Bidirectional transition luminescent material and preparation method thereof
A luminescent material, bidirectional conversion technology, applied in luminescent materials, chemical instruments and methods, wave energy or particle radiation treatment materials, etc., to achieve the effects of good biocompatibility, clear imaging, and strong conversion fluorescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment one, it comprises the following steps:
[0026] 1) Dissolve 1ml of allyl acrylate and 1ml of ethyl diazoacetate in 50ml of toluene, and stir at room temperature for 16 hours.
[0027] 2) Nitrogen gas was passed through the reacted solution for one hour, and heated to 70° C., then 5 mg of azobisisobutyronitrile was added, and reacted at 70° C. for 12 hours.
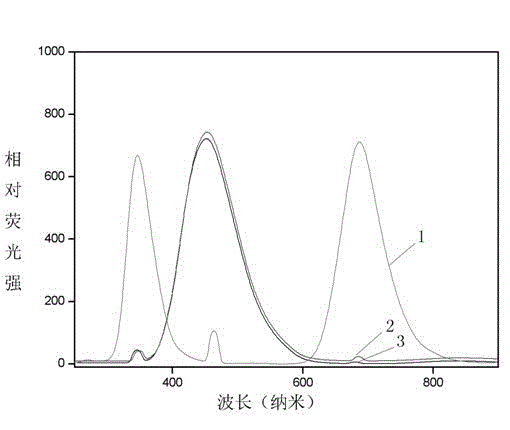
[0028] 3) The reacted solution was separated by column chromatography to obtain a polymer having a pyrazoline group in the side chain, which was dissolved in ethyl acetate to prepare a 1 mg / ml solution, and its fluorescence spectrum was measured.
Embodiment 2
[0029] Embodiment two, it comprises the following steps:
[0030] 1) Add 0.12 g of menthyl diazoacetate and 0.03 ml of acrylonitrile into 10 ml of chloroform, and stir at room temperature for 16 hours.
[0031] 2) After the solution after the reaction is rotary evaporated, the pyrazoline monomer containing menthol group is obtained by column chromatography separation
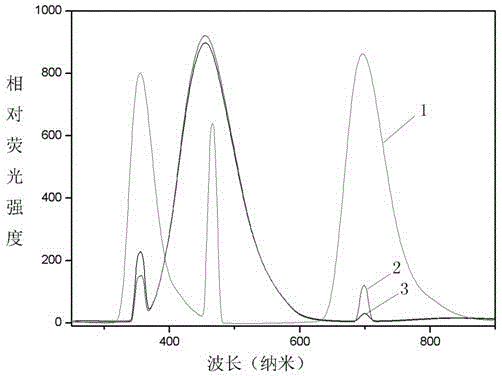
[0032] 3) The pyrazoline monomer in step 2 was dissolved in dimethyl sulfoxide to prepare a 7 mg / ml solution, and its fluorescence spectrum was measured.
Embodiment 3
[0033] Embodiment three, it comprises the following steps:
[0034] 1) Add 0.5 g of ethyl diazoacetate and 0.3 g of acrylonitrile into 10 ml of chloroform, and stir at room temperature for 16 hours.
[0035] 2) After the solution after the reaction is rotary evaporated, the pyrazoline monomer containing cyano group is obtained by column chromatography separation
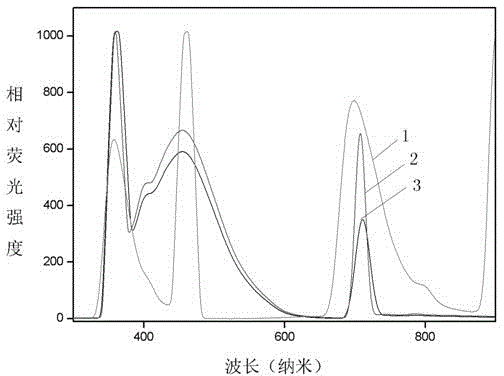
[0036] 3) The pyrazoline monomer in step 2 was dissolved in dimethyl sulfoxide to prepare a solution of 8 mg / ml, and its fluorescence spectrum was measured.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com