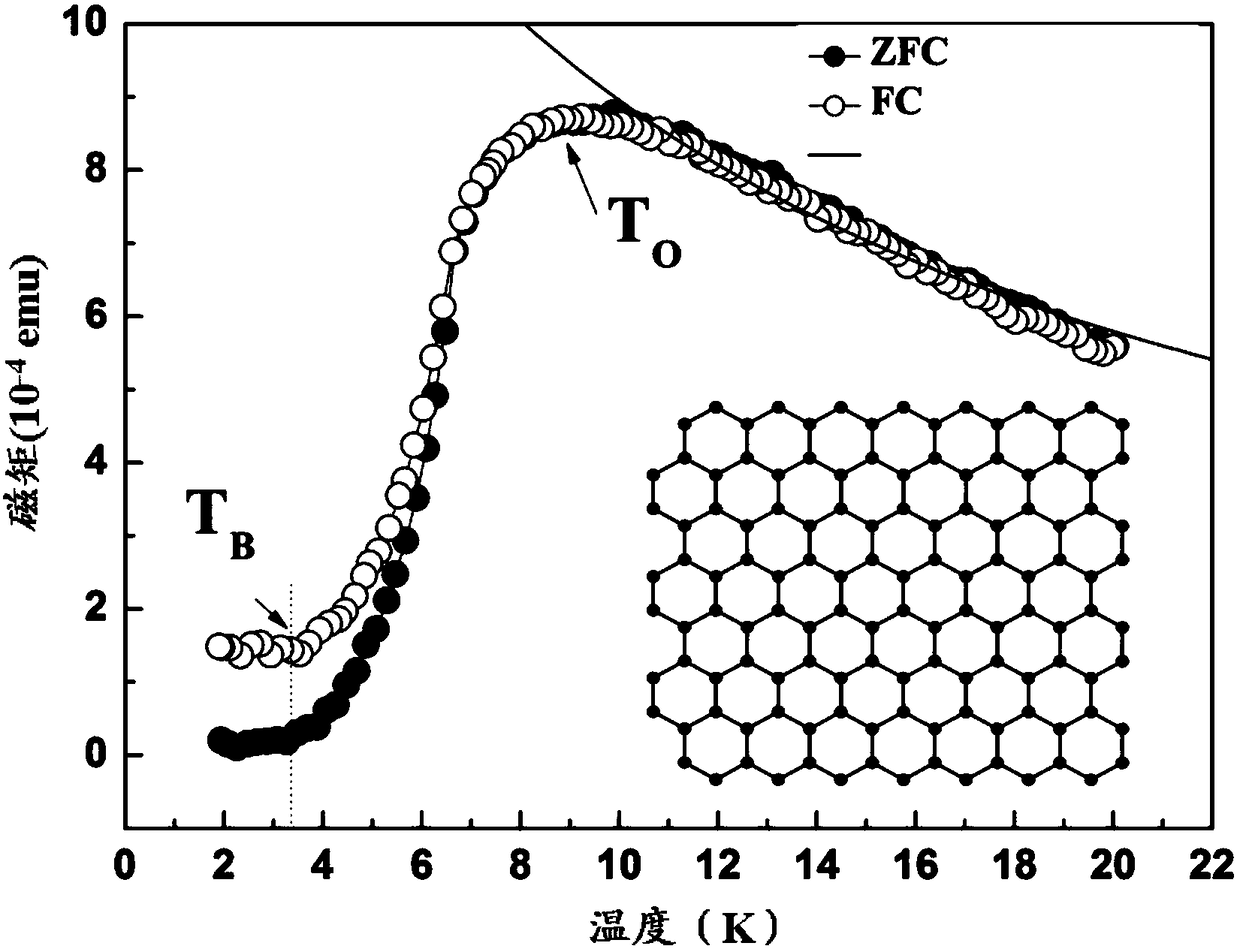
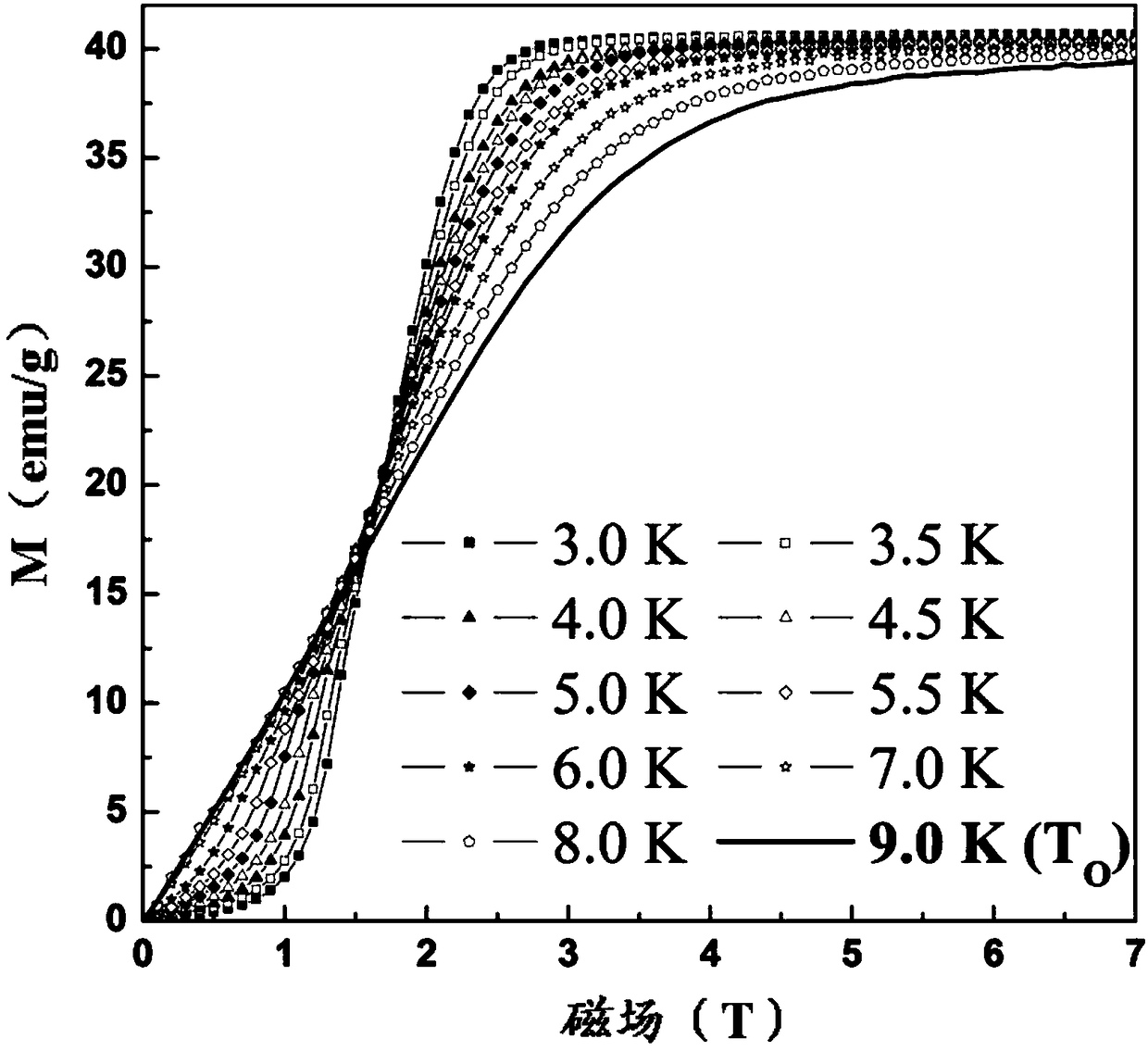
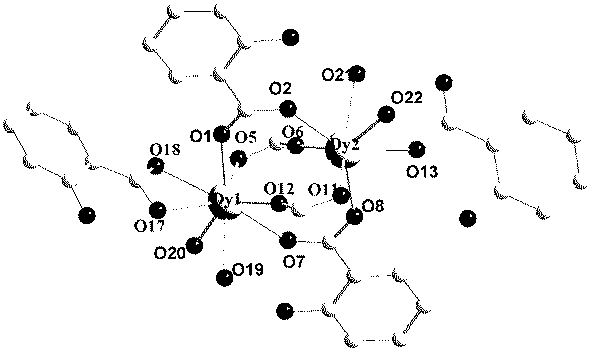
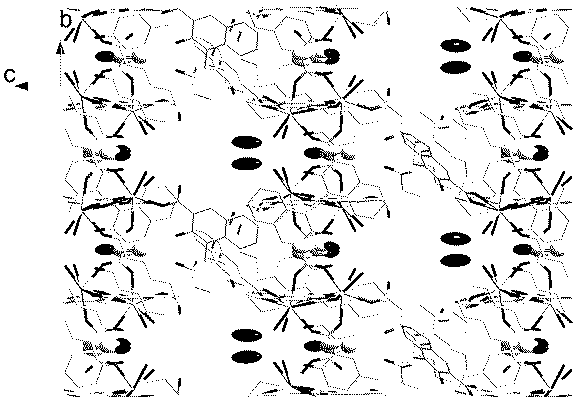
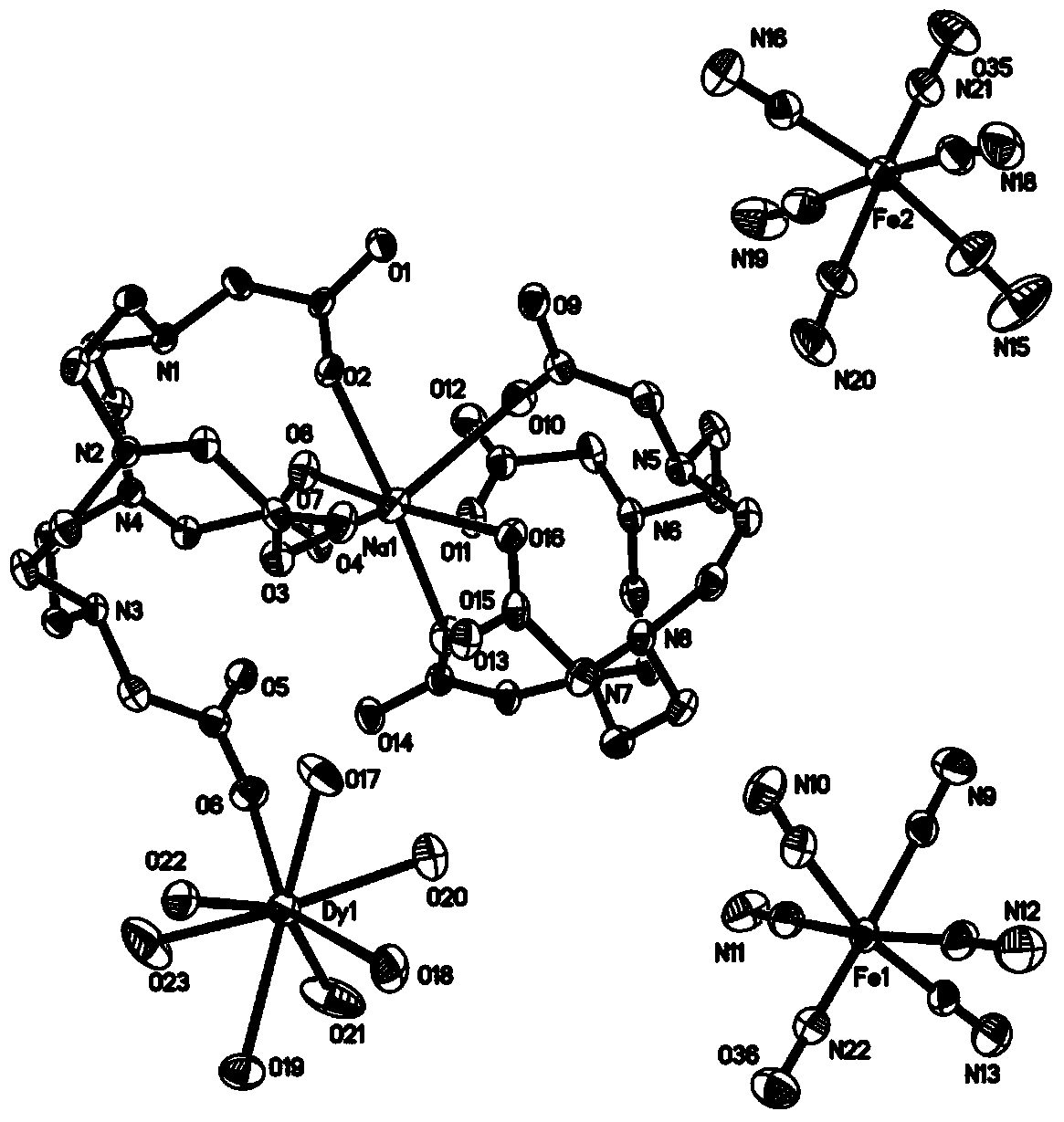
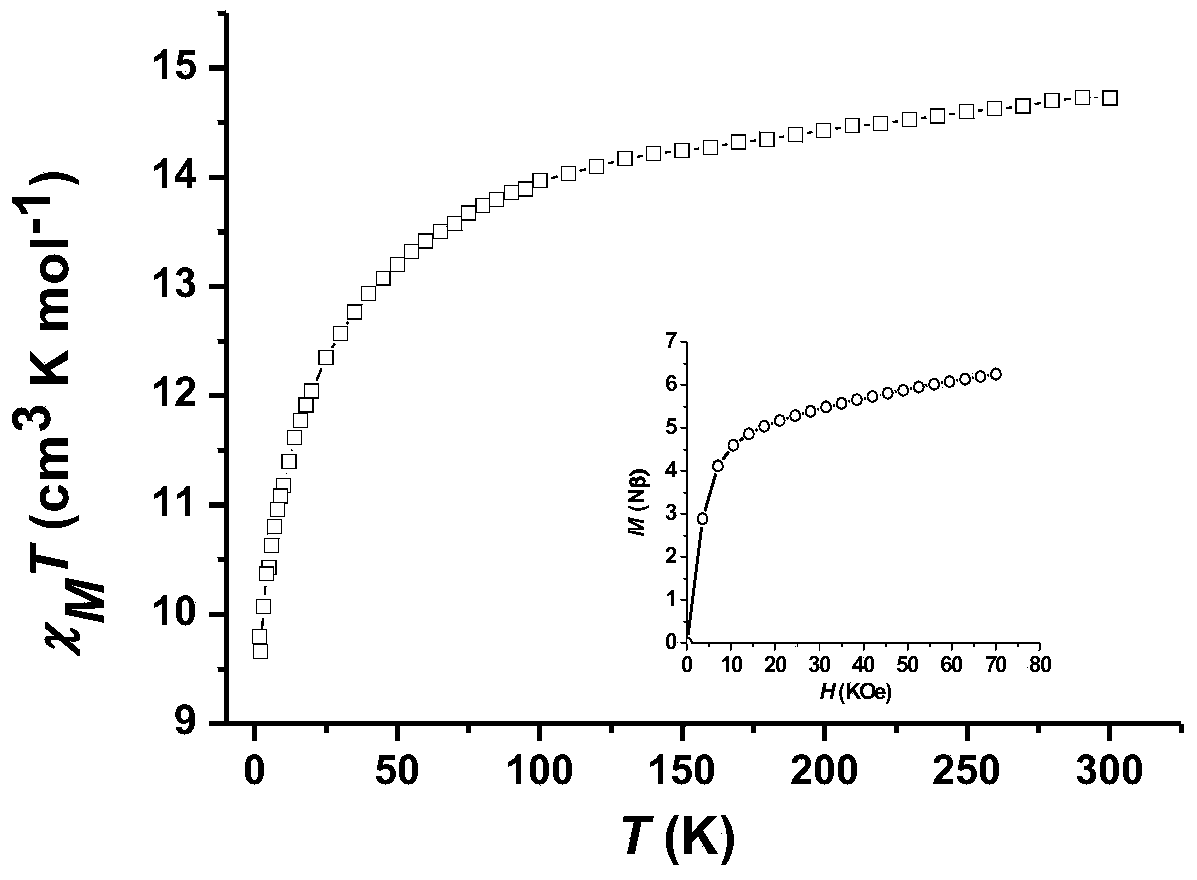
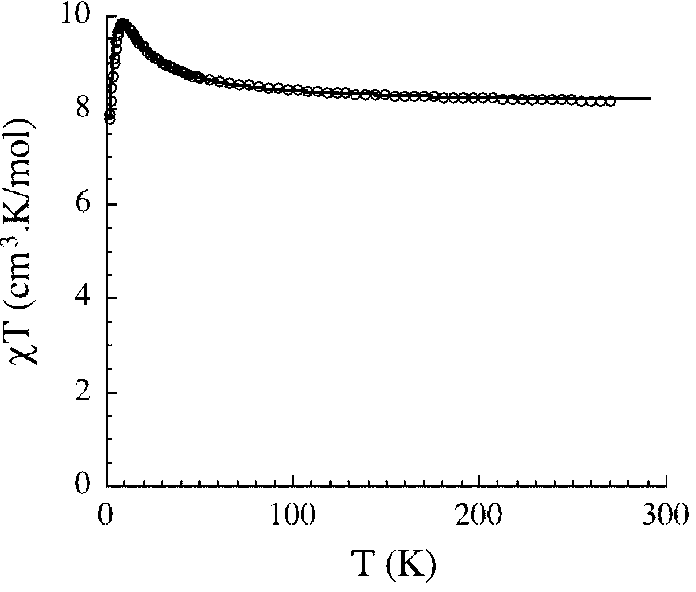
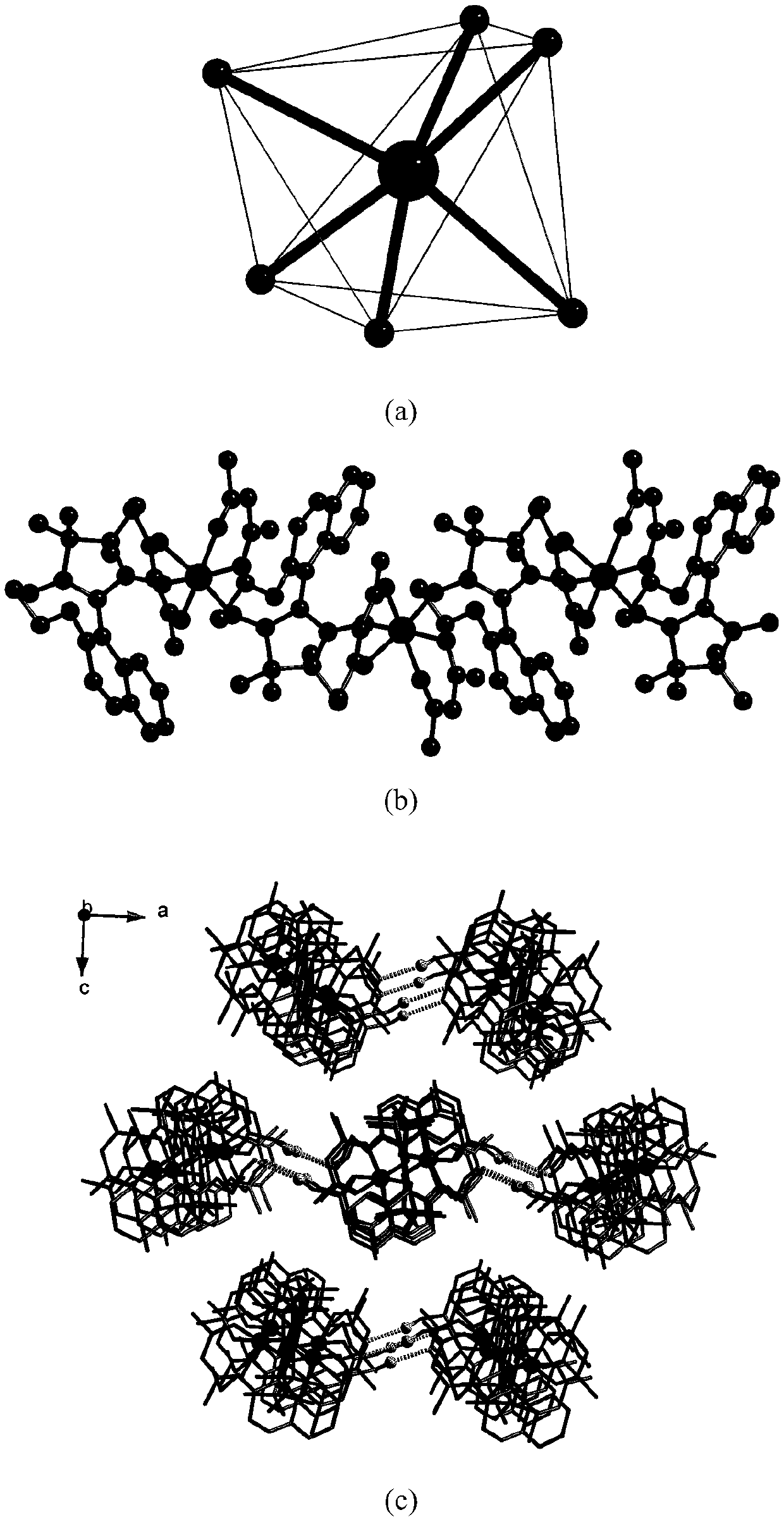
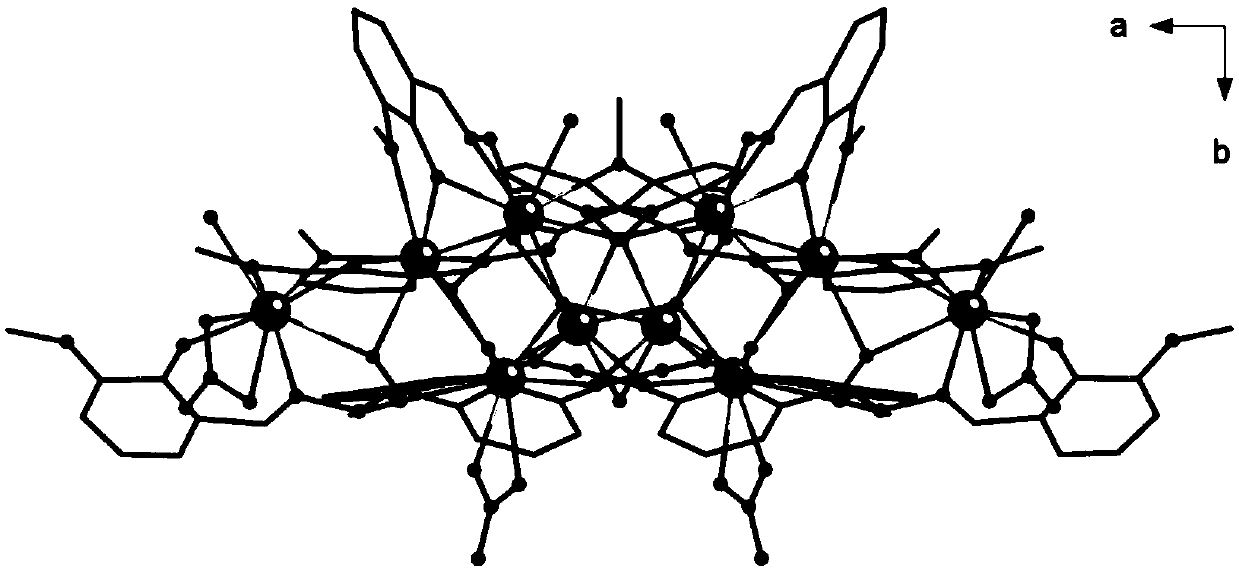
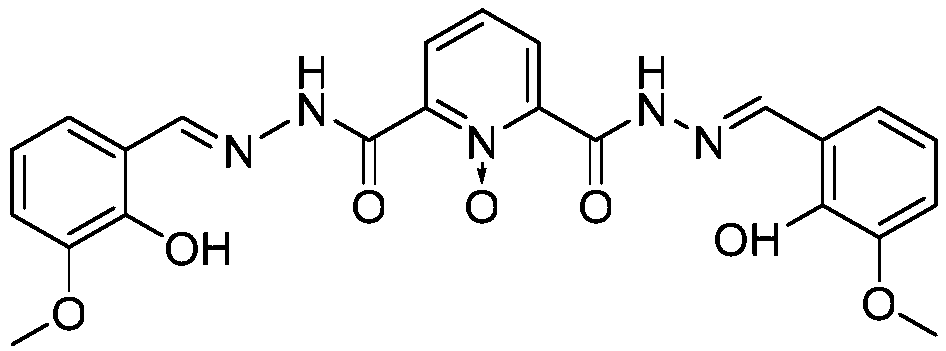
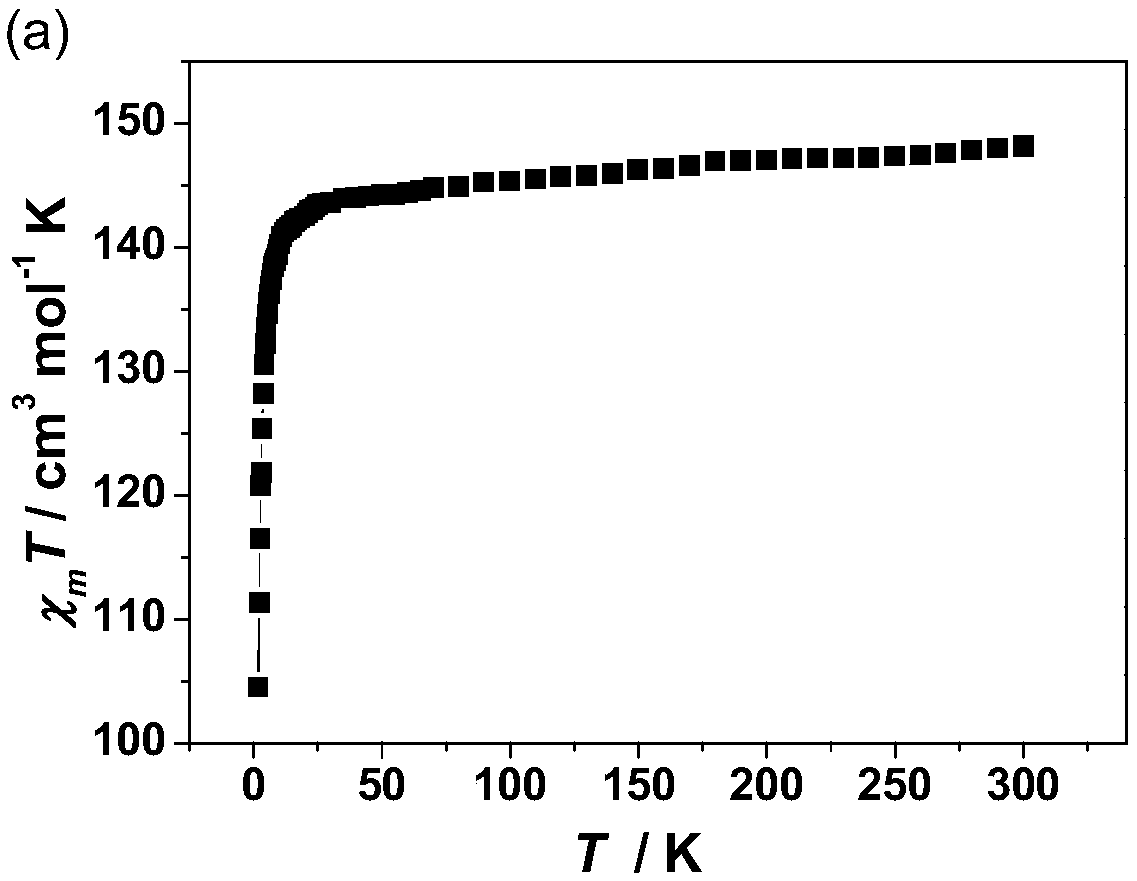
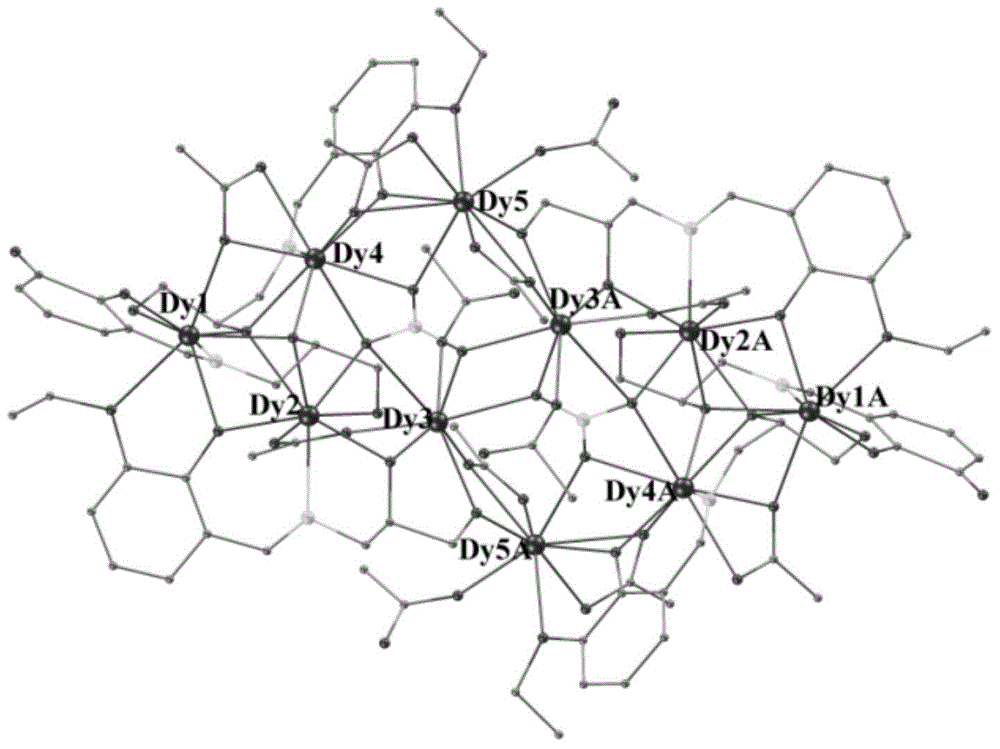
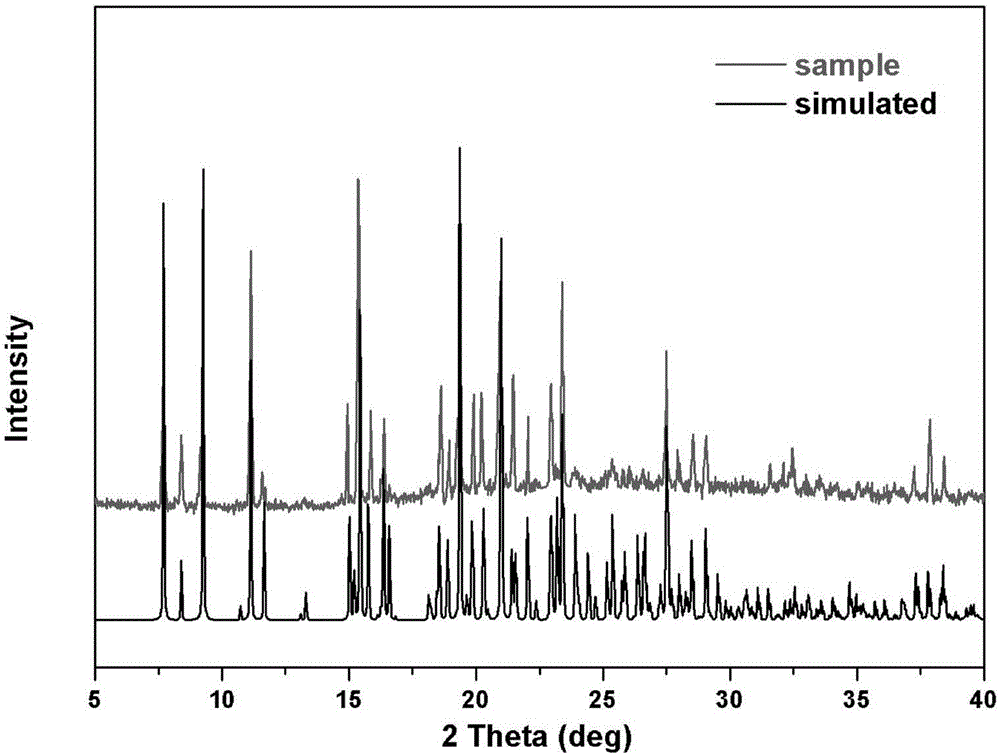
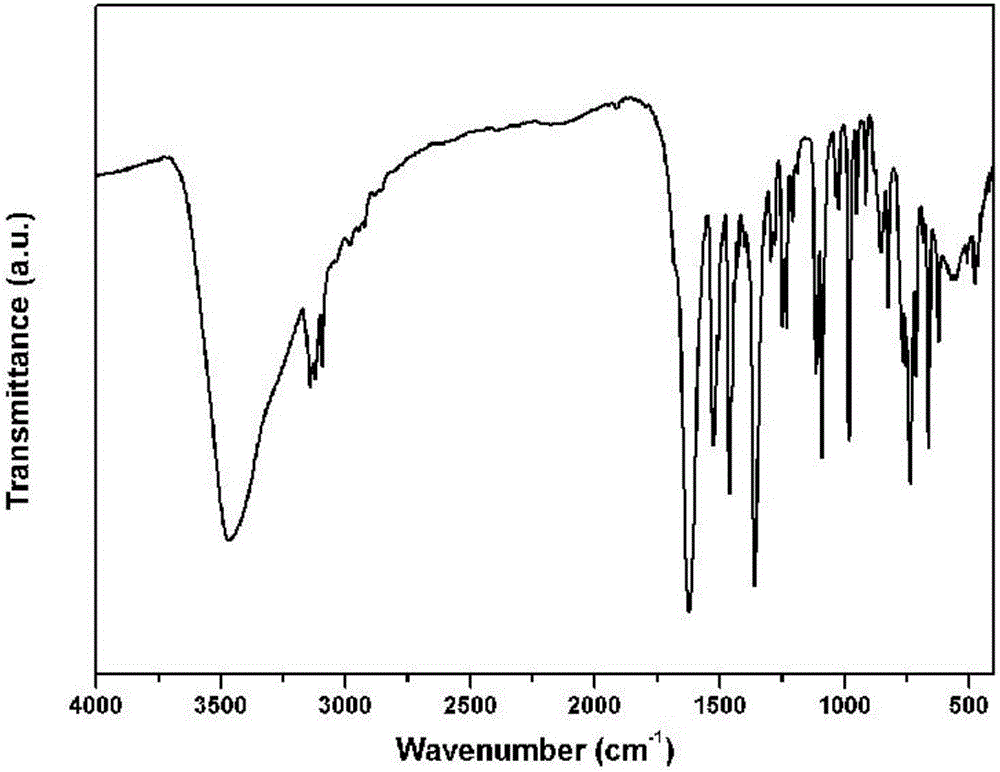
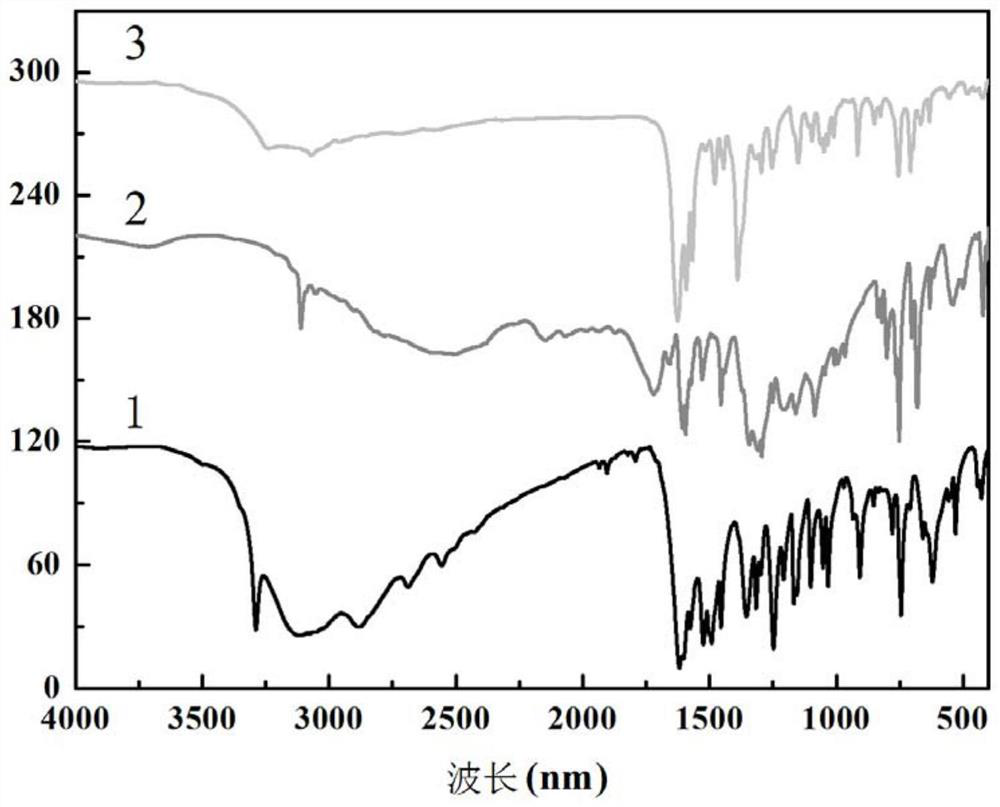
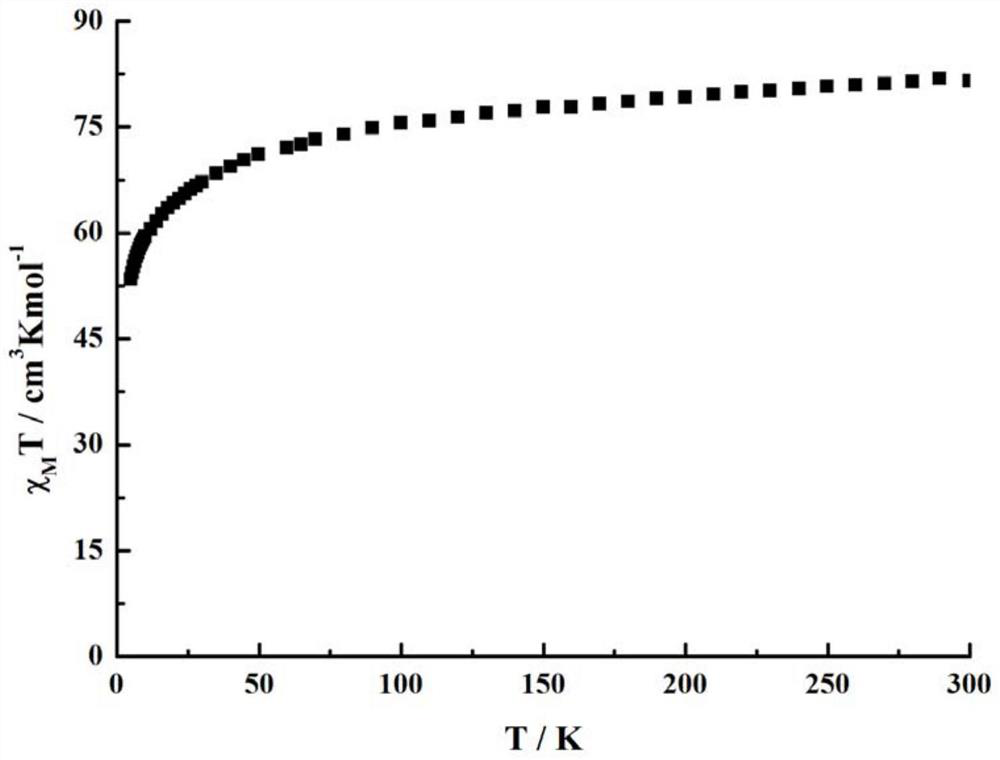
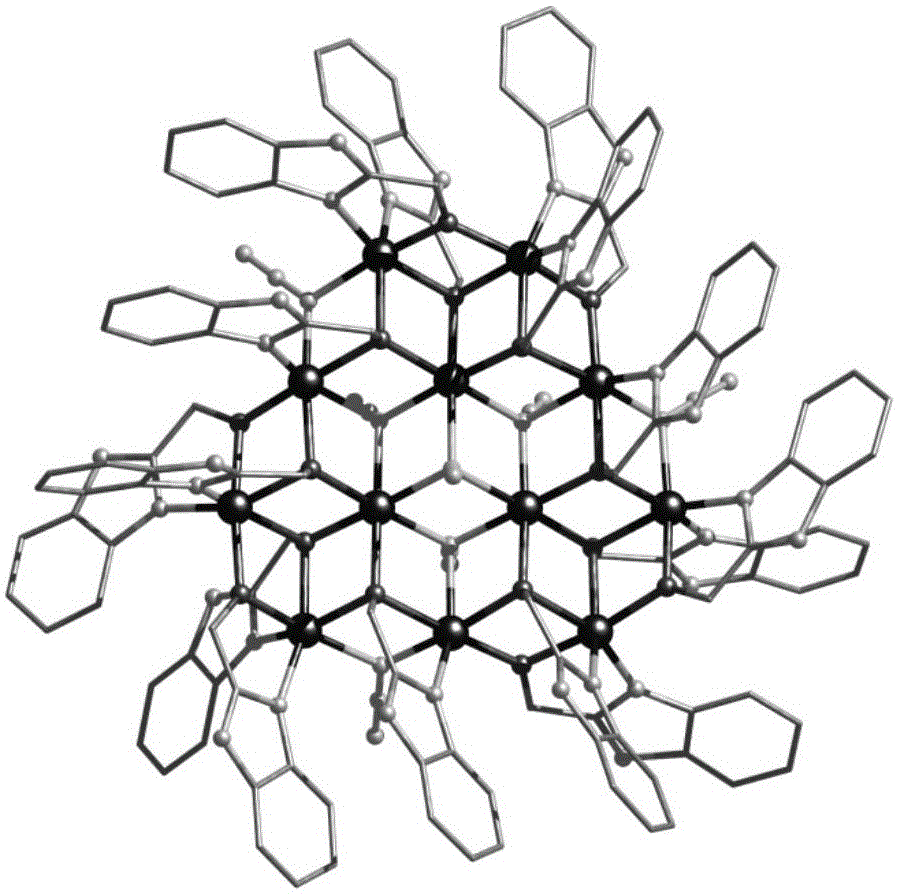
Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4]
InactiveCN104557994AStrong ferromagnetismThe synthesis method is simpleGroup 3/13 organic compounds without C-metal linkagesOrganic/organic-metallic materials magnetismSalicylaldehydeRare earth
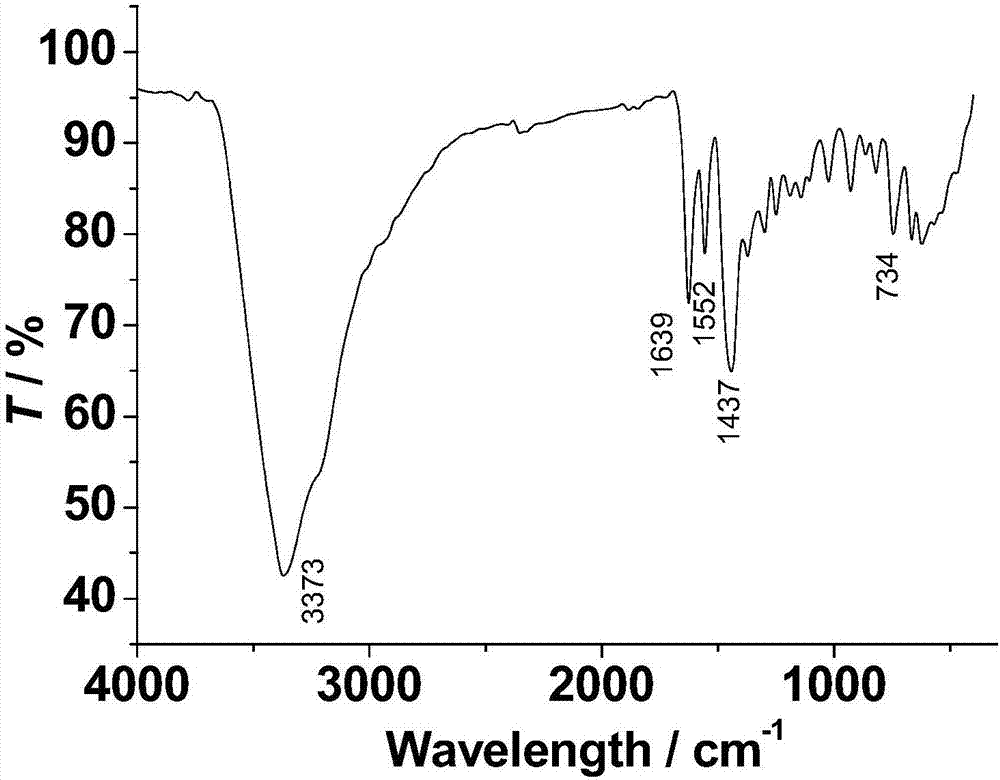
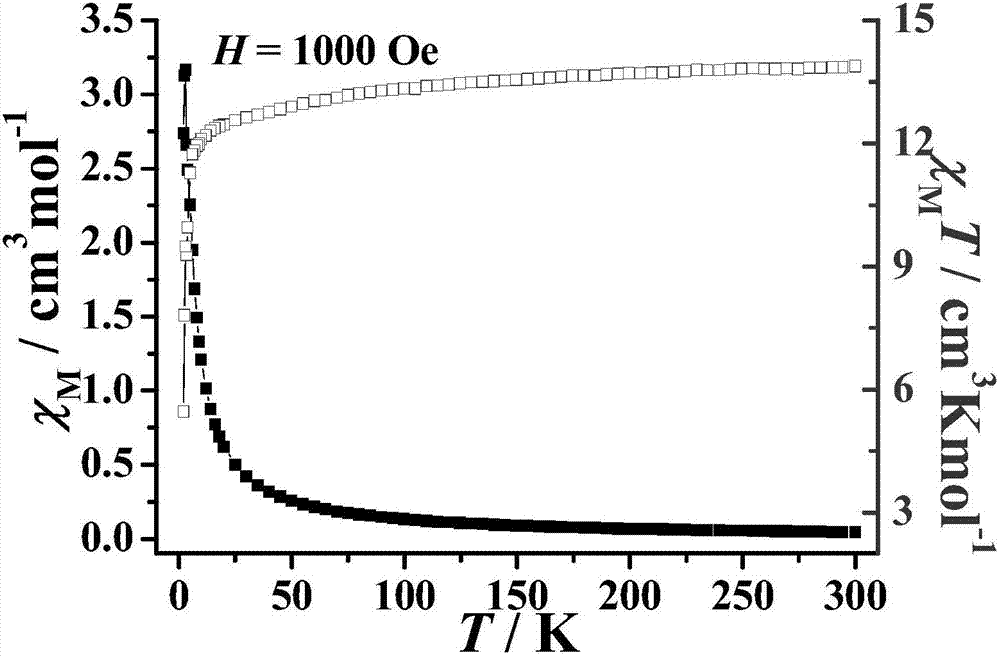
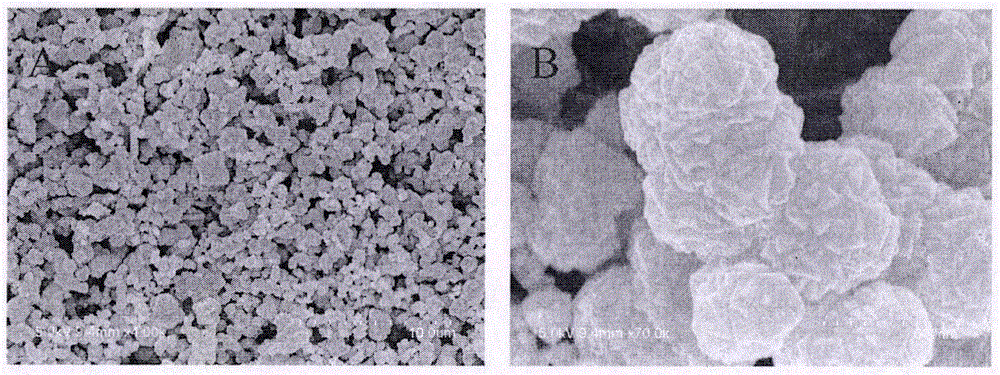
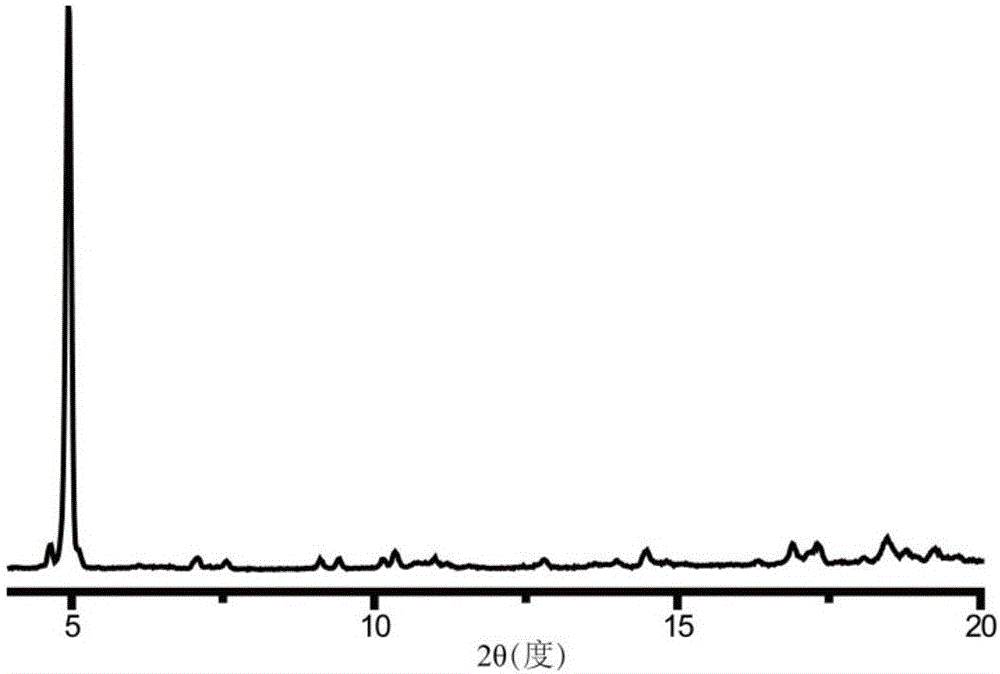
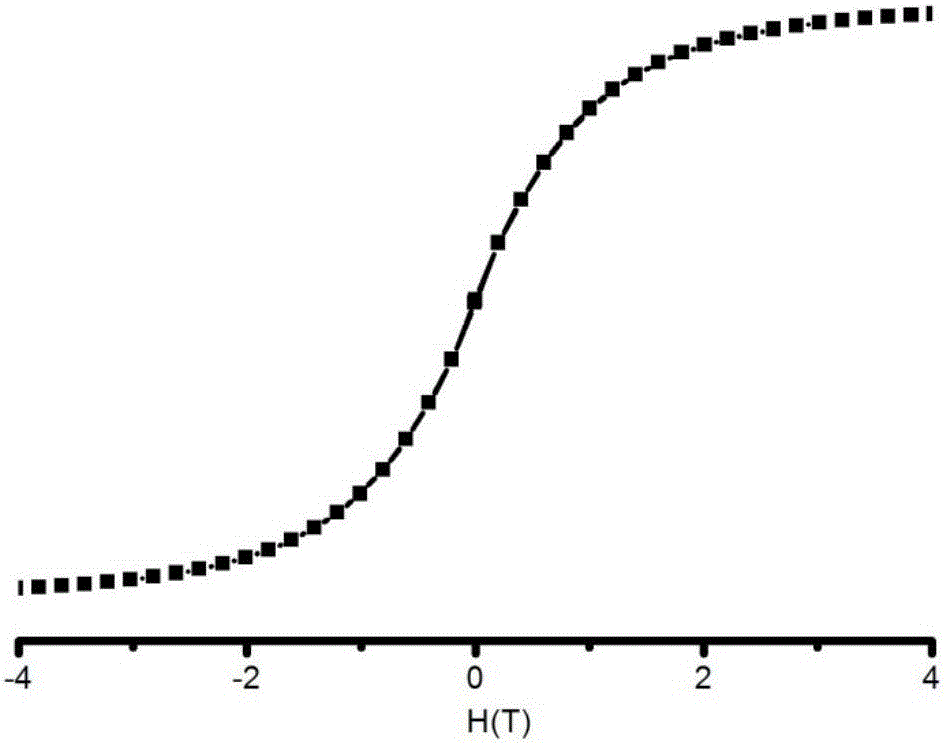
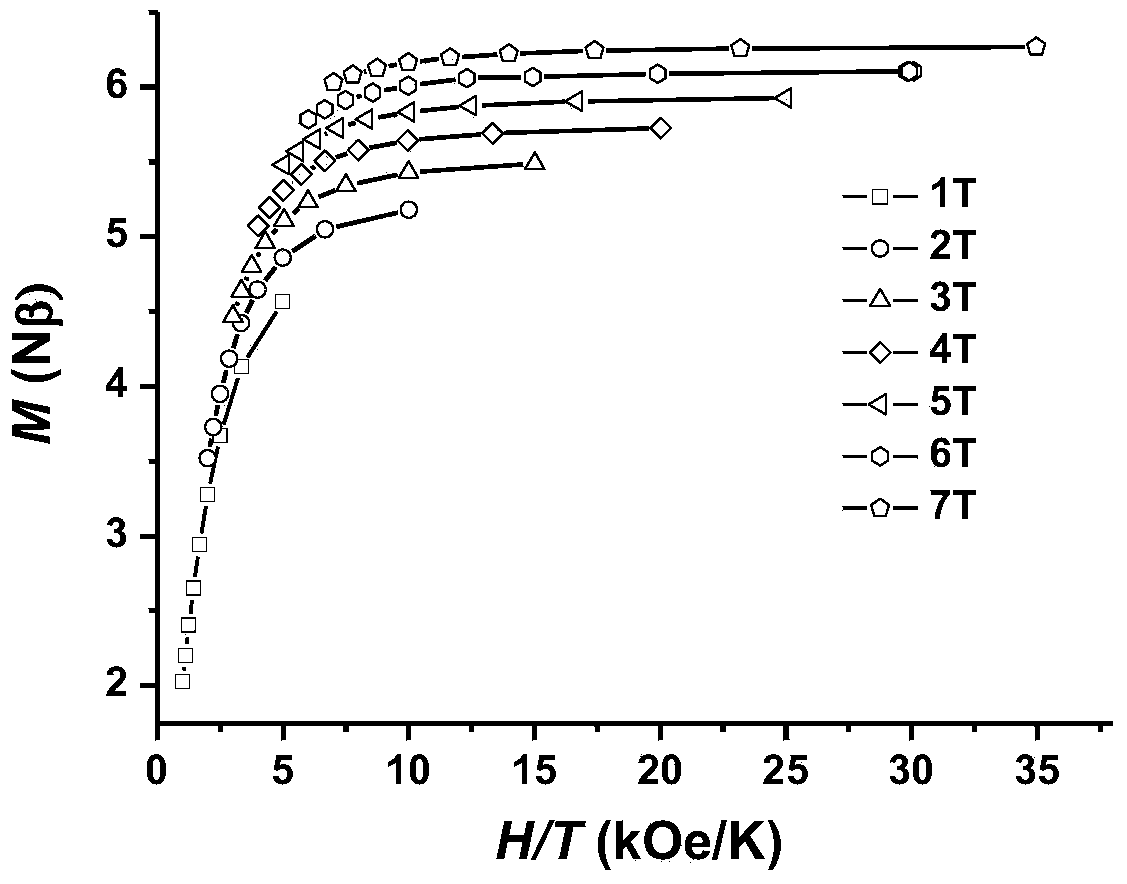
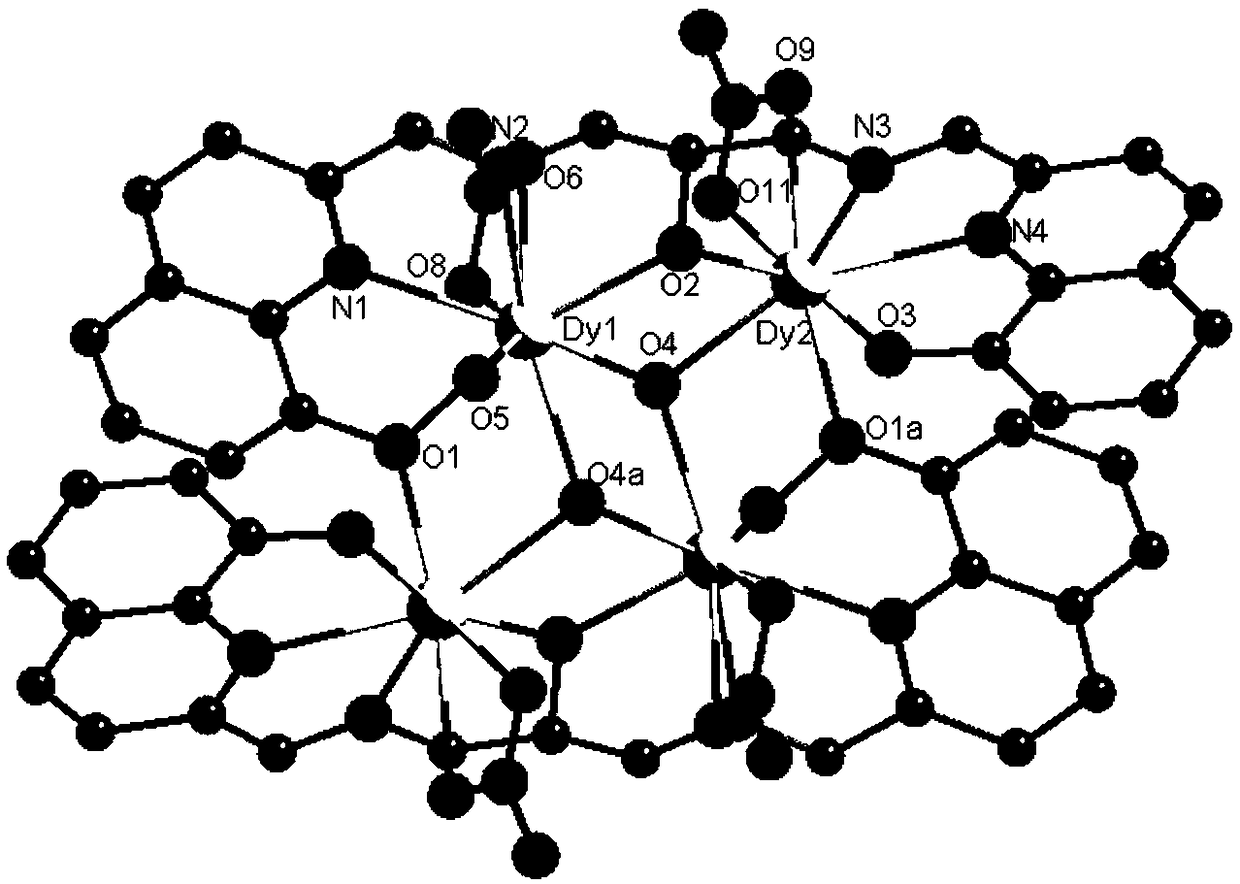
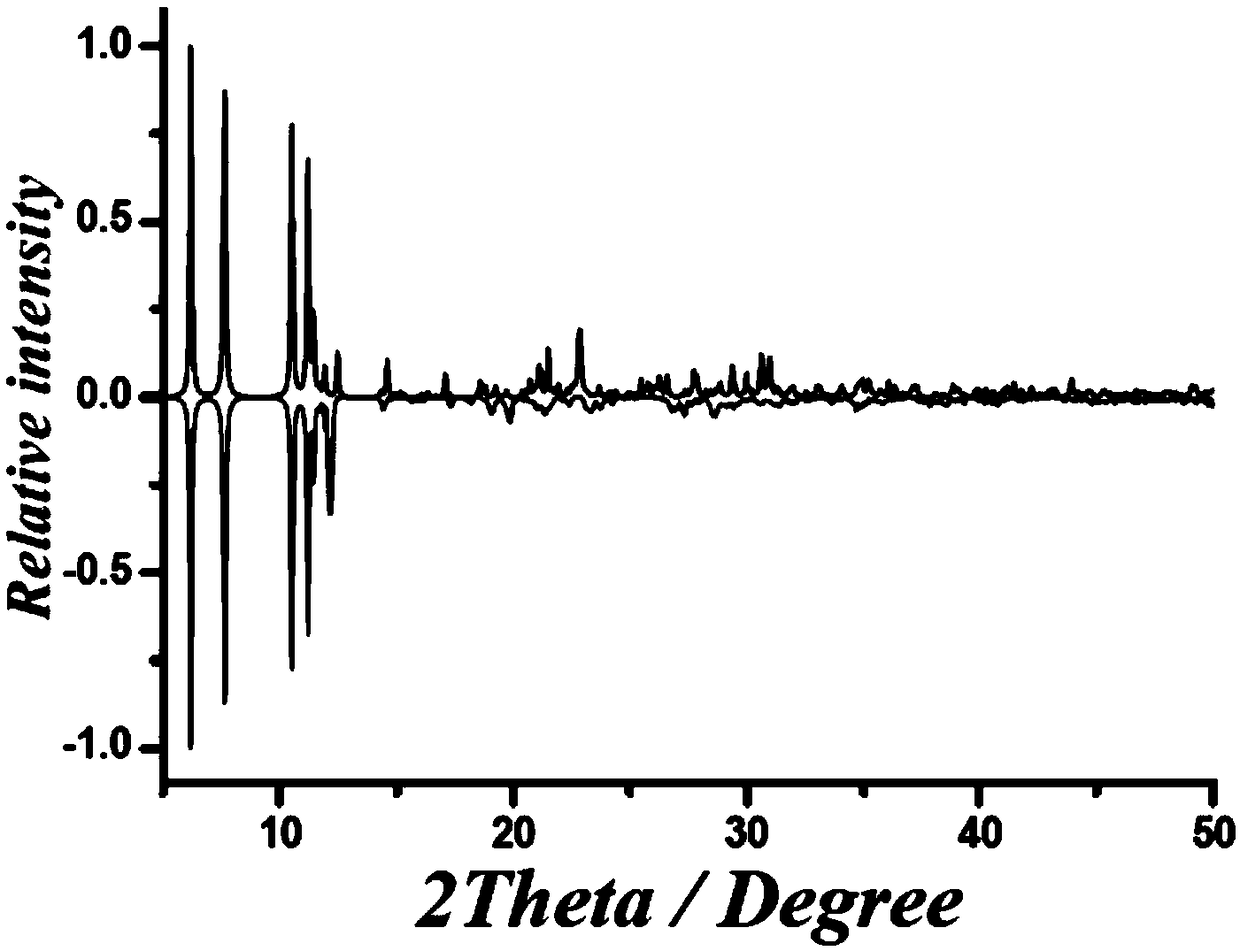
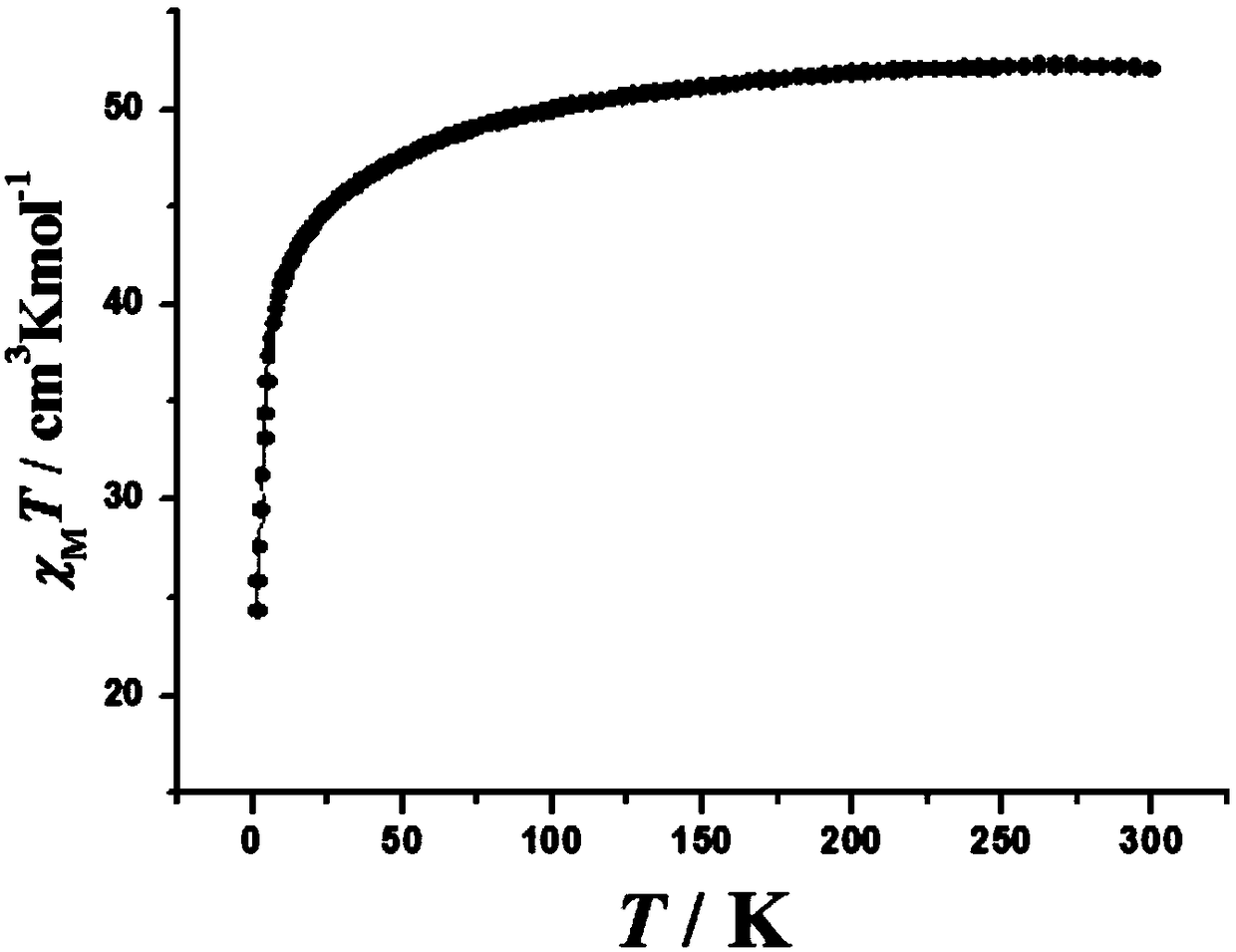
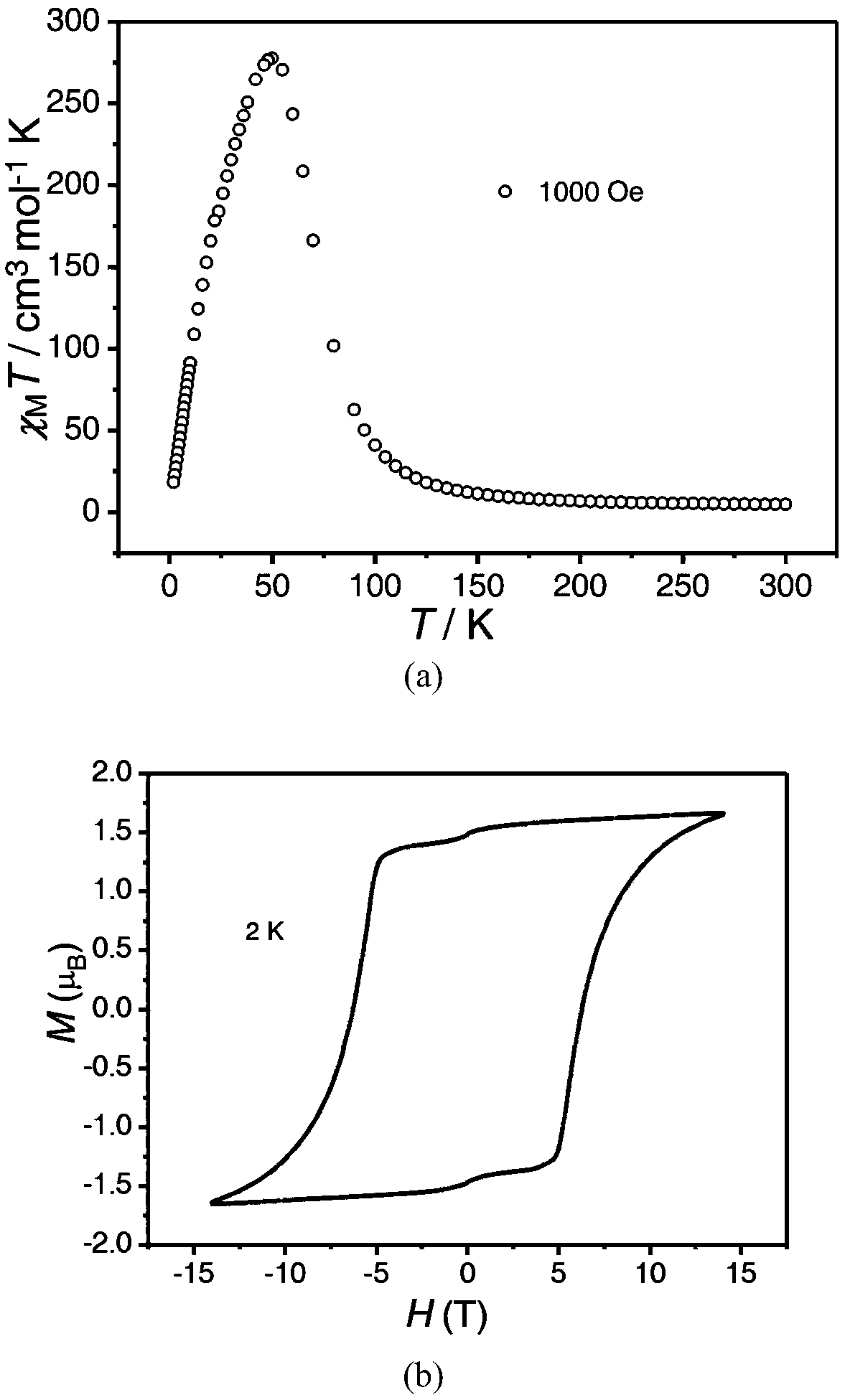
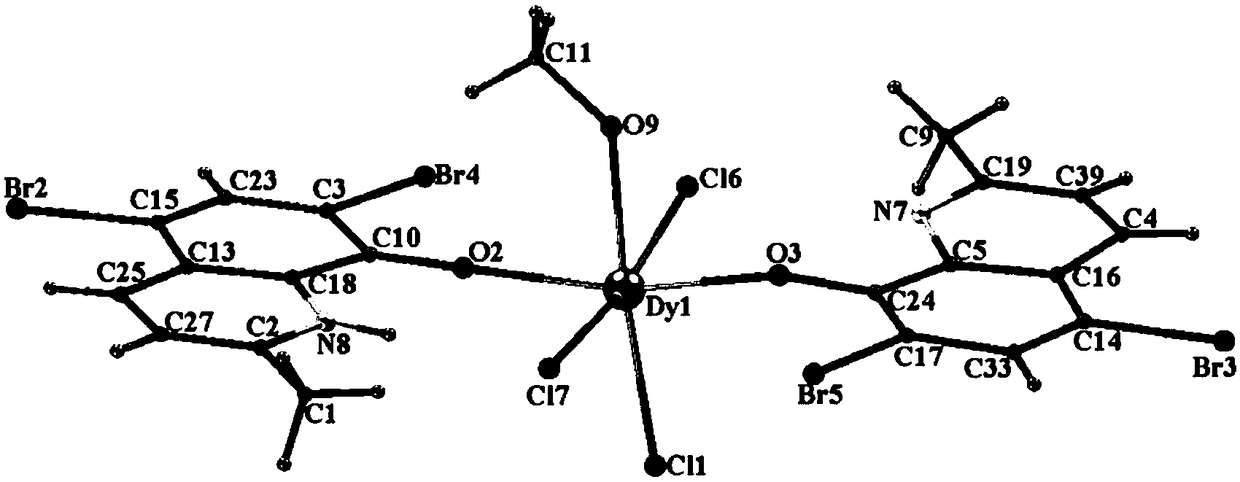
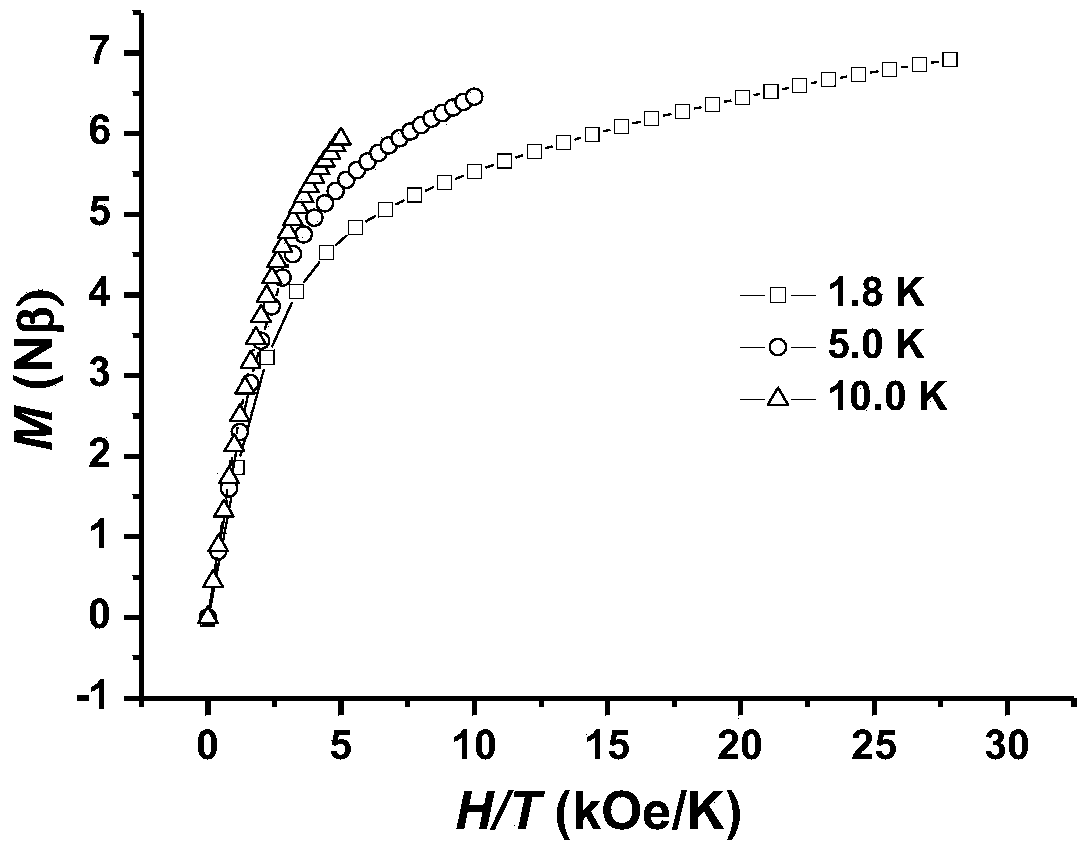
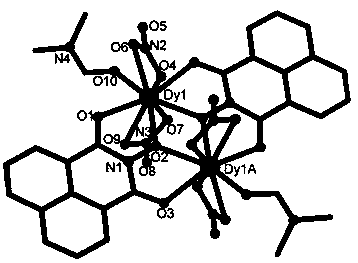
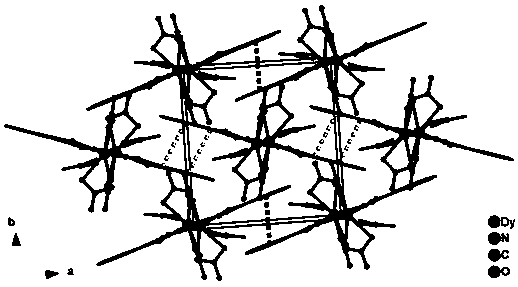
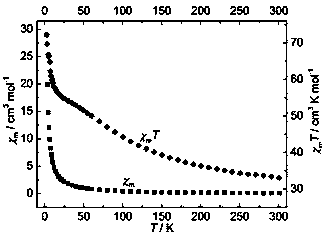
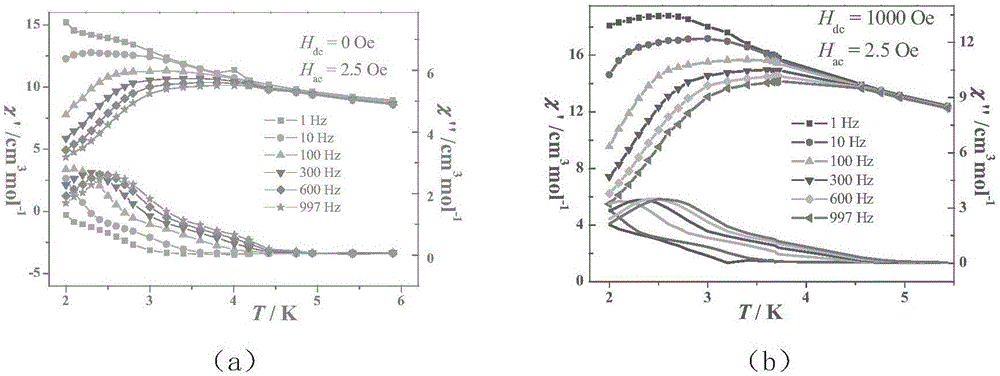
The invention discloses a method for preparing a single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4] and relates to a method for preparing a single-molecular magnet. The invention aims at solving the problems that the synthetic method of the conventional rare earth complex single-molecular magnet is low in yield and the synthetic method of the complex is complicated. The method comprises the following steps: dissolving o-aminophenol salicylaldehyde in acetonitrile, thereby obtaining a solution A; dissolving dysprosium nitrate in methanol, thereby obtaining a solution B; mixing the solution A and the solution B, adding a triethylamine solution into the mixed solution, stirring under room temperature condition, thereby obtaining a preform; and volatilizing a solvent out of the preform, thereby obtaining the complex. The [Dy2(saph)2(NO3)2(CH3OH)4] prepared by the method disclosed by the invention is a single-molecular magnet with good ferromagnetic properties, the yield of the preparation method disclosed by the invention is high and is over 52.84 percent, and the synthetic method of the single-molecular magnet is simple and high in repeatability. The invention belongs to the field of preparation of single-molecular magnets.
Owner:HEILONGJIANG UNIV
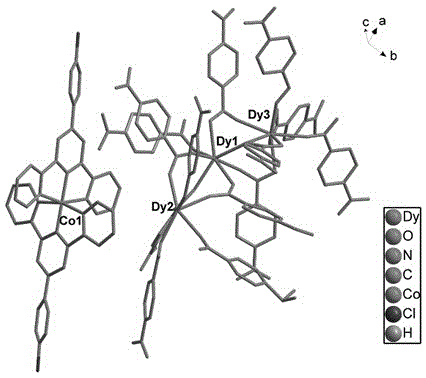
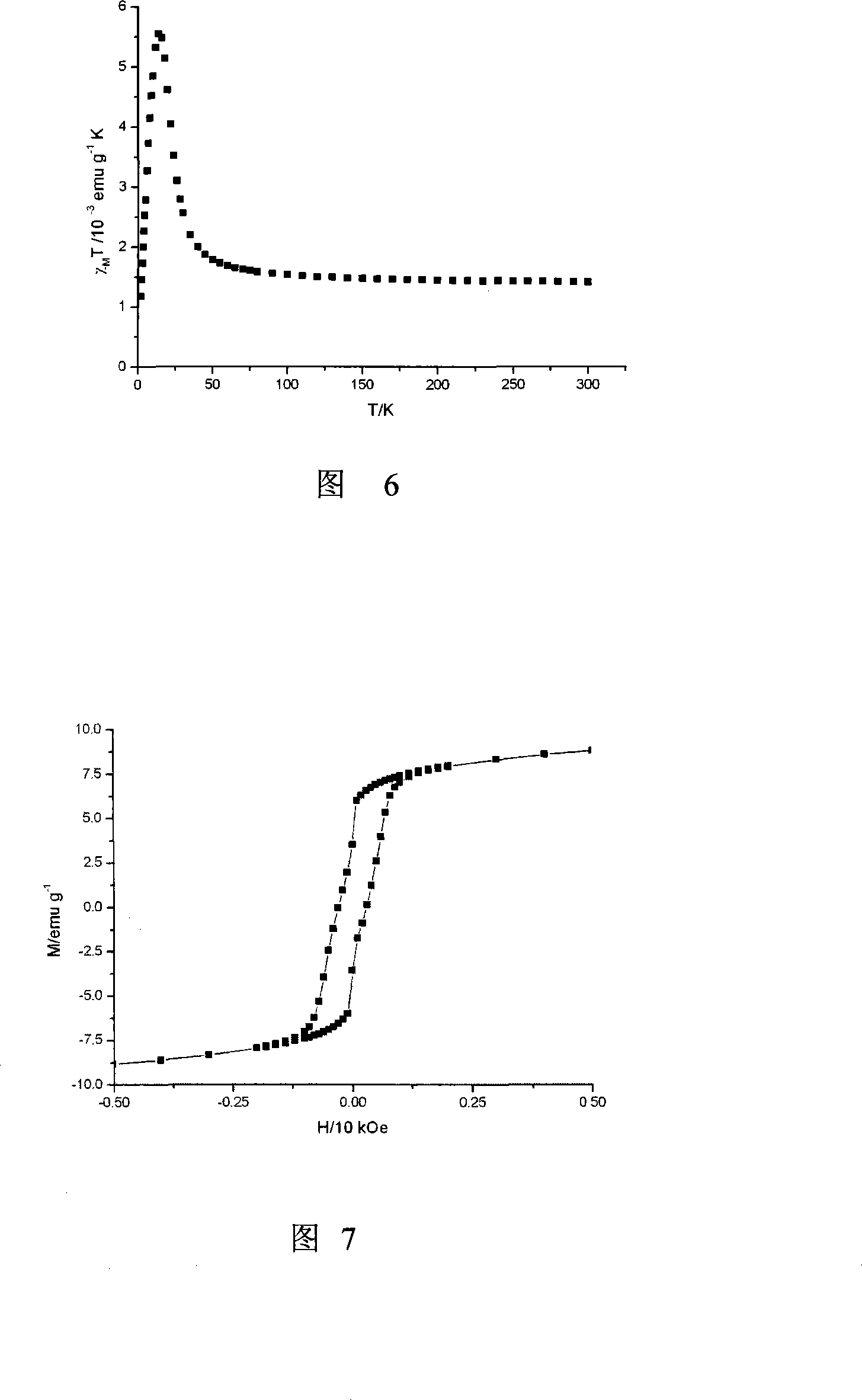
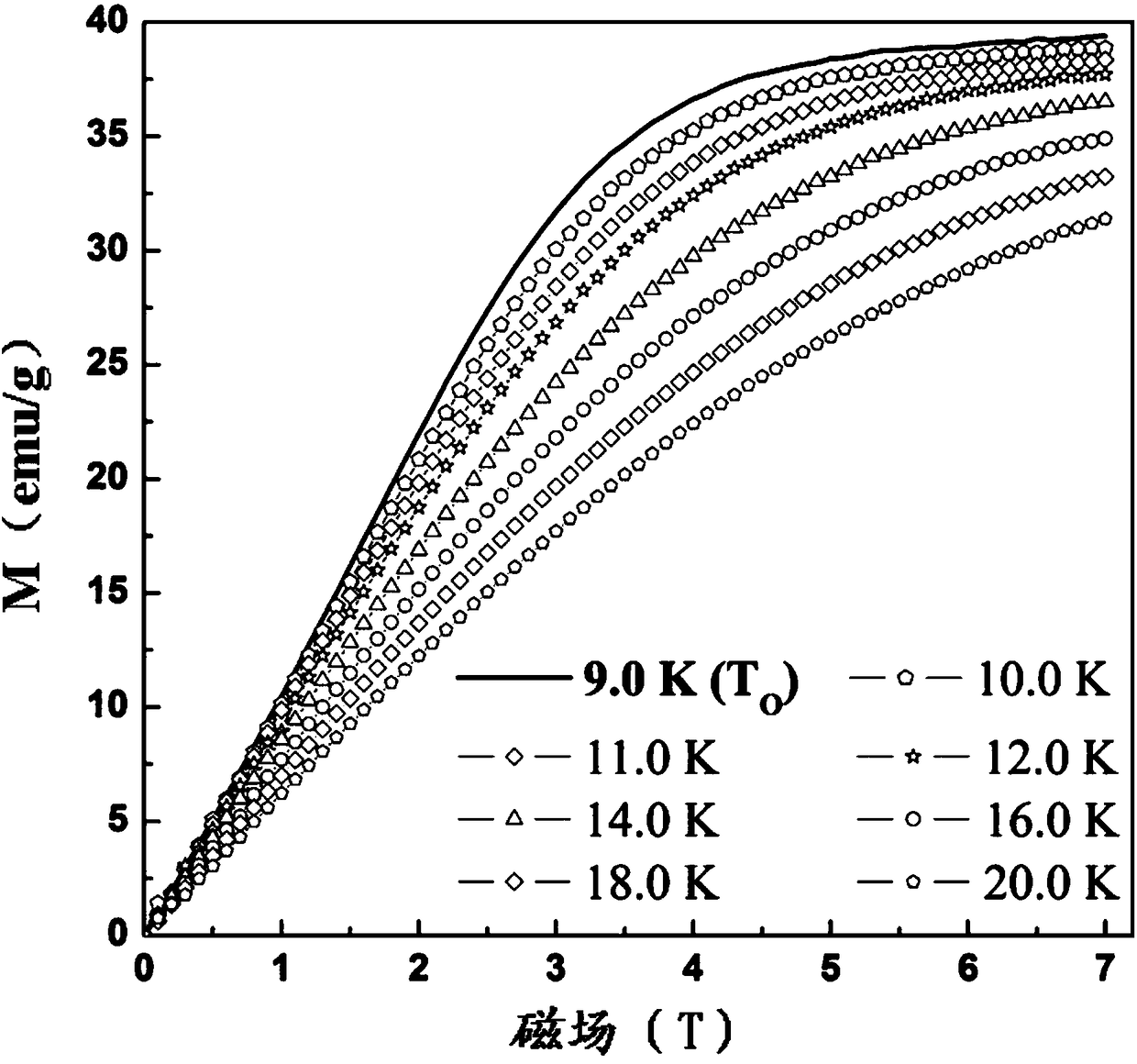
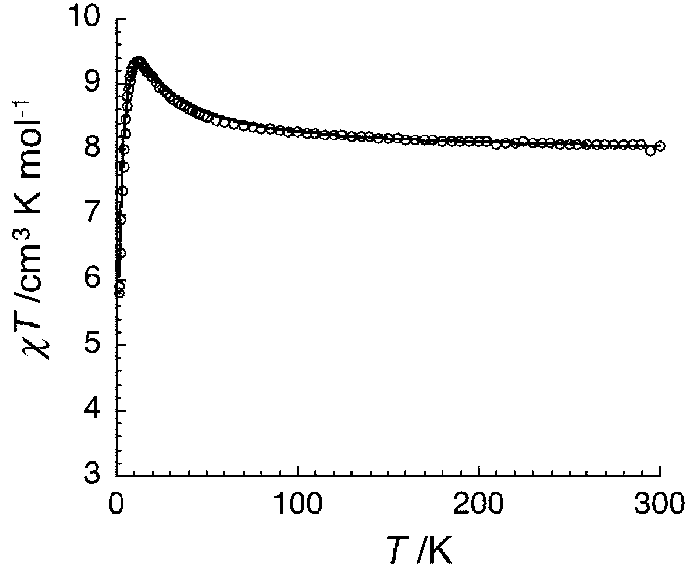
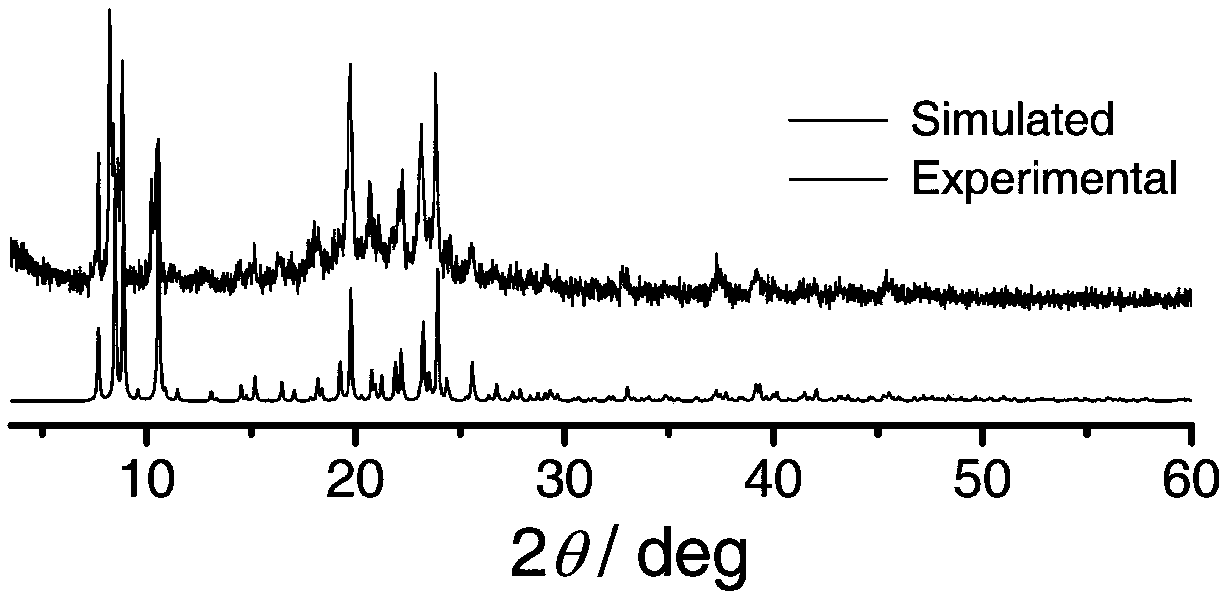
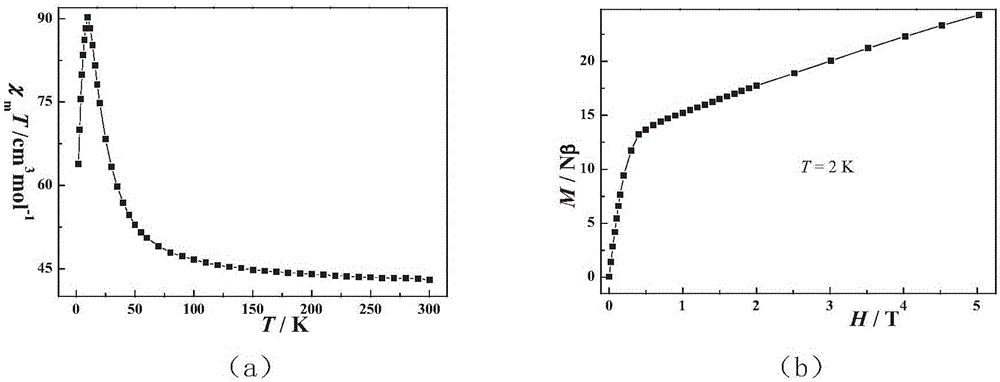
Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH
InactiveCN103641850AStrong ferromagnetismThe synthesis method is simpleOrganic/organic-metallic materials magnetismGroup 3/13 element organic compoundsSalicylaldehydeSynthesis methods
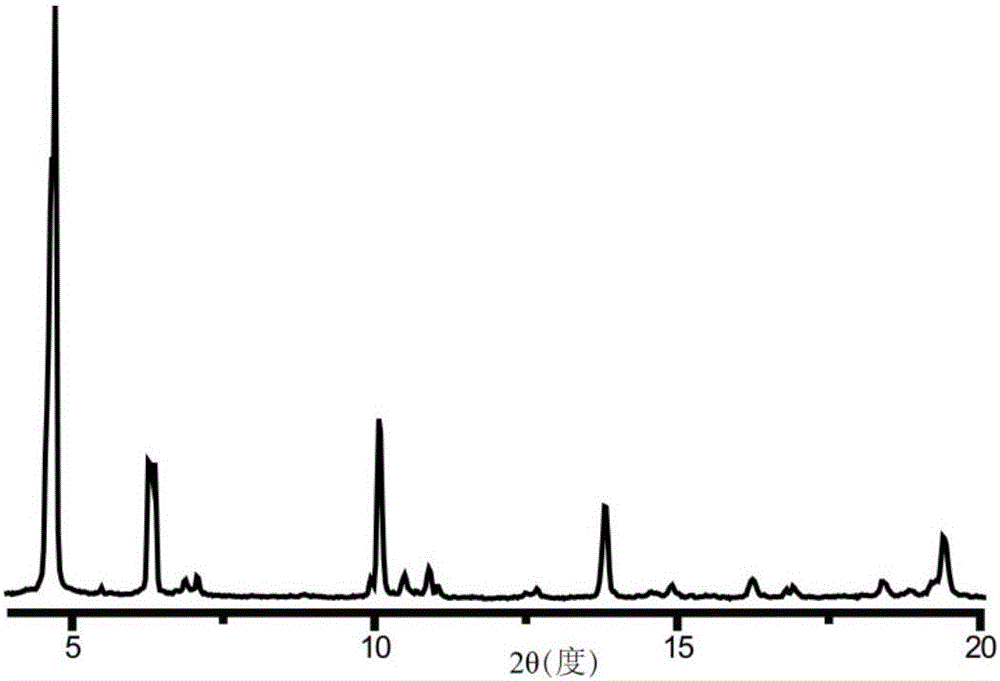
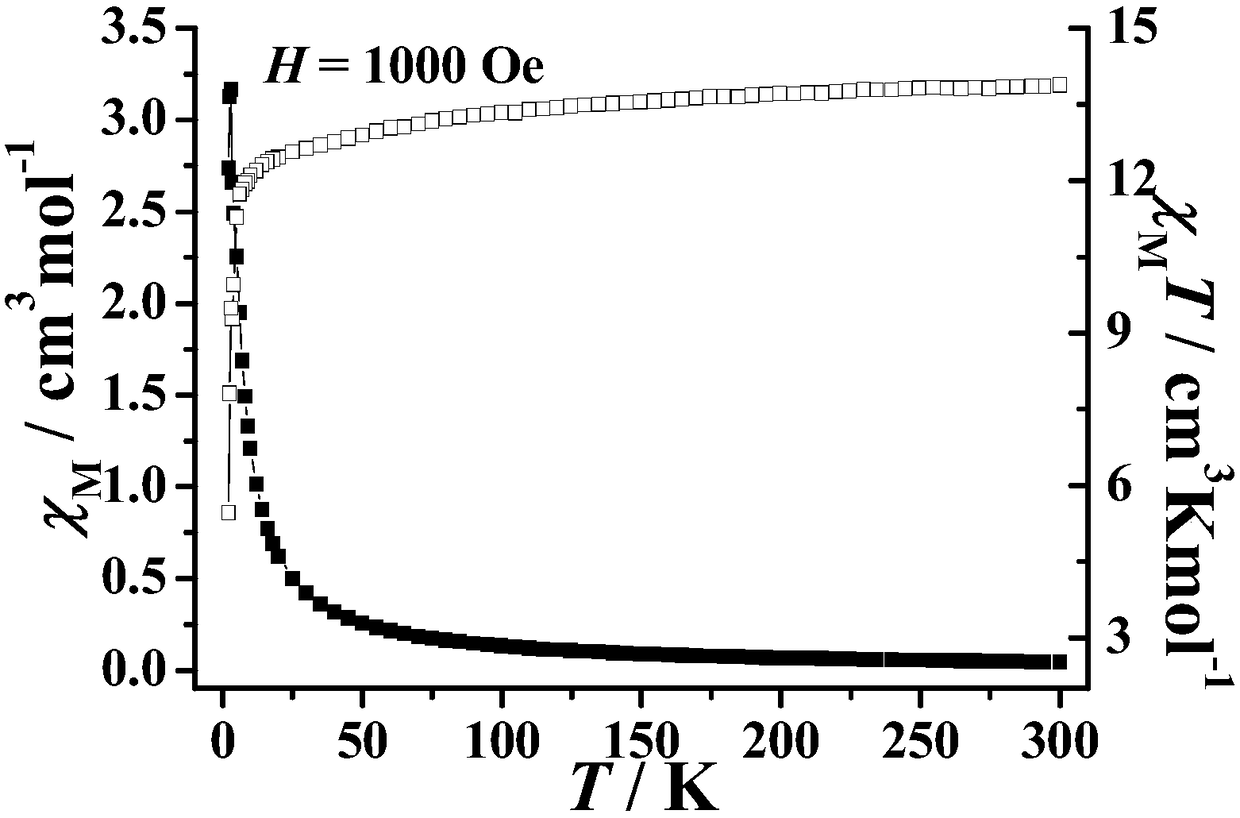
The invention discloses a preparation method of a single-molecular magnet [Dy2(saph)2Cl2].4CH3OH and relates to the preparation method of the single-molecular magnet [Dy2(saph)2Cl2].4CH3OH, which is used for solving the problems that an existing synthesis method for a rear-earth cluster compound single-molecular magnet is lower in yield, complex in synthesis method of a coordination compound and incapable of carrying out mass production. The preparation method comprises the following steps: I, dissolving o-aminophenol salicylaldehyde acetal into acetonitrile to obtain liquor A; dissolving dysprosium chloride into methanol to obtain liquor B; mixing the liquor A with the liquor B, adding triethylamine with concentration of 0.001mol / L to obtain mixed liquor; II, stirring the mixed liquor obtained in the step I under the room temperature to obtain a preform; and III, volatilizing the preform in the solvent to obtain the single-molecular magnet [Dy2(saph)2Cl2].4CH3OH. The preparation method disclosed by the invention is simple, and high in yield which reaches over 45.76%. The preparation method disclosed by the invention is applied to the field of preparing the single-molecular magnets.
Owner:HEILONGJIANG UNIV
Mononuclear dysprosium complex and preparation method and application thereof
ActiveCN107089999AEasy to prepareLow costOrganic chemistry methodsOrganic/organic-metallic materials magnetismAlcoholDysprosium
The invention discloses a mononuclear dysprosium complex and a preparation method and application thereof. The chemical formula of the complex is [Dy(HL)2(CH3OH)Cl3], wherein HL is 2-methyl-5,7-dibromo-8-hydroxyquinoline. The complex belongs to a monoclinic system and P21 / n space groups. The preparation method of the mononuclear dysprosium complex includes the steps that DyCl3.6H2O and 2-methyl-5,7-dibromo-8-hydroxyquinoline are taken and dissolved with a mixed solvent, the pH of the obtained solution is adjusted to range from 6.5 to 7.8, the obtained mixed liquid reacts under the heating condition, and then the complex is obtained, wherein the mixed solvent is a composition of methyl alcohol and acetonitrile. The preparation method of the complex is simple, the cost is low, good repeatability is achieved, magnetic properties of the complex are shown as field-induced single-molecular magnet behaviors, and the complex can be used for preparing magnetic materials.
Owner:GUANGXI NORMAL UNIV
Preparation method of novel micro-nano prussian blue particles
InactiveCN106745069AControl spawn rateEasy to prepareMaterial nanotechnologyIron cyanidesMicro nanoElectricity
The invention relates to a preparation method of novel micro-nano prussian blue particles, in particular to micro-nano prussian blue particles which are synthesized by mixing two precursor solutions, i. e. potassium ferricyanide and ferric trichloride of prussian blue, and then fully stirring the mixture under the condition of a proper pH value. A synthesis device adopted by the method is simple; a common beaker is taken as a reaction vessel, and a magnetic stirring apparatus having a stirring function is used. According to the synthesis technology, the micro-nano prussian blue particles are synthesized by controlling the conversion speed of the prussian blue in a solution. The micro-nano material can be used for the application fields such as solid state batteries, electrochromic devices, molecular magnets, and the like.
Owner:XUCHANG UNIV
Single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2 and preparation method of single molecular magnet
InactiveCN105037405AImprove propertiesThe synthesis method is simpleGroup 3/13 organic compounds without C-metal linkagesOrganic/organic-metallic materials magnetismCrystal systemReflux
The invention relates to a single molecular magnet and a preparation method of the single molecular magnet, namely to a single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2 and the preparation method of the single molecular magnet. The invention aims to solve the problems that the synthetic yield of rare earth complex single molecular magnet is relatively low, the synthetic method of the complex is complex, the batch production cannot be carried out and the like, which are faced at present. The molecular formula of the single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2 is C84H56Dy2F12N4O16S4, and the crystal system is a triclinic system. The preparation method comprises the following steps: Carrying out heating reflux on a mixed solution C, then cooling to the room temperature, and filtering to obtain a solution D; dispersing a mixed solution E into the solution D in a solution dispersal mode, and standing to obtain the single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2. The method is used for obtaining the single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2.
Owner:HEILONGJIANG UNIV
Double-functional molecular magnet material and synthesis method thereof
InactiveCN105097175APure enoughCobalt organic compoundsOrganic/organic-metallic materials magnetismBenzoic acidChlorobenzene
The invention discloses a double-functional molecular magnet material. The structural formula of the double-functional molecular magnet material is [Co (4-Cl-PH-terpy) <2>] [Dy<3> (p-NO<2>-PHCOO)<14>], wherein the Dy represents rare-earth meal dysprosium ions, the Co represents transition metal ions, the p-NO<2>-PHCOO represents p-nitrobenzoic acid negative ions, and the 4-Cl-pH-terpy represents 4-(4-chlorophenyl)-terpyridine. The double-functional molecular magnet material integrates two functions of slow relaxation and spin transition and is synthesized by a cobalt chloride hexahydrate (CoCl<2>6H<2>O), 4'-(4-chlorophenyl)-2,2':6',2''-terpyridine, dysprosium oxide (Dy<2>O<3>), nitrobenzoic acid and other raw materials. The double-functional molecular magnet material prepared by means of a preparation method has the advantages that the good slow relaxation behavior of the dysprosium (Dy) is retained, meanwhile the high and low spin transition of cobalt (Co) is achieved, and the double-functional molecular magnet material is unprecedented at home and abroad.
Owner:EAST CHINA UNIV OF TECH
PVP coated magnetic nano particle and its preparation method and application
InactiveCN101121830AInorganic material magnetismPigment treatment with macromolecular organic compoundsChemical compositionMagnetite Nanoparticles
The present invention relates to a PVP-coated magnetic nanoparticle, the preparation method and the application. The PVP-coated magnetic nanoparticles has the following chemical composition: KxMIIy [CrIII (CN) 6] z question mark nH2O question mark mPVP. Therein, the MII is FeII or CoII; PVP is the polyvinylpyrrolidone. The PVP-coated magnetic nanoparticle has the obvious size effectL with the increase of the content of the polymer surfactant, the size of the nanoparticle is gradually decreased; the magnetism has the dramatic changes. The present invention has the wide prospects to broaden the reorganization of people for the Prussian blue molecular magnet, to prepare and use the novel magnetic material.
Owner:NANKAI UNIV
Method for increasing performance of metallofullerene single-molecular magnet
InactiveCN106486239AIncreased magnetic anisotropyRaise the blocking temperatureOrganic/organic-metallic materials magnetismMetal-organic frameworkFullerene molecule
The invention relates to a method for increasing the performance of a metallofullerene single-molecular magnet. The method includes: dispersing the metallofullerene single-molecular magnet into the ducts of an organic porous material, wherein the organic porous material is a metal organic framework compound material or a covalent organic frame compound material. Magnetism test results show that the metallofullerene single-molecular magnet dispersed in the organic frame compound is high in blocking temperature and magnetization intensity. By the method, the performance of the metallofullerene single-molecular magnet can be increased effectively.
Owner:BEIJING FUNAKANG BIOTECH CO LTD
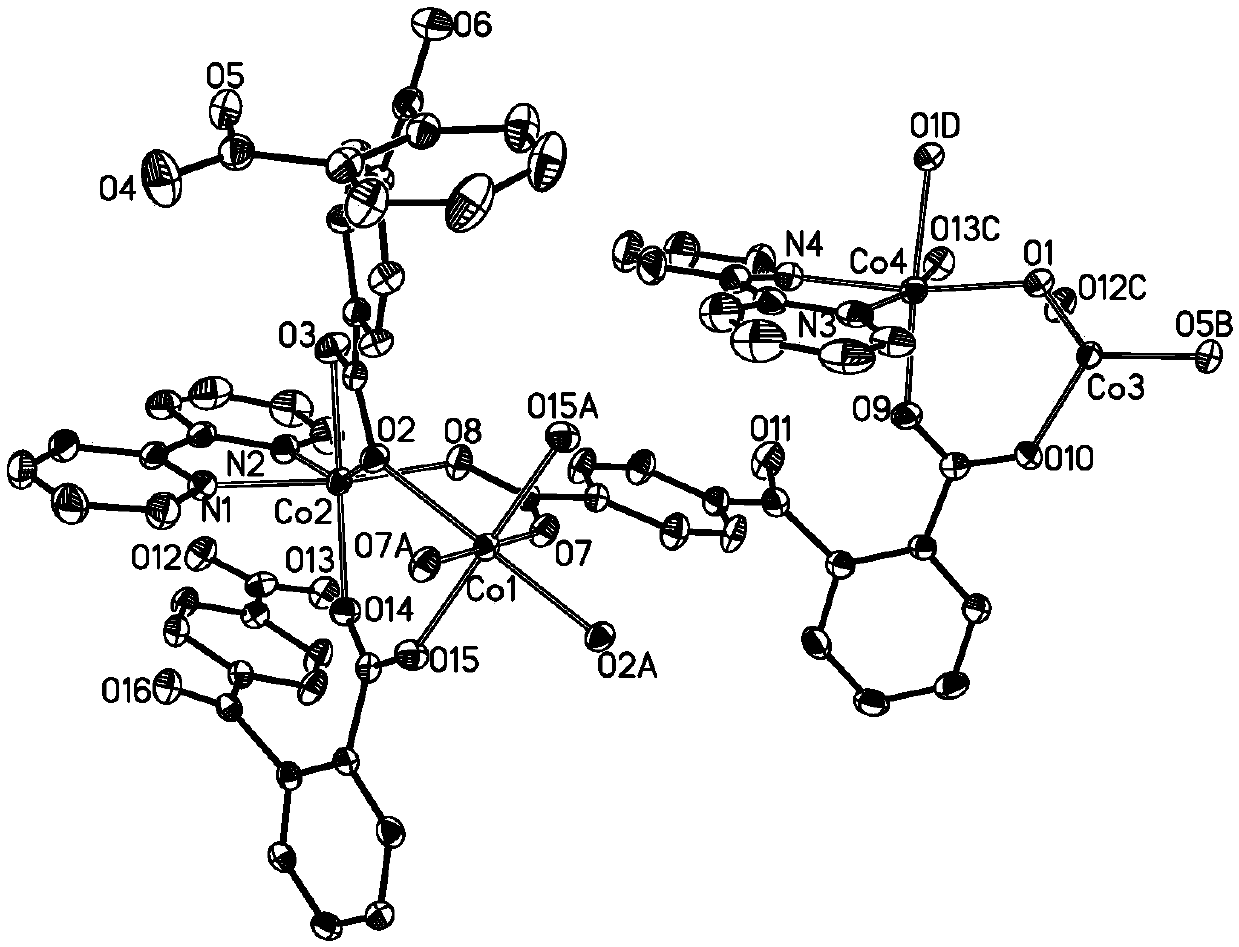
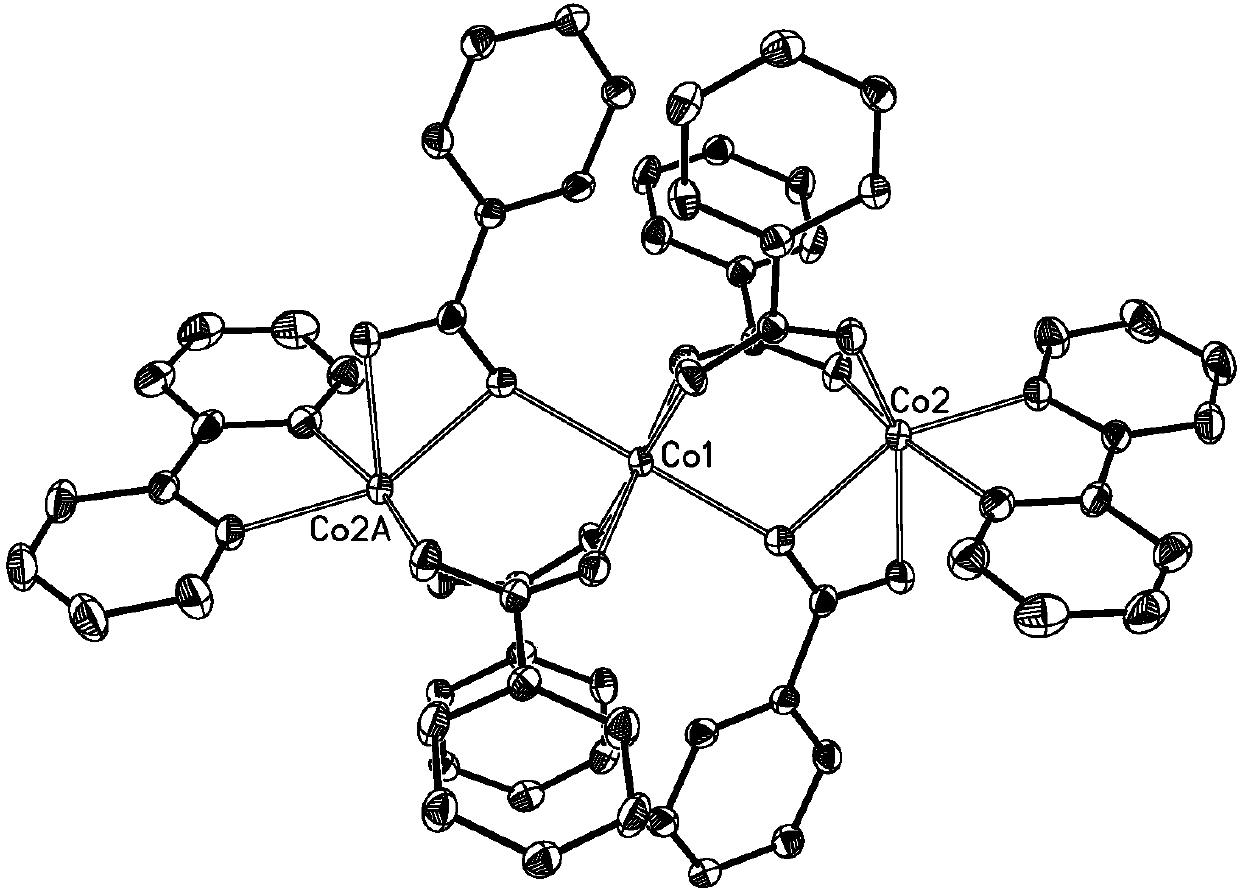
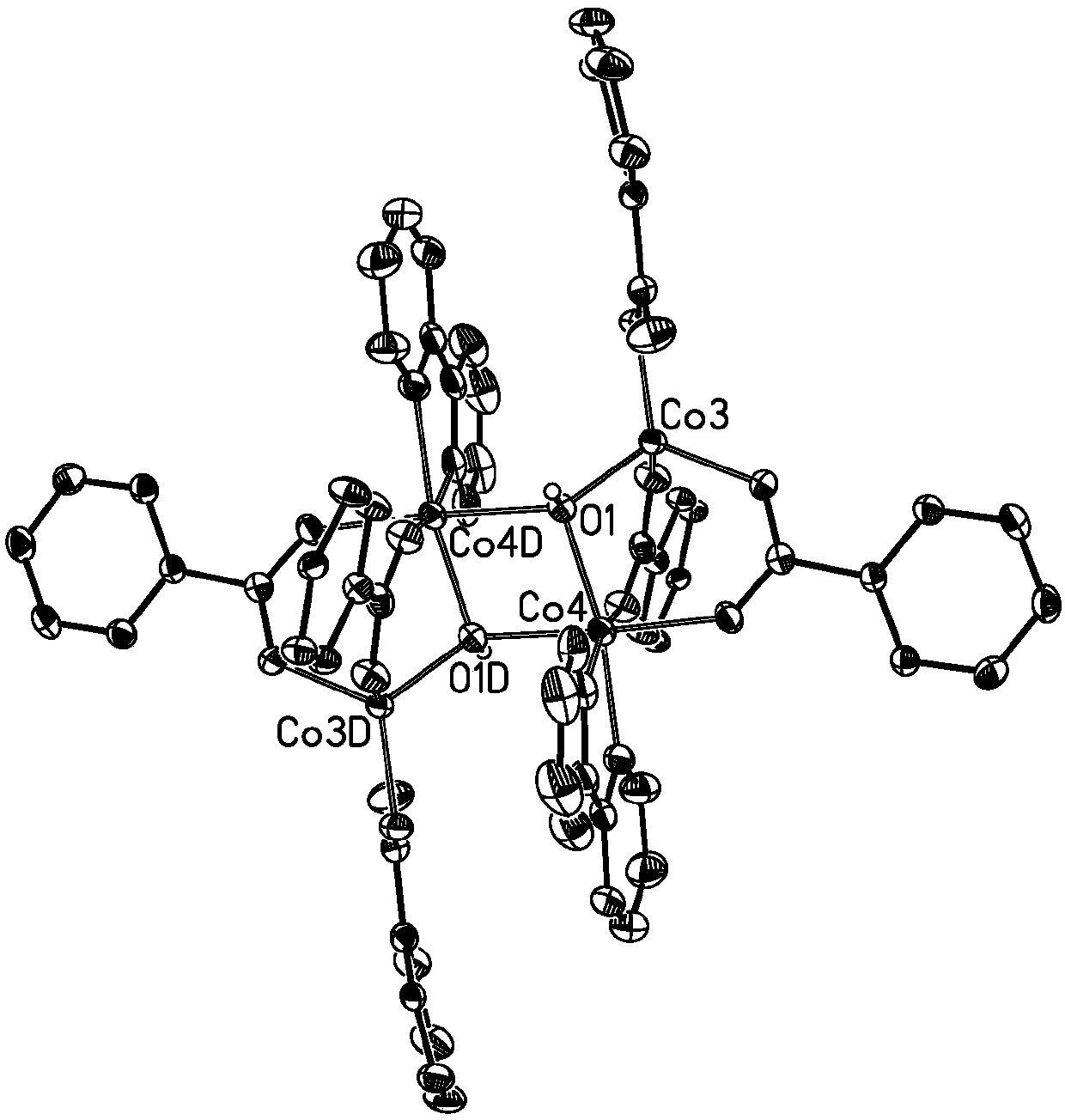
Three-core four-core mixed cobalt cluster coordination polymer as well as preparation method and application thereof
The invention belongs to the field of coordination polymers, and particularly relates to a three-core four-core mixed cobalt cluster coordination polymer as well as a preparation method and application thereof. A chemical formula of the three-core four-core mixed cobalt cluster coordination polymer is (C10H8N2)4(C15H8O5)6(OH)2Co7.(H2O)2, wherein C10H8N2 is 2,2-dipyridyl; C15H8O5 is benzophenone-2,4'-diformate. The three-core four-core mixed cobalt cluster coordination polymer has the advantages that the preparation is easy; the stability is high; the polymer is an antiferromagnetic three-corefour-core mixed cobalt cluster coordination polymer with good potential application prospects in the fields of adsorption separation, hydrogen and energy storage, molecular magnets, catalysis, sensingand / or molecular recognition, and the like. The problem that no three-core four-core mixed cobalt cluster coordination polymer exists in the prior art, and three-core four-core mixed cobalt cluster coordination polymer performance study cannot be performed is solved. The invention also provides the preparation method of the three-core four-core mixed cobalt cluster coordination polymer; the three-core four-core mixed cobalt cluster coordination polymer prepared by the method has small defects, high crystallization degree and high yield.
Owner:GUANGDONG UNIV OF TECH
Method for preparing metal crown ether single-molecular magnets
InactiveCN109705151ANovel structureGood antiferromagneticGroup 3/13 element organic compoundsOrganic/organic-metallic materials magnetismHydroxamic acidPyrazine
The invention discloses a method for preparing metal crown ether single-molecular magnets, and relates to a method for preparing single-molecular magnets. The invention aims to solve the problem of alow yield of a method for synthesizing metal crown ether rare earth complex single-molecular magnets in the prior art. The preparation method comprises the steps: dissolving salicyl hydroxamic acid, dysprosium nitrate and gallium nitrate in methanol so as to obtain a solution A, adding pyrazine to the solution A, performing stirring for a reaction so as to obtain a solution B, then adding pyridine, performing stirring at room temperature so as to obtain a preform, volatilizing the solvent of the preform so as to obtain the metal crown ether single-molecular magnets {Dy<3+>[Ga(III)MCshi]}. Themethod has the advantages that (1) the metal crown ether single-molecular magnets are single-molecular magnets with good antiferromagnetism, and (2) the preparation method is simple, and has high repeatability and a high yield which can reach 50% or above. The method is mainly applied to preparation of the metal crown ether single-molecular magnets {Dy<3+>[Ga(III)MCshi]}.
Owner:HEILONGJIANG UNIV
Low-temperature magnetic refrigeration material based on molecular magnet and preparation method and application thereof
ActiveCN108987026ALow blocking temperatureImprove air stabilityPolycrystalline material growthFrom normal temperature solutionsHeat sinkMagnetic refrigeration
The invention provides a low-temperature magnetic refrigeration material based on a molecular magnet, and a preparation method and application thereof. Molecular magnet [Mn3O (Et-Sao) _ 3 (ClO4) (OH)3] can be used for magnetic refrigeration in the temperature range of liquid helium because of its low blocking temperature and good air stability. The molecular magnet [Mn3O (EtSao) 3 (ClO4) (OH) 3]can be used for magnetic refrigeration in the temperature range of liquid helium. Secondly, by controlling the external magnetic field, the molecular magnet [Mn3O (EtSao) 3 (ClO4) (OH) 3] exhibits normal magnetocaloric effect or anti-magnetocaloric effect near the characteristic temperature to which can greatly improve the efficiency of the magnetic refrigeration cycle. The magnetization heating of conventional magnetic refrigeration materials can also be cooled by using the anti-magnetocaloric effect of molecular magnet [Mn3O (EtSao) 3 (ClO4) (OH) 3] as a heat sink.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Arrowhead complex material with solvent molecule-responded magnetic and ferroelectric properties and preparation method of arrowhead complex material
InactiveCN106928049AImprove ferroelectric propertiesHigh potential application valueOrganic compound preparationOrganic chemistry methodsSolvent moleculeCoordination complex
Owner:HENAN POLYTECHNIC UNIV
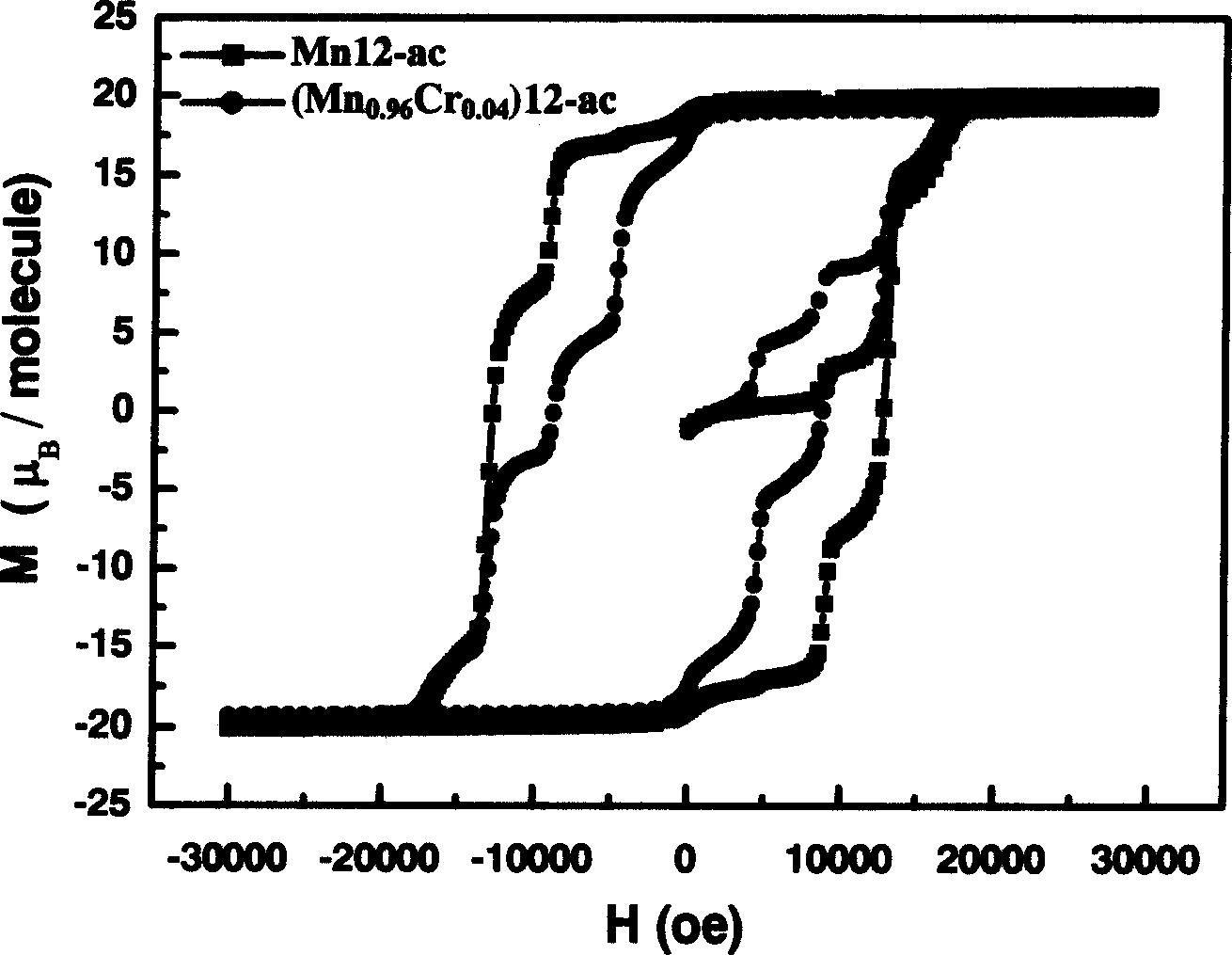
Method for preparing monomolecular magnet (Mn1-XCrX)12-ac
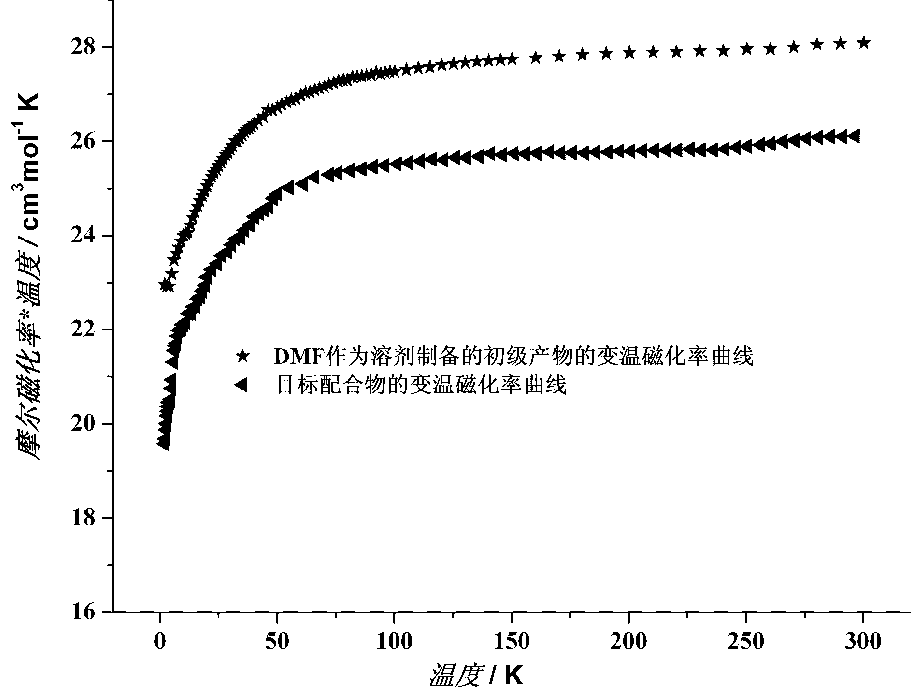
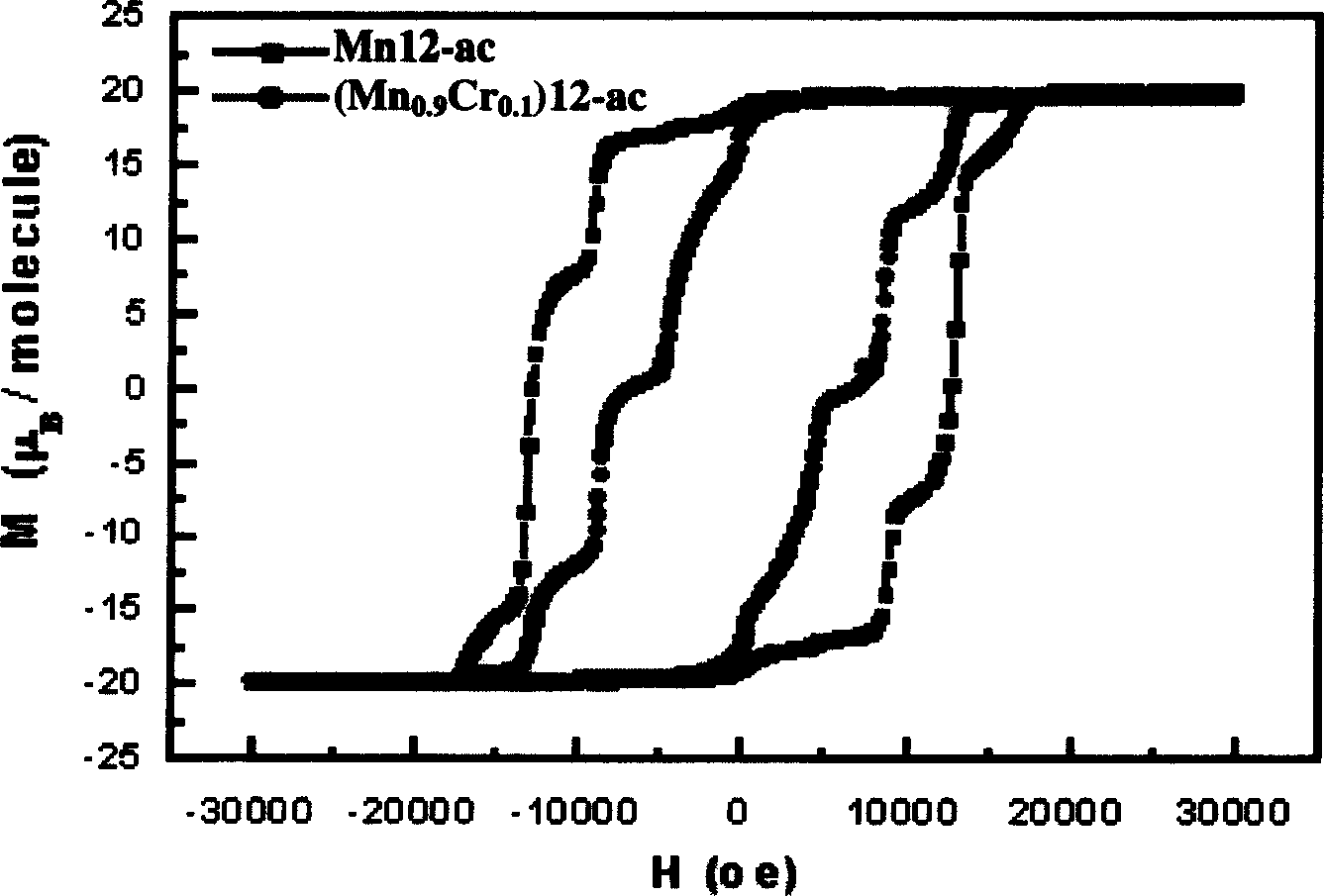
InactiveCN1564283AIncrease in sizeIncrease Quantum Storage CapacityDigital storageInductances/transformers/magnets manufactureWater bathsEngineering
The method includes steps: (1) dissolving Mn(CH3COO)2.4H2O,K2Cr O4 and KMnO4 in acetum fully under room temp, and continuous agitating; (2) Displacing reaction bulb to water bath jar, standing at room temp, coming up by using water bath method; (3) heating up to 318-328 K and keeping it at constant temperature; (4) cooling down to room temp naturally and standing at the state. The invention raises volume of unimolecule (Mn1-xCrx) 12-ac, and quanta storage capacity of crystal greatly, tunneling probability is 1-7.5 times larger than Mnl2-ac molecular magnet.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
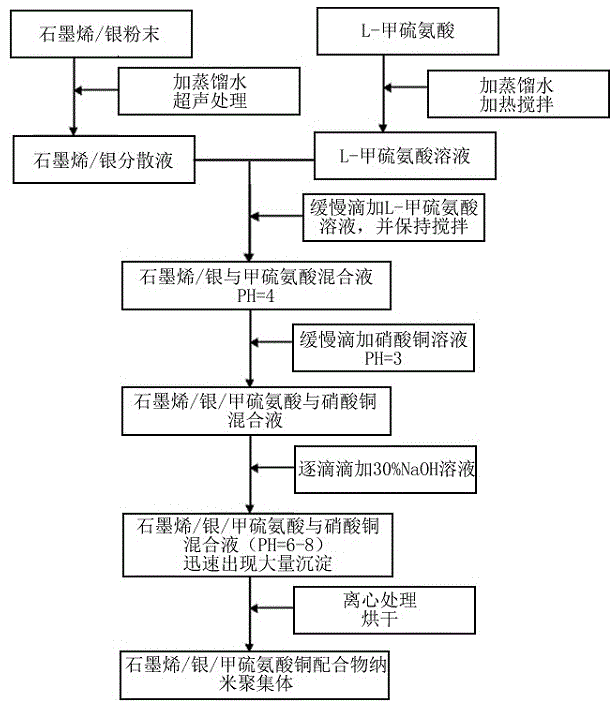
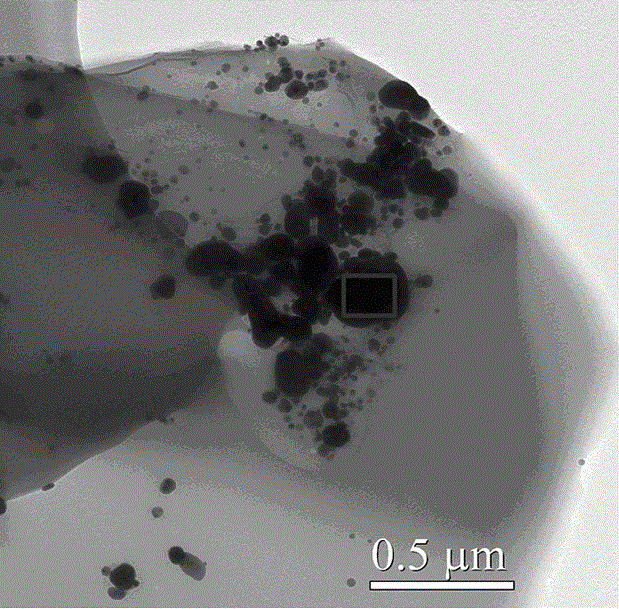
Method for preparing graphene/silver/methionine copper nano-aggregates
The invention relates to a method for preparing graphene / silver / methionine copper nano-aggregates by utilizing a liquid phase chemical reduction process and combining with coprecipitation through adjusting the pH value of a solution, and belongs to the technical field of nano-material preparation processes. The method is characterized by adjusting the pH valve of a mixed solution of graphene / silver, methionine and copper nitrate to facilitate heterogeneous nucleation of methionine and copper ions on a graphene / silver surface so as to fast prepare the nano-aggregates. The process disclosed by the invention is simple and easy, and is high in repeatability. The prepared nano-aggregates have potential applications in fields of biocompatibility electrodes, biosensors, molecular magnets and the like.
Owner:SHANGHAI UNIV
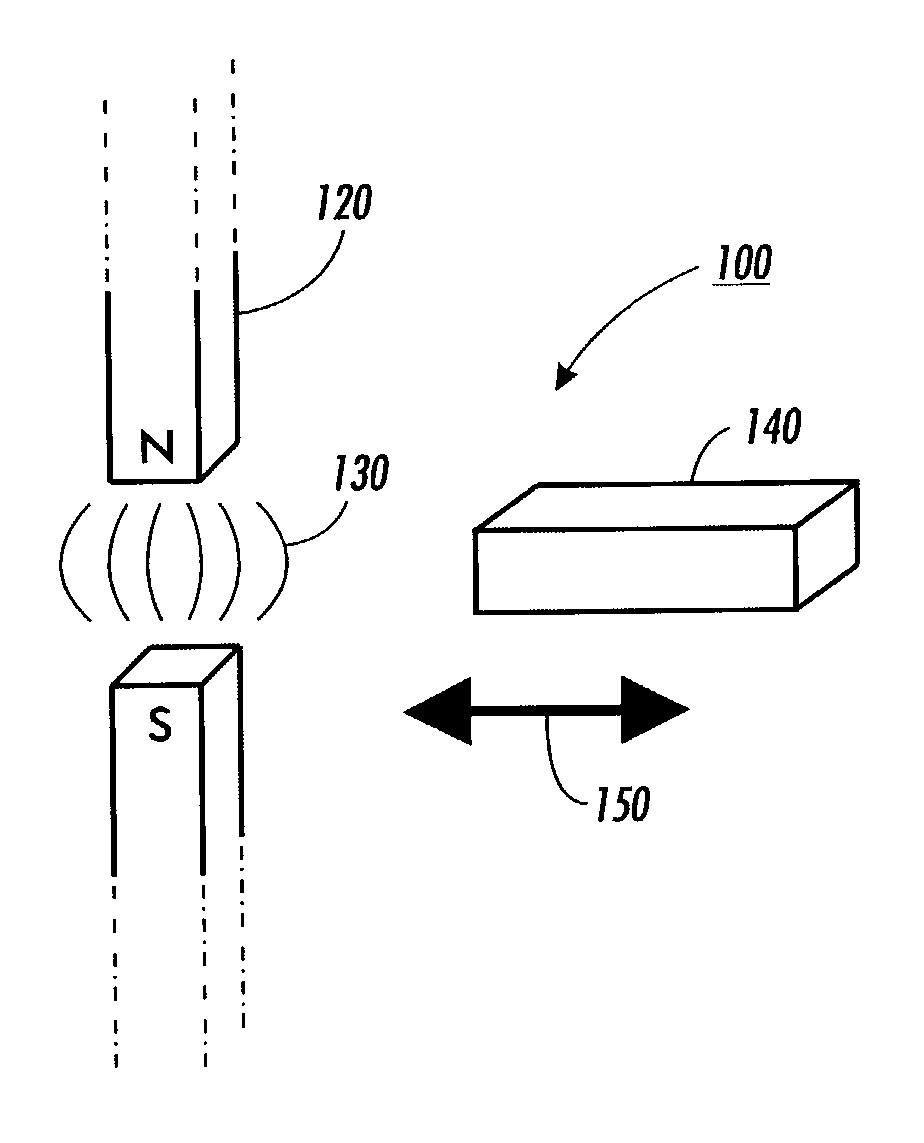
Systems and methods for producing superradiance using molecular magnets
InactiveUS20040100997A1Excitation process/apparatusSolid masersSuperradianceElectromagnetic spectrum
Superradiance is generated by generating coherent radiation in the 10 gigahertz (10<10>) to terahertz (10<12>) regions of the electromagnetic spectrum. The radiation is produced by pulsing a micro crystal of a molecular magnet in a cavity or between a pair of superconductor mirrors at Kelvin or milli-Kelvin temperatures. The coherence and source of the radiation result from enhanced quantum mechanical spin tunneling. Alternatively, the radiation may be obtained by moving the crystal in and out of the field of a permanent magnet.
Owner:XEROX CORP
Polyacid dysprosium single-molecular magnets and preparation method thereof
InactiveCN103994792ASimple methodEasy to implementOrganic chemistryInductances/transformers/magnets manufactureDodecaneMagnetic storage
The invention discloses polyacid dysprosium single-molecular magnets and a preparation method of the polyacid dysprosium single-molecular magnets and belongs to the field of magnetic storage materials. The chemical formula of the single-molecular magnets is [NaDy(DOTA)2 7H2O] [Fe(CN)5NO]2 11H2O, wherein DOTA is 2,2',2'',2'''-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetra) quadrol. The preparation method of the polyacid dysprosium single-molecular magnets includes the following steps that dysprosium nitrate, DOTA and sodium nitroprusside are prepared into a solution according to the molar ratio 1:1:1, the solution is mixed at normal temperature for one hour and then filtered, filtrate stands still at normal temperature, crystals are separated out, and the crystals are the polyacid dysprosium single-molecular magnets. The polyacid dysprosium single-molecular magnets can be applied to new magnetic storage materials. The preparation method of the polyacid dysprosium single-molecular magnets is simple, easy to achieve and low in cost.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
2-aldehyde-8-hydroxyquinoline-1,3-diamino-2-propyl alcohol Schiff base four-core dysprosium cluster compound and synthesis method thereof
ActiveCN108840880ANovel structureHave single-molecule magnet behaviorGroup 3/13 organic compounds without C-metal linkagesOrganic/organic-metallic materials magnetismSpace groupSynthesis methods
The invention discloses a 2-aldehyde-8-hydroxyquinoline-1,3-diamino-2-propyl alcohol Schiff base four-core dysprosium cluster compound and a synthesis method thereof. The cluster compound belongs to amonoclinic system, a P21 / n space group, and the chemical formula is [Dy4(C23H17N4O3)2(mu3-OH)2(NO3)4(H2O)2]. The synthesis method of the cluster compound comprises the following steps: dissolving 2-aldehyde-8-hydroxyquinoline, 1,3-diamino-2-propyl alcohol and Dy(NO3)3*6H2O into a mixed solvent, adjusting the pH of the obtained solution to be 9.4 to 10.0, performing reaction on the obtained mixedliquid under the heating condition, cooling a reactant and separating out crystals to obtain the cluster compound, wherein the mixed solvent is a composition of ethanol and acetonitrile. The cluster compound has field induced single-molecular magnet behavior and can be used for preparing a magnetic material; furthermore, the synthesis method is simple and easy to operate.
Owner:山东顺创新材料科技有限公司
Structure-fine controllable Mn<2+> paramagnetic compound and its preparation method and use
InactiveCN103232493AMild reaction conditionsLow costGroup 7/17 element organic compoundsHybrid materialMagnetic molecules
The invention discloses a structure-fine controllable Mn<2+> paramagnetic compound and its preparation method and use. A complex precursor ligand is modified and is introduced to the same multi-anion framework so that the structure-fine controllable Mn<2+> paramagnetic compound is obtained. The preparation method comprises synthesizing an Mn-Salen type dual-core compound, preparing an Mn-Salen solution, preparing a multi-anion aqueous solution, dropwisely adding the Mn-Salen solution into the multi-anion aqueous solution, and carrying out constant-speed stirring at a temperature of 40 to 50 DEG C for 4 to 10h to obtain the structure-fine controllable Mn<2+> paramagnetic compound. The structure-fine controllable Mn<2+> paramagnetic compound can be used for molecular magnets. The preparation method realizes fine control of a magnetic complex first and provides a novel approach for magnetic compound controllable synthesis. The preparation method has mild conditions and a low cost, further enriches types of organic and inorganic hybrid materials and provides a novel method for research on properties of a single magnetic molecule.
Owner:KUNMING UNIV
Cobalt-naphthalene ring nitrogen-oxygen free radical molecular magnet material with 6.3T coercive force and preparation method of molecular magnet material
InactiveCN109517011AEasy to prepareMild reaction conditionsCobalt organic compoundsOrganic/organic-metallic materials magnetismHysteresisOxygen
The invention relates to a cobalt-naphthalene ring nitrogen-oxygen free radical molecular magnet material with 6.3T coercive force and a preparation method of the molecular magnet material. The chemical formula of a molecular magnet is [Co(hfac)2(EtONapNIT)]n, wherein n is a natural number from 1 to positive infinity. The preparation method of the molecular magnet material comprises the followingsteps: enabling normal hexane suspension liquid of cobalt hexafluoroacetylacetonate to reflux for more than two hours, cooling and adding dichloromethane solution of EtONapNIT to react, and volatilizing for several days at room temperature to obtain a target product. The the molecular magnet material is simple in preparation method, mild in reaction conditions and high in yield, and has very goodair stability. The complex shows slow magnetic relaxation behavior in a zero field, and has a very large hysteresis loop at 2K, and the coercive field is close to 6.3T. The molecular magnet material with a large coercive field can effectively reduce the information loss conditions of an information storage device in environmental perturbation, and thus has a very high potential application value in the field of high-density information storage.
Owner:NANKAI UNIV
A kind of mononuclear dysprosium complex and its preparation method and application
ActiveCN107089999BEasy to prepareLow costOrganic chemistry methodsOrganic/organic-metallic materials magnetismAlcoholRuthenium
The invention discloses a mononuclear dysprosium complex and a preparation method and application thereof. The chemical formula of the complex is [Dy(HL)2(CH3OH)Cl3], wherein HL is 2-methyl-5,7-dibromo-8-hydroxyquinoline. The complex belongs to a monoclinic system and P21 / n space groups. The preparation method of the mononuclear dysprosium complex includes the steps that DyCl3.6H2O and 2-methyl-5,7-dibromo-8-hydroxyquinoline are taken and dissolved with a mixed solvent, the pH of the obtained solution is adjusted to range from 6.5 to 7.8, the obtained mixed liquid reacts under the heating condition, and then the complex is obtained, wherein the mixed solvent is a composition of methyl alcohol and acetonitrile. The preparation method of the complex is simple, the cost is low, good repeatability is achieved, magnetic properties of the complex are shown as field-induced single-molecular magnet behaviors, and the complex can be used for preparing magnetic materials.
Owner:GUANGXI NORMAL UNIV
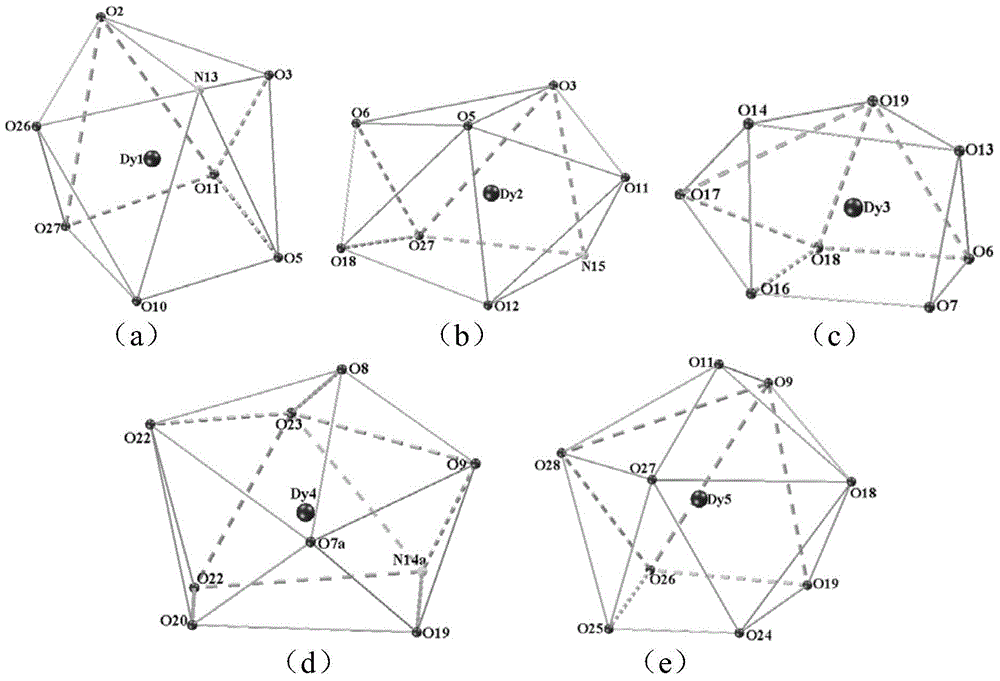
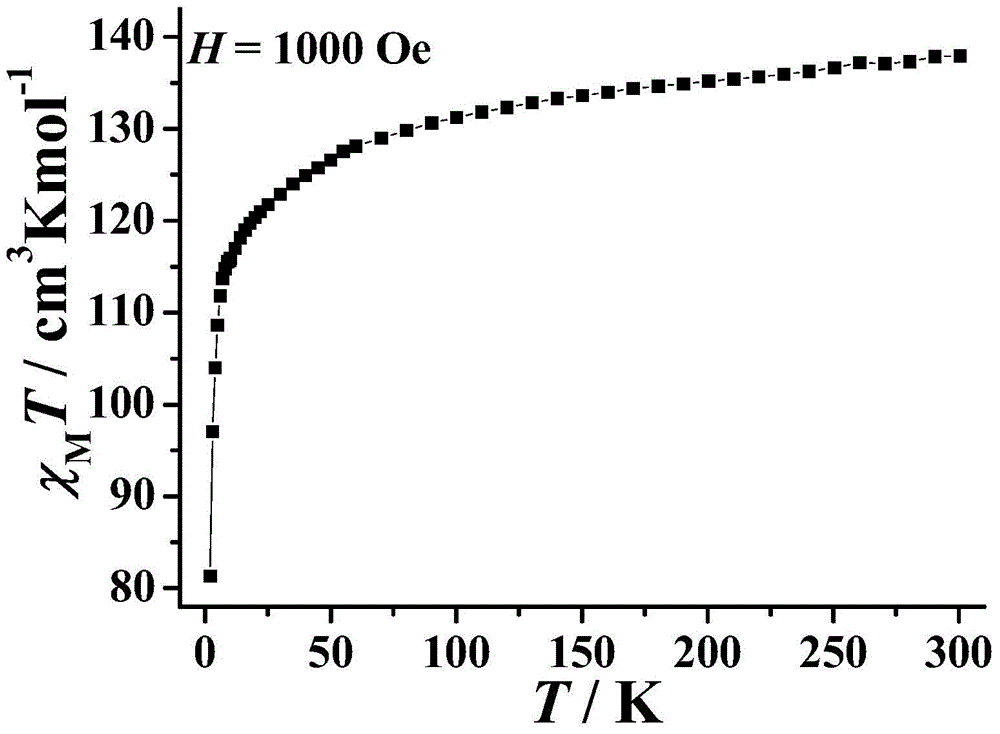
Ten-core dysprosium cluster compound single-molecular magnet and preparation method thereof
InactiveCN109053773AGroup 3/13 organic compounds without C-metal linkagesOrganic chemistry methodsSpace groupDual core
The invention discloses a ten-core dysprosium cluster compound single-molecular magnet and a preparation method thereof. The ten-core dysprosium cluster compound single-molecular magnet has the chemical formula as [Dy10(ovpho)4(NO3)4(CH3O)3(mu4-O)2(mu3-OH)2(mu2-OH)(H2O)4].8H2O. The ten-core dysprosium cluster compound single-molecular magnet is crystallized in an orthorhombic system Pbcn space group and is structurally formed by two edge-shaped {Dy4} tetrahedron elements and two {Dy2} double-core units located on two sides of the tetrahedron elements. The preparation method comprises the stepsof adopting an H4ovpho ligand and Dy(NO3)3.6H2O with the molar ratio being 1:3 as raw materials, adopting methanol and acetonitrile as solvents, adopting triethylamine as an alkaline matter, stirring, carrying out solvothermal reaction, separating, and washing solids. The ten-core dysprosium cluster compound single-molecular magnet provided by the invention is simple in process, low in cost, easyto control chemical components, good in repeatability and high in yield. Meanwhile, the cluster compound size reaches to the nano scale and is the rare high-core nano single-molecular magnet.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Rare earth based molecular magnet constructed by taking alkylol amine Schiff base generated in situ as ligand and preparation method of rare earth based molecular magnet
InactiveCN104952585AEasy to prepareHigh yieldOrganic/organic-metallic materials magnetismGroup 3/13 element organic compoundsBenzaldehydeRare earth
The invention discloses a rare earth based molecular magnet constructed by taking alkylol amine Schiff base generated in situ as a ligand and a preparation method of the rare earth based molecular magnet. The chemical formula of the rare earth based molecular magnet is [Dy10(L)4(HL)2([mu]5-NO3)2(CH3COO)12].8H2O, wherein the L represents that three hydroxyl hydrogen atoms are removed from 3-[(2-hydroxyl-3-oxethyl)-benzylideneamino]-1, 2-propylene glycol; the HL represents that two hydroxyl hydrogen atoms are removed from 3-[(2-hydroxyl-3-oxethyl)-benzylideneamino]-1, 2-propylene glycol. The preparation method of the rare earth based molecular magnet comprises the following steps: taking Dy(NO3)3.6H2O, Dy(CH3COO)3.6H2O, 3-oxethyl-2-hydroxy benzaldehyde and 3-amino-1, 2-propylene glycol, dissolving the materials by using a mixed solvent, adjusting the pH value, carrying out liquid nitrogen refrigeration, vacuumizing, fusion-sealing, and carrying out the heating reaction, so as to obtain the rare earth based molecular magnet. The molecular magnet provided by the invention is integrally paramagnetic.
Owner:GUANGXI NORMAL UNIV
Mixed metal ion single-molecular magnet and preparation method thereof
InactiveCN103980324ARaise the blocking temperatureHigh yieldOrganic chemistryMagnetsMagnetic storageSynthesis methods
The invention discloses a mixed metal ion single-molecular magnet and a synthesis method thereof, belonging to the field of magnetic storage materials. The chemical formula of the single-molecular magnet is D<y>(DMF)4(H2O)3Fe(CN)6.H2O, wherein DMF is N,N-dimethyl formamide. The preparation method comprises the following steps: evenly mixing dysprosium nitrate and a potassium ferricyanide solution, adding a DMF (dimethyl formamide) solution of quinoline-2-formic acid, stirring for one hour at normal temperature, filtering, standing a filtrate at the normal temperature, and separating out crystals, namely the mixed metal ion single-molecular magnet. The mixed metal ion single-molecular magnet can be applied to a novel magnetic storage material; the method is simple, easy to realize and low in cost.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
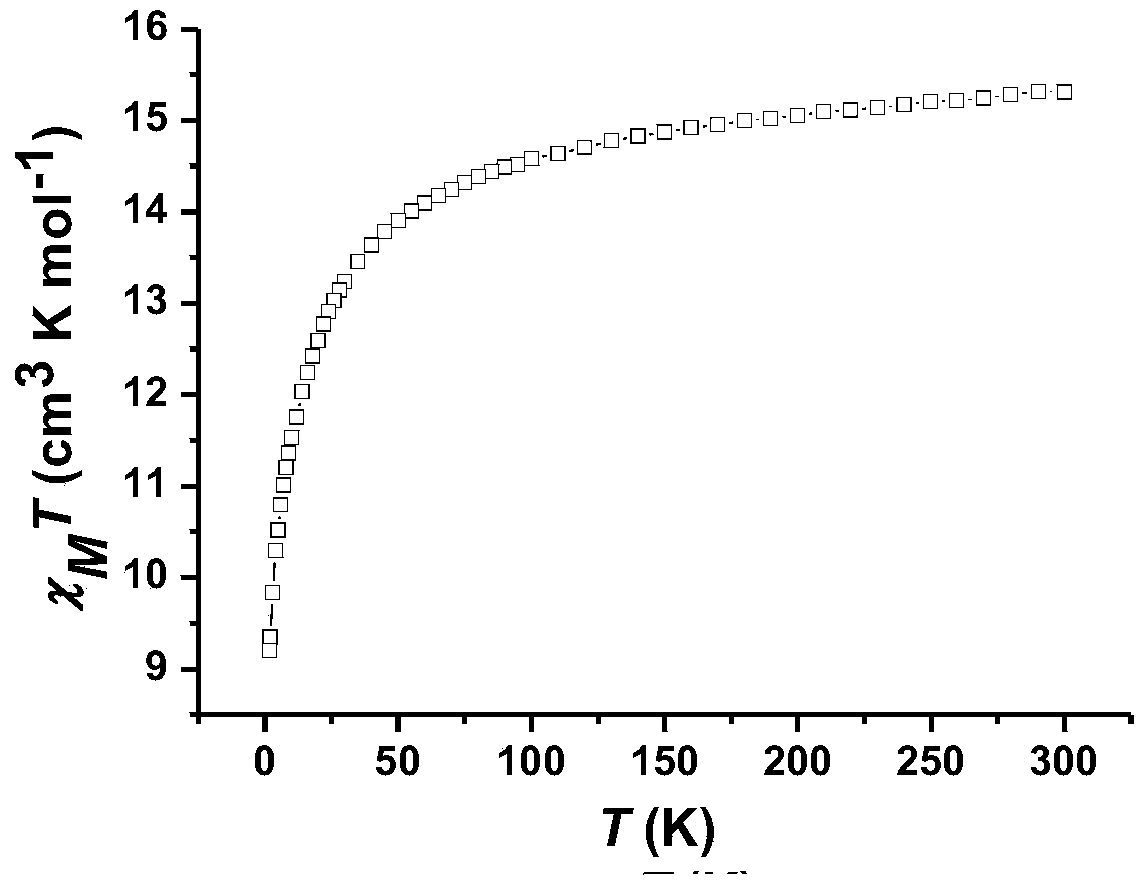
N-hydroxyphthalimide dysprosium coordination compound and preparation method thereof
ActiveCN110003252AImprove performanceOptimize the synthetic routeGroup 3/13 organic compounds without C-metal linkagesOrganic chemistry methodsSingle crystalSlow cooling
The invention provides a N-hydroxyphthalimide dysprosium coordination compound and a preparation method thereof. The coordination compound has a following chemical formula: [Dy2(HPI)2(NO3)4(DMF)2], wherein HPI is N-hydroxyphthalimide of which one hydrogen ion is removed. The preparation method comprises the following steps: weighing Dy(NO3)3.5H2O and N-hydroxy-1,8-naphthalimide in a hard glass tube, adding an organic solvent, performing uniform shaking, performing vacuum sealing, performing a reaction under heating condition at 60-80 DEG C for 1-3 days, taking out the reaction product, performing slow cooling to room temperature, performing washing, and selecting pure regular single crystals, and performing natural drying to obtain the N-hydroxyphthalimide dysprosium coordination compound.The method provided by the invention prepares the N-hydroxyphthalimide dysprosium coordination compound from selection of a rare earth ion, design of a single-molecular magnet and optimization of a synthetic route, and improves the performance of the rare earth single-molecular magnet; and the coordination compound is a 4f metal coordination compound with a slow relaxation behavior, and has potential application value in the fields of high-density magnetic memorizers and magnetic films.
Owner:SHAOYANG UNIV
Tetranuclear water cluster copper-containing coordination polymer with mixed ligand, and preparation method thereof
InactiveCN106831828AThe synthesis method is simpleGood repeatabilityCopper organic compoundsSpace groupWater cluster
The invention relates to the technical field of inorganic and organic hybrid materials and particularly discloses a preparation method of a tetranuclear water cluster copper-containing coordination polymer with a mixed ligand. According to the tetranuclear water cluster copper-containing coordination polymer with the mixed ligand, the molecular formula is C50H54CuF4N12O10, the polymer is a triclinic system, and a space group and the cell parameters are as shown in the specification. The tetranuclear water cluster copper-containing coordination polymer with the mixed ligand is capable of accurately measuring the positions of water molecules in crystal lattices through a single crystal X-ray diffractometer, so that various parameters of the hydrogen interaction among the water molecules can be accurately described and various physical and chemical behaviors of the water molecules in the coordination polymer can be more deeply understood. The tetranuclear water cluster copper-containing coordination polymer with the mixed ligand is easy to prepare, good in stability, few in defects of the synthetic material and high in crystallinity, and has a very good application prospect in the fields such as molecular magnets and catalysis.
Owner:QINGDAO UNIV OF SCI & TECH
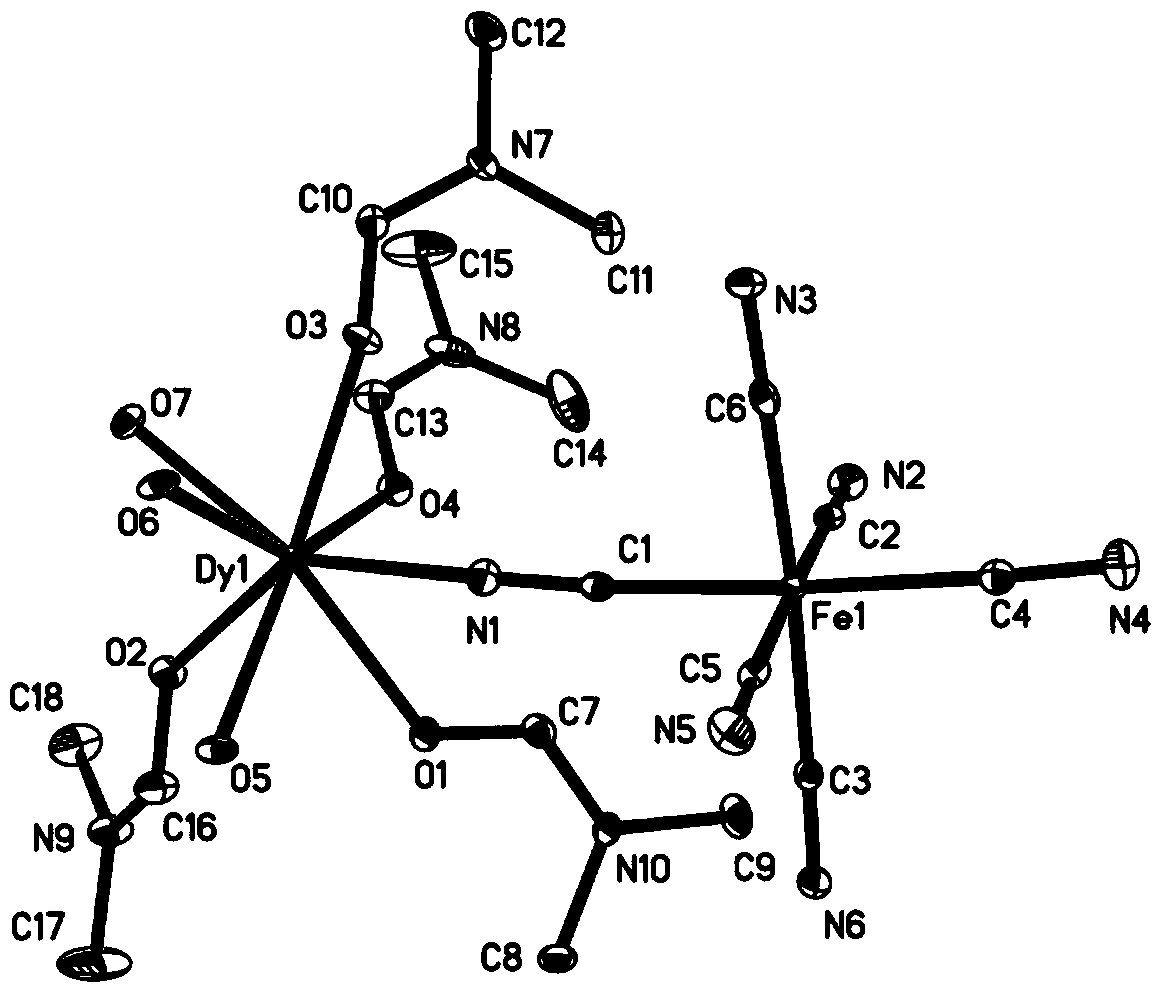
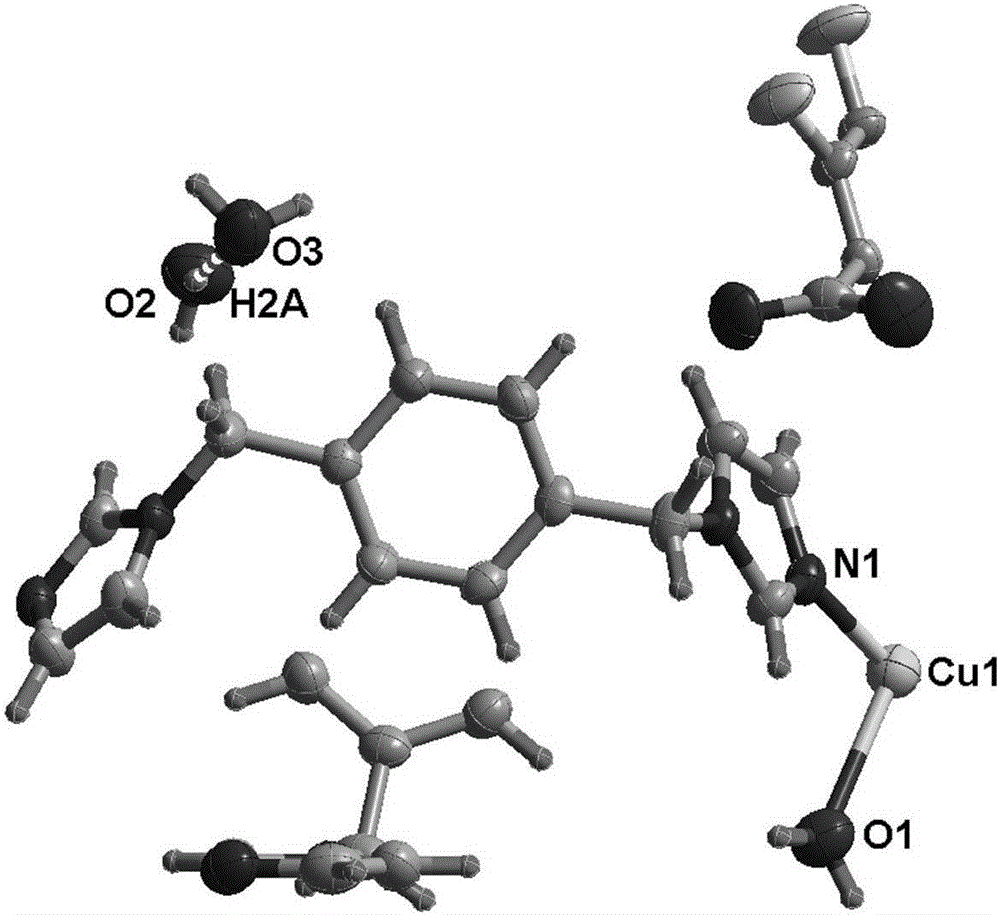
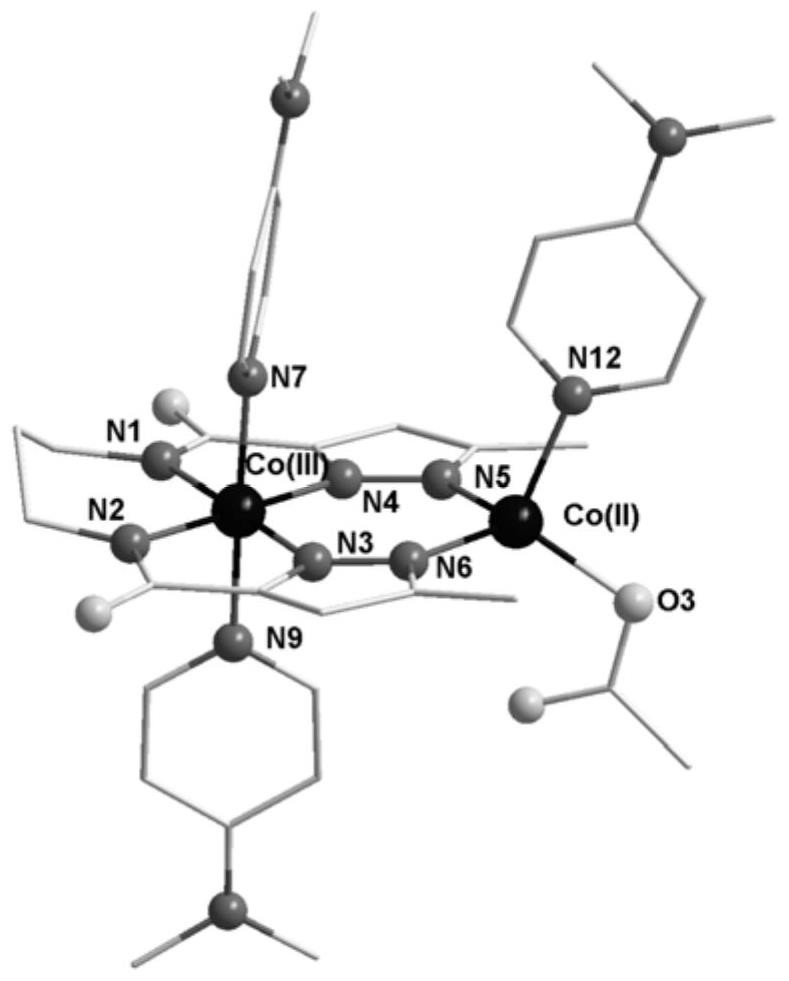
Co(III)-Co(II) binuclear cobalt single-molecular magnet, preparation method and application thereof
PendingCN113956297ATypical slow relaxation behaviorFeatures single-molecule magnetsCobalt organic compoundsOrganic/organic-metallic materials magnetismMethyl groupMaterials science
The invention discloses a Co(III)-Co(II) binuclear cobalt single-molecular magnet, a preparation method and application thereof, the chemical formula of the single-molecular magnet is [CoIIICoII(L)(DMAP)3(CH3COO)], wherein H4L is N, N'-bis(5-methylpyrazole-3-formyl)-1, 3-propane diamine, and the structure is as shown in the specification, DMAP is 4-dimethylaminopyridine, and the structure is as shown in the specification. Compared with the prior art, the Co(III)-Co(II) binuclear cobalt single-molecular magnet has the following advantages that: (1) the Co(III)-Co(II) binuclear cobalt single-molecular magnet can show typical slow relaxation behavior under an external magnetic field of 0.1T, has the characteristics of a single-molecular magnet, and can be used as a molecule-based magnetic material in novel high-density information storage equipment (such as an optical disk and a hard disk); and (2) the method is safe and simple in process, high in controllability and good in reproducibility.
Owner:JIANGSU UNIV OF SCI & TECH
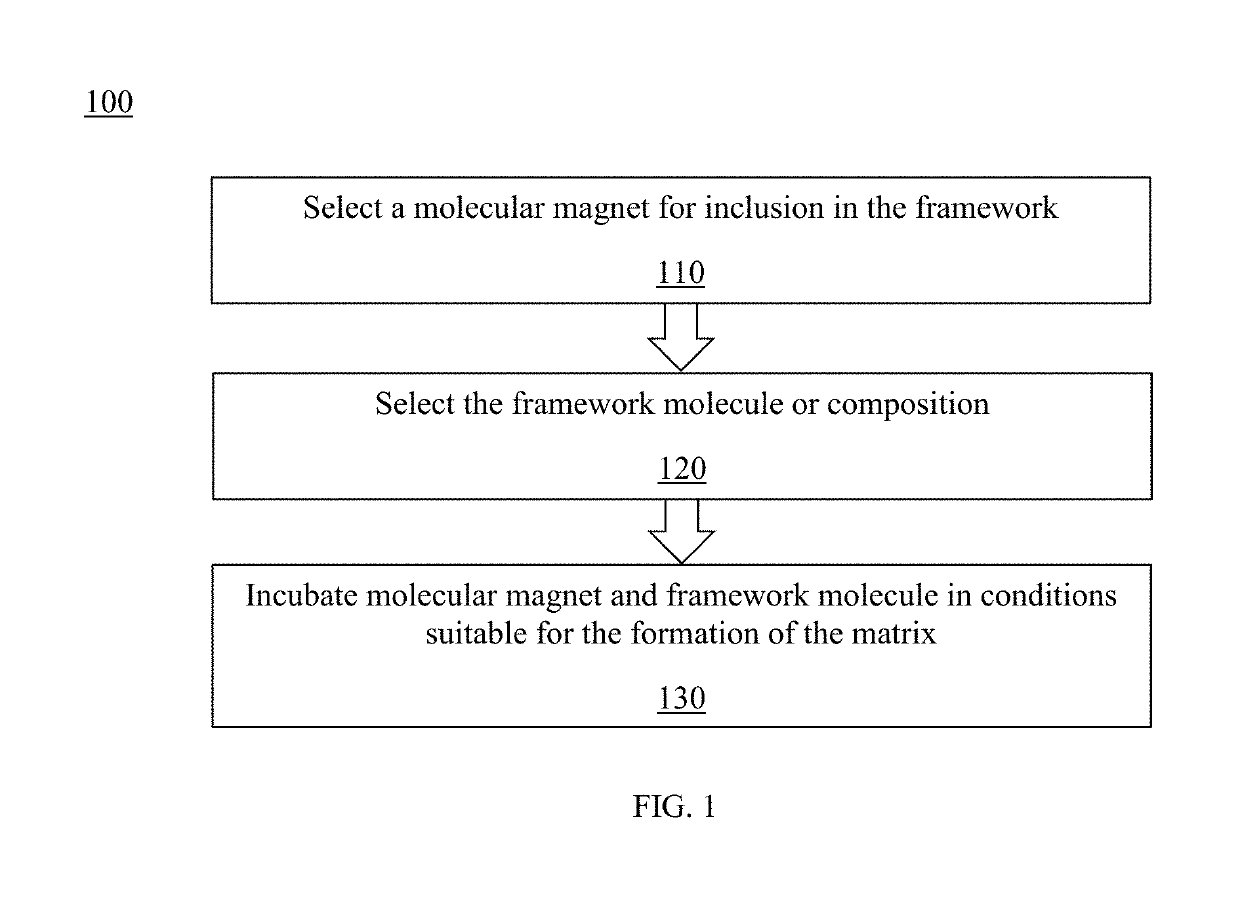
Method and system for controlled nanostructuring of nanomagnets
ActiveUS10340066B2Assembly precisionOrganic/organic-metallic materials magnetismGroup 3/13 element organic compoundsNanomagnetMagnetic molecules
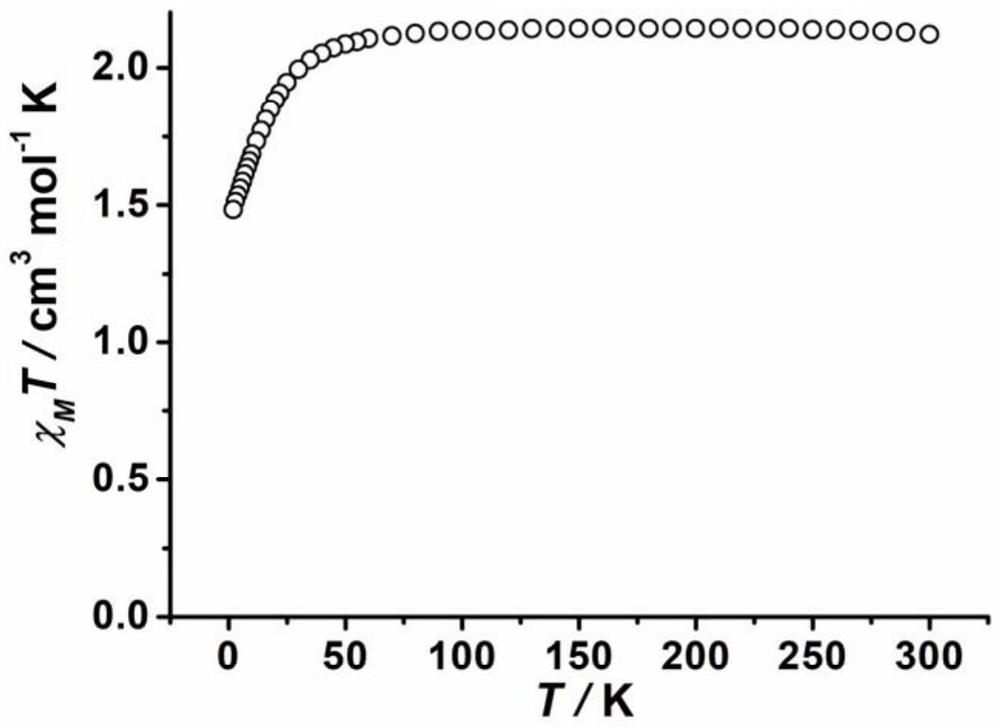
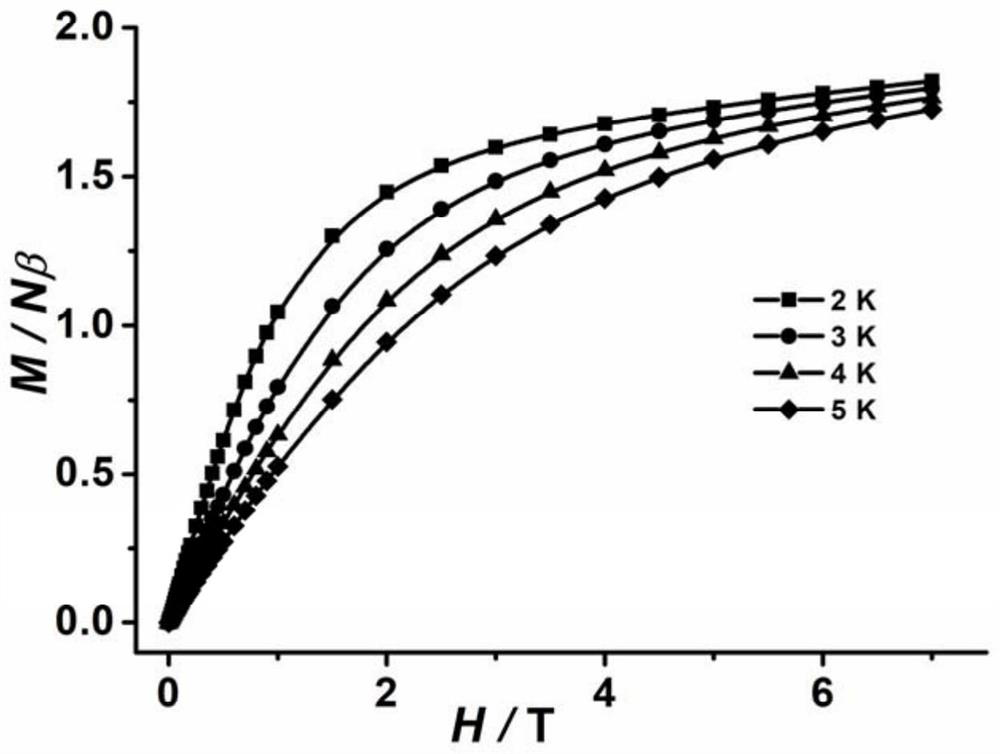
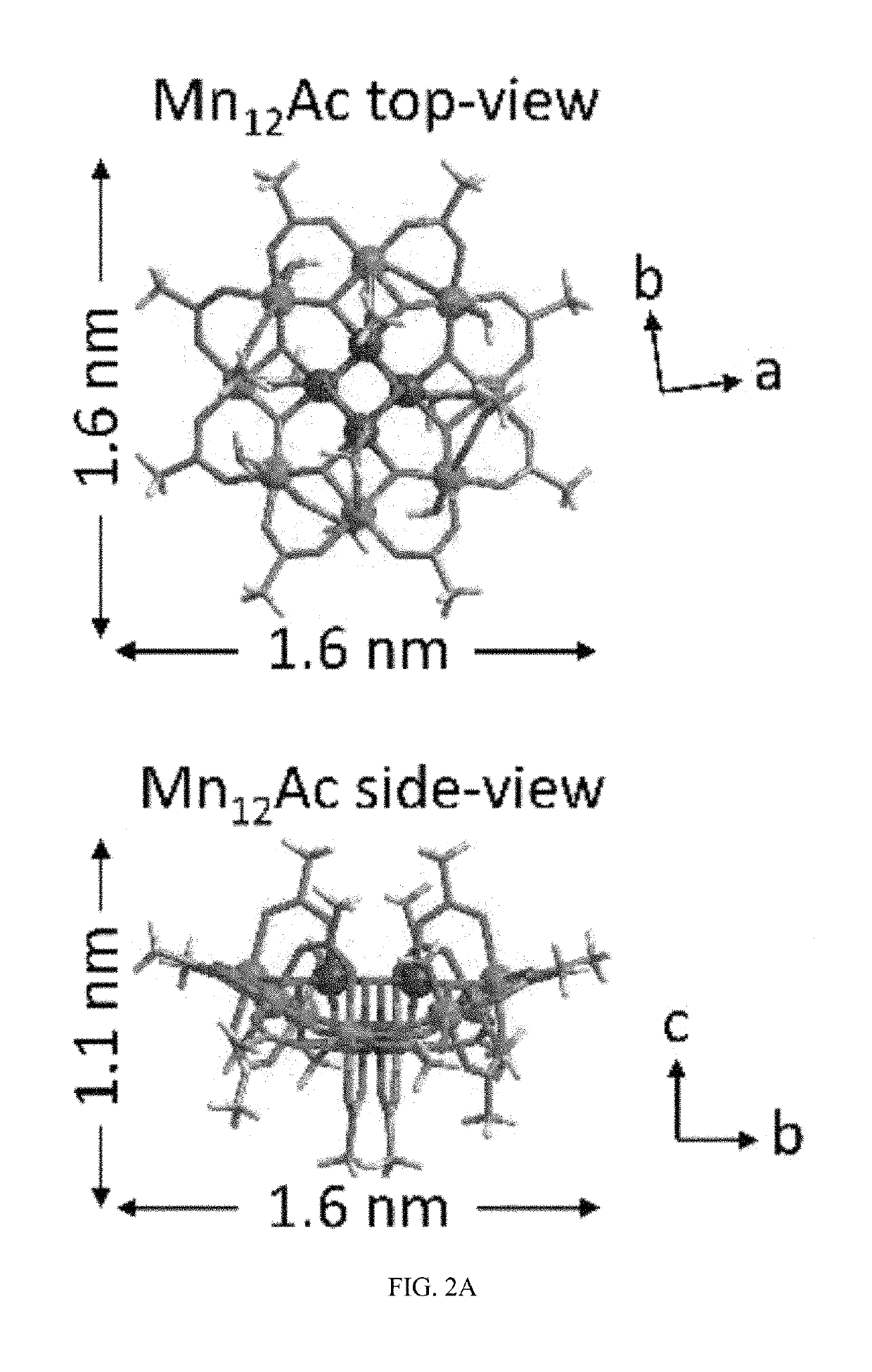
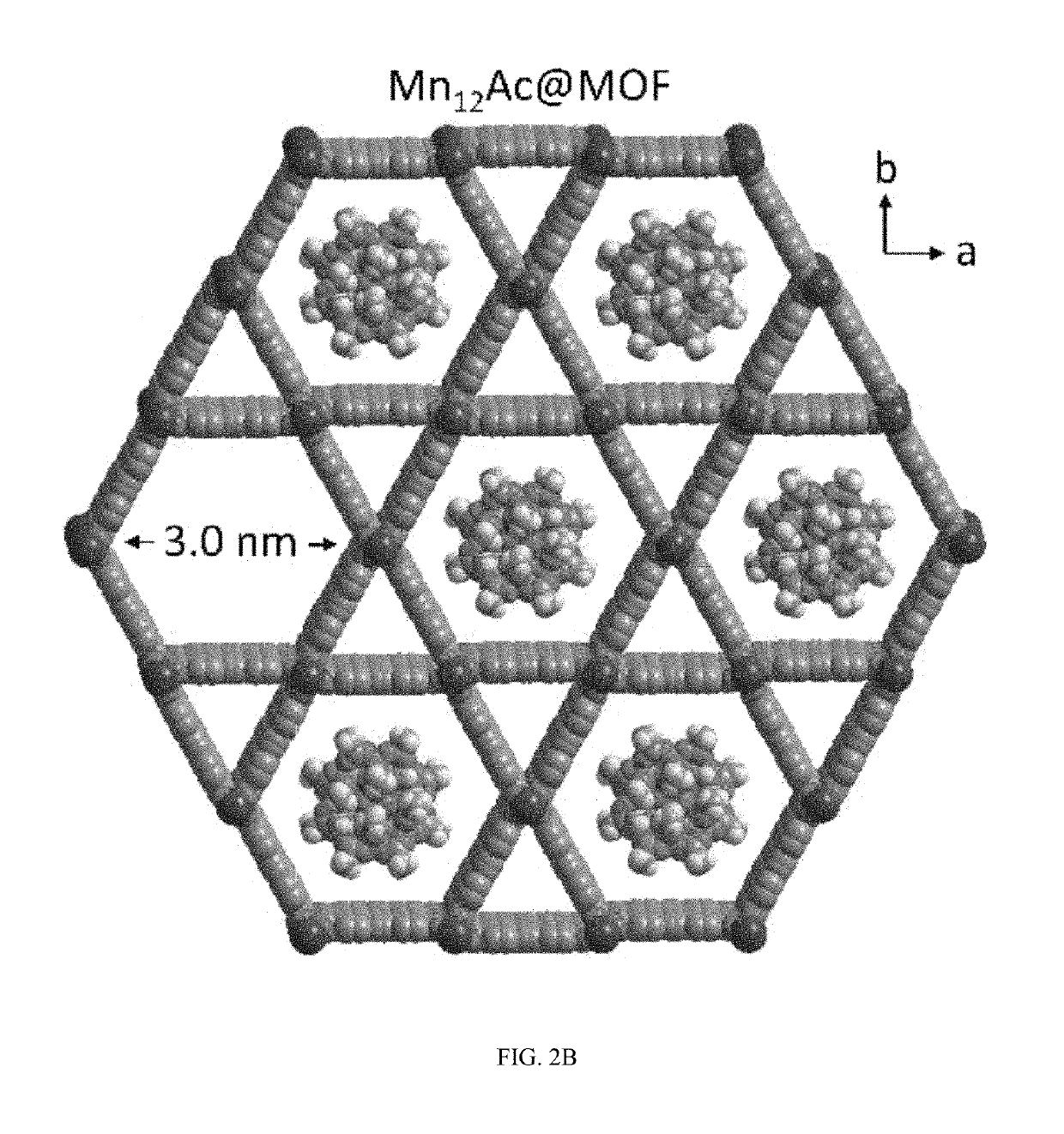
A composite magnetic matrix comprising a porous metal-organic framework (MOF) and a plurality of molecular magnets, where a plurality of pores of the MOF each comprise one of the plurality of molecular magnets, and where the each of the plurality of molecular magnets retains its magnetic properties in the matrix. The molecular magnet may be, for example, a single-molecule magnet or a single-chain magnet. For example, the composite magnetic matrix Mn12Ac@MOF comprises Mn12O12(O2CCH3)16(OH2)4 (Mn12Ac) as the single-molecule magnet and [Al(OH)(SDC)]n (H2SDC=4,4′-stilbenedicarboxylic acid) (CYCU-3) as the porous metal-organic framework.
Owner:CLARKSON UNIVERSITY
Organic-inorganic hybrid room temperature metallic molecular magnet and preparation method thereof
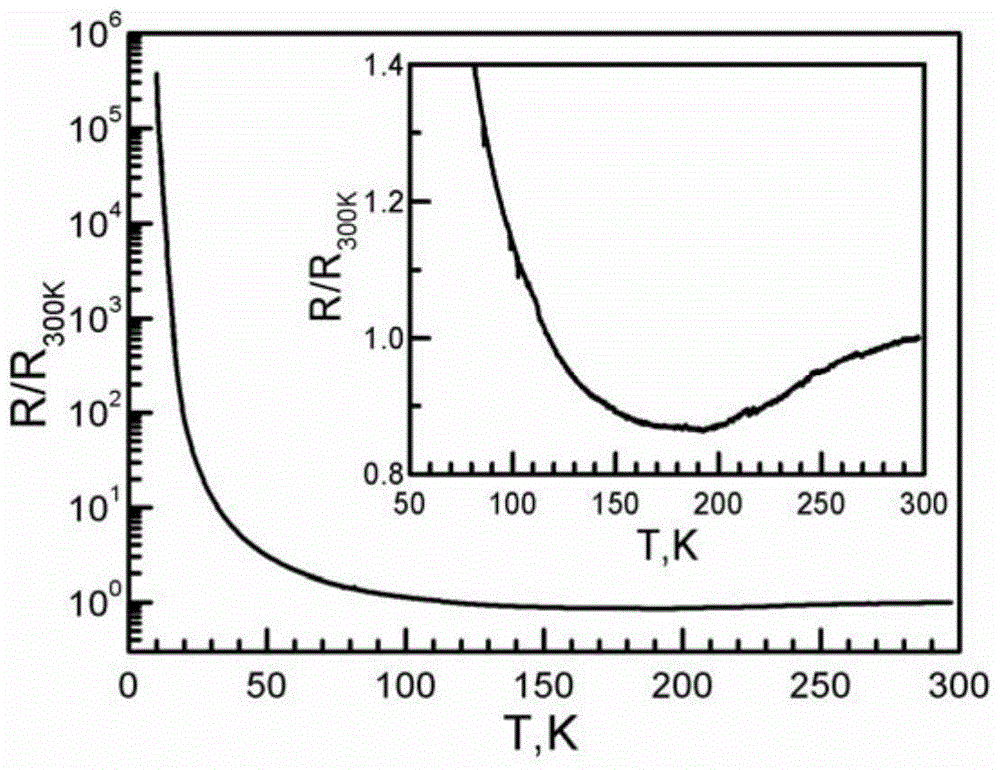
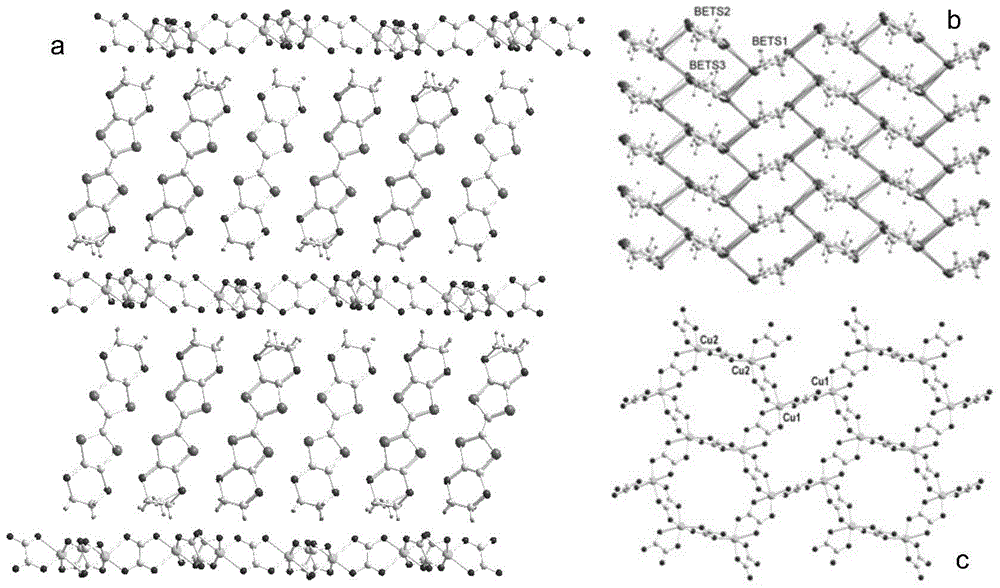
ActiveCN103864820BGroup 1/11 organic compounds without C-metal linkagesElectrolysis componentsElectrochemical responseSingle crystal
The invention discloses an organic-inorganic hybridization molecular magnet with room-temperature metallicity and a preparation method of the molecular magnet. The molecular formula of the molecular magnet with the room-temperature metallicity is BETS3[Cu2(C2O4)3]B2, wherein BETS represents disulfo-diethylene tetraselenafulvalene; and B represents an alcohol compound. The preparation method of the molecular magnet with the metallicity comprises the step of performing electrochemical reaction on an ammonium salt of cupric oxalate with BETS in an electrochemical tank by taking the alcohol compound as an electrolyte solution so as to obtain the molecular magnet with the room-temperature metallicity on an anode. According to the preparation method of the molecular magnet with the room-temperature metallicity, single crystal of the molecular magnet with room-temperature metallicity is prepared in an organic solvent system from an organic pi electron system and Jahn-Teller distorted magnetic anion. The molecular magnet with room-temperature metallicity, which is provided by the invention, is good in crystal quality, single in product and high in yield.
Owner:INST OF CHEM CHINESE ACAD OF SCI
A hexanuclear dysprosium cluster ring complex single-molecule magnet and its preparation method
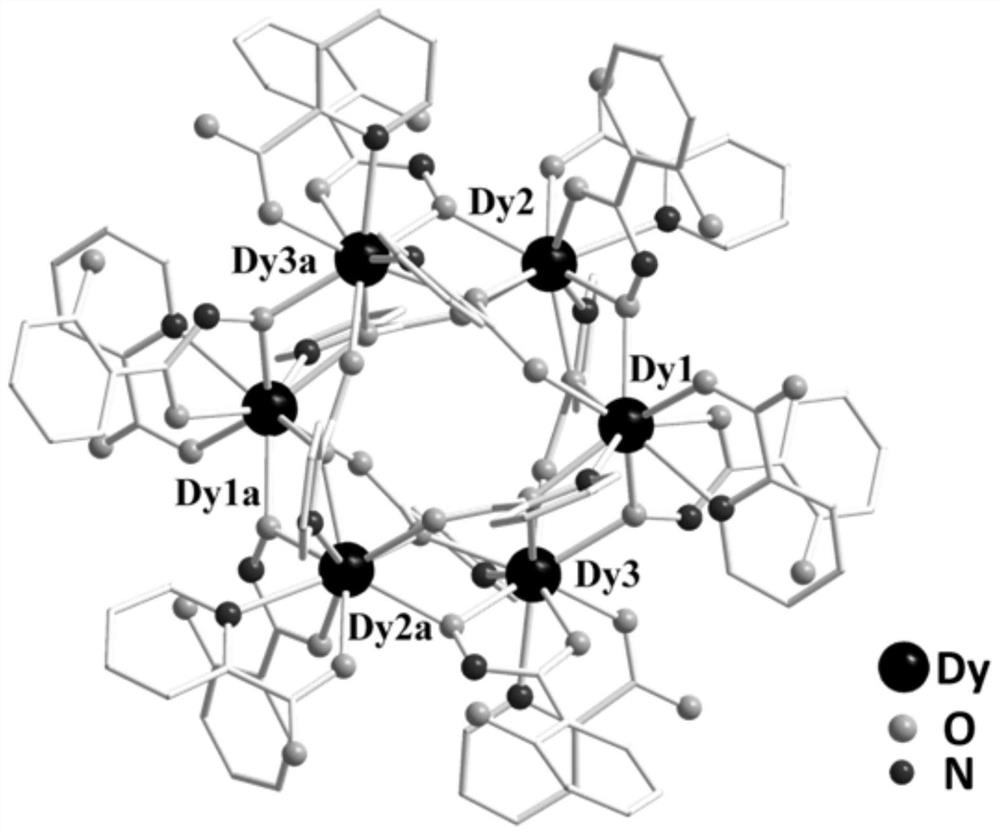
ActiveCN110294771BReduce quantum tunneling effectEasy to prepareGroup 3/13 organic compounds without C-metal linkagesOrganic chemistry methodsHydroxamic acidPhysical chemistry
A single-molecule magnet of a hexanuclear dysprosium cluster ring complex and a preparation method thereof, which relates to a single-molecule magnet and a preparation method thereof. The purpose of the invention is to solve the problems of complex synthesis method, low controllability and poor repeatability in the rare earth complex single-molecule magnet prepared by the existing method. The chemical formula of the hexanuclear dysprosium cluster ring complex single-molecule magnet is [Dy 6 (H 2 sh) 6 (2‑pyca) 6 (2‑pyca ‑ ) 6 ], molecular formula is C 114 h 90 Dy 6 N 18 o 42 . Method: Salicylhydroxamic acid, pyridine‑2‑carboxylic acid and Dy(NO 3 ) 3 ·6H 2 O is dissolved in the solvent, and a mixed solution is obtained by ultrasonication. The mixed solution is heated to reflux, and then pyridine is added to obtain a preform; the solvent is volatilized on the preform to obtain a hexanuclear dysprosium cluster ring complex single-molecule magnet. The invention can obtain a hexanuclear dysprosium cluster ring complex single-molecule magnet.
Owner:HEILONGJIANG UNIV
Cobalt-based single-molecular magnet synthesizing method
ActiveCN105070497ASimple and safe processGood reproducibilityInductances/transformers/magnets manufactureMagnetic materialsMicrowaveCobalt salt
The invention discloses a cobalt-based single-molecular magnet synthesizing method. Cobalt salt and 2-hydroxybenzimidazole ligand are adopted with participation of azides ions, and a cobalt-based single-molecular magnet is prepared under the solvothermal system or a microwave reaction condition. It is indicated by experiments that the process is safe and simple, the cost of raw materials is low, the defect that the reproducibility of a common solution method is poor is overcome, the obtained single-molecular magnet of the [Co12(L)15(N3)7](NO3)2.2(CH3OH).2(H2O) (L is the 2-hydroxybenzimidazole ligand ) is red hexagonal-prism-shaped crystal, the purity is high, the crystal size is large and can be controlled within 2*1*1.5 mm, and the yield is high and reaches over 50%.
Owner:GUANGXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4] Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/57537909-c307-494d-836f-821538d86323/HDA0000634118620000011.PNG)
![Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4] Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/57537909-c307-494d-836f-821538d86323/HDA0000634118620000012.PNG)
![Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4] Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/57537909-c307-494d-836f-821538d86323/HDA0000634118620000021.PNG)
![Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/692108a6-82a0-447f-bbdc-cab60749ad61/HDA0000447506500000011.PNG)
![Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/692108a6-82a0-447f-bbdc-cab60749ad61/HDA0000447506500000012.PNG)
![Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/692108a6-82a0-447f-bbdc-cab60749ad61/HDA0000447506500000021.PNG)