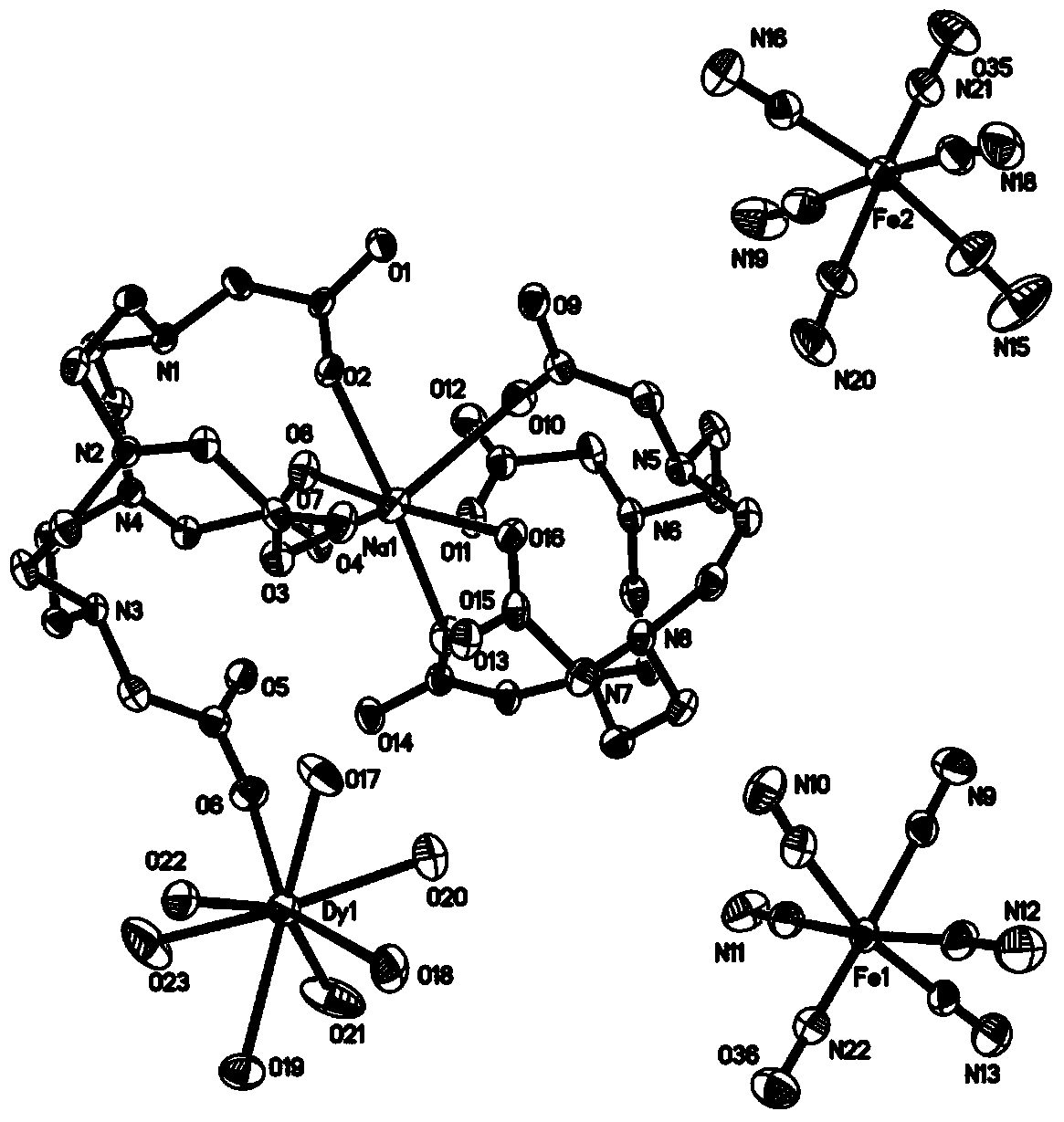
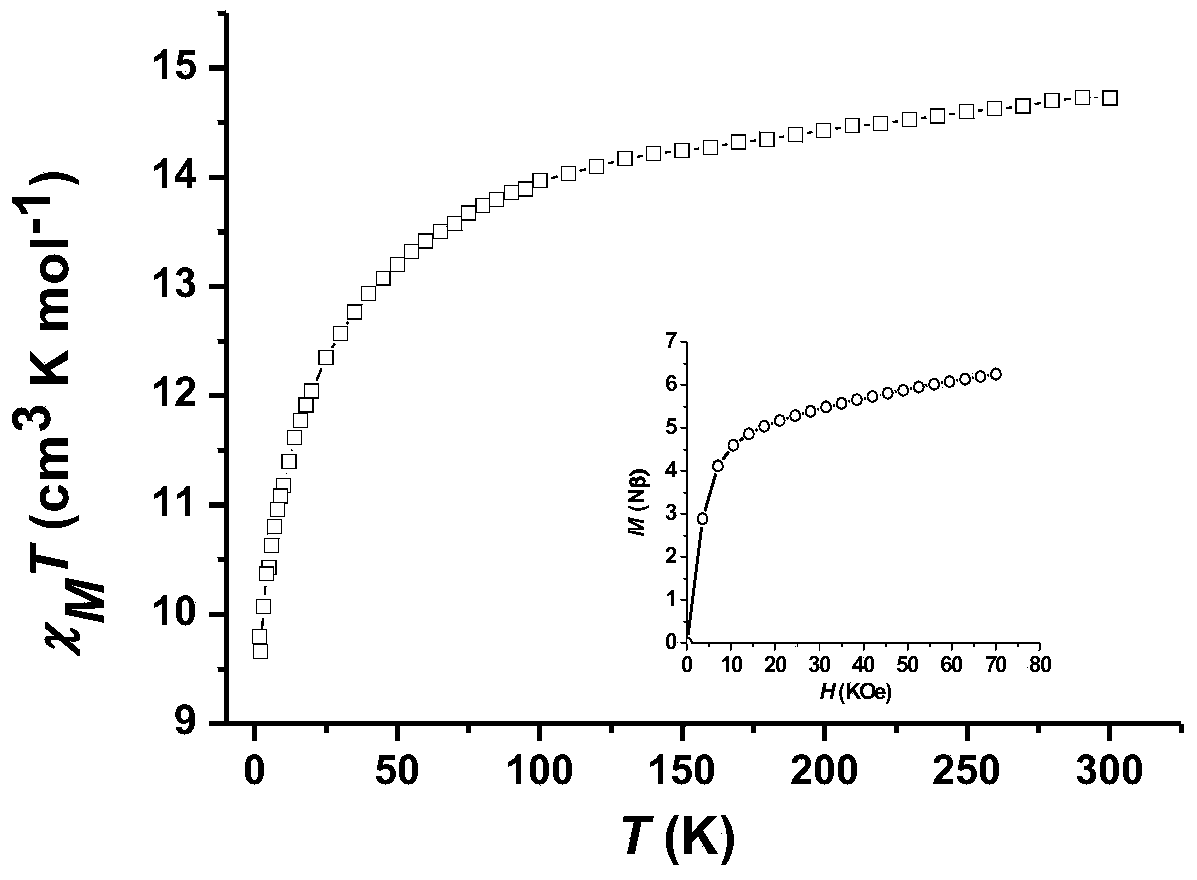
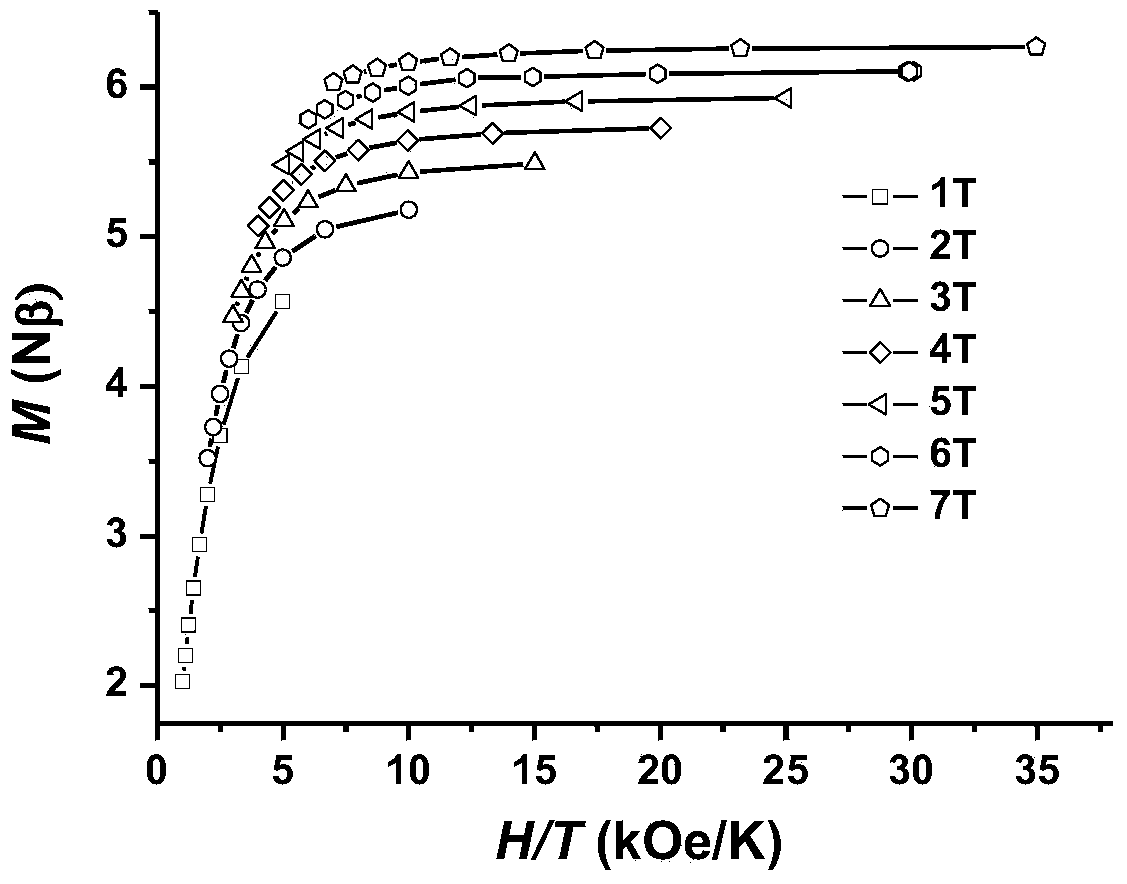
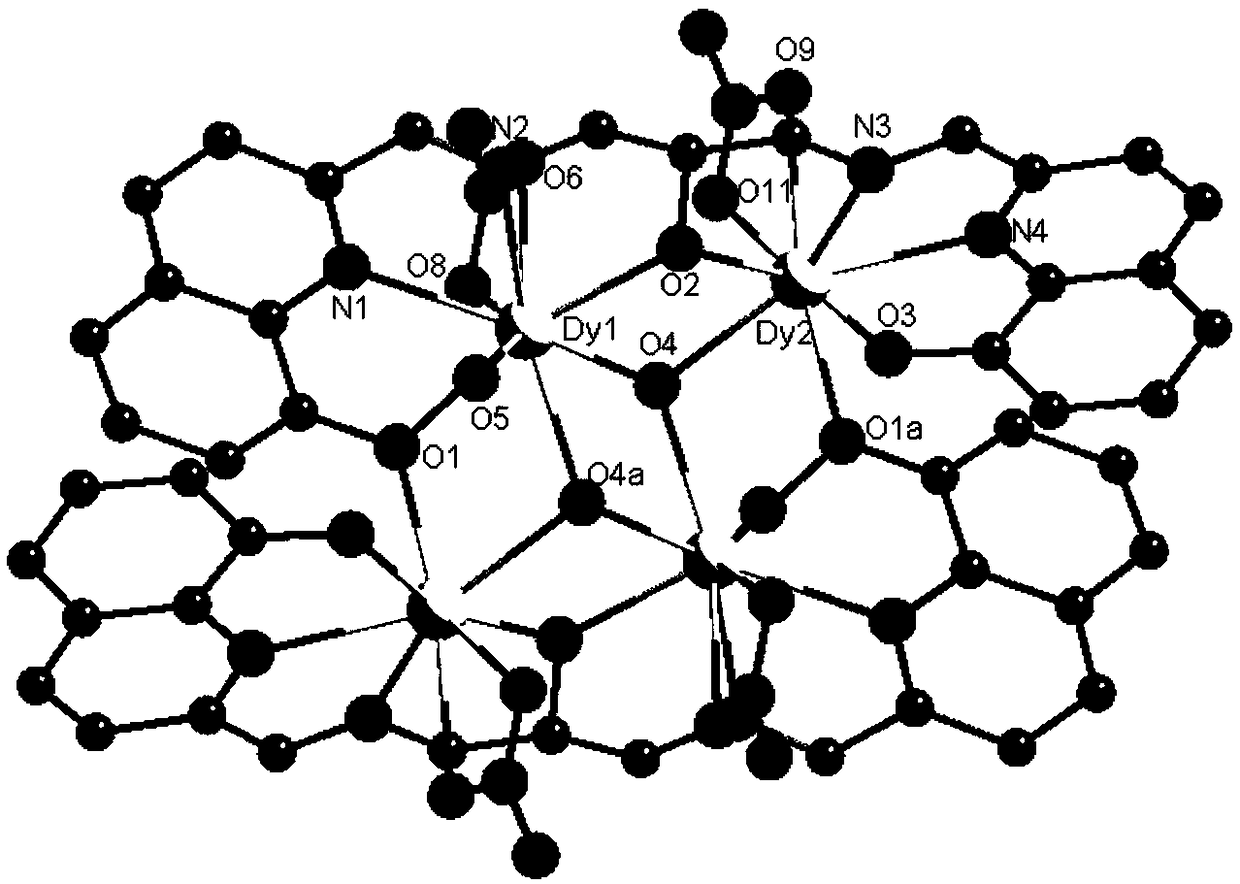
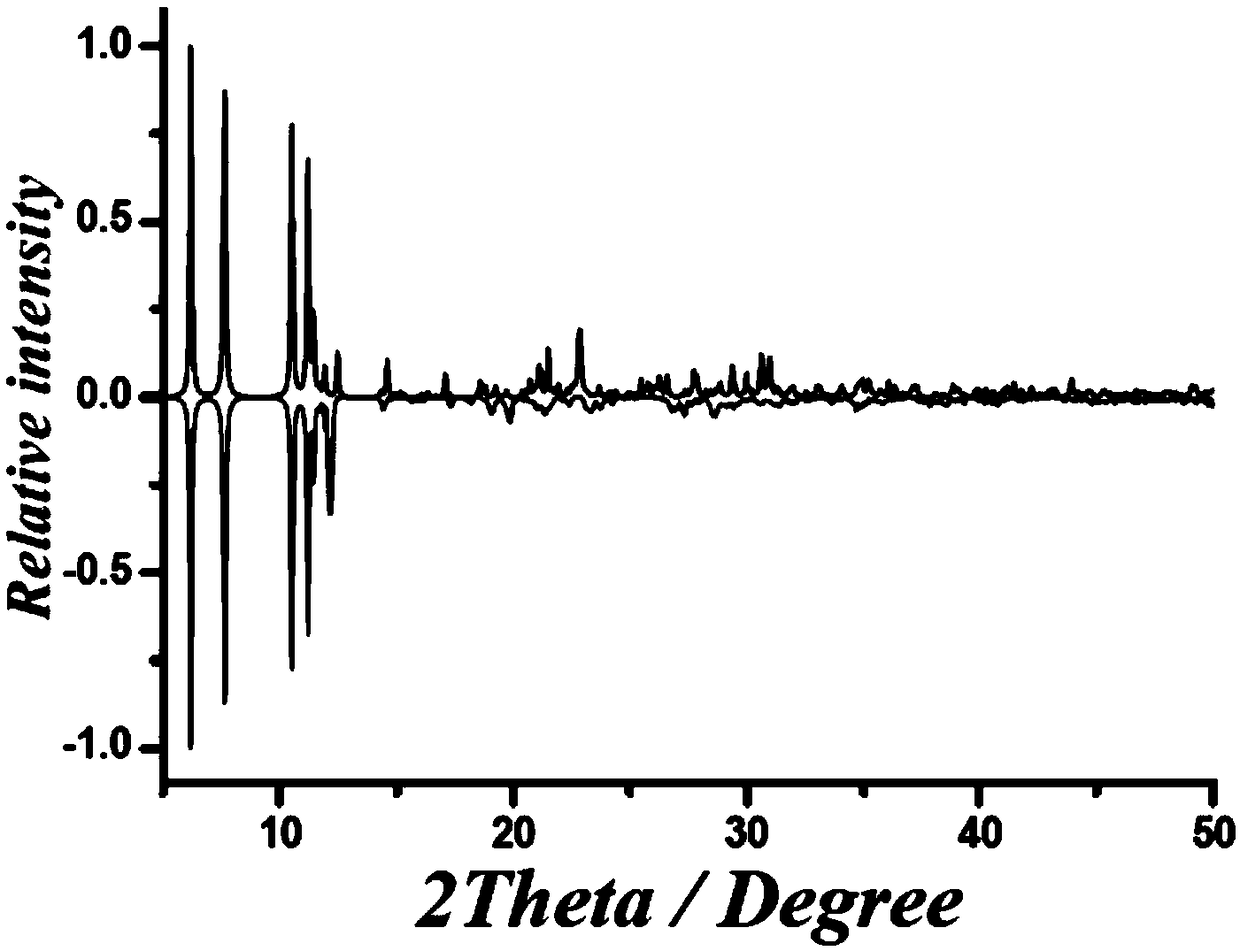
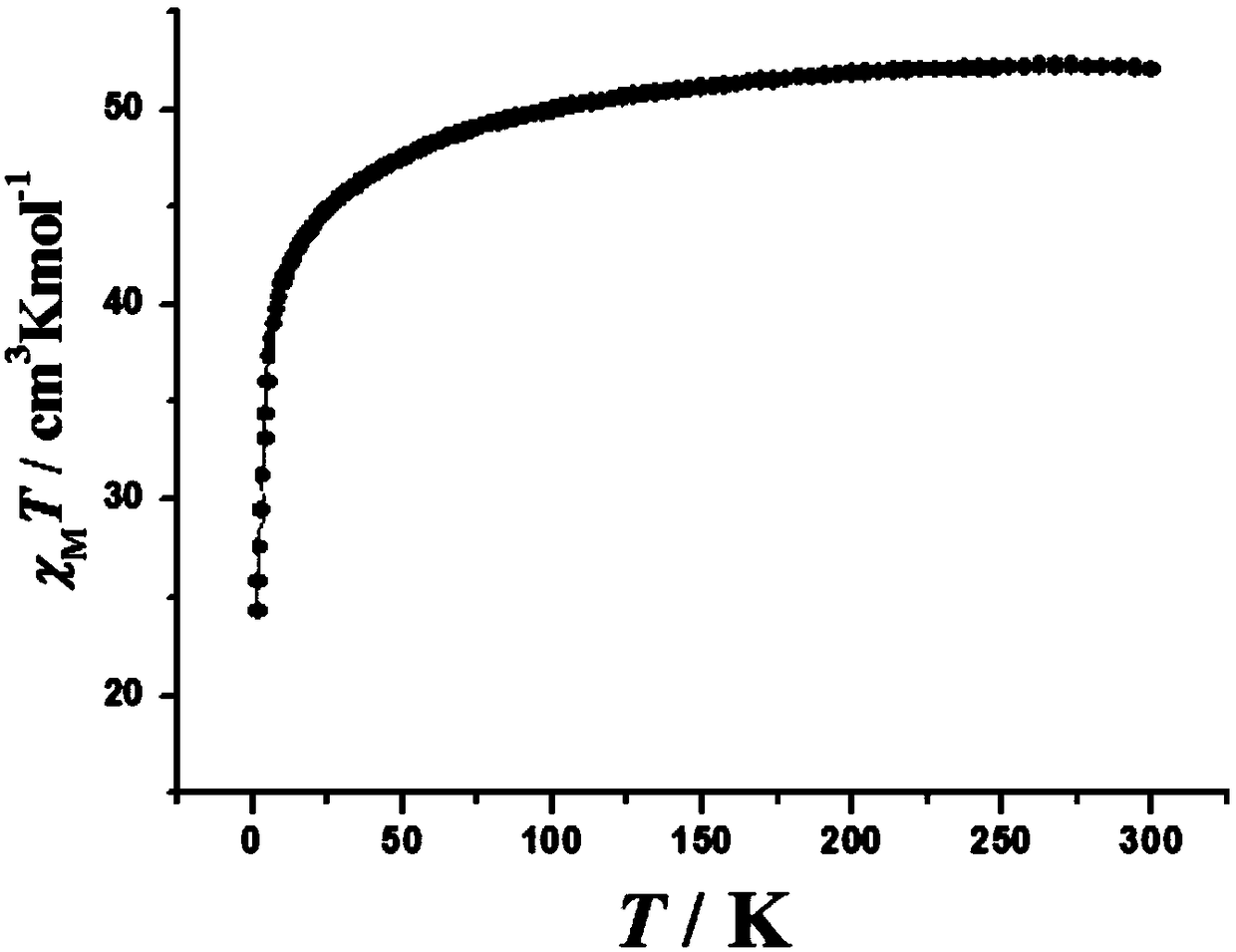
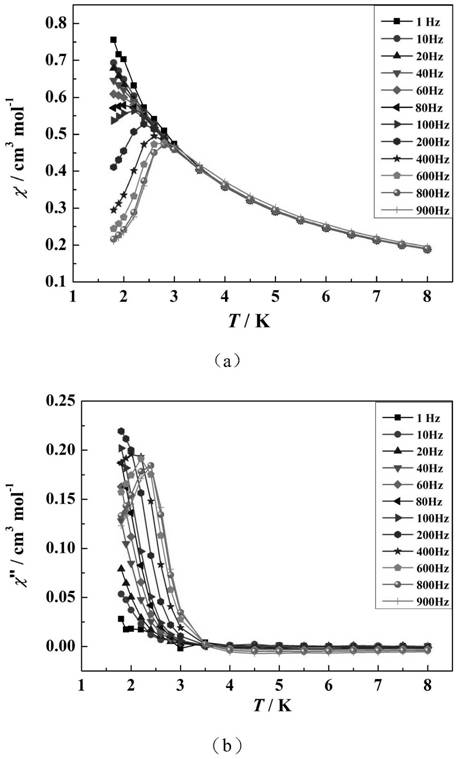
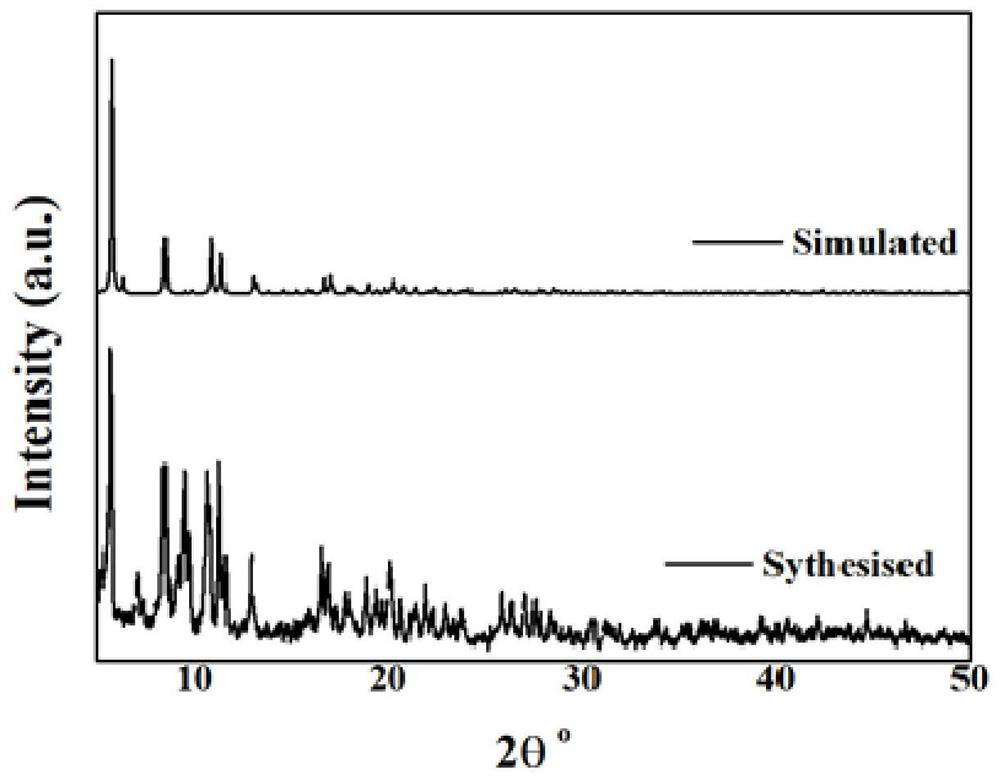
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
64 results about "Single-molecule magnet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A single-molecule magnet (SMM) is a metal-organic compound that has superparamagnetic behavior below a certain blocking temperature at the molecular scale. In this temperature range, a SMM exhibits magnetic hysteresis of purely molecular origin. In contrast to conventional bulk magnets and molecule-based magnets, collective long-range magnetic ordering of magnetic moments is not necessary.
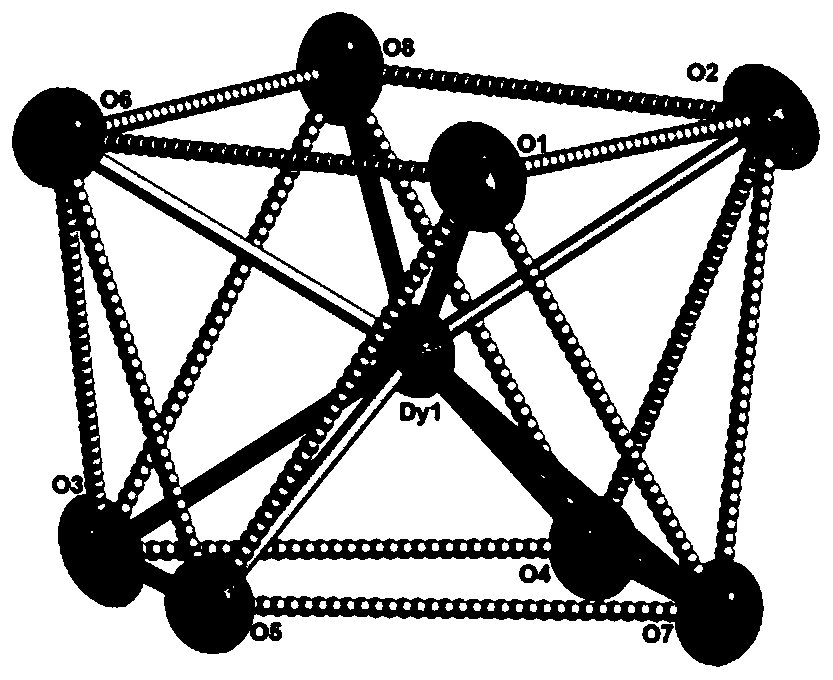
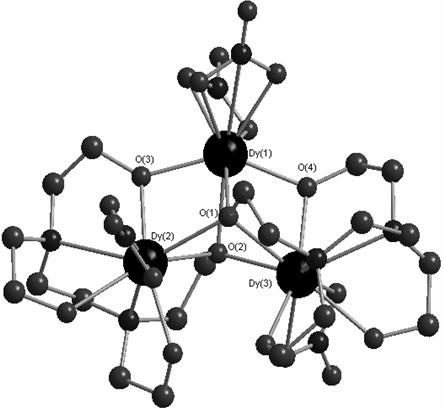
Dysprosium monomer magnet with dual functions of ferromagnetic and ferroelectric and preparation method thereof
InactiveCN102136339AHave spontaneous polarizationImprove ferroelectric propertiesMagnetsGroup 3/13 element organic compoundsSurface acoustic waveDysprosium nitrate
The invention discloses a dysprosium monomer magnet with the dual functions of ferromagnetic and ferroelectric, the chemical formula of which is represented as [Dy3(L)2(NO3)4].EtOH, wherein L is N, N, N', N'-tetrahydroxyethyl-ethylene diamine; and EtOH is ethanol. Three Dy atoms of different coordination environments exist in the dysprosium monomer magnet and form an unclosed triangle three-core Dy structure; a preparation method of the dysprosium monomer magnet is as follows: dysprosium nitrate hexahydrate is used as metal salt; L is used as a ligand, and LiOH is used as an alkaline substance to adjust the pH value to 7; and a mixed liquid is prepared by stirring, baking, separating and washing solids. The invention has the advantages that: the dysprosium monomer magnet not only shows rare dual relaxation monomer magnet behaviors, but also shows excellent ferroelectric properties; therefore, the material has high potential using values in solid devices like nanometer devices, low-temperature high-density storage devices, ferroelectric memorizers, infrared detectors, surface acoustic waves, and integrated ferroelectric devices, etc.
Owner:NANKAI UNIV
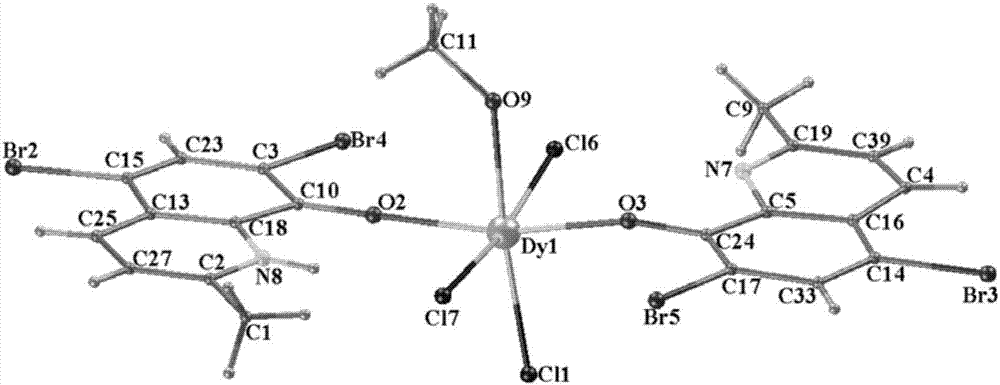
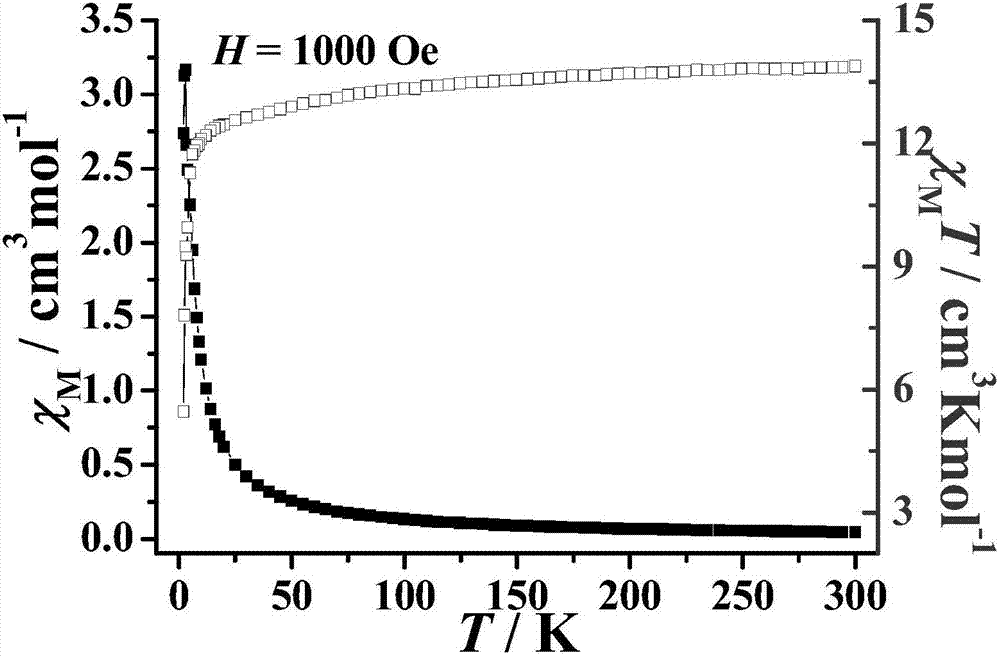
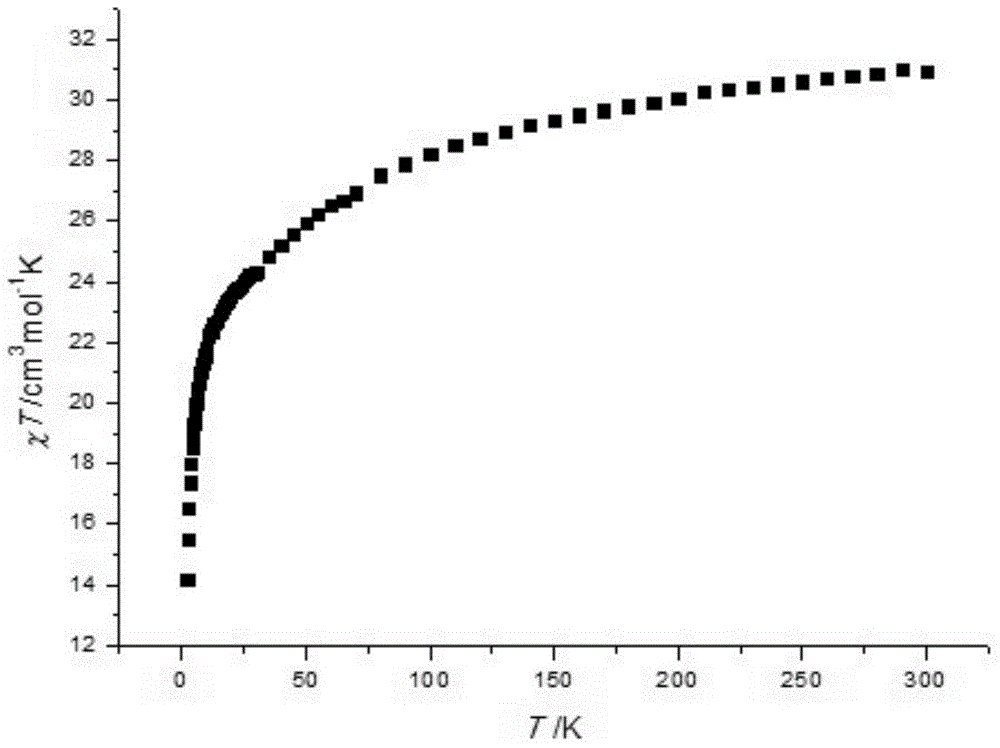
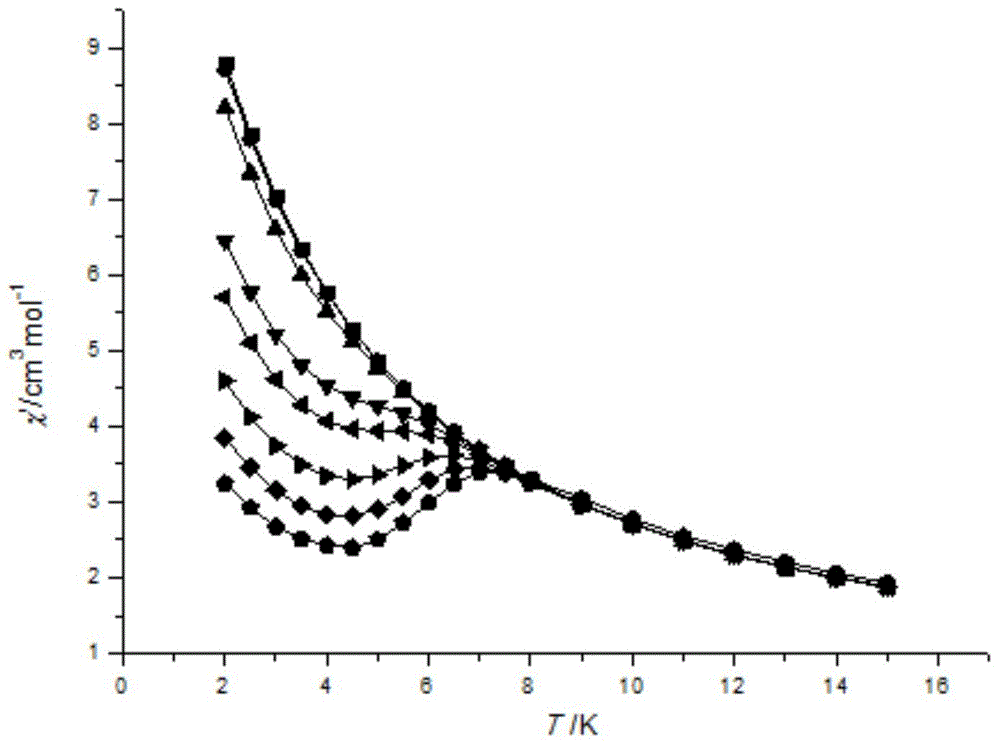
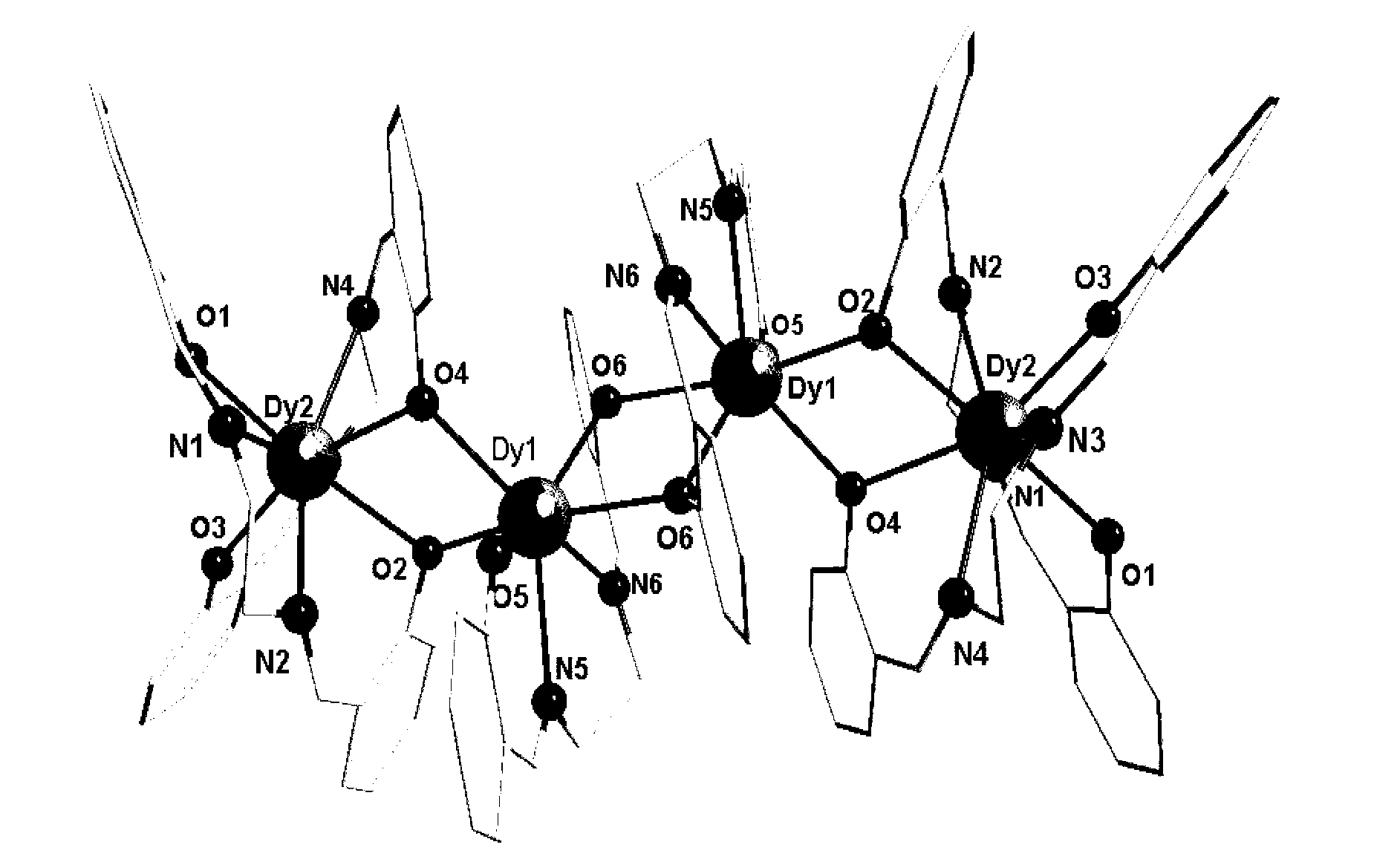
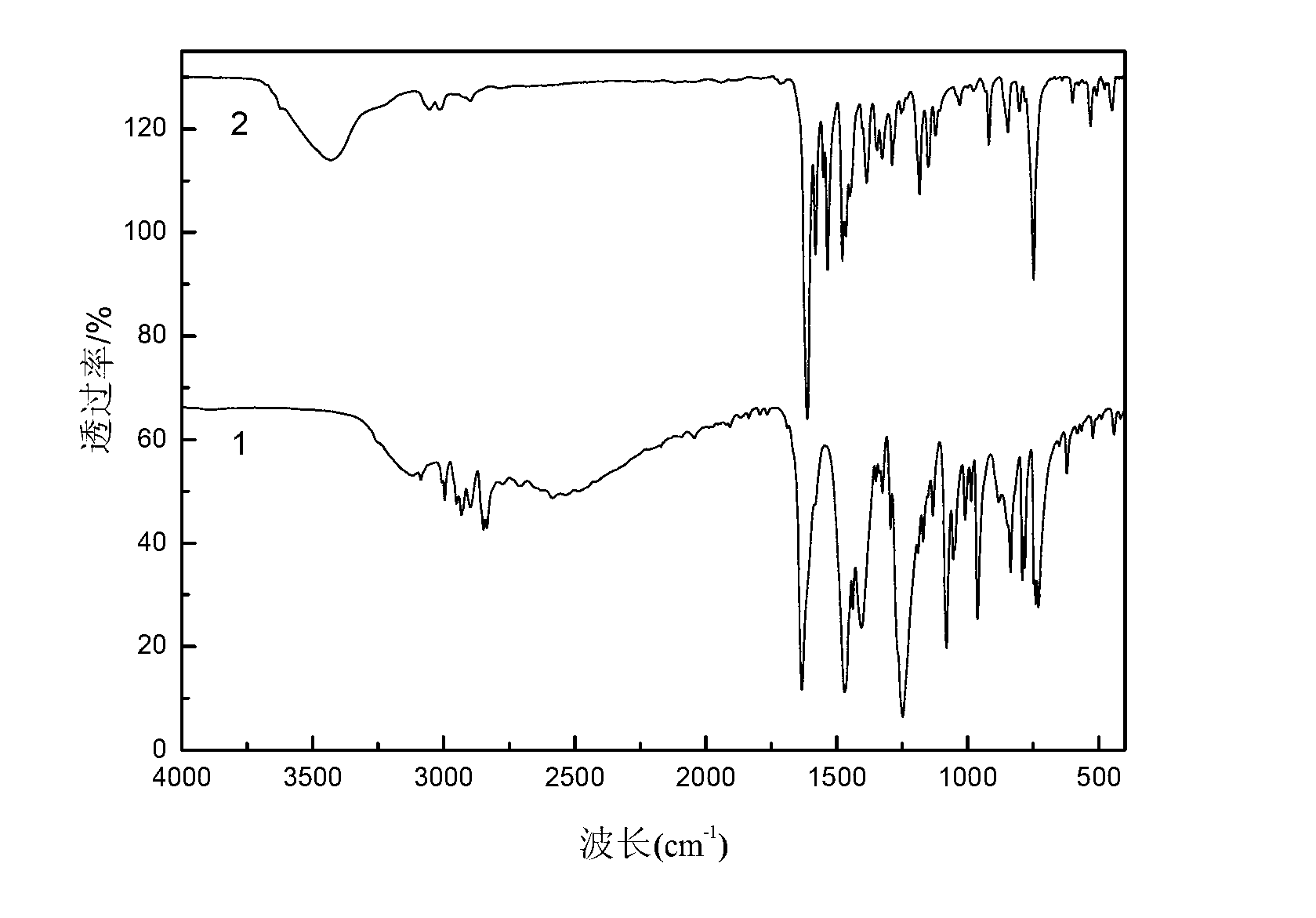
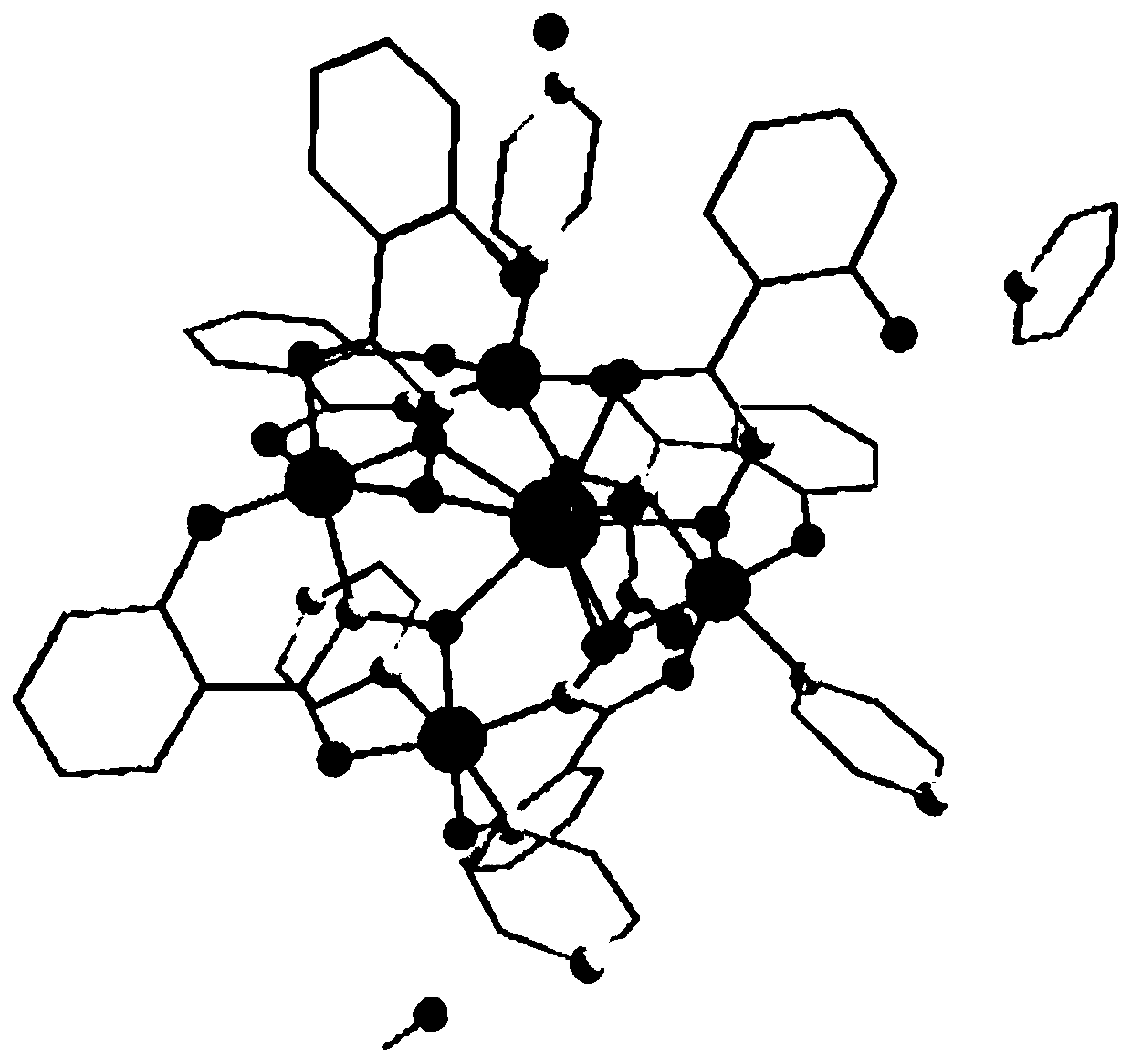
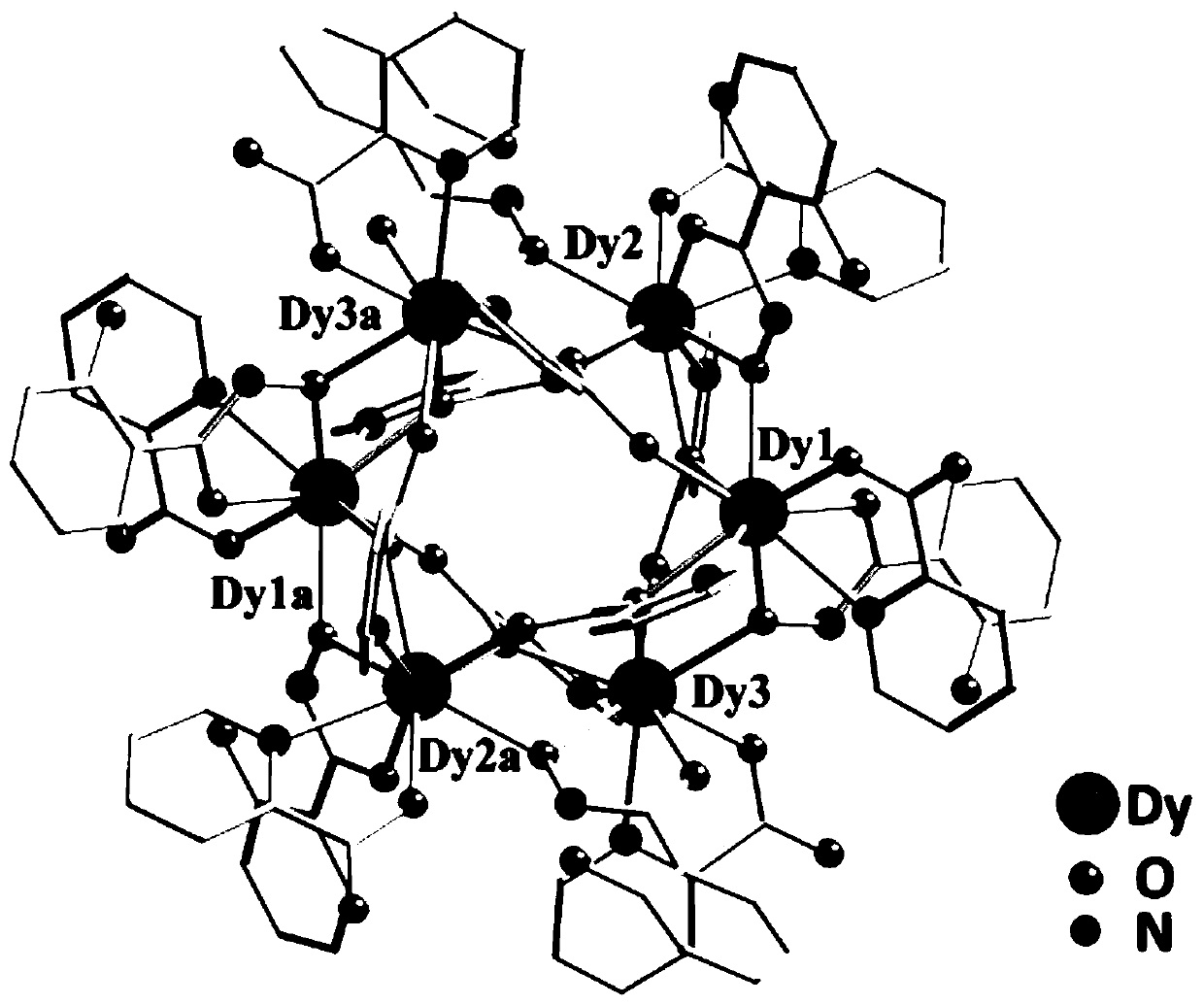
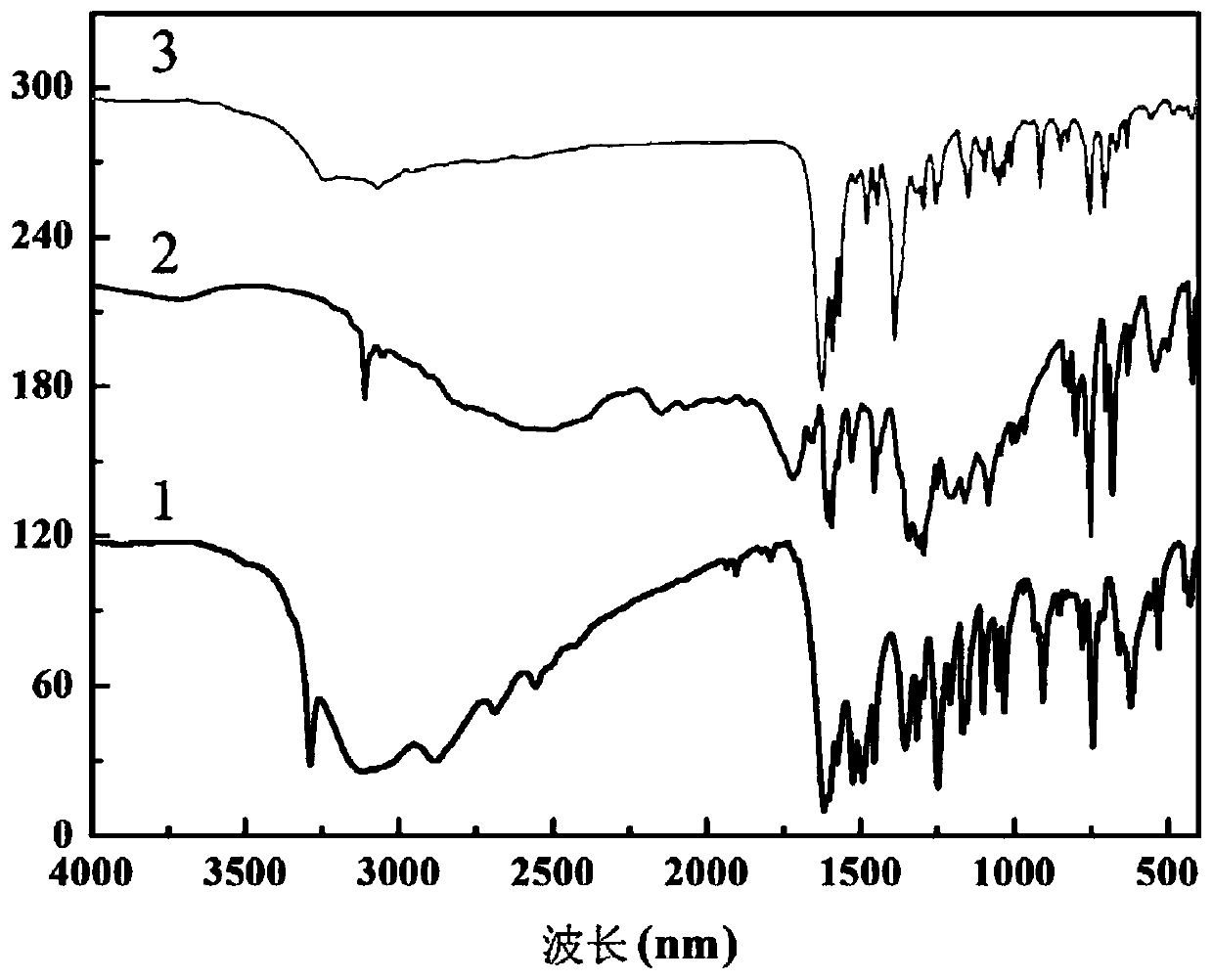
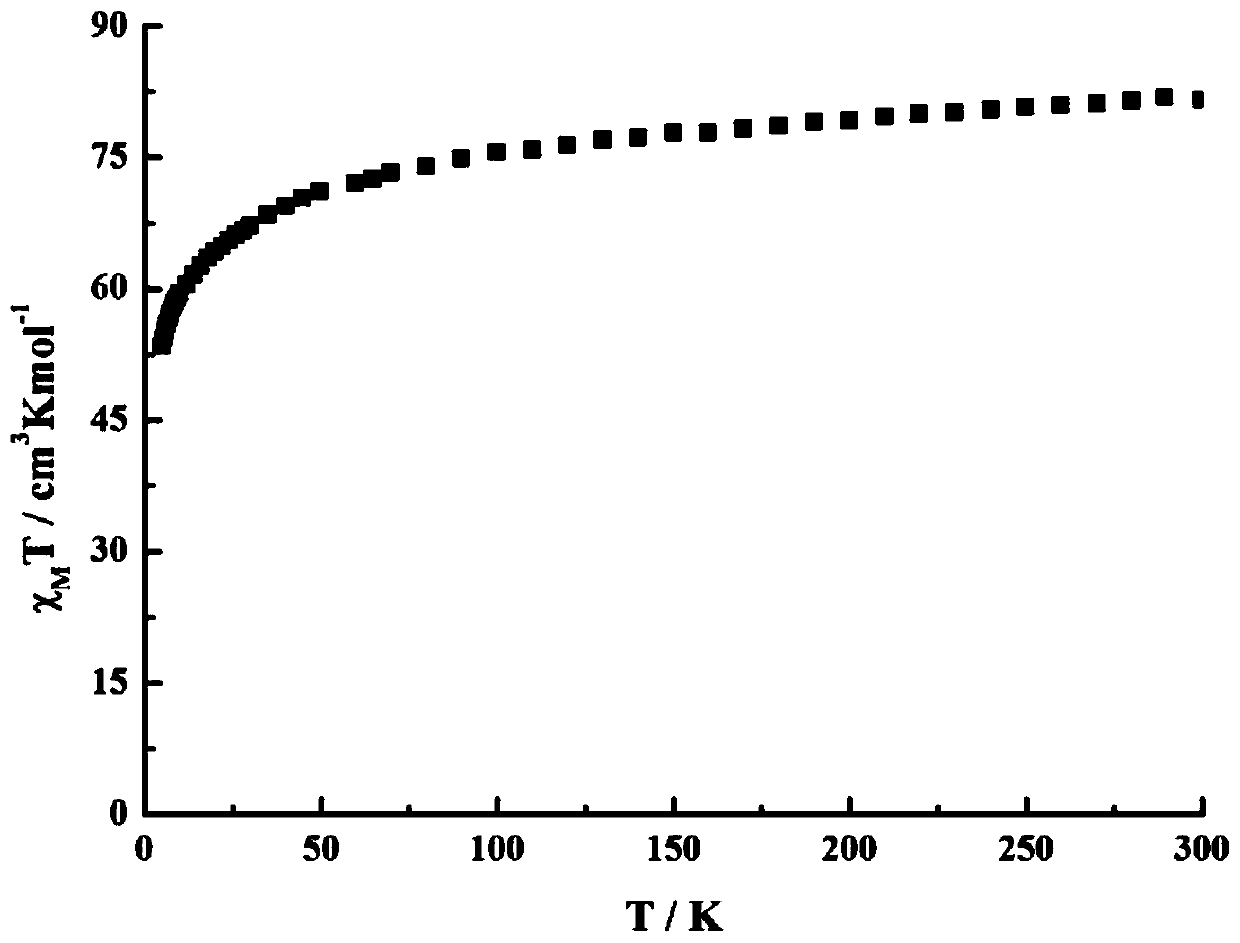
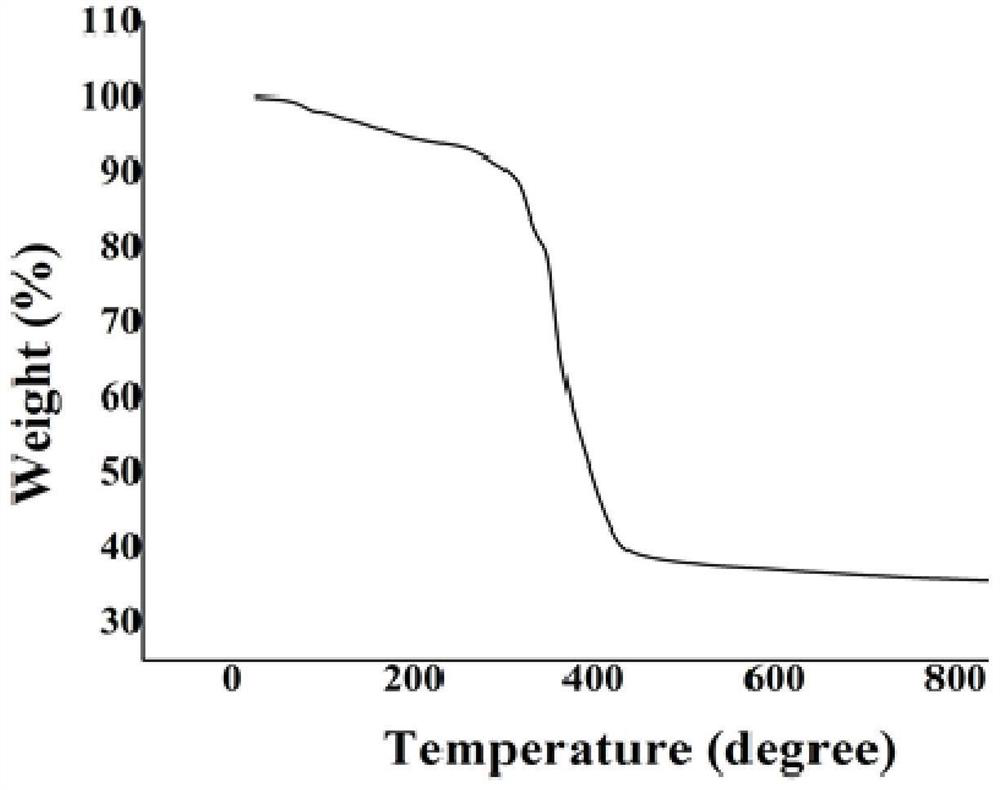
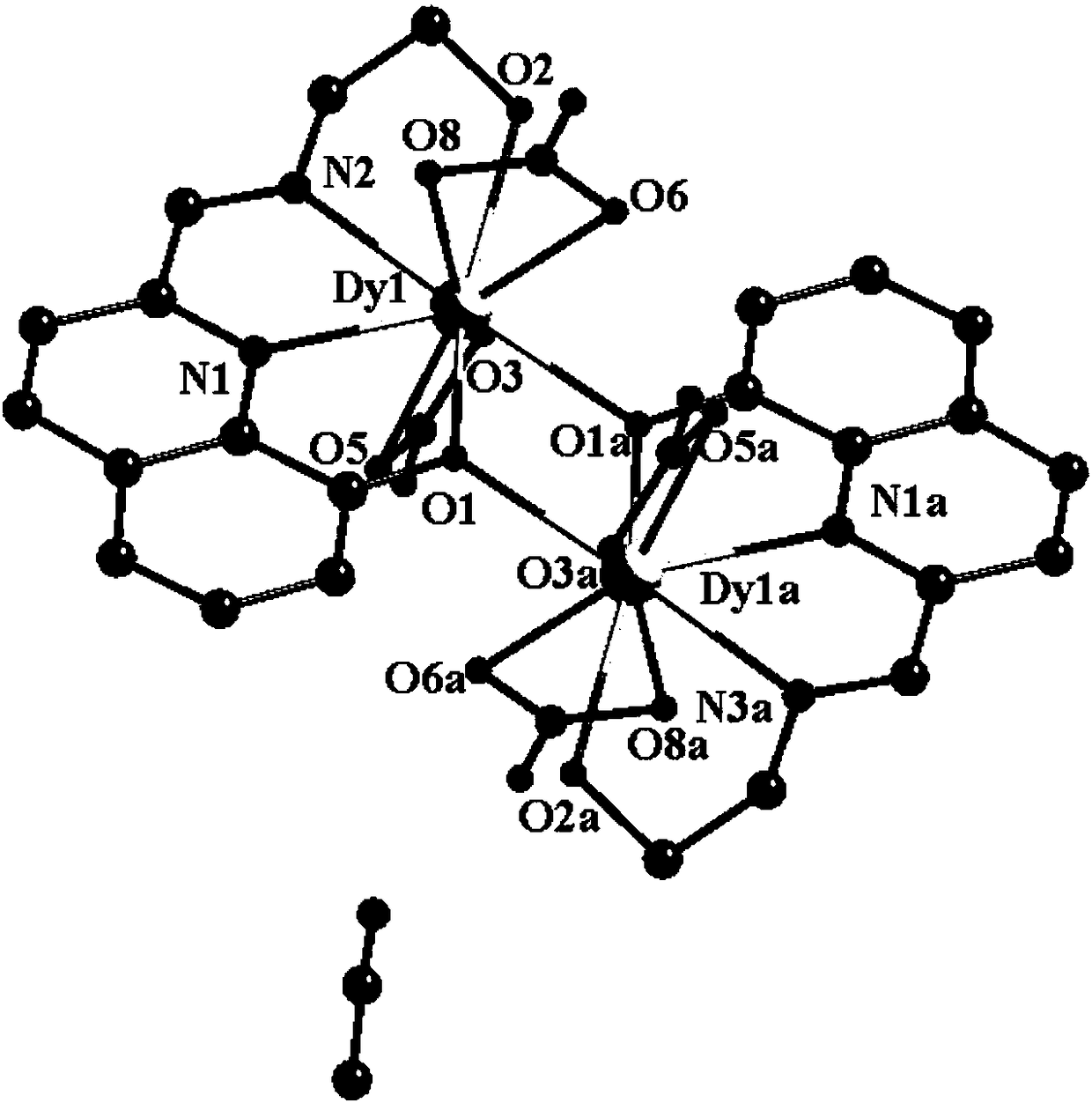
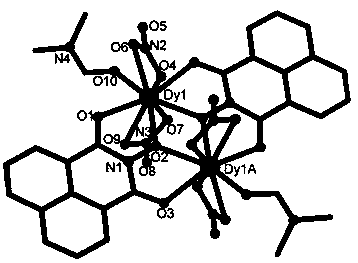
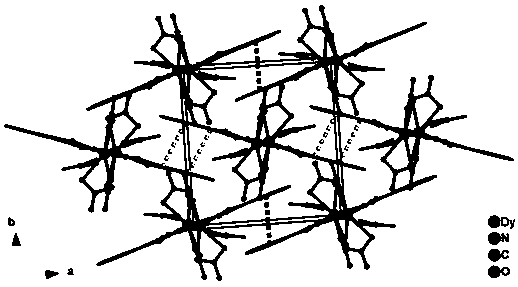
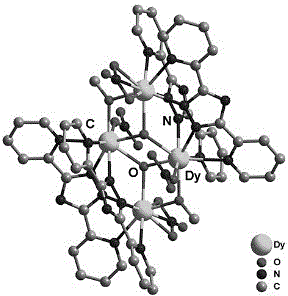
Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4]
InactiveCN104557994AStrong ferromagnetismThe synthesis method is simpleGroup 3/13 organic compounds without C-metal linkagesOrganic/organic-metallic materials magnetismSalicylaldehydeRare earth
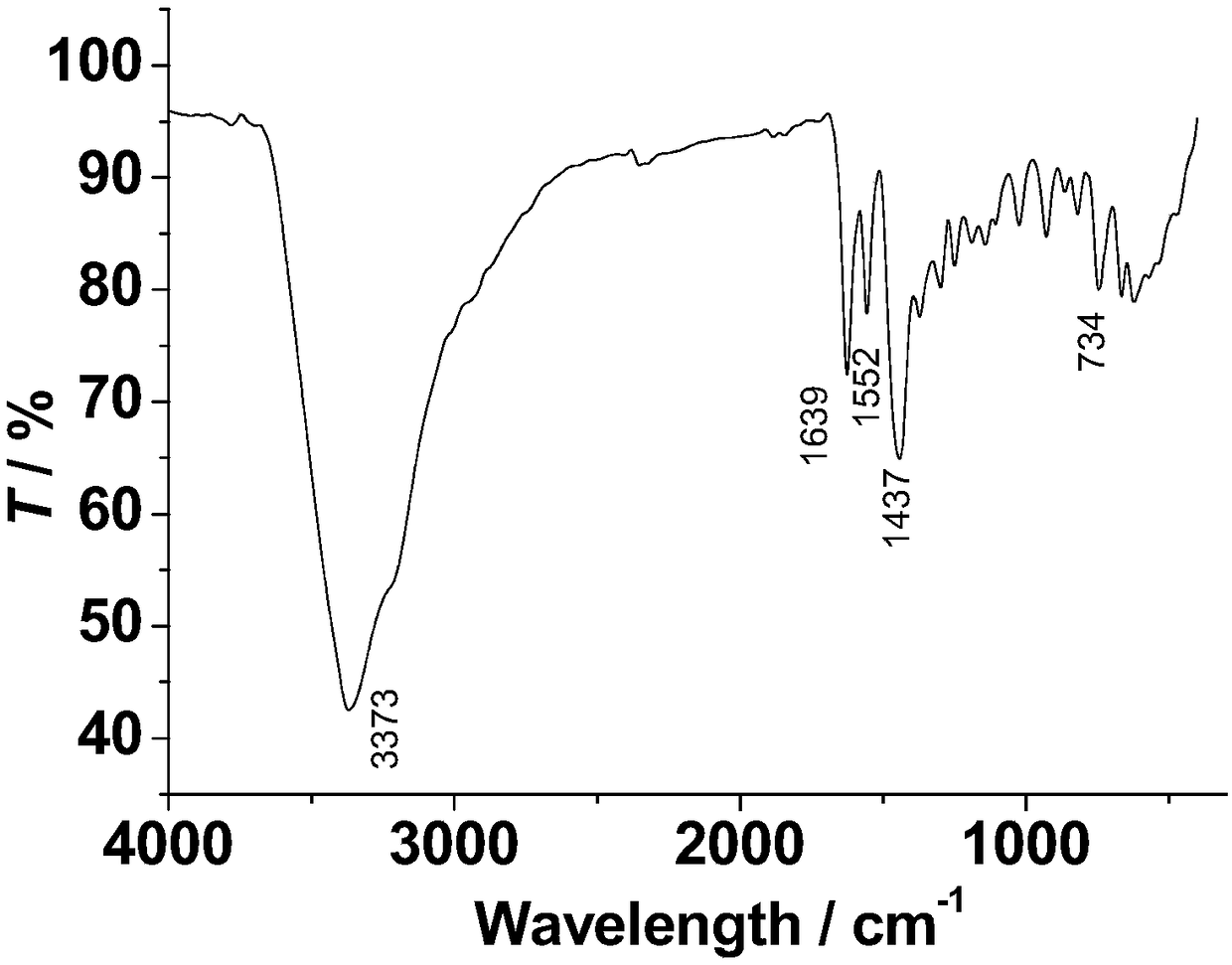
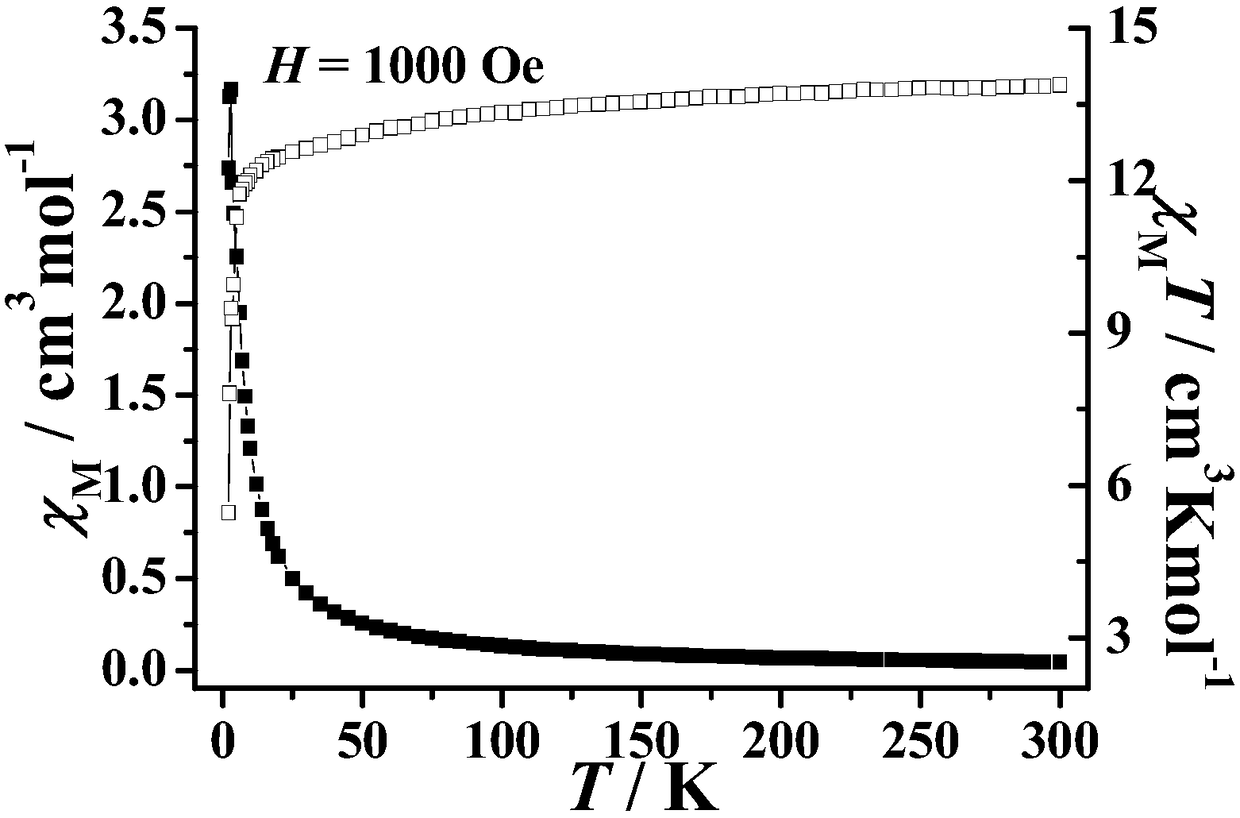
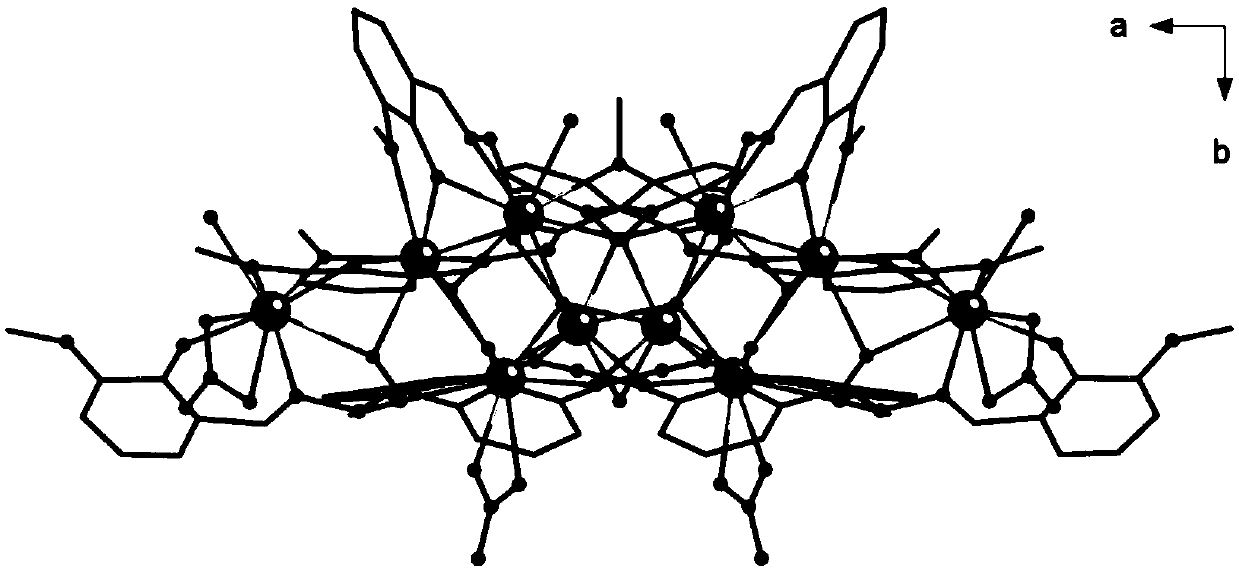
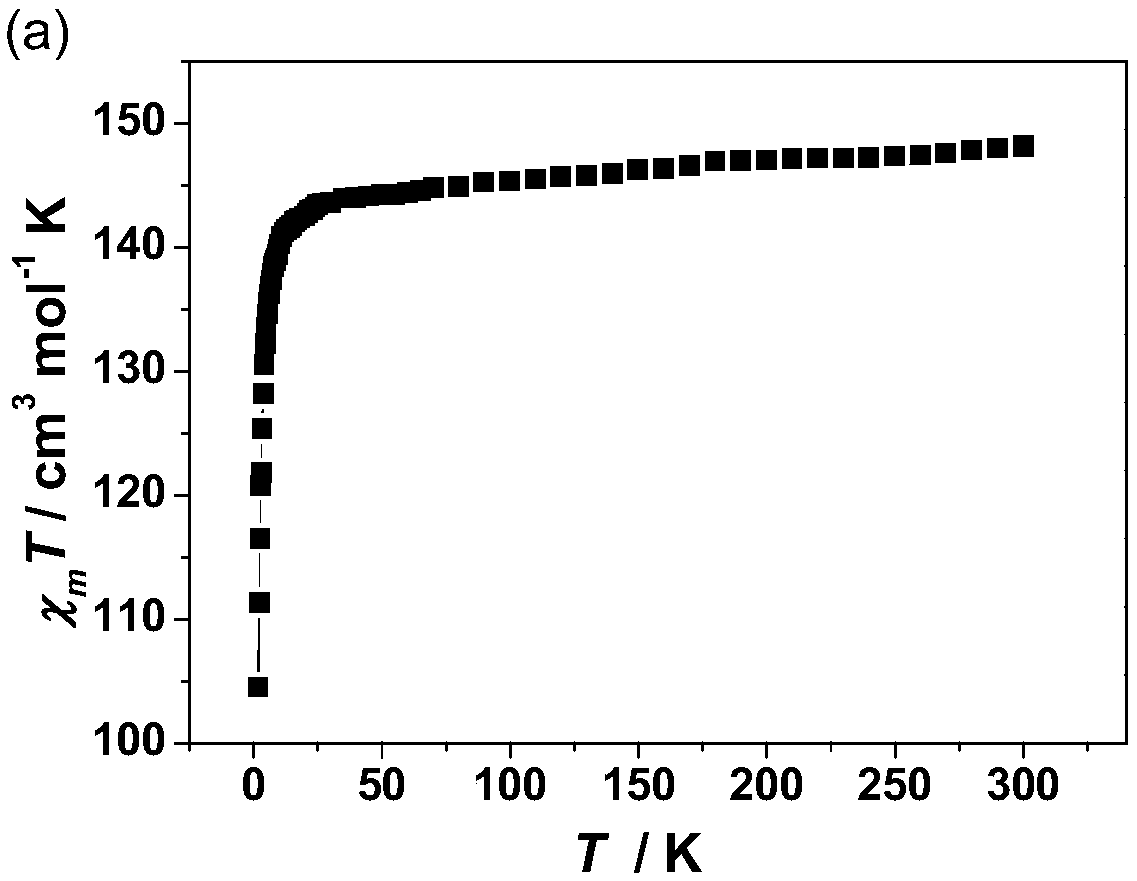
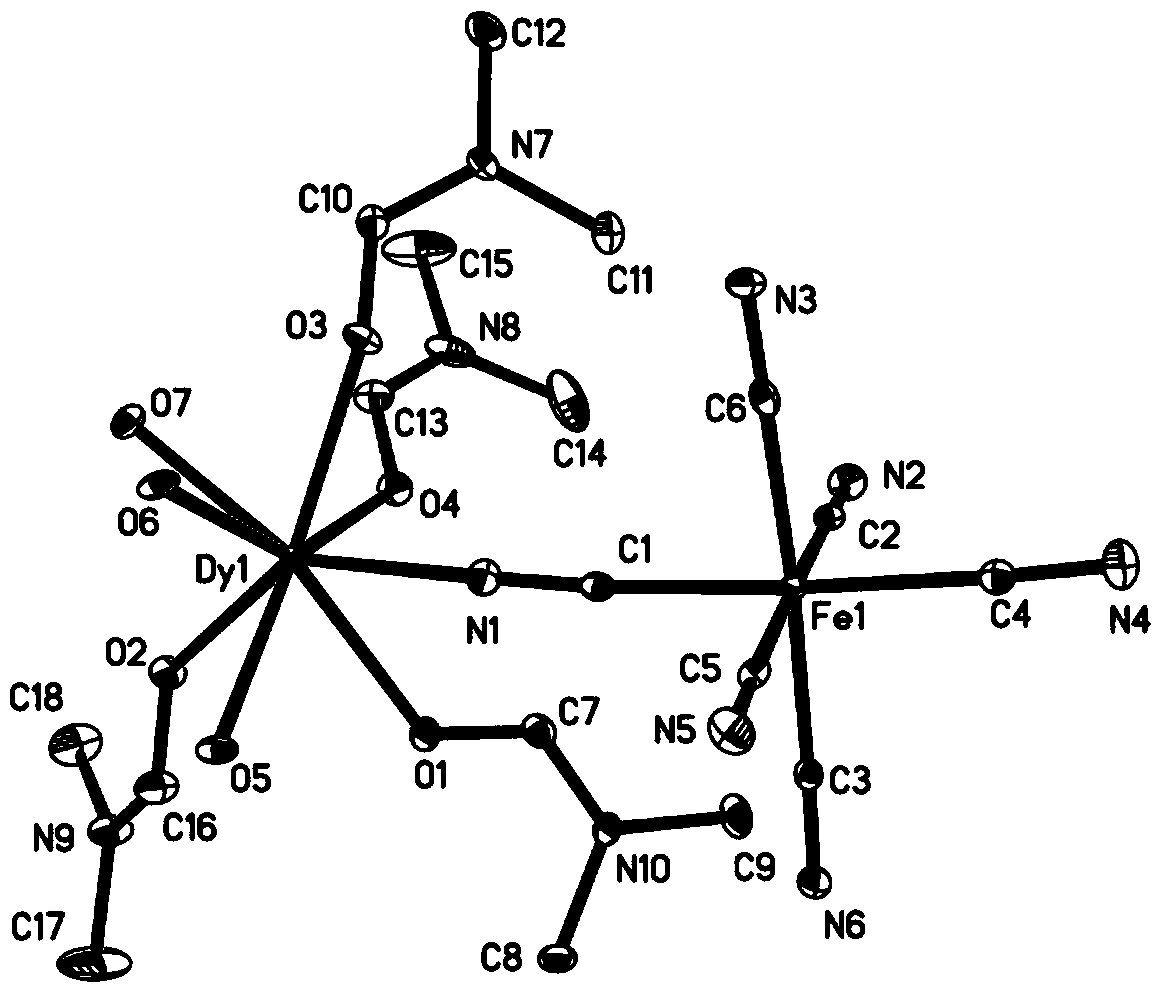
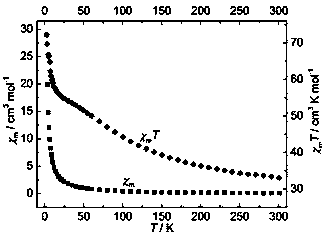
The invention discloses a method for preparing a single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4] and relates to a method for preparing a single-molecular magnet. The invention aims at solving the problems that the synthetic method of the conventional rare earth complex single-molecular magnet is low in yield and the synthetic method of the complex is complicated. The method comprises the following steps: dissolving o-aminophenol salicylaldehyde in acetonitrile, thereby obtaining a solution A; dissolving dysprosium nitrate in methanol, thereby obtaining a solution B; mixing the solution A and the solution B, adding a triethylamine solution into the mixed solution, stirring under room temperature condition, thereby obtaining a preform; and volatilizing a solvent out of the preform, thereby obtaining the complex. The [Dy2(saph)2(NO3)2(CH3OH)4] prepared by the method disclosed by the invention is a single-molecular magnet with good ferromagnetic properties, the yield of the preparation method disclosed by the invention is high and is over 52.84 percent, and the synthetic method of the single-molecular magnet is simple and high in repeatability. The invention belongs to the field of preparation of single-molecular magnets.
Owner:HEILONGJIANG UNIV
Dysprosium coordination polymer material with solvent molecule magnetic response and preparation method thereof
InactiveCN102993222ANovel structureSimple processRecord information storageGroup 3/13 element organic compoundsSolvent moleculeMagnetic response
The invention relates to a dysprosium coordination polymer material with solvent molecule magnetic response, which is a dysprosium (III) coordination polymer with unimolecular magnet behaviors. The chemical formula of the dysprosium coordination polymer material is {[Dy(INO)2(NO3)]}, each asymmetric unit contains dysprosium ions which exist in one coordination environment and stores three HINO ligands in connection modes, and dicaryon dysprosium units are further connected through two ligands to form a three-dimensional porous structure. The preparation method of the dysprosium coordination polymer material comprises the following steps of: mixing Dy (NO3)3.6H2O with HINO, and dissolving in a solvent; carrying out heating reaction, and then mixing solids obtained through filtering and washing with acetonitrile; and removing the acetonitrile after the heating reaction. The dysprosium coordination polymer material disclosed by the invention is novel in structure and based on an isonicotinic nitrogen oxide ligand, contains the nanometer-pore three-dimensional coordination polymer, shows the slow magnetic relaxation behavior and can be used as an information storage material; and the preparation method of the dysprosium coordination polymer material has the advantages of simple process, easiness for implementation and high productivity, and is conductive to large-scale popularization and application.
Owner:NANKAI UNIV
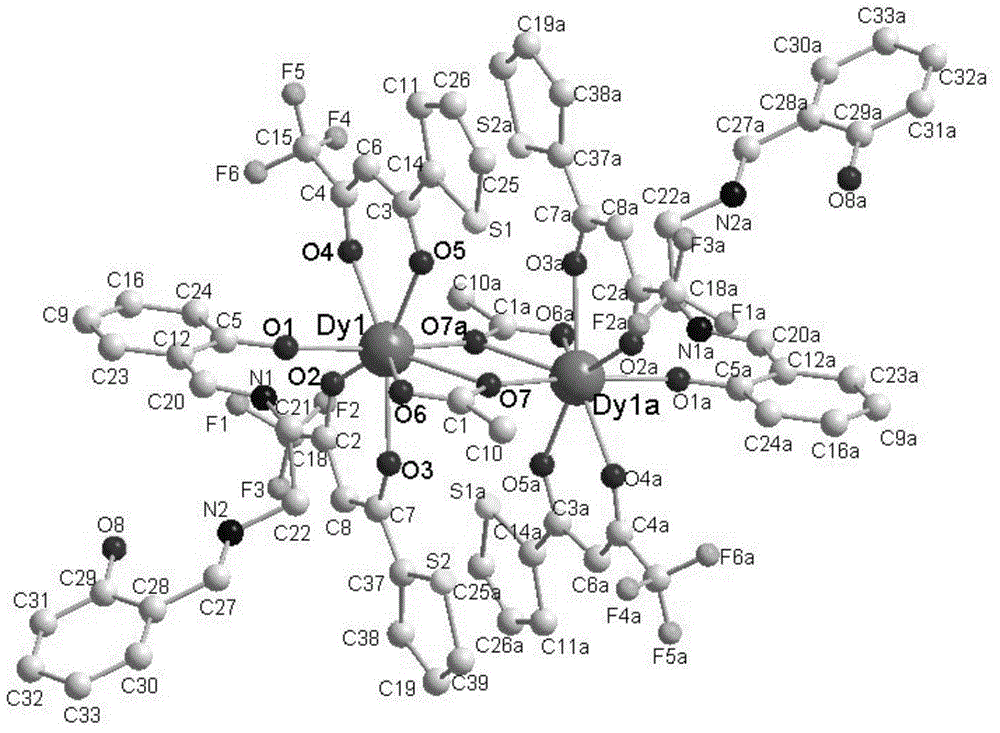
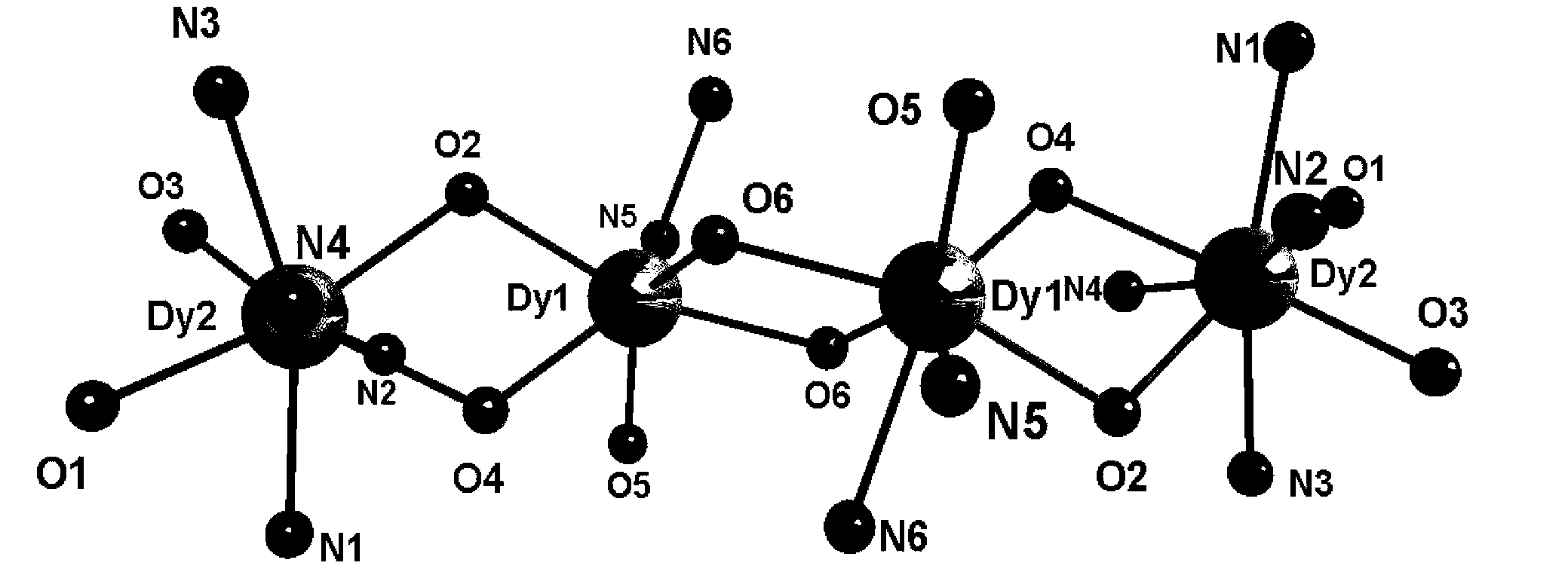
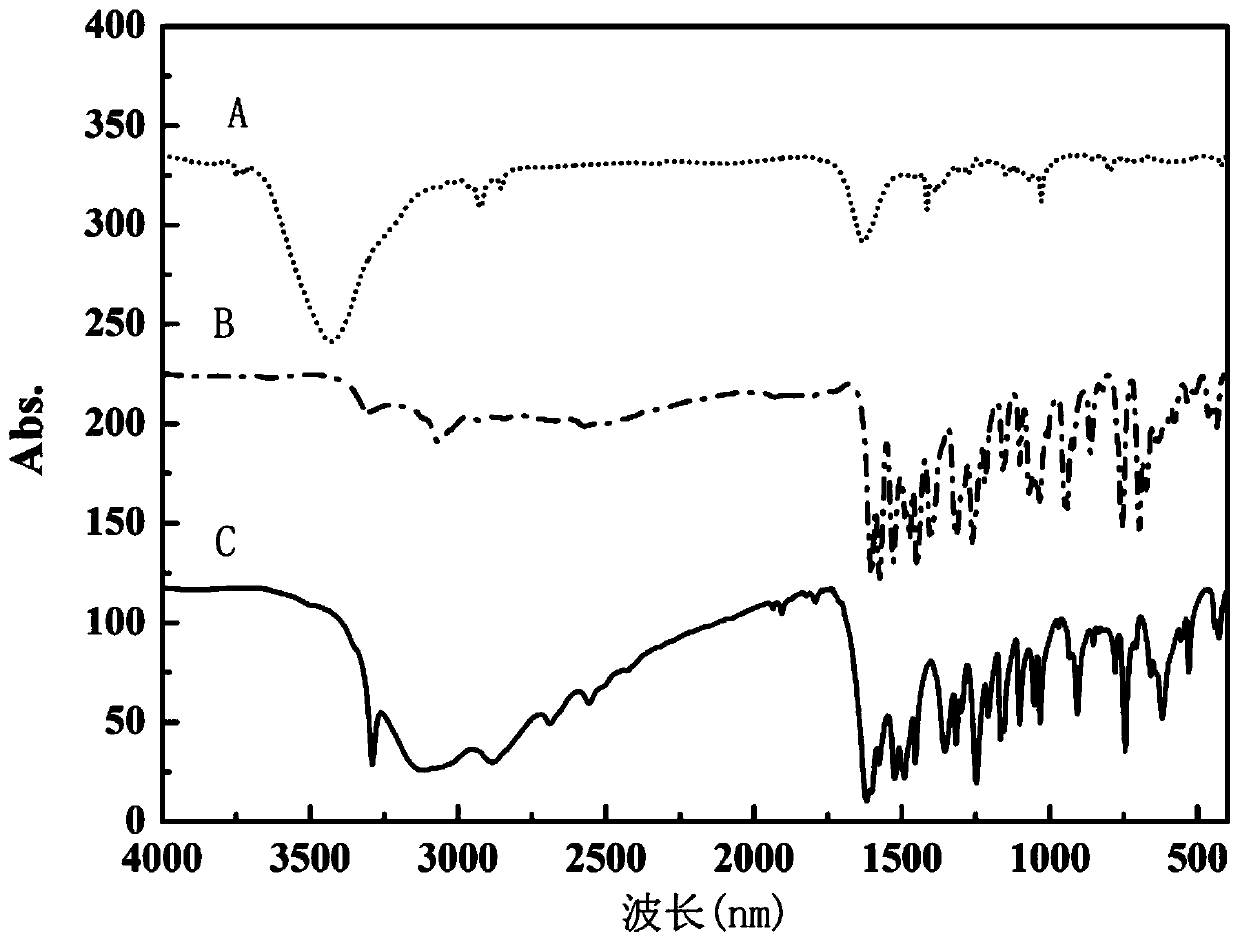
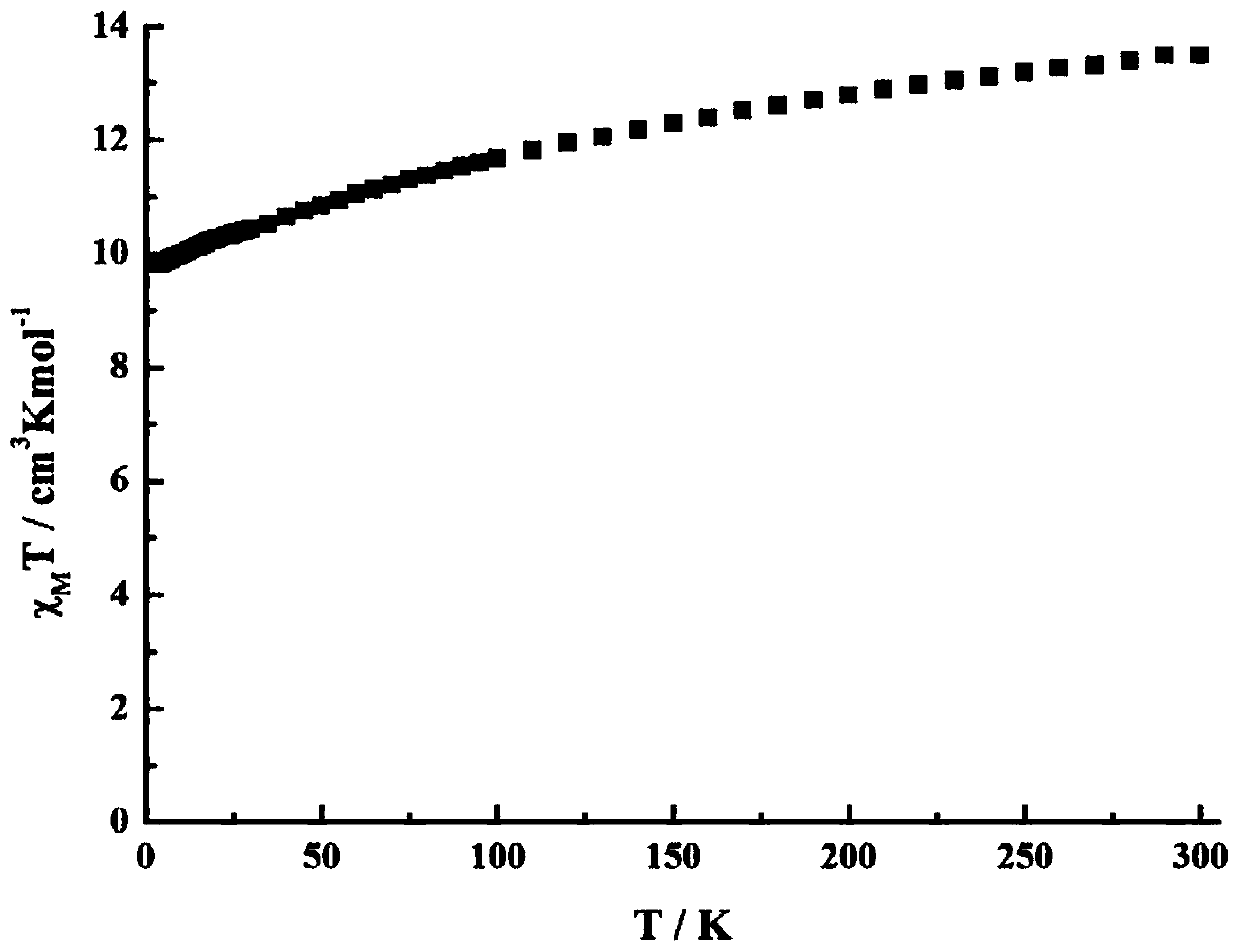
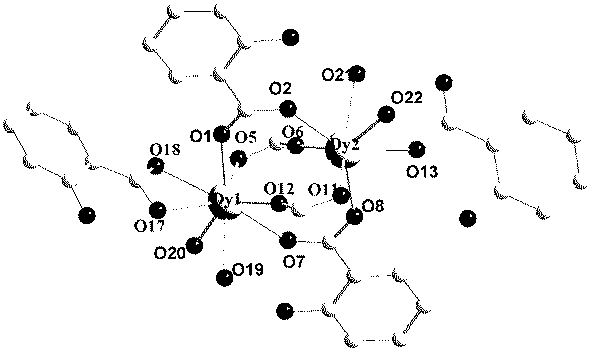
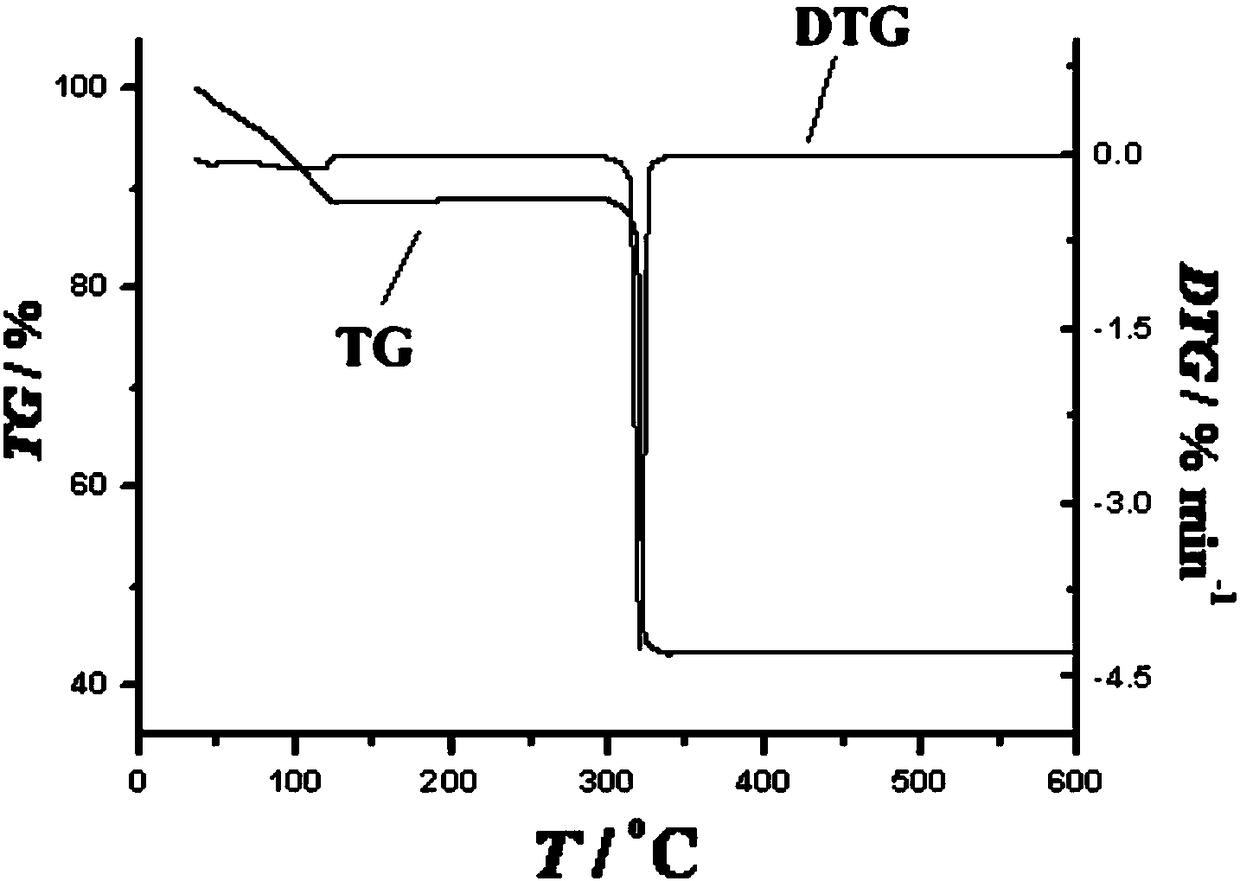
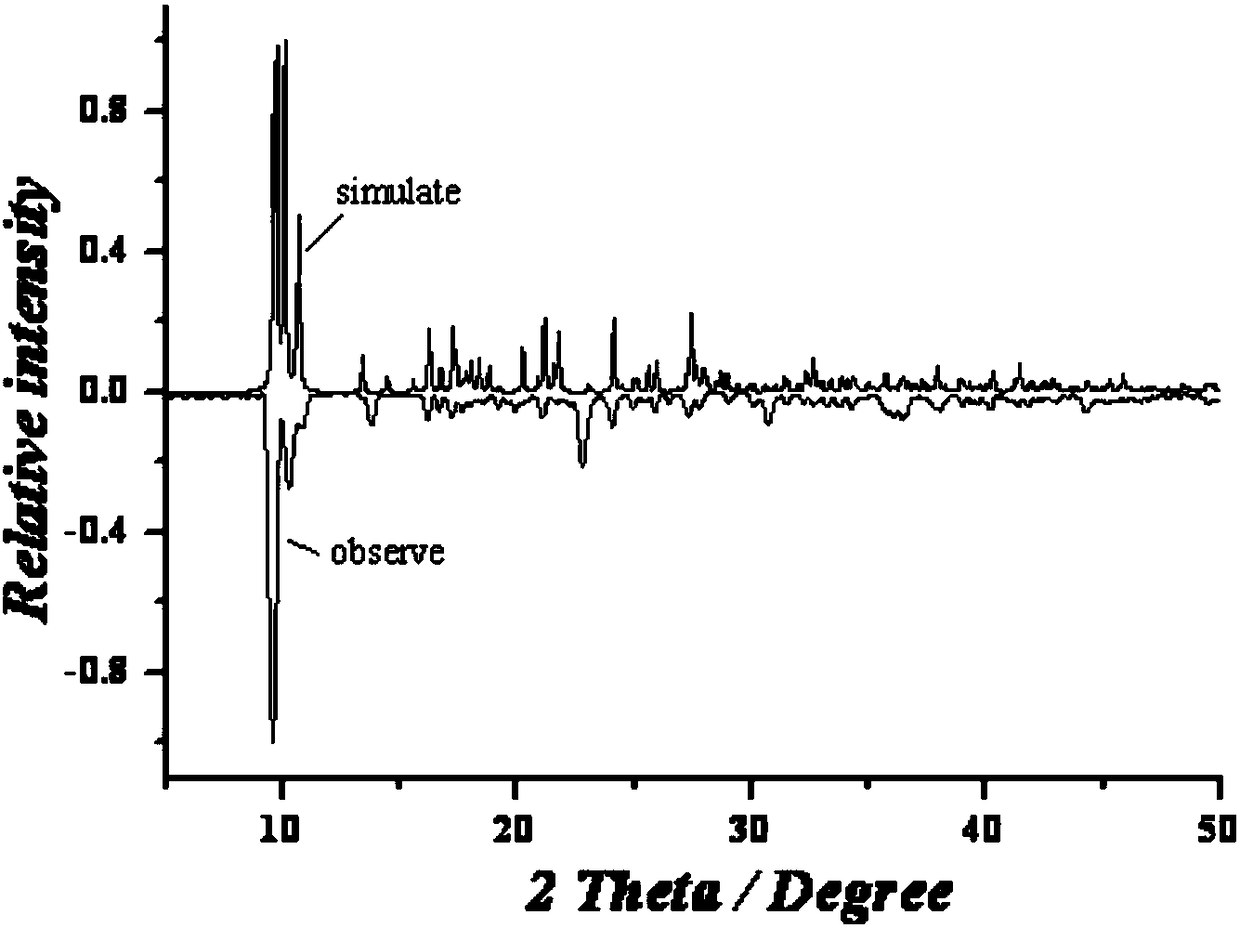
Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH
InactiveCN103641850AStrong ferromagnetismThe synthesis method is simpleOrganic/organic-metallic materials magnetismGroup 3/13 element organic compoundsSalicylaldehydeSynthesis methods
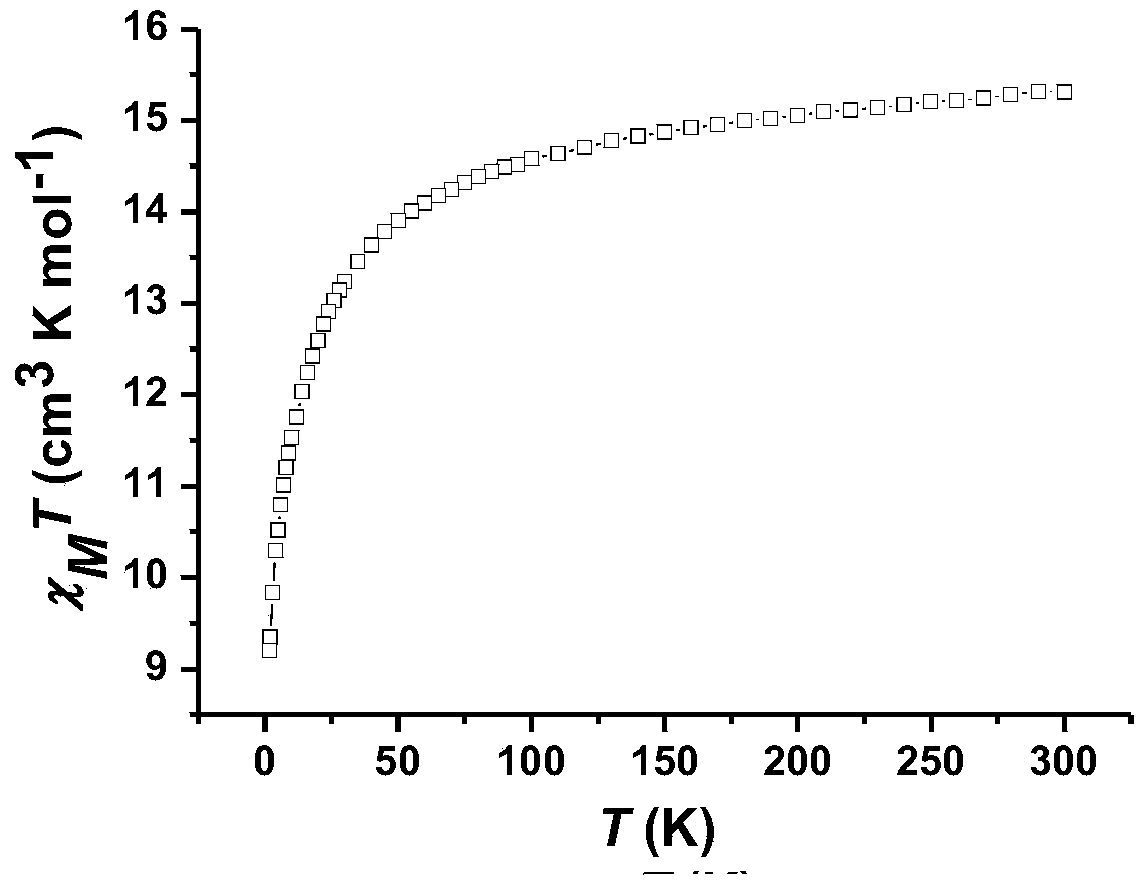
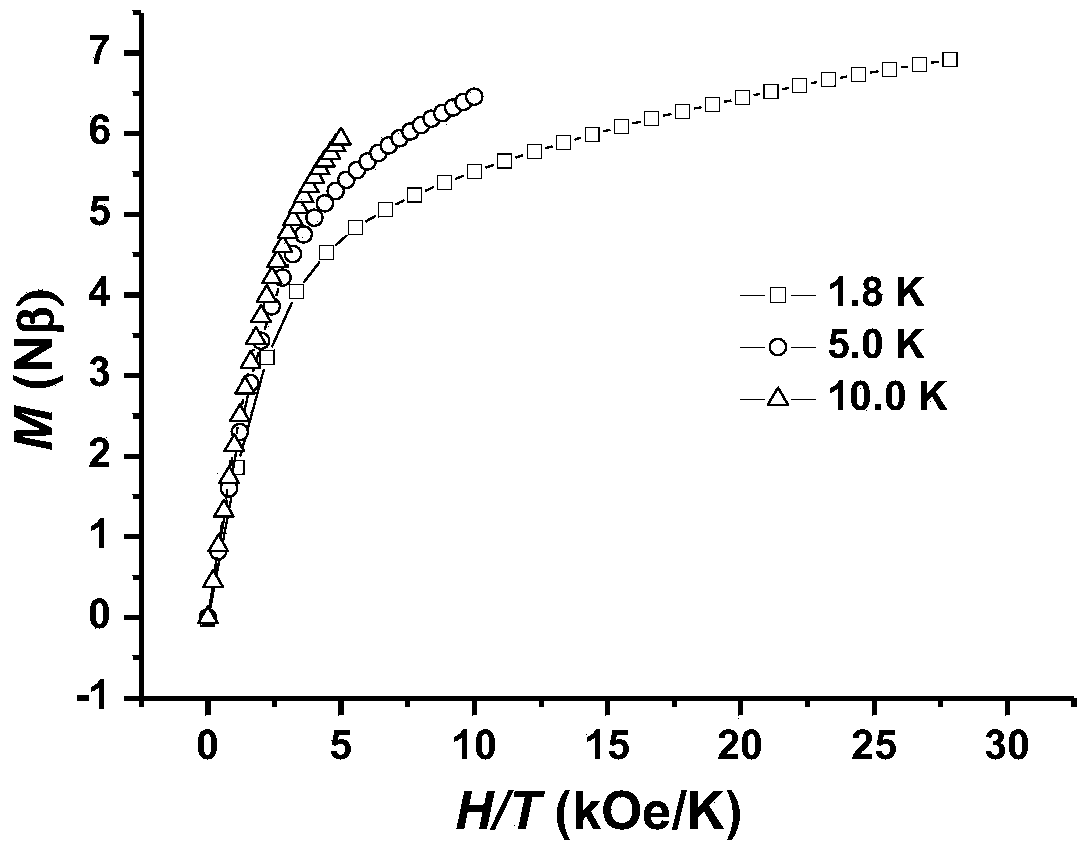
The invention discloses a preparation method of a single-molecular magnet [Dy2(saph)2Cl2].4CH3OH and relates to the preparation method of the single-molecular magnet [Dy2(saph)2Cl2].4CH3OH, which is used for solving the problems that an existing synthesis method for a rear-earth cluster compound single-molecular magnet is lower in yield, complex in synthesis method of a coordination compound and incapable of carrying out mass production. The preparation method comprises the following steps: I, dissolving o-aminophenol salicylaldehyde acetal into acetonitrile to obtain liquor A; dissolving dysprosium chloride into methanol to obtain liquor B; mixing the liquor A with the liquor B, adding triethylamine with concentration of 0.001mol / L to obtain mixed liquor; II, stirring the mixed liquor obtained in the step I under the room temperature to obtain a preform; and III, volatilizing the preform in the solvent to obtain the single-molecular magnet [Dy2(saph)2Cl2].4CH3OH. The preparation method disclosed by the invention is simple, and high in yield which reaches over 45.76%. The preparation method disclosed by the invention is applied to the field of preparing the single-molecular magnets.
Owner:HEILONGJIANG UNIV
Mononuclear dysprosium complex and preparation method and application thereof
ActiveCN107089999AEasy to prepareLow costOrganic chemistry methodsOrganic/organic-metallic materials magnetismAlcoholDysprosium
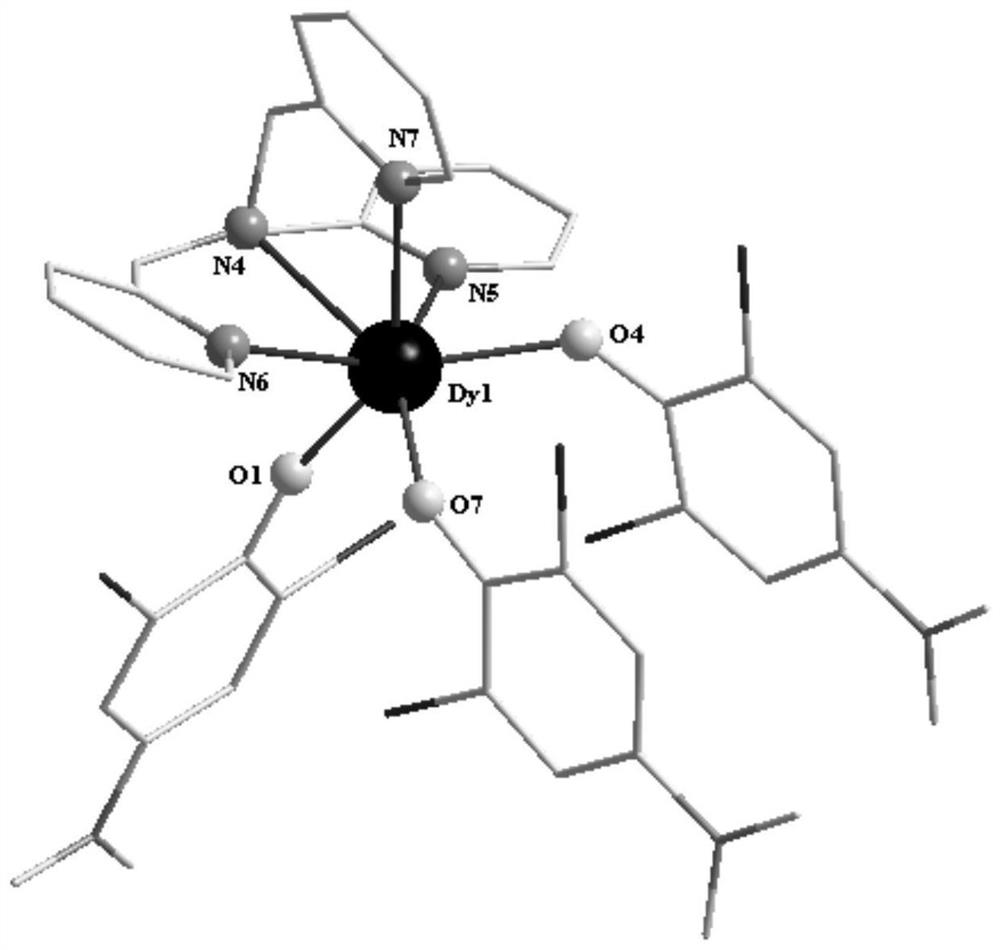
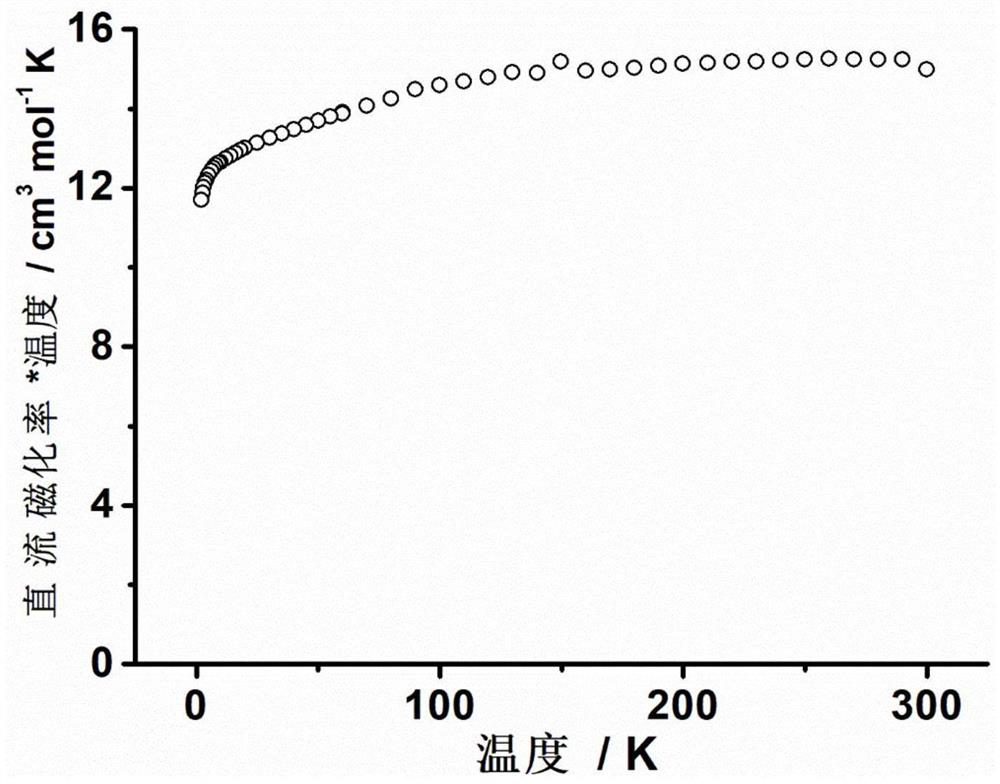
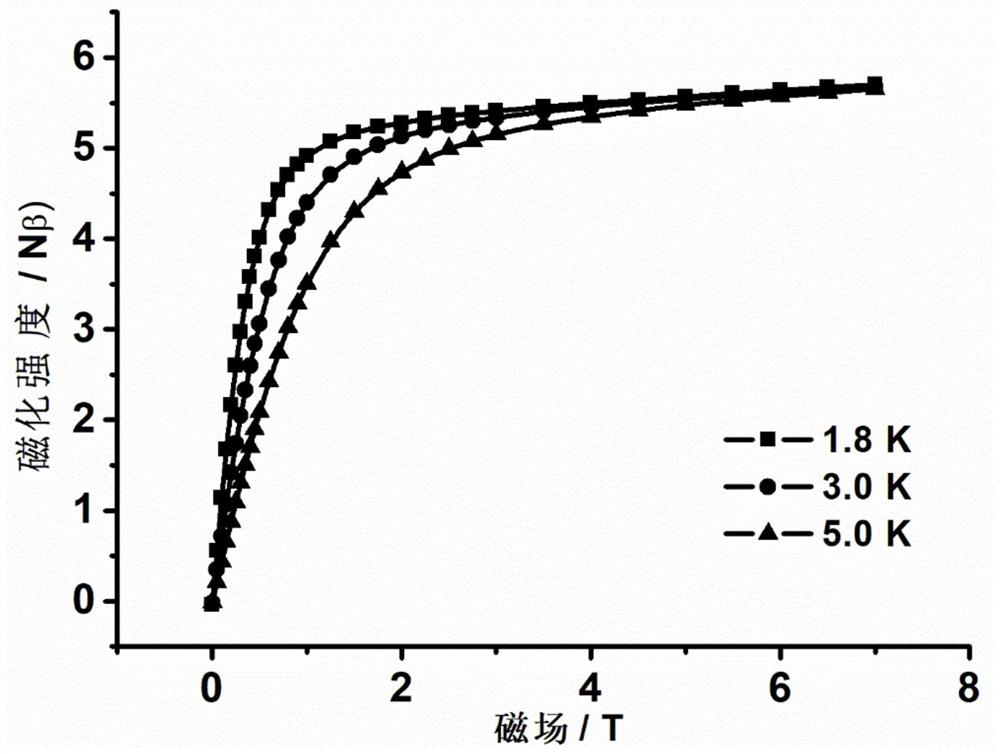
The invention discloses a mononuclear dysprosium complex and a preparation method and application thereof. The chemical formula of the complex is [Dy(HL)2(CH3OH)Cl3], wherein HL is 2-methyl-5,7-dibromo-8-hydroxyquinoline. The complex belongs to a monoclinic system and P21 / n space groups. The preparation method of the mononuclear dysprosium complex includes the steps that DyCl3.6H2O and 2-methyl-5,7-dibromo-8-hydroxyquinoline are taken and dissolved with a mixed solvent, the pH of the obtained solution is adjusted to range from 6.5 to 7.8, the obtained mixed liquid reacts under the heating condition, and then the complex is obtained, wherein the mixed solvent is a composition of methyl alcohol and acetonitrile. The preparation method of the complex is simple, the cost is low, good repeatability is achieved, magnetic properties of the complex are shown as field-induced single-molecular magnet behaviors, and the complex can be used for preparing magnetic materials.
Owner:GUANGXI NORMAL UNIV
Single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2 and preparation method of single molecular magnet
InactiveCN105037405AImprove propertiesThe synthesis method is simpleGroup 3/13 organic compounds without C-metal linkagesOrganic/organic-metallic materials magnetismCrystal systemReflux
The invention relates to a single molecular magnet and a preparation method of the single molecular magnet, namely to a single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2 and the preparation method of the single molecular magnet. The invention aims to solve the problems that the synthetic yield of rare earth complex single molecular magnet is relatively low, the synthetic method of the complex is complex, the batch production cannot be carried out and the like, which are faced at present. The molecular formula of the single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2 is C84H56Dy2F12N4O16S4, and the crystal system is a triclinic system. The preparation method comprises the following steps: Carrying out heating reflux on a mixed solution C, then cooling to the room temperature, and filtering to obtain a solution D; dispersing a mixed solution E into the solution D in a solution dispersal mode, and standing to obtain the single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2. The method is used for obtaining the single molecular magnet Dy2 (salen) 2 (tta) 4 (OAc) 2.
Owner:HEILONGJIANG UNIV
Preparation method of single-molecule magnetic body
InactiveCN103219148AThe synthesis method is simpleHigh yieldOrganic chemistryInductances/transformers/magnets manufactureRepeatabilityCoordination complex
The invention discloses a preparation method of a single-molecule magnetic body. The preparation method aims at solving the problem that an existing synthetic method is low in productivity, and a synthetic method of coordination compounds is complex. The preparation method comprises the following steps of preparing mixed solution; preparing a prefabricated body; and synthesizing. The preparation method is high in productivity, simple in synthetic method of the single-molecule magnetic body, strong in repeatability and capable of being used for preparing the single-molecule magnetic body.
Owner:HEILONGJIANG UNIV
Method for preparing metal crown ether single-molecular magnets
InactiveCN109705151ANovel structureGood antiferromagneticGroup 3/13 element organic compoundsOrganic/organic-metallic materials magnetismHydroxamic acidPyrazine
The invention discloses a method for preparing metal crown ether single-molecular magnets, and relates to a method for preparing single-molecular magnets. The invention aims to solve the problem of alow yield of a method for synthesizing metal crown ether rare earth complex single-molecular magnets in the prior art. The preparation method comprises the steps: dissolving salicyl hydroxamic acid, dysprosium nitrate and gallium nitrate in methanol so as to obtain a solution A, adding pyrazine to the solution A, performing stirring for a reaction so as to obtain a solution B, then adding pyridine, performing stirring at room temperature so as to obtain a preform, volatilizing the solvent of the preform so as to obtain the metal crown ether single-molecular magnets {Dy<3+>[Ga(III)MCshi]}. Themethod has the advantages that (1) the metal crown ether single-molecular magnets are single-molecular magnets with good antiferromagnetism, and (2) the preparation method is simple, and has high repeatability and a high yield which can reach 50% or above. The method is mainly applied to preparation of the metal crown ether single-molecular magnets {Dy<3+>[Ga(III)MCshi]}.
Owner:HEILONGJIANG UNIV
Six-nuclear dysprosium cluster cyclic complex unimolecular magnetic body and preparation method thereof
ActiveCN110294771AReduce quantum tunneling effectEasy to prepareGroup 3/13 organic compounds without C-metal linkagesOrganic chemistry methodsSynthesis methodsSolvent evaporation
The invention relates to a unimolecular magnetic body and a preparation method thereof, in particular to a six-nuclear dysprosium cluster cyclic complex unimolecular magnetic body and a preparation method thereof, and aims to solve the problem that a rare earth complex unimolecular magnetic body prepared by using an existing method is complicated in synthesis method, low in controllability and poor in repeatability. The chemical formula of the six-nuclear dysprosium cluster cyclic complex unimolecular magnetic body is [Dy6(H2shi)6(2-pyca)6(2-pyca-)6], and the molecular formula is C114H90Dy6N18O42. The method comprises the steps that salicylhydroxamic acid, picolinic acid and Dy(NO3)3 6H2O are dissolved into a solvent, and a mixed solution is obtained through ultrasonic treatment; the mixedsolution is subjected to heating reflux, pyridine is added, and a preform is obtained; the preform is subjected to solvent evaporation, and the six-nuclear dysprosium cluster cyclic complex unimolecular magnetic body is obtained. By means of the preparation method, the six-nuclear dysprosium cluster cyclic complex unimolecular magnetic body can be obtained.
Owner:HEILONGJIANG UNIV
Arrowhead complex material with solvent molecule-responded magnetic and ferroelectric properties and preparation method of arrowhead complex material
InactiveCN106928049AImprove ferroelectric propertiesHigh potential application valueOrganic compound preparationOrganic chemistry methodsSolvent moleculeCoordination complex
Owner:HENAN POLYTECHNIC UNIV
Method for preparing monomolecular magnet (Mn1-XCrX)12-ac
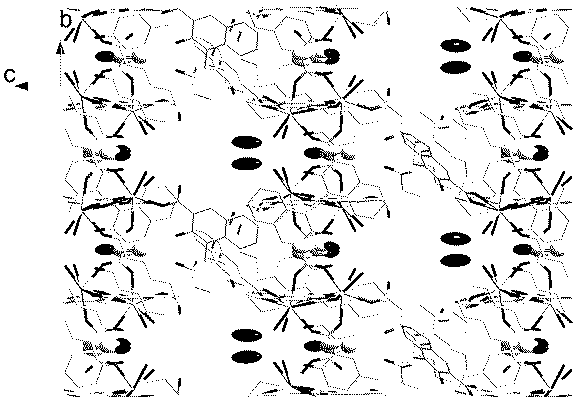
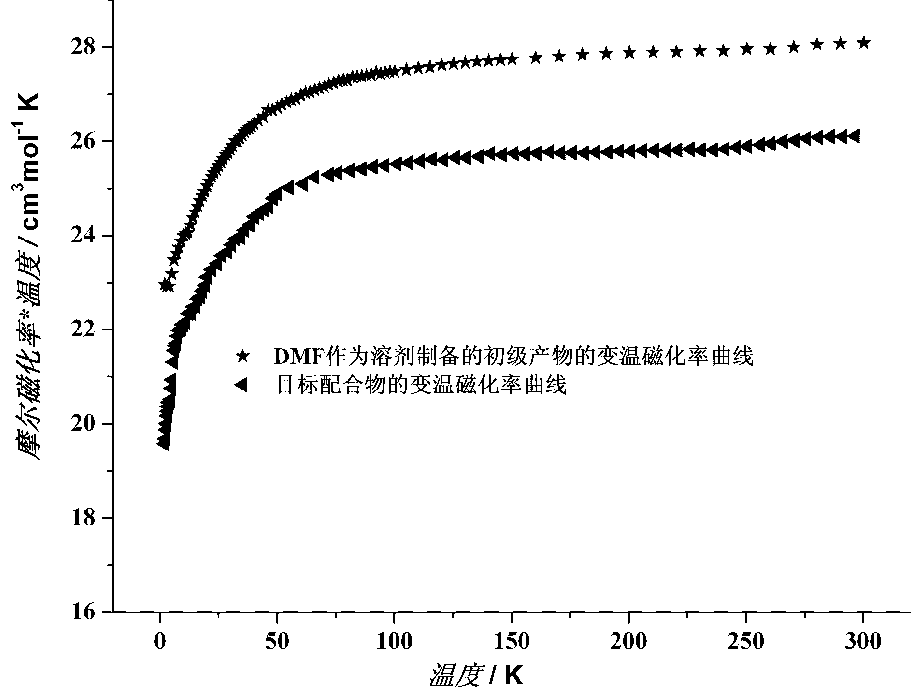
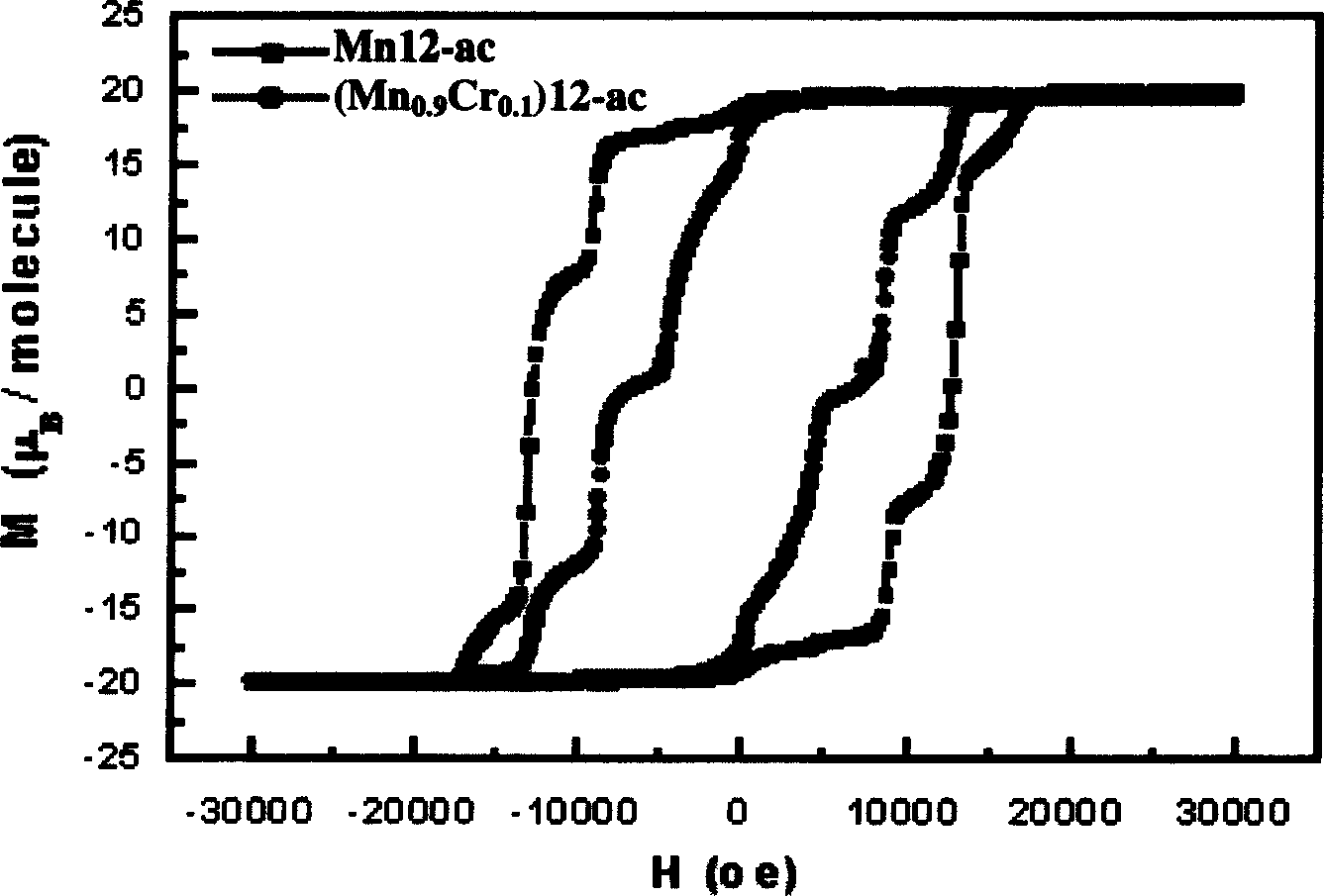
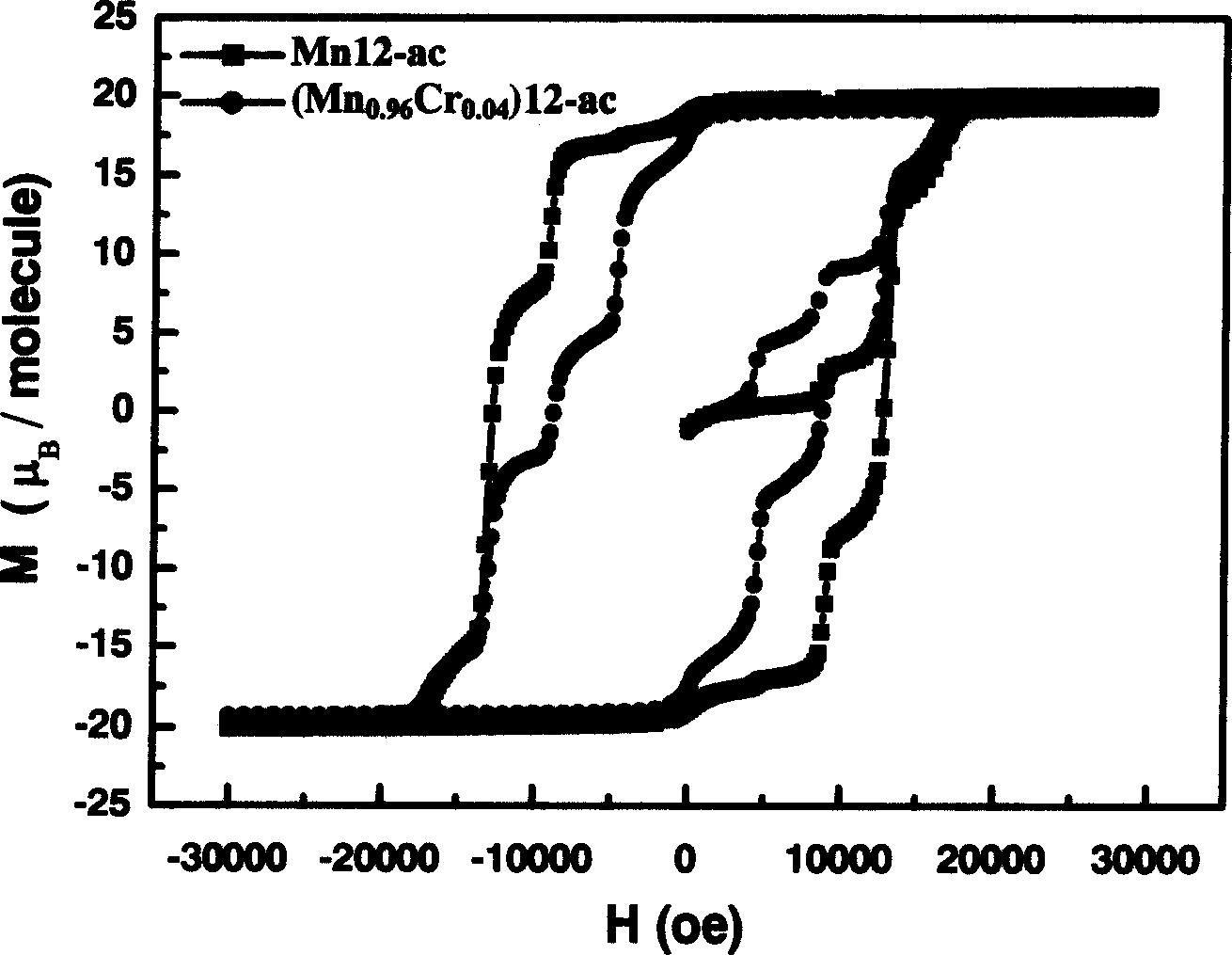
InactiveCN1564283AIncrease in sizeIncrease Quantum Storage CapacityDigital storageInductances/transformers/magnets manufactureWater bathsEngineering
The method includes steps: (1) dissolving Mn(CH3COO)2.4H2O,K2Cr O4 and KMnO4 in acetum fully under room temp, and continuous agitating; (2) Displacing reaction bulb to water bath jar, standing at room temp, coming up by using water bath method; (3) heating up to 318-328 K and keeping it at constant temperature; (4) cooling down to room temp naturally and standing at the state. The invention raises volume of unimolecule (Mn1-xCrx) 12-ac, and quanta storage capacity of crystal greatly, tunneling probability is 1-7.5 times larger than Mnl2-ac molecular magnet.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Polyacid dysprosium single-molecular magnets and preparation method thereof
InactiveCN103994792ASimple methodEasy to implementOrganic chemistryInductances/transformers/magnets manufactureDodecaneMagnetic storage
The invention discloses polyacid dysprosium single-molecular magnets and a preparation method of the polyacid dysprosium single-molecular magnets and belongs to the field of magnetic storage materials. The chemical formula of the single-molecular magnets is [NaDy(DOTA)2 7H2O] [Fe(CN)5NO]2 11H2O, wherein DOTA is 2,2',2'',2'''-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetra) quadrol. The preparation method of the polyacid dysprosium single-molecular magnets includes the following steps that dysprosium nitrate, DOTA and sodium nitroprusside are prepared into a solution according to the molar ratio 1:1:1, the solution is mixed at normal temperature for one hour and then filtered, filtrate stands still at normal temperature, crystals are separated out, and the crystals are the polyacid dysprosium single-molecular magnets. The polyacid dysprosium single-molecular magnets can be applied to new magnetic storage materials. The preparation method of the polyacid dysprosium single-molecular magnets is simple, easy to achieve and low in cost.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
2-aldehyde-8-hydroxyquinoline-1,3-diamino-2-propyl alcohol Schiff base four-core dysprosium cluster compound and synthesis method thereof
ActiveCN108840880ANovel structureHave single-molecule magnet behaviorGroup 3/13 organic compounds without C-metal linkagesOrganic/organic-metallic materials magnetismSpace groupSynthesis methods
The invention discloses a 2-aldehyde-8-hydroxyquinoline-1,3-diamino-2-propyl alcohol Schiff base four-core dysprosium cluster compound and a synthesis method thereof. The cluster compound belongs to amonoclinic system, a P21 / n space group, and the chemical formula is [Dy4(C23H17N4O3)2(mu3-OH)2(NO3)4(H2O)2]. The synthesis method of the cluster compound comprises the following steps: dissolving 2-aldehyde-8-hydroxyquinoline, 1,3-diamino-2-propyl alcohol and Dy(NO3)3*6H2O into a mixed solvent, adjusting the pH of the obtained solution to be 9.4 to 10.0, performing reaction on the obtained mixedliquid under the heating condition, cooling a reactant and separating out crystals to obtain the cluster compound, wherein the mixed solvent is a composition of ethanol and acetonitrile. The cluster compound has field induced single-molecular magnet behavior and can be used for preparing a magnetic material; furthermore, the synthesis method is simple and easy to operate.
Owner:山东顺创新材料科技有限公司
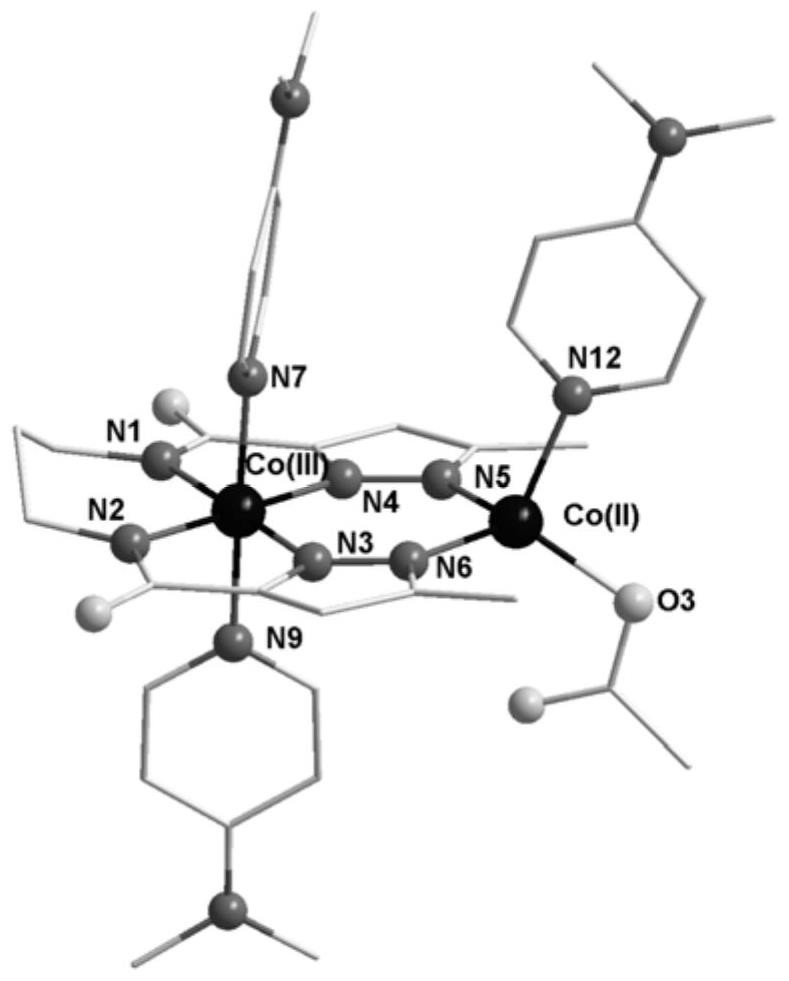
Novel cobalt (III)-sulfur cluster-based coordination polymer with single-molecule magnet property
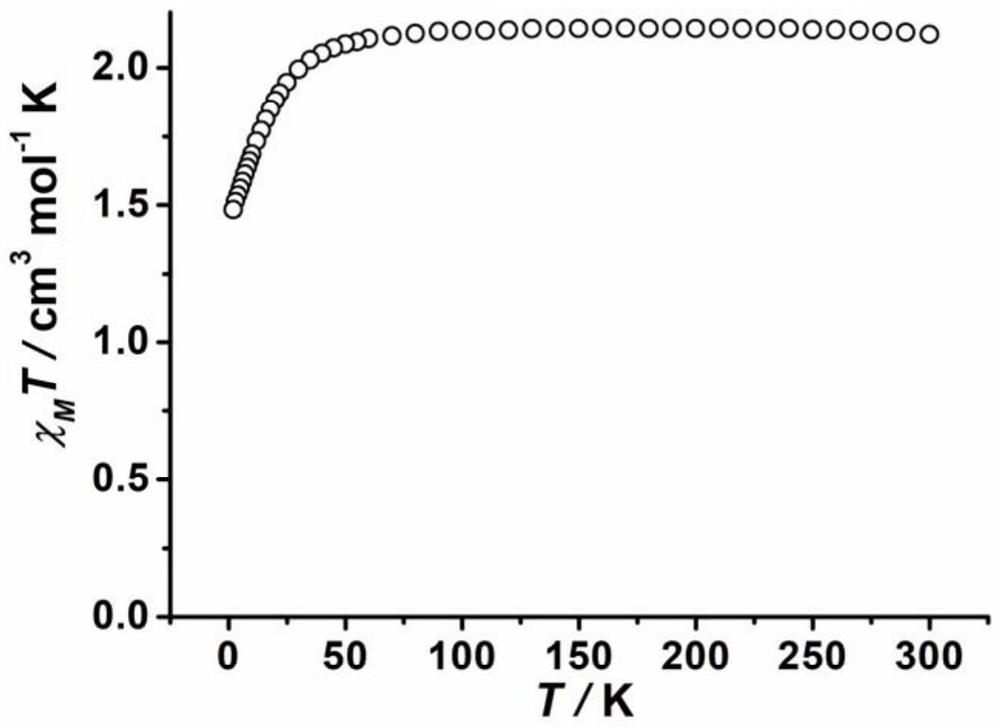
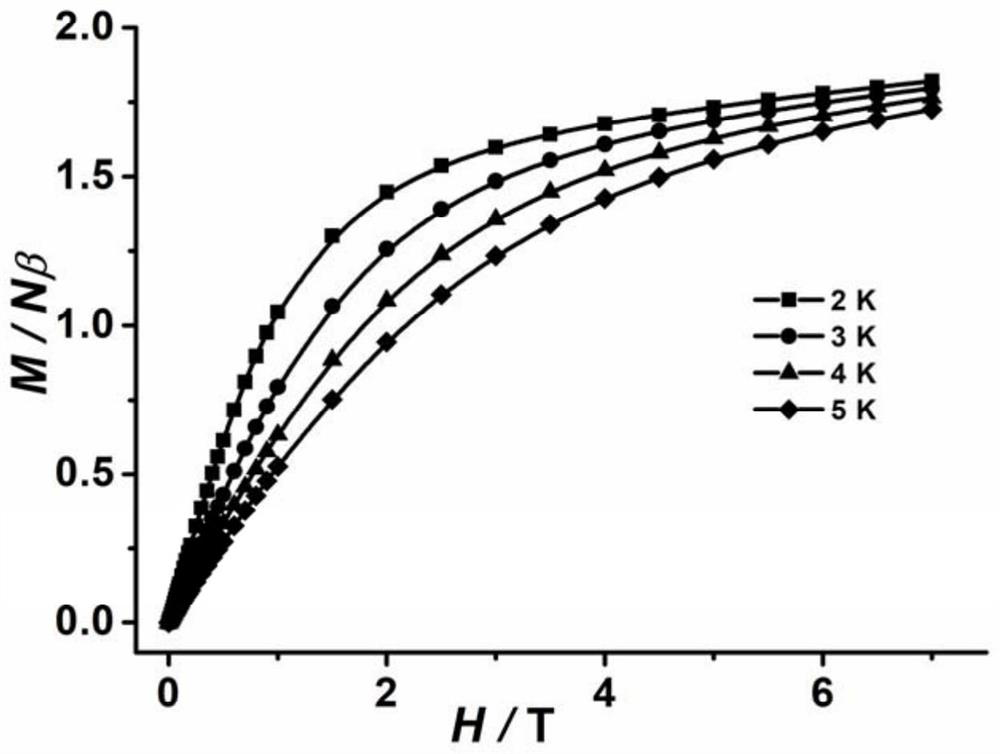
The invention belongs to the field of molecular magnetics, and particularly relates to a novel cobalt (III)-sulfur cluster-based coordination polymer with a single-molecule magnet property. The three-dimensional skeleton of the cobalt (III)-sulfur cluster-based coordination polymer provided by the invention is ([Co(III)3Co(II)2(mba)6(Hdtb)(H2O)4]n). Monocrystal XRD research shows that the polymer is composed of a trinuclear cobalt (III)-sulfur cluster structural unit and two mononuclear cobalt (II), a three-dimensional complex network structure is formed through bridging of a 2-mercaptobenzoic acid divalent anion ligand, and a cobalt (III) atom cluster unit is a trinuclear linear cobalt (III) cluster and serves as a construction unit in the coordination polymer. Magnetics research shows that the slow magnetic relaxation phenomenon of cobalt (II) ions is the characteristic behavior of a single-molecule magnet under the condition of no external direct-current magnetic field.
Owner:WENZHOU UNIVERSITY
Binuclear dysprosium cluster compound taking 2-aldehyde-8-hydroxyquinoline ethanolamine schiff base as ligand as well as synthesis methods and application thereof
InactiveCN108191896AHigh yieldThe synthesis method is simpleGroup 3/13 organic compounds without C-metal linkagesOrganic/organic-metallic materials magnetismSynthesis methodsDysprosium
The invention discloses a binuclear dysprosium cluster compound taking 2-aldehyde-8-hydroxyquinoline ethanolamine schiff base as a ligand as well as synthesis methods and application thereof. The chemical formula of the binuclear dysprosium cluster compound is [Dy2(C12H11N2O2)2(NO3)4].CH3CN; the cluster compound belongs to a triclinic system and a P-1 space group. The invention provides the two synthesis methods of the binuclear dysprosium cluster compound; the synthesis methods are simple, low in cost and good in repeatability. Research findings prove that the cluster compound has a field-induced single-molecule magnet behavior, thus being used for preparing magnetic materials.
Owner:GUANGXI NORMAL UNIV
Mononuclear dysprosium magnetic complex as well as preparation method and application thereof
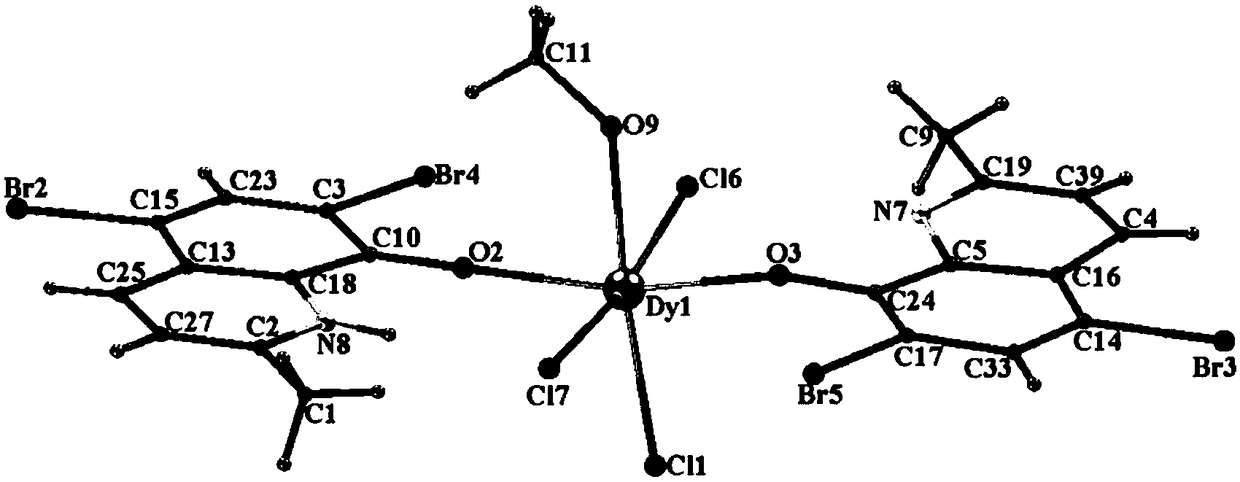
PendingCN112341481AHigh purityHigh yieldGroup 3/13 organic compounds without C-metal linkagesOrganic chemistry methodsNitrobenzeneStructural formula
The invention belongs to the technical field of rare earth metal coordination chemistry, and relates to a mononuclear dysprosium magnetic complex with a simple structural formula of Dy(TPA)(OPhCl2NO2)3, wherein TPA is tris(2-pyridylmethyl)amine, and HOPhCl2NO2 is 2, 6-dichloro-4-nitrophenol. The preparation method comprises the following steps: adding triethylamine into an acetonitrile solution containing 2, 6-dichloro-4-nitrophenol (HOPhCl2NO2), performing uniform stirring, adding the mixture into anhydrous alcohol containing dysprosium chloride hexahydrate (DyCl3.6H2O) and TPA, performing full reacting, completely dissolving the generated yellow precipitate into dichloromethane, slowly adding n-hexane, standing, and carrying out two-phase diffusion to obtain the seven-coordination mononuclear dysprosium complex. The preparation process is safe, simple, high in controllability and good in reproducibility, and the prepared complex is good in stability and high in purity and yield. Typical slow relaxation behaviors can be shown under an external magnetic field of 0.15 T, and the magnetic material has the characteristics of a single-molecule magnet and can be used as a molecular-based magnetic material in novel high-density information storage equipment (such as an optical disk and a hard disk).
Owner:JIANGSU UNIV OF SCI & TECH
A kind of mononuclear dysprosium complex and its preparation method and application
ActiveCN107089999BEasy to prepareLow costOrganic chemistry methodsOrganic/organic-metallic materials magnetismAlcoholRuthenium
The invention discloses a mononuclear dysprosium complex and a preparation method and application thereof. The chemical formula of the complex is [Dy(HL)2(CH3OH)Cl3], wherein HL is 2-methyl-5,7-dibromo-8-hydroxyquinoline. The complex belongs to a monoclinic system and P21 / n space groups. The preparation method of the mononuclear dysprosium complex includes the steps that DyCl3.6H2O and 2-methyl-5,7-dibromo-8-hydroxyquinoline are taken and dissolved with a mixed solvent, the pH of the obtained solution is adjusted to range from 6.5 to 7.8, the obtained mixed liquid reacts under the heating condition, and then the complex is obtained, wherein the mixed solvent is a composition of methyl alcohol and acetonitrile. The preparation method of the complex is simple, the cost is low, good repeatability is achieved, magnetic properties of the complex are shown as field-induced single-molecular magnet behaviors, and the complex can be used for preparing magnetic materials.
Owner:GUANGXI NORMAL UNIV
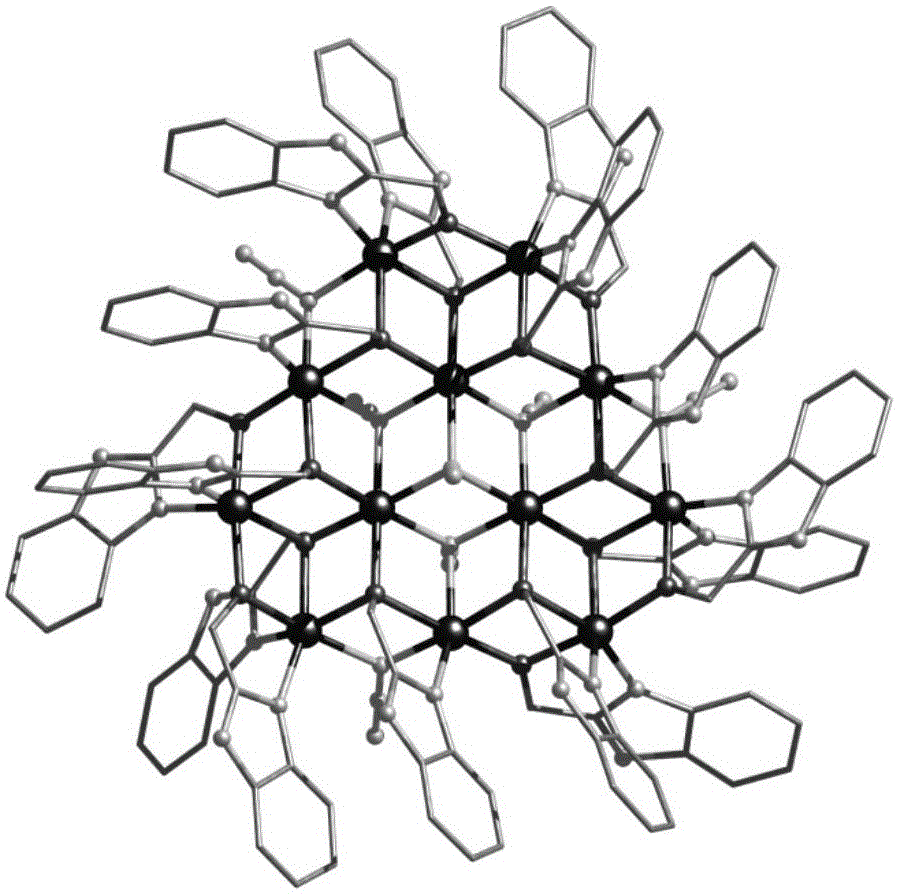
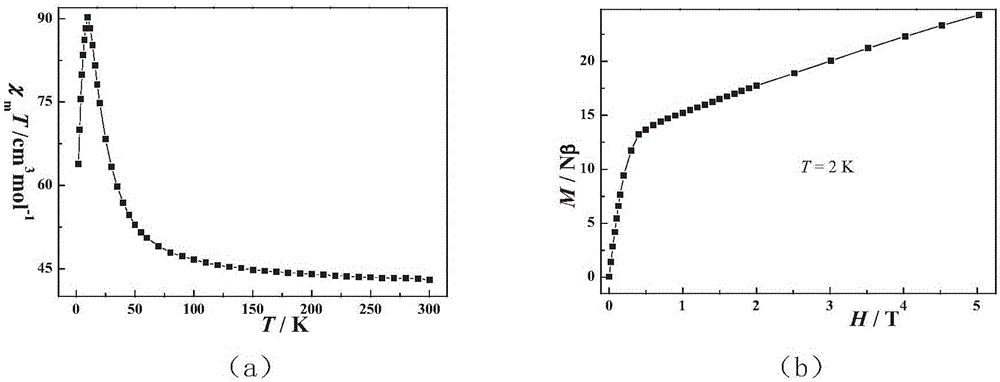
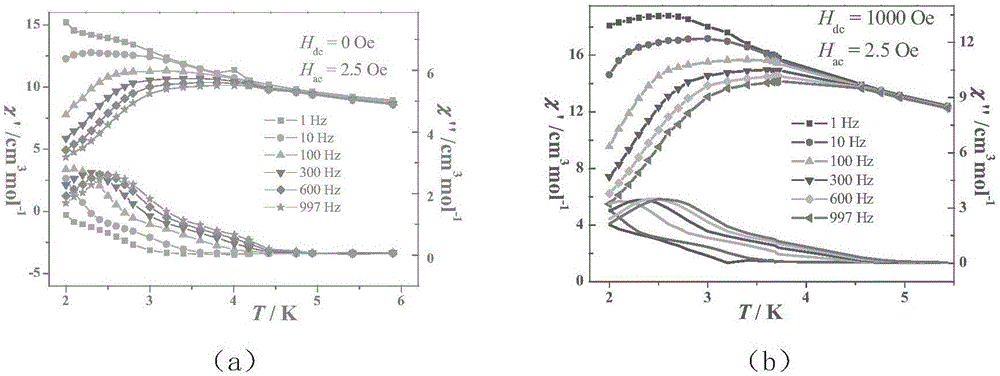
Ten-core dysprosium cluster compound single-molecular magnet and preparation method thereof
InactiveCN109053773AGroup 3/13 organic compounds without C-metal linkagesOrganic chemistry methodsSpace groupDual core
The invention discloses a ten-core dysprosium cluster compound single-molecular magnet and a preparation method thereof. The ten-core dysprosium cluster compound single-molecular magnet has the chemical formula as [Dy10(ovpho)4(NO3)4(CH3O)3(mu4-O)2(mu3-OH)2(mu2-OH)(H2O)4].8H2O. The ten-core dysprosium cluster compound single-molecular magnet is crystallized in an orthorhombic system Pbcn space group and is structurally formed by two edge-shaped {Dy4} tetrahedron elements and two {Dy2} double-core units located on two sides of the tetrahedron elements. The preparation method comprises the stepsof adopting an H4ovpho ligand and Dy(NO3)3.6H2O with the molar ratio being 1:3 as raw materials, adopting methanol and acetonitrile as solvents, adopting triethylamine as an alkaline matter, stirring, carrying out solvothermal reaction, separating, and washing solids. The ten-core dysprosium cluster compound single-molecular magnet provided by the invention is simple in process, low in cost, easyto control chemical components, good in repeatability and high in yield. Meanwhile, the cluster compound size reaches to the nano scale and is the rare high-core nano single-molecular magnet.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Mixed metal ion single-molecular magnet and preparation method thereof
InactiveCN103980324ARaise the blocking temperatureHigh yieldOrganic chemistryMagnetsMagnetic storageSynthesis methods
The invention discloses a mixed metal ion single-molecular magnet and a synthesis method thereof, belonging to the field of magnetic storage materials. The chemical formula of the single-molecular magnet is D<y>(DMF)4(H2O)3Fe(CN)6.H2O, wherein DMF is N,N-dimethyl formamide. The preparation method comprises the following steps: evenly mixing dysprosium nitrate and a potassium ferricyanide solution, adding a DMF (dimethyl formamide) solution of quinoline-2-formic acid, stirring for one hour at normal temperature, filtering, standing a filtrate at the normal temperature, and separating out crystals, namely the mixed metal ion single-molecular magnet. The mixed metal ion single-molecular magnet can be applied to a novel magnetic storage material; the method is simple, easy to realize and low in cost.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
N-hydroxyphthalimide dysprosium coordination compound and preparation method thereof
ActiveCN110003252AImprove performanceOptimize the synthetic routeGroup 3/13 organic compounds without C-metal linkagesOrganic chemistry methodsSingle crystalSlow cooling
The invention provides a N-hydroxyphthalimide dysprosium coordination compound and a preparation method thereof. The coordination compound has a following chemical formula: [Dy2(HPI)2(NO3)4(DMF)2], wherein HPI is N-hydroxyphthalimide of which one hydrogen ion is removed. The preparation method comprises the following steps: weighing Dy(NO3)3.5H2O and N-hydroxy-1,8-naphthalimide in a hard glass tube, adding an organic solvent, performing uniform shaking, performing vacuum sealing, performing a reaction under heating condition at 60-80 DEG C for 1-3 days, taking out the reaction product, performing slow cooling to room temperature, performing washing, and selecting pure regular single crystals, and performing natural drying to obtain the N-hydroxyphthalimide dysprosium coordination compound.The method provided by the invention prepares the N-hydroxyphthalimide dysprosium coordination compound from selection of a rare earth ion, design of a single-molecular magnet and optimization of a synthetic route, and improves the performance of the rare earth single-molecular magnet; and the coordination compound is a 4f metal coordination compound with a slow relaxation behavior, and has potential application value in the fields of high-density magnetic memorizers and magnetic films.
Owner:SHAOYANG UNIV
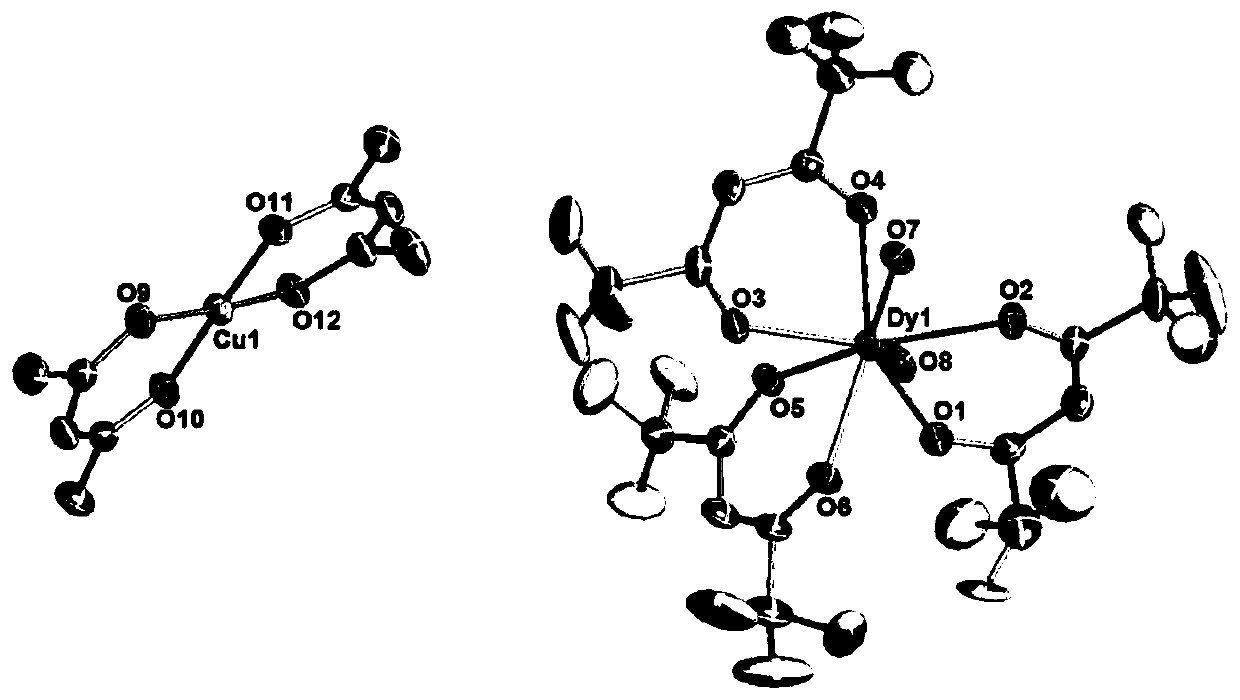
Dy (III)-Cu (II) eutectic monomolecular magnet and preparation method thereof
ActiveCN111116343ASimple filterSimple processOrganic chemistry methodsOrganic/organic-metallic materials magnetismMetallurgyQuantum chemical
The invention discloses a Dy (III)-Cu (II) eutectic monomolecular magnet and a preparation method thereof. The chemical formula of the Dy (III)-Cu (II) eutectic monomolecular magnet is [Dy(hfac)3(H2O)2]-[Cu(acac)2], wherein hfac is a hexafluoroacetylacetone anion, and acac is an acetylacetone anion. The preparation method comprises the following steps: adding a methanol solution dissolved with Dy(hfac)3(H2O)2 into a dichloromethane solution dissolved with Cu(acac)2, performing room-temperature stirring for 15-20 min, filtering to obtain a clear solution, naturally volatilizing the clear solution for 3 d to obtain dark green crystals, filtering, and washing and drying the dark green crystals to obtain the Dy (III)-Cu (II) eutectic monomolecular magnet. The Dy (III)-Cu (II) eutectic molecular material has the performance of a single-molecule magnet, and has a wide application prospect in the aspects of high-density information storage equipment, quantum chemical calculation, spintronicsand the like as a molecular-based magnetic material.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Manganese-Oxo Clusters as Contrast Agents for Magnetic Resonance Imaging
ActiveUS20120134932A1Enough timeNanomedicineDiagnostic recording/measuringNanoparticleMagnetic resonance imaging contrast medium
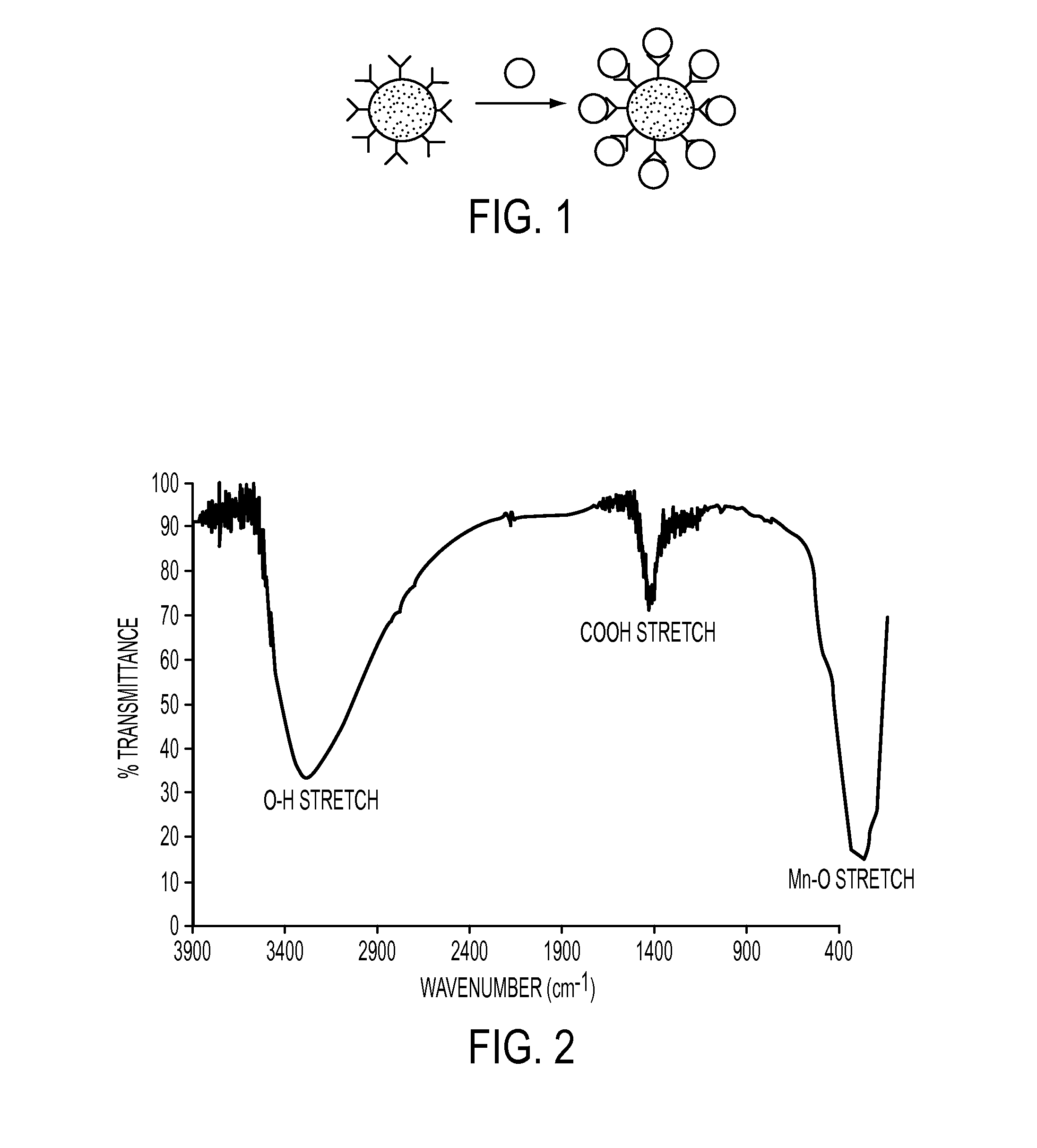
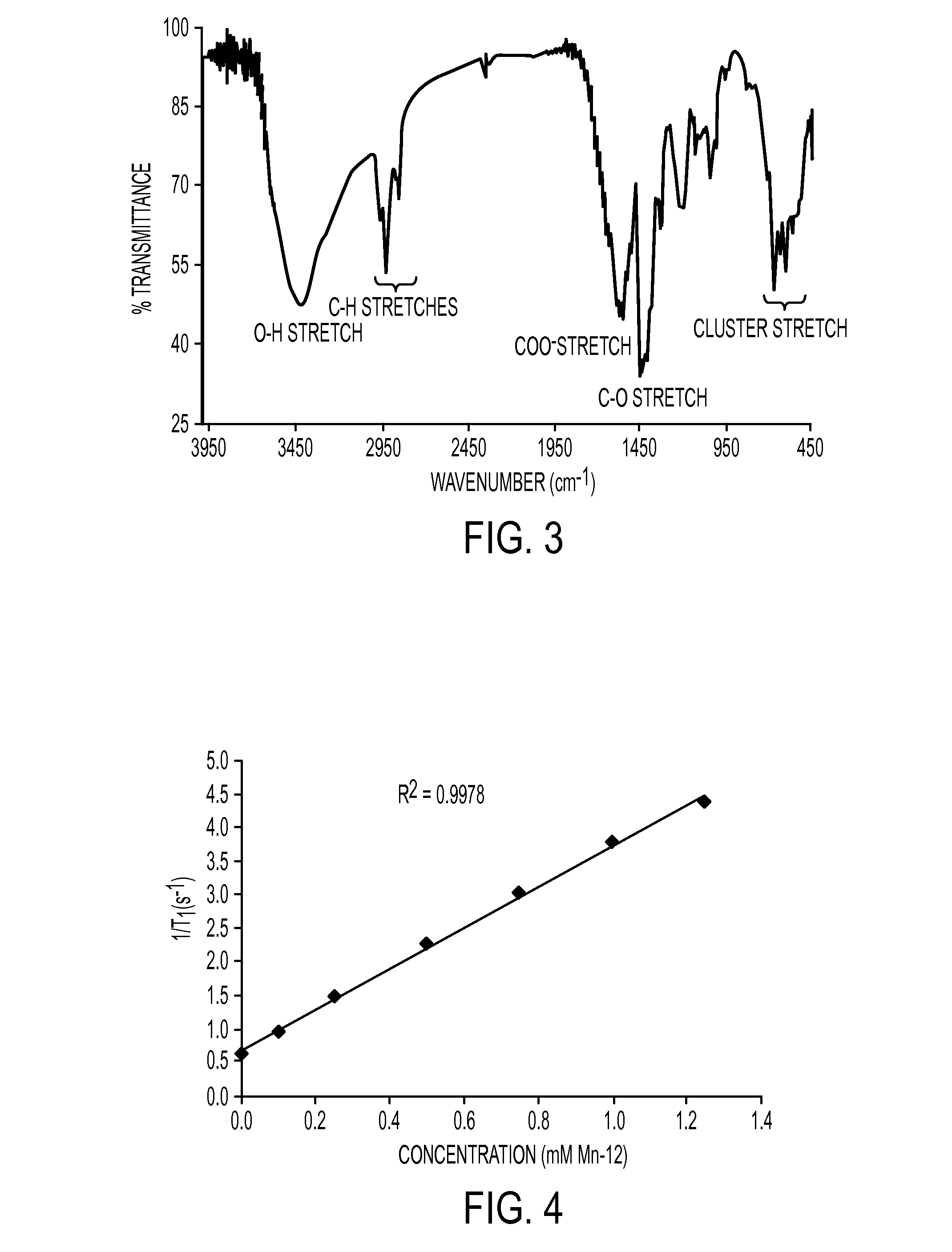
Nanoparticles for use as magnetic resonance imaging contrast agents are described. The nanoparticles are made up of a polymeric support and a manganese-oxo or manganses-iron-oxo cluster having magnetic properties suitable of a contrast agent. The manganese-oxo clusters may be Mn-12 clusters, which have known characteristics of a single molecule magnet. The polymer support may form a core particle which is coated by the clusters, or the clusters may be dispersed within the polymeric agent.
Owner:GEORGETOWN UNIV
Co(III)-Co(II) binuclear cobalt single-molecular magnet, preparation method and application thereof
PendingCN113956297ATypical slow relaxation behaviorFeatures single-molecule magnetsCobalt organic compoundsOrganic/organic-metallic materials magnetismMethyl groupMaterials science
The invention discloses a Co(III)-Co(II) binuclear cobalt single-molecular magnet, a preparation method and application thereof, the chemical formula of the single-molecular magnet is [CoIIICoII(L)(DMAP)3(CH3COO)], wherein H4L is N, N'-bis(5-methylpyrazole-3-formyl)-1, 3-propane diamine, and the structure is as shown in the specification, DMAP is 4-dimethylaminopyridine, and the structure is as shown in the specification. Compared with the prior art, the Co(III)-Co(II) binuclear cobalt single-molecular magnet has the following advantages that: (1) the Co(III)-Co(II) binuclear cobalt single-molecular magnet can show typical slow relaxation behavior under an external magnetic field of 0.1T, has the characteristics of a single-molecular magnet, and can be used as a molecule-based magnetic material in novel high-density information storage equipment (such as an optical disk and a hard disk); and (2) the method is safe and simple in process, high in controllability and good in reproducibility.
Owner:JIANGSU UNIV OF SCI & TECH
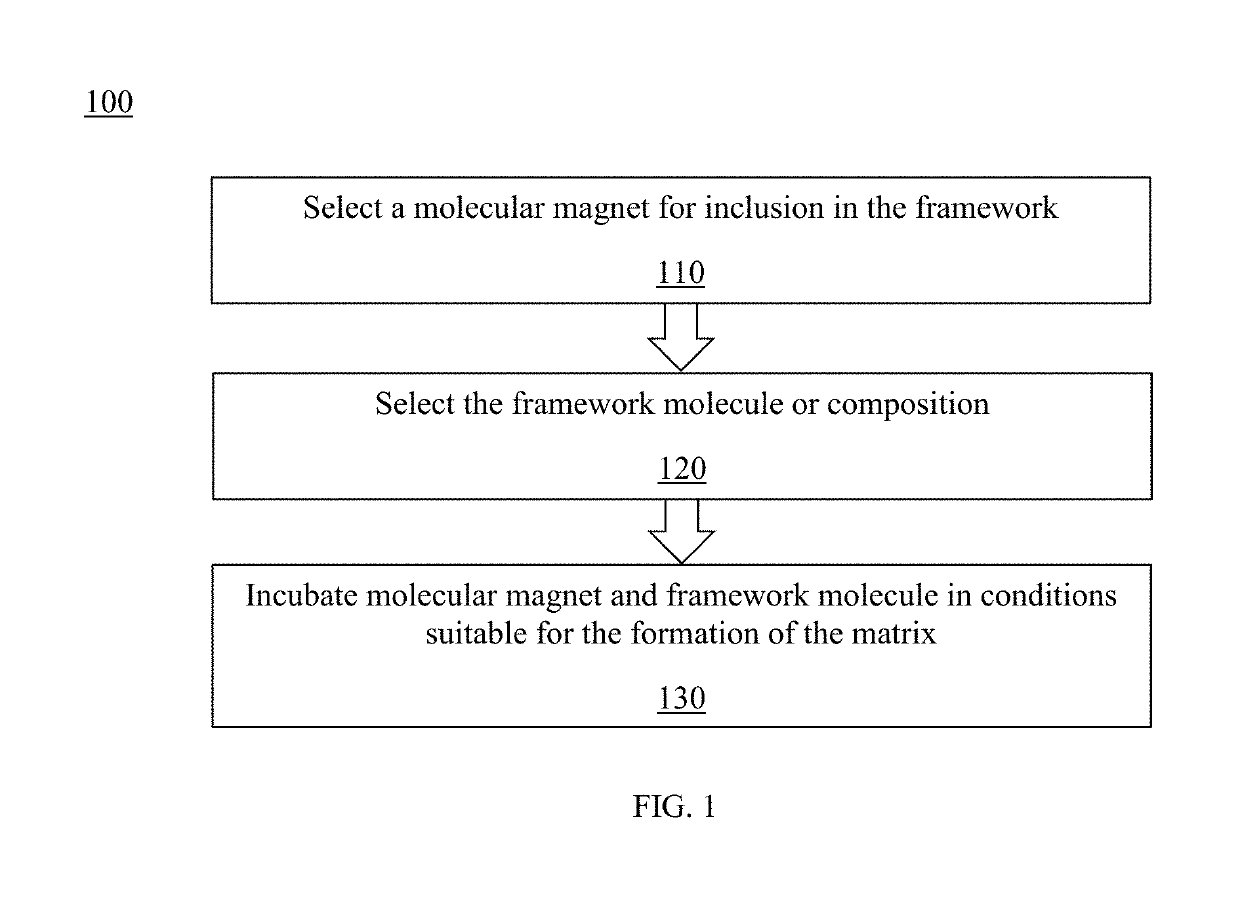
Method and system for controlled nanostructuring of nanomagnets
ActiveUS10340066B2Assembly precisionOrganic/organic-metallic materials magnetismGroup 3/13 element organic compoundsNanomagnetMagnetic molecules
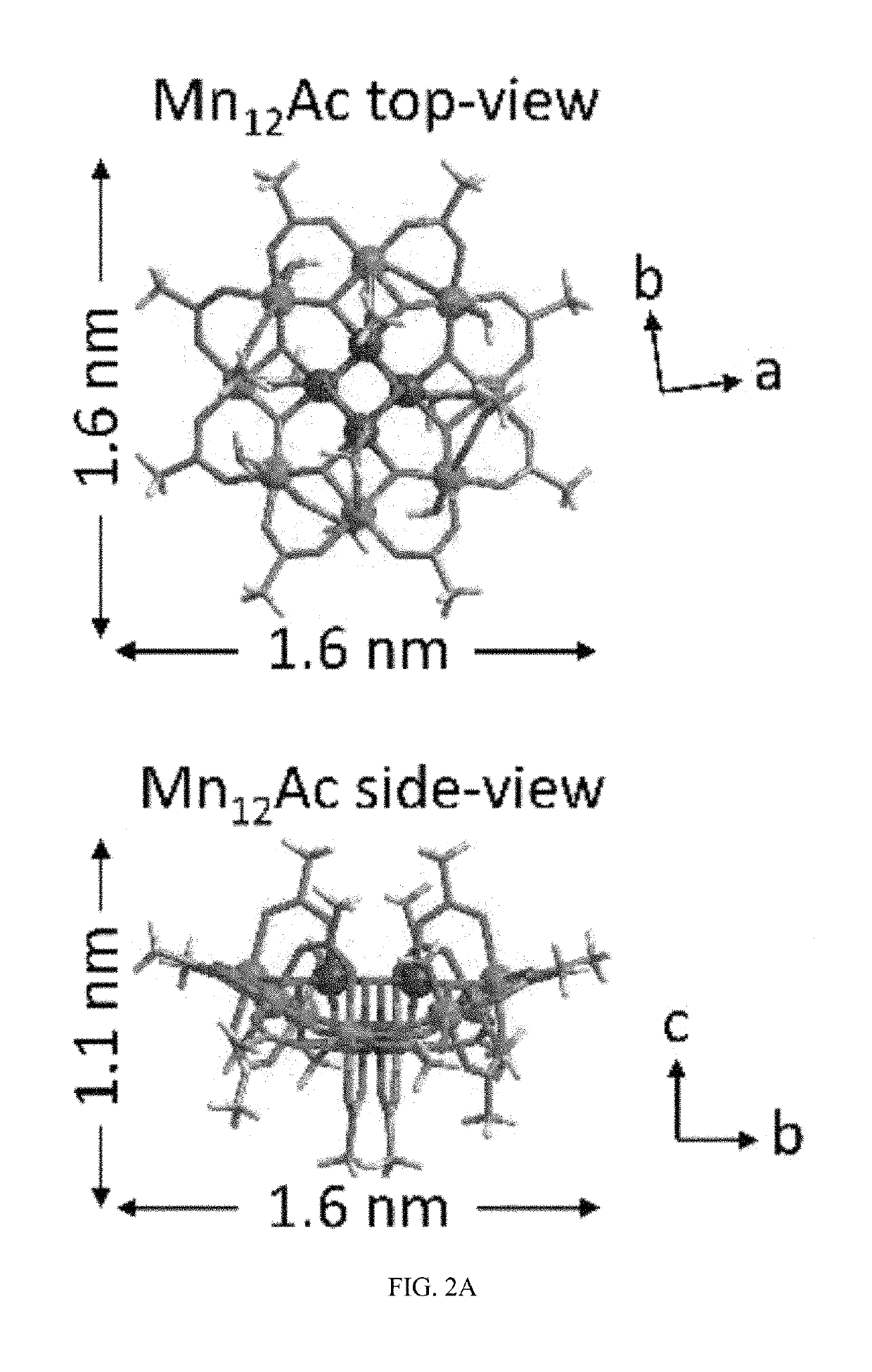
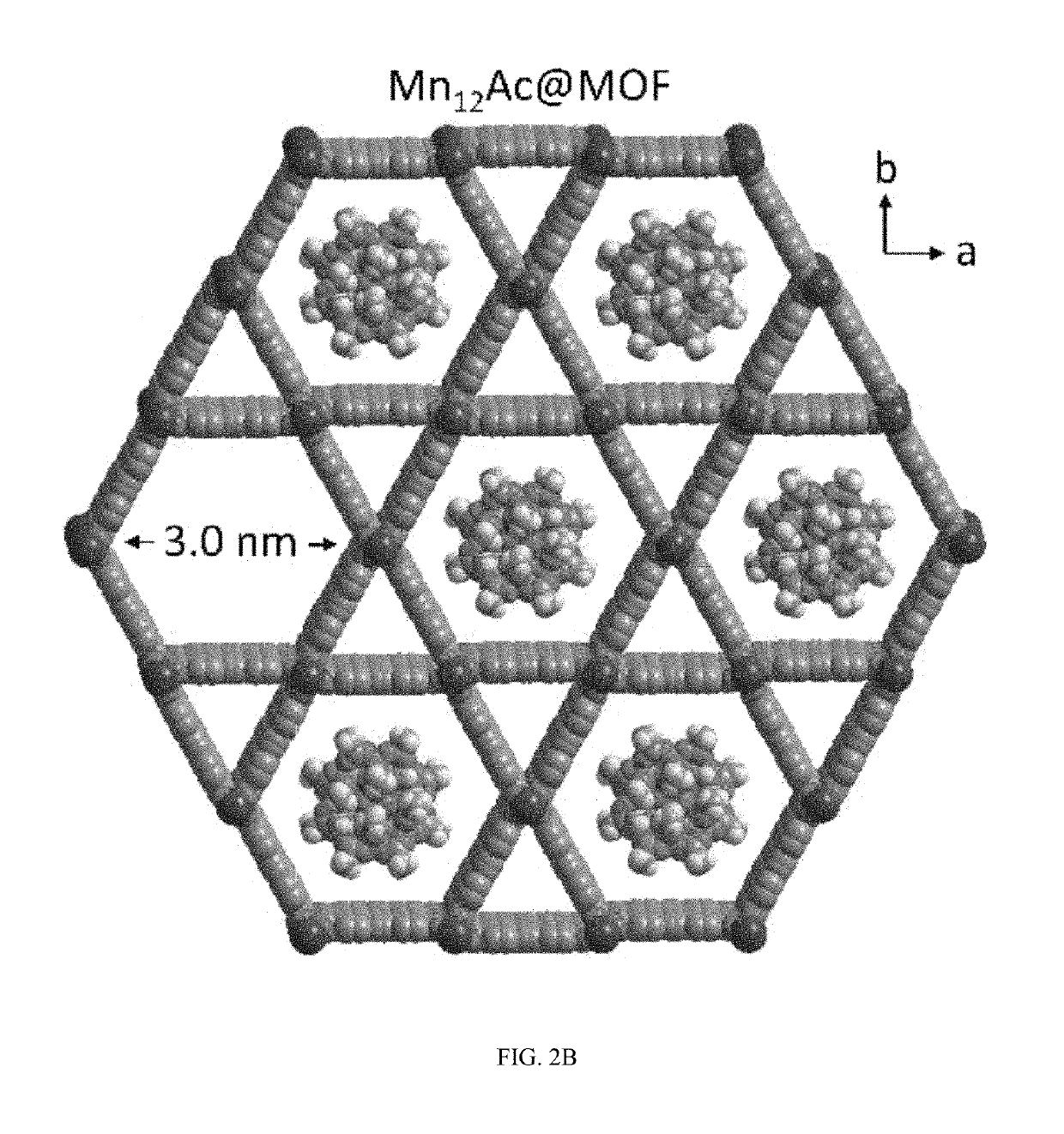
A composite magnetic matrix comprising a porous metal-organic framework (MOF) and a plurality of molecular magnets, where a plurality of pores of the MOF each comprise one of the plurality of molecular magnets, and where the each of the plurality of molecular magnets retains its magnetic properties in the matrix. The molecular magnet may be, for example, a single-molecule magnet or a single-chain magnet. For example, the composite magnetic matrix Mn12Ac@MOF comprises Mn12O12(O2CCH3)16(OH2)4 (Mn12Ac) as the single-molecule magnet and [Al(OH)(SDC)]n (H2SDC=4,4′-stilbenedicarboxylic acid) (CYCU-3) as the porous metal-organic framework.
Owner:CLARKSON UNIVERSITY
2-pyridinecarboxaldehyde-1,3-diamino-2-propanol Schiff base tetranuclear dysprosium cluster compound and synthesis method and application thereof
InactiveCN108440582AHave single-molecule magnet behaviorNovel structureGroup 3/13 organic compounds without C-metal linkagesOrganic/organic-metallic materials magnetismSynthesis methodsSolvent
The invention discloses a 2-pyridinecarboxaldehyde-1,3-diamino-2-propanol Schiff base tetranuclear dysprosium cluster compound and a synthesis method and application thereof. The cluster compound belongs to a tetragonal system and an I-4 space group, and the chemical formula is [Dy4(C15H15N4O)4(mu2-OH)4] 4ClO4-. The synthesis method for the cluster compound includes the steps of dissolving pyridine-2-carboxaldehyde, 1,3-diamino-2-propanol and Dy(ClO4)3 6H2O in a mixed solvent, adjusting the pH of the obtained solution to 7.8-8.5, and reacting the obtained mixed solution without heating to precipitate out crystals, wherein the mixed solvent is a combination of ethanol and acetonitrile. The cluster compound has field-induced single-molecule magnet behaviors and can be used for preparing magnetic materials; the whole reaction is carried out without heating, conditions are controllable, the synthesis method is simple and easy to operate, the yield is high, and the reproducibility is good.
Owner:GUANGXI NORMAL UNIV
A hexanuclear dysprosium cluster ring complex single-molecule magnet and its preparation method
ActiveCN110294771BReduce quantum tunneling effectEasy to prepareGroup 3/13 organic compounds without C-metal linkagesOrganic chemistry methodsHydroxamic acidPhysical chemistry
A single-molecule magnet of a hexanuclear dysprosium cluster ring complex and a preparation method thereof, which relates to a single-molecule magnet and a preparation method thereof. The purpose of the invention is to solve the problems of complex synthesis method, low controllability and poor repeatability in the rare earth complex single-molecule magnet prepared by the existing method. The chemical formula of the hexanuclear dysprosium cluster ring complex single-molecule magnet is [Dy 6 (H 2 sh) 6 (2‑pyca) 6 (2‑pyca ‑ ) 6 ], molecular formula is C 114 h 90 Dy 6 N 18 o 42 . Method: Salicylhydroxamic acid, pyridine‑2‑carboxylic acid and Dy(NO 3 ) 3 ·6H 2 O is dissolved in the solvent, and a mixed solution is obtained by ultrasonication. The mixed solution is heated to reflux, and then pyridine is added to obtain a preform; the solvent is volatilized on the preform to obtain a hexanuclear dysprosium cluster ring complex single-molecule magnet. The invention can obtain a hexanuclear dysprosium cluster ring complex single-molecule magnet.
Owner:HEILONGJIANG UNIV
Cobalt-based single-molecular magnet synthesizing method
ActiveCN105070497ASimple and safe processGood reproducibilityInductances/transformers/magnets manufactureMagnetic materialsMicrowaveCobalt salt
The invention discloses a cobalt-based single-molecular magnet synthesizing method. Cobalt salt and 2-hydroxybenzimidazole ligand are adopted with participation of azides ions, and a cobalt-based single-molecular magnet is prepared under the solvothermal system or a microwave reaction condition. It is indicated by experiments that the process is safe and simple, the cost of raw materials is low, the defect that the reproducibility of a common solution method is poor is overcome, the obtained single-molecular magnet of the [Co12(L)15(N3)7](NO3)2.2(CH3OH).2(H2O) (L is the 2-hydroxybenzimidazole ligand ) is red hexagonal-prism-shaped crystal, the purity is high, the crystal size is large and can be controlled within 2*1*1.5 mm, and the yield is high and reaches over 50%.
Owner:GUANGXI UNIV
A kind of multifunctional rare earth complex and its application
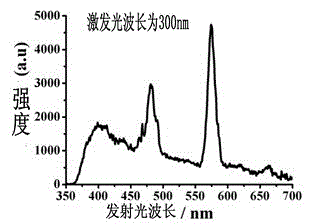
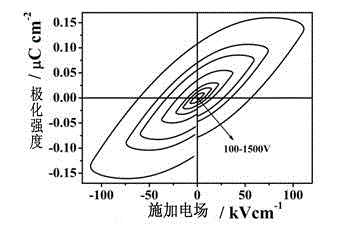
InactiveCN103193811BReduce manufacturing costImprove performanceGroup 3/13 element organic compoundsLight-emitting diodeMaterials science
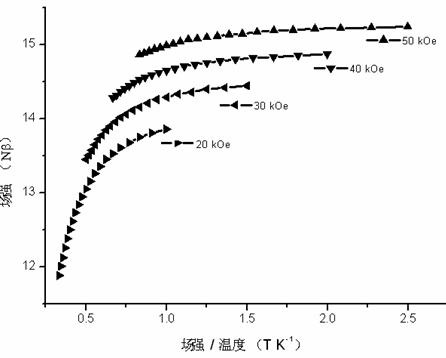
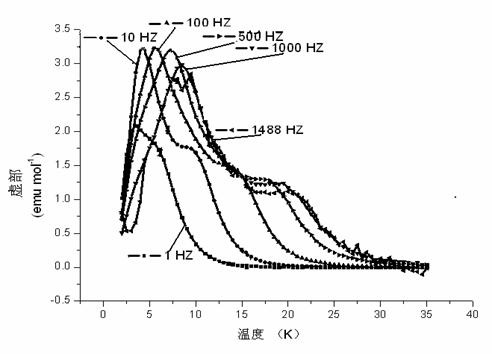
The invention provides a multifunctional rare-earth complex, a preparation method thereof and application of the complex in a white light LED (Light Emitting Diode), a ferroelectric and a monomolecular magnet. The chemical molecular formula of obtained dihydroxyl.tetranitro.tetra(3,5-di(2-pyridine)-1,2,4-triazole).dimethoxy tetra-dysprosium (III) is Dy4(bpt)4(NO3)4(OH)2(OCH3)2.3CH3OH. The multifunctional rare-earth complex provided by the invention can be used for emitting obvious white light at room temperature and normal pressure under exciting light of 210-310 nanometers and transforming a color coordinate CIE from (0.300, 0.310) to (0.308, 0.322), shows the ferroelectric behavior at room temperature and normal pressure under the action of an alternating electric field at 100-1500 V and 200 Hz, achieves the maximal polarization intensity of 0.16 muC.cm<-2> during 1500 V, shows the monomolecular magnet behavior at low temperature in zero external field under the action of an AC (Alternating Current) magnetic field at 1-1488 Hz and achieves the characteristic relaxation time of 2.91*10<-7> s and the effective energy barrier of 115.51 K.
Owner:SUN YAT SEN UNIV
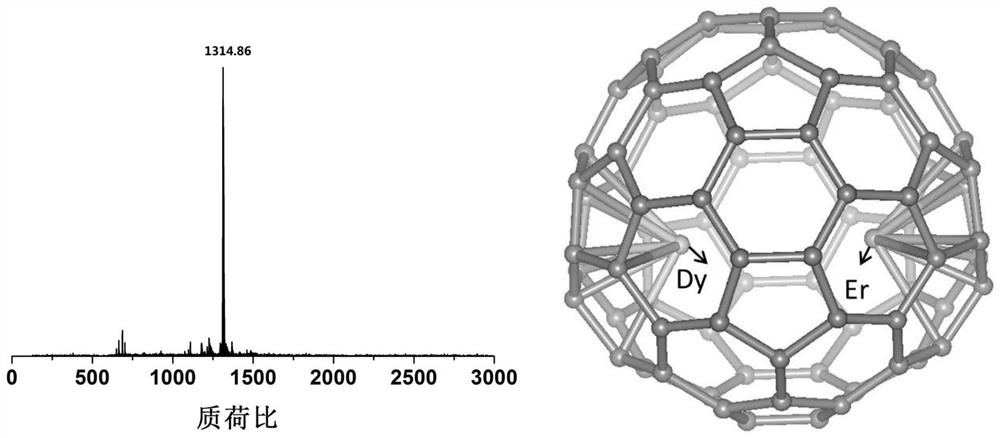
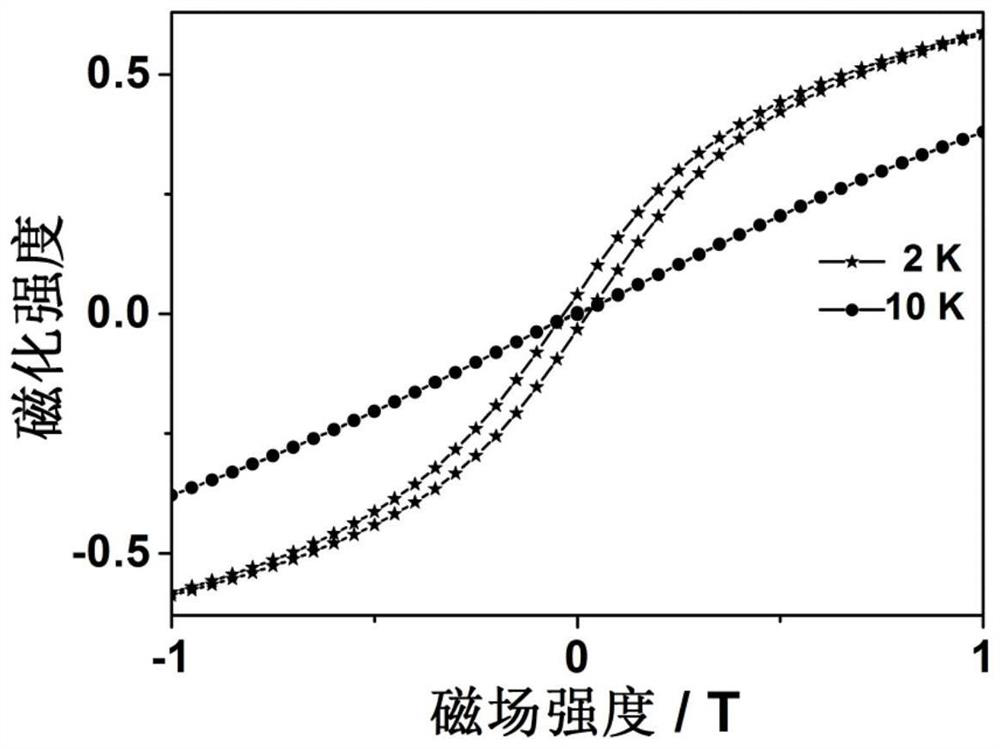
A photomagnetic functional material of metal fullerene and its preparation and application
ActiveCN111171817BStructure determinationEasy to operateFullerenesLuminescent compositionsPhotoluminescenceParticle physics
The invention provides a photomagnetic functional material of metallofullerene and its preparation method and application. The photomagnetic functional material of metallofullerene is in molecular form, with a size of about 1nm, a definite structure, easy manipulation, and performance It has single-molecule magnet performance and photoluminescence properties, and it has hysteresis phenomenon below 5K, and the metallofullerene containing Er has a luminescence wavelength in the range of 1400-1600nm, and the metallofullerene containing Y has a luminescence wavelength in the range of 700-800nm . Not only that, the photoluminescence wavelength or intensity of the optomagnetic functional material of the metal fullerene has modulation properties under the influence of an external magnetic field, that is, there is a coupling effect between magnetism and light, which can be used for quantum information storage or quantum computing. Material.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Zero field dysprosium single ion magnet and preparation method and application thereof
PendingCN114031634ATypical slow relaxation behaviorFeatures single-molecule magnetsOrganic compound preparationGroup 3/13 organic compounds without C-metal linkagesNitrobenzeneParticle physics
The invention discloses a zero field dysprosium single ion magnet and a preparation method and application thereof, the chemical formula of the dysprosium single ion magnet is [Dy (EO5) (OPhCl2NO2) 2] (OPhCl2NO2), EO5 is pentaethylene glycol, and HOPhCl2NO2 is 2, 6-dichloro-4-nitrophenol. Compared with the prior art, the zero field dysprosium single ion magnet has the following advantages: (1) the dysprosium single ion magnet can show typical slow relaxation behavior without adding a field, has the characteristics of a single molecule magnet, and can be used as a molecule based magnetic material in novel high-density information storage equipment (such as an optical disk, a hard disk and the like); (2) the dysprosium single ion magnet is not weathered in air and has good stability; and (3) the method is safe and simple in process, high in controllability and good in reproducibility.
Owner:JIANGSU UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4] Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/57537909-c307-494d-836f-821538d86323/HDA0000634118620000011.PNG)
![Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4] Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/57537909-c307-494d-836f-821538d86323/HDA0000634118620000012.PNG)
![Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4] Method for preparing single-molecular magnet [Dy2(saph)2(NO3)2(CH3OH)4]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/57537909-c307-494d-836f-821538d86323/HDA0000634118620000021.PNG)
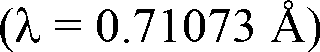
![Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/692108a6-82a0-447f-bbdc-cab60749ad61/HDA0000447506500000011.PNG)
![Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/692108a6-82a0-447f-bbdc-cab60749ad61/HDA0000447506500000012.PNG)
![Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH Preparation method of single-molecular magnet [Dy2(saph)2Cl2].4CH3OH](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/692108a6-82a0-447f-bbdc-cab60749ad61/HDA0000447506500000021.PNG)