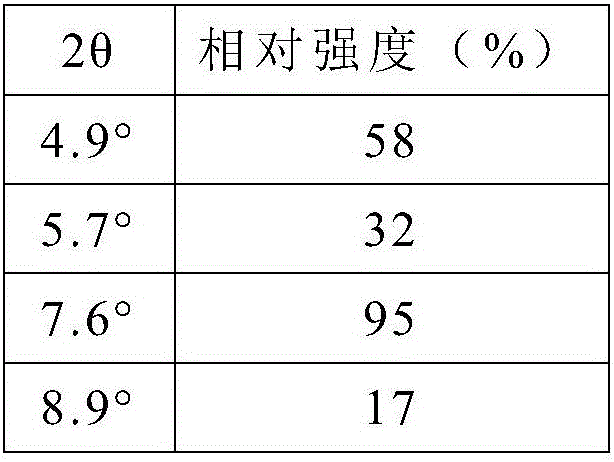
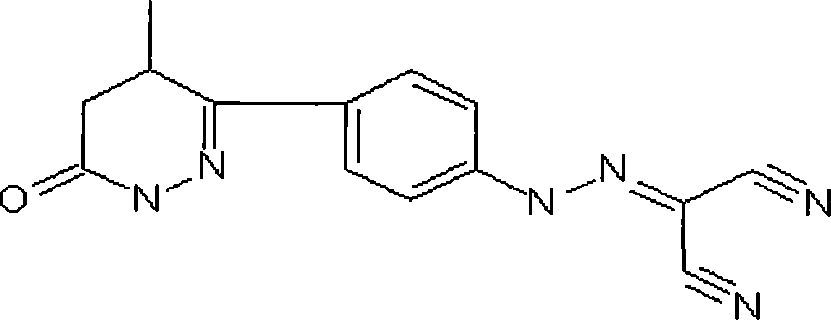
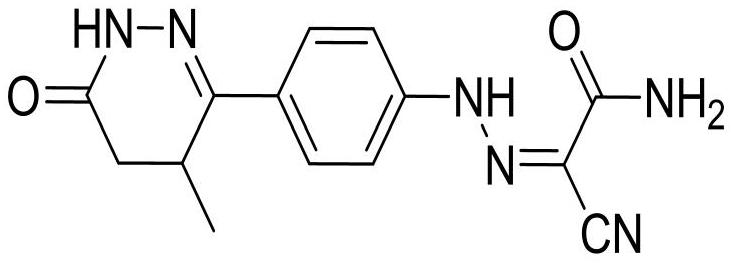
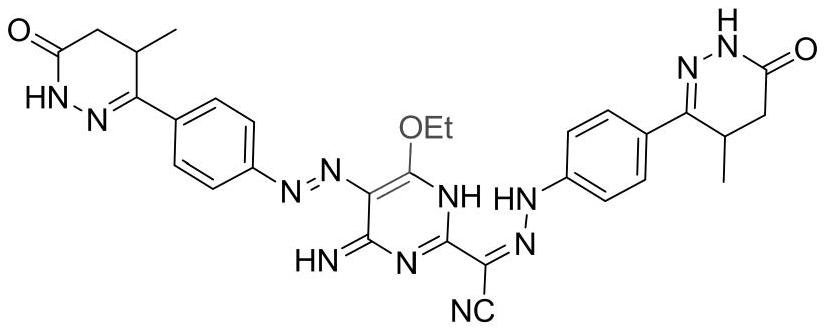
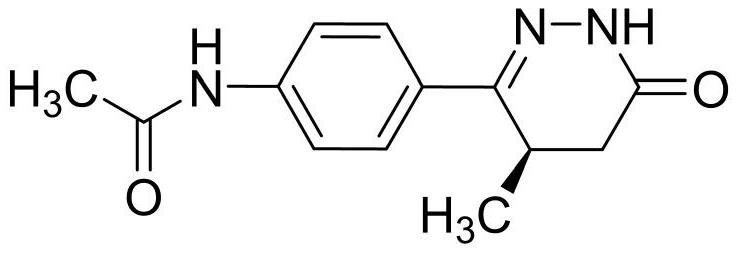
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Levosimendan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
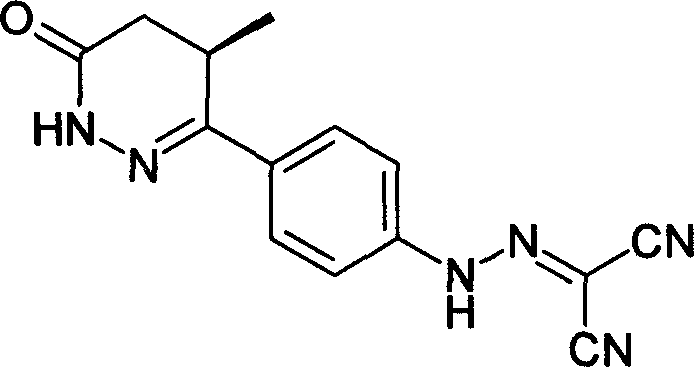
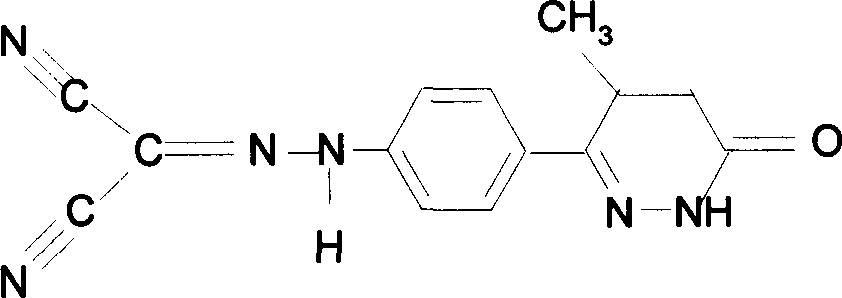
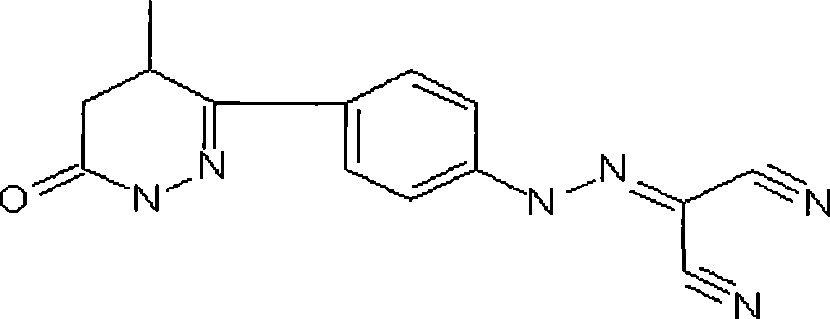
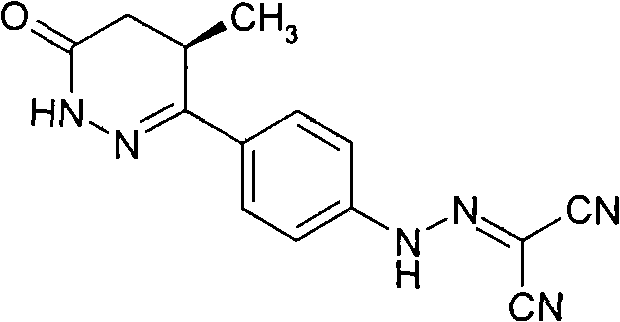
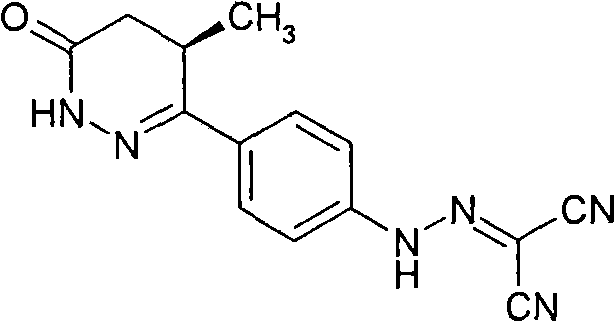
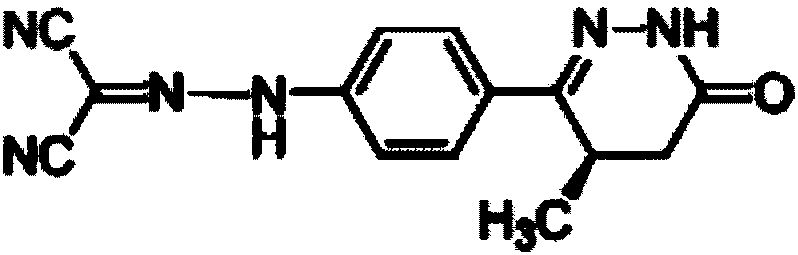
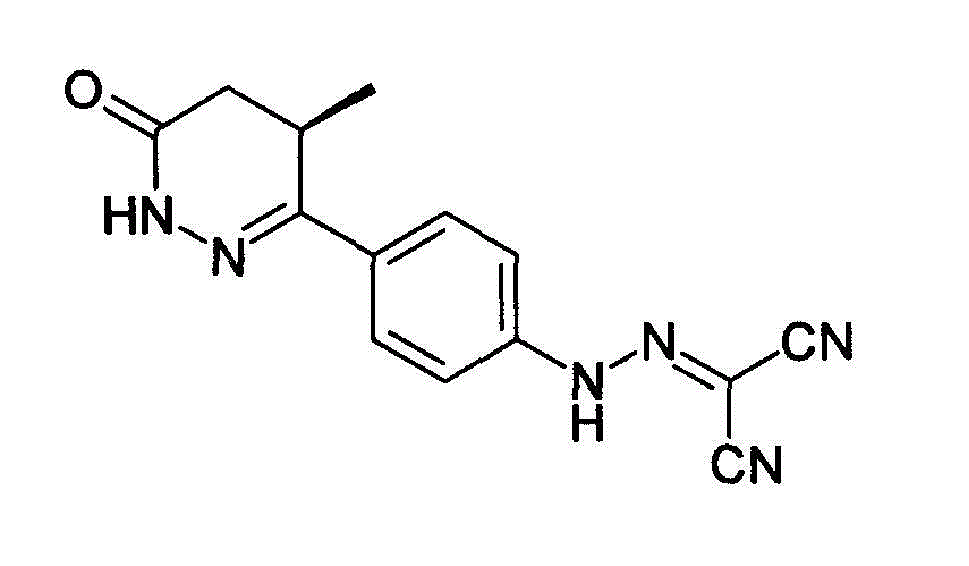
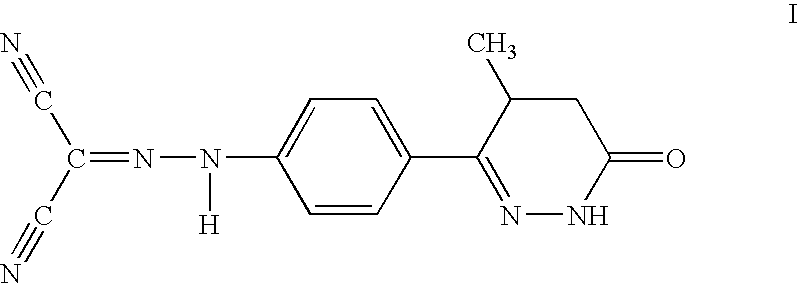
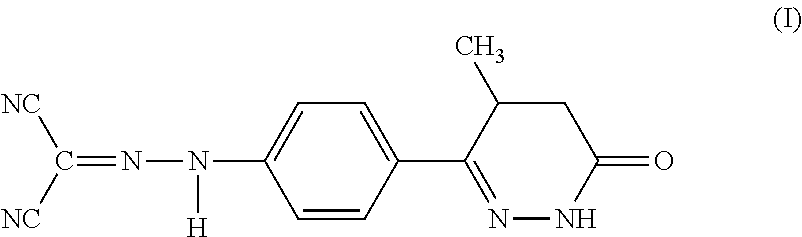
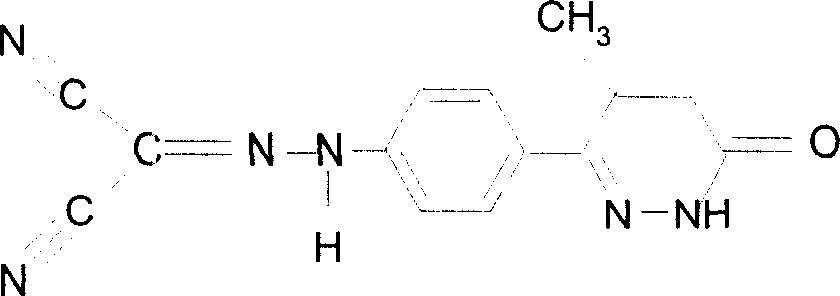
Levosimendan (INN) /ˌliːvoʊsaɪˈmɛndən/ is a calcium sensitiser used in the management of acutely decompensated congestive heart failure. It is marketed under the trade name Simdax (Orion Corporation).
Levosimendan freeze-dried preparation and preparing method
InactiveCN1626085AImprove solubilityImprove stabilityPowder deliveryLyophilised deliveryOrganic solventFreeze-drying
A freze-dried Zuoximengdan for treating heart failure is prepared from the pharmacologically acceptable pH regulator and water-soluble precipient, and the solution of Zuoximengdan or its pharmacologically acceptable salt through freeze drying. Its advantage is high stability.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Pharmaceutical composition containing levosimendan or its pharmaceutically acceptable salt as active ingredient
The invention relates to a pharmaceutical composition, wherein each unit dosage comprises: (1) 5-50mg of Levosimendan or its pharmaceutically acceptable salt, (2) 10-200mg of Solutol HS 15, (3) 0.1-1000ml of medicinal solvent.
Owner:成都英创科技发展有限责任公司 +1
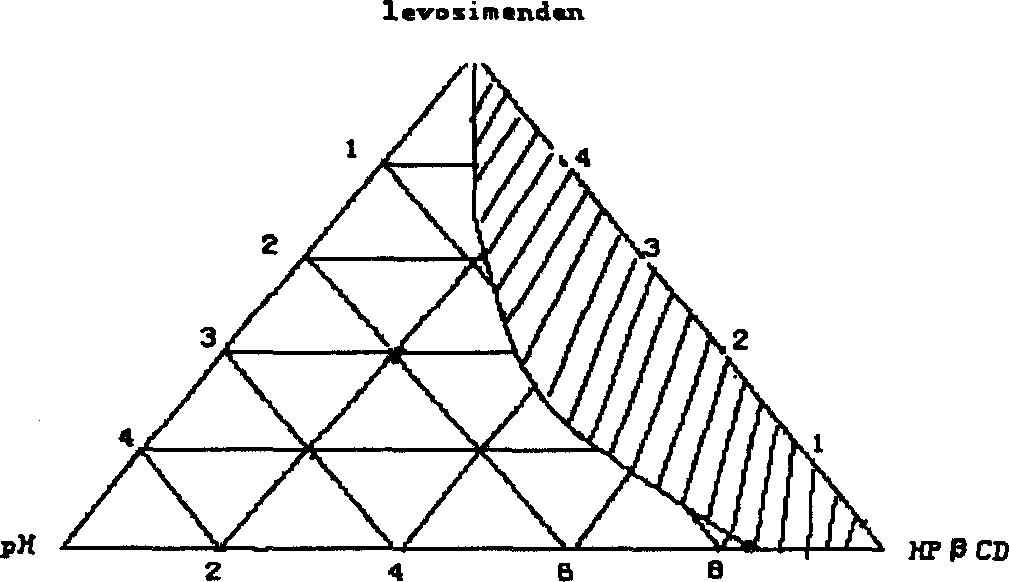
Inclusion preparation of levosimendan and beta cyclodextrin
InactiveCN1689573AEasy to operateMetabolism disorderPharmaceutical delivery mechanismBeta-CyclodextrinsPharmacology
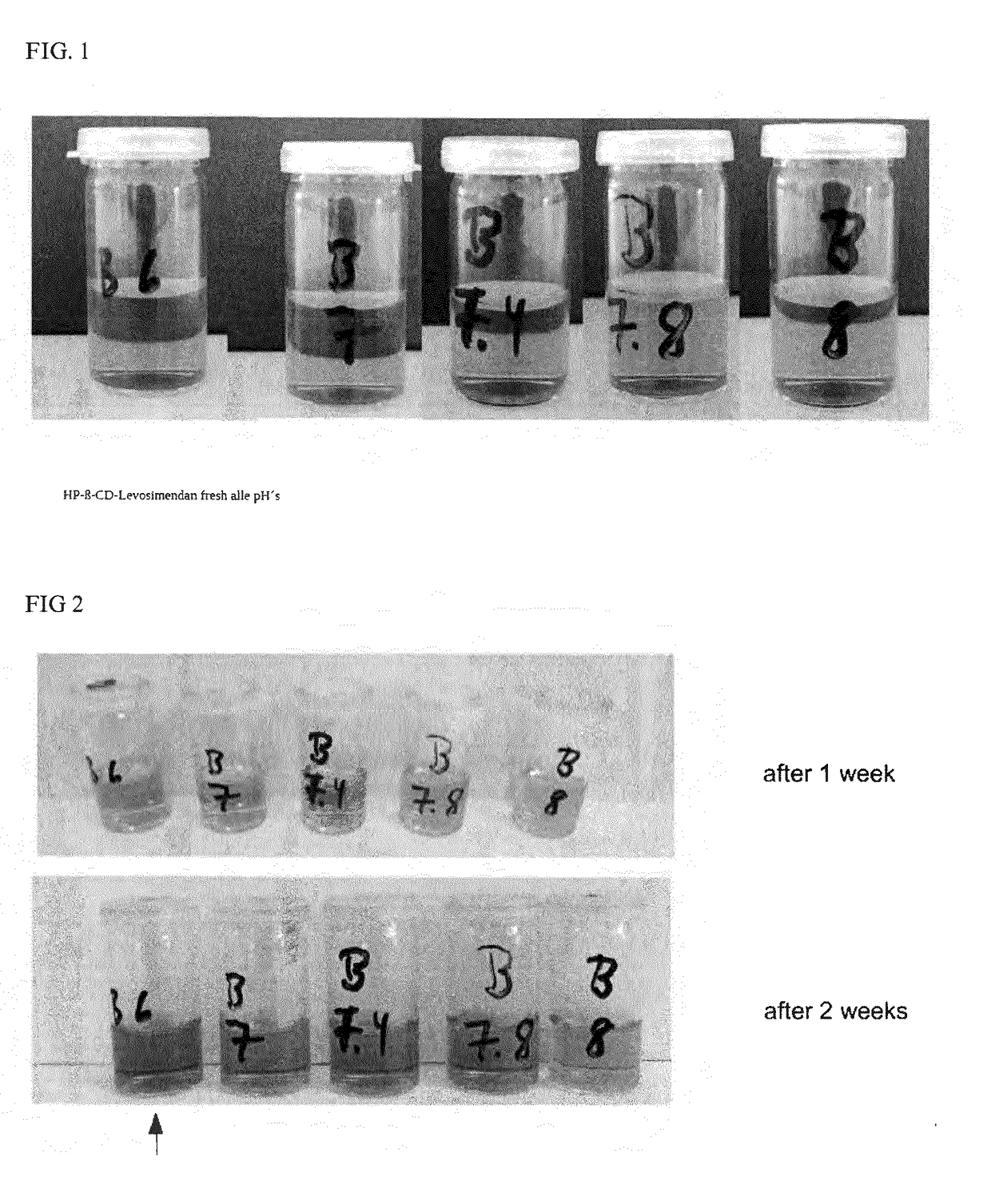
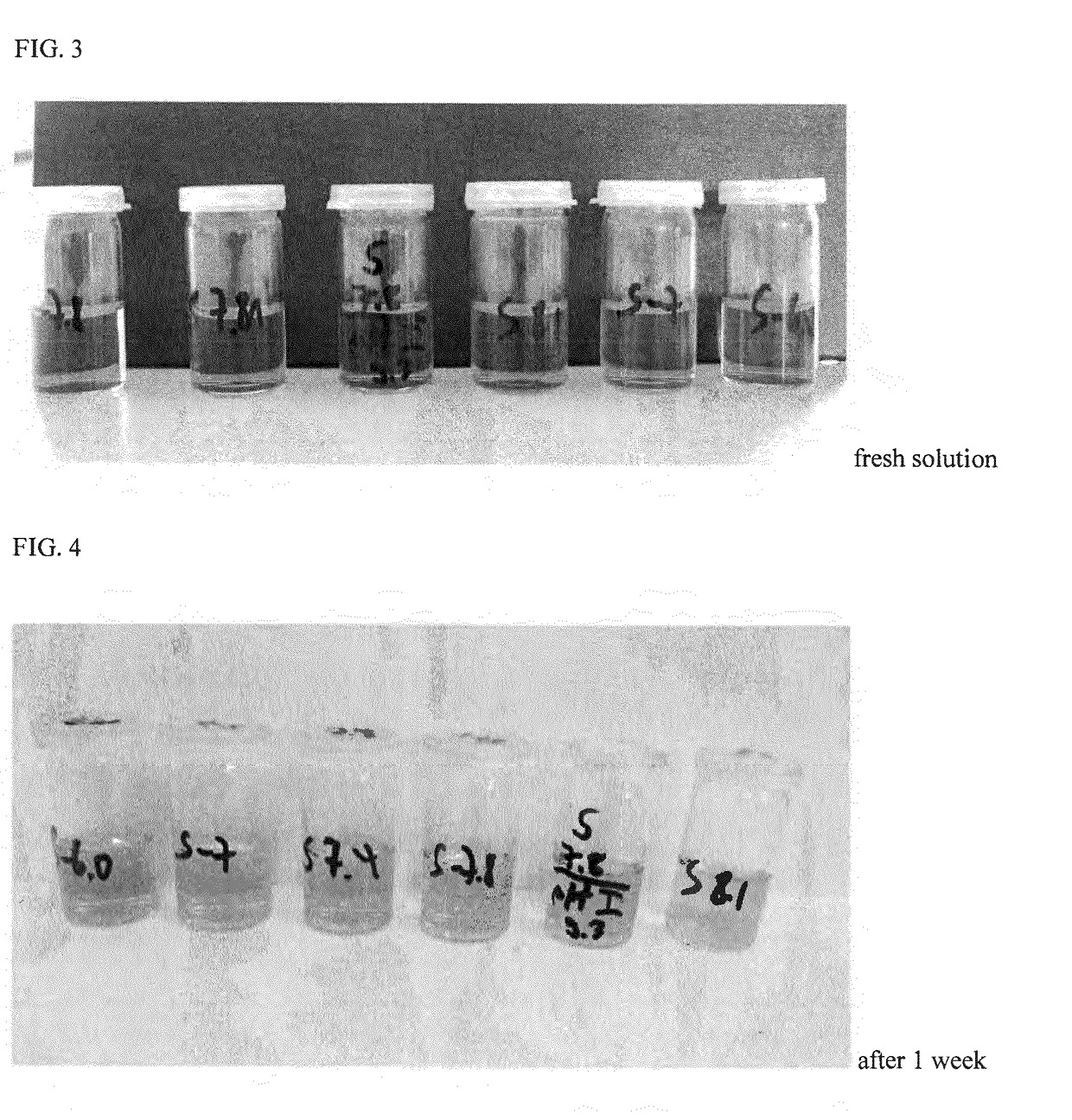
The present invention relates to one kind of heart failure resisting medicine, cyclodextrin or its derivative included preparation of levosimendan. The preparation has calcium-sensitive positive muscle medicine levosimendan as main component, and beta-cyclodextrin or its derivative, such as hydroxypropyl beta-cyclodextrin as including matter.
Owner:王思清
Stable Levosimendan pharmaceutical composition and preparation method thereof
ActiveCN101411708AEasy to transportSimple preparation processPharmaceutical delivery mechanismCardiovascular disorderOrganic solventMedicine
The invention relates to a stable levosimendan medicine composition. The medicine composition consists of two chambers, wherein a medicine chamber is filled with the levosimendan or a pharmaceutically acceptable salt thereof; and a solvent chamber is filled with a pharmaceutically usable solvent. The solvent contains a pharmaceutically acceptable organic solvent and solubilizing agent, and also can be added with a pharmaceutically acceptable acid. The composition can be used for intravenous injection, or taken orally. The invention also relates to a method for preparing the composition.
Owner:QILU PHARMA CO LTD
Combination treatment for acute myocardial infarction
InactiveUS20050255096A1Reduce mortalityBeneficial synergistic effect on the mortalityBiocidePeptide/protein ingredientsThrombusMortality rate
A combination therapy for the treatment of acute myocardial infarction comprises administering a combination of a thrombolytic agent and levosimendan or a pharmaceutically acceptable salt thereof to a patient. The combination synergistically reduces mortality of patients with acute myocardial infarction.
Owner:ORION CORPORATION
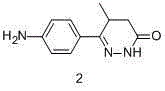
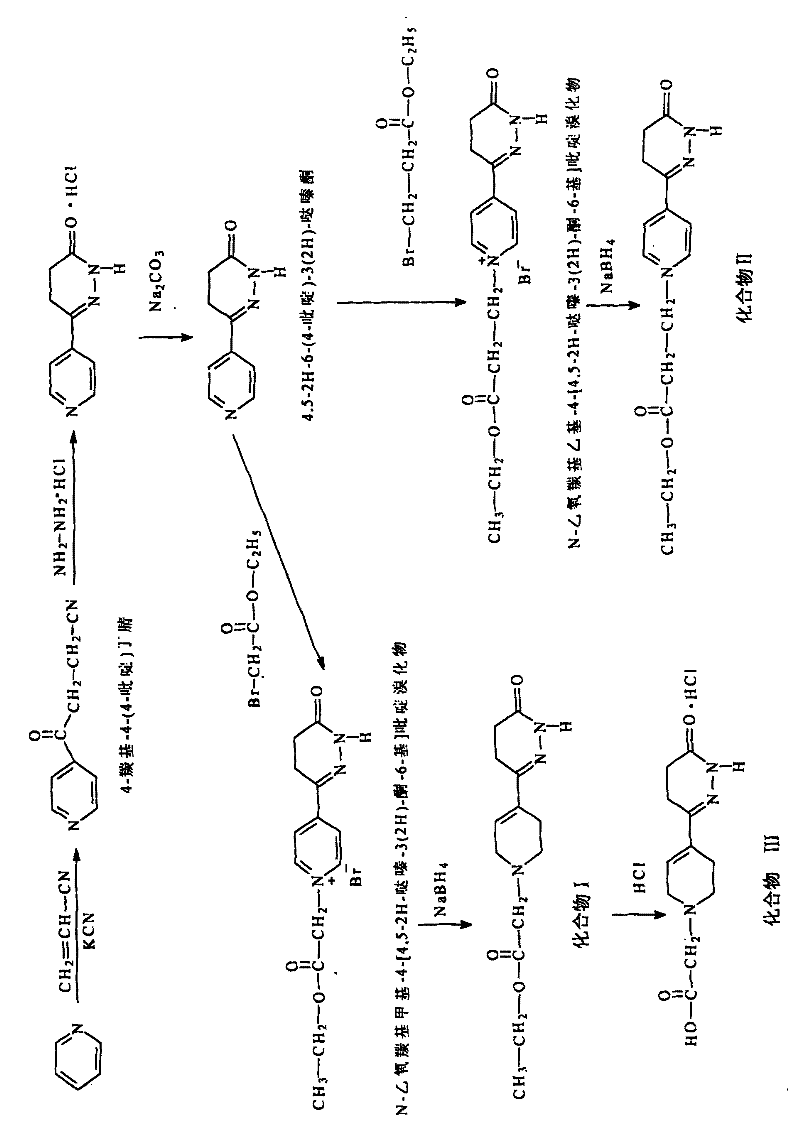
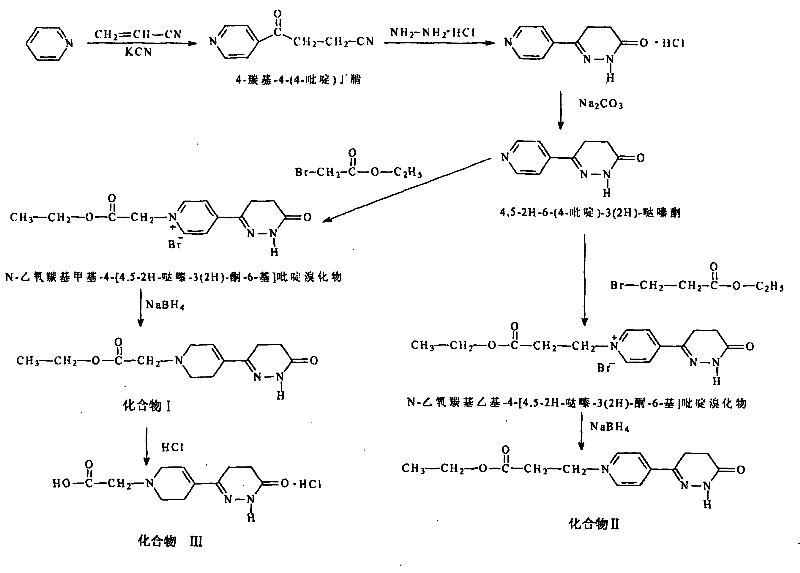
Racemization method of 6-4-(4-amino pheny)-4, 5-dihydro-5-methyl-3-(2H)-pyridazinone
The present invention relates to the processing method of 6-(4-amino phenyl)-4, 5-dihydro-5-methyl-3-(2H)-pyridazinone as the intermediate material for levosimendan and pimobendan as cardiac and cerebral vascular diseases medicine. The processing includes the first mixing of 6-(4-amino phenyl)-4, 5-dihydro-5-methyl-3-(2H)-pyridazinone dextroisomer with alkali or acid in 50 %, dissolving with solvent and reflux, evaporation to dry, water washing, and drying to obtain product. The said method can raise material utilization rate and lower product cost, and through four times one racemization and five times of resolution, the accumulated yield may reach 30-40 %, with the single time resolution yield being 8-12 %.
Owner:GUANGXI UNIV
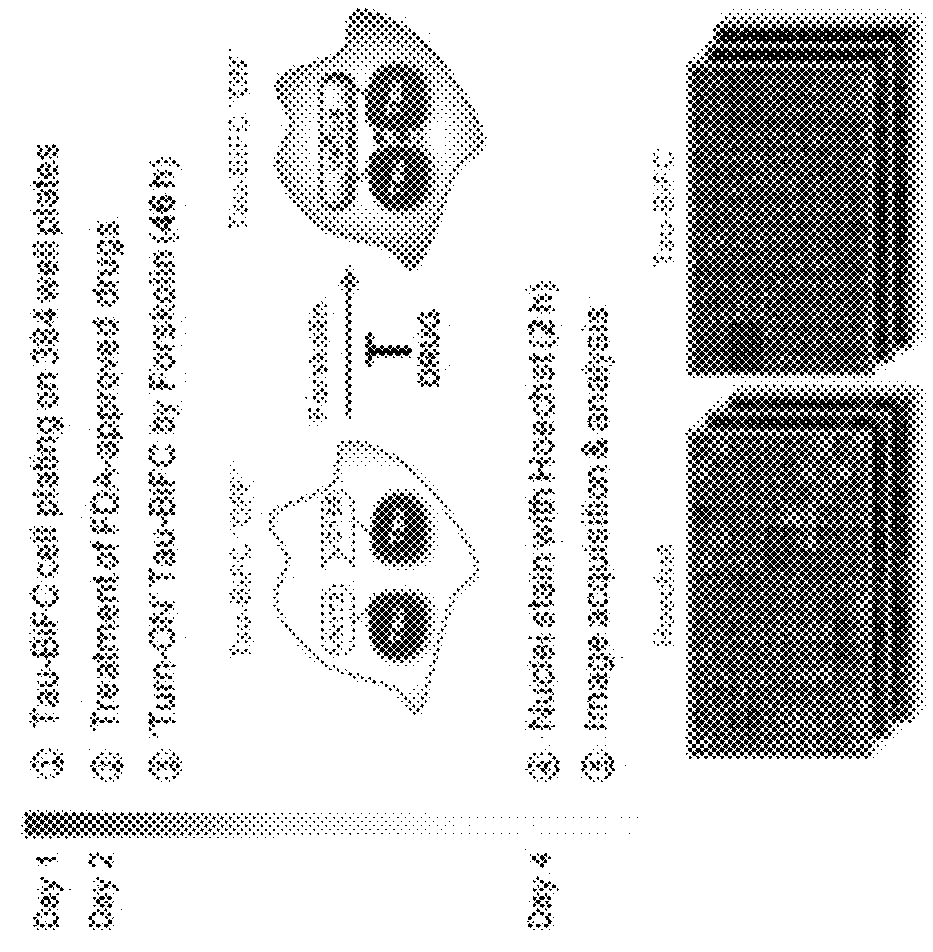
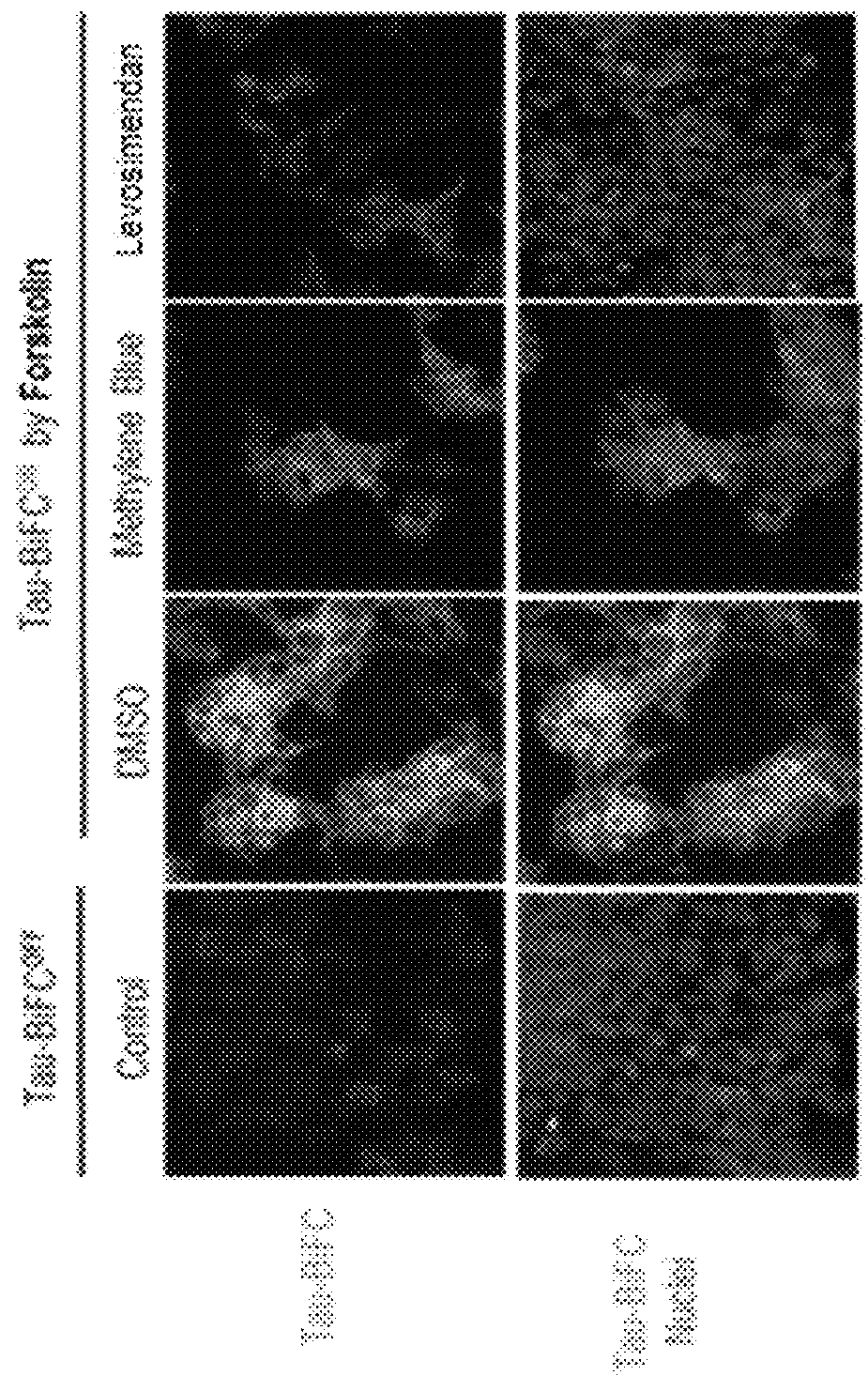
Levosimendan compound for preventing or treating tau-related diseases
ActiveUS9962384B1Promote aggregationOrganic chemistryPharmaceutical non-active ingredientsDiseasePhosphorylation
Disclosed is a levosimendan compound, an optically active isomer thereof, or a pharmaceutically acceptable salt thereof, which is used for the prevention and / or treatment of diseases caused by tau aggregation or phosphorylation, such as neurodegenerative diseases (e.g., Alzheimer disease). In accordance with yet another aspect of the present invention, there is provided a health functional food for preventing or treating tau aggregation-related diseases, containing a levosimendan compound or a pharmaceutically acceptable salt, stereoisomer or solvate thereof.
Owner:KOREA INST OF SCI & TECH
Medicaments solution
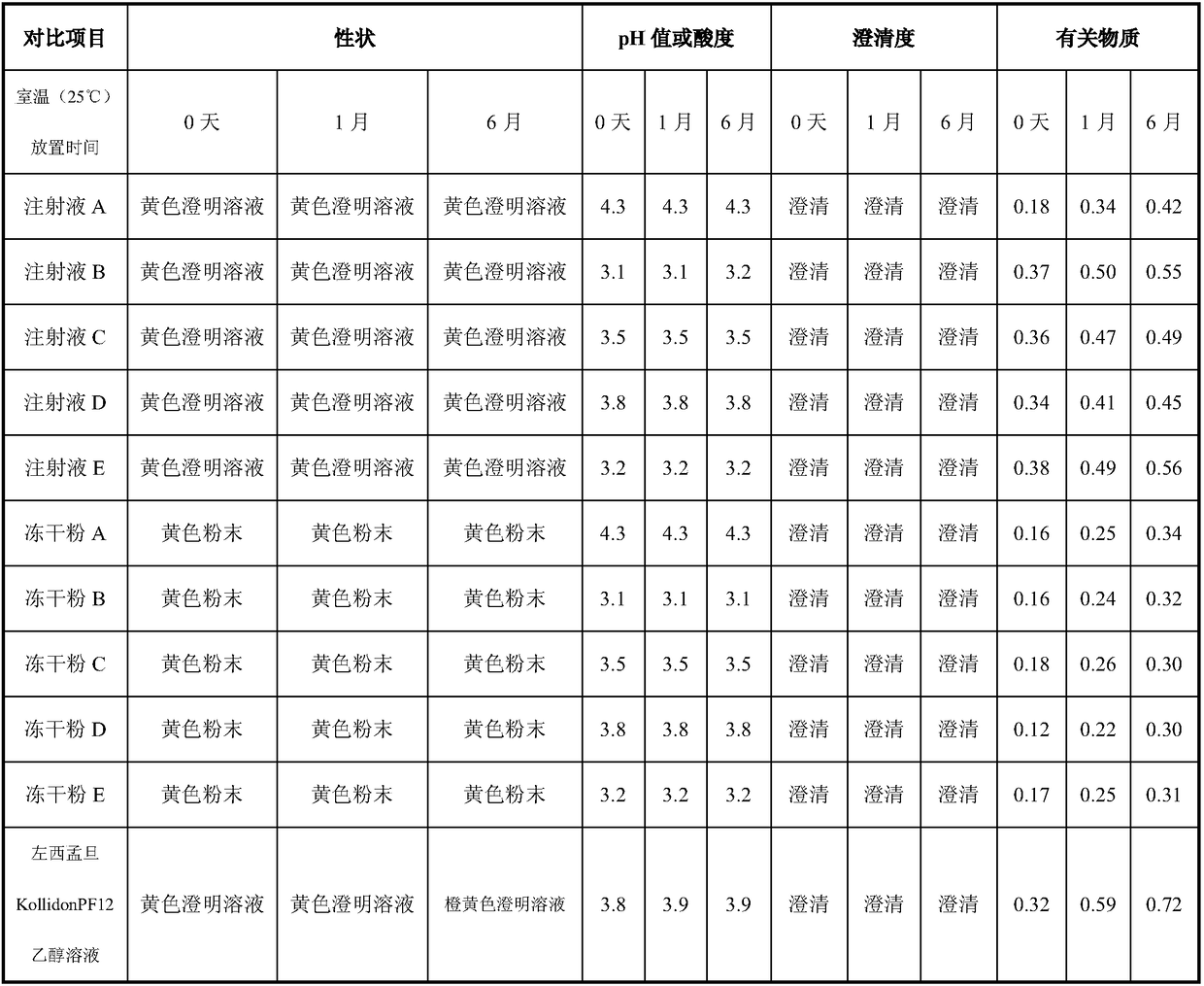
InactiveCN101305984AGood solubilization effectPharmaceutical delivery mechanismPharmaceutical non-active ingredientsDrugs solutionAlcohol
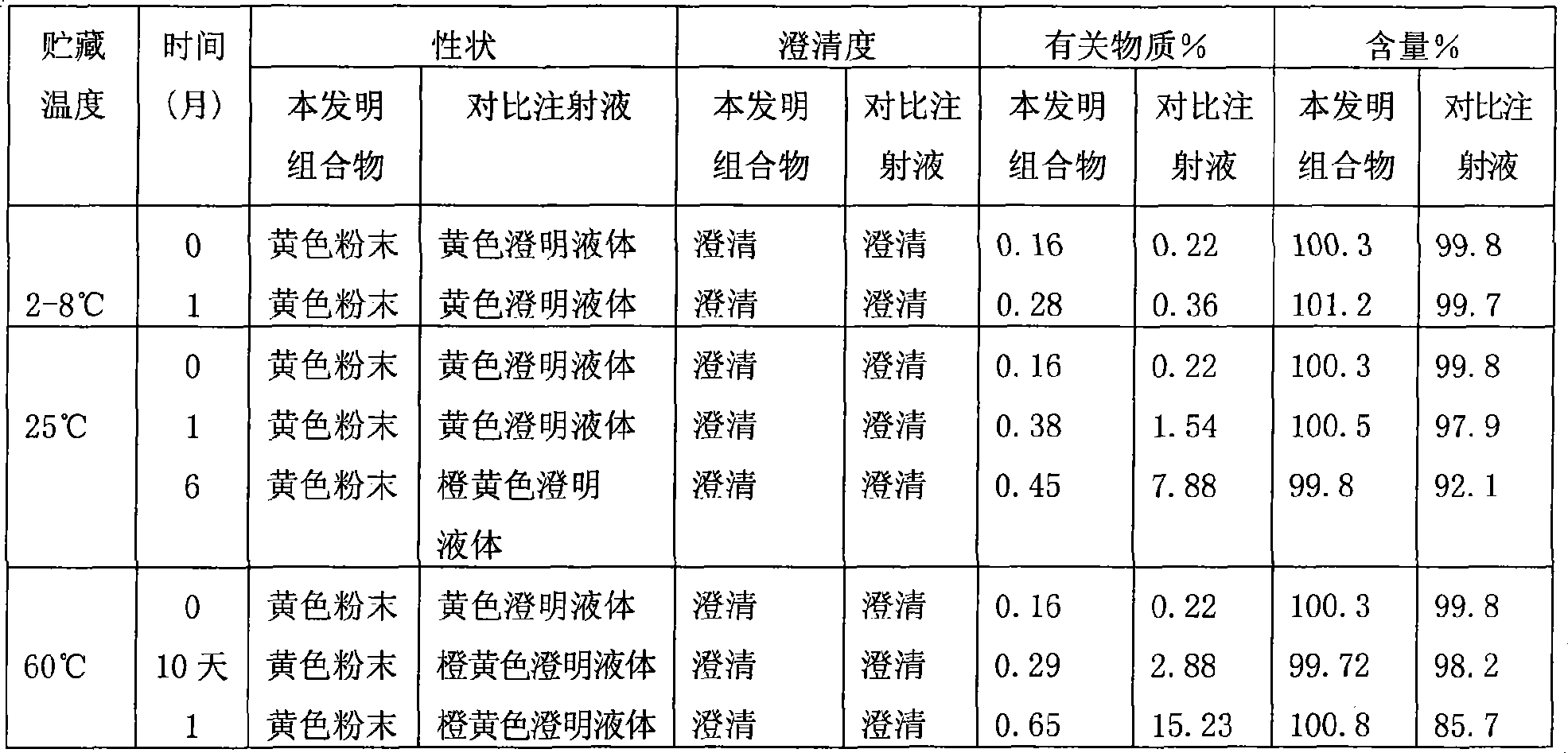
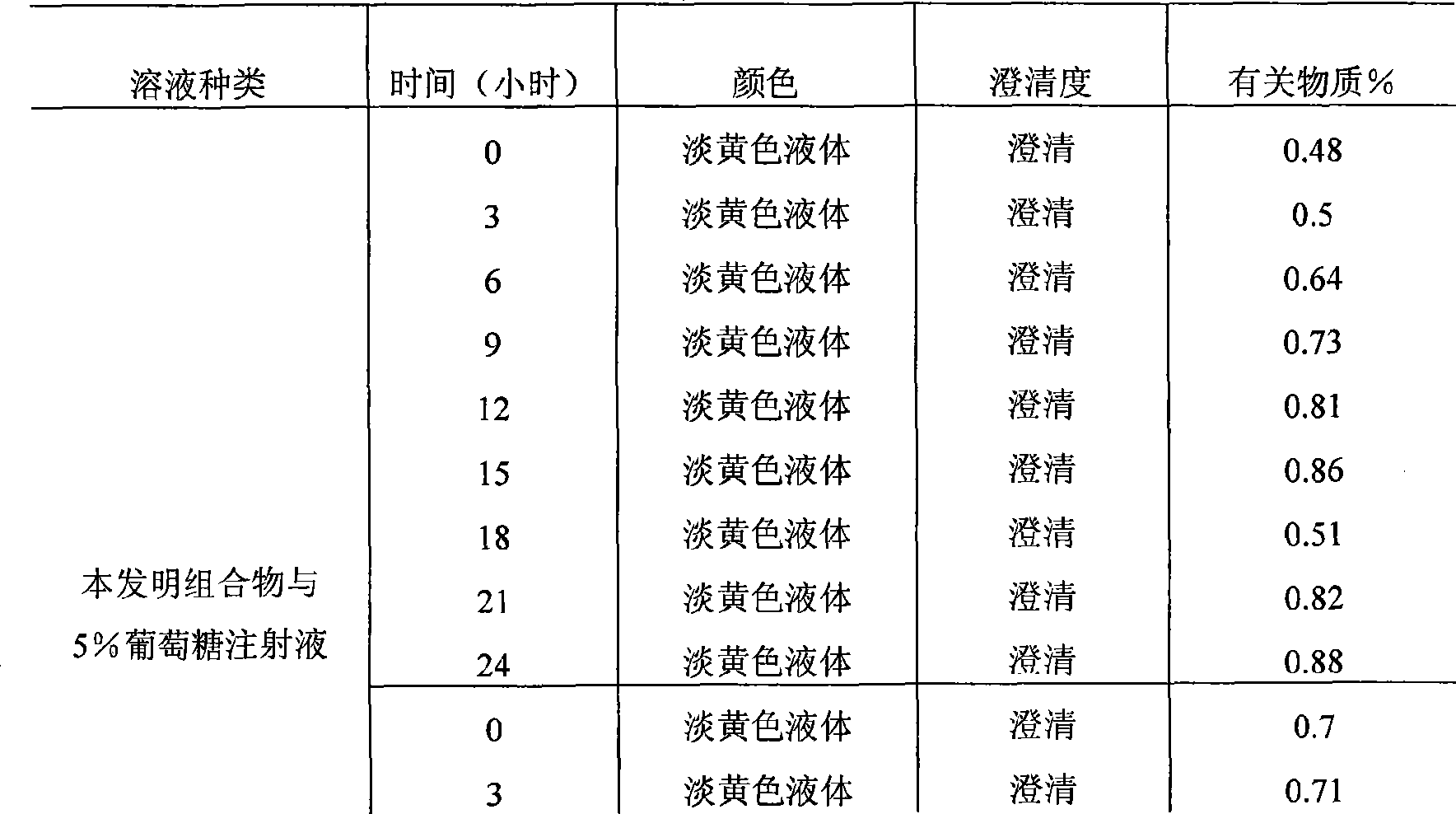
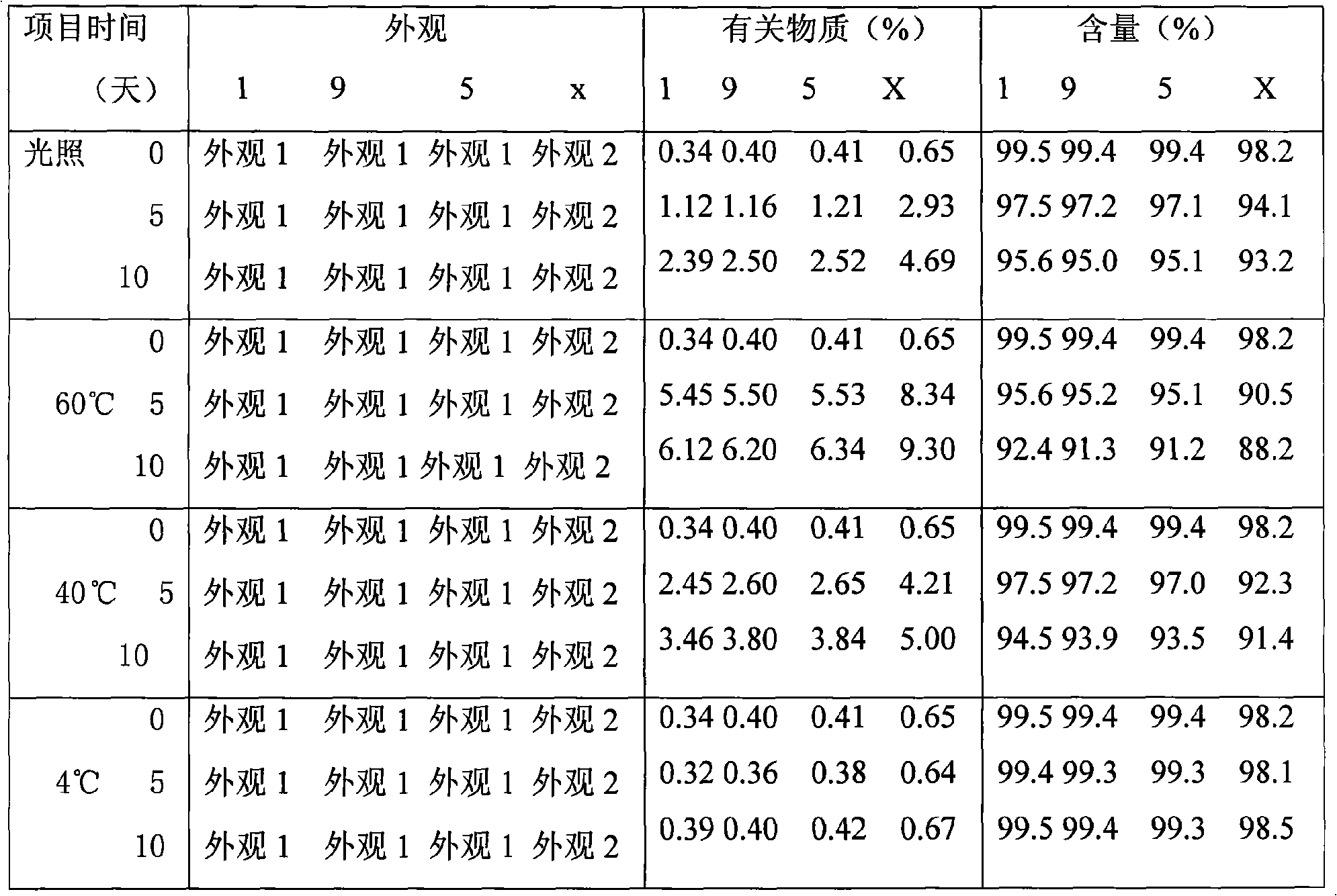
The invention discloses a pharmaceutical solution which takes levosimendan or medicine salt thereof as an active ingredient, takes absolute ethyl alcohol as a solvent, and also comprises maleic acid and solubilizer polyvidone. The pH valve of the pharmaceutical solution ranges from 3 to 4; wherein, the weight-to-volume ratio of the levosimendan or the medicine salt thereof is 0.2 to 0.3 percent, and 0.25 percent is optimized; the weight-to-volume ratio of the maleic acid is 0.001 to 0.01 percent, and the range from 0.005 to 0.009 percent is optimized; the weight-to-volume ratio of the polyvidone is 0.5 to 2 percent, and 1 percent is optimized. A stability experiment shows that pharmaceutical solution has better stability.
Owner:NANJING YOKO PHARMA
Methods for treating a mammal before, during and after cardiac arrest
Methods for treating mammals before, during and after cardiac arrest are disclosed. Pharmaceutical compositions comprising levosimendan useful for such treatment also are disclosed.
Owner:ABBOTT LAB INC
Improved formulations of levosimendan for intravenous administration as infusion or injection and of infusion concentrate
ActiveUS20180318210A1Improve abilitiesPowder deliveryGranular deliveryCongestive heart failure chfSolvent
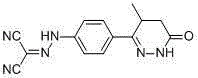
The present invention relates to improved formulations of Levosimendan for pharmaceutical use, and particularly for intravenous administration as infusion or injection and of infusion concentrates. The present invention therefore relates to pharmaceutical compositions comprising Levosimendan, in which Levosimendan ispresent in a solubilized form. The formulations have therapeuticallyand commercial useful concentrations of Levosimendan. The solutions of the invention have enhanced ability at physiological pH (pH 7.4) and are particular useful as infusion or injection solutions or infusion concentrates. The composition according to the present invention can also be spray-dried or lyophilized to obtain a dried powder which is very stable and which powder forms the original solutionafter reconstitution in wateror an aqueous solvent. Levosimendan or (-)-[[4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazi-nyl)phenyl]hydrazono]propanedinitrile is useful in the treatment of congestive heart failure.
Owner:CARINOPHARM
Levosimendan-containing injection medicine preparation and preparation method thereof
InactiveCN108261398AAvoid inconvenienceImprove securityPowder deliveryLyophilised deliveryFreeze-dryingBULK ACTIVE INGREDIENT
The invention provides a levosimendan-containing injection medicine preparation and a preparation method thereof, and belongs to the field of medicine preparations. The preparation can be injection solution or freeze-dried powder and is mainly used for administrating medicines by injection. The injection solution comprises levosimendan serving as an active component or medicinal derivative of thelevosimendan and sulfobutyl beta cyclodextrin serving as a solubilizing stabilizer or medicinal salt of the sulfobutyl beta cyclodextrin, the weight ratio of the active component to the solubilizing stabilizer is 1:10-300, preferably, the weight ratio is 1:40-100, and most preferably, the weight ratio is 1:64-100. According to the preparation, the accessory sulfobutyl beta cyclodextrin has betterwater solubility, less hemolytic action and low renal toxicity as compared with hydroxypropyl beta cyclodextrin and is firstly used as a clathration material of the levosimendan or the medicinal derivative of the levosimendan. Compared with marketed similar injection solution, the injection solution takes water as a solvent, does not contain ethyl alcohol and is better in safety, and a freeze-dried preparation is more stable and easily stored at the room temperature.
Owner:QILU PHARMA CO LTD
Methods for treating a mammal before, during and after cardiac arrest
InactiveUS20060293395A1Reduce frequencyReducing defibrillation energyBiocideNervous disorderCardiorespiratory arrestLevosimendan
Methods for treating mammals before, during and after cardiac arrest are disclosed. Pharmaceutical compositions comprising levosimendan useful for such treatment also are disclosed.
Owner:ORION CORPORATION
Method for the treatment of heart failure
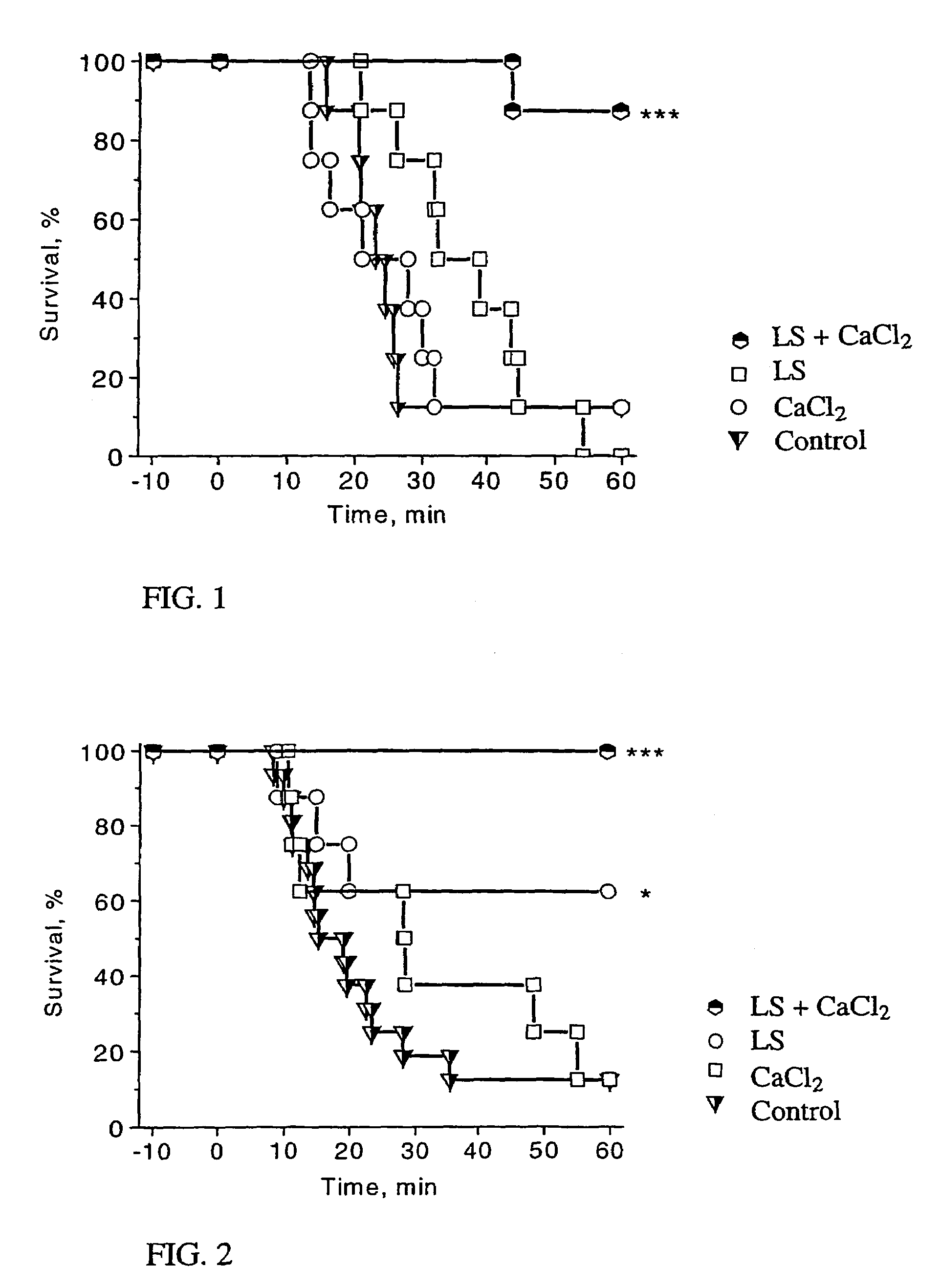
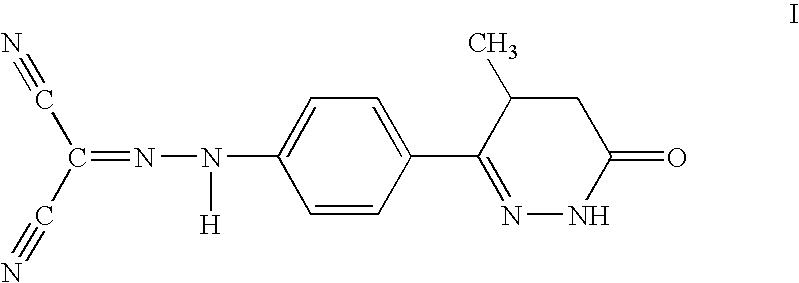
InactiveUS7279479B2Elevated level of intracellular calciumMarked synergistic effect on the survival in acute heart failureBiocideMetabolism disorderHeart failure cellCombination therapy
A combination therapy for the treatment of heart failure, particularly acute heart failure, comprises administering a combination of levosimendan or a pharmaceutically acceptable salt thereof and a calcium ion source and / or an intracellular calcium increasing agent to a patient. The combination shows a synergistic effect even at doses having no effect if administered alone.
Owner:ORION CORPORATION
Synthesis process of levosimendan
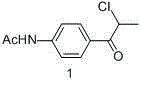
The invention provides a synthesis process of levosimendan. The process includes: taking acetanilide and 2-chloropropionylchloride as the starting materials, conducting Friedel-Crafts acylation to obtain N-[4-(2-chloropropionylchloride)phenyl]acetamide (compound 1), then synthesizing 6-(4-aminophenyl)-5-methyl-4, 5-dihydropyridazine-3(2H)-one (compound 2) by one-pot method, and further condensing the compound 2 with malononitrile to obtain levosimendan (compound 3). The process provided by the invention has the characteristics of few step, simple operation, easy separation and purification of product, high yield and low cost, and employs common chemical materials as the reagents, thus being a new process suitable for industrial production.
Owner:QINGDAO AGRI UNIV
Medicine production equipment and method capable of improving yield
InactiveCN105125409AIncrease productivityImprove yieldPowder deliveryPharmaceutical product form changeMicrocomputerMicrocontroller
The invention provides medicine production equipment capable of improving yield. The medicine production equipment comprises a stirring device, a material adding device, a vacuum drying device and a control device, wherein a plurality of flow guide pipelines are arranged between the material adding device and the stirring device. With the adoption of the equipment, a stirring process during the production of levosimendan is controlled through a singlechip microcomputer, materials are automatically added into the stirring device according to input preset stirring time, stirring speed and the desired temperature during stirring, and are stirred according to the required stirring time at the corresponding stirring speed under the preset temperature value, the temperature and the pH inside the stirring device are monitored so that the temperature and the pH are within the preset range, the highest yield is achieved, and finally the production efficiency of levosimendan is improved.
Owner:BEIJING HAIHETIAN TECH DEV
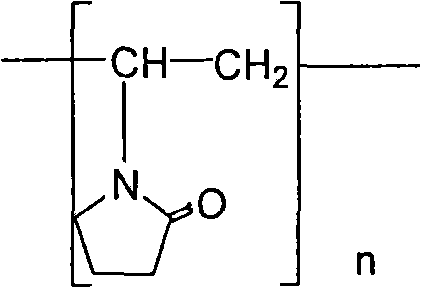
Levosimendan-containing medicine composition
InactiveCN104414963AImprove solubilityImprove stabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsAdditive ingredientBULK ACTIVE INGREDIENT
The invention relates to a medicine composition containing levosimendan or the pharmaceutically acceptable salts thereof as active ingredients. The composition comprises the following ingredients: (1) 5-50mg of levosimendan or the pharmaceutically acceptable salts per dose; (2) 10-500mg of povidone K17 per dose; (3) 0.1-100ml of medicinal solvent per dose.
Owner:迈洋致达(北京)科技有限公司
Method for administering levosimendan
InactiveUS20070032557A1Safe and effective short-term and long-term treatmentBiocideKetone active ingredientsCompound (substance)Cardiac muscle
The invention relates to intermittent administration of an inotropic agent (such as, but not limited to a levosimendan compound) or a pharmaceutically acceptable salt thereof in the treatment of cardiovascular disorders, such as, pulmonary hypertension, myocardial ischemia, acute heart failure, chronic heart failure or acute on chronic heart failure.
Owner:KIVIKKO MATTI +3
Pyridazinone derivative and synthetic method thereof
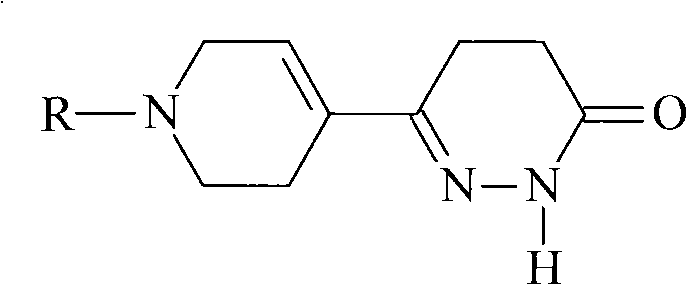
The invention provides a pyridazinone derivative and a synthetic method thereof. The method comprises the steps of: (a) synthesizing 4-carbonyl-4(4-pyridine) butyronityile; (b) synthesizing 4,5-2H-6-(4-pyridine)-3(2H)-pyridazinone; (c) preparing N-ethoxycarbonyl methyl-4-(4,5-pyridazine-3(2H)-ketone-6-yl) pyridine bromide; (d) preparing the target object 4,5-2H-6-((1-ethoxycarbonyl methyl)-1,2,5,6-tetrahydropyridine-4-yl)-3(2H)-pyridazinone. The derivative provided in the invention has an obvious positive inotropic action like levosimendan, therefore, the derivative can be used to research and develop new medicaments treating cardiac insufficiency.
Owner:HEBEI MEDICAL UNIVERSITY
Method for treating renal failure
InactiveUS20060166994A1Reduce mortalityBiocideUrinary disorderFAILURE KIDNEYCongestive heart failure chf
Levosimendan or its active metabolite (II), which have been previously suggested for the treatment of congestive heart failure, are useful in the treatment of renal failure.
Owner:ORION CORPORATION
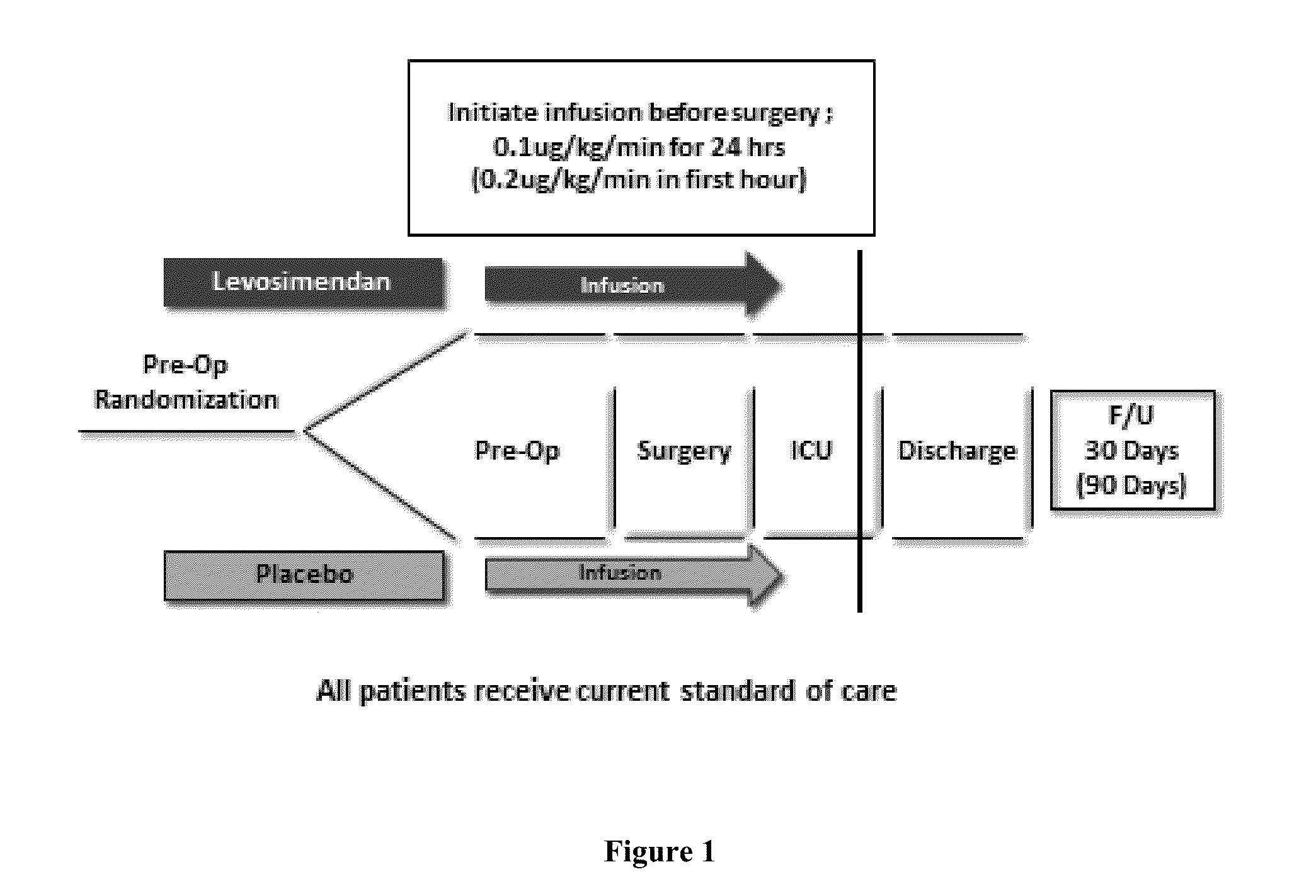
Use of Levosimendan to Treat Left Ventricular Systolic Dysfunction in Patients Undergoing Cardiac Surgery Requiring Cardiopulmonary Bypass
InactiveUS20150374689A1Prevent and reduce riskReduce doseBiocideAnimal repellantsLeft ventricular sizeMortality rate
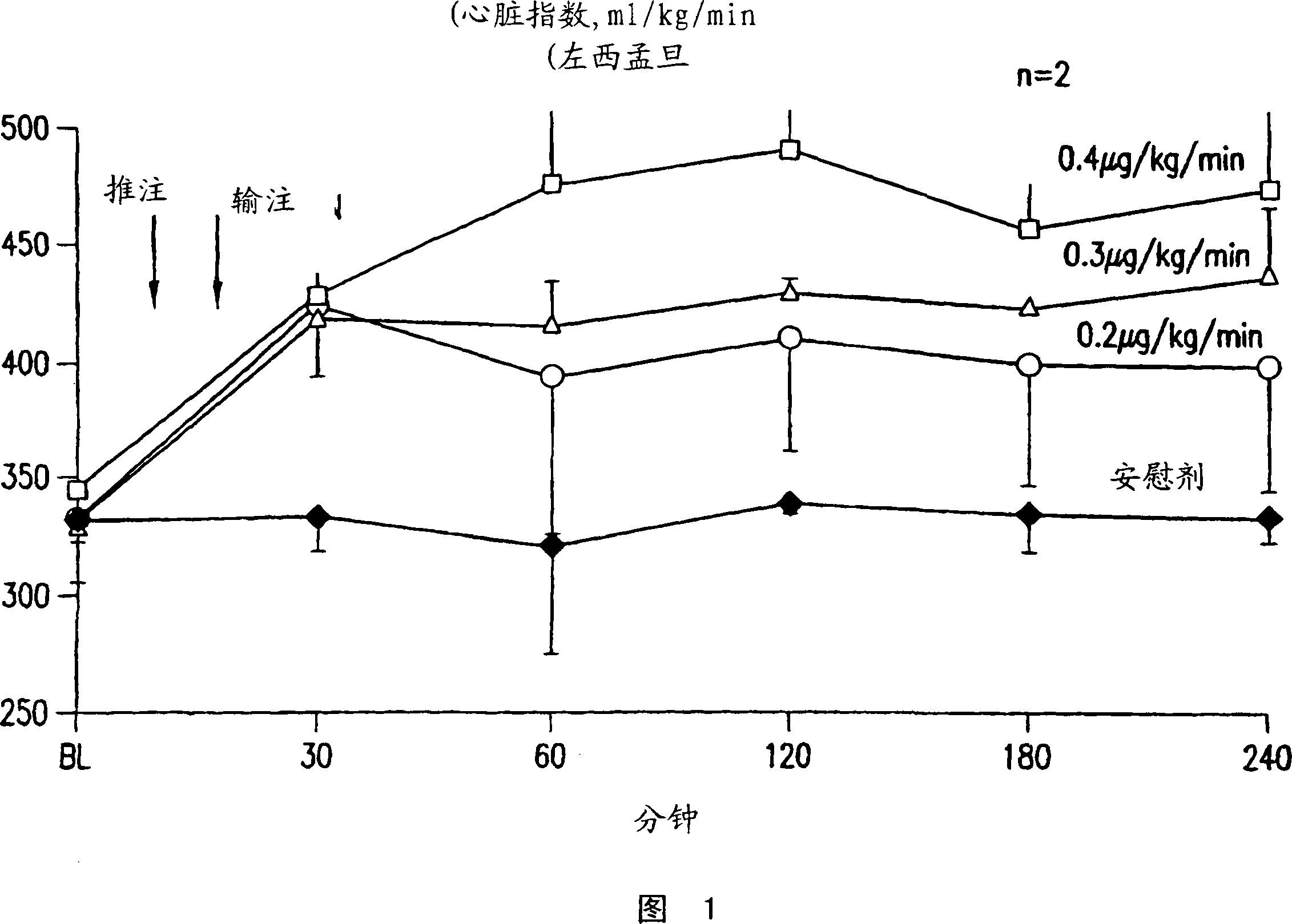
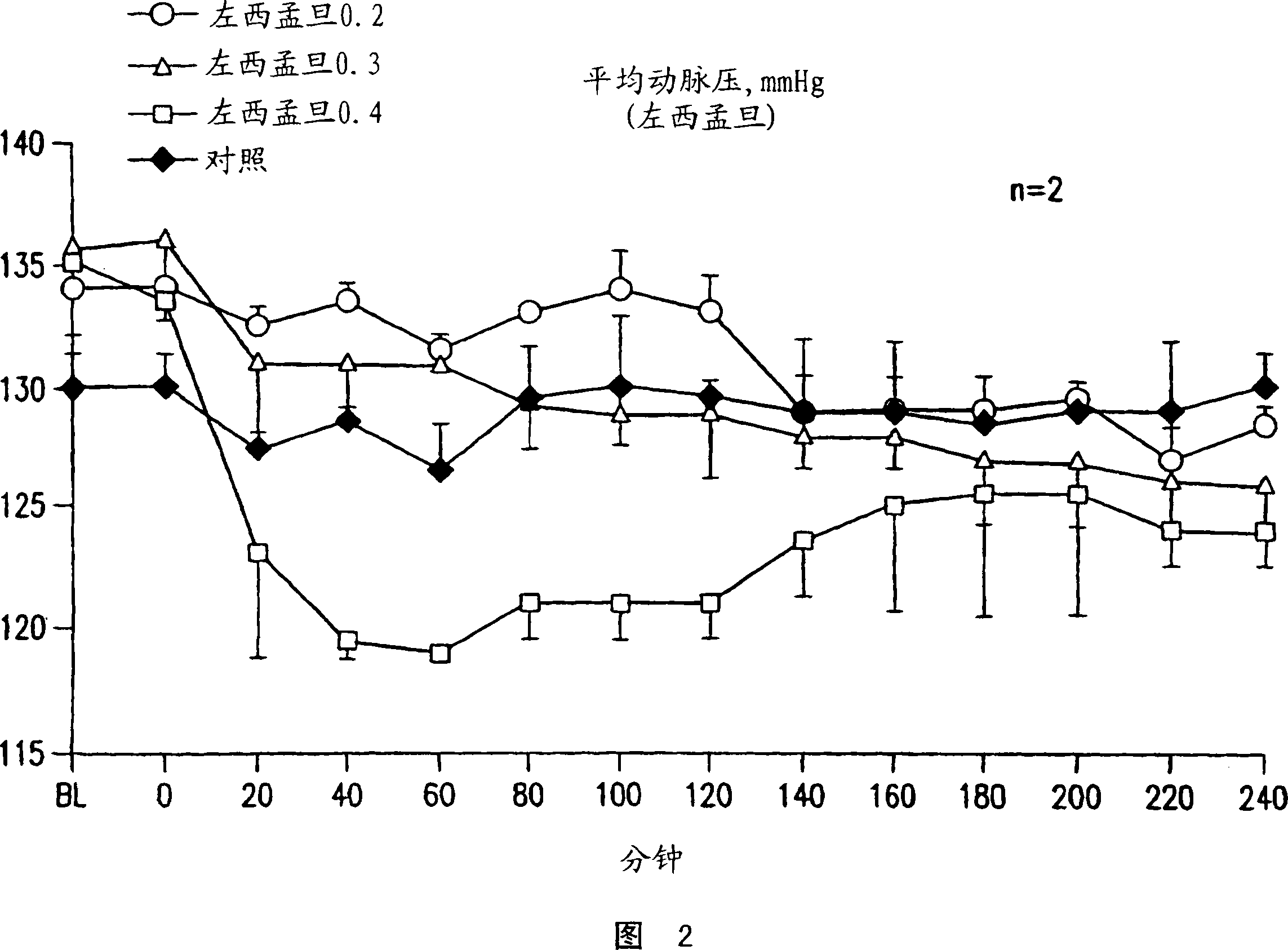
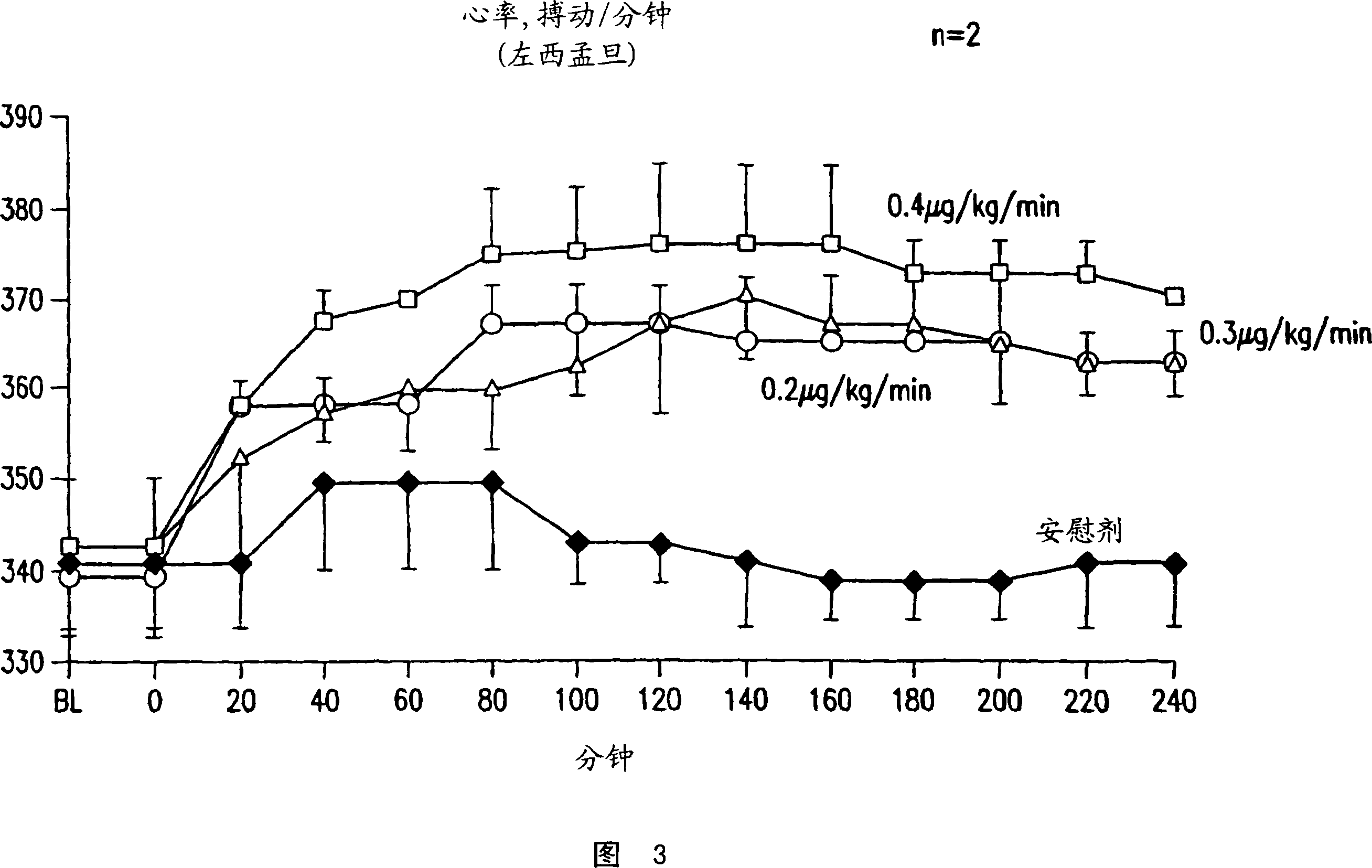
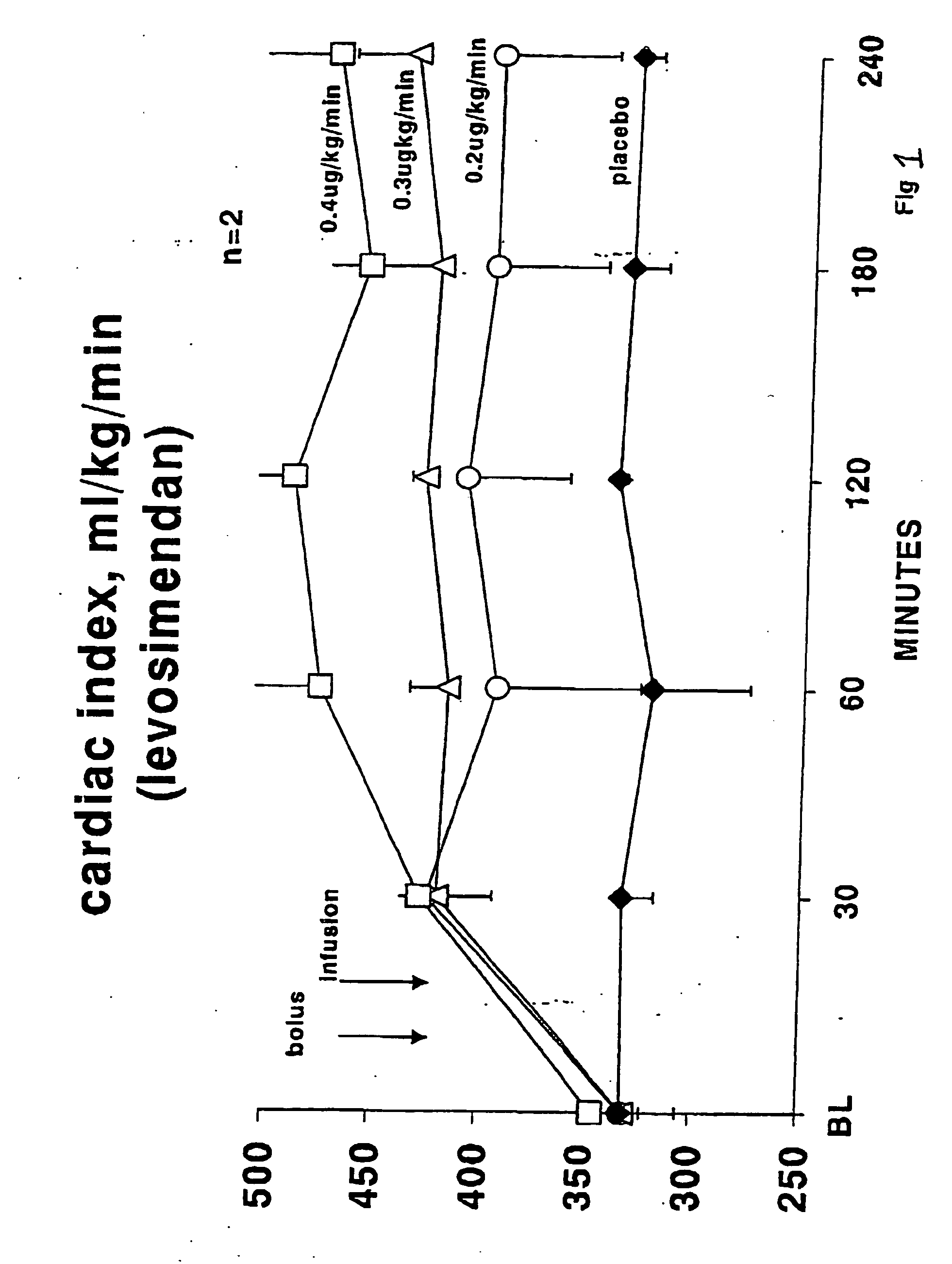
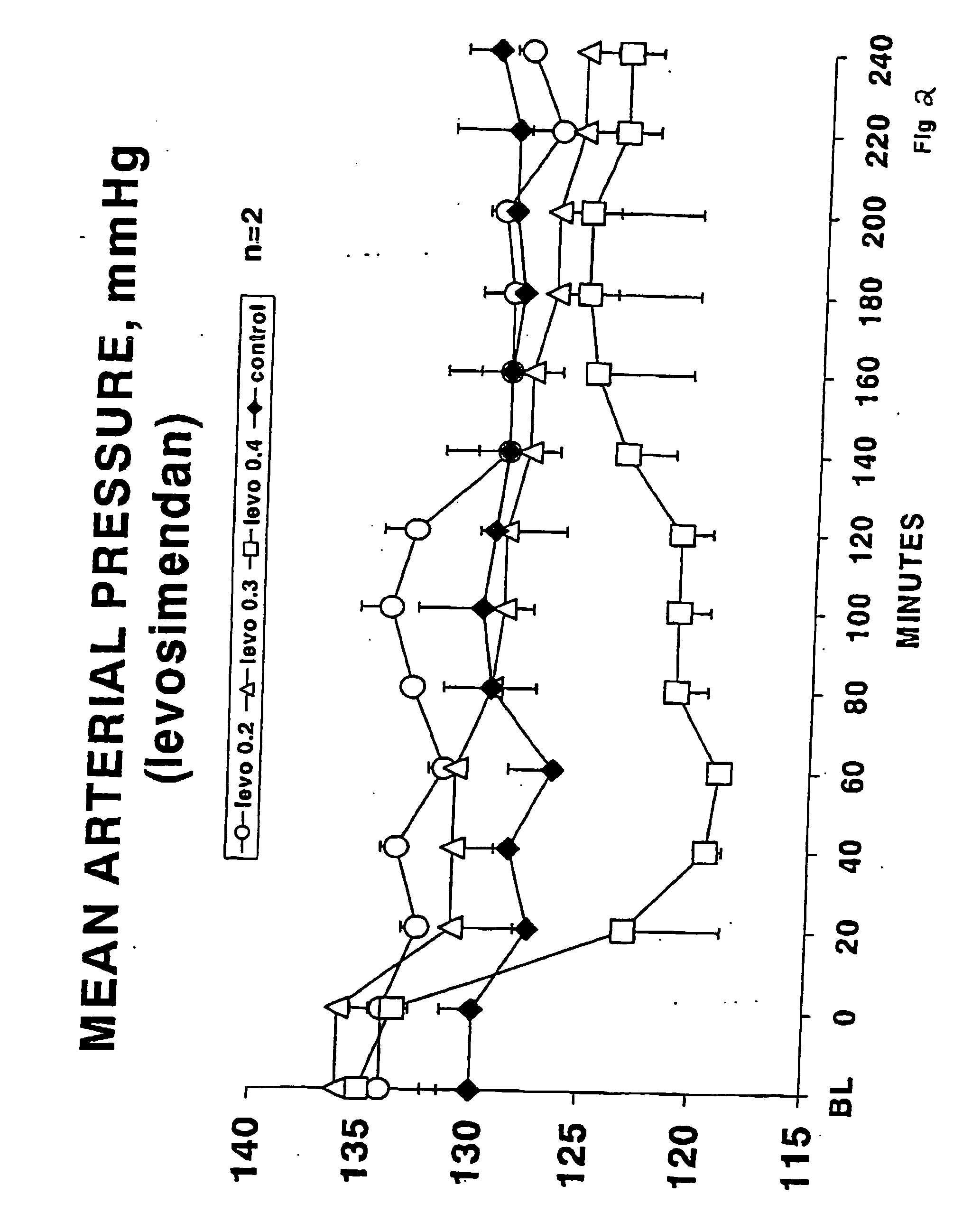
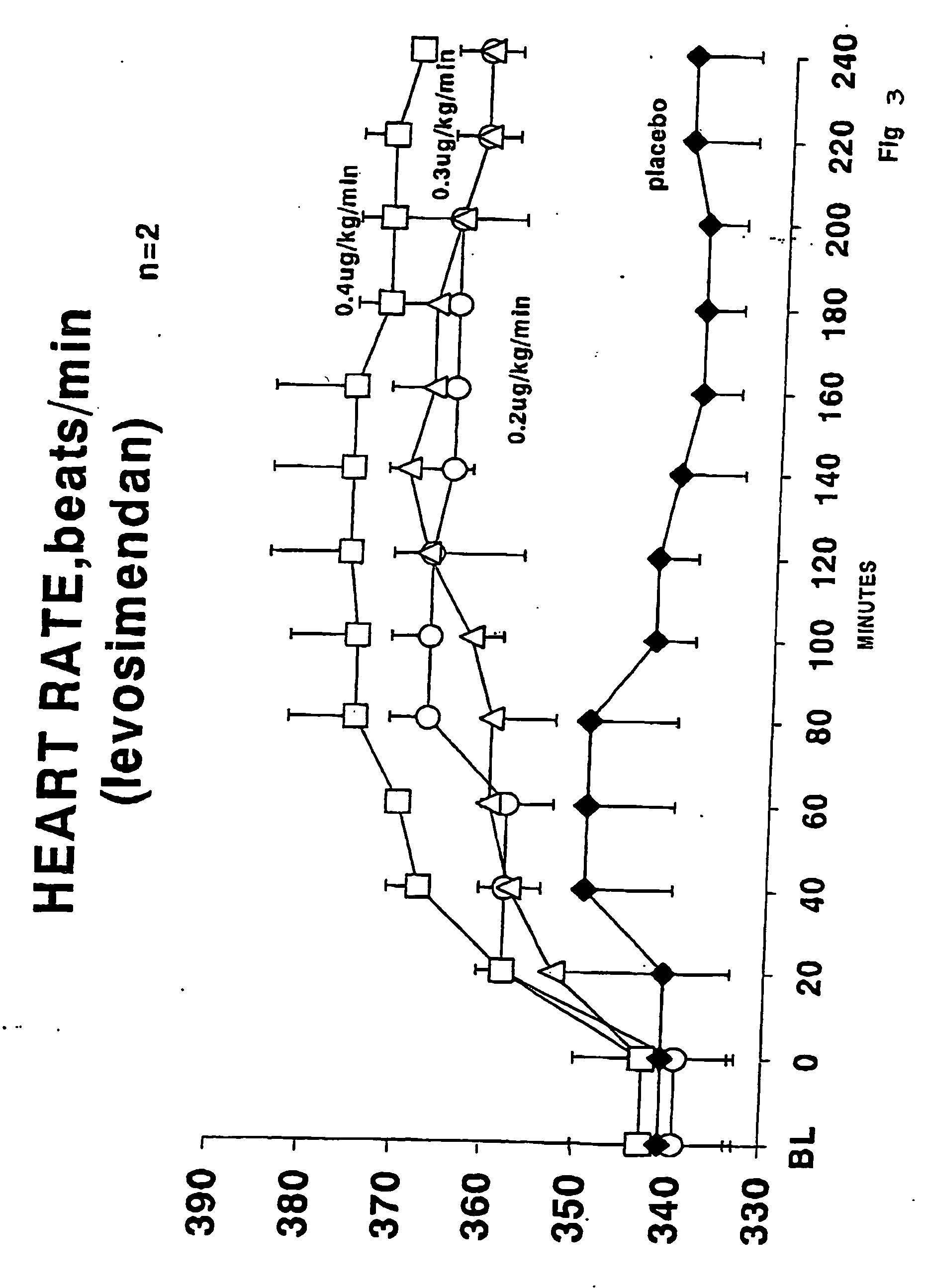
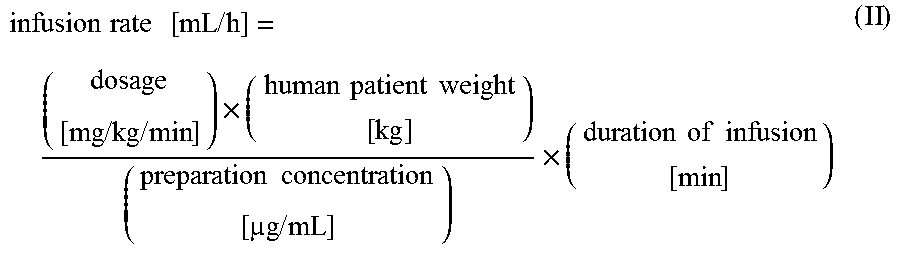
Methods of (i) reducing morbidity and / or mortality in a human patient undergoing cardiac surgery; (ii) preventing, or reducing the risk of development of, LCOS in a human patient undergoing cardiac surgery; or (iii) reducing the risk of, intensity of, or occurrence of, one or more postoperative adverse events in a human patient undergoing cardiac surgery. The methods can involve (a) a first period of administering to the patient levosimendan for about 1 hour, in which the administration of levosimendan during the first period is initiated: (i) before skin incision for the cardiac surgery, and (ii) at an infusion dose of about 0.2 μg / kg / min; and (b) a second period of administering to the patient levosimendan for about 23 hours, in which the administration of levosimendan during the second period is initiated at an infusion dose of about 0.1 μg / kg / min.
Owner:TENAX THERAPEUTICS INC
Levosimendan emulsion for intravenous injection and preparation thereof
InactiveCN101322710ALess irritatingImprove securityEmulsion deliveryOil/fats/waxes non-active ingredientsIrritationMedicine
The invention relates to a levosimendan emulsion or a levosimendan salt emulsion for intravenous injection and a preparation method thereof. The invention is a stable levosimendan oil-in-water emulsion which comprises the active components such as levosimendan or levosimenda salt, oil for injection, emulsifier and water for injection. The emulsion alleviates the irritation to blood vessels while ethanol is taken as solvent, reduces the adverse reaction, improves stability of the emulsion, ensures the safety in the clinical application of the emulsion, and enhances the compliance of patients with the emulsion.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Controlled release peroral compositions of levosimendan
InactiveCN1301163APharmaceutical non-active ingredientsPill deliveryControlled releaseCongestive heart failure chf
The invention relates to peroral pharmaceutical compositions which release levosimendan in a controlled fashion with reduced occurrence of undesired effects. Levosimendan, or (-)-[[4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl]-hydrazono]propanedinitrile, is useful in the treatment of congestive heart failure.
Owner:ORION CORPORATION
Combination treatment
InactiveUS20100249103A1Reducing hypertensive complicationReduce riskBiocideNervous disorderAngiotensin-converting enzymeCombined treatment
A combination of a levosimendan compound or a pharmaceutically acceptable salt thereof and an angiotensin II receptor antagonist or an angiotensin converting enzyme (ACE) inhibitor shows synergistic effect in the prevention of stroke. Pharmaceutical compositions and medical kits comprising as a first active ingredient a levosimendan compound or a pharmaceutically acceptable salt thereof and as a second active ingredient an angiotensin II receptor antagonist or an angiotensin converting enzyme (ACE) inhibitor are provided.
Owner:ORION CORPORATION
Inclusion preparation of levosimendan and beta cyclodextrin
InactiveCN100367964CEasy to operatePharmaceutical delivery mechanismMacromolecular non-active ingredientsBeta-CyclodextrinsLevosimendan
The present invention relates to one kind of heart failure resisting medicine, cyclodextrin or its derivative included preparation of levosimendan. The preparation has calcium-sensitive positive muscle medicine levosimendan as main component, and beta-cyclodextrin or its derivative, such as hydroxypropyl beta-cyclodextrin as including matter.
Owner:王思清
Method for the prevention of thromboembolic disorders
InactiveUS8017609B2Reduce incidenceReduce volumeBiocideNervous disorderMetaboliteThromboembolic disorder
A method for the prevention of thrombotic, embolic and / or hemorrhagic disorders, such as cerebral infarction (stroke) or myocardial infarction, by administering levosimendan or its metabolite (II) or any of their pharmaceutically acceptable salts to a mammal in need of such prevention.
Owner:ORION CORPORATION
Compound of angiotensin receptor blocker and levosimendan and use of compound
ActiveCN106214680AGood treatment effectConvenient treatmentMetabolism disorderHeterocyclic compound active ingredientsAngiotensin receptorMedicine
The invention provides a compound of an angiotensin receptor blocker and levosimendan and use of the compound. The compound is composed of the angiotensin receptor blocker and levosimendan, which can be mixed directly or be connected indirectly through a hydrogen bond, wherein a co-crystallized sodium hydrate formed by connection through hydrogen bond is more stable in property; and the pharmacokinetic properties are improved significantly; the action mechanisms of the angiotensin receptor blocker and the levosimendan are different, but the formed compound has an unexpected synergistic effect and has positive application prospects in the anti-heart failure and anti-hypertensive treatment field.
Owner:赛隆药业集团股份有限公司(长沙)医药研发中心
Stable Levosimendan pharmaceutical composition and preparation method thereof
ActiveCN101411708BEnsure safetyPharmaceutical delivery mechanismHeterocyclic compound active ingredientsOrganic solventMedicine
The invention relates to a stable levosimendan medicine composition. The medicine composition consists of two chambers, wherein a medicine chamber is filled with the levosimendan or a pharmaceutically acceptable salt thereof; and a solvent chamber is filled with a pharmaceutically usable solvent. The solvent contains a pharmaceutically acceptable organic solvent and solubilizing agent, and also can be added with a pharmaceutically acceptable acid. The composition can be used for intravenous injection, or taken orally. The invention also relates to a method for preparing the composition.
Owner:QILU PHARMA CO LTD
Formulations of levosimendan for intravenous administration as infusion or injection and of infusion concentrate
The present invention relates to improved formulations of Levosimendan for pharmaceutical use, and particularly for intravenous administration as infusion or injection and of infusion concentrates. The present invention therefore relates to pharmaceutical compositions comprising Levosimendan, in which Levosimendan is present in a solubilized form. The formulations have therapeutically and commercial useful concentrations of Levosimendan. The solutions of the invention have enhanced ability at physiological pH (pH 7.4) and are particular useful as infusion or injection solutions or infusion concentrates. The composition according to the present invention can also be spray-dried or lyophilized to obtain a dried powder which is very stable and which powder forms the original solution after reconstitution in water or an aqueous solvent. Levosimendan or (−)-[[4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazi-nyl)phenyl]hydrazono]propanedinitrile is useful in the treatment of congestive heart failure.
Owner:CARINOPHARM
Levosimendan sodium pharmaceutical composition for acute decompensated heart failure symptoms and preparation method
ActiveCN111514147BImprove solubilitySimple production processPowder deliveryInorganic non-active ingredientsOfficinalMedicine
The invention discloses a pharmaceutical composition of levosimendan sodium for acute decompensated heart failure symptoms and a preparation method. The preparation of the invention can be an injection or a dry powder, and the dry powder is reconstituted to form an original solution. Mainly used for administration by injection route, the pharmaceutical composition of the present invention comprises: levosimendan sodium as an active ingredient, and excipients. The invention solves the problems of insolubility of levosimendan free base in water and instability of impurities degraded by hydrolysis during marketing and storage, so that levosimendan sodium can be prepared into a stable injection with water as a solvent.
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Combination Treatment for Enhancing Diuresis
A combination therapy for promoting diuresis in a patient comprises administering levosimendan or its active metabolite or any of their pharmaceutically acceptable salts in conjunction with a diuretic to a patient. The combination therapy provides diuretic effect also in patients who are refractory to standard diuretic therapy.
Owner:ORION CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com