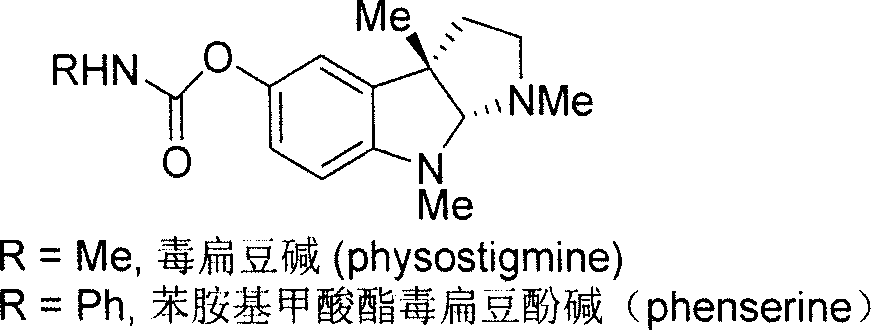
Synthesis for natural medicament physostigmine for resisting senile dementia disease and phenylaminoformic acid ester phenserine
A technology of phenol phenyl carbamate and physostigmine, which is applied in the field of synthesis of natural medicine physostigmine and its derivative anicarbamate physostigmine for anti-senile dementia, and can solve the problem of physostigmine Problems such as short action time, limited use of physostigmine, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The preparation of embodiment 1 compound 5
[0015]
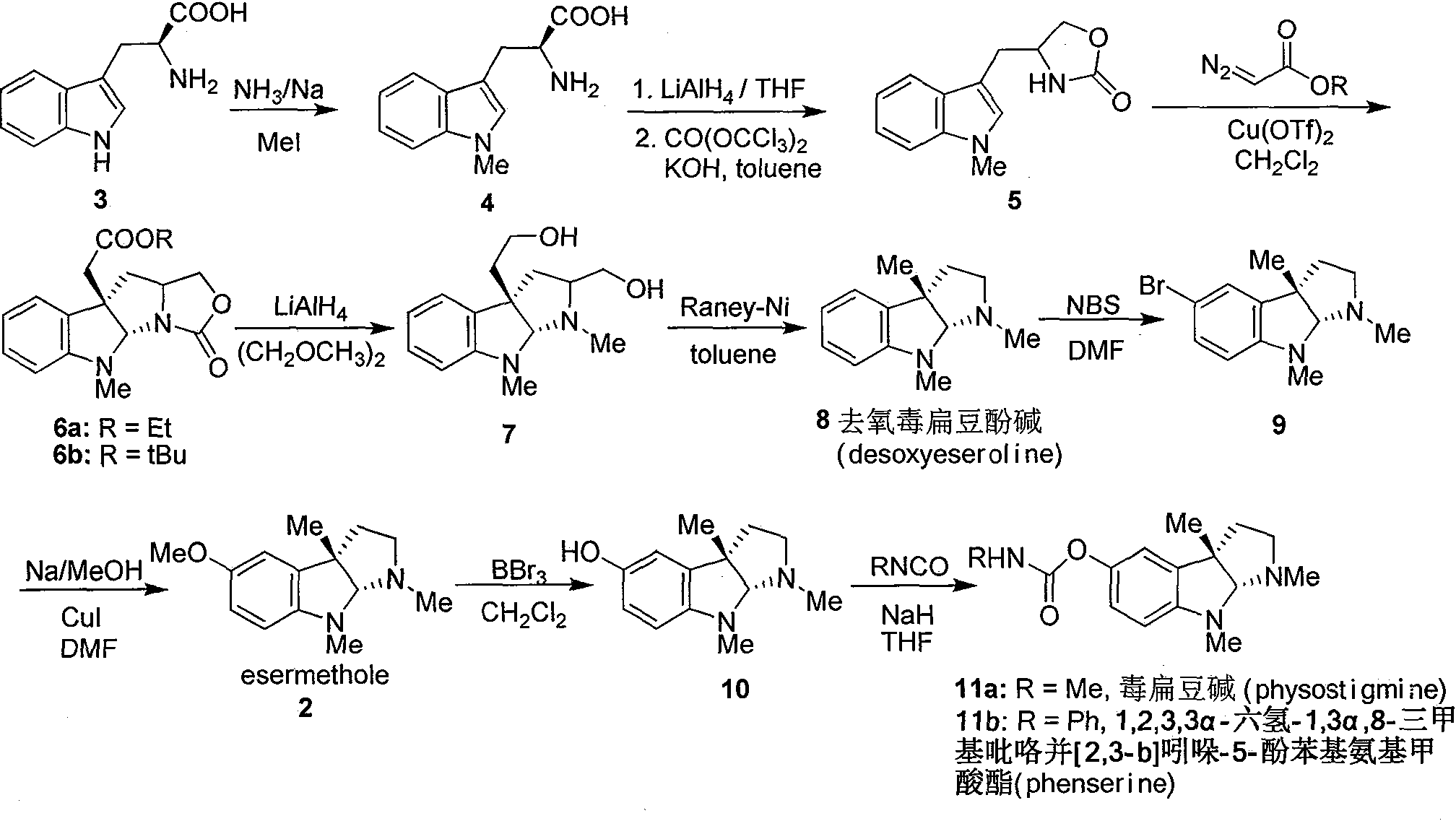
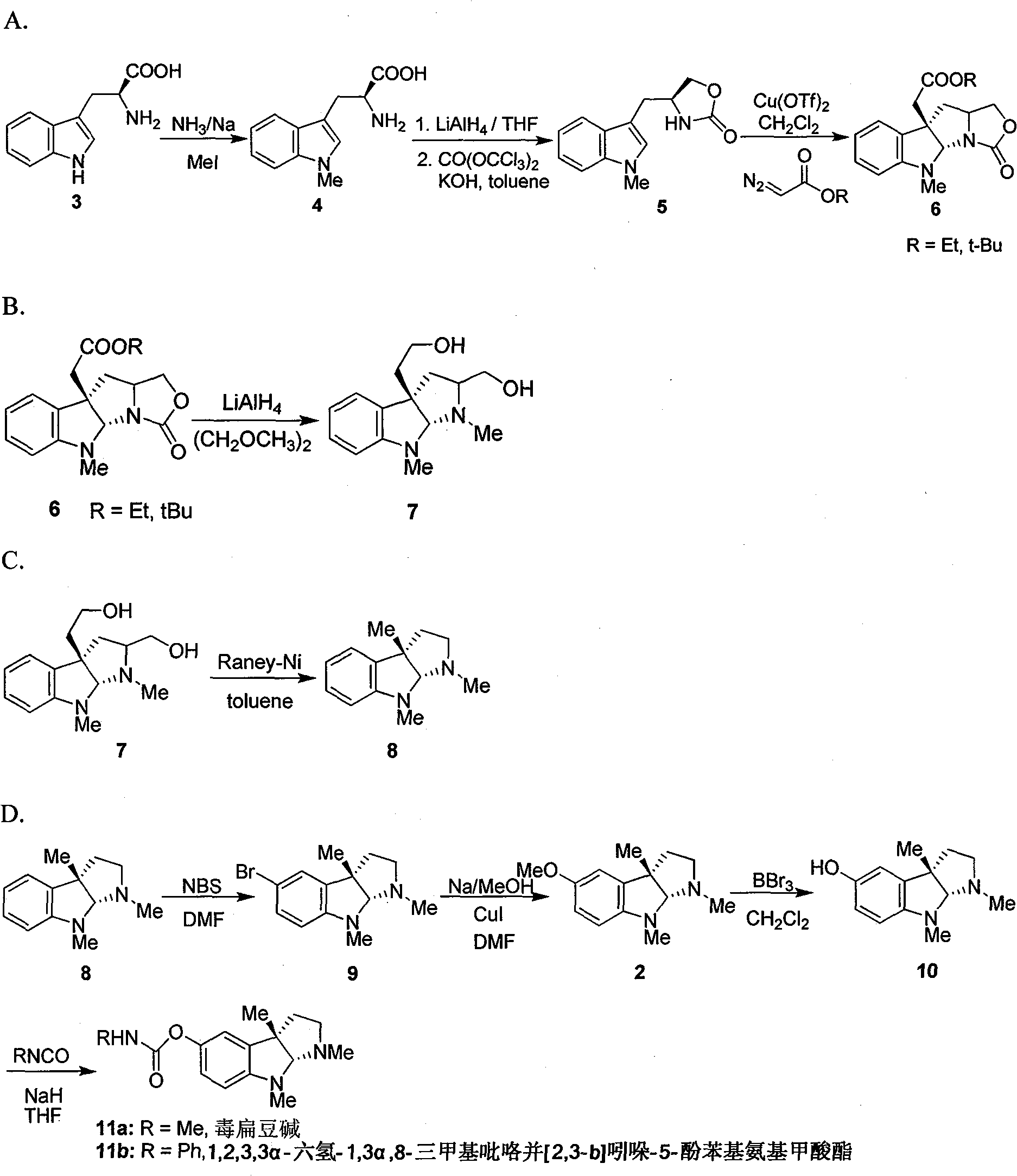
[0016] Add metal Na (3.5g, 0.15mol) into 150mL liquid ammonia, stir at -60°C until the metal Na completely disappears, add L-tryptophan 3 (20.4g, 0.1mol), stir at -60°C After 15 minutes, MeI (14.2 g, 0.1 mol) was slowly added dropwise, and after stirring at -60° C. for 2 h, the reaction solution was naturally warmed to room temperature. 100 mL of water was added to dissolve the reaction solution, and the pH was adjusted to 5 with glacial acetic acid. A large amount of white solids were precipitated, and the solids were filtered to obtain 20.1 g of crude compound 4. The crude product was dissolved in 150 mL of dry THF, and LiAlH was slowly added in batches under ice-water bath conditions. 4 (5.2g, 0.14mol), after the addition was complete, the reaction solution was stirred at room temperature for 0.5h. Add saturated Na 2 SO 4 solution until the white precipitate no longer increases. Remove the precipitate by f...
Embodiment 2
[0017] The preparation of embodiment 2 compound 6
[0018]
[0019] Compounds 6a and 6b were prepared by using compound 5 as a raw material according to the literature method, through intermolecular asymmetric cyclopropanation-ring-opening-ring and three-step one-pot waterfall reaction, and reacting with diazoacetate to prepare (Qin, Y.et al. Org. Lett. 2006, 8, 2187). 6a: [α] 20 D =-175° (c 1.0, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ7.15(td, J=7.6, 1.2Hz, 1H), 7.08(dd, J=7.6, 0.8Hz, 1H), 6.69(td, J=7.6, 0.8Hz, 1H), 6.42(d, J =8.0Hz, 1H), 5.38(s, 1H), 4.40(t.J=8.0Hz, 1H), 4.09-4.19(m, 3H), 3.74-3.77(m, 1H), 2.92(s, 3H), 2.80 (d, J=16.0Hz, 1H), 2.74(d, J=15.6Hz, 1H), 2.56(dd, J=12.0, 5.2Hz, 1H), 2.05(t, J=6.8Hz, 1H), 1.21 (t, J=7.6Hz, 3H)ppm.
[0020] 6b: [α] 20 D = -150° (c 1.0, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ7.16(td, J=7.6, 1.2Hz, 1H), 7.10(dd, J=7.2, 0.8Hz, 1H), 6.70(td, J=7.6, 1.2Hz, 1H), 6.43(d, J =7.6Hz, 1H), 5.41(s, 1H), 4.41(t, J=8.0Hz, 1H), 4.18(dd,...
Embodiment 3
[0021] The preparation of implementation example 3 compound 7
[0022]
[0023] LiAlH 4 (2.5g, 64mmol) was added to 50mL of dry ethylene glycol dimethyl ether solution, and after refluxing for 5 minutes, compound 6a (5.0g, 16mmol) or 6b (5.7g, 16mmol) was dissolved in 100mL of ethylene glycol dimethyl ether The solution was slowly added dropwise into the reaction solution, and after the dropwise addition was completed, it was refluxed for 10 minutes. After the reaction solution was cooled to room temperature, saturated Na 2 SO 4 solution until the white precipitate no longer increases. Remove the precipitate by filtration, wash the filter cake with 200 mL of ethyl acetate, combine the filtrate, separate the organic layer, extract the aqueous phase with ethyl acetate (3 × 100 mL), combine the organic phase, anhydrous Na 2 SO 4 Dry, filter, evaporate the solvent under reduced pressure, and the crude product is separated and purified by silica gel column (CH 2 Cl 2 :MeO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com