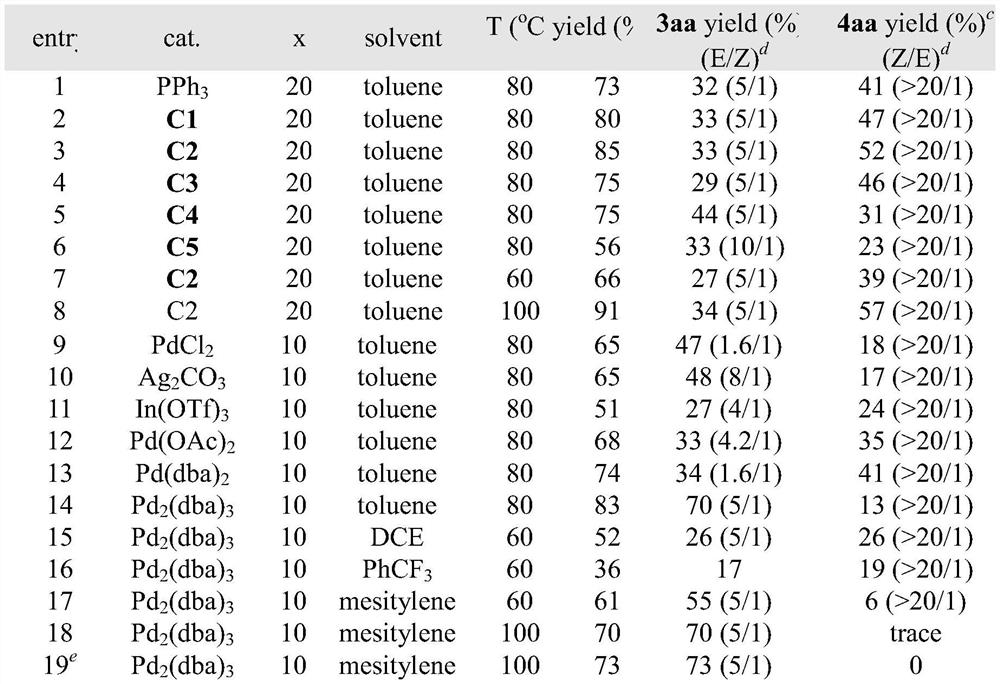
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Diazoacetic ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Catalyst for cyclopropanizing reaction of olefine and its preparing process
A carried catalyst used for cyclopropanizing 2,5-dimethyl-2,4-hexadiene contains the active component (20-90%) chosen from Fe, Ca, Ni, Ca, Zn, Cr, Mo or Ti, the assistant (0.1-5%) chosen from L, Na or K, and the carrier chosen from alumina silica gel and activated carbon. It is prepared through premodifying carrier, and immersing while adding active component and the organic compound containing hydroxy or carboxyl group and oxygen. Its advantages are low dosage, high output rate, little coking and easy separation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Adsorbent, preparation method thereof, and adsorption device for blood perfusion
ActiveCN105126784AReduce manufacturing costImprove efficiencyOther blood circulation devicesOther chemical processesDiseasePhosphate
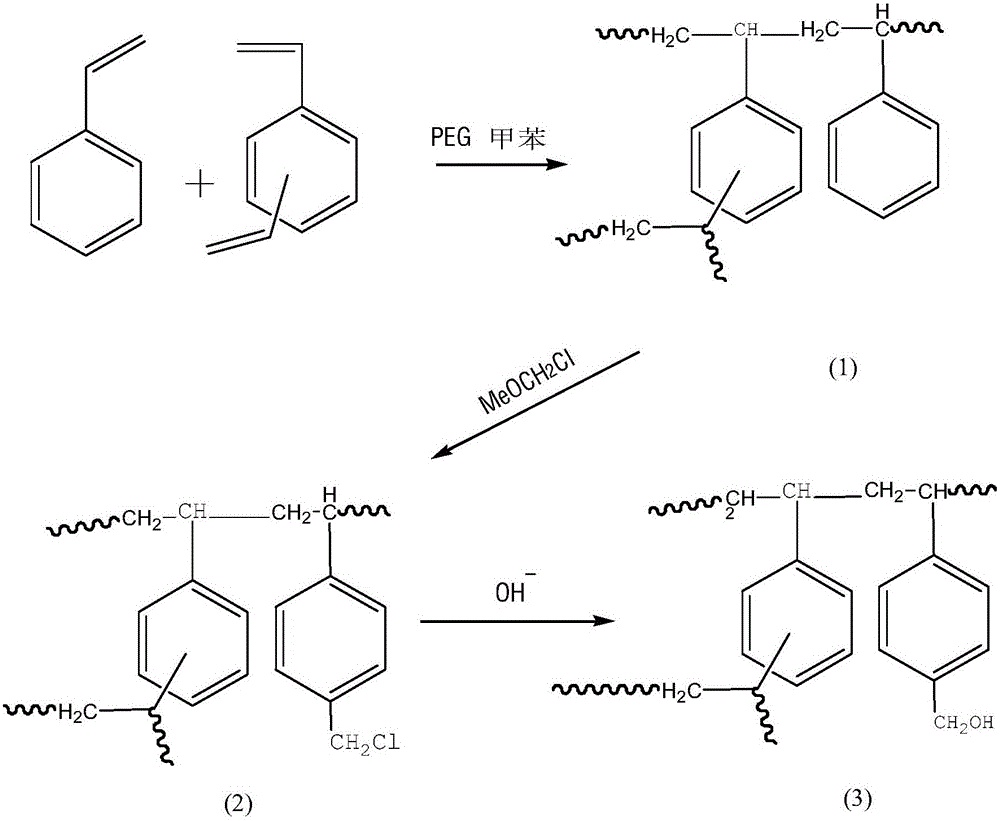
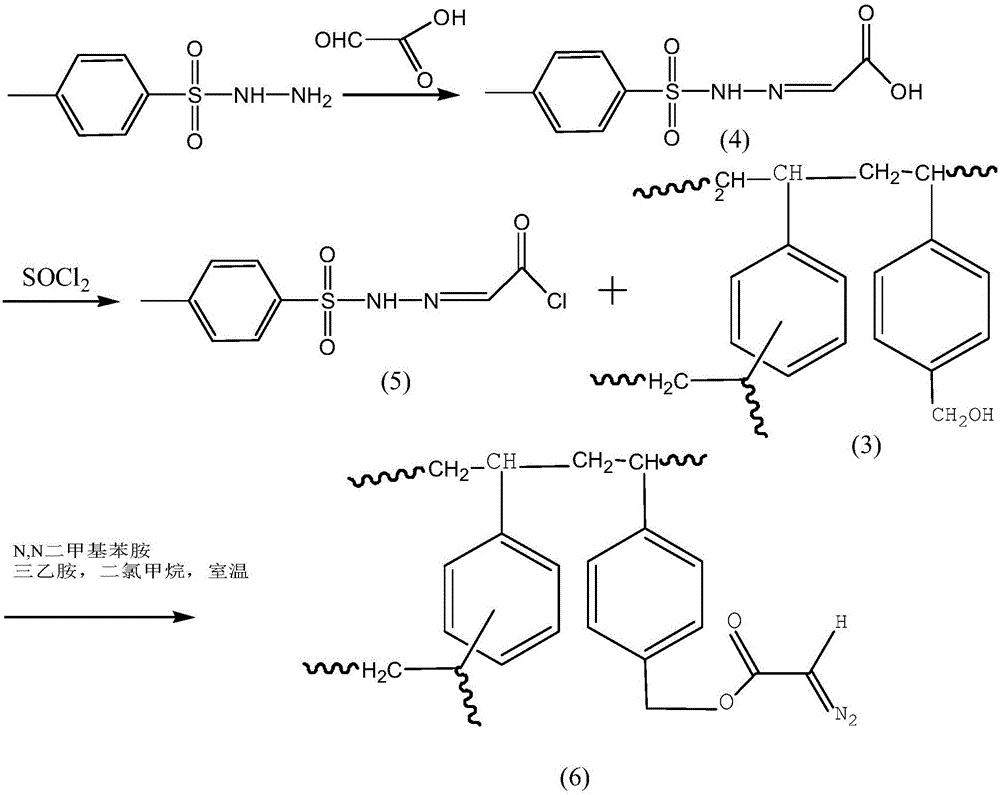
The invention provides an adsorbent, a preparation method thereof, and an adsorption device for blood perfusion. The adsorbent is prepared by bonding phosphate group in DNA molecules to diazo acetic acid esterified polystyrene-divinyl benzene microspheres through covalent bonds. The preparation method comprises the following steps: preparing polystyrene-divinyl benzene microspheres, carrying out chloromethylation, carrying out alcoholization, and performing diazo acetic acid esterification to obtain the adsorbent. The adsorption device comprises the provided adsorbent. The adsorbent has a specific absorbing performance on anti-ds-DNA antibody. Through the covalent bond, the DNA can be more firmly loaded on the solid carriers, the efficiency is higher, and the DNA will not fall off from the carrier during the process of sterilization, storage, and using. The adsorbent can be applied to SLE and can also applied to autoimmune diseases such as myasthenia gravis, Guillain-Barre syndrome, rheumatoid arthritis, hypersensitive organ transplant patients, and the like.
Owner:JAFRON BIOMEDICAL
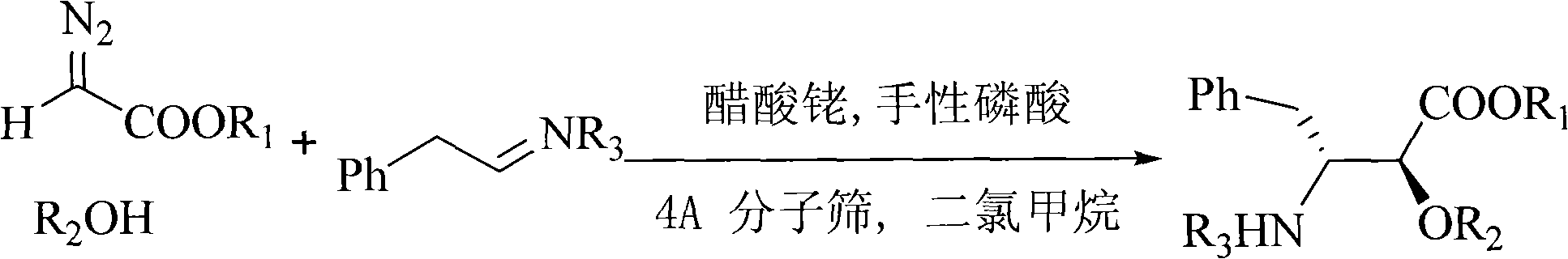
Method for synthesizing optically active alpha-hydroxyl-beta-phenmethyl-beta-amino acid derivative
InactiveCN101538226AHigh atomic economyHigh selectivityCarbamic acid derivatives preparationOrganic compound preparationSolventCoordination complex
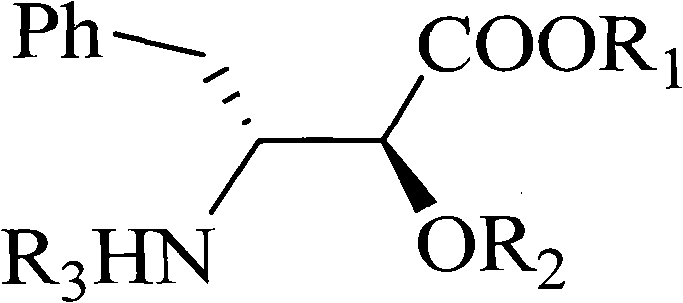
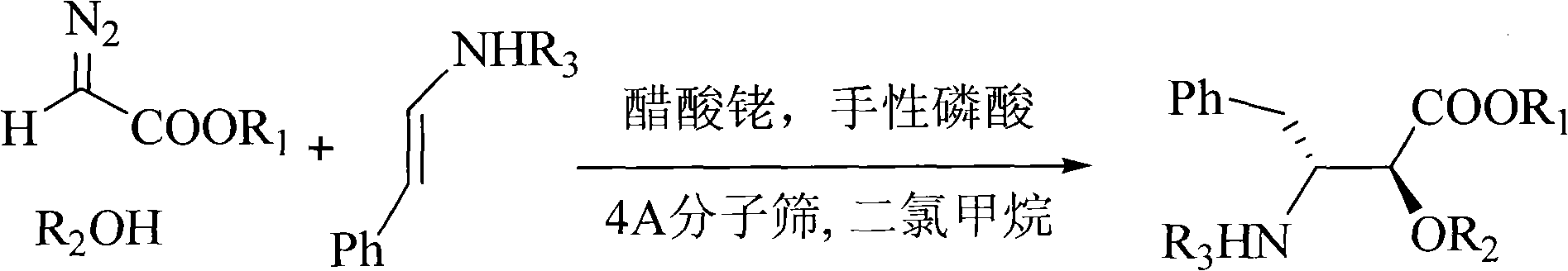
The invention relates to a method for synthesizing optically active alpha-hydroxyl-beta-phenmethyl-beta-amino acid derivative, relating to a process for synthesizing the alpha-hydroxyl-beta-phenmethyl-beta-amino acid derivative. The method adopts ethyl diazoacetate, alcohol and styrylamine or benzyl imine as raw materials, chiral phosphoric acid and rhodium carboxylic acid or chiral phosphoric acid and copper (I) metal complex as catalyst, organic solvent as solvent, and 4molecular sieve, or 3 molecular sieve, or 5 molecular sieve as activating agent; and after one step of reaction, the dissolvent is removed to obtain a crude product. The crude product is processed by the operation of column chromatography by a solution in which the volume ratio of ethyl acetate to sherwood oil ranges from 1:50 to 1:20 to obtain the optically active alpha-hydroxyl-beta-phenmethyl-beta-amino acid derivative. The mol ratio of the components is as follows: diazocompound to alcohol to styrylamine or benzyl imine to chiral phosphoric acid to rhodium carboxylic acid or copper(I) metal complex is equal to 1.1:1:1.2:0.02:0.02; and the proportion of the activating agent is 2 to 5g per mmol diazocompound. The method has the advantages of high atom economy, selectivity and yield, and easy and safe operation.
Owner:EAST CHINA NORMAL UNIV
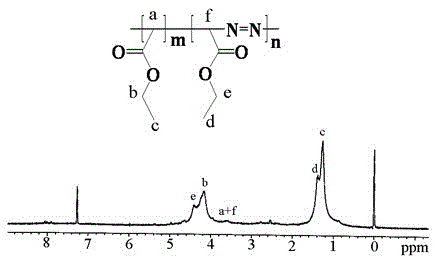
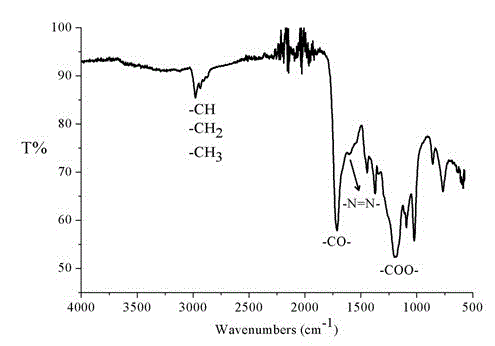
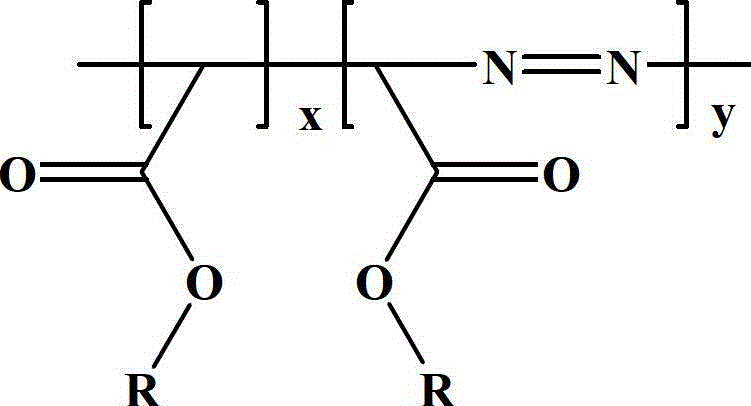
Diazoacetate-ethoxycarbonyl carbene copolymer and preparation method thereof
The invention discloses a diazoacetate-ethoxycarbonyl carbene copolymer and a synthesis method of the copolymer. The main chain of the polymer comprises carbon atoms and nitrogen atoms, wherein the carbon atoms are prepared by carbene polymerization reaction, and the nitrogen atoms are added into the main chain of the polymer by double-free-radical polymerization. The invention also provides a preparation method of the copolymer, which takes diazoacetate as raw material, and comprises the following steps: putting the diazoacetate into a microwave oven, and carrying out microwave irradiation on the diazoacetate for a period of time under the protection of argon; and finally, carrying out reprecipitation and vacuum drying to obtain the product. The method does not use catalyst and solvent, and is simple and convenient in steps as well as simple in reaction and aftertreatment; and the prepared polyester with the novel structure can be applied to the wide fields such as optical study and the like.
Owner:WUHAN UNIV
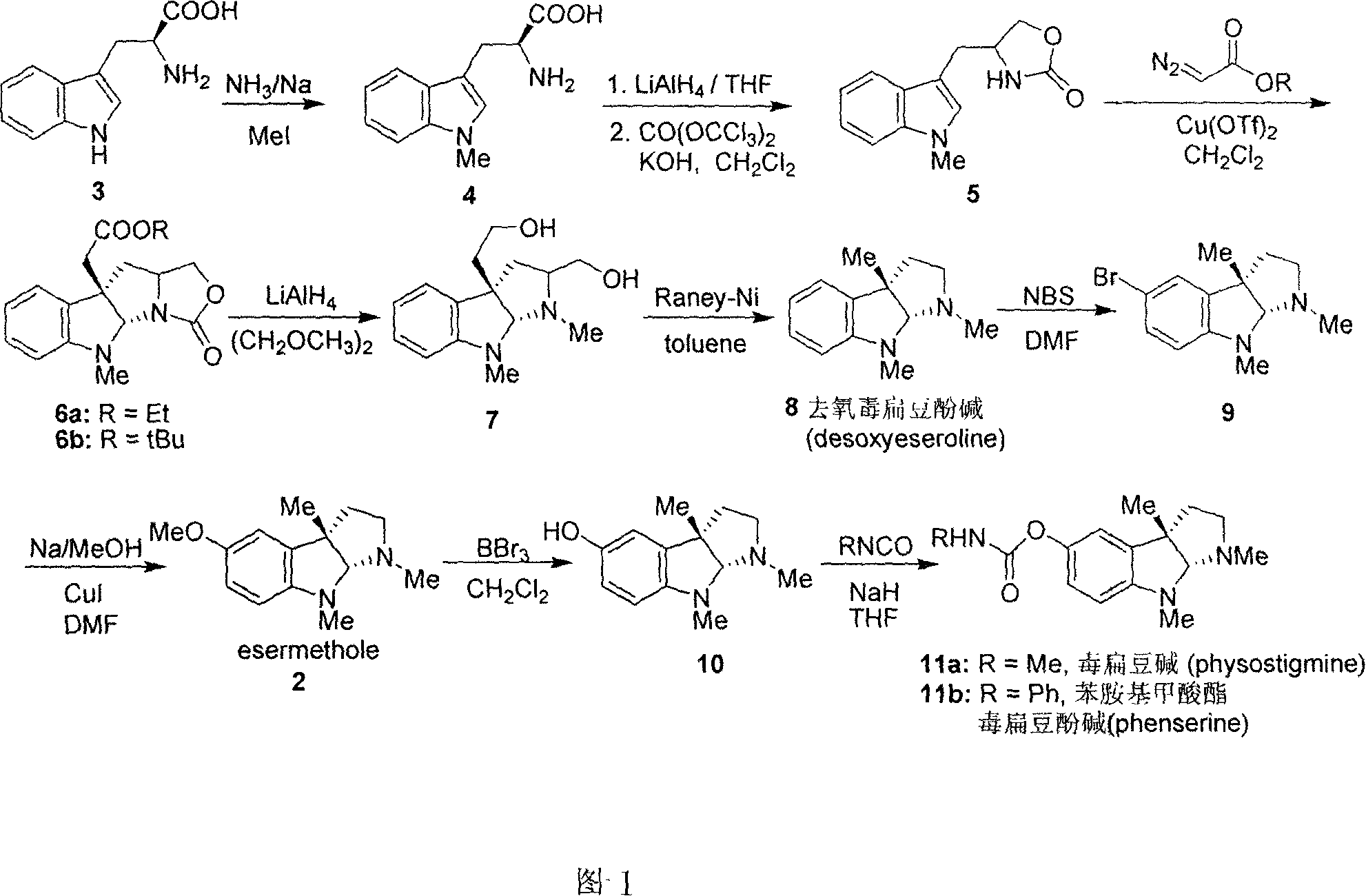
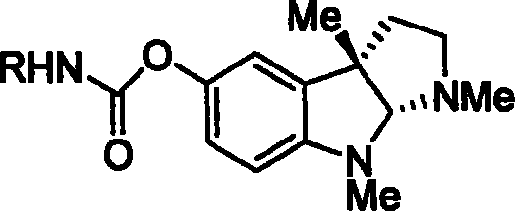
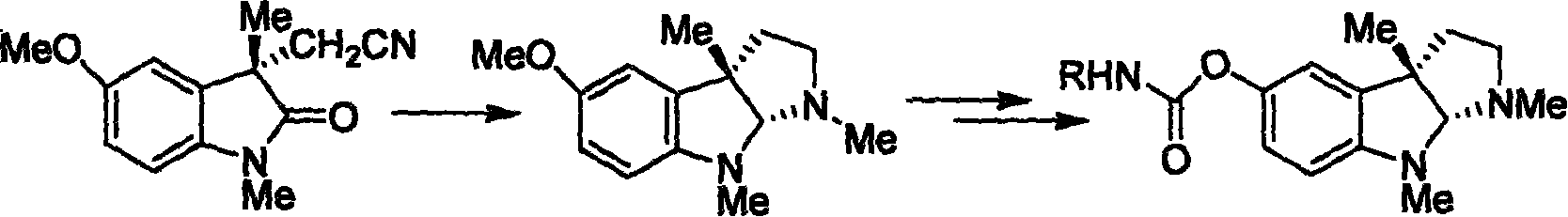
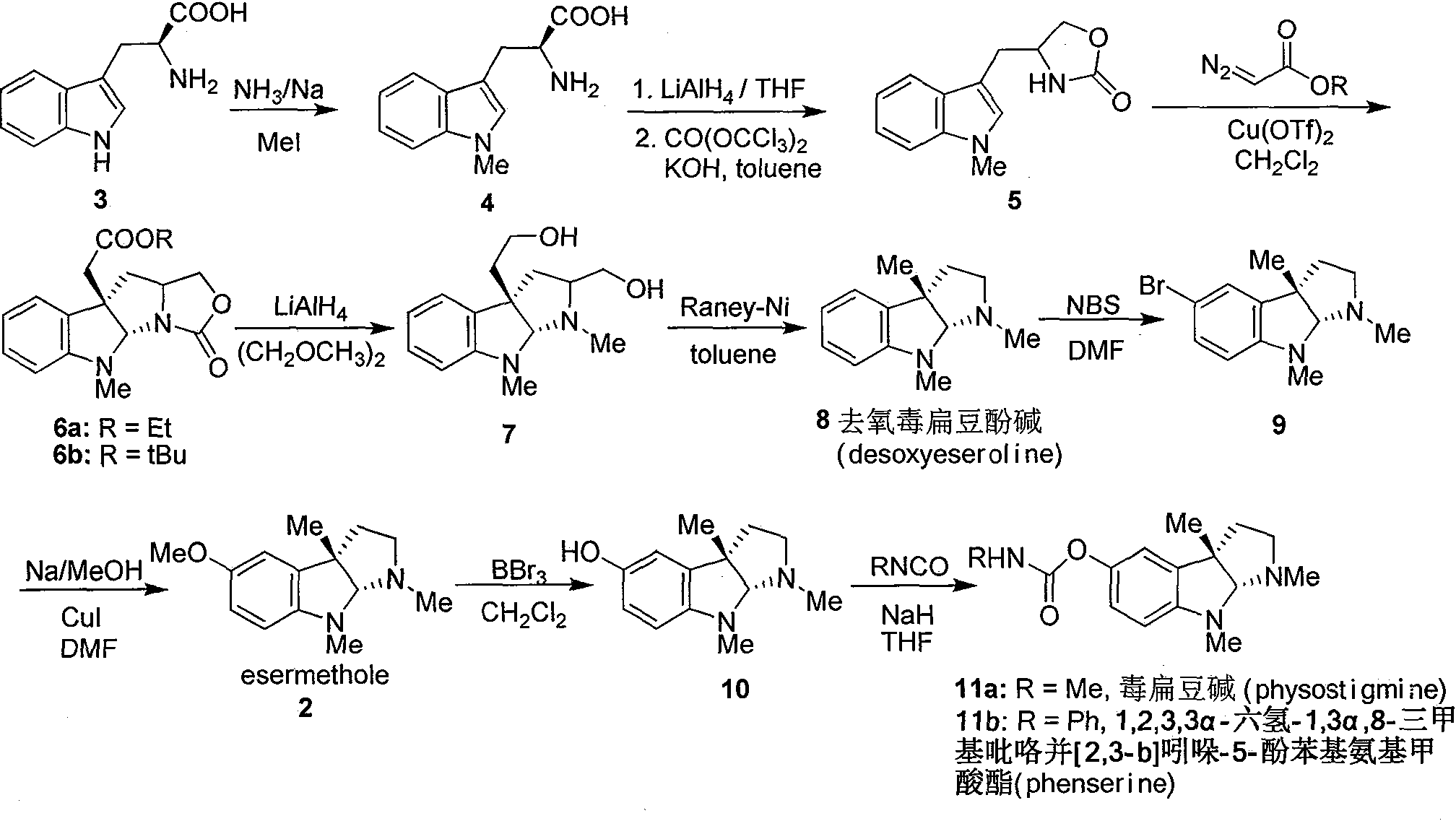
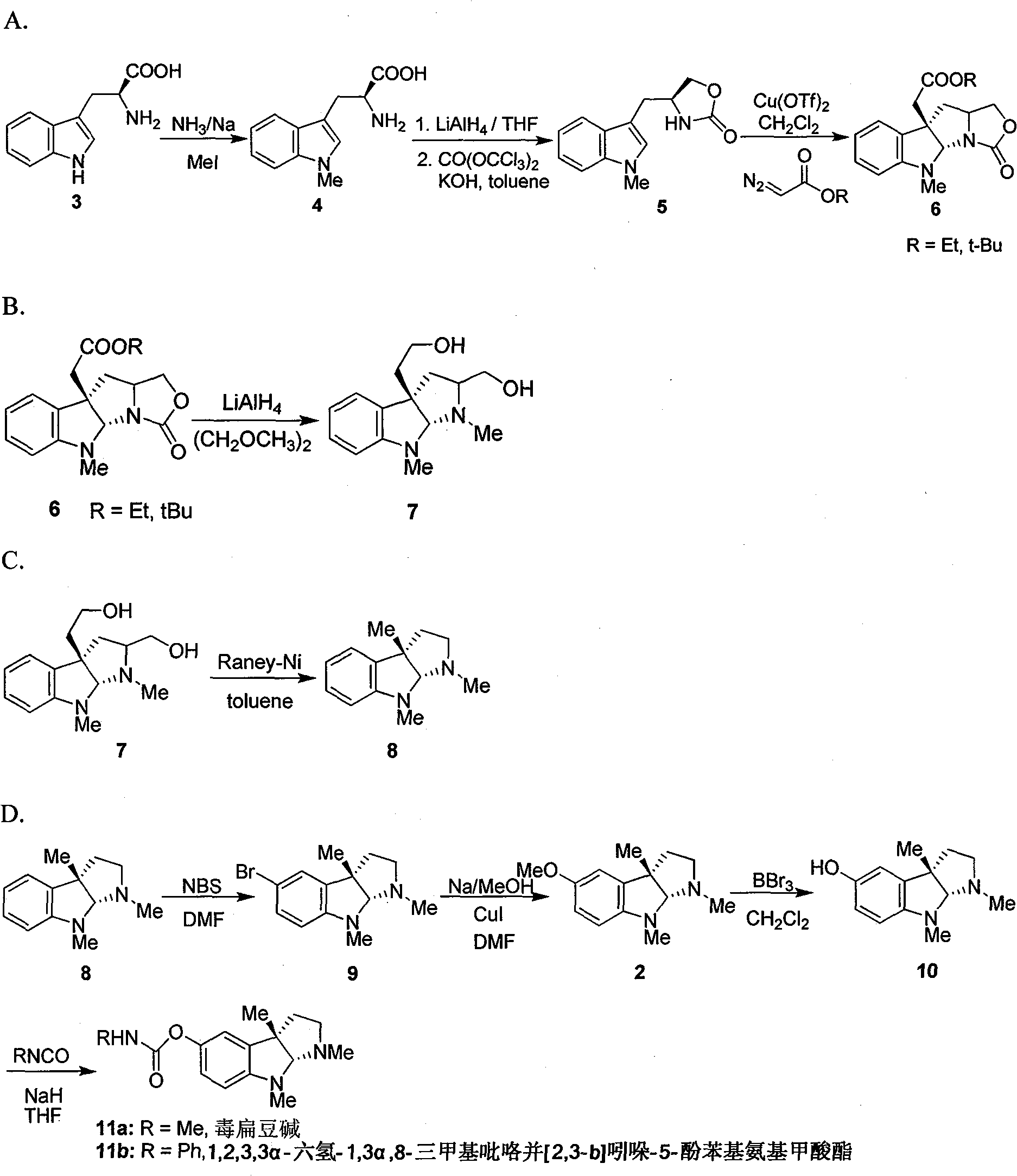
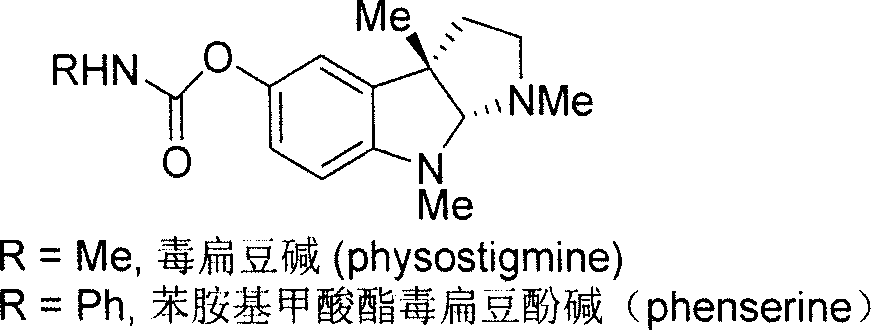
Synthesis for natural medicament physostigmine for resisting senile dementia disease and phenylaminoformic acid ester phenserine
The invention belongs to the field of drug synthesis, and relates to a new method for synthesizing physostigmine, a natural drug against senile dementia, and its derivative, anilinoformate, phenserine. The present invention uses L-tryptophan 3 as raw material, firstly prepares chiral oxazolone intermediate 5, and then through intermolecular asymmetric cyclopropionation-ring-opening-ring and three-step one-pot waterfall reaction, and diazonium Acetate reaction was prepared to obtain optically pure 3-substituted tetrahydropyrroloindole skeleton 6, which was reduced by lithium aluminum hydride to obtain diol-substituted intermediate 7, and intermediate 7 was treated with Raney nickel (Raney-Ni ) after removing the hydroxymethylene group to obtain the intermediate 8 deoxy physostigmine (desoxyeseroline), and the intermediate 8 is obtained by electrophilic bromination reaction, methyl etherification, demethylation and carbonamidation four-step reaction with high yield. Completed the synthesis of physostigmine and its derivative, anilinoformate, physostigmine, a natural drug against Alzheimer's disease. The method has the advantages of high key reaction yield, simple operation, short reaction steps, etc., and has the advantages of solving the problem of raw material source of the marketed drug physostigmine and its derivative anilinoformate phenserine. important practical significance.
Owner:SICHUAN UNIV
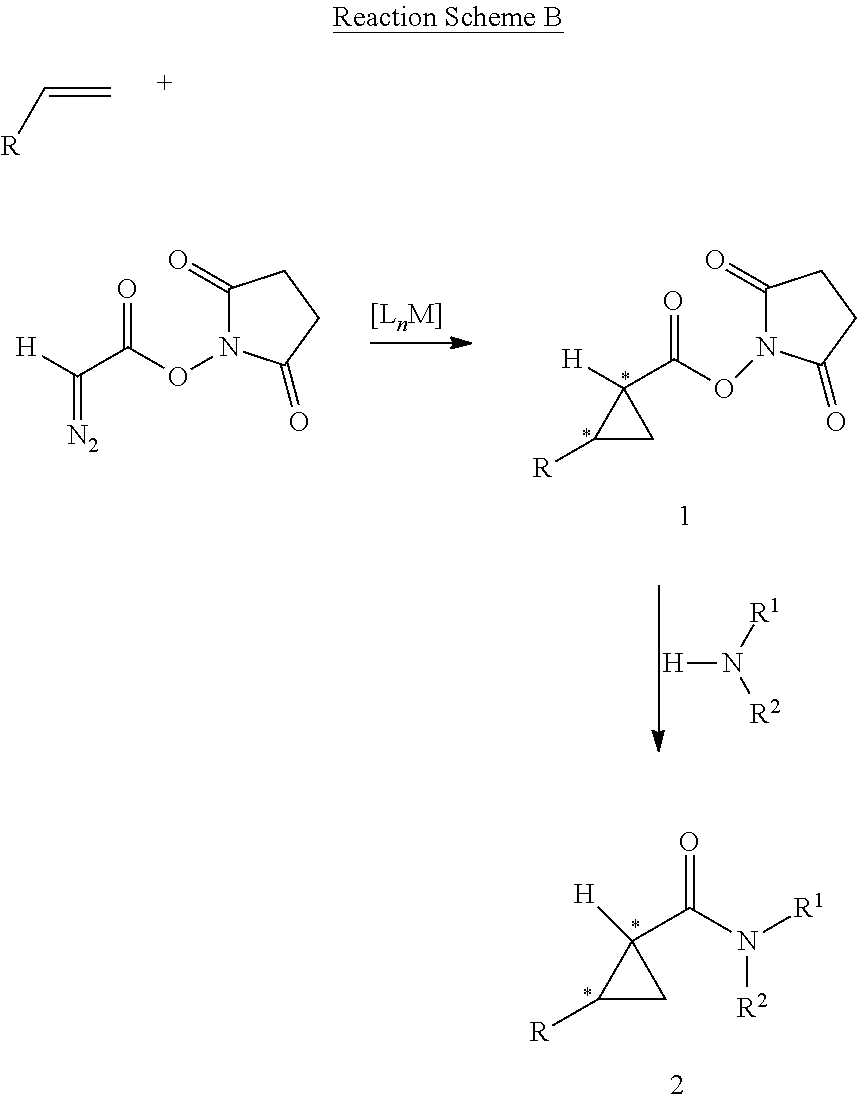
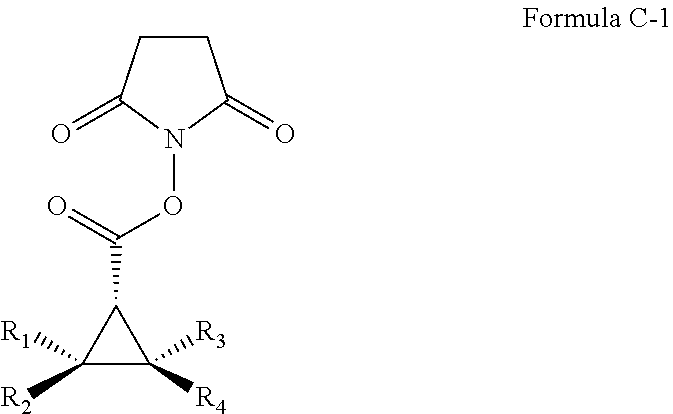
Asymmetric Cobalt-Catalyzed Cyclopropanation With Succinimidyl Diazoacetate
InactiveUS20120077959A1Excellent diastereoExcellent enantioselectivitiesSugar derivativesOrganic compound preparationPtru catalystSynthon
Cobalt(II) complexes of the D2-symmetric chiral porphyrins are effective catalysts for asymmetric cyclopropanation reactions with succinimidyl diazoacetate. The Co-catalyzed reaction is suitable for various olefins, providing the corresponding cyclopropane succinimidyl esters in high yields and excellent diastereo- and enantio-selectivity. The resulting enantioenriched cyclopropane succinimidyl esters can serve as convenient synthons for the general synthesis of optically active cyclopropyl carboxamides through mild reactions with a wide range of amine derivatives, including unprotected peptides and amino sugars
Owner:UNIV OF SOUTH FLORIDA
Terpolymer of diazoacetate, carbethoxy cabbeen and cyclic lactone and preparation method thereof
The invention discloses a method for copolymerizing diazoacetate and cyclic lactone. The specific preparation method comprises the following steps of: reacting the diazoacetate and the cyclic lactone as monomers for a period of time under microwave irradiation in the presence of an initiator and under the condition of inert gas protection, thereby obtaining the polymer. The method is free of catalyst and solvent, simple and convenient to prepare, easy in parameter control and fast in reaction, and the prepared polymer is large in molecular weight and a brand new path is provided for the copolymerization reaction of the cyclic lactone and free radicals. The polymer prepared by the method provided by the invention is expected to be widely applied to fields of rubber, plastics and the like.
Owner:WUHAN UNIV
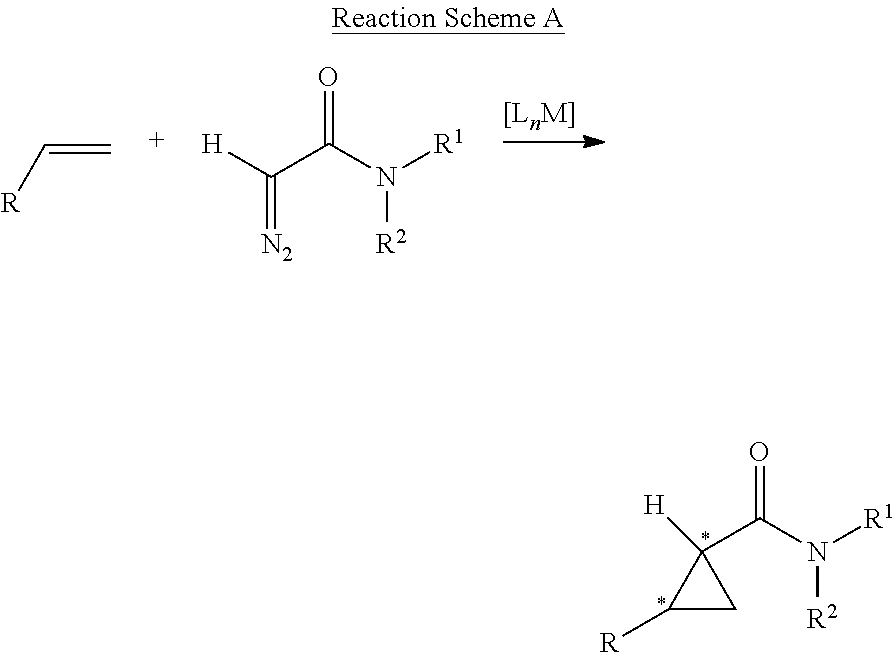
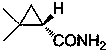
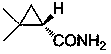
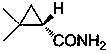
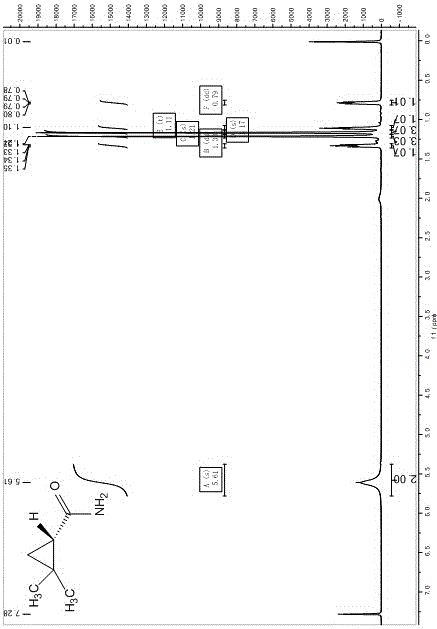
Preparation method of chiral dimethyl cyclopropyl carboxamide
InactiveCN104193645AOrganic compound preparationCarboxylic acid amide separation/purificationAlkyl transferDiazoacetic ester
The invention discloses a preparation method of chiral dimethyl cyclopropyl carboxamide. The method comprises a step of asymmetric cyclopropyl alkylation and a step of catalytic amidation of cyclopropyl formic ether, wherein in the step of asymmetric cyclopropyl alkylation, a cyclopropyl alkylation reaction is carried out on ethyl diazoacetate and isobutene under the catalysis of a chiral ligand complex of a cuprous salt so as to obtain (S)-dimethyl cyclopropyl formate; and in the step of catalytic amidation of cyclopropyl formic ether, an ammonolysis reaction is carried out on the (S)-dimethyl cyclopropyl formate by one step so as to directly obtain (S)-2,2-dimethyl cyclopropyl carboxamide, and refining the carboxamide with an alcohol so as to obtain the chiral dimethyl cyclopropyl carboxamide with chemical purity being greater than 99.5% and an e.e. value being greater than 99.5%. Thus, the method used for synthesizing the (S)-2,2-dimethyl cyclopropyl carboxamide is environment-friendly, simple, rapid and efficient.
Owner:SHANGHAI INST OF TECH
Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether
The invention relates to the preparation of cyclopropane carboxylate, in particular to a method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether under the mild condition of the catalysis of non-noble metal. The method comprises the following steps of: taking thiophene and ethyl diazoacetate as raw materials, adopting a compound of Cu or Co as a catalyst, adopting a compound which does not contain an alcoholic hydroxyl group, a carboxylic acid group, a primary amino radical and a secondary amino group as a solvent, dripping the ethyl diazoacetate or a solution thereof into the mixture of the catalyst and the thiophene, stirring, reacting for 5-24min at the temperature of 0-120 DEG C until a reaction mixture does not discharge gas any more, then distilling off the solvent andthe excessive thiophene and separating by the methods of column chromatography or reduced pressure distillation and the like to obtain the 4-thio-bicyclo[3.1.0]-2-hexene-6-formic ether, wherein calculated by molar weight, the use quantity of the thiophene in the reaction is 1-200 times that of the ethyl diazoacetate, the use quantity of the catalyst is 0.0001-100mol% of that of the ethyl diazoacetate, and the volume of the solvent is 0-50 times the volume of the thiophene. The invention has low cost, mild condition, better selectivity and higher economic value.
Owner:DALIAN UNIV
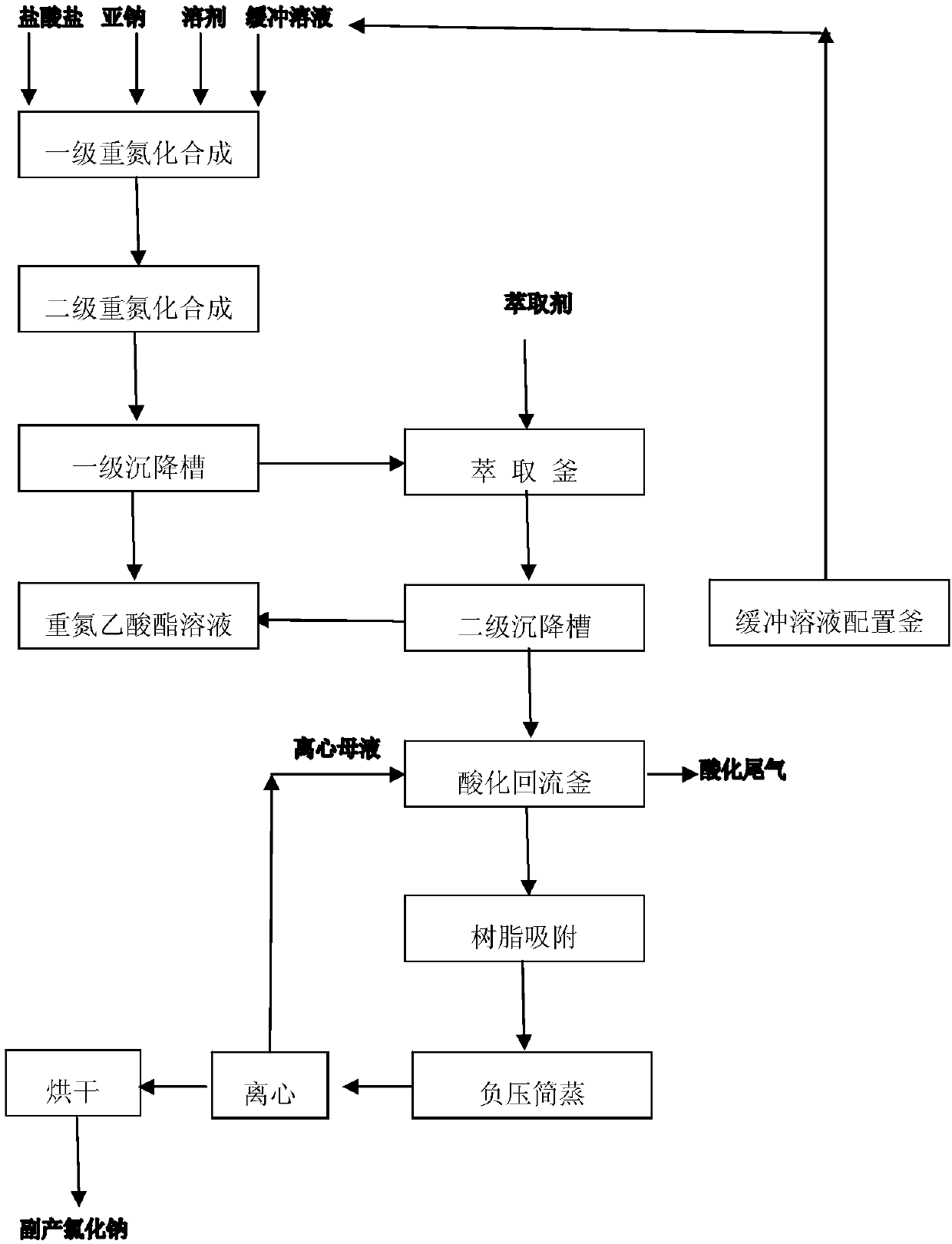
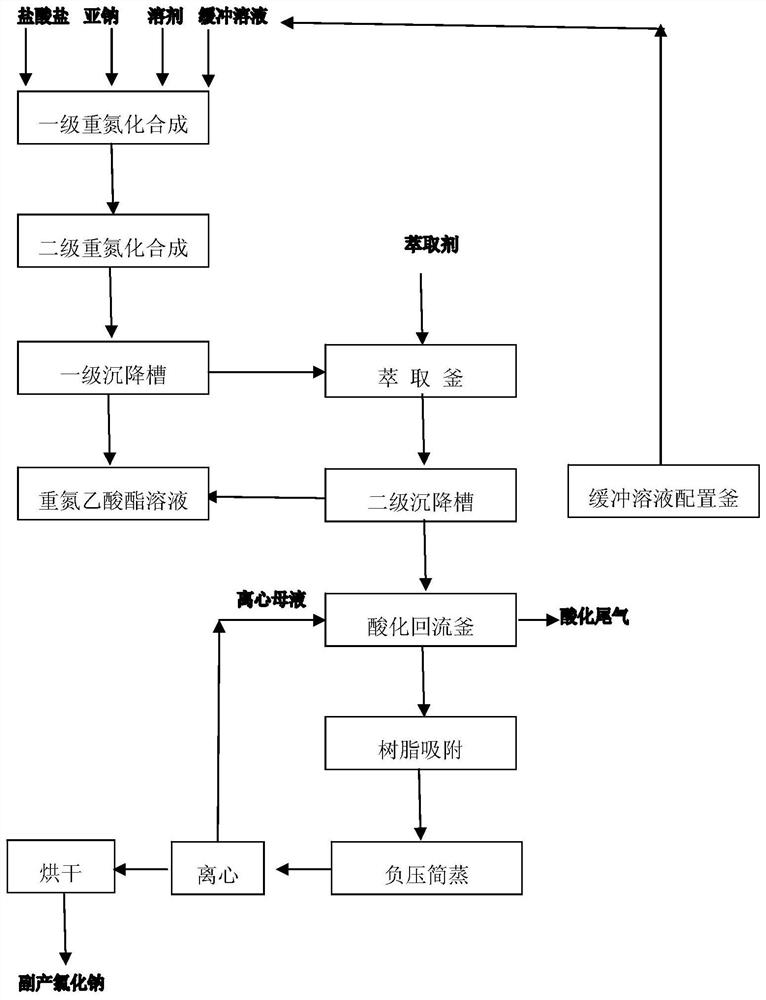
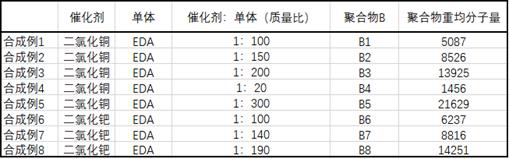
Continuous synthesis method of diazoacetate
ActiveCN109776351ARealize continuous productionHigh yieldOrganic chemistrySalt-wastingSynthesis methods
The invention discloses a continuous synthesis method of diazoacetate. The method comprises the following steps: performing a two-stage diazotization synthesis reaction on glycine ester hydrochlorideaqueous solution, sodium nitrite aqueous solution and an organic solvent, performing still standing on a product for layering, enabling an oil layer to enter a diazonium liquid low tank and enabling awater layer to enter an extraction kettle; and adding an extraction agent into the extraction kettle for extraction, continuously performing still standing for layering, enabling a water layer to enter an acidification backflow kettle, enabling an oil layer to enter the diazonium liquid low tank for combination, so as to obtain diazoacetate solution. Acidification backflow, cooling, resin adsorption, negative pressure steaming, dehydration, concentration and centrifugation are performed on the water layer in the acidification backflow kettle, so as to obtain a sodium chloride wet basis and centrifugal mother liquid; the centrifugal mother liquid flows back to the acidification backflow kettle for use; meanwhile, high salt waste water generated by reaction is treated for recycling sodium chloride; and in addition, continuous production of the whole process is achieved, and clean production without discharge of waste water, waste gas and waste residues is achieved.
Owner:JIANGSU YOUTH CHEM +1
Bidirectional transition luminescent material and preparation method thereof
InactiveCN104403660AUp and down conversion fluorescence performance is strongGood biocompatibilityOrganic chemistryEnergy modified materialsSide chainDouble bond
The invention discloses a bidirectional transition luminescent material and a preparation method thereof. Diazoacetate and alkene of which double bonds connected to an electrophilic group undergo 1,3-dipolar cycloaddition reaction to produce the bidirectional transition luminescent material. The bidirectional transition luminescent material is a polymer of which the side chain is connected to a pyrazoline group with fluorescence performances or a pyrazoline monomer contains other groups so that the material has special bidirectional transition luminescence performances. The bidirectional transition luminescent material can emit fluorescence with the same wavelength, can image in organisms under the actions of near infrared excitation and near ultraviolet excitation, can produce dual wavelength-excited fluorescence with intensity higher than that of fluorescence produced by single wavelength excitation and can further improve fluorescence imaging sharpness.
Owner:WUHAN UNIV
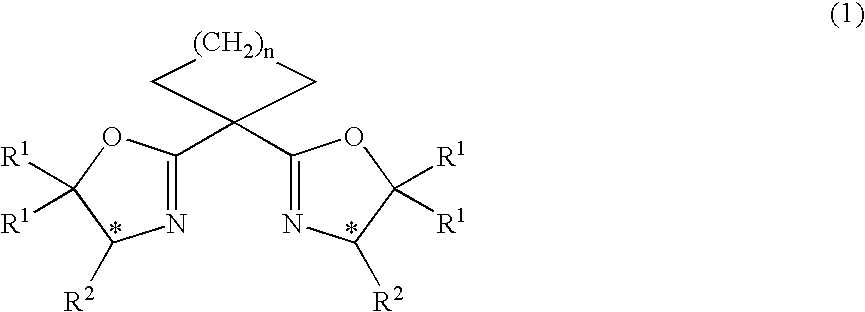
Process for producing optically active cyclopropane compound and asymmetric copper complex for use in the same
InactiveUS20070032659A1Efficiently obtainedGroup 1/11 organic compounds without C-metal linkagesOrganic compound preparationHalogenHydrogen atom
A process for producing an optically active cyclopropane compound represented by the formula (4): wherein R3, R4, R5 and R6 are the same or different, and represent a hydrogen atom, a halogen atom, a substituted or unsubstituted alkyl group, and so on; and R7 represents a C1-6 alkyl group; and * represents an asymmetric carbon atom, which comprises reacting a prochiral olefin represented by the formula (2): wherein R3, R4, R5, and R6 are as described above, with a diazoacetic acid ester represented by the formula (3):N2CHCO2R7 (3)wherein R7 is as defined above, in the presence of an asymmetric copper complex prepared from an optically active cycloalkylidenebisoxazoline compound represented by the formula (1): wherein R1 represents a hydrogen atom, a C1-6 alkyl group, and so on; R2 represents a C1-6 alkyl group and so on; and n represents an integer of 0 to 3; provided that, two R1s may be bonded each other together with the carbon atom to which they are bonded to form a ring; and * represents an asymmetric carbon atom, and a copper compound, is provided.
Owner:SUMITOMO CHEM CO LTD
A kind of preparation method of chiral dimethyl cyclopropanamide
InactiveCN104193645BOrganic compound preparationCarboxylic acid amide separation/purificationAlkyl transferAlcohol
The invention discloses a preparation method of chiral dimethyl cyclopropyl carboxamide. The method comprises a step of asymmetric cyclopropyl alkylation and a step of catalytic amidation of cyclopropyl formic ether, wherein in the step of asymmetric cyclopropyl alkylation, a cyclopropyl alkylation reaction is carried out on ethyl diazoacetate and isobutene under the catalysis of a chiral ligand complex of a cuprous salt so as to obtain (S)-dimethyl cyclopropyl formate; and in the step of catalytic amidation of cyclopropyl formic ether, an ammonolysis reaction is carried out on the (S)-dimethyl cyclopropyl formate by one step so as to directly obtain (S)-2,2-dimethyl cyclopropyl carboxamide, and refining the carboxamide with an alcohol so as to obtain the chiral dimethyl cyclopropyl carboxamide with chemical purity being greater than 99.5% and an e.e. value being greater than 99.5%. Thus, the method used for synthesizing the (S)-2,2-dimethyl cyclopropyl carboxamide is environment-friendly, simple, rapid and efficient.
Owner:SHANGHAI INST OF TECH
Method for synthesizing imino benzotriazole compound under photocatalysis condition
PendingCN114835652AProtonation improvementWith cleanOrganic chemistryChemical recyclingDiazoacetic esterBenzotriazole
The invention belongs to the technical field of compound preparation, and discloses a method for synthesizing an imino benzotriazole compound under a photocatalytic condition, which comprises the following steps: by taking benzotriazole as shown in a formula I and diazoacetate as shown in a formula II as raw materials, reacting under the action of an additive and a solvent, thereby obtaining the imino benzotriazole compound. And reacting under the conditions of inert atmosphere and illumination to synthesize the imino benzotriazole compound as shown in the formula III. Under the illumination condition, benzotriazole, diazoacetate and nitriles are synthesized into the imino benzotriazole compound in one step. The raw materials used in the method are wide in source and low in price, and do not need to be treated too much. The whole reaction is carried out under an illumination condition, needs to be heated at a relatively low temperature, and is green and environment-friendly.
Owner:HENAN UNIVERSITY
Terpolymer of diazoacetate, carbethoxy cabbeen and cyclic lactone and preparation method thereof
The invention discloses a method for copolymerizing diazoacetate and cyclic lactone. The specific preparation method comprises the following steps of: reacting the diazoacetate and the cyclic lactone as monomers for a period of time under microwave irradiation in the presence of an initiator and under the condition of inert gas protection, thereby obtaining the polymer. The method is free of catalyst and solvent, simple and convenient to prepare, easy in parameter control and fast in reaction, and the prepared polymer is large in molecular weight and a brand new path is provided for the copolymerization reaction of the cyclic lactone and free radicals. The polymer prepared by the method provided by the invention is expected to be widely applied to fields of rubber, plastics and the like.
Owner:WUHAN UNIV
The method for synthesizing 2-thiabicyclo[3.1.0]-3-hexene-6-carboxylate
The invention discloses a method for synthesizing 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate. The method comprises the following steps: adding diazo acetate or a diazo acetate solution to a mixture of a catalyst, thiophene and a solvent in a dropwise mode under stirring at 0-140DEG C, and reacting until a gas is completely discharged; filtering to remove the catalyst, and distilling off excel thiophene and solvent; and finally separating to obtain 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate, wherein the catalyst is a supported Cu catalyst which comprises a Cu active component, an assistant and a carrier. According to the invention, the cheap supported Cu catalyst is adopted, application amounts of reactants are clarified, preparation conditions of the catalyst are regulated, and the assistant is added, so performances of the catalyst are changed, and the reaction selectivity is improved; and the catalyst has the advantages of easy preparation and low cost, cyclopropanation reaction conditions are mild, the reaction selectivity and the product yield are high, and the catalyst is easy to separate after the reaction. The method is suitable for massive synthesis of 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate.
Owner:DALIAN UNIV
A kind of synthetic method of pyrazoline nucleoside analog with quaternary carbon center
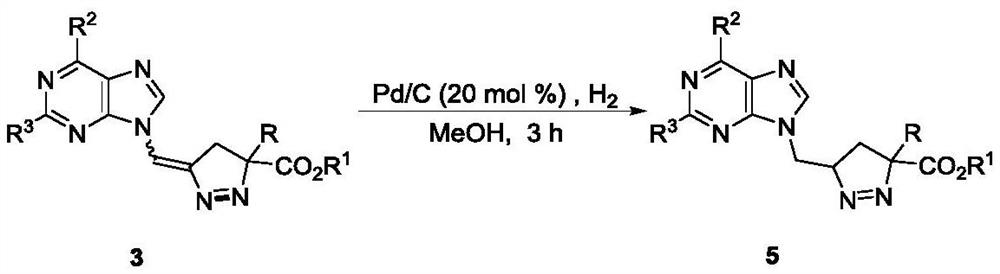
The invention discloses a method for synthesizing pyrazoline nucleoside analogs with a quaternary carbon center, belonging to the technical field of organic chemistry. Starting from 9-alkenyl purine 1 and α-alkyl / aryl diazoacetate 2, DPPB or Pd 2 (dba) 3 As a catalyst, after the reaction, a pyrazoline-like purine nucleoside analog 3 with a quaternary carbon center is obtained, and the reaction yield is moderate to excellent. In the present invention, the diversity and singleness of pyrazoline products can be effectively controlled by changing the catalytic system, and the pyrazoline-like purine nucleoside analog 5 can be obtained after the product is further hydrogenated.
Owner:HENAN NORMAL UNIV
Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether
The invention relates to the preparation of cyclopropane carboxylate, in particular to a method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether under the mild condition of the catalysis of non-noble metal. The method comprises the following steps of: taking thiophene and ethyl diazoacetate as raw materials, adopting a compound of Cu or Co as a catalyst, adopting a compound which does not contain an alcoholic hydroxyl group, a carboxylic acid group, a primary amino radical and a secondary amino group as a solvent, dripping the ethyl diazoacetate or a solution thereof into the mixture of the catalyst and the thiophene, stirring, reacting for 5-24min at the temperature of 0-120 DEG C until a reaction mixture does not discharge gas any more, then distilling off the solvent andthe excessive thiophene and separating by the methods of column chromatography or reduced pressure distillation and the like to obtain the 4-thio-bicyclo[3.1.0]-2-hexene-6-formic ether, wherein calculated by molar weight, the use quantity of the thiophene in the reaction is 1-200 times that of the ethyl diazoacetate, the use quantity of the catalyst is 0.0001-100mol% of that of the ethyl diazoacetate, and the volume of the solvent is 0-50 times the volume of the thiophene. The invention has low cost, mild condition, better selectivity and higher economic value.
Owner:DALIAN UNIV
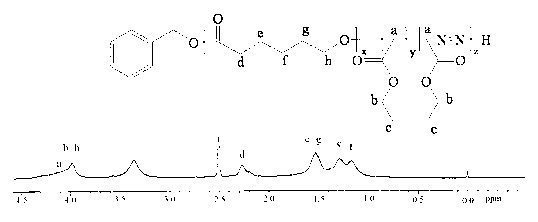
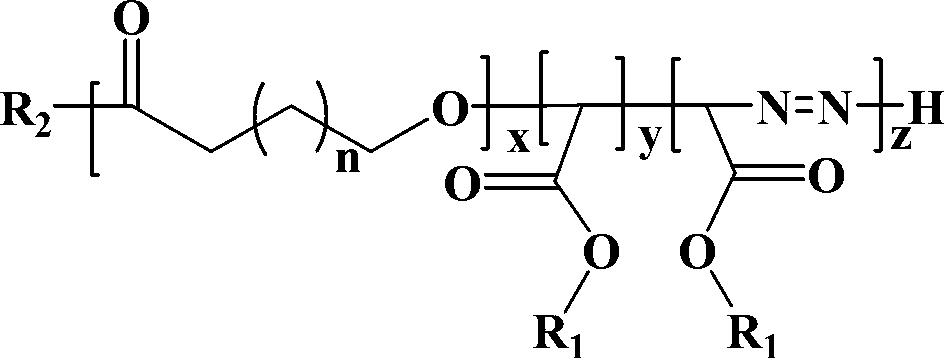
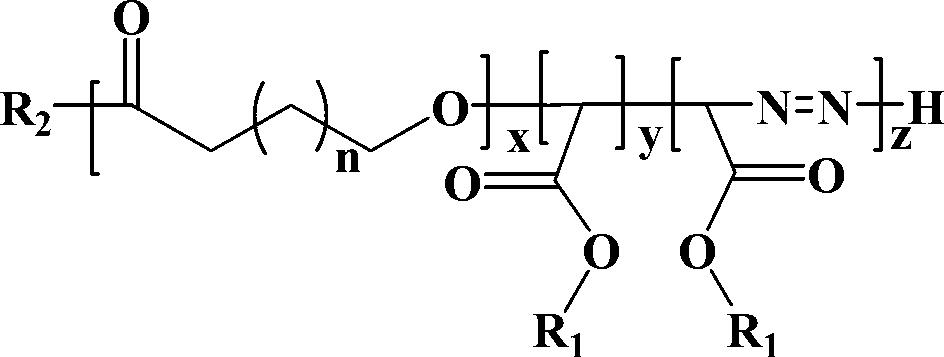
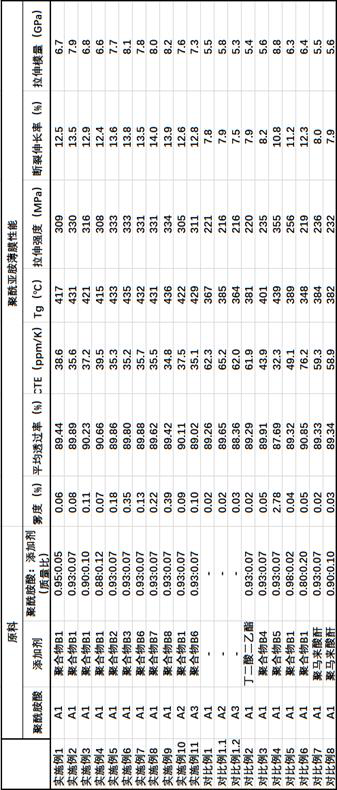
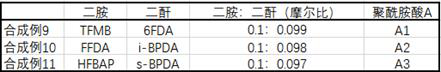
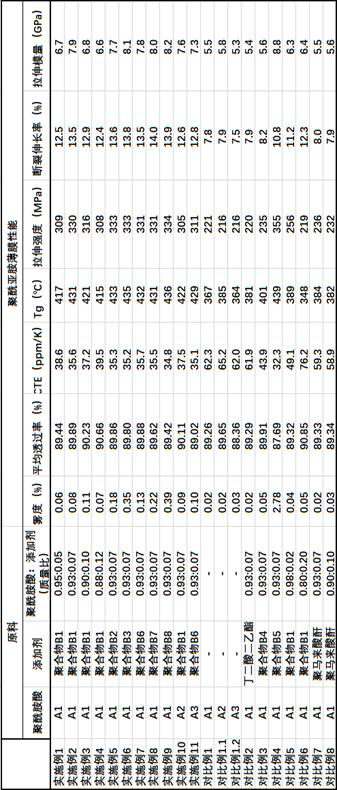
Display device, polyimide precursor composition, polyimide film, and laminated body
ActiveCN113061340BImprove transmittanceHigh glass transition temperatureNon-linear opticsIdentification meansPolymer scienceDisplay device
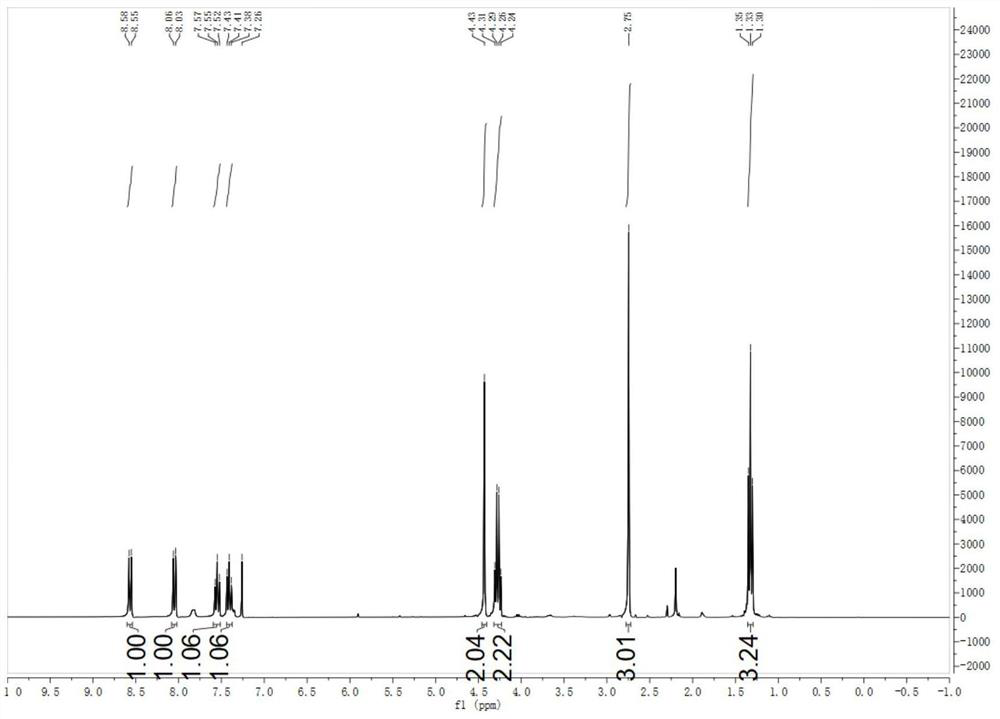
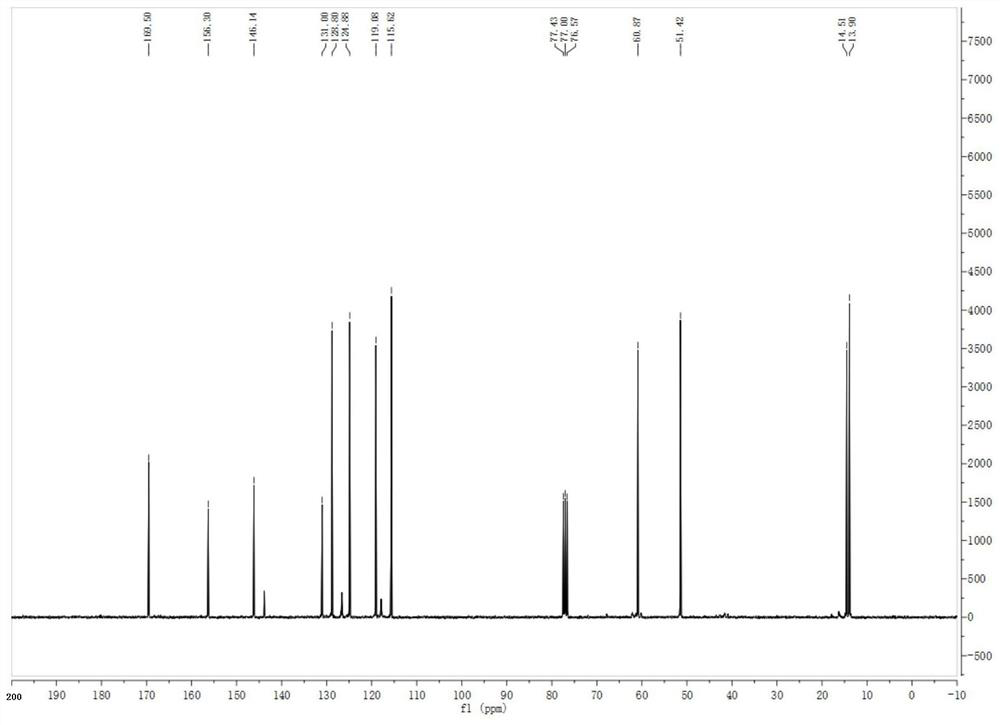
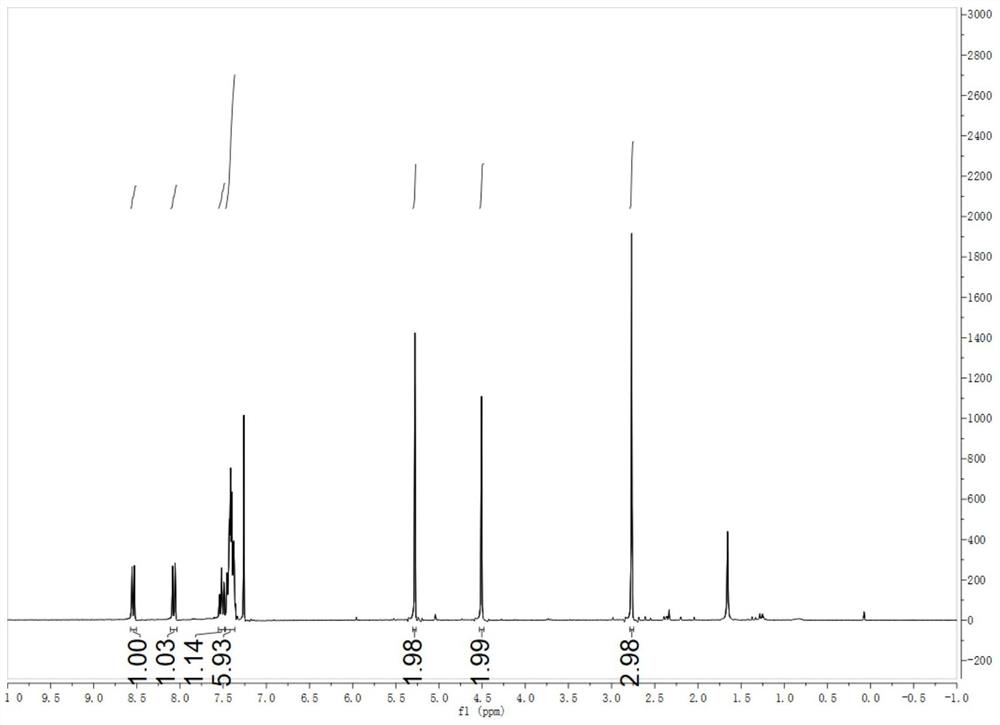
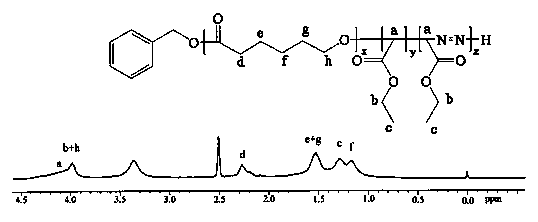
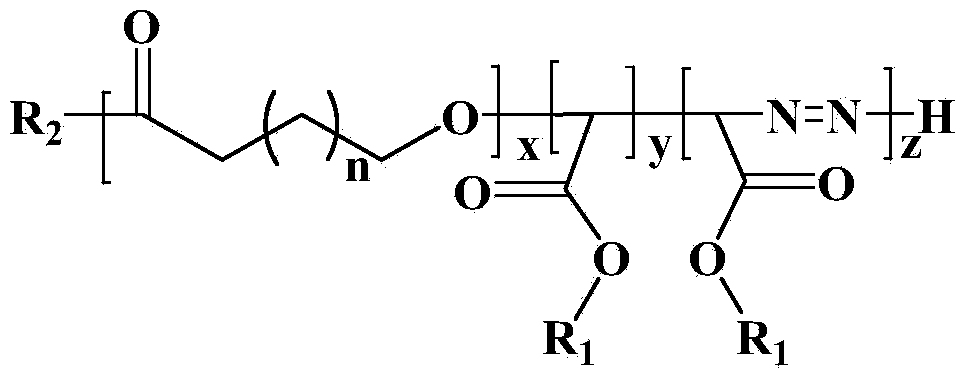
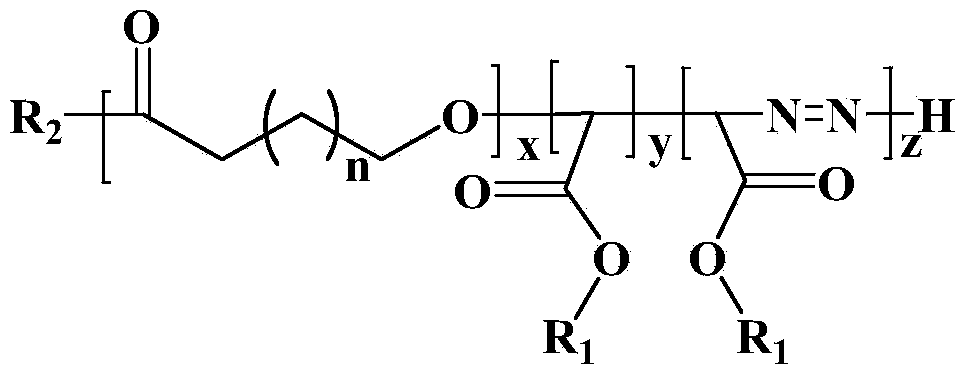
The invention discloses a display device, a polyimide precursor composition, a polyimide film and a laminated body related to the field of photoelectric technology. The display device includes a substrate and a display unit, the substrate includes a polyimide film formed from a polyimide precursor composition, the polyimide precursor composition includes polyamic acid and a polymer, and the polymer The compound contains the repeating unit shown in formula (1): in formula (1) in formula (1), n is an integer of 2-200, R 1 Represents a hydrogen atom, a monovalent organic group, R 2 Represents any one or more monovalent organic groups. Through the introduction of diazoacetate polymers, the present invention not only improves the transmittance of polyimide film, but also improves its glass transition temperature and mechanical properties, and obtains polyimide film with low haze and lower CTE. Amine film.
Owner:武汉柔显科技股份有限公司
Preparing process of catalyst for cyclopropanizing reaction of 2,5-dimethyl-2,4-hexadiene
A carried catalyst used for cyclopropanizing 2,5-dimethyl-2,4-hexadiene contains the active component (20-90%) chosen from Fe, Ca, Ni, Ca, Zn, Cr, Mo or Ti, the assistant (0.1-5%) chosen from L, Na or K, and the carrier chosen from alumina silica gel and activated carbon. It is prepared through premodifying carrier, and immersing while adding active component and the organic compound containing hydroxy or carboxyl group and oxygen. Its advantages are low dosage, high output rate, little coking and easy separation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A kind of continuous synthesis method of diazoacetate
ActiveCN109776351BRealize continuous productionHigh yieldOrganic chemistrySodium nitriteSodium nitrate
The invention discloses a continuous synthesis method of diazoacetate, in which glycine ester hydrochloride aqueous solution, sodium nitrite aqueous solution, and organic solvent are subjected to two-stage diazotization synthesis reaction, the product is separated and separated, and the oil layer is deweighted Nitrogen liquid low level tank, water layer to the extraction tank; add extractant to the extraction tank for extraction and continue to stand for stratification, the water layer goes to the acidification reflux tank, and the oil layer goes to the diazo liquid low level tank to combine to obtain a diazoacetate solution . The water layer in the acidification reflux kettle is subjected to acidification reflux, cooling, resin adsorption, negative pressure simple steaming, dehydration and concentration, and centrifugation to obtain the sodium chloride wet base and centrifuged mother liquor. The centrifuged mother liquor is returned to the acidification reflux kettle for use mechanically. The wet base is dried to obtain industrial grade sodium chloride. The method of the invention enables the reaction to be fully carried out so as to obtain a high yield, and at the same time, the high-salt wastewater produced by the reaction is treated to recover sodium chloride; and the continuous production of the whole process is tested. Realize clean production without waste water, waste gas and waste residue discharge.
Owner:JIANGSU YOUTH CHEM +1
Synthesis for natural medicament physostigmine for resisting senile dementia disease and phenylaminoformic acid ester phenserine
Owner:SICHUAN UNIV
Display device, polyimide precursor composition, polyimide film, and laminate
ActiveCN113061340AImprove transmittanceHigh glass transition temperatureNon-linear opticsIdentification meansPolymer scienceDiazoacetic ester
The invention discloses a display device, a polyimide precursor composition, a polyimide film and a laminate, and relates to the technical field of photoelectricity. The display device includes a substrate and a display unit; the substrate comprises a polyimide film formed by a polyimide precursor composition comprising a polyamic acid and a polymer, the polymer comprises a repeating unit represented by a formula (1) defined in the specification: in the formula (1), n is an integer of 2-200, R1 represents a hydrogen atom or a monovalent organic group, and R2 represents any one or more monovalent organic groups. By introducing a diazoacetate polymer, the transmittance of the polyimide film is improved, the glass transition temperature of the polyimide film is increased, the mechanical property of the polyimide film is improved, and the polyimide film with low haze and low CTE is obtained.
Owner:武汉柔显科技股份有限公司
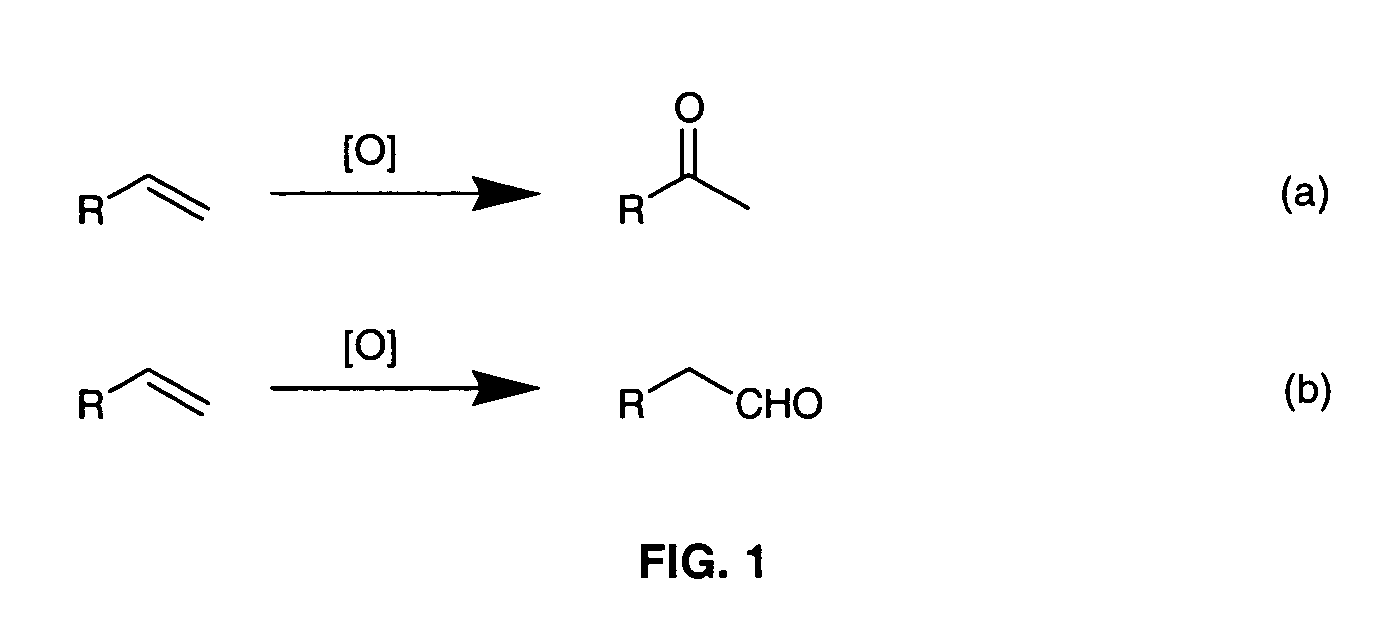
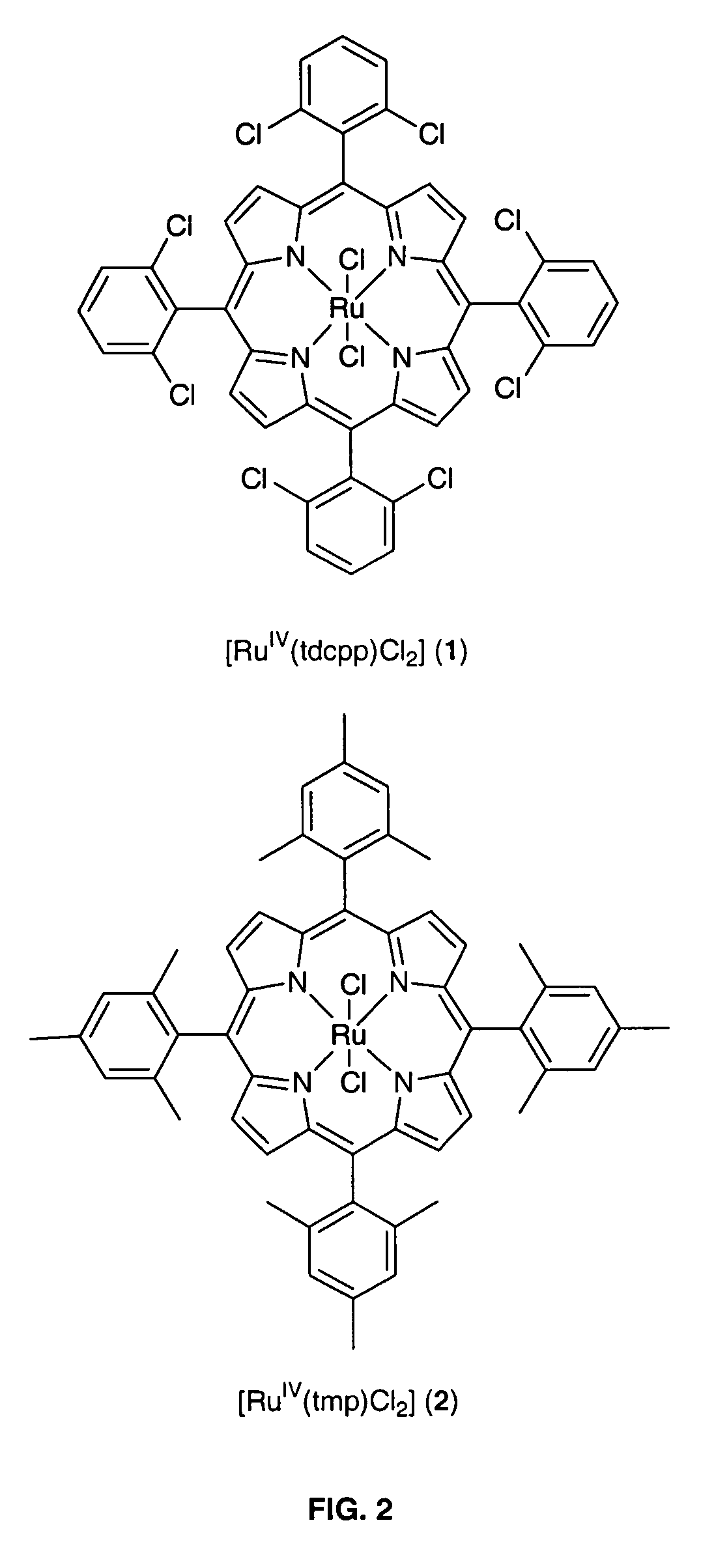
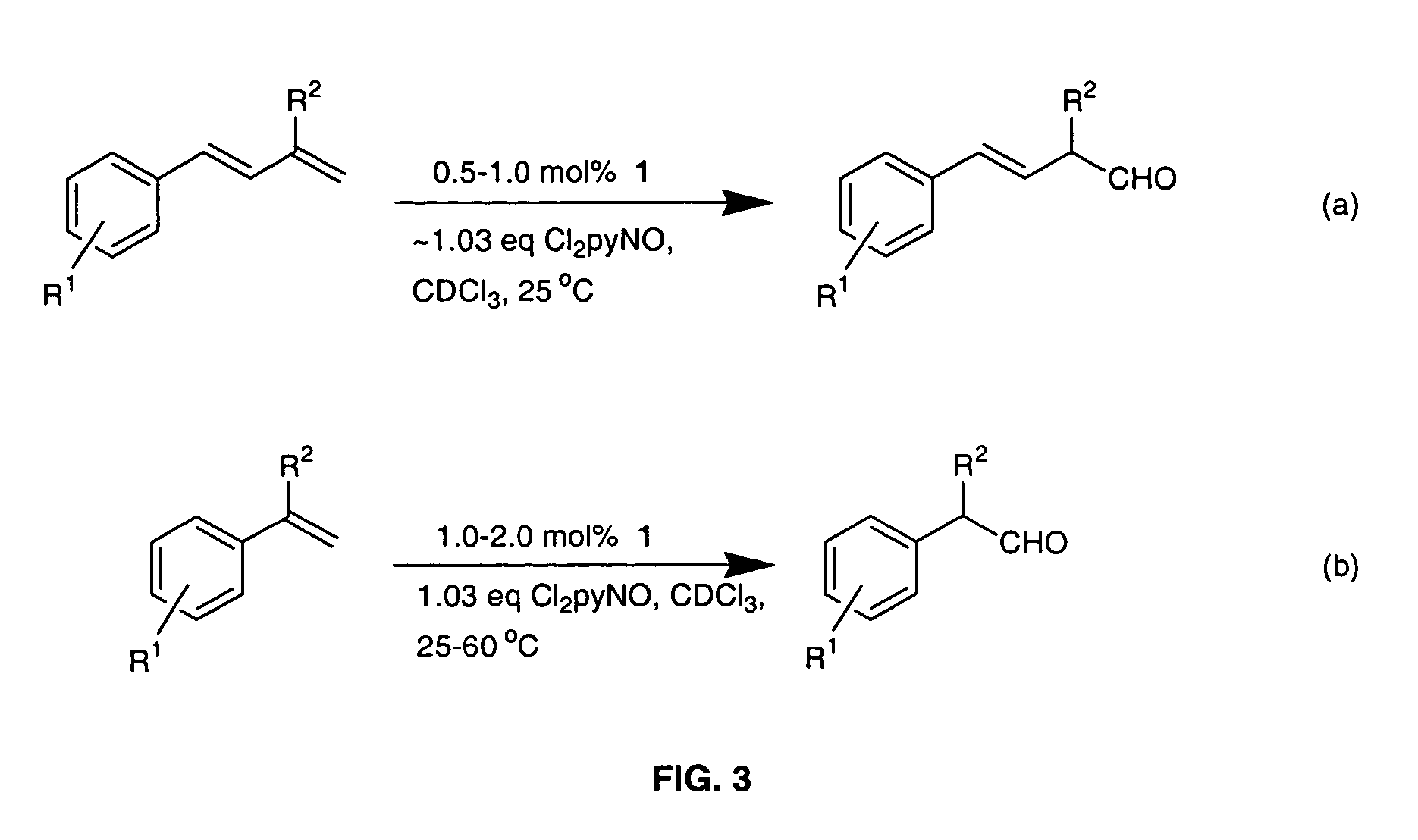
Method for conversion of terminal alkenes to aldehydes using ruthenium (IV) porphyrin catalysts
ActiveUS7582750B2Good-to-excellent yieldOrganic compound preparationCarboxylic acid esters preparationAcetic acidDiazoacetic ester
Aldehydes were obtained in excellent yields from ruthenium-porphyrin-catalyzed oxidation of various terminal alkenes with 2,6-dichloropyridine N-oxide under mild conditions. The aldehydes generated from these ruthenium-catalyzed alkene oxidation reactions can be used in-situ for olefination reactions with ethyl diazoacetate in the presence of PPh3, leading to one-pot diazoacetate olefination starting from alkenes.
Owner:HONG KONG THE UNIV OF
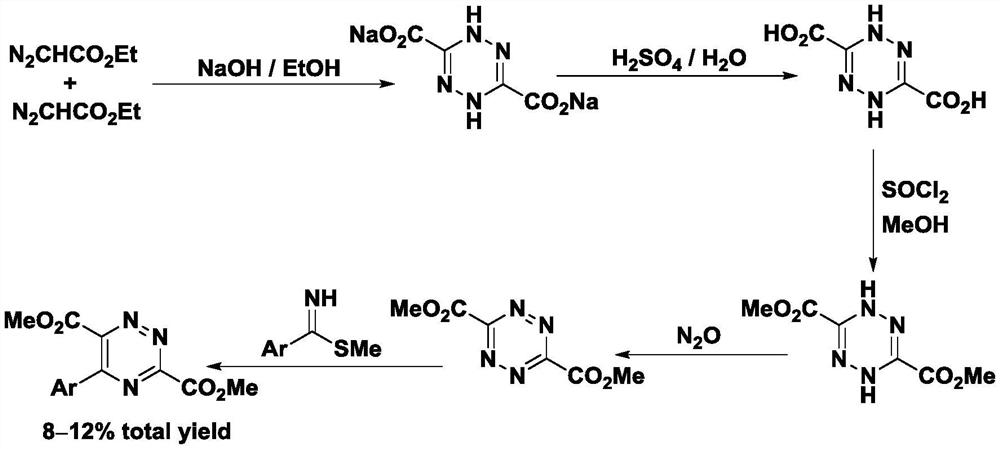
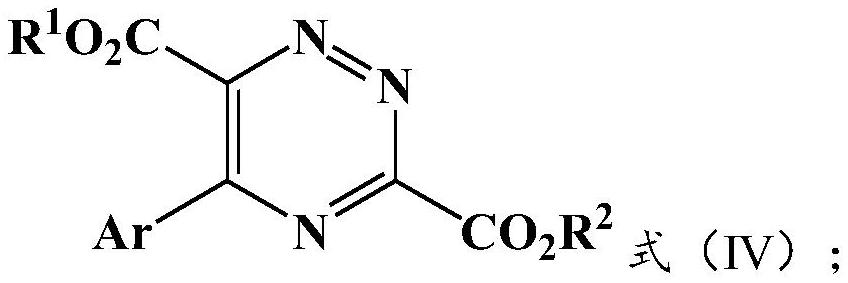
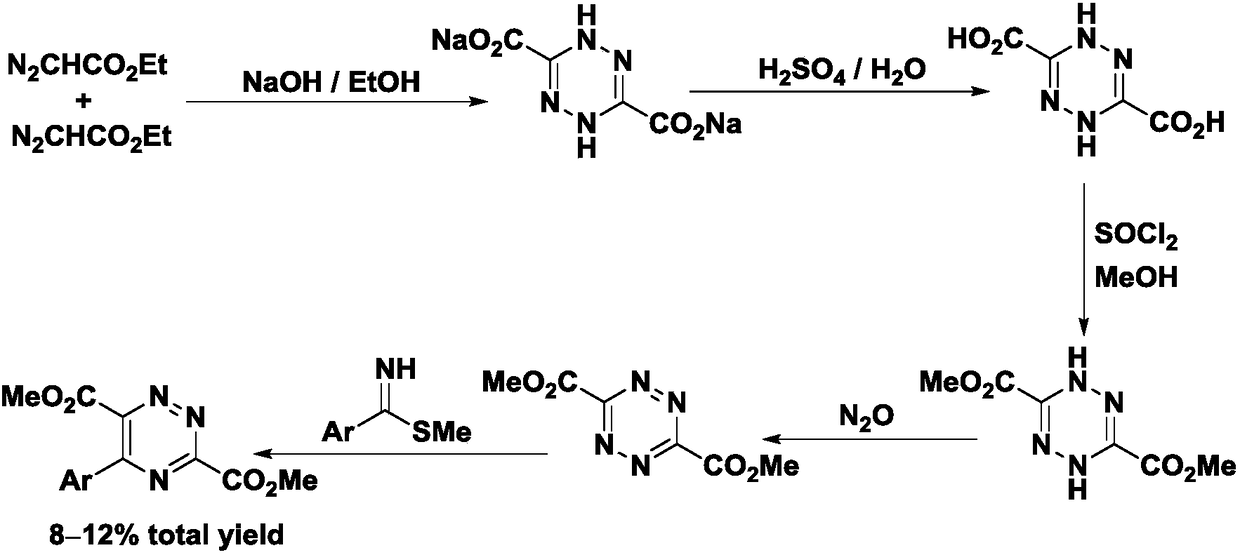
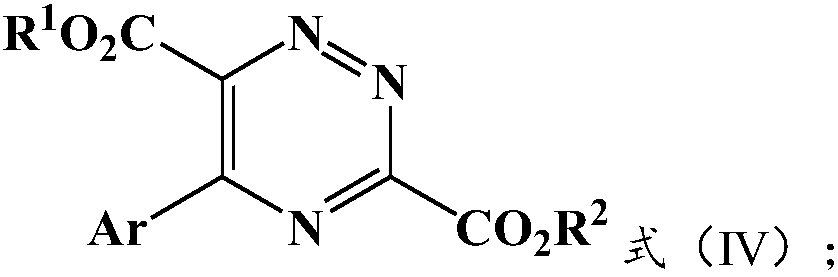
Preparation method and application of 5-aryl-1,2,4-triazazine-3,6-dicarboxylate
The invention provides a preparation method of 5-aryl-1,2,4-triazine-3,6-diformate. The method comprises the following steps: S1) allowing diazoacetate of the formula (I) to react with glycine ester aromatic aldimines of the formula (II) in the presence of a silver salt catalyst and an inorganic base, so that an intermediate represented by formula (III) is obtained; S2) mixing the intermediate represented by the formula (III) with an oxidizing agent to carry out oxidation reaction to obtain 5-aryl-1,2,4-triazine-3,6-diformate represented by formula (IV). Compared with the prior art, the methodhas the advantages of less reaction steps and relatively high area selectivity and yield.
Owner:TIANJIN UNIV
Preparation method of 5-aryl-1,2,4-triazine-3,6-diformate and application thereof
The invention provides a preparation method of 5-aryl-1,2,4-triazine-3,6-diformate. The method comprises the following steps: S1) allowing diazoacetate of the formula (I) to react with glycine ester aromatic aldimines of the formula (II) in the presence of a silver salt catalyst and an inorganic base, so that an intermediate represented by formula (III) is obtained; S2) mixing the intermediate represented by the formula (III) with an oxidizing agent to carry out oxidation reaction to obtain 5-aryl-1,2,4-triazine-3,6-diformate represented by formula (IV). Compared with the prior art, the methodhas the advantages of less reaction steps and relatively high area selectivity and yield.
Owner:TIANJIN UNIV
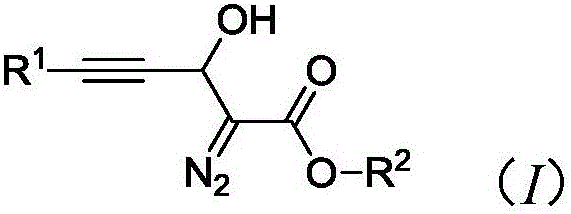
Synthesis method of 3-hydroxyl diazoester intermediate alkyne compound
The invention provides a synthesis method of a 3-hydroxyl diazoester intermediate alkyne compound. The synthesis method comprises the following steps: with a diazoacetate compound and various kinds of substituted propiolaldehyde as raw materials, synthesizing the 3-hydroxyl diazoester intermediate alkyne compound in a mild alkaline environment. The synthesis method provided by the invention has the advantages of simple operation, mild reaction conditions, cheap and available raw materials, wide reaction substrate adaptability, high product selectivity and yield, environmental friendliness and the like and has a good industrial application prospect.
Owner:HUBEI UNIV OF SCI & TECH
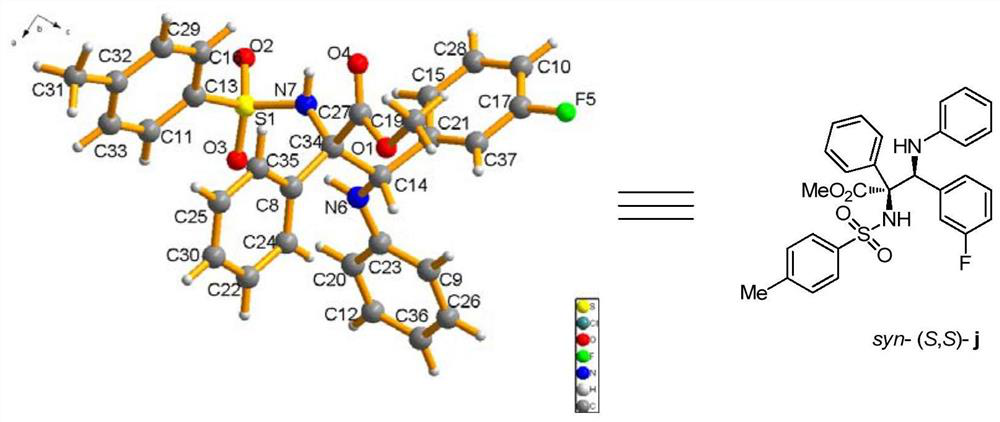
A kind of chiral sulfonamide derivative and its preparation method and application
ActiveCN109134402BMild reaction conditionsRapid responseSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationO-Phosphoric AcidEthylic acid
The invention discloses a chiral sulfonamide derivative, the structural formula is as follows, wherein, R is an aryl group, an alkyl group, a cycloalkyl group, a heterocyclic group, a substituted aryl group, a substituted alkyl group, or a substituted heterocyclic group; Ar 1 Aryl, substituted aryl; Ar 2 Aryl, substituted aryl, heterocyclic aryl; Ar 3 Aryl, substituted aryl, heterocyclic aryl; the present invention uses sulfonamide, aryl diazoacetate, imine as raw material, 4Å molecular sieve as water absorbing agent, rhodium acetate, chiral phosphoric acid to form a catalytic system, In an organic solvent, the chiral sulfonamide derivatives are obtained through one-step reaction. The synthesis method of the invention has the advantages of high atom economy, high selectivity and high yield, and has mild reaction conditions and simple and safe operation. The chiral sulfonamide derivatives with two quaternary carbon centers of the present invention are potential drug active molecules, are widely used in the field of medicine, and have great application prospects.
Owner:SUN YAT SEN UNIV
Fluorocarbon chain-free hydrophobic fabric as well as preparation method and application thereof
ActiveCN114427167AAvoid pollutionGood chemical repellencyLiquid repellent fibresVegetal fibresFiberPolymer science
The invention discloses a fluorocarbon-chain-free hydrophobic fabric and a preparation method and application thereof.The preparation method comprises the steps that a fabric is sequentially soaked in alkali liquor and acid liquor, and a pretreated fabric is obtained; then reacting the pretreated fabric with bromoacetyl bromide to obtain a treated fabric; reacting the treated fabric with 1, 2-bis (p-toluenesulfonyl) hydrazine to obtain a diazotized fabric; reacting the diazotized fabric with a diazoacetate monomer to obtain a fluorine-free carbon chain hydrophobic fabric; the diazoacetate monomer is butyl diazoacetate, hexyl diazoacetate, octyl diazoacetate, dodecyl diazoacetate, tetradecyl diazoacetate or octadecyl diazoacetate. According to the invention, diazoacetate is taken as a monomer, different fiber grafting modification processes are adopted, and structures with different properties are formed on the fiber surface; the comprehensive properties such as thermal stability, air permeability and breaking strength of the finished fabric are tested, the heat resistance and breaking strength of the finished fabric are reduced, and the air permeability is good.
Owner:SUZHOU UNIV
Method for synthesizing 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate
The invention discloses a method for synthesizing 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate. The method comprises the following steps: adding diazo acetate or a diazo acetate solution to a mixture of a catalyst, thiophene and a solvent in a dropwise mode under stirring at 0-140DEG C, and reacting until a gas is completely discharged; filtering to remove the catalyst, and distilling off excel thiophene and solvent; and finally separating to obtain 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate, wherein the catalyst is a supported Cu catalyst which comprises a Cu active component, an assistant and a carrier. According to the invention, the cheap supported Cu catalyst is adopted, application amounts of reactants are clarified, preparation conditions of the catalyst are regulated, and the assistant is added, so performances of the catalyst are changed, and the reaction selectivity is improved; and the catalyst has the advantages of easy preparation and low cost, cyclopropanation reaction conditions are mild, the reaction selectivity and the product yield are high, and the catalyst is easy to separate after the reaction. The method is suitable for massive synthesis of 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate.
Owner:DALIAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/475f2366-3302-4de2-a7f5-a5c681c9df33/F2009101883222C00011.PNG)
![Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/475f2366-3302-4de2-a7f5-a5c681c9df33/F2009101883222C00021.PNG)
![Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/475f2366-3302-4de2-a7f5-a5c681c9df33/F2009101883222C00022.PNG)


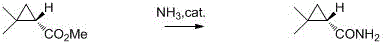
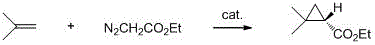
![The method for synthesizing 2-thiabicyclo[3.1.0]-3-hexene-6-carboxylate The method for synthesizing 2-thiabicyclo[3.1.0]-3-hexene-6-carboxylate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3867b3a9-bbb6-45d2-b081-de925d082169/FDA0000758317910000011.PNG)
![The method for synthesizing 2-thiabicyclo[3.1.0]-3-hexene-6-carboxylate The method for synthesizing 2-thiabicyclo[3.1.0]-3-hexene-6-carboxylate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3867b3a9-bbb6-45d2-b081-de925d082169/FDA0000758317910000021.PNG)
![The method for synthesizing 2-thiabicyclo[3.1.0]-3-hexene-6-carboxylate The method for synthesizing 2-thiabicyclo[3.1.0]-3-hexene-6-carboxylate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3867b3a9-bbb6-45d2-b081-de925d082169/GDA0000758317920000011.PNG)
![Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5a99fc4b-3eca-4459-98a0-9964be09f3b5/FSB00001045499000011.PNG)
![Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5a99fc4b-3eca-4459-98a0-9964be09f3b5/FSB00001045499000021.PNG)
![Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether Method for synthesizing 4-thio-bicyclo [3.1.0]-2-hexene-6-formic ether](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5a99fc4b-3eca-4459-98a0-9964be09f3b5/FSB00001045499000022.PNG)
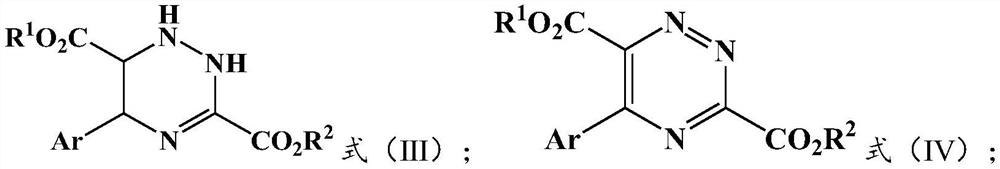
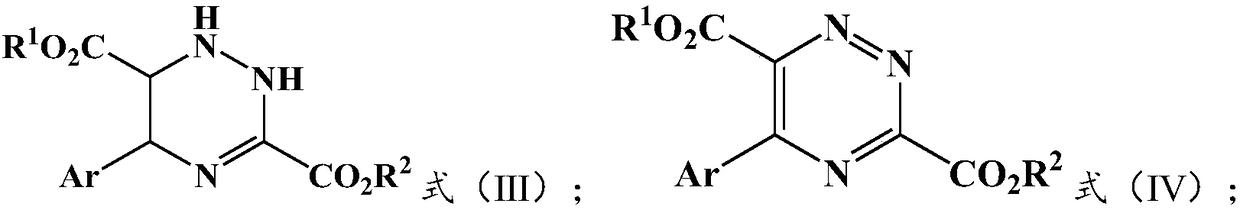
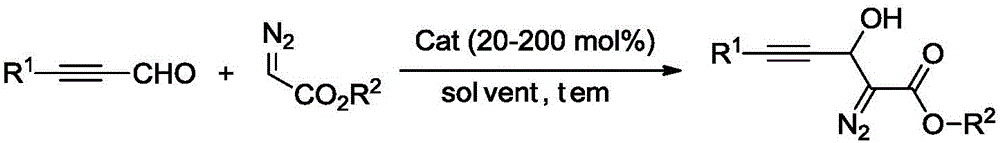
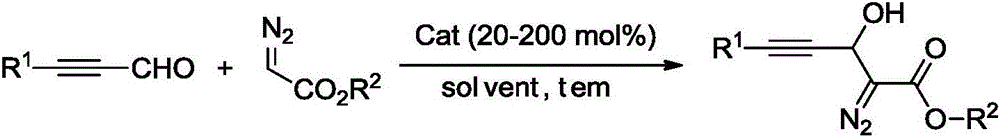
![Method for synthesizing 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate Method for synthesizing 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d33a3a0d-1556-437a-b1e3-fa641157c137/BDA0000045560590000011.PNG)
![Method for synthesizing 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate Method for synthesizing 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d33a3a0d-1556-437a-b1e3-fa641157c137/FDA0000045560580000011.PNG)
![Method for synthesizing 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate Method for synthesizing 2-thiabicyclo[3.1.0]-3-hexenyl-6-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d33a3a0d-1556-437a-b1e3-fa641157c137/FDA0000045560580000021.PNG)