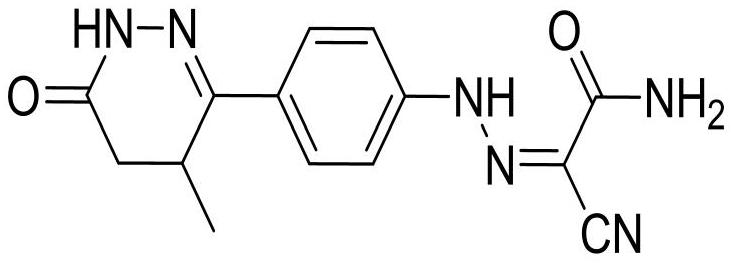
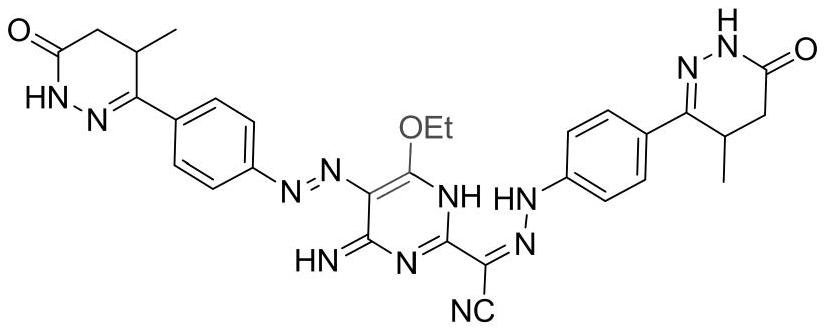
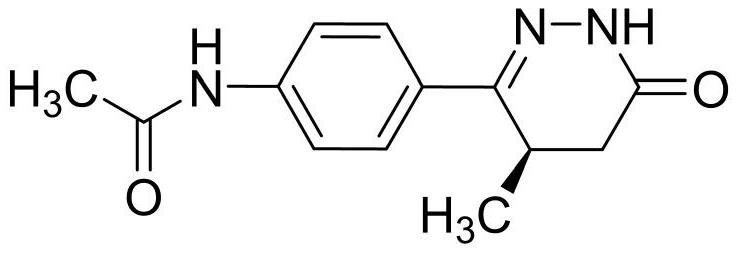
Levosimendan sodium pharmaceutical composition for acute decompensated heart failure symptoms and preparation method
A composition and compound technology, applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of tumor formation, tumor-like reaction, insoluble particles and particles increase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The preparation of embodiment 1 levosimendan sodium
[0077] Add 2.9g of sodium hydroxide and 120ml of purified water into a 250ml flask, stir to dissolve. Add 20 g of levosimendan and stir to dissolve levosimendan. Stir and crystallize at 5-10°C for 2 hours. Filter and wash the filter cake with 30ml ethanol. Dry under reduced pressure at 60°C to obtain 17.8 g of thermally stable form A levosimendan sodium, with a weight yield of 89.0%.
[0078] The 2θ angles of diffraction peaks of the thermally stable crystalline form of levosimendan sodium: 12.1°±0.2°, 15.1°±0.2°, 18.3°±0.2°, 19.4°±0.2°, 22.9°±0.2°, 24.3° ±0.2°, 27.4°±0.2°, 29.4°±0.2°, 32.3°±0.2°, 33.9°±0.2°.
Embodiment 2
[0081] (1) Take 270mg of levosimendan sodium and dissolve it in water for injection with stirring, then add 2.0g of mannitol and stir to dissolve, then add water for injection, adjust the pH to pH 7.8-8.2 with sodium hydroxide or hydrochloric acid to form solution A;
[0082] (2) Use a 0.22 μm polyethersulfone material filter membrane to filter solution A to sterilize, divide into bottles (bottles are vials, each vial contains 5ml of solution A), and use conventional freeze-drying production process to dry after bottling to control the moisture content in the bottle. 2% or less;
[0083] (3) Each vial is filled with nitrogen and sealed with a gland to obtain a sterile freeze-dried powder.
[0084] In this embodiment, the total amount of water for injection is 100ml.
[0085] The sterile freeze-dried powder of this example was tested for appearance, reconstitution time, solution particles, pH value and OR-1420 impurities, and the results are shown in Table 1 below.
Embodiment 3
[0087] (1) Stir and dissolve 270mg of levosimendan sodium in water for injection, then add 3.0g of lactose and 100mg of anhydrous sodium acetate, stir and dissolve, add injection water, adjust the pH to pH 7.8-8.2 with acetic acid, and form solution B;
[0088] (2) Use a 0.22 μm polyethersulfone material filter membrane to filter solution B to sterilize, divide into bottles (bottles are vials, each vial contains 5ml of solution B), and use conventional freeze-drying production process to dry after bottling to control the moisture content in the bottle. 2% or less;
[0089] (3) Each vial is filled with nitrogen and sealed with a gland to obtain a sterile freeze-dried powder.
[0090] In this embodiment, the total amount of water for injection is 100ml.
[0091] The sterile freeze-dried powder of this example was tested for appearance, reconstitution time, solution particles, pH value and OR-1420 impurities, and the results are shown in Table 1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com