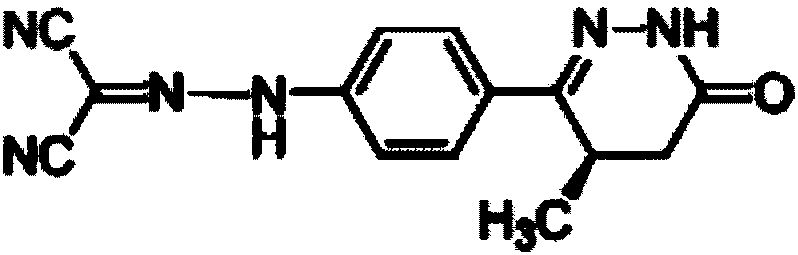
Levosimendan-containing injection medicine preparation and preparation method thereof
A technology of levosimendan and injection, applied in the field of pharmaceutical preparations, can solve the problems of carcinogenicity, toxicity and side effects, and achieve the effects of reducing toxicity and side effects, less hemolysis and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Add to 50ml
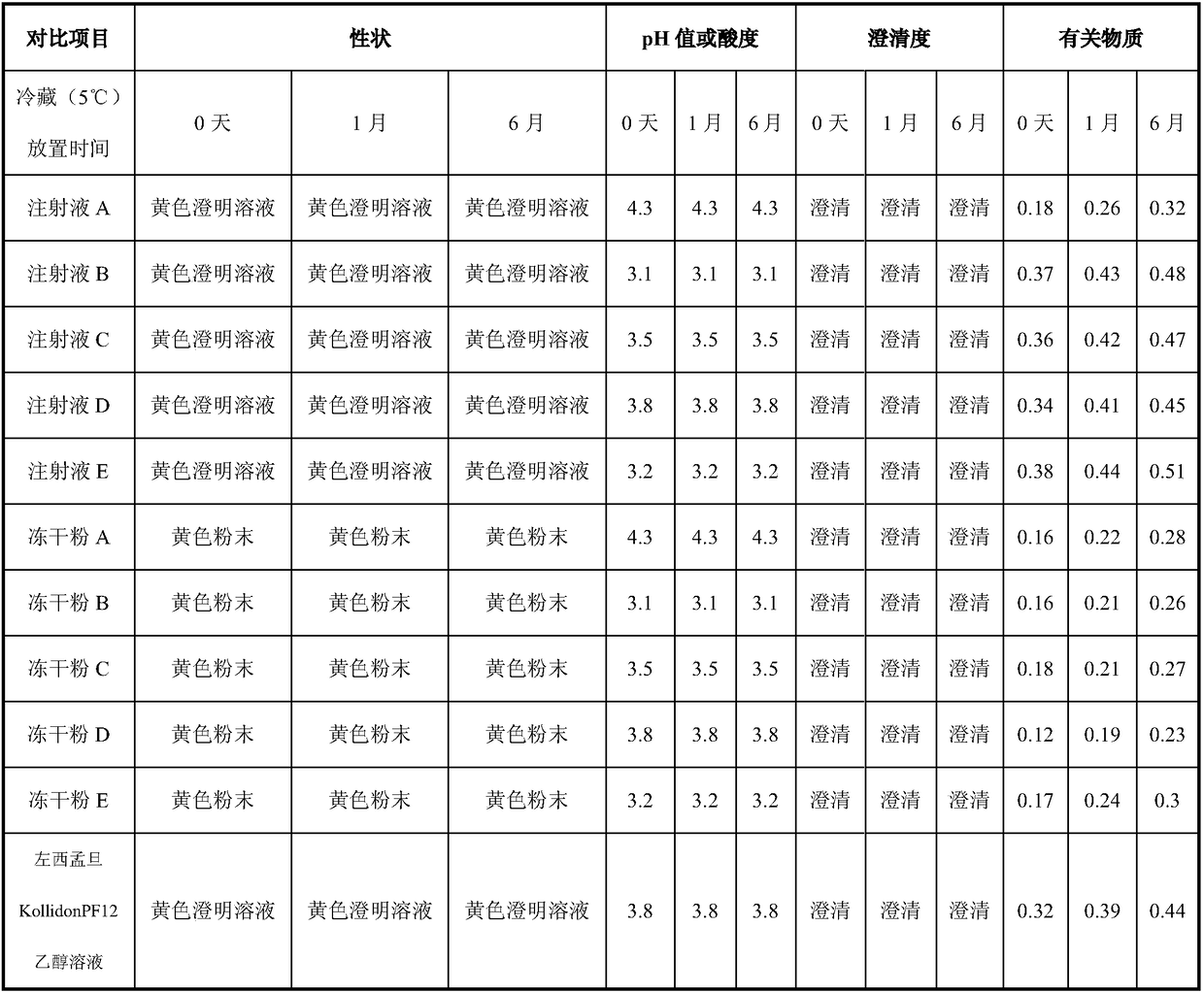
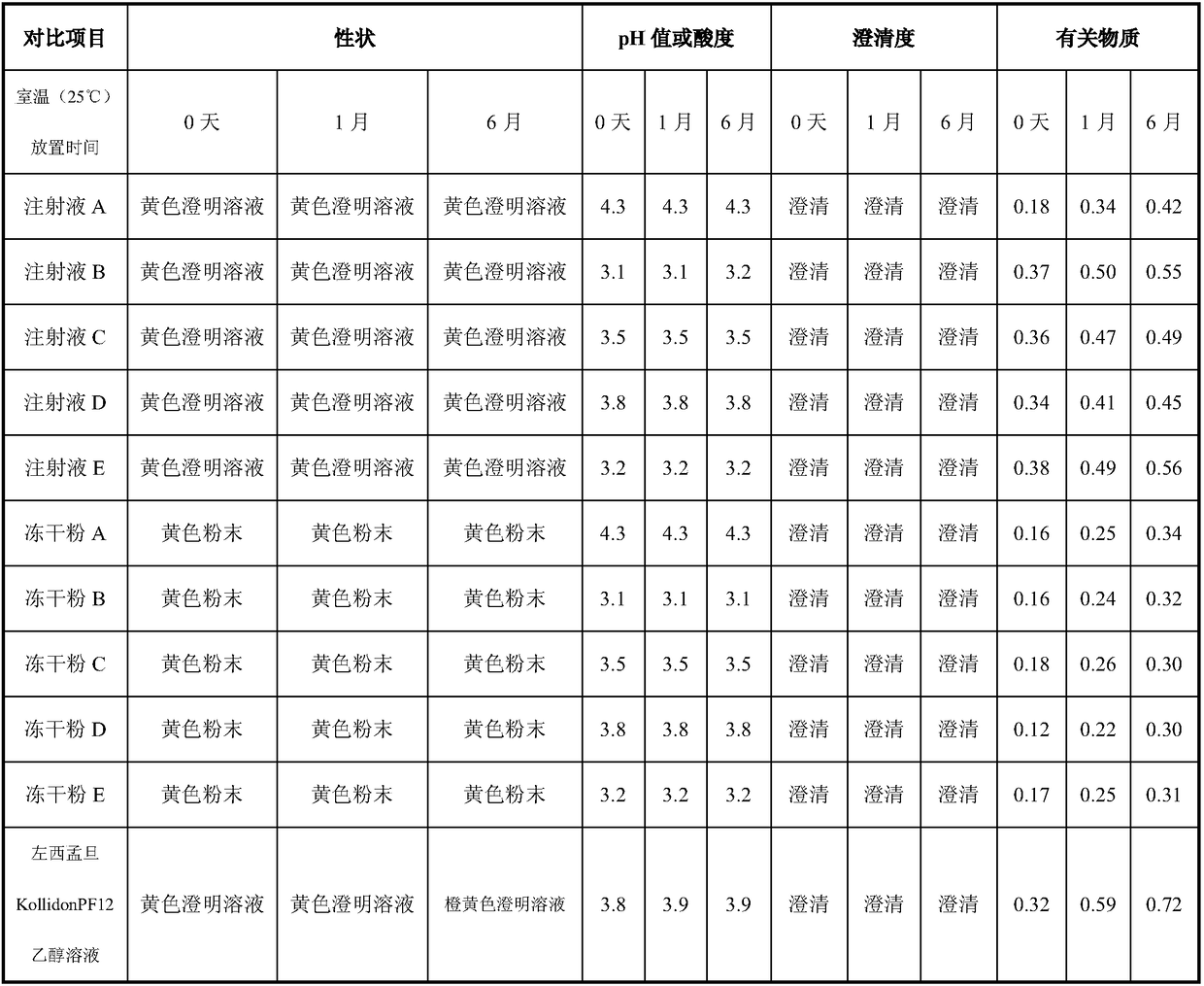
[0038] Take 8.0 g of sulfobutylbeta cyclodextrin, dissolve it in 40 ml of water for injection, add 125 mg of levosimendan, stir until the drug is completely dissolved, add water for injection to 50 ml, and obtain 2.5 mg / ml of levosimendan The quibeta cyclodextrin solution was sterilized by filtration through a 0.22 μm filter membrane, filled with 5ml / bottle, stoppered, and capped to obtain the injection A;
[0039] Or take 8.0g of sulfobutylbeta cyclodextrin, dissolve it in 40ml of water for injection, add 125mg of levosimendan, stir until the drug is completely dissolved, add water for injection to 50ml, and get 2.5mg / ml of levosimendan The butyl beta-cyclodextrin solution is sterilized by filtration through a 0.22 μm filter membrane, filled and semi-stoppered according to 5ml / bottle, freeze-dried, plugged, and capped. The yellow levosimendan freeze-dried powder A was obtained.
Embodiment 2
Add to 50ml
[0042] Take 2.5g of mannitol, 100mg of anhydrous citric acid, and 5.0g of sulfobutylbeta cyclodextrin, dissolve them in 40ml of water for injection, add 125mg of levosimendan, ultrasonicate until the drug is completely dissolved, add water for injection to 50ml, Obtain 2.5 mg / ml levosimendan sulfobutyl beta-cyclodextrin solution, filter and sterilize through a 0.22 μm filter membrane, fill with 5 ml / bottle, stopper, press the cap, and then sterilize at 121°C for 15 minutes, Get injection B;
[0043]Or take 2.5g of mannitol, 100mg of anhydrous citric acid, and 5.0g of sulfobutylbeta-cyclodextrin, dissolve in 40ml of water for injection, add 125mg of levosimendan, ultrasonically until the drug is completely dissolved, add water for injection to 50ml , to obtain 2.5 mg / ml levosimendan-sulphur-butyl-beta-cyclodextrin solution, which was sterilized by filtration through a 0.22 μm filter membrane, filled and semi-stoppered according to 5ml / bottle, freeze-dried, p...
Embodiment 3
Add to 50ml
[0046] Take 2.5g of mannitol, 100mg of anhydrous citric acid, and 12.5g of sulfobutylbeta cyclodextrin, dissolve in 40ml of water for injection, add 125mg of levosimendan, stir until the drug is completely dissolved, add water for injection to 50ml, Obtain 2.5 mg / ml levosimendan sulfobutyl beta-cyclodextrin solution, filter and sterilize through a 0.22 μm filter membrane, fill with 5 ml / bottle, stopper, press the cap, and then sterilize at 121°C for 15 minutes, Obtain injection C;
[0047] Or take 2.5g of mannitol, 100mg of anhydrous citric acid, and 12.5g of sulfobutylbeta cyclodextrin, dissolve in 40ml of water for injection, add 125mg of levosimendan, stir until the drug is completely dissolved, add water for injection to 50ml , to obtain 2.5 mg / ml levosimendan-sulphur-butyl-beta-cyclodextrin solution, which was sterilized by filtration through a 0.22 μm filter membrane, filled and semi-stoppered according to 5ml / bottle, freeze-dried, plugged, and capped...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com